Abstract
Chromosome inversions have fascinated the scientific community, mainly because of their role in the rapid adaption of different taxa to changing environments. However, the ecological traits linked to chromosome inversions have been poorly studied. Here, we investigated the roles played by 23 chromosome inversions in the adaptation of the four major African malaria mosquitoes to local environments in Africa. We studied their distribution patterns by using spatially explicit modeling and characterized the ecogeographical determinants of each inversion range. We then performed hierarchical clustering and constrained ordination analyses to assess the spatial and ecological similarities among inversions. Our results show that most inversions are environmentally structured, suggesting that they are actively involved in processes of local adaptation. Some inversions exhibited similar geographical patterns and ecological requirements among the four mosquito species, providing evidence for parallel evolution. Conversely, common inversion polymorphisms between sibling species displayed divergent ecological patterns, suggesting that they might have a different adaptive role in each species. These results are in agreement with the finding that chromosomal inversions play a role in Anopheles ecotypic adaptation. This study establishes a strong ecological basis for future genome-based analyses to elucidate the genetic mechanisms of local adaptation in these four mosquitoes.
Keywords: chromosome inversions, local adaptation, Anopheles, parallel evolution, ecological divergence
Introduction
Chromosome inversions have been considered by pioneering geneticists as the fingerprints of evolutionary processes in many different species (Krimbas and Powell 1992; Hoffmann and Rieseberg 2008). Over the last century, theoretical and experimental works have positioned chromosome inversions as key actors of chromosome architecture, local adaptation, sex evolution and speciation (Coghlan et al. 2005; Feuk et al. 2005; van Doorn and Kirkpatrick 2007; Bhutkar et al. 2008; Hoffmann and Rieseberg 2008; Sharakhova et al. 2011). Evidence that natural selection acts on chromosomal inversions has been gathered in many taxa, from mice (Lyon 2003), humans (Stefansson et al. 2005), Drosophila (Hoffmann et al. 2004) and monkeyflowers (Lowry and Willis 2010) to mosquitoes (Fouet et al. 2012; Ayala et al. 2013). Evolutionarily, chromosomal inversions are important because they reduce and, consequently, substantially alter recombination in heterozygotes. This characteristic together with their size (spanning hundreds or even thousands of genes) facilitate capturing favourable combinations of locally adapted alleles. Indeed, recent theoretical models (Kirkpatrick and Barton 2006; Manoukis et al. 2008; Schaeffer 2008) and ecological evidence support their role in local adaptation (Coluzzi et al. 1979b; Rodriguez-Trelles et al. 1996; Coluzzi et al. 2002; Balanya et al. 2003; Hoffmann et al. 2004; Ayala et al. 2011; Ayala et al. 2014). Although other processes like limited gene flow and genetic drift can also explain inversion clines (Dobzhansky and Wright 1943), the finding that chromosome inversion frequencies change at different collection sites was the first hint that they could contribute to local adaptation (Dobzhansky and Sturtevant 1938). Specifically, the relative frequencies of paracentric inversion polymorphisms (i.e., when a segment of a chromosome arm, which does not include the centromere, breaks and is reinserted in the reverse orientation) change in concert with the variation of different biotic and non-biotic factors. This feature allowed studying the natural selection forces that drive the evolution of inversions towards fixation (i.e., inversion frequency close to 100%) or stable polymorphism (i.e., inversion frequency stable across time) (Kirkpatrick and Barton 2006; Feder et al. 2011). However, the molecular basis of the adaptive role of these inversions remains barely known. Kirkpatrick and Kern (Kirkpatrick and Kern 2012) wrote that “the money is on the inversions” to suggest where to find genes involved in local adaptation. Heliconius butterflies offer a compelling example of how an inversion captures and protect several adaptive loci linked to mimicry (Joron et al. 2011). In Drosophila, the classical animal model for studying chromosome rearrangements, inversion frequencies and some genes within the inversion vary along environmental clines (Umina et al. 2005; Collinge et al. 2006; Kolaczkowski et al. 2011; Kapun et al. 2016). In Anopheles gambiae, comparative genomic studies on carriers of the 2La inversion along an environmental aridity gradient led to the identification of divergent genes within the inversion (Cheng et al. 2012). These genes mainly encode signalling molecules, gustatory receptors or ion-channel genes. Interestingly, a remarkable correspondence between orthologues and their functions was observed between Drosophila and An. gambiae in comparable environmental clines, providing evidences of potential parallel evolution (Kolaczkowski et al. 2011; Cheng et al. 2012). Nevertheless, studies remain scarce. The main obstacle to phenotypic experiments lies in the difficulty of identifying the local adaptation drivers (ecological, behavioural, sexual, etc.) that model the inversion distribution in a population along a cline. For example, in sub-Saharan Africa, the An. gambiae 2La inversion frequency varies significantly along an aridity gradient, from complete absence in the humid rainforest of Central Africa to fixation in the arid savannas of West and East Africa (Coluzzi et al. 1985; Simard et al. 2009). In an attempt to validate the hypothesis that aridity tolerance is linked to this inversion, the thermal tolerance and desiccation resistance of 2La inversion carriers and non-carriers was tested in laboratory conditions. The results confirmed that 2La inversion carriers exhibit a higher desiccation and thermal resistance (Gray et al. 2009; Rocca et al. 2009; Fouet et al. 2012). Moreover, genome-wide expression analyses before and after exposure to thermal stress revealed that a large number of stress-linked genes were up-regulated in larvae carrying the inversion, compared with non-carriers (Cassone et al. 2011). However, only two of these up-regulated genes were in common with those found along the latitudinal cline (Cheng et al. 2012). Unfortunately, this is the only inversion where both phenotypic and molecular analyses have been carried out. Therefore, to understand the genetic mechanism of local adaptation, it is essential to identify the specific adaptive traits that shaped inversion frequencies in any species.
The availability of polytene chromosomes in Anopheles is a unique opportunity for studying chromosome evolution (Green and Hunt 1980; Coluzzi et al. 2002; Sharakhov et al. 2002; Sharakhova et al. 2006; Sharakhova et al. 2010a; Sharakhova et al. 2010b; Sharakhova et al. 2011; Sharakhova et al. 2013). In Africa, four Anopheles species have received most attention: An. gambiae, An. coluzzii, An. arabiensis and An. funestus. The first three species belong to the same complex, although An. gambiae and An. coluzzii have been only recently proposed as separate species (Coetzee et al. 2013). Until the development of molecular diagnostic tools in the 1990s, An. arabiensis was distinguished from the other sibling species in the Anopheles gambiae complex on the basis of post-zygotic barriers and five fixed inversions on chromosome X (Davidson 1964; Davidson and Hunt 1973; Coluzzi et al. 2002). These four species can thrive in a wide range of environments and live in sympatry in many sub-Saharan Africa regions (Ayala et al. 2009; Sinka et al. 2012). Their ecological plasticity and their anthropophilic habits (resting, feeding and breeding preferences) make them most efficient malaria vectors. Chromosomal rearrangements have been directly involved in Anopheles ecological and behavioural plasticity (Ayala et al. 2014). However, little is known about the specific environmental requirements of chromosomal inversions and how they promote local adaptation in these malaria mosquitoes.
In this study, we investigated, from a macro-ecological perspective, the role played by 23 chromosome inversion polymorphisms in the adaptation of the four major African malaria vectors (Anopheles gambiae, An. coluzzii, An. arabiensis and An. funestus) to local environments in West-Central Africa. We studied the distribution patterns of such inversion polymorphisms by using spatially explicit modelling, and characterized the ecogeographical determinants of each inversion range. We then performed hierarchical clustering and constrained ordination analyses to assess the spatial and ecological similarities of the 23 inversions. Our results show that most inversions are environmentally structured, suggesting that they are involved in processes of local adaptation to a wide range of habitats. The eco-geographical distribution of some inversions exhibited similar patterns among the four mosquito species, providing evidence for parallel evolution. Conversely, inversion polymorphisms that were considered to be common between sibling species displayed divergent ecological patterns, suggesting that they might have a different adaptive role in each species. These results are in agreement with the view that chromosomal inversions play a role in Anopheles ecotypic adaptation, by making them more easily adaptable and, therefore, more ubiquitous and potent malaria vectors.
Materials and Methods
Chromosome inversion frequency data
The full karyotypes of 35,618 mosquitoes were used in this study, among which 16% of specimens represented unpublished data (Table 1, Table S1–S4). In total, 23 polymorphic inversions in An. gambiae, An. coluzzii, An. arabiensis and An. funestus were reviewed and analysed (see Table 1). We characterized the inversions in each species, even if they were considered as common, to avoid potential biases in the analysis. Mosquito sampling was carried out in 27 countries in Africa and Saudi Arabia and included the dry savannahs of Senegal and South Africa, the Highlands of Kenya and Madagascar, and the rainforests of Cameroon and Ivory Coast (Figure 1). This vast territory is highly heterogeneous, thus increasing the genetic diversity of mosquito species (Lehmann et al. 2003; Michel et al. 2005).
Table 1.
Summary of the chromosome inversions in the four malaria vectors Anopheles gambiae, An. coluzzii, An. arabiensis and An. funestus.
| Specimens | Villages | Inversions | Inversions names | |
|---|---|---|---|---|
| An. gambiae | 7949 | 799 | 6 | 2La; 2Rb, 2Rc, 2Rd, 2Ru, 2Rj |
| An. coluzzii | 5000 | 528 | 5 | 2La; 2Rb, 2Rc, 2Rd, 2Ru |
| An. arabiensis | 12836 | 125 | 5 | 2Ra, 2Rb, 2Rc, 2Rd1; 3Ra |
| An. funestus | 9833 | 165 | 7 | 2Ra, 2Rh, 2Rab, 2Rt; 3Ra, 3Rb; 3La |
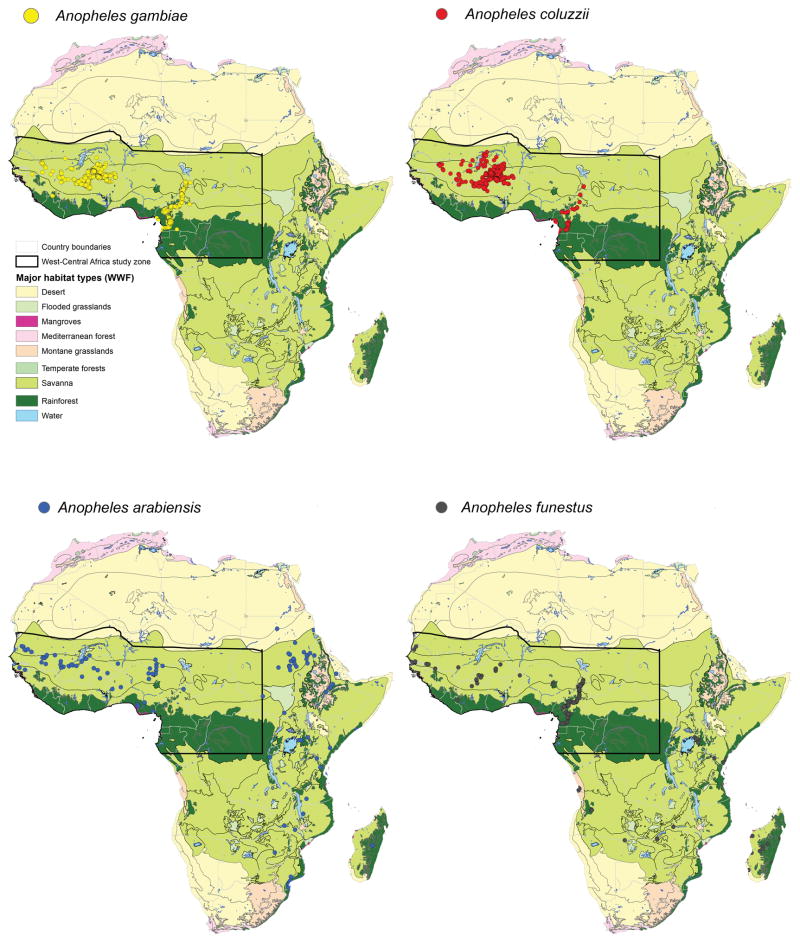
Figure 1. Sampling villages.
Map of the main African habitat types (Olson et al. 2001) showing the distribution of the villages where the four Anopheles species were sampled: Anopheles gambiae (yellow dots), An. coluzzii (red dots), An. arabiensis (blue dots) and An. funestus (grey dots). A detailed representation of the West-Central Africa area that was selected to plot the predicted maps and carry out the similarity analyses is shown. Species’ points out of the study area were used for calibration purposes (see Materials and Methods)
Mosquitoes were collected from 1979 to 2007. We obtained the frequencies of each inversion in each sampled locality (n=1,617 villages) and for each species (Table 1). Only localities providing the frequencies of all inversions included in this study were retained. Inversions in Anopheles are arbitrarily classified as standard or inverted. To avoid any potential bias, we included the frequencies of both inverted and standard forms in our analyses. The geographical coordinates of the original sampling localities were recorded using a hand-held GPS receiver. Unknown geographical coordinates of localities reported in the literature were validated through the National Geospatial-Intelligence Agency [http://geonames.nga.mil]. For modelling purposes, we used 5 km × 5 km grid squares as territorial units on the basis of the mosquito dispersal capabilities (Costantini et al. 1996; Ayala et al. 2013). Mosquito information was then assigned to the square in which the sampling village was located.
Karyotyping analysis was identical for the four mosquito species. Briefly, ovaries of half-gravid females were dissected and stored in Carnoy’s fixative solution (three parts 100% ethanol: one part glacial acetic acid, by volume). They were subsequently prepared according to standard protocols to obtain the polytene chromosomes (della Torre 1997). Paracentric inversions were identified and scored according to their respective chromosome maps (Coluzzi et al. 2002; Sharakhov et al. 2004). Only the most common polymorphic inversions were used to ensure sufficient statistical power for the subsequent analyses (Table 1).
Ecogeographical predictors
On the basis of their potential predictive power and according to previous studies on mosquitoes (Ayala et al. 2009; Costantini et al. 2009; Simard et al. 2009; Sinka et al. 2010), we selected 11 ecogeographical predictors as potential drivers of the distribution patterns of each chromosomal inversion. Predictors fell within three categories: spatial (four predictors), land (four predictors) and climate (three predictors). All information was transferred to the territorial units by using zonal statistics tools.
Spatial variables
Spatial predictors were investigated to uncover geographical trends in the distributions associated with historical events, or species – inversions – population dynamics (Real et al. 2003). We investigated four spatial predictors: latitude, longitude, the product of latitude and longitude, and distance to the equator (measured as the absolute value of latitude) of each territorial unit, to account for the data spatial structure (Legendre 1998; Kennington et al. 2006).
Land variables
The importance of land use in explaining insect distribution patterns is well known (Acevedo et al. 2010; Hortal et al. 2010), including for Anopheles species (Manoukis et al. 2008; Simard et al. 2009). Here, we considered the Normalized Difference Vegetation Index (NDVI) and its seasonal variations (Acevedo et al. 2010). NDVI is a measure of the amount and vigour of vegetation on the land surface directly related to soil moisture, and has been successfully used to highlight changes in land cover (Nicholson et al. 1990; Nicholson and Farrar 1994). The NDVIs were derived from a monthly imaging dataset (http://modis.gsfc.nasa.gov/data/dataprod/mod13.php) over a 13-year period, from 1998 to 2010, at a spatial resolution of ~1 km. Four different NDVI-derived variables were quantified on the basis of their importance in mosquito phenology (Clements 1999): yearly mean, yearly variation, wettest quarter mean (May to October), wettest quarter variation (May to October). Variations were quantified as the variation coefficients of yearly/quarterly means in the 13-year period. We selected the wettest quarter on the basis of mosquito population dynamics (Molineaux and Gramiccia 1980) and because mosquitoes are mainly captured during this season (Lindsay et al. 1998).
Climate variables
Three predictors were selected from the range of topoclimatic predictors associated with inversion distributions in Diptera: temperature, rainfall and elevation (Petrarca et al. 2000; Hoffmann et al. 2004; Ayala et al. 2011). In our study, the mean temperature and rainfall were quantified for the wettest quarter of the year, because this is the most important period for mosquito population dynamics (Moffett et al. 2007). Data on bioclimatic variables and altitude are available from the Worldclim project database (see (Hijmans et al. 2005) for details) at a spatial resolution of ~5 km.
Statistical Analysis
Ecogeographical models
Using an inductive approach we determined the macro-ecological requirements of the chromosomal inversions at the locations where they occurred (Corsi et al. 2000). We related the frequency of each inversion to the predictors using generalized linear models (GLM) with binomial distribution (number of inversions relative to the number of sampled mosquitoes per sampling locality, see below) and a logistic link function (Hosmer et al. 1989). To obtain the most parsimonious model for each polymorphic rearrangement we used a forward-backward stepwise model-selection procedure. All steps were assessed to decrease the Akaike Information Criterion (AIC) (Akaike 1974). Models were built on a subset of randomly selected sampling localities for each inversion (80%) and then projected to the whole study area. The remaining sampling localities (20%) were used as independent data to evaluate the predictive performance of the models. To this aim, calibration plots (Pearce and Ferrier 2000) and Pearson’s correlations were employed to statistically determine the relationship between the predicted probabilities and the observed frequencies (Zheng and Agresti 2000). Bins with n<15 specimens were not included in this evaluation, because this is the minimum sample size required to estimate a frequency with acceptable accuracy (Jovani and Tella 2006). We restricted the ecogeographical models to West-Central Africa to avoid the inclusion of sparsely sampled areas in eastern and southern Africa (Figure 1) where the uncertainty of the model predictions could not be properly assessed (Heikkinen et al. 2012). Finally, potential confounding effects between spatial and environmental factors can be expected in models of species spatial distribution (Dormann 2007). To rule out this modelling bias, we investigated spatial autocorrelation in the residuals by estimating the Moran’s I index (a measure of global spatial autocorrelation) for each model (Table S6).
Hierarchical clustering analysis
Clustering methods are a powerful tool for classifying similar objects in groups and are particularly useful for selecting species with similar biogeographical patterns (Kreft and Jetz 2010; Olivero et al. 2011) or with genetic/molecular similarities (Heard et al. 2005). We investigated similarities among the ecogeographical patterns of the polymorphic inversions in West-Central Africa. To carry out the hierarchical clustering analysis we randomly selected 1666 evaluation points in accordance with our sampling design (Legendre 1998), each of which was attributed with the probability of occurrence of each inversion according to the models’ predictions.
To avoid mismatching due to inversion arrangements (standard versus inverted), we used the absolute value of the Pearson’s correlation coefficient to estimate pairwise distances between the predicted probability of occurrence of each inversion. This index indicates similarity in shape between two (or more) profiles, and fits perfectly with the aim of clustering together inversions that respond similarly to ecogeographical gradients and species ranges. To maximize similarity within groups, we used the unweighted pair-group method with arithmetic averages (UPGMA) as agglomeration method. This produces less distortion from the original similarities than complete or single linkages and is consistently the best performing clustering algorithm for biogeographical classifications (Kreft and Jetz 2010). We then searched for chorotypes, defined as clusters of inversions the probabilities of occurrence of which are similarly distributed within the group and/or dissimilarly in relation to other inversions. Chorotype detection was performed as described by Olivero et al. (Olivero et al. 2011). A cluster was considered a quantitative chorotype if: (a) IH = 1; or else (b) IH was positive, had higher values than those of the other clusters including the distributions involved, and the proportion of “+” signs between the cluster and the most similar cluster was significantly lower than the proportion of “+” signs within the cluster (evaluated using a G-test of independence; (Sokal and Rohlf 1981)).
Canonical Ordination
The canonical correspondence analysis (CCA) is a statistical method for ordering species along canonical axes according to their (ecological) optima (Ter Braak 1987). We used this technique to relate the probability of inversion occurrence (on the evaluation points, Figure S2) with the set of ecological predictors (in this case, excluding spatial predictors to account only for ecological similarity). This allows for fairly easy ecological interpretation of inversion assemblages (Ayala et al. 2011). The statistical significance of the canonical axes and environmental predictors was assessed with permutation tests (10,000 times). To improve the CCA ecological comprehension, we plotted the major habitat boundaries according to (Olson et al. 2001). To determine the number of components to interpret from our CCA, we used the broken stick model (based on eigenvalues from random data) and the Kaiser-Guttman rule (based on the average value of eigenvalues) procedures (Jackson 1993).
All computational statistics were performed using the IBM-SPSS 19.0 software (IBM Corporation, New York, USA) and “R” v3.0.1 (R Development Core Team, http://cran.r-project.org/), with the addition of the “amap” (Lucas 2011), “vegan” (Oksanen et al. 2011), and ggvegan (https://github.com/gavinsimpson/ggvegan) libraries.
Results
Data on the inversions and Anopheles species retrieved from the literature and from our unpublished work and used for this study are summarized in Table 1, Figure 1 and Supplementary Material (Tables S1–S4).
Biogeographical patterns of chromosomal inversions
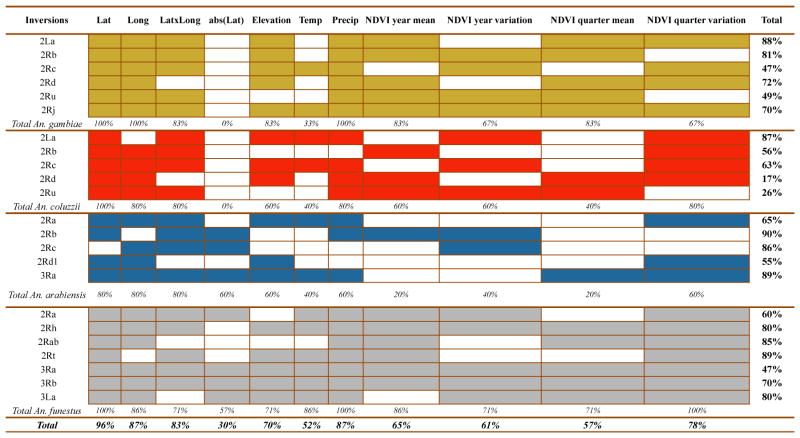
Using a generalized linear model approach, the frequency of each inversion was correlated with several ecogeographical predictors to calculate the inversion ecogeographical favourability throughout the study area (West-Central Africa, highlighted in Figure 1). Table 2 summarizes the predictors that were found to drive inversion frequency variations and the explained deviance (goodness-of-fit) for each model (see also Table S5). Despite the variability between models, latitude and precipitation were repeatedly the most important predictors of the inversion distribution patterns in the four mosquito species. Conversely, distance to the equator was the least significant variable in the final models. Overall, the models’ predictive performance was high. Indeed, predictions were highly correlated with the observed values in the datasets used for the independent validations (Table S5 and Figure S1). However in some cases (for instance, 2Rt in An. funestus), model performance was adequate according to the calibration plots, but could not be statistically evaluated due to insufficient independent data. Moreover, the inversion models did not exhibit a significant spatial autocorrelation bias as indicated by the Moran’s I value close to zero. This means that the residuals of the models for each inversion show a random spatial pattern (Table S6).
Table 2.
Summary of the models for each polymorphic inversion
Ecogeographical predictors selected in the final models for each inversion and Anopheles species. Species are coded by colour: An. gambiae (yellow), An. coluzzii (red), An. arabiensis (blue) and An. funestus (grey). Solid cells represent selected variables for each model according to AIC and open cells non-selected ones. The percentage of models in which each predictor was selected is indicated in the last row of each species. The last column represents the total explained deviance of the final model for each inversion. Lat: latitude; Long: longitude; Lat × Long: the product of latitude and longitude; abs(Lat): distance to equator expressed as absolute latitude value; Temp: mean temperature of the wettest quarter of the year; Precip: mean precipitation of the wettest quarter of the year; NDVI (normalized difference vegetation index) is numerical indicator that uses remote sensing measurements of live green vegetation (NDVI yearly mean, yearly variation, quarterly mean and variation and wettest quarter mean and variation for the period included in the study).
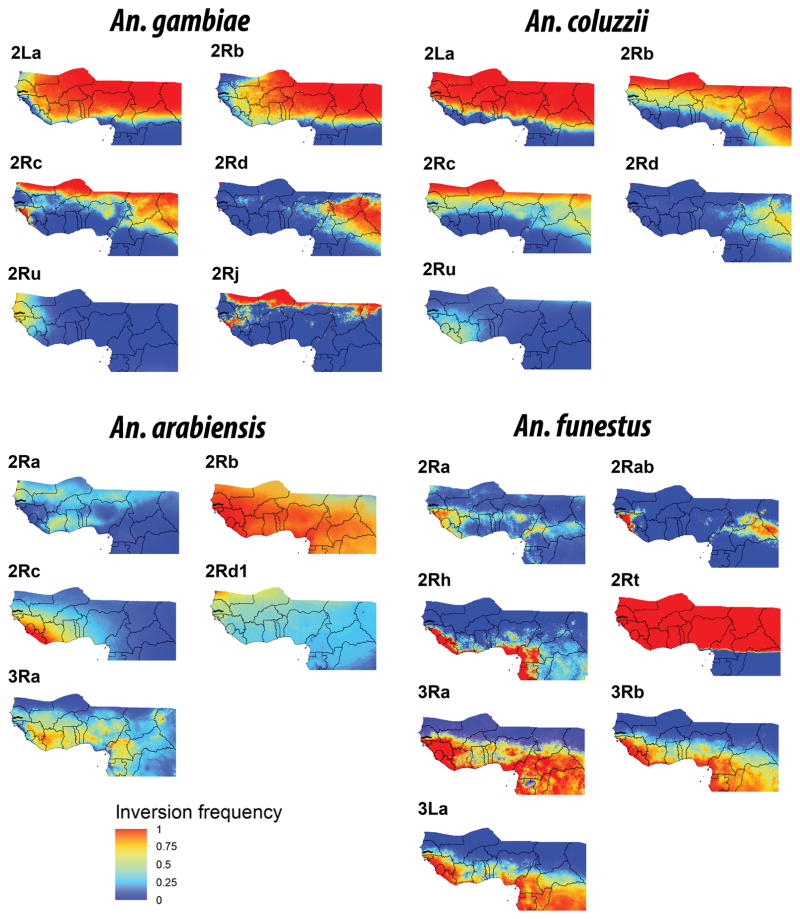
Next, the statistical models were represented in geographical space to obtain cartographic models of the expected probability of occurrence of a given inversion, on the basis of the local ecogeographical conditions (Figure 2). The predicted frequencies were subject to the local occurrence of each species and, accordingly, these maps should be interpreted as the predicted inversion frequency of the target species in a given locality.
Figure 2.
Maps of the predicted inversion frequencies for Anopheles gambiae, An. coluzzii, An. arabiensis and An. funestus. Predicted inversion distributions were passively plotted in the West-Central Africa study area. Blue represents a probability of 100% for the standard inversion, red represents a probability of 100% for the inverted inversion form, according to the literature data for each species (Green and Hunt 1980; Coluzzi et al. 2002). To improve their representativity, probabilities were reclassified in four classes: 0.00–0.25; 0.25–0.50; 0.50–0.75; 0.75–1.0.
Hierarchical clustering analysis
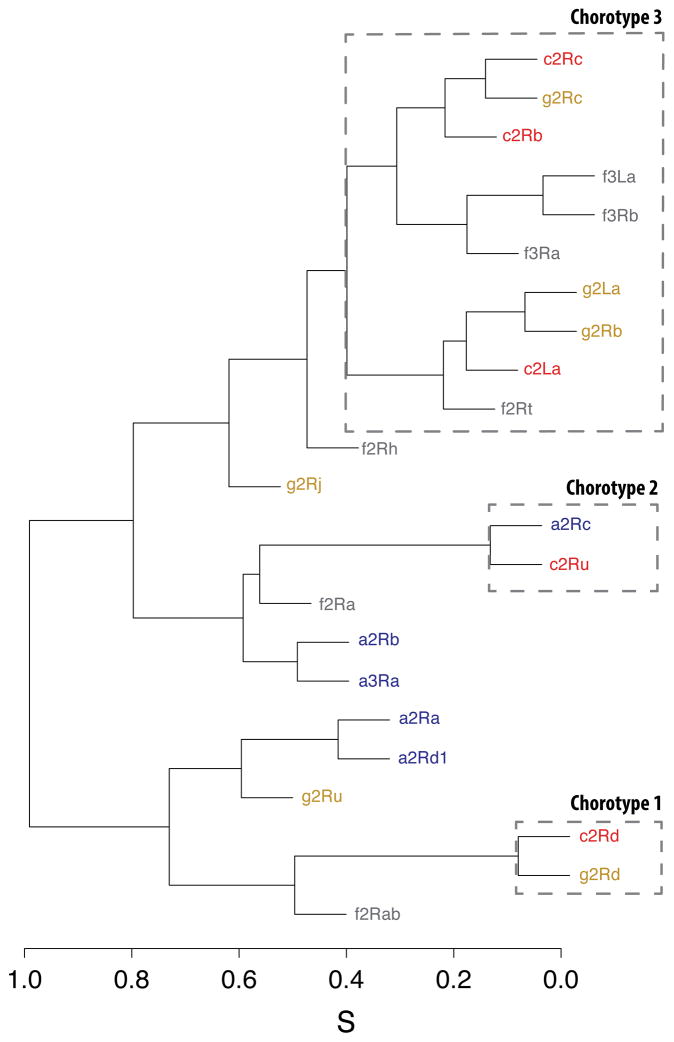
The predicted inversion frequencies throughout the study area were then correlated with each other to find similar eco-geographical patterns. Inversions that showed a significantly similar environmental distribution were grouped in chorotypes. We identified three significant inversion chorotypes in the study area (Figure 3). The first chorotype contained the 2Rd inversion in An. gambiae and An. coluzzii; the second contained the inversions 2Ru in An. coluzzii and 2Rc in An. arabiensis; the third comprised the inversions 2La, 2Rb and 2Rc in An. gambiae and An. coluzzii, and 2Rt, 3Ra, 3Rb and 3La in An. funestus. Altogether, the dendrogram highlighted important features concerning the inversion spatial patterns. Overall, chromosome inversions in An. gambiae and An. coluzzii showed a significant common spatial pattern. Moreover, chromosome inversions in An. funestus, An. gambiae and An. coluzzii were associated with similar environmental clines, revealing the presence of significantly correlated eco-geographical patterns. Finally, some inversions exhibited a very specific spatial pattern, such as 2Rj in An. gambiae.
Figure 3. Dendrogram of the predicted inversion frequency distribution in West-Central Africa showing similar environmental patterns in Anopheles species.
Inversions included in the three chorotype clusters (Chorotype 1, Chorotype 2 and Chorotype 3) are enclosed in squares. Anopheles spp. are coded by letters and colours: g (yellow): An. gambiae; c (red): An. coluzzii; a (blue): An. arabiensis; f (grey): An. funestus. In x-axis, S corresponds to the Baroni-Urbani and Buser similarity index (Olivero et al. 2011). Chorotype 1 and Chorotype 2 have both a IH =1, while Chorotype 3 has a IH = 0.822 (G-test, p-value<0.001) (Olivero et al. 2011).
Environmental drivers of inversions
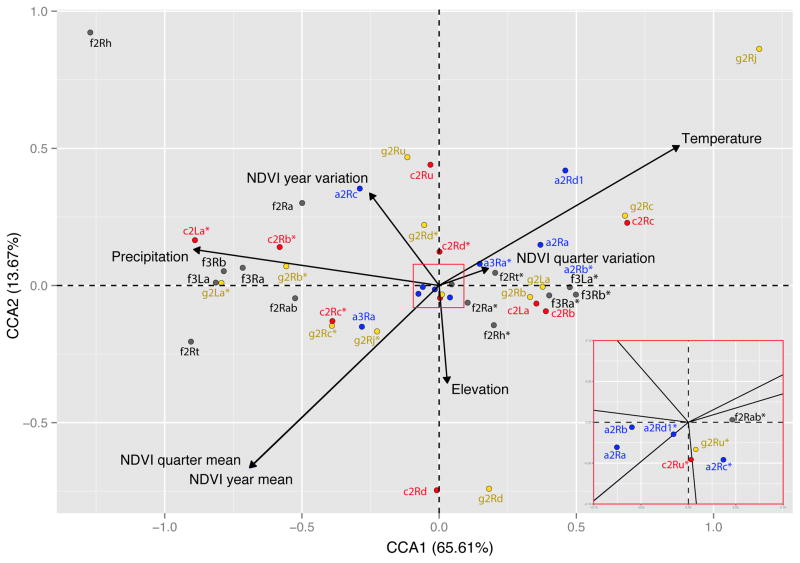
The maximum correlations between the set of land and climate predictors (spatial predictors were excluded to account only for ecological similarity) and the inversion frequencies were then determined using a canonical correspondence analysis (CCA) method. This statistical approach allowed us to define the ecological optimum of each inversion and to represent the overall contribution of the tested ecological predictors to the chromosomal polymorphism frequency among species (Figure 4). The seven CCA axes were all statistically significant (ANOVA, p< 0.001). In accordance with the broken stick model and the Kaiser–Guttman rule, only the first two CCA axes, which accounted for almost 80% of the total variance in the inversion dataset, were retained (Jackson 1993). The first axis explained 65.61% (ANOVA, F=1219, p<0.001) and the second 13.67% of the total variance (ANOVA F = 254, p<0.001). The other five CCA axes represent 10.3%, 6.1%, 2.6, 0.9% and 0.7%, respectively. The significance of all tested ecological predictors was assessed by permutation tests (ANOVA, p<0.001). The first axis could be ascribed to an aridity gradient (Figure S2A) and structured chromosomal inversions according to their tolerance to aridity, relative to the habitat boundaries. The second CCA axis represented an environmental gradient mostly influenced by vegetation productivity patterns and elevation (Figure S2B). Here, inversions with an ecological optimum in the savanna biome showed the highest frequency variations.
Figure 4. Canonical correspondence analysis of the inversion ecological distribution throughout West-Central Africa to highlight local adaptation patterns among chromosome inversions and mosquito species.
CCA diagram showing the ordination of the chromosomal inversions (standard and inverted; red crosses indicate their ecological optima) for each species along the first two canonical axes (CCA1 and 2) that, together, explain ~80% of variance. Anopheles spp. are coded by letters and colours: g (yellow): An. gambiae; c (red): An. coluzzii; a (blue): An. arabiensis; f (grey): An. funestus. Asterisks represent the standard form of each inversion. Ecological predictors are passively plotted on the graph: elevation, temperature (mean temperature of the wettest quarter of the year), precipitations (mean precipitations of the wettest quarter of the year) and NDVI variables (yearly mean, yearly variation, quarterly variation and wettest quarter variation for the period included in the study). An inset of the main Figure is provided in the bottom-righ corner for clarity purposses, showing the inversions encompassed in the red square.
Discussion
This study elucidates the ecological basis of the spatially structured distribution of 23 chromosomal inversions in the four major malaria vectors in Africa. Three major outcomes emerge: i) each chromosome inversion shows a specific and unique eco-geographical pattern, possibly explaining how mosquito species can extend their habitat ranges; ii) some of the inversion polymorphisms in the four mosquito species exhibit common ecogeographical patterns, suggesting that they are involved in the adaption to similar local pressures; and iii) some inversion polymorphisms, presumably shared by sibling species, exhibit contrasting ecological patterns, suggesting a different adaptive role. Altogether, our results establish a strong ecological basis for future genomic studies to elucidate the genetic bases of inversion contribution to local adaptation in these four malaria vectors.
The role of inversions in environmental adaptation
Chromosome inversions have often been considered as major drivers in the geographical expansion of many species (Krimbas and Powell 1992), including An. gambiae, An. coluzzii, An. arabiensis and An. funestus. Our study shows that the frequency of most of the studied inversions could be explained by the tested ecogeographical gradients (Table 2). An important novelty of our study is that we could compare and pull together not only different inversions from the same species but among species. This fact allow to expand our knowledge about inversions, permitting to interpret patterns between alternative arrangements and inversions. Only for two inversions (2Rd and 2Ru in An. coluzzii), the models could not explain their ecogeographical variation (only 17% and 26%, respectively). On the other hand, each main ecogeographical gradient had a different weight on each inversion (Table 2, Figure 2). The CCA identified the major environmental variables that influenced the distribution of each inversion and provided evidence that chromosomal rearrangements are linked to different environmental gradients (Figure 4). To date, chromosomal rearrangements have been exclusively linked to specific ecogeographical predictors, such as latitude (Hoffmann and Rieseberg 2008), but our analyses provide a broader picture of the habitats where these inversions play an important role for local adaptation. For instance, inversion 2Rd in An. gambiae and An. coluzzii plays a robust role in the adaptation to elevation. It is not the first time that an inversion has been associated with altitudinal clines (Collinge et al. 2006). Unfortunately, previous studies on the risk of malaria transmission at high altitude in Africa did not characterize the chromosome polymorphisms of the adapted populations (Tchuinkam et al. 2010). However, the major ecological challenge for these four mosquito species is the adaptation to humid conditions. Many of the inversions in An. coluzzii, An. gambiae and An. funestus are thought to have a savanna origin (Ayala and Coluzzi 2005; Ayala et al. 2009; Kamali et al. 2014) and have been linked to local acclimatization to rainforest habitat (Figure 4, Figure 3 – Chorotype 3). At least for An. coluzzii and An. gambiae, breakpoints analyses have confirmed that species with the inverted 2La karyotype (arid savanna) originated from the introgression from the arid adapted species An. arabiensis (White et al. 2009b), predate species with the standard 2La karyotype (forest) (Sharakhov et al. 2006; Fontaine et al. 2015). This is in agreement with the crucial role played by these rearrangements in malaria expansion to rainforest habitats (Annan et al. 2007). Similarly, inversion 3Ra in An. arabiensis appears to be associated with the adaption to the mosaic forest-savannah biome. Despite the many evidences, we cannot conclude that all the observed inversion distribution patterns have an exclusive ecological basis. Indeed, in Anopheles, like in other organisms, chromosomal inversions have been correlated also with non eco-geographical factors (Hoffmann and Rieseberg 2008), such as resting behavior (Coluzzi et al. 1977; Bryan et al. 1987; Costantini et al. 1999), host preference (Coluzzi et al. 1979b; Petrarca and Beier 1992), insecticide resistance (Brooke et al. 2002) and Plasmodium infection (Petrarca and Beier 1992). Therefore, although ecological forces seem to be the major drivers of inversion distribution (Table 2), other behavioural and/or physiological traits could have a non-negligible effect on their frequency patterns. Another plausible explanation is that demographic forces are responsible for the clinal patterns observed. However, several factors lead us to think that clinal variation is related to the action of natural forces (Endler 1977). Firstly, many inversions exhibit parallel clines across the continent (Table S1–S4). For instance, in An. gambiae, Simard et al., (Simard et al. 2009) and Coluzzi et al., (Coluzzi et al. 1979a) found identical clinal patterns in Cameroon and Nigeria, respectively. Secondly, another important factor is migration. Strong gene flow between populations would quickly make dissappear any inversion gradient in absence of selection. According to neutral genetic markers, An. gambiae/An. coluzzii and An. funestus exhibit parallel genetic structure across Africa coherent with a common expansion (Lehmann et al. 2003; Michel et al. 2005). Both continental studies highlight the strong gene flow between natural populations of these mosquitoes, denoting weak population structure. On the other hand, scattered countrywide studies in An. arabiensis revealed as well important gene flow between populations of this vector (Donnelly et al. 1999; Simard et al. 2000). Finally, the large effective population size in all these mosquitoes reinforce the assumptions of one panmictic population through the continent with limited gene flow barriers (Donnelly et al. 1999; Lehmann et al. 2003; Michel et al. 2005). Overall, these evidences are the strongest indications that environmental selection forces, and not demographic forces, are responsible for the observed environmental gradients of most of our inversions.
A key feature in the evolution of chromosome polymorphisms is the additive (i.e., the sum) or epistatic (i.e., the interaction) effect of inversions (Schaeffer et al. 2003). Our hierarchical and ordination analyses revealed common environmental patterns across inversions within species (i.e., 2Rb and 2La in An. gambiae and An. coluzzii; 3Ra, 3Rb and 3La in An. funestus). The existence of ecotypes– chromosomal inversion forms – within An. gambiae (Coluzzi 1982) and An. funestus (Costantini et al. 1999) has been determined on the basis of stable combinations of polymorphic rearrangements. These combinations exhibit strong linkage disequilibrium (LD) and are persistent in different habitats (Costantini et al. 2009; Simard et al. 2009; Ayala et al. 2011). Theoretical models (Schaeffer 2008; Burger and Akerman 2011) and empirical evidence from Drosophila (Schaeffer et al. 2003) and Heliconius (Joron et al. 2011) support the hypothesis that epistatic effects among inversions may maintain LD in a heterogeneous environment. However, epistatic interactions between inversions will certainly interfere with the model assumption that inversion frequencies depend on ecogeographical variables, but not on the frequency of other inversions. Unfortunately, our dataset did not provide information on the entire karyotype to enable us to build models with different sets of inversions and therefore to investigate the recombinant fitness for each biome, as already done in Heliconius (Le Poul et al. 2014). On the other hand, the high performance of most of our models reveals that if an epistatic effect exists, it should be less important that the environmental conditions. Nevertheless, it would be important to investigate whether and how these inversions in An. gambiae, An. coluzzii, An. arabiensis or An. funestus work together to implement fitness in different habitats.
Same patterns, same causes: parallel chromosome evolution in Anopheles
The hierarchical clustering analysis showed that chorotype 3 included inversions from three different species: An. gambiae, An. coluzzii and An. funestus. This cluster can be interpreted as a biogeographical pattern with strong internal similarity. Moreover, the CCA confirmed that these inversions have similar ecological gradients (Figure 4). These three species live in sympatry throughout much of their geographical range in Africa (Gillies and Coetzee 1987). In An. gambiae and An. coluzzii, the origin of the inversions 2La, 2Rb and 2Rc predates their divergence (White et al. 2009a; Lawniczak et al. 2010; Fontaine et al. 2015); therefore, we could expect similar patterns among their shared ancestral polymorphisms. On the other hand, An. funestus and An. gambiae diverged ~35 Mya (Krzywinski and Besansky 2003; Neafsey et al. 2015). Therefore, any common spatial pattern could be attributed to similar environmental pressures (Ayala et al. 2009; Sinka et al. 2010). One hypothesis is that these inversions captured similar sets of genes. Several authors found nearly perfect synteny preservation between arms when analysing whole chromosome arms (Sharakhov et al. 2002; Neafsey et al. 2015). This means that if inversions captured similar sets of genes, they should be on homologous arms. In a key paper, Sharakhova et al. (Sharakhova et al. 2011) investigated the non-random distribution of genes along homologous arms of malaria vectors. They found significant non-random gene combinations on An. gambiae 2Rb and on An. funestus 2Rh and 2Rd. Here, we found that inversions 2Rb and 2Rh are both strongly associated with rainforest habitat (Figure 2 and Figure 4). Unfortunately, the 2Rd inversion frequencies in An. funestus were too low and uneven to be included in the analysis. However, this rearrangement has been consistently associated with forest areas in Cameroon (Cohuet et al. 2005). Therefore, environmental adaptation to very humid conditions could have preserved specific gene combinations within these three inversions in the two species. Moreover, Sharakhova et al. (Sharakhova et al. 2011) found an almost significant (p = 0.07) association between 2La in A. gambiae and 3Rb in An. funestus. The very similar patterns of the main environmental gradients for 2La and 3Rb in the study area (Figure 2 and Figure 4) indicate their common role in adaptation to an aridity cline. However, these two inversions, together with 3Ra in An. funestus, show limited co-linearity, leaving strong doubts that they captured comparable large blocks of genes (Sharakhov et al. 2002). Nevertheless, we cannot exclude the possibility that smaller segments and/or few genes are shared within the inversions 2La and 3Ra/3Rb. Theoretically, the inversion would only need to capture one locally adapted locus to increase its frequency (Kirkpatrick and Barton 2006). The availability of the An. gambiae, An. coluzzii and An. funestus genomes will make possible to identify genes common to these species (Neafsey et al. 2015). In conclusion, despite the fast evolution of autosomal arms and gene shuffling, natural selection may preserve specific gene combinations within polymorphic inversions, particularly in distant species, such as An. gambiae-An. coluzzii and An. funestus, that are subject to similar environmental pressures. Thus, the most plausible scenario is that inversions in these species occurred independently (i.e., parallel evolution) and captured locally adapted genes in homologous arms. This knowledge may be very useful for identifying the gene signatures of local adaptation in these malaria vectors (Neafsey et al. 2015).
Common, but ecologically divergent inversions
Our analyses revealed some degree of ecogeographical divergence among hypothetically common inversions in species of the An. gambiae complex. Although Anopheles arabiensis, An. gambiae and An. coluzzii are characterized by different fixed chromosomal rearrangements on the X chromosome, they potentially share three chromosome inversions: 2La, which is fixed in An. arabiensis and polymorphic in An. gambiae and An. coluzzii, as well as 2Rb and 2Rc, which are polymorphic in all three species (Table 1, (Coluzzi et al. 2002)). Our predictive models, dendrogram and CCA revealed very different ecogeographical distribution patterns for 2Rb and 2Rc between An. gambiae/An. coluzzii and An. arabiensis (Figure 2, Figure 3, and 4; Table 2). The most plausible explanation is that the ecological and local adaptation patterns (and thus, captured genes) of these inversions are not the same or have rapidly evolved after speciation among mosquito species. Originally, they were characterized by the presence of a banding pattern (Coluzzi et al. 2002) and therefore, a potential mismatch in breakpoint recognitions is highly possible. Moreover, there is extensive evidence for breakpoint reuse and inversion recycling in other Diptera (Ranz et al. 2007). Indeed, differences in gene expression patterns might be expected if breakpoints are different between species (Puig et al. 2004). No molecular information is available for 2Rc. On the other hand, 2Rb breakpoints have been molecularly characterized in recent years. Lobo et al. (Lobo et al. 2010) argued that the homozygous 2Rb inverted form has a single common origin in all three sibling species, while the homozygous standard form (2Rb+) may have arisen twice through breakpoint reuse. In our models, the inversion 2Rb+ shows contrasting ecological patterns in An. gambiae-An. coluzzii and An. arabiensis. On the basis of molecular data, White et al. (White et al. 2009b) hypothesized that introgression from An. arabiensis brought the 2Rb arrangement into An. gambiae, while introgression in the opposite direction introduced 2Rb+ into An. arabiensis. The occurrence of the last introgression was supported by Fontaine et al., (Fontaine et al. 2015), who confirmed the ancestral status of the standard 2Rb+ form. Recent statistical models have reinforced the hypothesis that inversions can act as “cassettes of genes that can accelerate adaptation by crossing species boundaries” (Kirkpatrick and Barrett 2015). On the basis of our results, we hypothesize that a new 2Rb+ inversion might have appeared through breakpoint reuse in An. gambiae after speciation of An. arabiensis (Lobo et al. 2010). This new 2Rb+ could have brought new mutations that favoured (together with other inversions, see above) the colonization of rainforest habitats by An. gambiae/An. coluzzii (Sharakhov et al. 2006). New molecular and phenotypic analyses of the inversion 2Rb+ across species and along their geographical range might confirm this last hypothesis.
Conclusions and implications for malaria control
To date, vector control is the main approach for reducing malaria transmission (Enayati and Hemingway 2010). However, environmental and behavioural diversity within vector populations constitute serious challenges to the efficacy of any malaria control strategy (Ferguson et al. 2010). Much efforts is now required to understand the adaptive mechanisms of vector species for improving such control strategies (Boete 2005; Windbichler et al. 2011).
Our study reveals the potential roles played by inversion polymorphisms in the ecological success of the four major malaria mosquitoes. The importance of chromosome inversions in adaptation is attested by the strong, significant correlations between their frequencies and ecogeographical predictors, and by the strong, spatially-structured patterns identified in the study area. Therefore, inversion polymorphisms may have enabled considerable geographical expansion of these mosquitoes, with major consequences for malaria parasite transmission. Moreover, the extensive reshuffling of gene orders confirms, to some extent, that this type of chromosomal rearrangement is very common and a frequent local adaptation mechanism in Anopheles (Kamali et al. 2012). On the other hand, the converging evolution of inversions in An. gambiae, An. coluzzii and An. funestus may provide a suitable basis for comparative studies to identify the genes responsible for environmental adaptation. Understanding the genetic mechanisms that enable these vectors to extend their geographical range will have a profound impact on malaria epidemiology. The complete genome of An. funestus (Besansky 2008; Neafsey et al. 2015) will now provide considerable opportunities for comparative genomic studies with An. gambiae and will help elucidating the common mechanisms involved in ecological adaptation. Finally, besides the adaptive role of inversions, the recognition of ecological divergence within populations of insect vectors has a direct impact on the efficacy of any vector-borne disease control strategy (Ferguson et al. 2010). Ecological and behavioural diversification within species of the An. gambiae complex have expanded malaria transmission spatially and temporally, compromising the efficacy of malaria control efforts (Molineaux and Gramiccia 1980). Surveillance of genetic and ecological divergence within vector populations will ultimately lead to more effective malaria vector control interventions.
Supplementary Material
(A): An. gambiae; (B): An. coluzzii; (C): An. arabiensis; (D): An. funestus. Open circles indicate bins with more than 15 specimens. Full circle, bins with less than 15 specimens.
(A) CCA 1. (B) CCA 2. Dots indicate the evaluation points where the probability of occurrence of each inversion was predicted by the models. Colour varies in function of the CCA value.
(A): An. gambiae; (B): An. coluzzii; (C): An. arabiensis; (D): An. funestus
Values shown in the diagrams are the percentages of variation in inversions’ distribution explained exclusively by spatial gradients (Spatial), land cover (Land) and climatic variables (Climate) and by the combined effect of these factors. See Table S5 for details about variables included in each of the above-mentioned factors.
Tables S1–S4. Chromosomal inversion data used in this study.
Table S1: Anopheles gambiae; Table S2: Anopheles coluzzii; Table S4: Anopheles arabiensis; Table S3: Anopheles funestus.
Lat: latitude decimal degrees; Long: longitude decimal degrees; Lat+X : latitude + X to obtain all positive values; Long+ X : longitude + X to obtain all positive values; Lat+ X *Long+X : the product of latitude (lat X ) and longitude (long X ); abs(Lat): absolute latitude value; Elevation (in m); Mean temperature of the wettest quarter of the year (in C); Mean precipitation of the wettest quarter of the year (in mm); NDVI yearly mean and yearly variation for the period included in the study; NDVI quarterly mean: NDVI wettest quarter mean for the period included in the study; NDVI quarterly variation: NDVI wettest quarter variation for the period included in the study; Sample size: Number of mosquitoes karyotyped in the study (in An. arabiensis, missing numbers for some villages were arbitrarily replaced by 20); Inv_XX: inversion frequency.
Table S5. Complete inversion models.
Table S6. Spatial auto-correlation among the inversion models.
Acknowledgments
We thank Mark Kirkpatrick, Patrik Nosil and Francois Rousset for their insightful comments, which helped us to improve the manuscript. We thank Daniel Couret for preliminary GIS assistance, and Ana L. Márquez for assistance with chorotype detection. Fieldwork was supported by the Institut de Recherche pour le Développement and the National Institutes of Health grant R01-AI063508 awarded to Nora J. Besansky. P. Acevedo is funded by a MINECO and Universidad de Castilla-La Mancha (UCLM) through “Ramon y Cajal” contract (RYC-2012-11970). Finally, we specially thank Ray Gaab for his continuous support and advices.
Bibliographic references
- Acevedo P, Ruiz-Fons F, Estrada R, Luz Marquez A, Angel Miranda M, Gortazar C, Lucientes J. A Broad Assessment of Factors Determining Culicoides imicola Abundance: Modelling the Present and Forecasting Its Future in Climate Change Scenarios. PLoS ONE. 2010:5. doi: 10.1371/journal.pone.0014236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H. New look at statistical-model identification. Ieee Transactions on Automatic Control. 1974;AC19:716–723. [Google Scholar]
- Annan Z, Durand P, Ayala FJ, Arnathau C, Awono-Ambene P, Simard F, Razakandrainibe FG, Koella JC, Fontenille D, Renaud F. Population genetic structure of Plasmodium falciparum in the two main African vectors, Anopheles gambiae and Anopheles funestus. Proc Natl Acad Sci U S A. 2007;104:7987–7992. doi: 10.1073/pnas.0702715104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala D, Costantini C, Ose K, Kamdem G, Antonio-Nkondjio C, Agbor JP, Awono-Ambene P, Fontenille D, Simard F. Habitat suitability and ecological niche profile of major malaria vectors in Cameroon. Malaria Journal. 2009;8:307. doi: 10.1186/1475-2875-8-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala D, Fontaine MC, Cohuet A, Fontenille D, Vitalis R, Simard F. Chromosomal inversions, natural selection and adaptation in the malaria vector Anopheles funestus Mol. Biol Evol. 2011;28:745–758. doi: 10.1093/molbev/msq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala D, Guerrero RF, Kirkpatrick M. Reproductive isolation and local adaptation quantified for a chromosome inversion in a malaria mosquito. Evolution. 2013;67:946–958. doi: 10.1111/j.1558-5646.2012.01836.x. [DOI] [PubMed] [Google Scholar]
- Ayala D, Ullastres A, González J. Adaptation through chromosomal inversions in Anopheles. Frontiers in Genetics. 2014:5. doi: 10.3389/fgene.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala FJ, Coluzzi M. Chromosome speciation: humans, Drosophila, and mosquitoes. Proc Natl Acad Sci U S A. 2005;102(Suppl 1):6535–6542. doi: 10.1073/pnas.0501847102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balanya J, Serra L, Gilchrist GW, Huey RB, Pascual M, Mestres F, Sole E. Evolutionary pace of chromosomal polymorphism in colonizing populations of Drosophila subobscura: an evolutionary time series. Evolution: International Journal of Organic Evolution. 2003;57:1837–1845. doi: 10.1111/j.0014-3820.2003.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Besansky N. Genome analysis of vectorial capacity in major Anopheles vectors of malaria parasites. Vectorbase. 2008 http://www.vectorbase.org.
- Bhutkar A, Schaeffer SW, Russo SM, Xu M, Smith TE, Gelbart WM. Chromosomal rearrangement inferred from comparisons of 12 Drosophila genomes. Genetics. 2008;179:1657–1680. doi: 10.1534/genetics.107.086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boete C. Malaria parasites in mosquitoes: laboratory models, evolutionary temptation and the real world. Trends Parasitol. 2005;21:445–447. doi: 10.1016/j.pt.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Brooke BD, Hunt RH, Chandre F, Carnevale P, Coetzee M. Stable chromosomal inversion polymorphisms and insecticide resistance in the malaria vector mosquito Anopheles gambiae (Diptera: Culicidae) J Med Entomol. 2002;39:568. doi: 10.1603/0022-2585-39.4.568. [DOI] [PubMed] [Google Scholar]
- Bryan JH, Petrarca V, di Deco MA, Coluzzi M. Adult behaviour of members of the Anopheles gambiae complex in the Gambia with special reference to An. melas and its chromosomal variants. Parassitologia (Rome) 1987;29:221–249. [PubMed] [Google Scholar]
- Burger R, Akerman A. The effects of linkage and gene flow on local adaptation: A two-locus continent-island model. Theoretical Population Biology. 2011;80:272–288. doi: 10.1016/j.tpb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone BJ, Molloy MJ, Cheng C, Tan JC, Hahn MW, Besansky NJ. Divergent transcriptional response to thermal stress by Anopheles gambiae larvae carrying alternative arrangements of inversion 2La. Molecular Ecology. 2011;20:2567–2580. doi: 10.1111/j.1365-294X.2011.05114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, White BJ, Kamdem C, Mockaitis K, Costantini C, Hahn MW, Besansky NJ. Ecological Genomics of Anopheles gambiae Along a Latitudinal Cline: A Population-Resequencing Approach. Genetics. 2012;190:1417–1432. doi: 10.1534/genetics.111.137794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes: Development, Nutrition and Reproduction v.1. CABI Publishing; 1999. [Google Scholar]
- Coetzee M, Hunt RH, Wilkerson R, Della Torre A, Coulibaly MB, Besansky NJ. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa. 2013;3619:246–274. [PubMed] [Google Scholar]
- Coghlan A, Eichler EE, Oliver SG, Paterson AH, Stein L. Chromosome evolution in eukaryotes: a multi-kingdom perspective. Trends in Genetics. 2005;21:673–682. doi: 10.1016/j.tig.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Cohuet A, Dia I, Simard F, Raymond M, Rousset F, Antonio-Nkondjio C, Awono-Ambene PH, Wondji CS, Fontenille D. Gene flow between chromosomal forms of the malaria vector Anopheles funestus in Cameroon, Central Africa, and its relevance in malaria fighting. Genetics. 2005;169:301–311. doi: 10.1534/genetics.103.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge JE, Hoffmann AA, McKechnie SW. Altitudinal patterns for latitudinally varying traits and polymorphic markers in Drosophila melanogaster from eastern Australia. Journal of Evolutionary Biology. 2006;19:473–482. doi: 10.1111/j.1420-9101.2005.01016.x. [DOI] [PubMed] [Google Scholar]
- Coluzzi M. Spatial distribuion of chromosomal inversions and speciation in anopheline mosquitoes. In: BC, editor. Mechanisms of Speciation. Alan R Liss; New York: 1982. pp. 113–115. [PubMed] [Google Scholar]
- Coluzzi M, Petrarca V, Di Deco MA. Chromosomal inversion intergradation and incipient speciation in Anopheles gambiae. Bollettino di Zoologia. 1985;52:45–63. [Google Scholar]
- Coluzzi M, Sabatini A, della Torre A, Di Deco MA, Petrarca V. A polytene chromosome analysis of the Anopheles gambiae species complex. Science. 2002;298:1415. doi: 10.1126/science.1077769. [DOI] [PubMed] [Google Scholar]
- Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Behavioural divergences between mosquitoes with different inversion karyotypes in polymorphic populations of the Anopheles gambiae complex. Nature. 1977;266:832–833. doi: 10.1038/266832a0. [DOI] [PubMed] [Google Scholar]
- Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979a:73. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- Coluzzi M, Sabatini A, Petrarca V, Dideco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1979b;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- Corsi F, de Leeuw J, Skidmore A. Modeling species distribution with GIS 2000 [Google Scholar]
- Costantini C, Ayala D, Guelbeogo W, Pombi M, Some C, Bassole I, Ose K, Fotsing JM, Sagnon NF, Fontenille D, Besansky N, Simard F. Living at the edge: biogeographic patterns of habitat segregation conform to speciation by niche expansion in Anopheles gambiae. BMC Ecology. 2009;9:16. doi: 10.1186/1472-6785-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini C, Li SG, della Torre A, Sagnon NF, Coluzzi M, Taylor CE. Density, survival and dispersal of Anopheles gambiae complex mosquitoes in a west African Sudan savanna village. Med Vet Entomol. 1996;10:203–219. doi: 10.1111/j.1365-2915.1996.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Costantini C, Sagnon N, Ilboudo-Sanogo E, Coluzzi M, Boccolini D. Chromosomal and bionomic heterogeneities suggest incipient speciation in Anopheles funestus from Burkina Faso. Parassitologia. 1999;41:595–611. [PubMed] [Google Scholar]
- Davidson G. The five mating-types in the Anopheles gambiae complex. Rivista di Malariologia. 1964:43. [PubMed] [Google Scholar]
- Davidson G, Hunt RH. The crossing and chromosome characteristics of a new 6th species in the Anopheles gambiae complex. Parassitologia. 1973;15:121–128. [PubMed] [Google Scholar]
- della Torre A. Polytene chromosome preparation from Anopheline mosquitoes. In: Crampton JM, Beard CB, Louis C, editors. Molecular Biology of Insect Disease Vectors: a Methods Manual. Chapman & Hall; London: 1997. pp. 329–336. [Google Scholar]
- Dobzhansky T, Sturtevant AH. Inversions in the chromosomes of Drosophila pseudoobscura. Genetics. 1938;23:28–64. doi: 10.1093/genetics/23.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T, Wright S. Genetics of natural populations. X Dispersion rates in Drosophila pseudoobscura. Genetics. 1943;28:304–340. doi: 10.1093/genetics/28.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly MJ, Cuamba N, Charlwood JD, Collins FH, Townson H. Population structure in the malaria vector, Anopheles arabiensis Patton, in East Africa. Heredity. 1999;83:408–417. doi: 10.1038/sj.hdy.6885930. [DOI] [PubMed] [Google Scholar]
- Dormann CF. Effects of incorporating spatial autocorrelation into the analysis of species distribution data. Global Ecology and Biogeography. 2007;16:129–138. [Google Scholar]
- Enayati A, Hemingway J. Malaria Management: Past, Present, and Future. Annual Review of Entomology. 2010;55:569–591. doi: 10.1146/annurev-ento-112408-085423. [DOI] [PubMed] [Google Scholar]
- Endler JA. Geographic Variation, Speciation, and Clines. Princetown University Press; Princetown, New Yersey: 1977. [PubMed] [Google Scholar]
- Feder JL, Gejji R, Powell THQ, Nosil P. Adaptive chromosomal divergence driven by mixed geographic mode of evolution. Evolution. 2011;65:2157–2170. doi: 10.1111/j.1558-5646.2011.01321.x. [DOI] [PubMed] [Google Scholar]
- Ferguson HM, Dornhaus A, Beeche A, Borgemeister C, Gottlieb M, Mulla MS, Gimnig JE, Fish D, Killeen GF. Ecology: A Prerequisite for Malaria Elimination and Eradication. PLos Med. 2010;7:7. doi: 10.1371/journal.pmed.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuk L, MacDonald JR, Tang T, Carson AR, Li M. Discovery of human inversion polymorphisms by comparative analysis of human and chimpanzee DNA sequence assemblies. PloS Genet. 2005;1:489. doi: 10.1371/journal.pgen.0010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine MC, Pease JB, Steele A, Waterhouse RM, Neafsey DE, Sharakhov IV, Jiang X, Hall AB, Catteruccia F, Kakani E, Mitchell SN, Wu YC, Smith HA, Love RR, Lawniczak MK, Slotman MA, Emrich SJ, Hahn MW, Besansky NJ. Extensive introgression in a malaria vector species complex revealed by phylogenomics. Science. 2015;347:42. doi: 10.1126/science.1258524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouet C, Gray E, Besansky NJ, Costantini C. Adaptation to Aridity in the Malaria Mosquito Anopheles gambiae: Chromosomal Inversion Polymorphism and Body Size Influence Resistance to Desiccation. PLoS ONE. 2012:7. doi: 10.1371/journal.pone.0034841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies MT, Coetzee MC. A Supplement to the Anophelinae of Africa South of the Sahara (Afrotropical region) The South African Institute for Medical Research; Johannesburg: 1987. [Google Scholar]
- Gray EM, Rocca KAC, Costantini C, Besansky NJ. Inversion 2La is associated with enhanced desiccation resistance in Anopheles gambiae. Malaria Journal. 2009:8. doi: 10.1186/1475-2875-8-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CA, Hunt RH. Interpretation of variation in ovarian polytene chromosomes of Anopheles funestus Giles, Anopheles parensis Gillies, and Anopheles aruni. Genetica. 1980;51:187–195. [Google Scholar]
- Heard NA, Holmes CC, Stephens DA, Hand DJ, Dimopoulos G. Bayesian coclustering of Anopheles gene expression time series: Study of immune defense response to multiple experimental challenges. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16939–16944. doi: 10.1073/pnas.0408393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen RK, Marmion M, Luoto M. Does the interpolation accuracy of species distribution models come at the expense of transferability? Ecography. 2012;35:276–288. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- Hoffmann AA, Rieseberg LH. Revisiting the impact of inversions in evolution: from population genetic markers to drivers of adaptive shifts and speciation? Annual Review of Ecology, Evolution, and Systematics. 2008;39:21–42. doi: 10.1146/annurev.ecolsys.39.110707.173532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Sgro CM, Weeks AR. Chromosomal inversion polymorphisms and adaptation. Trends in Ecology & Evolution. 2004;19:482–488. doi: 10.1016/j.tree.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Hortal J, Roura-Pascual N, Sanders NJ, Rahbek C. Understanding (insect) species distributions across spatial scales. Ecography. 2010;33:51–53. [Google Scholar]
- Hosmer DW, Jovanovic B, Lemeshow S. BEST SUBSETS LOGISTIC-REGRESSION. Biometrics. 1989;45:1265–1270. [Google Scholar]
- Jackson DA. STOPPING RULES IN PRINCIPAL COMPONENTS-ANALYSIS - A COMPARISON OF HEURISTIC AND STATISTICAL APPROACHES. Ecology. 1993;74:2204–2214. [Google Scholar]
- Joron M, Frezal L, Jones RT, Chamberlain NL, Lee SF, Haag CR, Whibley A, Becuwe M, Baxter SW, Ferguson L, Wilkinson PA, Salazar C, Davidson C, Clark R, Quail MA, Beasley H, Glithero R, Lloyd C, Sims S, Jones MC, Rogers J, Jiggins CD, Ffrench-Constant RH. Chromosomal rearrangements maintain a polymorphic supergene controlling butterfly mimicry. Nature. 2011;477:203–206. doi: 10.1038/nature10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovani R, Tella JL. Parasite prevalence and sample size: misconceptions and solutions. Trends in Parasitology. 2006;22:214–218. doi: 10.1016/j.pt.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Kamali M, Marek PE, Peery A, Antonio-Nkondjio C, Ndo C, Tu Z, Simard F, Sharakhov IV. Multigene Phylogenetics Reveals Temporal Diversification of Major African Malaria Vectors. PLoS ONE. 2014:9. doi: 10.1371/journal.pone.0093580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali M, Xia A, Tu ZJ, Sharakhov IV. A New Chromosomal Phylogeny Supports the Repeated Origin of Vectorial Capacity in Malaria Mosquitoes of the Anopheles gambiae Complex. Plos Pathogens. 2012:8. doi: 10.1371/journal.ppat.1002960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapun M, Fabian DK, Goudet J, Flatt T. Genomic Evidence for Adaptive Inversion Clines in Drosophila melanogaster. Mol Biol Evol. 2016 doi: 10.1093/molbev/msw016. [DOI] [PubMed] [Google Scholar]
- Kennington WJ, Partridge L, Hoffmann AA. Patterns of diversity and linkage disequilibrium within the cosmopolitan inversion In(3R)Payne in Drosophila melanogaster are indicative of coadaptation. Genetics. 2006;172:1655–1663. doi: 10.1534/genetics.105.053173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M, Barrett B. Chromosome inversions, adaptive cassettes and the evolution of species’ ranges. Molecular Ecology. 2015;24:2046–2055. doi: 10.1111/mec.13074. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Barton N. Chromosome inversions, local adaptation and speciation. Genetics. 2006;173:419. doi: 10.1534/genetics.105.047985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M, Kern A. Where’s the Money? Inversions, Genes, and the Hunt for Genomic Targets of Selection. Genetics. 2012;190:1153–1155. doi: 10.1534/genetics.112.139899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowski B, Kern AD, Holloway AK, Begun DJ. Genomic Differentiation Between Temperate and Tropical Australian Populations of Drosophila melanogaster. Genetics. 2011;187:245–260. doi: 10.1534/genetics.110.123059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft H, Jetz W. A framework for delineating biogeographical regions based on species distributions. Journal of Biogeography. 2010;37:2029–2053. [Google Scholar]
- Krimbas C, Powell J. Drosophila inversion polymorphism. CRC Press; Boca Raton: 1992. [Google Scholar]
- Krzywinski J, Besansky NJ. Molecular systematics of Anopheles: from subgenera to subpopulations. Annual Review of Entomology. 2003;48:111–139. doi: 10.1146/annurev.ento.48.091801.112647. [DOI] [PubMed] [Google Scholar]
- Lawniczak MKN, Emrich SJ, Holloway AK, Regier AP, Olson M, White B, Redmond S, Fulton L, Appelbaum E, Godfrey J, Farmer C, Chinwalla A, Yang S-P, Minx P, Nelson J, Kyung K, Walenz BP, Garcia-Hernandez E, Aguiar M, Viswanathan LD, Rogers Y-H, Strausberg RL, Saski CA, Lawson D, Collins FH, Kafatos FC, Christophides GK, Clifton SW, Kirkness EF, Besansky NJ. Widespread Divergence Between Incipient Anopheles gambiae Species Revealed by Whole Genome Sequences. Science. 2010;330:512–514. doi: 10.1126/science.1195755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poul Y, Whibley A, Chouteau M, Prunier F, Llaurens V, Joron M. Evolution of dominance mechanisms at a butterfly mimicry supergene. Nature Communications. 2014:5. doi: 10.1038/ncomms6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P. Numercial ecology. Elsevier; Amsterdam: 1998. [Google Scholar]
- Lehmann T, Licht M, Elissa N, Maega BT, Chimumbwa JM, Watsenga FT, Wondji CS, Simard F, Hawley WA. Population Structure of Anopheles gambiae in Africa. Journal of Heredity. 2003;94:133–147. doi: 10.1093/jhered/esg024. [DOI] [PubMed] [Google Scholar]
- Lindsay SW, Parson L, Thomas CJ. Mapping the ranges and relative abundance of the two principal African malaria vectors, Anopheles gambiae sensu stricto and An-arabiensis, using climate data. Proceedings of the Royal Society B-Biological Sciences. 1998;265:847–854. doi: 10.1098/rspb.1998.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo N, Sangare D, Regier A, Reidenbach K, Bretz D, Sharakhova M, Emrich S, Traore S, Costantini C, Besansky N, Collins F. Breakpoint structure of the Anopheles gambiae 2Rb chromosomal inversion. Malaria Journal. 2010;9:293. doi: 10.1186/1475-2875-9-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry DB, Willis JH. A Widespread Chromosomal Inversion Polymorphism Contributes to a Major Life-History Transition, Local Adaptation, and Reproductive Isolation. PLoS Biology. 2010:8. doi: 10.1371/journal.pbio.1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A. R package version 0.8-7. 2011. amap: Another Multidimensional Analysis Package. [Google Scholar]
- Lyon MF. Transmission ratio distortion in mice. Annu Rev Genet. 2003;37:393. doi: 10.1146/annurev.genet.37.110801.143030. [DOI] [PubMed] [Google Scholar]
- Manoukis NC, Powell JR, Toure MB, Sacko A, Edillo FE, Coulibaly MB, Traore SF, Taylor CE, Besansky NJ. A test of the chromosomal theory of ecotypic speciation in Anopheles gambiae. Proceedings of the National Academy of Sciences. 2008;105:2940–2945. doi: 10.1073/pnas.0709806105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel AP, Ingrasci MJ, Schemerhorn BJ, Kern M, Le Goff G, Coetzee M, Elissa N, Fontenille D, Vulule J, Lehmann T, Sagnon N, Costantini C, Besansky NJ. Rangewide population genetic structure of the African malaria vector Anopheles funestus. Molecular Ecology. 2005;14:4235–4248. doi: 10.1111/j.1365-294X.2005.02754.x. [DOI] [PubMed] [Google Scholar]
- Moffett A, Shackelford N, Sarkar S. Malaria in Africa: Vector Species’ Niche Models and Relative Risk Maps. PLoS ONE. 2007;2:e824. doi: 10.1371/journal.pone.0000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molineaux L, Gramiccia G. Bulletin of the World Health Organization. 1980. The Garki Project. Research on Epidemiology and Control of Malaria in the Sudan Savannah of West Africa. [Google Scholar]
- Neafsey DE, Waterhouse RM, Abai MR, Aganezov SS, Alekseyev MA, Allen JE, Amon J, Arca B, Arensburger P, Artemov G, Assour LA, Basseri H, Berlin A, Birren BW, Blandin SA, Brockman AI, Burkot TR, Burt A, Chan CS, Chauve C, Chiu JC, Christensen M, Costantini C, Davidson VLM, Deligianni E, Dottorini T, Dritsou V, Gabriel SB, Guelbeogo WM, Hall AB, Han MV, Hlaing T, Hughes DST, Jenkins AM, Jiang X, Jungreis I, Kakani EG, Kamali M, Kemppainen P, Kennedy RC, Kirmitzoglou IK, Koekemoer LL, Laban N, Langridge N, Lawniczak MKN, Lirakis M, Lobo NF, Lowy E, MacCallum RM, Mao C, Maslen G, Mbogo C, McCarthy J, Michel K, Mitchell SN, Moore W, Murphy KA, Naumenko AN, Nolan T, Novoa EM, O’Loughlin S, Oringanje C, Oshaghi MA, Pakpour N, Papathanos PA, Peery AN, Povelones M, Prakash A, Price DP, Rajaraman A, Reimer LJ, Rinker DC, Rokas A, Russell TL, Sagnon NF, Sharakhova MV, Shea T, Simao FA, Simard F, Slotman MA, Somboon P, Stegniy V, Struchiner CJ, Thomas GWC, Tojo M, Topalis P, Tubio JMC, Unger MF, Vontas J, Walton C, Wilding CS, Willis JH, Wu Y-C, Yan G, Zdobnov EM, Zhou X, Catteruccia F, Christophides GK, Collins FH, Cornman RS, Crisanti A, Donnelly MJ, Emrich SJ, Fontaine MC, Gelbart W, Hahn MW, Hansen IA, Howell PI, Kafatos FC, Kellis M, Lawson D, Louis C, Luckhart S, Muskavitch MAT, Ribeiro JM, Riehle MA, Sharakhov IV, Tu Z, Zwiebel LJ, Besansky NJ. Highly evolvable malaria vectors: The genomes of 16 Anopheles mosquitoes. Science. 2015;347:43. doi: 10.1126/science.1258522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson SE, Davenport ML, Malo AR. A comparison of the vegetation response to rainfall in the sahel and East-Africa, using normalized difference vegetation index from NOAA AVHRR. Climatic Change. 1990;17:209–241. [Google Scholar]
- Nicholson SE, Farrar TJ. The influence of soil type on the relationships between ndvi, rainfall, and soil-moisture in semiarid botswana .1. ndvi response to rainfall. Remote Sensing of Environment. 1994;50:107–120. [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H. R package version 2.0-2. 2011. vegan: Community Ecology Package. [Google Scholar]
- Olivero J, Real R, Marquez AL. Fuzzy Chorotypes as a Conceptual Tool to Improve Insight into Biogeographic Patterns. Systematic Biology. 2011;60:645–660. doi: 10.1093/sysbio/syr026. [DOI] [PubMed] [Google Scholar]
- Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC, D’Amico JA, Itoua I, Strand HE, Morrison JC, Loucks CJ, Allnutt TF, Ricketts TH, Kura Y, Lamoreux JF, Wettengel WW, Hedao P, Kassem KR. Terrestrial ecoregions of the worlds: A new map of life on Earth. Bioscience. 2001;51:933–938. [Google Scholar]
- Pearce J, Ferrier S. Evaluating the predictive performance of habitat models developed using logistic regression. Ecological Modelling. 2000;133:225–245. [Google Scholar]
- Petrarca V, Beier JC. Intraspecific chromosomal polymorphism in the Anopheles gambiae complex as a factor affecting malaria transmission in the Kisumu area of Kenya. Am J Trop Med Hyg. 1992;46:229–237. doi: 10.4269/ajtmh.1992.46.229. [DOI] [PubMed] [Google Scholar]
- Petrarca V, Nugud AD, Ahmed MAE, Haridi AM, Di Deco MA, Coluzzi M. Cytogenetics of the Anopheles gambiae complex in Sudan, with special reference to An. arabiensis: relationships with East and West African populations. Medical and Veterinary Entomology. 2000;14:149–164. doi: 10.1046/j.1365-2915.2000.00231.x. [DOI] [PubMed] [Google Scholar]
- Puig M, Caceres M, Ruiz A. Silencing of a gene adjacent to the breakpoint of a widespread Drosophila inversion by a transposon-induced antisense RNA. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9013–9018. doi: 10.1073/pnas.0403090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranz Jé, Maurin MD, Chan YS, von Grotthuss M, Hillier LW, Roote J, Ashburner M, Bergman CM. Principles of Genome Evolution in the Drosophila melanogaster Species Group. PLoS Biology. 2007;5:1366–1381. doi: 10.1371/journal.pbio.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real R, Barbosa AM, Porras D, Kin MS, Marquez AL, Guerrero JC, Palomo LJ, Justo ER, Vargas JM. Relative importance of environment, human activity and spatial situation in determining the distribution of terrestrial mammal diversity in Argentina. Journal of Biogeography. 2003;30:939–947. [Google Scholar]
- Rocca KAC, Gray EM, Costantini C, Besansky NJ. 2La chromosomal inversion enhances thermal tolerance of Anopheles gambiae larvae. Malaria Journal. 2009:8. doi: 10.1186/1475-2875-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Trelles F, Alvarez G, Zapata C. Time-series analysis of seasonal changes of the O inversion polymorphism of Drosophila subobscura. Genetics. 1996;142:179–187. doi: 10.1093/genetics/142.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer SW. Selection in heterogeneous environments maintains the gene arrangement polymorphism of Drosophila pseudoobscura. Evolution. 2008;62:3082–3099. doi: 10.1111/j.1558-5646.2008.00504.x. [DOI] [PubMed] [Google Scholar]
- Schaeffer SW, Goetting-Minesky MP, Kovacevic M, Peoples JR, Graybill JL. Evolutionary genomics of inversions in Drosophila pseudoobscura: Evidence for epistasis. Proc Natl Acad Sci USA. 2003;100:8319. doi: 10.1073/pnas.1432900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharakhov I, Braginets O, Grushko O, Cohuet A, Guelbeogo WM, Boccolini D, Weill M, Costantini C, Sagnon N, Fontenille D, Yan G, Besansky NJ. A microsatellite map of the African human malaria vector Anopheles funestus. Journal of Heredity. 2004;95:29–34. doi: 10.1093/jhered/esh011. [DOI] [PubMed] [Google Scholar]
- Sharakhov IV, Serazin AC, Grushko OG, Dana A, Lobo N, Hillenmeyer ME, Westerman R, Romero-Severson J, Costantini C, Sagnon N, Collins FH, Besansky NJ. Inversions and gene order shuffling in Anopheles gambiae and Anopheles funestus. Science. 2002;298:182–185. doi: 10.1126/science.1076803. [DOI] [PubMed] [Google Scholar]
- Sharakhov IV, White BJ, Sharakhova MV, Kayondo J, Lobo NF. Breakpoint structure reveals the unique origin of an interspecific chromosomal inversion (2La) in the Anopheles gambiae complex. Proc Natl Acad Sci USA. 2006;103:6258. doi: 10.1073/pnas.0509683103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharakhova MV, George P, Brusentsova IV, Leman SC, Bailey JA, Smith CD, Sharakhov IV. Genome mapping and characterization of the Anopheles gambiae heterochromatin. BMC Genomics. 2010a:11. doi: 10.1186/1471-2164-11-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharakhova MV, Peery A, Antonio-Nkondjio C, Xia A, Ndo C, Awono-Ambene P, Simard F, Sharakhov IV. Cytogenetic analysis of Anopheles ovengensis revealed high structural divergence of chromosomes in the Anopheles nili group. Infection Genetics and Evolution. 2013;16:341–348. doi: 10.1016/j.meegid.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharakhova MV, Xia A, Leman SC, Sharakhov IV. Arm-specific dynamics of chromosome evolution in malaria mosquitoes. BMC Evolutionary Biology. 2011;11:1–17. doi: 10.1186/1471-2148-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharakhova MV, Xia A, McAlister SI, Sharakhov IV. A standard cytogenetic photomap for the mosquito Anopheles stephensi (Diptera: Culicidae): application for physical mapping. J Med Entomol. 2006;43:861–866. doi: 10.1603/0022-2585(2006)43[861:ascpft]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sharakhova MV, Xia A, Tu ZJ, Shouche YS, Unger MF, Sharakhov IV. A Physical Map for an Asian Malaria Mosquito, Anopheles stephensi. American Journal of Tropical Medicine and Hygiene. 2010b;83:1023–1027. doi: 10.4269/ajtmh.2010.10-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard F, Ayala D, Kamdem G, Pombi M, Etouna J, Ose K, Fotsing J-M, Fontenille D, Besansky N, Costantini C. Ecological niche partitioning between Anopheles gambiae molecular forms in Cameroon: the ecological side of speciation. BMC Ecology. 2009;9:17. doi: 10.1186/1472-6785-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard F, Lehmann T, Lemasson J-J, Diatta M, Fontenille D. Persistence of Anopheles arabiensis during the severe dry season conditions in Senegal: an indirect approach using microsatellite loci. Insect Molecular Biology. 2000;9:467–479. doi: 10.1046/j.1365-2583.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, Patil AP, Temperley WH, Gething PW, Kabaria CW, Okara RM, Van Boeckel T, Godfray HCJ, Harbach RE, Hay SI. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasites & Vectors. 2010:3. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka ME, Bangs MJ, Manguin S, Rubio-Palis Y, Chareonviriyaphap T, Coetzee M, Mbogo CM, Hemingway J, Patil AP, Temperley WH, Gething PW, Kabaria CW, Burkot TR, Harbach RE, Hay SI. A global map of dominant malaria vectors. Parasites & Vectors. 2012:5. doi: 10.1186/1756-3305-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. Freeman & Co; New York: 1981. [Google Scholar]
- Stefansson H, Helgason A, Thorleifsson G, Steinthorsdottir V, Masson G. A common inversion under selection in Europeans. Nature Genetics. 2005;37:129. doi: 10.1038/ng1508. [DOI] [PubMed] [Google Scholar]
- Tchuinkam T, Simard F, Lele-Defo E, Tene-Fossog B, Tateng-Ngouateu A, Antonio-Nkondjio C, Mpoame M, Toto JC, Njine T, Fontenille D, Awono-Ambene HP. Bionomics of Anopheline species and malaria transmission dynamics along an altitudinal transect in Western Cameroon. Bmc Infectious Diseases. 2010:10. doi: 10.1186/1471-2334-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Braak C. Principal Component Analysis and Redundancy Analysis. Microcomputer Power; Ithaca, NY: 1987. CANOCO - A FORTRAN Program for Canonical Community Ordination by Canonical Correspondance Analysis. [Google Scholar]
- Umina PA, Weeks AR, Kearney MR, McKechnie SW, Hoffmann AA. A rapid shift in a classic clinal pattern in Drosophila reflecting climate change. Science. 2005;308:691–693. doi: 10.1126/science.1109523. [DOI] [PubMed] [Google Scholar]
- van Doorn GS, Kirkpatrick M. Turnover of sex chromosomes induced by sexual conflict. Nature. 2007;449:909–912. doi: 10.1038/nature06178. [DOI] [PubMed] [Google Scholar]
- White BJ, Cheng C, Sangare D, Lobo NF, Collins FH, Besansky NJ. The population genomics of trans-specific inversion polymorphisms in Anopheles gambiae. Genetics. 2009a:183. doi: 10.1534/genetics.109.105817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BJ, Cheng C, Sangare D, Lobo NF, Collins FH, Besansky NJ. The population genomics of trans-specific inversion polymorphisms in Anopheles gambiae. Genetics. 2009b;183:275–288. doi: 10.1534/genetics.109.105817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windbichler N, Menichelli M, Papathanos PA, Thyme SB, Li H, Ulge UY, Hovde BT, Baker D, Monnat RJ, Burt A, Crisanti A. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature. 2011;473:212. doi: 10.1038/nature09937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng BY, Agresti A. Summarizing the predictive power of a generalized linear model. Statistics in Medicine. 2000;19:1771–1781. doi: 10.1002/1097-0258(20000715)19:13<1771::aid-sim485>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A): An. gambiae; (B): An. coluzzii; (C): An. arabiensis; (D): An. funestus. Open circles indicate bins with more than 15 specimens. Full circle, bins with less than 15 specimens.
(A) CCA 1. (B) CCA 2. Dots indicate the evaluation points where the probability of occurrence of each inversion was predicted by the models. Colour varies in function of the CCA value.
(A): An. gambiae; (B): An. coluzzii; (C): An. arabiensis; (D): An. funestus
Values shown in the diagrams are the percentages of variation in inversions’ distribution explained exclusively by spatial gradients (Spatial), land cover (Land) and climatic variables (Climate) and by the combined effect of these factors. See Table S5 for details about variables included in each of the above-mentioned factors.
Tables S1–S4. Chromosomal inversion data used in this study.
Table S1: Anopheles gambiae; Table S2: Anopheles coluzzii; Table S4: Anopheles arabiensis; Table S3: Anopheles funestus.
Lat: latitude decimal degrees; Long: longitude decimal degrees; Lat+X : latitude + X to obtain all positive values; Long+ X : longitude + X to obtain all positive values; Lat+ X *Long+X : the product of latitude (lat X ) and longitude (long X ); abs(Lat): absolute latitude value; Elevation (in m); Mean temperature of the wettest quarter of the year (in C); Mean precipitation of the wettest quarter of the year (in mm); NDVI yearly mean and yearly variation for the period included in the study; NDVI quarterly mean: NDVI wettest quarter mean for the period included in the study; NDVI quarterly variation: NDVI wettest quarter variation for the period included in the study; Sample size: Number of mosquitoes karyotyped in the study (in An. arabiensis, missing numbers for some villages were arbitrarily replaced by 20); Inv_XX: inversion frequency.
Table S5. Complete inversion models.
Table S6. Spatial auto-correlation among the inversion models.