Abstract
In 1998, Drs. Claudio Ronco and Ronaldo Bellomo published an article in Nephrology, Dialysis and Transplantation (NDT) entitled “Critical Care Nephrology: the time has come” [1]; in that article they advocated for improvements in the multifaceted care of critically ill patients with kidney disease by promoting more training, enhanced collaboration, and improved clinical interactions between specialties. Today —almost 2 decades later— the need for nephrology expertise in the care of critically ill patients is more important than ever; yet it is uncertain whether the collaboration and training envisioned by Drs. Ronco and Bellomo have been adequately realized.
Critically ill patients are surviving despite the enhanced severity of their illness, and our understanding about the epidemiology and pathophysiology of acute kidney injury (AKI) has changed in such manner that AKI is no longer thought as a marker of the death process, but instead, an active contributor to this process [2]. The role and methods of renal replacement therapy (RRT) has greatly expanded allowing for provision of effective therapies with less complications. RRT is no longer used as a last resort for indications such as severe uremia; instead, earlier support affords the ability to maintain fluid, acid/base and electrolyte homeostasis during critical illness [3]. Critical Care Nephrology has become an established specialty, with teams that perform research, clinical care, and education strictly in this arena. Great strides have been made through ongoing collaborations between critical care physicians and nephrologist to improve the way we care for patients in the ICU, educate multi-disciplinary team members, and build programs designed to maximize the delivery of care.
Unfortunately, there is great variability in the care for critically ill patients with AKI, with large variations across institutions, as the field lacks consensus on how and when to begin RRT in ICU patients [4–6]. Many institutions have less-than-ideal collaborations between nephrologists and intensivists, some programs fail to offer adequate educational training of nurses, and only a few programs have incorporated quality improvement programs designed to maximize the effectiveness and minimize complications of RRT delivery.
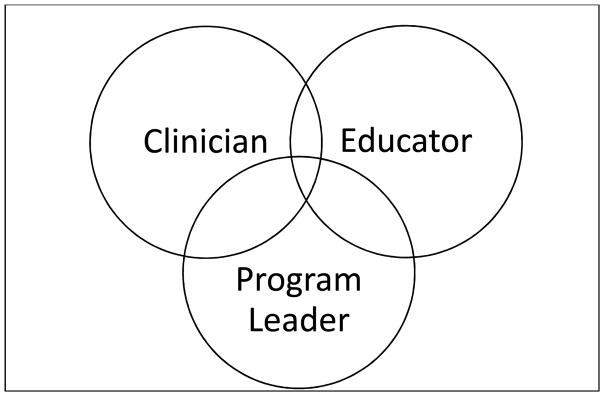
With the rapid growth of critical care medicine, the role of the nephrologist in the ICU has evolved. He/she must possess the necessary knowledge-base to practice effectively in the ICU, lead education efforts to optimize care, and we suggest that the nephrologist is the best representative to ensure that the programmatic aspects of RRT are optimized. This special article will provide insights into the role of nephrologist as the leader of the acute care nephrology program, highlight the role of the nephrologist as clinician for critically ill patients, and argue that nephrologists must help educate all members of the team who provide care to these vulnerable patients (Figure 1 and Table 1).
Keywords: Critical care nephrology, quality Improvement, acute kidney injury, education, RRT
NEPHROLOGIST AS LEADERS FOR ACUTE RRT PROGRAMS
RRT program leadership
In addition to the key clinical and educational roles described above, the nephrologist must establish an administrative role as the leader for the hospital acute RRT program. This is analogous to the medical director role for outpatient dialysis facilities, and requires a similar attention to regulatory, clinical, and quality improvement details [7,8]. The nephrologist needs dedicated time to support infrastructure, engage leadership from multiple intensive care units, develop and oversee quality assessments, and create an appropriate culture to maximize success. Since the requirements to adequately fulfill these efforts can be substantial, “buy-in” from hospital leadership is essential, and the health care system should provide resources and protected time for personnel to accomplish these roles.
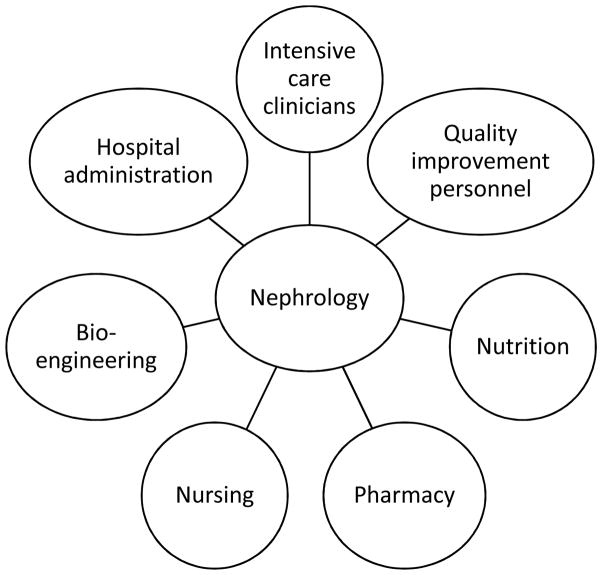
A RRT program requires a core of strong, engaged, multidisciplinary team members who are accountable to high-quality and cost-effective delivery of care across the institution, and the nephrologist may be the best suited to lead this team (Figure 2). Unlike the outpatient dialysis setting, the hospital setting is not solely the nephrologists’ domain, thus a successful acute RRT program must include personnel from nephrology, nursing, ICU, and pharmacy. Good attitude and disciplined teamwork within the leadership team are key elements to a successful program; conversely, making perceived unilateral decisions from a nephrology perspective can result in a pushback from our ICU partners and increase difficulty in implementing process improvements [4]. This multi-disciplinary team should meet regularly to allow for more timely assessment of safety incidents, assessment of methods to reduce harm, review protocols and procedures, and disseminate information. In addition, the team should conduct regular reviews of staff training, as well as assessments of competencies, reduction of costs, staff satisfaction, streamlining care and mitigating complications. Several nursing models have been successful in CRRT programs —e.g., utilization of only dialysis nurses, only intensive care unit (ICU) nurses or a combination of both— and each program needs to decide the model that suits best their situation [9].
Figure 2.
Participants in an ideal RRT program
Development of protocols and procedures
Once the decision to initiate RRT has been made, the initiation and delivery of CRRT must be done in a timely, efficient and safe manner. A dedicated team and providers adhering to structured and accepted hospital protocols and procedures for this service should be mandatory. A consistent process of care for RRT is a movement toward improved quality of RRT because standardization allows for consistency in care, improved communication and a basis for program quality evaluation. Early evidence suggest that a consistent protocol and procedures may be beneficial for patient outcome [10]. Protocols should be written to provide consistent care in the majority of situations, while allowing for flexibility for specific individual needs. When deviation from protocol occurs, it provides the opportunity to communicate with all involved team members and clear documentation highlighting the thought process for deviation.
The use of RRT in conjunction with other therapies (i.e., plasmapheresis [11], extra-corporeal membrane oxygenation [12], molecular absorbent recirculating systems [13]) is increasingly being implemented across pediatric and adult ICU’s. Future extracorporeal devices show great promise as adjuncts to critical care (e.g. renal assist devices [14]). Multi-disciplinary discussion about the best processes for care, with clearly demarcated policies and procedures are critical to the success of CRRT in conjunction with other therapies.
CRRT hardware regulation
The past fifty years of dialysis research have resulted in a significant evolution in the technology used to deliver CRRT [15]. The nephrologist must lead the team to determine which machines, filters, anti-coagulations, dialysis/replacement solutions will be available in their hospital. Factors that will affect these decisions include size of the program, resources, frequency of treatments, patient characteristics, ancillary support, and cost. Invested nephrologists should understand the specifics of optimal catheter use (i.e., size, location, and depth of insertion) and also be a resource to the physicians who place these catheters. The team needs to determine which solutions to carry, and whether modifications locally will occur. The expanding array of available CRRT filters requires a detailed knowledge of the benefits and risks of each product. Understanding how to quantify dose, ideal flows, limits of access pressures, monitoring filter patency, and optimization of filtration fraction are aspects of CRRT that should be mastered by nephrologists [16]. In addition, nephrologists need to decide which method of anticoagulation is best suited for their institution and take measures to limit potential complications. Having more than one form of anticoagulation can allow a more tailored plan specific to the individual, but will bring complexity to program structure and delivery of care.
Development of quality improvement programs
Depending on the institution, RRT may be performed relatively infrequently, or could be a common procedure across multiple ICU’s at one single institution. Anecdotal history from individual institutions suggests wide-ranging variability in the delivery of CRRT in all phases —initiation, maintenance, and discontinuation. As quality improvement science becomes more consistently focused on both the process of care and the consistency of care, CRRT has become a target for quality improvement [9,17]. The nephrologist is ideally placed to lead a team dedicated to improving CRRT quality across the entire institution: ensuring the use of a regular, systematic, and consistent process for how CRRT provision is assessed and delivered.
Quality monitoring and improvement of a RRT program is challenging for several reasons. First, CRRT is often delivered across different ICUs within the same institution. Each ICU varies in the approach to collaboration with nephrology, criteria for RRT initiation, nursing expertise, availability and turnover, patient characteristics, and patient volume. This further underlines the importance of a program to oversee care across different settings. Second, there are no established standards for defining nursing competency (either initial or maintenance) for RRT care [18,19]. Third, RRT is performed in acutely ill patient populations that inherently have high mortality, and therefore, errors related to RRT care can be difficult to recognize due to the overall complexity of the clinical situation. Lastly, at present there are few well-defined metrics to define quality of RRT care, and because of the large variation in CRRT practices between institutions, not all metrics may be equally applicable.
Management thinker Peter Drucker is often quoted as saying that “you can’t manage what you can’t measure”. A systematic process for delivering, capturing, analyzing and reporting data is a critical first step to quality improvement. Table 2 lists some potential quality metrics that may be appropriate for RRT care. While mortality is an obvious metric that should be tracked, it may be difficult to ascribe survival from only one procedure in these critically ill complex patients. Given the known high mortality in this population, additional metrics examining surrogate outcomes or areas such as process of care are needed [20]. One of the few evidence-based metrics available for CRRT is the delivered effluent dose, for which it has been established target of 20–25 mL/kg/hr in adults [21–23]. Identification of factors that contribute to poor delivery of the effluent dose may be addressed at a systematic level.
Table 2.
Potential quality metrics in RRT
| Patient Outcomes | RRT Prescription | Process/Efficiency | Competency Assessment |
|---|---|---|---|
| Mortality | Prescribed and delivered effluent dose | Time between CRRT order and initiation | Initial training program (nurses and physicians) |
| Patient safety events/medical errors | Filter circuit life | Causes of delayed initiation | Maintenance standards |
| Dialysis catheter related bloodstream infections | Filter clotting/unplanned filter exchanges | ||
| Achievement of fluid removal goals | |||
| Electrolyte supplementation requirements |
Premature loss of a RRT circuit contributes to lower delivered effluent dose, potential blood loss, increased nursing workload, and increased costs. Circuit filter life is one of the only metrics in RRT for which quality improvement processes have been shown to be successful [17,24]. Although there is still no agreement on specific targets, circuit life is a key parameter to assess the entire program —was the right catheter placed?, was anti-coagulation appropriate?, was the CRRT prescription optimal to sustain circuit patency?, was the nurse able to troubleshoot access pressures?.
Monitoring of complications (e.g., bleeding, citrate lock, infections) and patient safety events are additional key aspect of the quality improvement process. RRT centers should have an established review process that incorporates principles of root cause analysis for any potential safety event. In pediatric patients and others who may not tolerate CRRT initiation well, evaluation of the cardiovascular changes that occur should be tracked.
In the future, as more centers will continue to perform quality improvement in their CRRT programs, benchmarks can be set, and processes that show consistent efficiency, safety and benefit can be adapted to other programs, allowing to stablish targeted benchmarks for care.
Acquiring and maintaining competency in RRT
Physician and nursing competency for CRRT has not been defined, and there is a broad range of training requirements between institutions [19]. Nonetheless, each center should seek to establish internal standards, monitor progress, and periodically reassess needs [2].
THE ROLE OF THE NEPHROLOGIST AS A CRITICAL CARE CLINICIAN
All hospital systems who care for critically ill patients must develop strategies to provide timely AKI diagnosis, minimize complications related to AKI, and provide safe and effective renal support therapies. The nephrologist must be the expert on a multitude of situations that may arise to ensure that the team is providing high quality, timely, efficient, and safe clinical care for patients who have renal dysfunction. In order to provide patient and family-centered acute nephrology care for critically ill patients, the nephrology consultants need to be committed acquiring and maintaining: 1) fundamental knowledge of renal physiology, 2) expertise in AKI diagnosis and management, 3) understanding benefits/drawbacks of different acute RRT modalities, and 4) comprehension of fundamental aspects of critical care medicine, including how RRT affects the overall care of the patient (i.e., drug dosing and nutrition support needs during acute RRT, etc.).
Fundamental knowledge of renal physiology and its understanding in critical illness
The clinician must possess fundamental renal physiology knowledge to determine the etiology of kidney disease, correct the underlying problems, and provide homeostasis of fluids, electrolytes, acid/base, calcium/phosphorous.
AKI Diagnosis
Understanding the etiology of AKI will serve as a basis for an individualized treatment plan in hopes of limiting renal damage, ameliorating AKI, and fostering recovery. Frequently, one single cause cannot be identified, and a combination of factors contribute to AKI development. Urine sediment evaluation by the nephrologist and calculating the urine sediment score remains essential to establish a diagnosis of acute tubular necrosis (ATN) [25,26]. These data and other features from the history, exam, and laboratory data will help discern ATN from other etiologies of AKI such as acute interstitial nephritis (AIN), acute glomerulonephritis (GN), intra-abdominal & venous hypertension, nephrotoxins, thrombotic microangiopathy, and urinary obstruction. Timely evaluation of AKI and processes to mitigate its duration and effects can have lasting impact on long-term morbidity and mortality.
Tests such as fractional excretion of sodium (FENa) and renal Doppler ultrasound may have limited value in distinguishing between causes of AKI in critically ill patients [27–29]. Since novel urine biomarkers are now being used across the world to discern whether there is a functional change (e.g., a rise in serum creatinine) or kidney damage (e.g., a rise in urine biomarkers) [30], the nephrologist needs to be an active participant in helping teams determine which patients need these tests, as well as in the interpretation of the data obtained. Similarly, the integration and interpretation of a standardized furosemide stress test may be useful in early stages of AKI to predict progression and the need for dialysis [29].
Non-dialytic management of AKI
The complications that stem from AKI may have a significant impact on patient survival and hospital expenditures. The nephrologist adds value by providing evidence-based assistance regarding drug dosing in AKI and identification of nephrotoxic medications that should be halted/replaced to avoid further complications. Given the negative consequences ascribed to the complications of AKI —such as acidemia, hyperphosphatemia, hypocalcemia and hyperkalemia—, the nephrologist can anticipate and help avoid/treat these complications early in the disease course. As growing evidence suggest that volume overload leads to poor patient outcomes, the nephrologist plays a key role in achieving volume homeostasis by addressing the provision of fluids, as well as assisting to implement strategies to increase urine output and initiation of RRT to prevent further cumulative fluid overload [32]. The Acute Dialysis Quality Initiative recommends that volume removal should be guided by the phase of critical illness [33], and the nephrologist has an active role in ensuring that ICUs follow these recommendations. In addition to these traditional complications of AKI, the practicing nephrologist should be aware of many of the non-traditional complications of AKI that likely contribute to mortality, including impaired immunity, and organ cross talk to kidney, heart, brain and other organs [34].
Acute RRT expert technician/clinician
Ideally the nephrologist should be engaged in patient care early enough to participate in in decisions to “maximize medical management”, and concomitantly, discussion on criteria for escalation of care with RRT should occur early in the course of AKI. This will allow the nephrologist to begin conversations with the critical care team and the family about options for care early in the disease course. Early nephrology referral will help maximize medical management, and if RRT is indicated, it will ease the coordination of care.
Understanding the strengths and weaknesses of the broad array of acute RRT options —from optimized intermittent hemodialysis (IHD), to prolonged intermittent RRT, and continuous RRT— allows for successful application of the most appropriate therapy to meet tailored daily care for each patient. The nephrologist must help balance the risk and benefits of starting vs. waiting to start RRT with the critical care team. When the decision to begin RRT is made, the nephrologist must provide the right prescription (i.e., blood flow, dose of clearance, mode of RRT, rate of ultrafiltration, type of anti-coagulation etc.) that will lead to maximal efficacy and minimal complications. The nephrologist must provide recommendations on pharmacokinetics of life-saving drugs (e.g., antibiotics), and the need for increased protein/vitamin and micronutrients required during RRT. Optimal delivery of care requires a systematic team-based goal-oriented approach agreed upon by nephrology & ICU teams. The team will need to know how to adapt to specific, and sometimes complex, scenarios (e.g., with extra-corporeal membrane oxygenation, heart assist devices, or therapeutic plasma exchange). Patients with hepatic failure, burns, high intra-cranial pressure, trauma, dysnatremias, intoxications, and inborn errors of metabolism required specialized considerations as well.
Management of end-stage renal disease in the ICU
The role of the nephrologist around the care of critically ill patients with end-stage renal disease (ESRD) and renal transplant is self-evident, as these patients already have pre-established connections with nephrology practice prior to their ICU admission. The nephrologist must provide guidance on vascular access, chronic medications, fluid balance, electrolytes homeostasis, immunosuppressive therapies, and adjustment of drug dose, which requires careful assessment based on residual renal function and drug elimination by RRT.
ROLE OF THE NEPHROLOGIST AS EDUCATOR
The nephrologist must be able to serve as an educator for ICU patients with severe electrolyte disorders, AKI, CKD, and ESRD. The nephrologist should be promoting awareness regarding AKI, and should be educating their colleagues about the impact of the institutional and financial burdens of AKI [35–39]. These should be repeatedly addressed through educational curricula, clinical rounds and personal communications.
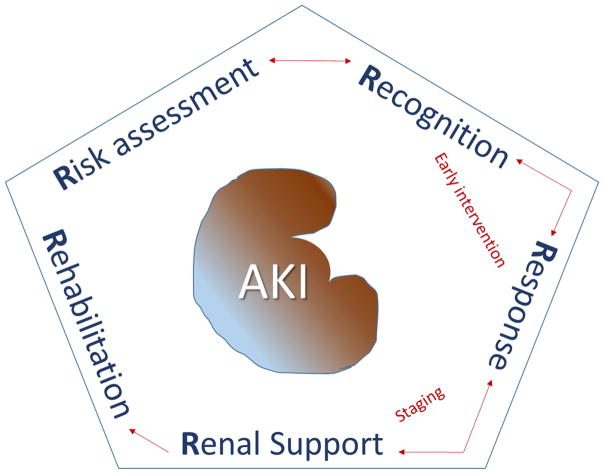
A recent review on the available teaching opportunities available to nephrologists highlighted eight unique roles for the nephrologist as an educator [40]. Recently, a framework for education regarding AKI has been proposed using the “5 R’s” concept. The 5 R’s include the following steps: “Risk assessment”, “Recognition”, “Response”, “Renal support”, and “Rehabilitation” (Figure 3). Recent developments in AKI that need to be emphasized include the development of consensus AKI definitions, the independent association between fluid overload and mortality, and the long-term complications of AKI [41–45]. Nephrologists should educate about these and other emerging concepts —for example, the clinical use of AKI biomarkers [46–48].
Figure 3.
Framework for education on AKI based on 5 R’s
The nephrologist must be able to educate on issues related to RRT including timing of starting and stopping RRT, modality selection, and specifics on the RRT prescription (i.e., access, blood flow, clearance dose, filtration fraction, anti-coagulation, ultrafiltration rates) as well as complications related to RRT [49–52]. Nephrologists must educate ICU providers regarding the specific care for the ESRD population, including issues related to choosing and maintaining vascular access, dialysis adequacy, and continuation of chronic medications, including chronic immunosuppressive medications. The added cardiovascular and infectious risks that CKD and renal transplant patients have should be also emphasized.
Critical care nephrology programs must also ensure that nurses can provide the safest and most effective therapy as possible, with an emphasis on the delivery of care and how acute care therapies (RRT and non-RRT) impact their patients. Nephrologists and nursing champions should provide didactic and hands-on teaching to all nursing staff who are asked to provide RRT. The use of high-fidelity simulation as an intervention during annual evaluation of knowledge skills, increases nurse satisfaction, and improves understanding of CRRT principles as well as critical thinking skills [17]. Nephrologists should play an active role in the ongoing education of pharmacists and pharmacy support staff to ensure understanding of drug dosing in the setting of dynamic changes in renal function and clearance of drugs during RRT. Similarly, education on nutritional support for ICU patients with kidney disease, regardless of whether it is enteral or parenteral nutrition, is imperative.
Importantly, the scientific evidence that informs these topics, and particularly AKI, is not limited to nephrology journals, as some of the most cited articles have been published in high-impact medicine journals (e.g., New England Journal of Medicine, Journal of the American Medical Association, Critical Care Medicine, Annals of Surgery). An often over-looked but important aspect of the educational role of the nephrologist is the interpretation of the critical care literature from a nephrology perspective. This may include articles that examine the impact of certain treatment or therapeutic approaches on renal outcomes in the critically ill; but also includes fundamental ideas such as the assessment of intravascular volume, the use of diuretics or choice of intravenous fluid selection [53–56]. These intersection of the “worlds” of critical care and nephrology should serve as the nest for collaborative educational opportunities [57].
In order to provide the best education on AKI and RRT, providers (particularly RRT program leaders) should engage in available practical and didactic programs (Table 3) on an ongoing bases. Attending these courses during nephrology fellowships provide students and the program with invaluable tools and knowledge.
Table 3.
Example of multi-disciplinary training opportunities available for critical care nephrology
|
CONCLUSIONS
The role of the nephrologist in the ICU has evolved as the practice of critical medicine has changed. He/she must have adequate knowledge and skills to diagnose and safely care for patients with complex diagnoses. In addition, the nephrologist has an important role in the education of those who care for critically ill patients in their institution. Finally, the nephrologist must help develop a program for RRT that strives to assess quality and improve care.
Figure 1.
Role of the nephrologist in the ICU
Table 1.
Practical considerations of the role of the critical care nephrologist
| Programmatic aspects of Critical Care Nephrology |
|
| Care of critically ill patients |
|
| Education of the ICU team |
|
Acknowledgments
Emma Perez-Costas, PhD (Department of Pediatrics, University of Alabama at Birmingham) performed the technical editing and proofreading of this manuscript.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicting interests that could affect the data and views presented in this manuscript. All authors had full final responsibility for the decision to publish. This manuscript has not been published previously in whole or part.
Voluntary disclosure of other non-conflicting activities: Dr. Askenazi is a speaker for the Acute Kidney Injury (AKI) foundation, Baxter, and BTG International Ltd; He also currently receives research funding from the National Institutes of Health (NIH) [R01–DK103608, R18–hs023763, R01–FD005092], Octapharma Pharmazeutika Produktionsges.m.b.H, as well as from several sources within University of Alabama at Birmingham [i.e., Center for Clinical and Translational Science (CCTS), Impact Funds for the Pediatric and Infant Center for Acute Nephrology (PICAN) from the Department of Pediatrics in conjunction with UAB School of Medicine and Childrens of Alabama]. Dr. Koyner has received funds from NxStage Medical Inc. as well as from Astute Medical Inc.; Dr. Bihorac has receive consulting fees from AtoxBio, she has received research grants from the Society of Critical Care Medicine, and is supported by R01 GM110240 and P50 GM-111152 from NIH–National Institute of General Medical Sciences; Dr. Vijayan is a speaker for NxStage Medical Inc.
References
- 1.Ronco C, Bellomo R. Critical care nephrology: the time has come. Nephrol Dial Transplant. 1998;13(2):264–267. doi: 10.1093/oxfordjournals.ndt.a027816. [DOI] [PubMed] [Google Scholar]
- 2.Srisawat N, Kellum JA. Acute kidney injury: definition, epidemiology, and outcome. Curr Opin Crit Care. 2011;17(6):548–555. doi: 10.1097/MCC.0b013e32834cd349. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein SL. Fluid management in acute kidney injury. J Intensive Care Med. 2014;29(4):183–9. doi: 10.1177/0885066612465816. [DOI] [PubMed] [Google Scholar]
- 4.Davenport A, Bouman C, Kirpalani A, et al. Delivery of renal replacement therapy in acute kidney injury: what are the key issues? CJASN. 2008;3(3):869–875. doi: 10.2215/CJN.04821107. [DOI] [PubMed] [Google Scholar]
- 5.Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Eng J Med. 2016 doi: 10.1056/NEJMoa1603017. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Winkelmayer WC. Early to dialyze: Healthy and wise? JAMA. 2016;315(20):2171–2172. doi: 10.1001/jama.2016.6210. [DOI] [PubMed] [Google Scholar]
- 7.Maddux FW, Nissenson AR. The evolving role of the medical director of a dialysis facility. CJASN. 2015;10(2):326–330. doi: 10.2215/CJN.04920514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiller B. The medical director and quality requirements in the dialysis facility. CJASN. 2015;10(3):493–499. doi: 10.2215/CJN.05810614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rewa O, Mottes T, Bagshaw SM. Quality measures for acute kidney injury and continuous renal replacement therapy. Curr Opin Crit Care. 2015;21(6):490–499. doi: 10.1097/MCC.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 10.Kee YK, Kim EJ, Park KS, et al. The effect of specialized continuous renal replacement therapy team in acute kidney injury patients treatment. Yonsei Medical J. 2015;56(3):658–665. doi: 10.3349/ymj.2015.56.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eding DM, Jelsma LR, Metz CJ, et al. Innovative techniques to decrease blood exposure and minimize interruptions in pediatric continuous renal replacement therapy. Crit Care Nurse. 2011;31(1):64–71. doi: 10.4037/ccn2011757. [DOI] [PubMed] [Google Scholar]
- 12.Askenazi DJ, Selewski DT, Paden ML, et al. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. CJASN. 2012;7(8):1328–1336. doi: 10.2215/CJN.12731211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrera-Gutierrez ME, Seller-Perez G, Lebron-Gallardo M, et al. Safety and efficacy of the MARS therapy applied by continuous renal replacement therapy (CRRT) monitors. Med Intensiva. 2007;31(7):367–374. doi: 10.1016/s0210-5691(07)74841-6. [DOI] [PubMed] [Google Scholar]
- 14.Song JH, Humes HD. The bioartificial kidney in the treatment of acute kidney injury. Curr Drug Targets. 2009;10(12):1227–1234. doi: 10.2174/138945009789753273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ronco C, Bellomo R. Continuous renal replacement therapy: evolution in technology and current nomenclature. Kidney Int Suppl. 1998;66:S160–164. [PubMed] [Google Scholar]
- 16.Ronco C, Ricci Z, De Backer D, Kellum JA, et al. Renal replacement therapy in acute kidney injury: controversy and consensus. Crit Care. 2015;19:146. doi: 10.1186/s13054-015-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mottes T, Owens T, Niedner M, et al. Improving delivery of continuous renal replacement therapy: impact of a simulation-based educational intervention. Pediatr Crit Care Med. 2013;14(8):747–754. doi: 10.1097/PCC.0b013e318297626e. [DOI] [PubMed] [Google Scholar]
- 18.Przybyl H, Androwich I, Evans J. Using High-Fidelity Simulation to Assess Knowledge, Skills, and Attitudes in Nurses Performing CRRT. Nephrol Nurs J. 2015;42(2):135–147. quiz 148. [PubMed] [Google Scholar]
- 19.Graham P, Lischer E. Nursing issues in renal replacement therapy: organization, manpower assessment, competency evaluation and quality improvement processes. Semin Dial. 2011;24(2):183–187. doi: 10.1111/j.1525-139X.2011.00835.x. [DOI] [PubMed] [Google Scholar]
- 20.Benfield CB, Brummond P, Lucarotti A, et al. Applying lean principles to continuous renal replacement therapy processes. Am J Health Syst Pharm. 2015;72(3):218–223. doi: 10.2146/ajhp140257. [DOI] [PubMed] [Google Scholar]
- 21.VA/NIH Acute Renal Failure Trial Network. Palevsky PM, Zhang JH, O'Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renal Replacement Therapy Study Investigators. Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 23.Chow NH, Liu HS, Lee EI, et al. Significance of urinary epidermal growth factor and its receptor expression in human bladder cancer. Anticancer Res. 1997;17(2B):1293–1296. [PubMed] [Google Scholar]
- 24.Kleger GR, Fässler E. Can circuit lifetime be a quality indicator in continuous renal replacement therapy in the critically ill? In J Artif Organs. 2010;33(3):139–146. doi: 10.1177/039139881003300302. [DOI] [PubMed] [Google Scholar]
- 25.Perazella MA. The urine sediment as a biomarker of kidney disease. Am J Kidney Dis. 2015;66(5):748–755. doi: 10.1053/j.ajkd.2015.02.342. [DOI] [PubMed] [Google Scholar]
- 26.Perazella MA, Coca SG, Hall IE, et al. Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2010;5(3):402–408. doi: 10.2215/CJN.06960909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pons B, Lautrette A, Oziel J, et al. Diagnostic accuracy of early urinary index changes in differentiating transient from persistent acute kidney injury in critically ill patients: multicenter cohort study. Crit Care. 2013;17(2):R56. doi: 10.1186/cc12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bagshaw SM, Langenberg C, Bellomo R. Urinary biochemistry and microscopy in septic acute renal failure: a systematic review. Am J Kidney Dis. 2006;48(5):695–705. doi: 10.1053/j.ajkd.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Keyserling HF, Fielding JR, Mittelstaedt CA. Renal sonography in the intensive care unit: when is it necessary? J Ultrasound Med. 2002;21(5):517–520. doi: 10.7863/jum.2002.21.5.517. [DOI] [PubMed] [Google Scholar]
- 30.Endre ZH, Kellum JA, Di Somma S, et al. Differential diagnosis of AKI in clinical practice by functional and damage biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:30–44. doi: 10.1159/000349964. [DOI] [PubMed] [Google Scholar]
- 31.Koyner JL, Davison DL, Brasha-Mitchell E, et al. Furosemide Stress Test and Biomarkers for the Prediction of AKI Severity. J Am Soc Nephrol. 2015;26(8):2023–2031. doi: 10.1681/ASN.2014060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. 2014;10(1):37–47. doi: 10.1038/nrneph.2013.232. [DOI] [PubMed] [Google Scholar]
- 33.Hoste EA, Maitland K, Brudney CS, et al. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113(5):740–747. doi: 10.1093/bja/aeu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faubel S, Shah PB. Immediate Consequences of Acute Kidney Injury: The Impact of Traditional and Nontraditional Complications on Mortality in Acute Kidney Injury. Adv Chronic Kidney Dis. 2016;23(3):179–185. doi: 10.1053/j.ackd.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Teixeira C, Garzotto F, Piccinni P, et al. Fluid balance and urine volume are independent predictors of mortality in acute kidney injury. Crit Care. 2013;17(1):R14. doi: 10.1186/cc12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76(4):422–27. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 37.Hobson CE, Ozrazgat-Baslanti T, Kuxhausen A, et al. Cost and Mortality Associated With Postoperative Acute Kidney Injury. Ann Surg. 2015;261(6):1207–1214. doi: 10.1097/SLA.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hobson CE, Singhania G, Bihorac A. Acute kidney injury in the surgical patient. Crit Care Clin. 2015;31(4):705–723. doi: 10.1016/j.ccc.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozrazgat-Baslanti T, Thottakkara P, Huber M, et al. Acute and Chronic Kidney Disease and Cardiovascular Mortality After Major Surgery. Ann Surg. 2016 doi: 10.1097/SLA.0000000000001582. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jhaveri KD, Perazella MA. Nephrologists as Educators: Clarifying Roles, Seizing Opportunities. Clin J Am Soc Nephrol. 2016;11(1):176–189. doi: 10.2215/CJN.12151214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 42.Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18(4):1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 43.KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl (2011) 2012;2(1):1–141. [Google Scholar]
- 44.Bihorac A, Brennan M, Ozrazgat-Baslanti T, et al. National surgical quality improvement program underestimates the risk associated with mild and moderate postoperative acute kidney injury. Crit Care Med. 2013;41(11):2570–2583. doi: 10.1097/CCM.0b013e31829860fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korenkevych D, Ozrazgat-Baslanti T, Thottakkara P, et al. The Pattern of Longitudinal Change in Serum Creatinine and 90-Day Mortality After Major Surgery. Ann Surg. 2016;263(6):1219–1227. doi: 10.1097/SLA.0000000000001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17(1):R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koyner JL, Parikh CR. Clinical utility of biomarkers of AKI in cardiac surgery and critical illness. Clin J Am Soc Nephrol. 2013;8(6):1034–1042. doi: 10.2215/CJN.05150512. [DOI] [PubMed] [Google Scholar]
- 48.Bihorac A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189(8):932–939. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 49.Vaara ST, Reinikainen M, Wald R, et al. Timing of RRT based on the presence of conventional indications. Clin J Am Soc Nephrol. 2014;9(9):1577–1585. doi: 10.2215/CJN.12691213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wald R, Adhikari NK, Smith OM, et al. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int. 2015;88(4):897–904. doi: 10.1038/ki.2015.184. [DOI] [PubMed] [Google Scholar]
- 51.Renal Replacement Therapy Study Investigators. Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 52.VA/NIH Acute Renal Failure Trial Network. Palevsky PM, Zhang JH, O'Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 54.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 55.Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012;255(5):821–829. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 56.Chawla LS, Davison DL, Brasha-Mitchell E, et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17(5):R207. doi: 10.1186/cc13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bihorac A. Guiding AKI prevention using biomarkers. Society of Critical Care Medicine. Critical Connections. 2015;14(2):1. [PMC free article] [PubMed] [Google Scholar]