Abstract
Background
Reprogramming of cardiac fibroblasts into induced cardiomyocyte-like cells (iCMs) in situ represents a promising strategy for cardiac regeneration. A combination of three cardiac transcription factors, Gata4, Mef2c and Tbx5 (GMT), can convert fibroblasts into iCMs, albeit with low efficiency in vitro.
Methods
We screened 5,500 compounds in primary cardiac fibroblasts to identify the pathways that can be modulated to enhance cardiomyocyte reprogramming.
Results
We found that a combination of the transforming growth factor (TGF)-β inhibitor SB431542 and the WNT inhibitor XAV939 increased reprogramming efficiency eight-fold when added to GMT-overexpressing cardiac fibroblasts. The small-molecules also enhanced the speed and the quality of cell conversion, as we observed beating cells as early as 1 week after reprogramming compared to 6–8 weeks with GMT alone. In vivo, mice exposed to GMT, SB431542, and XAV939 for 2 weeks after myocardial infarction showed significantly improved reprogramming and cardiac function compared to those exposed to only GMT. Human cardiac reprogramming was similarly enhanced upon TGF-β and WNT inhibition and was achieved most efficiently with GMT plus Myocardin.
Conclusions
Thus, TGF-β and WNT inhibitors jointly enhance GMT-induced direct cardiac reprogramming from cardiac fibroblasts in vitro and in vivo and provide a more robust platform for cardiac regeneration.
Keywords: cardiac differentiation, heart regeneration, heart, calcium, transcription factors, reprogramming
Introduction
Heart failure affects 23 million people worldwide and is typically caused by loss of cardiomyocytes or dysfunction of existing cardiomyocytes 1. A common cause of cardiomyocyte loss is ischemic heart disease leading to myocardial infarction, and, due to the limited ability of the heart to regenerate, damage is permanent and progressive. Despite advances in medical therapy, there is currently no strategy to restore muscle mass other than orthotopic heart transplant, which is limited in number and long-term efficacy. Cell-based therapies used in human trials to date have demonstrated that transplanted cells do not become cardiomyocytes and fail to persist in the heart 2. Transplant of pluripotent stem cell–derived cardiomyocytes is being tested and could be of value if issues of survival, maturity and electrophysiological integration can be overcome 3. In situ reprogramming of cells using lineage-enriched transcription factors (TFs) into the cell type lost in disease represents a promising alternative to cell-based approaches to tissue regeneration 4. In the heart, an abundant pool of non-myocytes, largely cardiac fibroblasts, can be harnessed for conversion into induced cardiomyocyte-like cells (iCMs) using a combination of TFs 4.
In rodents, direct intramyocardial viral introduction of three core cardiac transcription factors, Gata4, Mef2c and Tbx5 (GMT), after coronary ligation results in conversion of non-myocytes into iCMs that electrically couple with existing cardiomyocytes, improve cardiac function and decrease scar size 5. However, the efficiency remains limited, particularly in vitro, where the majority of CMs are only partially reprogrammed 4. The signals present in vivo resulting in improved quality of reprogramming remain unknown, but suggest that alteration of culture conditions or signaling pathways could enhance in vitro, and possibly in vivo, cardiac reprogramming.
Since the first successful generation of induced cardiomyocyte-like cells (iCMs) 4, we and others have taken a candidate approach to identify combinations of genes or conditions that enhance cardiac reprogramming. By altering the stoichiometry of GMT 6, including additional factors, or manipulating signaling pathways, the quality and efficiency of iCMs generated in vitro can be improved 7–14. In most cases, improvements in efficiency were largely found in mouse embryonic fibroblasts (MEFs) in the presence of GMT plus additional transcription factors, with limited improvement in primary cardiac fibroblasts. Although recent siRNA-mediated knockdown of Bmi1 improved efficiency of cardiac fibroblasts in vitro 15, it remains to be determined if any of the above approaches enhance in vivo reprogramming in mice or influence reprogramming of human cardiac fibroblasts, both of which are critical to future translation.
Here we report the first high-throughput chemical screening in primary mouse cardiac fibroblasts and reveal pathways that can be modulated to enhance cardiomyocyte reprogramming from cardiac fibroblasts using the minimal combination of GMT. Chemical screening converged on TGF-β and WNT signaling pathways as barriers to reprogramming. We show that chemically inhibiting both pathways together boosts the efficiency, quality, and speed of converting postnatal mouse or human cardiac fibroblasts to cardiomyocyte-like cells in vitro. Most importantly, in vivo delivery of these inhibitors along with GMT in an acute model of mouse myocardial infarction (MI) improved cardiac function, generation of iCMs and scarring compared to GMT alone. These findings provide the first demonstration of a combined gene therapy and drug approach to cardiac regeneration in vivo and pave the way for new translational approaches for heart failure.
Materials and Methods
Tissue Collection and Fibroblast Isolation
The animal procedures followed were in accordance with the institutional guidelines and approved by the University of California, San Francisco Institutional Animal Care and Use Committee. Mouse cardiac fibroblasts were isolated from P0-P4 αMHC-GFP transgenic neonates using the migration method as previously described 4, 16. Heart tissue was isolated, minced, and cultured on gelatin-coated plates in fibroblast explant media (20% fetal bovine serum (FBS) in IMDM) for one week at 37°C. Migrated cells were washed twice with phosphate buffered saline (PBS), digested in 0.05% Trypsin for 5 minutes, and quenched with fibroblast explant media. Tissues were filtered through a 70-µM filter and pelleted. Pelleted cells were stained for 20 minutes with Thy-1-APC (Ebioscience, anti-mouse/rat CD90.1 thy-1.1 #17-0900-82) and washed twice with PBS as previously described. APC+ cells were isolated by fluorescence activated cell sorting (FACS), plated onto 10-cm gelatin-coated plates, and used fresh (without freezing) for all studies. All cell preparations were tested for mycoplasma contamination.
Reprogramming of Mouse Cardiac Fibroblasts to iCMs
Direct conversion of Thy1+ cardiac fibroblasts to iCMs was completed as previously described 16. pMXs-Gata4, pMXs-Mef2c, pMXs-Tbx5, polycystronic pMXs-Mef2c-Gata4-Tbx5 (GMT polycystronic) or pMXs-dsRed were constructed as previously described 4, 17. Retroviral vectors were packaged using Fugene HD (Roche) and delivered in OptiMEM (10 µg) to 15-cm plates containing ~80% confluent PlatE cells in fibroblast explant media, as previously described 5. Viral supernatant was collected 48 hours post-transfection and used to infect cardiac fibroblasts with the addition of 0.6 µg/ml polybrene (Chemicon) and added to cardiac fibroblasts at day −1. After 24 hours, the culture medium was replaced with cardiomyocyte culture medium (iCM medium) 16 at day 0, and replaced every 3–4 days. We used the three separate Gata4, Mef2c, and Tbx5 retroviruses in the initial drug screening and the in vivo experiments; however, for further in vitro experiments following the initial screening we used a GMT polycystronic retrovirus.
Please see Supplementary Methods for more details regarding for Drug Screening, FACS Analyses and Sorting, Western Blotting, Real-time PCR, RNAseq Analyses, Animal experiments, MRI, Isolation of adult CMs, Calcium-transient assessment, Action potential recordings, and human cardiac reprogramming.
Statistical analyses
Differences between groups were examined for statistical significance using unpaired Student’s t-tests or ANOVA. A P-value < 0.05 was regarded as significant. Error bars indicate standard error of the mean (s.e.m.).
Results
SB431542 and XAV939 combinatorially enhance efficiency of cardiac reprogramming
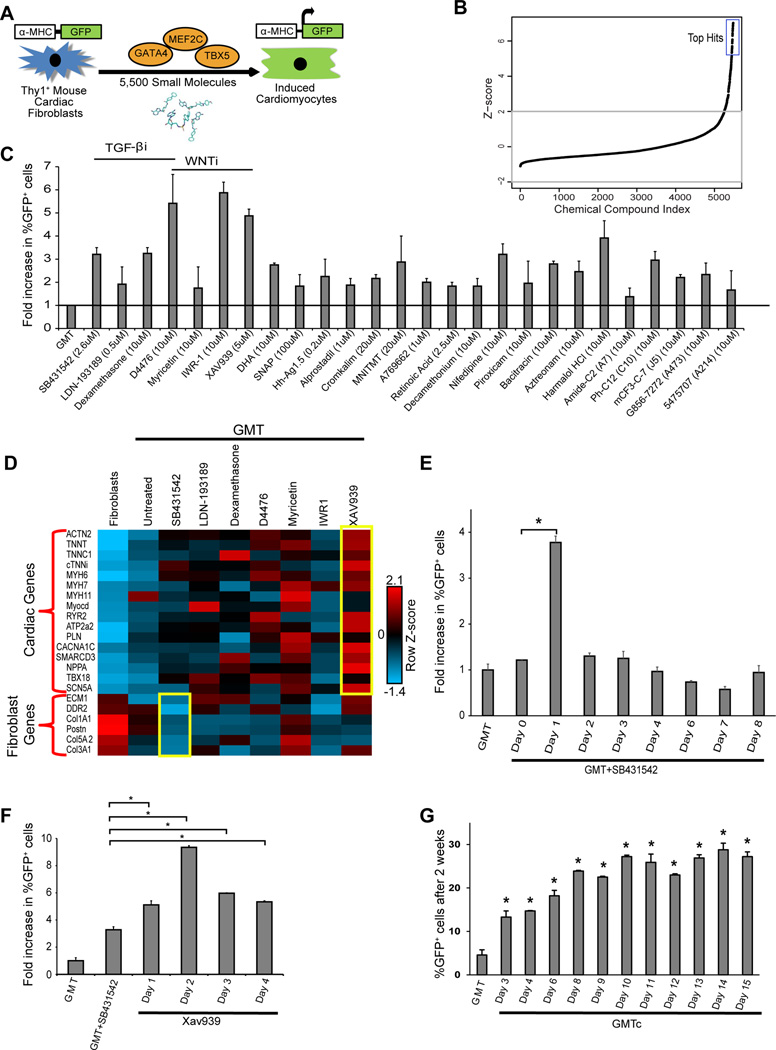
To identify biological pathways that could be manipulated to improve cardiac reprogramming, we used a chemical biology approach involving an unbiased small molecule library screen. We purified Thy1+ mouse cardiac fibroblasts from transgenic mice containing green fluorescent protein (GFP) under control of the cardiomyocyte-specific α-myosin heavy chain (α-MHC) promoter 4. Cardiac reprogramming was induced with a polycystronic GMT retrovirus (Mef2c-P2A-Gata4-T2A-Tbx5), which optimizes stoichiometry of each TF 6. Potential iCMs were detected by activation of the α-MHC-driven GFP reporter. After optimizing this method of direct reprogramming in a 384-well format, we screened a total of 5500 compounds from libraries of toxicologically tested compounds (e.g., Ding lab 18, LOPAC 19, TOCRIS 20, and SPECTRUM 21 small molecule libraries) using high-throughput, high-content imaging (Figure 1A). Compounds were added one day after GMT transduction, and α-MHC-GFP+ cells were quantified after 2 weeks. We identified 26 top hits with a Z score over 5 (Figure 1B) and following validation, these hits increased the percentage of GFP+ iCMs by two- to six-fold (Figure 1C). The top hits included three molecules that inhibit TGF-β signaling (SB431542 22, LDN-193189 23, and dexamethasone 24–26), three that inhibit WNT signaling (XAV939 27, IWR1 28, and myricetin 29), and one that inhibits both WNT and TGF-β signaling (D4476) 30, 31 (Figure 1C). The multiple chemical modulators of the same pathways suggested these signaling pathways reliably impact cardiac reprogramming and we focused further analysis in this area.
Figure 1. High-throughput small-molecule screening reveals barriers to direct cardiac reprogramming.
(A) Schematic for the small molecule-screening strategy using primary mouse cardiac fibroblasts (Thy1+ cells) from α-MHC-GFP transgenic mice. (B) Z-score plot for the 5500 compounds showing the top hits with Z-score over 5. (C) Bar graph showing validation of the hits from our small molecule screen, seven of which are annotated to inhibit TGF-β or WNT signaling (n=3). (D) Row-normalized Z-score heat map representing expression of major cardiac and fibroblast genes as determined by qRT-PCR of RNA extracted from fibroblasts (Control), iCMs reprogrammed for 2 weeks with GMT (GMT) or GMT + the indicated compounds. Bar graphs show that the effect of SB431542 (E) or XAV939 (F) in enhancing reprogramming is time dependant (n=3, *p<0.05). (G) % α-MHC-GFP+ cells two weeks after GMT infection with or without SB431542 + XAV939 for the number of days indicated (GMTc) (n=3, *p<0.05).
To identify which of the putative WNT or TGF-β inhibitor compounds most effectively improve GMT-reprogramming quality and to exclude false positives, we conducted qRT-PCR for a panel of endogenous cardiac and fibroblast genes 2 weeks after initiating conversion with each compound. SB431542 was the most efficient of the TGF-β inhibitors at downregulating fibroblast gene expression and XAV939 was the most efficient of the WNT inhibitors at activating cardiac gene expression at 2 weeks of reprogramming (Figure 1D). We also found that SB431542 (2.6 µM) (Figure S1A) was most effective if added 24 hours after GMT infection (day 1 of reprogramming) (Figure 1E). We tested various doses and timing of XAV939 to identify its optimal timing and concentration. We found that 5 µM was the most effective dose and resulted in similar enhancement when added at any time during the first 8 days of reprogramming (Figure S1B, C). Upon combining the two small molecules, we found that maximum reprogramming efficiency was achieved by adding XAV939 at day 2 (48 hours) of reprogramming, after adding SB431542 at day 1. This protocol resulted in greater than eight-fold increase in cardiac reprogramming, as indicated by the increase in α-MHC-GFP+ iCMs (Figure 1F). Furthermore, removal of compounds at serial days of reprogramming revealed that the compounds were dispensable after ~1 week of reprogramming (Figure 1G).
SB431542 and XAV939 enhance the efficiency, speed, and quality of iCM generation
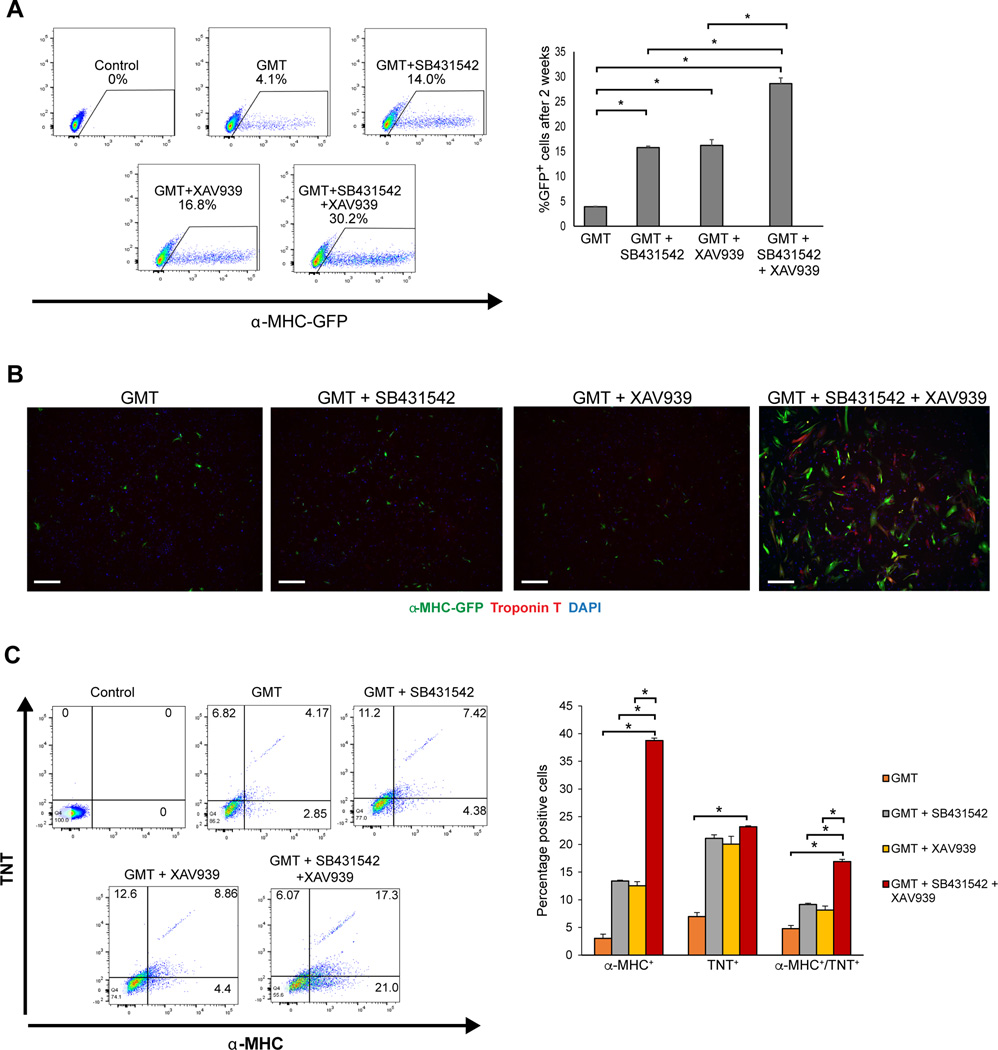
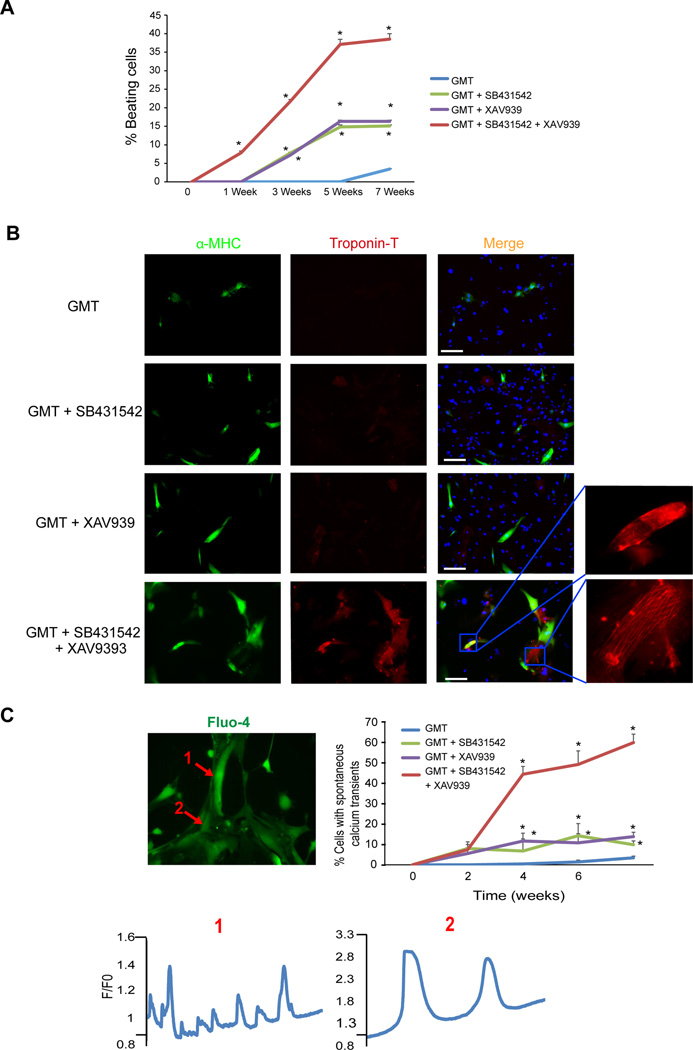
After validating and optimizing the timing, dose, and conditions for adding small molecules to the reprogramming cocktail, we treated GMT-overexpressing fibroblasts with SB431542 (2.6 µM) at day 1 and XAV939 (5 µM) at day 2. This combination of SB431542 at day 1 and XAV939 at day 2 significantly increased GMT-induced reprogramming efficiency within two weeks to ~30% α-MHC-GFP+ iCMs from primary cardiac fibroblasts compared to ~15% in the presence of either compounds and 4% without any compounds (Figure 2A, B). After 4 weeks of reprogramming, each compound alone resulted in a doubling of the number of iCMs positive for α-MHC and cardiac Troponin-T (cTNT), while reprogramming in the presence of both compounds resulted in a 4-fold increase in the number of double positive iCMs compared to GMT alone (Figure 2C). With SB431542 or XAV939, beating cells appeared as early as 3 weeks after GMT infection. Remarkably, with the combination of SB431542 and XAV939, beating cells appeared as early as 1 week, compared to 6–8 weeks with GMT alone (Figure 3A, Movies S1–2). We also observed clear cTNT staining and advanced sarcomere organization in iCMs after only 2 weeks of reprogramming with GMT + SB431542/XAV939 versus 4 weeks with GMT + either compound alone 16 (Figure 3B). Moreover, over ~50% of the cells possessed spontaneous calcium transients within 4 weeks of reprogramming in the presence of the two compounds (Figure 3C and Movie S3).
Figure 2. SB431542 and XAV939 enhance the quantity of cardiac reprogramming in vitro.
(A) Representative FACS plots for iCMs labelled with the live cell reporter, α-MHC-GFP+, after two weeks of reprogramming and quantification (n=3, *p<0.05). (B) Representative images of immunostaining for cardiac markers αMHC (Green) and TNT (Red) in cardiac fibroblasts treated with either GMT, GMT+SB431542, GMT+XAV939 or GMT+SB431542+XAV939 for two weeks (scale bar 200 µm). (C) Representative FACS plots and quantification for cells immuno-stained for Troponin-T (TNT) and α-MHC cardiac marker after 4 weeks of reprogramming (n=3, *p<0.05).
Figure 3. SB431542 and XAV939 enhance the quality and speed of cardiac reprogramming in vitro.
(A) Quantification of percent beating cardiomyocytes over time showing that SB431542 and XAV939 accelerate the appearance of spontaneously beating cells as early as 1 week and increase the number of beating cells (n=3 independent experiment, *p<0.05, GMT (Blue), GMT + SB431542 (Green), GMT + XAV939 (Purple), GMT + SB431542 + XAV939 (Red)) (B) Within 2 weeks of reprogramming, troponin T and enhanced sarcomere organization were observed in the GMT + SB431542 + XAV939 group, but not in other settings (scale bar 50 µm). (C) Calcium transients from spontaneously contracting iCMs loaded with Fluo-4 calcium dye (Movie S3) (1 and 2 from two adjacent cells that beat at different rates) after 3 weeks of reprogramming, and quantification of the percentage of iCMs that exhibit spontaneous calcium transients over 8 weeks of reprogramming (n=200 cells analyzed at each time point from two independent experiments, *p<0.05 compared to GMT).
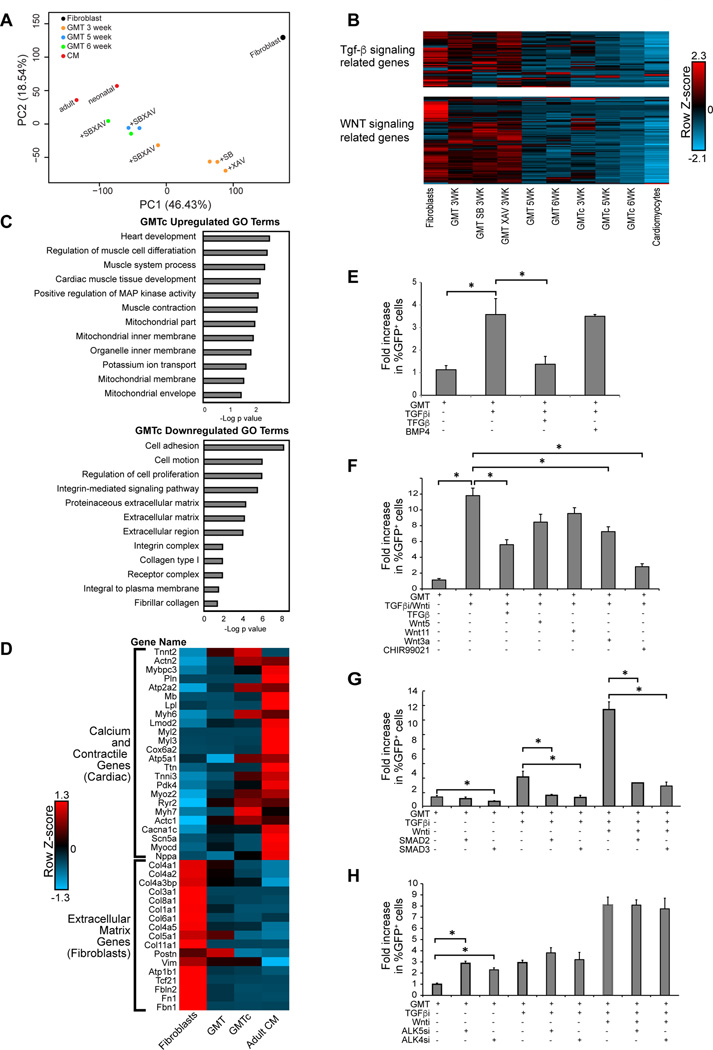
We analyzed gene expression profiles in iCMs 1, 2, 3, 5 and 6 weeks after cardiac reprogramming using RNAseq. The data analysis was performed on the average values of fragments per kilobase of exon per million fragments mapped (FPKM) of 2 replicates in each group. 1, 2 and 3 week iCMs treated with SB431542 and XAV939 (GMTc) had a more cardiomyocyte-like transcriptional profile compared to GMT iCMs as reflected by principal component analysis (PCA) and analysis of the cardiomyocyte and fibroblast genes that were inadequately regulated with GMT alone (Figure S2D). iCMs treated for 3 weeks with GMT + SB431542 and XAV939, but not iCMs treated with a single compound, had a more cardiomyocyte-like transcriptional profile as reflected by PCA (Figure 4A). Reprogramming with GMTc for 5 or 6 weeks resulted in gene expression profiles closer to adult mouse ventricular cardiomyocytes than reprogramming with GMT alone (Figure 4A). Furthermore, iCMs reprogrammed with GMTc for 3, 5 or 6 weeks significantly downregulated genes with Gene Ontology (GO) terms related to TGF-β and WNT signaling, similar to cardiomyocytes (Figure 4B). Comparing gene expression profiles between GMT and GMTc at 5 weeks, we found that the top differentially regulated GO terms were related to regulation of extracellular matrix, ion channels, and muscle formation (Figure 4C). Upon closer analysis of the most highly expressed cardiac-contractile, ion-handling, and extracellular matrix genes (Figure 4D), we found that the compounds brought the expression of these genes very close to that of adult cardiomyocytes, albeit not completely. Thus, SB431542 and XAV939 together increased not only the quantity, but also the quality and speed of cell-fate conversion in vitro.
Figure 4. B431542 and XAV939 enhance reprogramming by inhibiting TGF-β and canonical WNT signalling, respectively.
(A) Principal component analysis of RNA-seq data showing that GMT-reprogrammed fibroblasts are at an intermediate state between fibroblasts and cardiomyocytes. Addition of SB431542 and XAV939 (+SBXAV) to GMT-transduced fibroblasts resulted in an advanced state of reprogramming, closer to the cardiomyocyte (CM) state, compared to GMT alone. Neither SB431542 (+SB) or XAV939 (+XAV) alone with GMT showed this effect. (B) Heat map showing differential expression of genes with GO terms related to TGF-β (upper panel) and WNT (lower panel) signaling. (C) Bar graph for the top gene ontology (GO) terms of the differentially expressed genes between GMT and GMTc iCMs at 5 weeks by RNA-seq. (D) Heat map of genes expressed in cardiomyocytes or fibroblasts showing that SB431542 and XAV939 (GMTc) enhanced conversion toward an adult cardiomyocyte gene program compared to GMT alone (GMT). (E) Excess TGFβ1 (TGF-β) ligand during reprogramming reversed the effect of SB431542 (TGFβi); BMP4 did not have a significant effect. (F) The glycogen synthase kinase 3 beta (GSK3β) inhibitor CHIR99021, which promotes canonical WNT signaling, largely reversed the effects of SB431542 (TGFβi) and XAV939 (WNTi); activating the non-canonical WNT pathway through WNT5 or WNT11 only had an insignificant effect (n=3, *p<0.05). (G) Overexpression of constitutively active SMAD2 or SMAD3 abolished enhanced cardiac reprogramming by SB431542 (TGFβi) (n=3, *p<0.05). (H) Knocking down either ALK4 or ALK5 receptors using siRNA enhanced cardiac reprogramming similar to SB431542 (TGFβi), without affecting the efficiency of reprogramming with SB431542 (n=3, *p<0.05).
SB431542 and XAV939 function by reducing TGF-β- and WNT-dependent barriers to cardiac reprogramming
Next, we investigated the mechanisms by which SB431542 and XAV939 function, specifically testing if they affect reprogramming through TGF-β and WNT signaling, respectively. Phosphorylation of SMAD2/3 (indicator of TGF-β activation) and the active form of β catenin (indicator of WNT activation) were significantly reduced (Figure S2A, B) in the GMTc setting compared to GMT alone. We also observed that activating TGF-β or canonical WNT signaling reversed the reprogramming efficiency gains induced by SB431542 or XAV939, respectively. Adding excess TGF-β1 ligand during reprogramming reversed the effect of SB431542 and partially inhibited the combined effect of SB431542 and XAV939; however, BMP4, which regulates other aspects of TGF-β signaling, did not significantly affect cardiac reprogramming (Figure 4E). Moreover, overexpression of constitutively active SMAD2 or SMAD3 (TGF-β signaling effectors) abolished cardiac reprogramming enhancement by SB431542 (Figure 4G). Because SB431542 inhibits activin receptor-like kinase (ALK) 4 and ALK5 receptors 22, we tested the effect of knocking down ALK4 or ALK5 with siRNA on direct cardiac reprogramming. An ~80% reduction of ALK4 or ALK5 (Figure S2C) enhanced direct cardiac reprogramming, similar to SB431542 (Figure 4H). Similarly, WNT3a, which activates canonical WNT signaling, partially blocked the effects of SB431542 and XAV939 on cardiac reprogramming; however, activating the non-canonical WNT pathway through WNT5 or WNT11 showed moderate, but not statistically significant, reduction in reprogramming. Furthermore, adding the glycogen synthase kinase 3β (GSK3β) inhibitor CHIR99021, which activates the WNT pathway, completely reversed the combined effect of SB431542 and XAV939 (Figure 4F). Thus, SB431542 and XAV939 appear to enhance cardiac reprogramming by inhibiting TGF-β and WNT signaling, respectively.
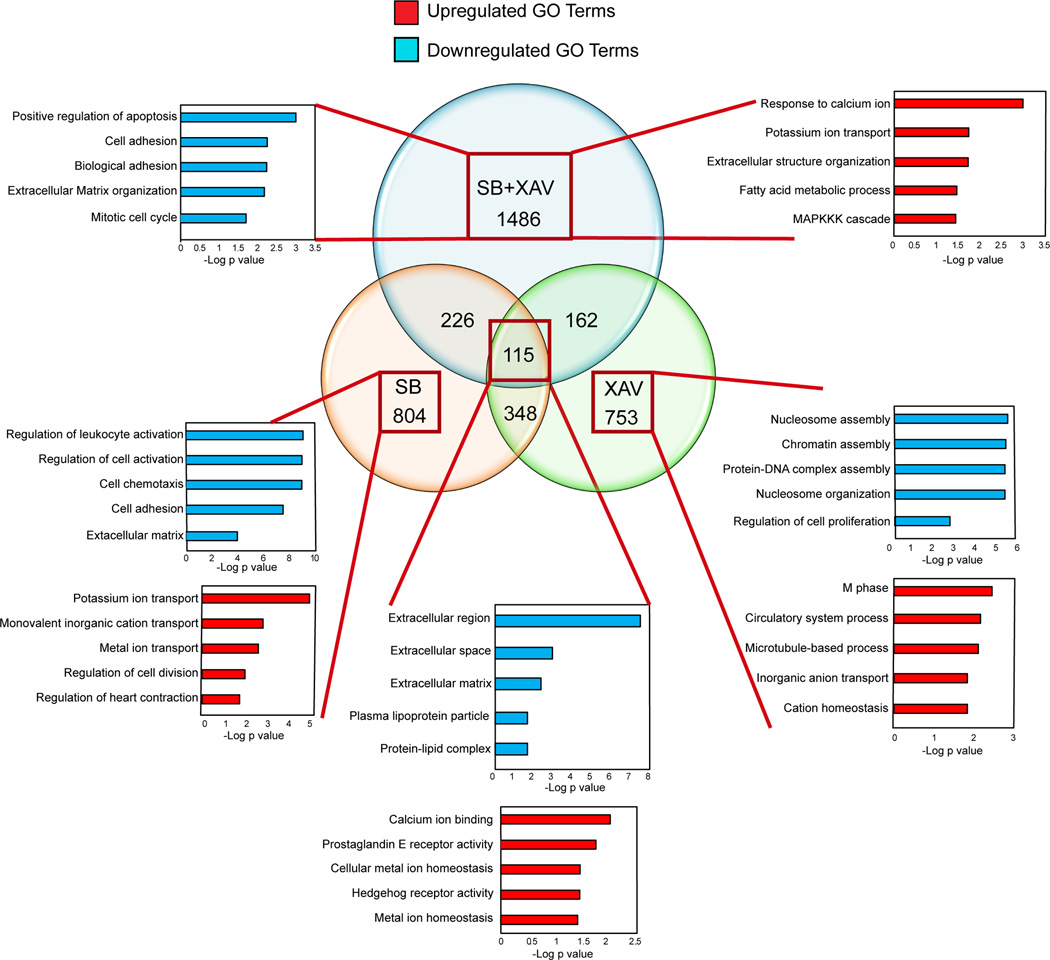
Our primary GO enrichment analysis of the RNA-seq data from 3 week reprogrammed iCMs in the presence of SB431542, XAV939 or SB431542+XAV939 highlighted the major downstream modification by the compounds to increase the efficiency of reprogramming (Figure 5). SB431542 reprogrammed iCMs showed downregulation in genes that are enriched in GO terms related to fibrotic signal and extra cellular matrix formation, consistent with what has been reported in other reprogramming settings 13, 14. However, XAV939 reprogrammed iCMs possessed downregulation in genes primarily involved in chromatin modulation, DNA packaging and nucleosome organization, which indicates that inhibition of WNT signaling may act on chromatin modulation to facilitate GMT chromatin binding at the cardiac gene sites, as previously reported for WNT signaling in other cell types 32. However, iCMs reprogrammed in the presence of both SB431542 and XAV939 showed specific upregulation of GO terms related to calcium handling, ion channels, fatty acid metabolism and MAPKK signaling, all consistent with a more differentiated cardiomyocyte state (Figure 5).
Figure 5. Enriched GO terms for differentially regulated genes in iCMs treated with either SB431542, XAV939 or SB431542 + XAV939 for 3 weeks.
Venn diagram shows the number of differentially expressed genes by at least 5-fold between iCMs reprogrammed with GMT alone compared to iCMs reprogrammed with GMT + SB431542 (SB) or GMT + XAV939 (XAV) or GMT + SB431542 + XAV939 (SB+XAV) for 3 weeks. The bar graphs shows –log p values for the top enriched GO terms for the upregulated (red) or downregulated (blue) genes under each condition.
TGF-β and WNT inhibition enhance cardiac reprogramming in vivo
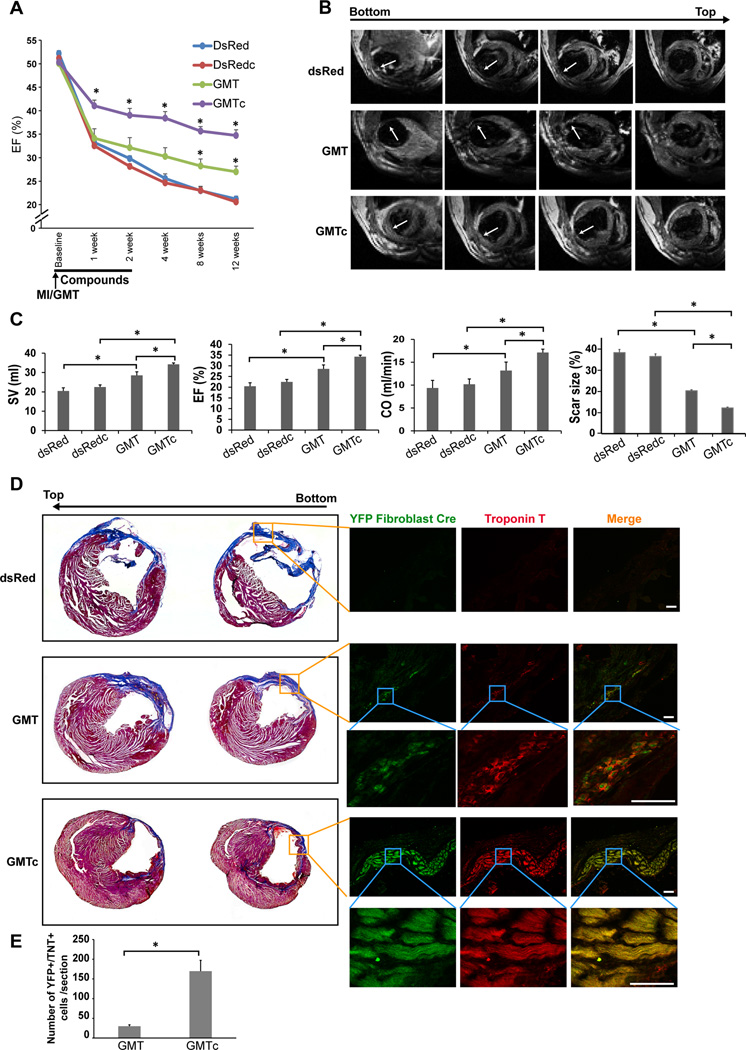
We previously showed that intramyocardial injection of GMT successfully reprogrammed fibroblasts into iCMs in vivo, increased heart function, and decreased scar size after injury, but improvement in the technology is likely necessary for further in vivo translation 5. We tested the effects of inhibiting TGF-β and WNT signaling on cardiac reprogramming in vivo by injecting SB431542 (10 mg/kg/day) 33 and XAV939 (2.5 mg/kg/day) 34 intraperitoneally every day for 2 weeks after coronary ligation and intramyocardial injection of GMT-encoding retrovirus (GMTc). All the surgeries, echocardiography, and analyses were conducted blindly and animals decoded after all data was collected. GMTc significantly enhanced cardiac function compared to treatment with GMT alone, as reflected by changes in the ejection fraction (EF) assessed by echocardiography (Figure 6A). The improved function occurred as early as 1 week after MI, consistent with our in vitro observations showing an acceleration of reprogramming with beating cells at 1 week, and the functional improvement persisted over 12 weeks. The inhibitors alone did not significantly affect cardiac function. 12 weeks after MI, we conducted blinded magnetic resonance imaging (MRI) to evaluate heart structure and function, as it is the most accurate form of measurement. Thick muscle within the infarct region was observed only in the group treated with GMTc, even at the apex of the heart (Figure 6B). Heart function revealed by MRI was significantly improved in all animals treated with GMTc compared to GMT alone, as assessed by changes in stroke volume (SV), EF, and cardiac output (CO) (Figure 6C).
Figure 6. SB431542 and XAV939 enhance in vivo reprogramming with GMT.
(A) Changes in ejection fraction (EF), assessed by echocardiography at 1, 2, 4, 8 and 12 weeks, showed that treatment with GMTc significantly improved cardiac function compared to animals treated with GMT alone; the compounds alone did not affect cardiac function (dsRed or dsRedc) (n=5–8, *p<0.05). (B) Representative magnetic resonance imaging (MRI) after 12 weeks of reprogramming shows the infarct site (white arrows) in the three groups. (C) Stroke volume (SV), ejection fraction (EF), and cardiac output (CO) measured by MRI, and scar size quantified by histology, were significantly improved in GMTc-treated mice compared to GMT-treated or control mice (dsRed or dsRedc) (n=6–7, *p<0.05). (D) Representative histological sections with Masson’s Trichrome staining or immunofluorescence staining showing that the remuscularization around the infarct area was due to newly formed iCMs (stained positive for troponin T (red) and the YFP reporter (green) when experiments performed in Periostin-Cre:Rosa-YFP transgenic mouse (scale bar 50 µm). (E) Quantification of the reprogrammed cell number of in vivo reprogrammed iCM number in multiple heart sections (n= 5 animals in each group, *p<0.05).
We conducted histological analyses to quantify the scar size and detect the presence of muscle within the infarct area of treated hearts. Consistent with our in vivo imaging observations, we found that scar size was significantly decreased further with GMTc compared to GMT alone (Figure 6C). We also observed threads of myocytes that developed within the infarct site of hearts treated with GMT alone, similar to our previous report 5; however, in hearts isolated from animals treated with GMTc, we observed thick bands of myocytes throughout the infarct zone (Figure 6D and Figure S3A). To investigate whether this remuscularization was due to reprogramming of resident fibroblasts, we crossed ROSA-Lox-Stop-Lox-YFP mice with Periostin-Cre mice to generate ROSA-YFP/Periostin-Cre lineage-tracing mice. These mice express YFP only in non-myocytes—largely fibroblasts—and in their descendants, and thereby distinguish reprogrammed cardiomyocytes from endogenous cardiomyocytes (Figure S3B). We previously validated this system, evaluating for potential of false positive data from cell-cell fusion events or leakiness of the Cre 5. We found that the remuscularization around the infarct area was due to newly formed iCMs, as these cells stained positive for Troponin-T and the Periostin-YFP reporter (Figure 6D and Figure S3D). However, we did not find any YFP+ cells in the control groups or in areas distal to the infarct site (Figure 6D and Figure S3C).
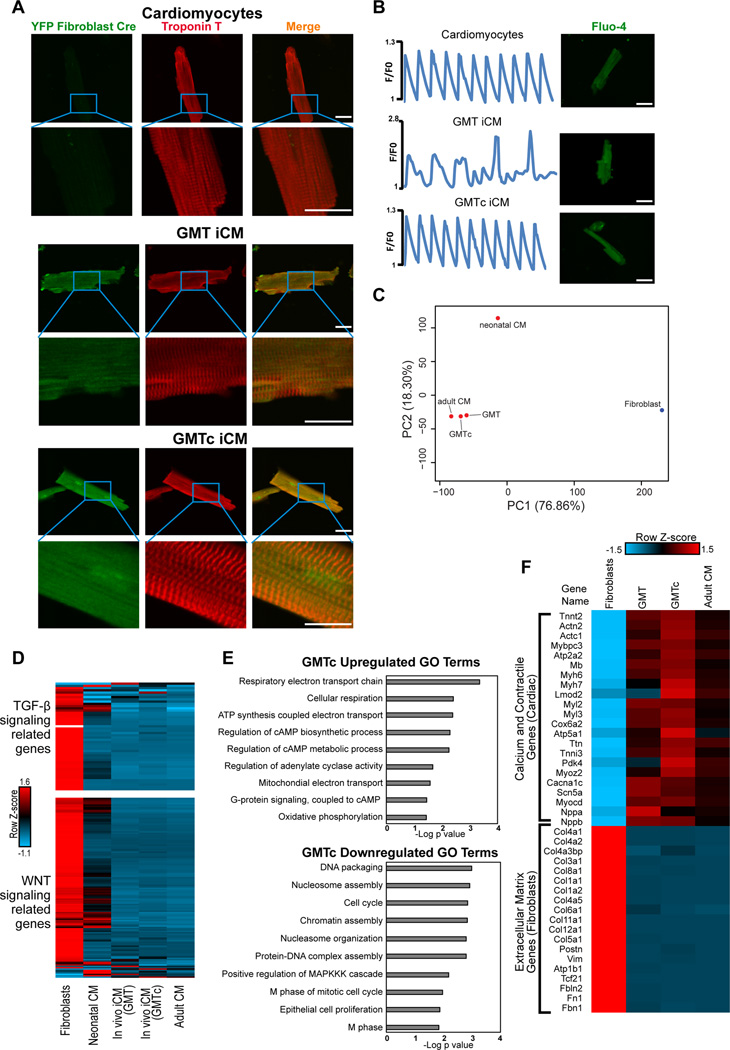
To assess the functionality and quality of the in vivo iCMs, we used a Langendorff preparation to isolate iCMs from ROSA-YFP/Periostin-Cre mice. The number of YFP+ iCMs significantly increased by five-fold in animals treated with GMTc compared to GMT alone (Figure 6E). Almost all iCMs isolated from either GMT- or GMTc-treated mice were rod shaped and formed well-organized sarcomere structures, similar to adult cardiomyocytes (Figure 7A). All cells isolated from GMT-treated mice exhibited spontaneous calcium transients, while endogenous cardiomyocytes were quiescent until electrically stimulated, reflecting their greater hyperpolarization. In contrast, over 70% of the cells isolated from GMTc mice were quiescent but became contractile upon electrical stimulation, indicating a greater similarity to endogenous cardiomyocytes (Figure 7B and Movies S4–6). In addition, analysis of the calcium transients revealed that the calcium amplitude and the time constant of calcium decay (Tau) of GMTc iCMs were more similar to control cardiomyocytes than GMT iCMs (Figure S4A). Although electrophysiological analysis of the action potential of GMT iCMs and GMTc iCMs by patch-clamp showed similar maximum diastolic potential at ~-80 mV, the action potential amplitude as well as its duration were significantly greater in GMTc iCMs compared to GMT iCMs, and similar to endogenous CMs (Figure S4B). These data indicate that GMTc iCMs are closer to adult control cardiomyocytes in terms of functionality compared to GMT iCMs.
Figure 7. SB431542 and XAV939 enhanced the quality of in vivo cardiac reprogramming.
(A) Representative image of in vivo reprogrammed iCMs isolated from either GMT- or GMTc-treated mice showing rod shapes and well-organized sarcomere structures similar to adult cardiomyocytes (red, troponin T; green, YFP-Cre) (scale bar, 25 µm). (B) Calcium-transient traces from cells labelled with the Fluo-4 calcium dye and isolated from GMT-treated mice (middle panel), which exhibited spontaneous calcium transients. Cells isolated from GMTc mice (bottom panel) did not beat spontaneously and synchronized with external electrical stimulation at 1 Hz, similar to the control CM (top panel). (scale bar, 25 µm). (C) Principal component analysis plot for the global transcriptome of fibroblasts, neonatal mouse cardiomyocytes (CM), GMT iCMs in vivo (GMT), GMTc iCMs in vivo (GMTc), and adult ventricular cardiomyocytes assessed by RNA-seq (n=2 biological replicates for each group). (D) Heat map showing differential expression of genes with GO terms related to TGF-β (upper panel) and WNT (lower panel) signaling. (E) Bar graph for the top GO terms for the differentially expressed genes from RNA-sequencing data between GMT and GMTc iCMs in vivo. (F) Row normalized Z-score heat map showing the relative gene expression of major cardiac and fibroblast genes in GMT and GMTc iCMs in vivo compared with control adult ventricular cardiomyocytes. (n=3 for all samples).
We also conducted RNA-seq to compare whole-transcriptome changes between endogenous cardiomyocytes, GMT-iCMs, and GMTc-iCMs isolated from in vivo reprogrammed hearts. The gene expression signatures of GMT- or GMTc-iCMs were more similar to adult ventricular cardiomyocytes than neonatal cardiomyocytes, with GMTc-iCMs being closer to adult cardiomyocytes as reflected by PCA analysis (Figure 7C). Additionally, compared to neonatal CMs, GMT-iCMs and GMTc-iCMs displayed more fully downregulated genes with GO terms related to TGF-β and WNT signaling, similar to adult cardiomyocytes (Figure 7D). However, GO analysis for the genes that were differentially regulated by at least two-fold between GMT- and GMTc-iCMs revealed downregulation of genes involved in cell division and mitosis in GMTc, and upregulation of metabolic genes and cAMP related genes, consistent with a more differentiated state (Figure 7E). Focusing on changes in highly expressed major cardiac and fibroblast genes, we found that GMTc iCMs displayed a more complete upregulation of cardiac genes compared to GMT iCMs (Figure 7F).
TGF-β and WNT inhibitors enhance reprogramming of human adult cardiac fibroblasts
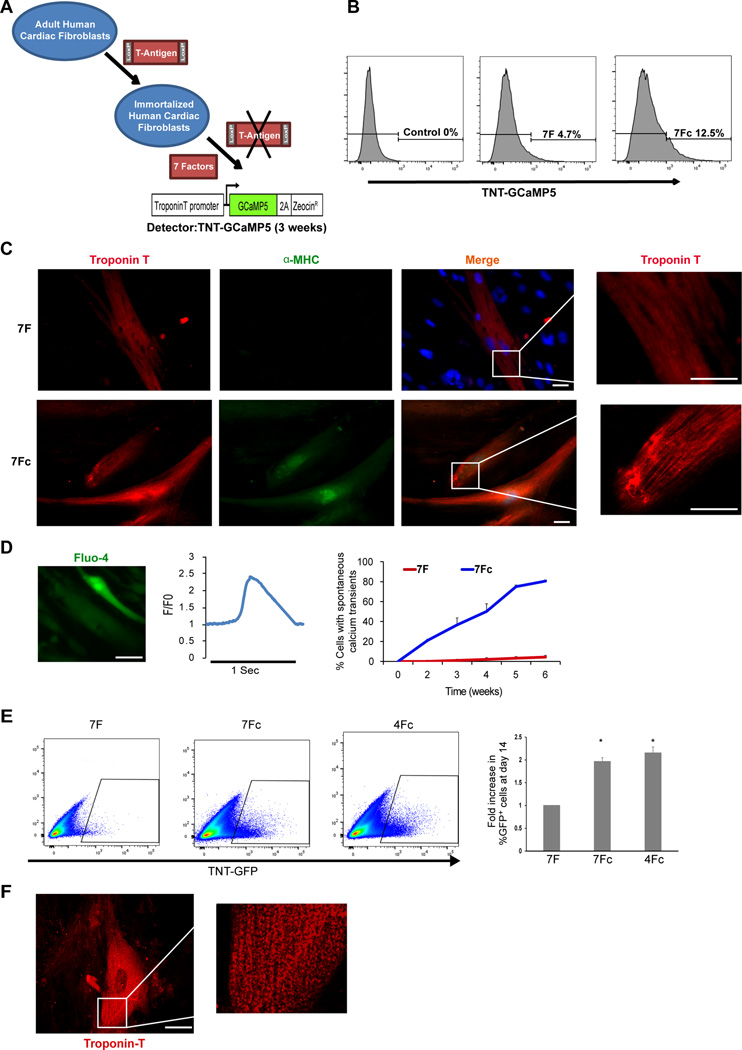
We previously reported that GMT was insufficient to reprogram human fibroblasts, but addition of ESRRG and MESP1 resulted in generation of iCMs, and inclusion of Myocardin and ZFPM2 (7F) could reprogram human cells to similar quality as in mouse cells in vitro 35. To test the effects of SB431542 and XAV939 on human cardiac reprogramming induced by 7F in the most relevant type of fibroblasts, we generated a reversibly immortalized cell line of adult human cardiac fibroblasts. We did this by introducing a puromycin resistant floxed T-antigen into primary adult cardiac fibroblasts and selecting for puromycin resistance. Viral introduction of Cre recombinase into the cell line resulted in efficient removal of the T-antigen and reversal of the proliferative phenotype (Figure S5A, B). Using the cardiac TNT-GCaMP reporter to detect cells reprogrammed to the point of having calcium transients (Figure 8A), we found that 7F plus SB431542 and XAV939 (7Fc) doubled the percentage of iCMs reprogrammed by 7F (Figure 8B). iCMs reprogrammed with 7Fc also exhibited sarcomere formation as early as 3 weeks (Figure 8C). Furthermore, calcium sparks occurred after just 10 days of reprogramming with 7Fc (Movie S7), and within 3 weeks of reprogramming, these calcium transients became more homogenous throughout the cell. Within 4 weeks, over 50% of 7Fc reprogrammed cells exhibited spontaneous calcium transients compared to less than 5% with 7F (Figure 8D and Movie S8).
Figure 8. SB431542 and XAV939 enhanced the efficiency of adult human cardiac fibroblast reprogramming.
(A) Schematic representation of the strategy for generating a reversibly immortalized cell line of human cardiac fibroblasts using a floxed T-Antigen introduced into human adult cardiac fibroblasts. (B) Representative FACS plots shows the effect of the compounds on cardiac reprogramming with 7 factors (after deletion of T-Antigen) using a TNT-GCaMP reporter 3 weeks after reprogramming. (C) Representative immunofluorescence showing that iCMs reprogrammed with SB431542 and XAV939 exhibited sarcomere formation as early as 3 weeks (red, troponin T; green, α-MHC); (scale bar, 25 µm). (D) Spontaneous Fluo-4calcium transients within 3 weeks of reprogramming (left panel) and quantification of the percentage of cells that exhibited spontaneous calcium transients at 2, 4, 6, and 8 weeks of reprogramming (right panel) (n=100 cell in each group, *p<0.05). (E) Representative FACS plots and quantification shows that 4F with the compounds are producing a similar percent of GFP+ cells as 7Fc using cTnT-GFP as a reporter (n=3, *p<0.05). (F) Representative immunofluorescence showing that iCMs reprogrammed with 4Fc exhibited sarcomere formation as early as 3 weeks (Red: Troponin T) although they were less organized compare to 7Fc.
To assess the quality of reprogrammed cells, we performed RNA-seq on TNT-GFP positive iCMs after 4 weeks of 7F-induced reprogramming with or without chemicals. The gene-expression profile changes in 7Fc iCMs was improved compared to 7F iCMs, as indicated by expression levels of the major cardiac and fibroblast genes in TNT-GFP positive iCMs. We found that the chemicals enhanced the 7F effect on expression of cardiac genes and suppressed the expression of fibroblast genes (Figure S5C). Furthermore, we performed GO analysis of the genes that were differentially regulated between the 7F and 7Fc iCMs by at least two-fold and found that these genes were mostly involved in downregulation of extracellular-matrix formation and collagens, as well as upregulation in calcium and ion transport related genes, similar to that observed in mouse reprogramming (Figure S5D).
We tested how removal of one or more of the TFs in 7Fc would affect gene expression in human cardiac fibroblasts expressing the remaining factors. By removing 1, 2, or 3 factors at a time (Figure S6) and using qRT-PCR Taqman assays (Supplementary table 1) to assess the gene expression of cardiac and fibroblast gene, we found that Mesp1, Zfpm2 and Esrrg were dispensable in the presence of TGF-β and WNT inhibition, and we could achieve a similar degree of gene expression change with just 4 factors (Gata4, Mef2c, Tbx5, and Myocardin) plus chemicals (4Fc) (Figure S6). FACS analysis showed that we obtained a similar percentage of TnT+ iCMs with 4Fc and 7F (Figure 8E), and immunofluorescence revealed highly organized sarcomere organization in 4Fc iCMs, comparable to 7Fc iCMs as early as 3 weeks (Figure 8F). Furthermore, 4Fc iCMs showed calcium transients similar to 7Fc-induced iCMs (Movie S9).
Discussion
We performed an unbiased, high-throughput screen of small molecules in primary cardiac fibroblasts to identify barriers to cardiac cell–fate conversion that could be overcome to enhance efficiency and quality of GMT-mediated cardiac reprogramming in vivo. We discovered that combinatorial inhibition of TGF-β and WNT signaling with SB431542 and XAV939 potently enhances the efficiency, quality and speed of reprogrammed iCMs generated upon delivery of the minimal transcription factor cocktail, GMT, into postnatal cardiac fibroblasts. Most importantly, these small molecules significantly improved in vivo reprogramming and cardiac function in mice compared to GMT alone and reduced the number of TFs needed for human reprogramming.
Recent reports explored various combinations of TFs 7–10, growth factors 36, kinases 12, small molecules 13, 14, and microRNAs 11 to improve GMT-induced reprogramming in vitro. However, each of these reports utilized mouse embryonic fibroblasts (MEFs) for screening, and although improved reprogramming efficiency was found in MEFs, the effect on post-natal mouse or human cardiac fibroblasts was limited and no in vivo enhancement has been reported. In our study, we utilized primary cardiac fibroblasts for screening purposes and thereby report the first small-molecule cocktail that enhances direct cardiac reprogramming with the minimal TF cocktail in postnatal human and mouse cardiac fibroblasts in vitro and in vivo in a mouse cardiac injury model. Furthermore, in the presence of TGF-β and WNT inhibitors we were able to reduce the number of transcription factors needed for human cardiac reprogramming to only 4 factors. Increasing the efficiency, speed and quality of direct cardiac reprogramming and reducing the number of TFs needed for human reprogramming will facilitate further clinical application for the direct cardiac reprogramming strategy in heart failure treatment.
The multiple chemical compounds annotated to impact TGF-β or WNT signaling pathways at distinct steps provided confidence that these pathways were involved in cardiac reprogramming. The most robust TGF-β inhibitor, SB431542, selectively inhibits ALK5 (the TGF-β type I receptor), ALK4, and ALK7 22. The observation that TGF-β ligand reverses SB431542’s effects, ALK5 siRNA mimics the effects, and the transcriptome data demonstrating suppression of the TGF-β pathway by the inhibitor, together support TFG-β signaling as the relevant target of SB431542. Previous reports showed that SB431542 enhances in vitro direct cardiac reprogramming of MEFs induced by a TF cocktail containing GMT, Hand2, and Nkx2–5, but has little effect on postnatal cardiac fibroblasts 13, 14. The additional TFs may limit the function of SB431542 when introduced into cardiac fibroblasts compared to MEFs.
WNT signaling occurs through three major pathways: canonical, noncanonical planar–cell polarity, and noncanonical WNT/calcium. In the canonical pathway, WNT binds frizzled to disrupt the function of a complex that targets β-catenin for ubiquitination and degradation in the proteasome. Interestingly, biphasic modulation of canonical WNT signaling during stem-cell differentiation into cardiomyocytes promotes mesoderm differentiation and produces a high yield of pure cardiomyocytes 37, 38. The most potent WNT inhibitor, XAV939, is a tankyrase inhibitor that inhibits Wnt signaling via axin stabilization, resulting in degradation of β-catenin. The reversal of XAV939’s effects by WNT activation using GSK3β inhibitor (CHIR99021) supports the notion that inhibition of Wnt signaling promotes cardiac reprogramming. In another context, CHIR99021, which enhances Wnt signaling, has been shown to cooperate with additional small molecules to replace the induced pluripotent stem cell (iPSC) reprogramming factors (OCT4, SOX2, cMYC, and KLF4) as a means to induce an epigenetically unstable state. Subsequent treatment with cardiogenic factors (BMP/Activin/VEGF) enabled cardiogenesis and produced cardiomyocyte like cells 18, 39. However, the cellular path involving reprogramming toward pluripotency is fundamentally different compared to the direct reprogramming to a cardiomyocyte in the presence of GMT, perhaps explaining the enhancement of cardiac reprogramming with Wnt inhibition rather than Wnt activation.
Ongoing genome-wide studies will reveal how Smad and TCF proteins, TFs that mediate TGF-β and WNT signaling, respectively, function cooperatively with GMT to regulate the conversion of fibroblasts to the cardiac fate. However, the primary analysis of GO terms enrichment analysis of the RNA-seq data from reprogrammed iCMs in the presence of SB431542, XAV939 or SB431542+XAV939 highlighted the major downstream modification by the compounds to increase the efficiency of reprogramming. SB431542 reprogrammed iCMs mainly showed downregulation in fibrotic signal and extracellular matrix formation, consistent with previous reports in other reprogramming settings 13, 14. In contrast, XAV939 reprogrammed iCMs showed downregulation in genes involved in chromatin modulation, DNA packaging and nucleosome organization similar to previous reports in other cell types 32.
Interestingly, our RNA-seq data revealed that in vitro and in vivo GMTc iCMs have a gene expression profile more consistent with adult ventricular cardiomyocytes than neonatal cardiomyocytes. Surprisingly, the RNA expression levels of certain cardiac genes in GMTc-iCMs in vitro and in vivo were actually higher than in isolated control endogenous cardiomyocytes (e.g., TNNT, ACTC1, ACTN2, MYH7 and RYR2, Figure 4D, 7F). This unexpected observation could reflect that iCMs are still undergoing reprogramming and therefore are actively transcribing higher levels of these lineage-specific genes to support their cell-fate transformation. Alternatively, these genes may represent targets that are particularly sensitive to the sustained activity of exogenous GMT and would suggest that transient expression would be preferable as translation of this technology progresses.
Direct cardiac reprogramming is an innovative approach to cardiac regeneration that has potential to become a novel therapy for heart failure. However, many challenges remain that will require ongoing innovation, including improved delivery systems, regulation of timing for expression of reprogramming factors, and testing in more chronic conditions. In the current study, we present the translational promise of this technology with the help of small molecules in vivo and in human cells. Although our experiments showed the first proof of principle of the small molecule enhancement of in vivo reprogramming, further studies are needed to optimize the dosages and timing for the small molecules to achieve the greatest outcome. Currently, several TGF-β and WNT inhibitors are in clinical trials 40, 41, which will aid the translational use of these inhibitors for cardiac reprogramming in the future. Together, these findings may facilitate combined gene and small molecule therapy for heart failure.
Supplementary Material
Clinical Perspective.
What is new?
Using a high-throughput chemical screen in postnatal mouse cardiac fibroblasts, we found that TGF-β and WNT inhibition enhances transcription factor-based direct reprogramming of cardiac fibroblasts to induced cardiomyocyte-like cells in vitro and in vivo.
A combination of TGF-β and WNT chemical inhibitors increases quality, quantity and speed of direct reprogramming, resulting in improved cardiac function after injury as early as one week after treatment.
These chemical inhibitors enhanced human cardiac reprogramming and reduced the number of transcription factors needed for human cardiac reprogramming to 4 factors.
What are the clinical implications?
The enhancement in quality and quantity of cardiac reprogramming with the help of small molecules in vivo and in human cells moves this technology closer to translation.
These findings, if validated in large animals, could facilitate a combined gene therapy and small molecule approach to heart failure.
Acknowledgments
We thank A. Williams, A. Foley, L. Liu, and M. Calvert for technical assistance and helpful suggestions. We also thank B. Taylor for help in preparing the figures and editing the manuscript. We would like to thank the Gladstone Institutes core facilities, including the Genomics, Flow Cytometry, Histology and the Bioinformatics Core.
Funding:
This work was supported by NIH grants R01 HL057181 (D.S.), U01 HL098179 (D.S.), and U01 HL100406 (D.S.) and AHA Scientist Development Grant 16SDG29950012 (T.M.A.M.). D.S. was supported by the L.K. Whittier, William H. Younger Family, and Eugene Roddenberry Foundations and the California Institute for Regenerative Medicine.
Footnotes
Author contributions:
TMAM: conception and design, collection and analysis of data, manuscript writing, final approval of manuscript; NS, EB, ER, YH, KP, YSA, PY, HY, ST, SM: collection and analysis of data; SD, KI: interpretation, analysis, and manuscript writing; DS: conception and design, financial support, manuscript writing, final approval of manuscript.
Disclosures:
D.S. is a scientific co-founder of Tenaya Therapeutics; K.N.I and T.M.A.M hold equity in Tenaya Therapeutics.
Data and materials availability:
All RNAseq data were deposited in Gene Expression Omnibus database under accession number GSE81809.
References
- 1.Liu L, Eisen HJ. Epidemiology of heart failure and scope of the problem. Cardiol Clin. 2014;32:1–8. doi: 10.1016/j.ccl.2013.09.009. vii. [DOI] [PubMed] [Google Scholar]
- 2.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chong JJ, Murry CE. Cardiac regeneration using pluripotent stem cells--progression to large animal models. Stem Cell Res. 2014;13:654–665. doi: 10.1016/j.scr.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Liu Z, Yin C, Asfour H, Chen O, Li Y, Bursac N, Liu J, Qian L. Stoichiometry of Gata4, Mef2c, and Tbx5 influences the efficiency and quality of induced cardiac myocyte reprogramming. Circ Res. 2015;116:237–244. doi: 10.1161/CIRCRESAHA.116.305547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Protze S, Khattak S, Poulet C, Lindemann D, Tanaka EM, Ravens U. A new approach to transcription factor screening for reprogramming of fibroblasts to cardiomyocyte-like cells. J Mol Cell Cardiol. 2012;53:323–332. doi: 10.1016/j.yjmcc.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Christoforou N, Chellappan M, Adler AF, Kirkton RD, Wu T, Addis RC, Bursac N, Leong KW. Transcription factors MYOCD, SRF, Mesp1 and SMARCD3 enhance the cardio-inducing effect of GATA4, TBX5, and MEF2C during direct cellular reprogramming. PLoS ONE. 2013;8:e63577. doi: 10.1371/journal.pone.0063577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Addis RC, Ifkovits JL, Pinto F, Kellam LD, Esteso P, Rentschler S, Christoforou N, Epstein JA, Gearhart JD. Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success. J Mol Cell Cardiol. 2013;60:97–106. doi: 10.1016/j.yjmcc.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110:1465–1473. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou H, Dickson ME, Kim MS, Bassel-Duby R, Olson EN. Akt1/protein kinase B enhances transcriptional reprogramming of fibroblasts to functional cardiomyocytes. Proc Natl Acad Sci U S A. 2015;112:11864–11869. doi: 10.1073/pnas.1516237112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ifkovits JL, Addis RC, Epstein JA, Gearhart JD. Inhibition of TGFbeta signaling increases direct conversion of fibroblasts to induced cardiomyocytes. PloS one. 2014;9:e89678. doi: 10.1371/journal.pone.0089678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Londono P, Cao Y, Sharpe EJ, Proenza C, O’Rourke R, Jones KL, Jeong MY, Walker LA, Buttrick PM, McKinsey TA, Song K. High-efficiency reprogramming of fibroblasts into cardiomyocytes requires suppression of pro-fibrotic signalling. Nat Commun. 2015;6:8243. doi: 10.1038/ncomms9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Wang L, Vaseghi HR, Liu Z, Lu R, Alimohamadi S, Yin C, Fu JD, Wang GG, Liu J, Qian L. Bmi1 Is a Key Epigenetic Barrier to Direct Cardiac Reprogramming. Cell Stem Cell. 2016;18:382–395. doi: 10.1016/j.stem.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian L, Berry EC, Fu JD, Ieda M, Srivastava D. Reprogramming of mouse fibroblasts into cardiomyocyte-like cells in vitro. Nature protocols. 2013;8:1204–1215. doi: 10.1038/nprot.2013.067. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Liu Z, Yin C, Asfour H, Chen O, Li Y, Bursac N, Liu J, Qian L. Stoichiometry of Gata4, Mef2c, and Tbx5 influences the efficiency and quality of induced cardiac myocyte reprogramming. Circ Res. 2015;116:237–244. doi: 10.1161/CIRCRESAHA.116.305547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Cao N, Spencer CI, Nie B, Ma T, Xu T, Zhang Y, Wang X, Srivastava D, Ding S. Small molecules enable cardiac reprogramming of mouse fibroblasts with a single factor, Oct4. Cell Rep. 2014;6:951–960. doi: 10.1016/j.celrep.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quintavalle M, Elia L, Price JH, Heynen-Genel S, Courtneidge SA. A cell-based high-content screening assay reveals activators and inhibitors of cancer cell invasion. Sci Signal. 2011;4 doi: 10.1126/scisignal.2002032. ra49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hattori H, Subramanian KK, Sakai J, Jia Y, Li Y, Porter TF, Loison F, Sarraj B, Kasorn A, Jo H, Blanchard C, Zirkle D, McDonald D, Pai SY, Serhan CN, Luo HR. Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc Natl Acad Sci U S A. 2010;107:3546–3551. doi: 10.1073/pnas.0914351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preuett B, Leeder JS, Abdel-Rahman S. Development and Application of a High-Throughput Screening Method to Evaluate Antifungal Activity against Trichophyton tonsurans. J Biomol Screen. 2015;20:1171–1177. doi: 10.1177/1087057115594751. [DOI] [PubMed] [Google Scholar]
- 22.Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 23.Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazaki Y, Tsukazaki T, Hirota Y, Yonekura A, Osaki M, Shindo H, Yamashita S. Dexamethasone inhibition of TGF beta-induced cell growth and type II collagen mRNA expression through ERK-integrated AP-1 activity in cultured rat articular chondrocytes. Osteoarthritis Cartilage. 2000;8:378–385. doi: 10.1053/joca.1999.0313. [DOI] [PubMed] [Google Scholar]
- 25.Potchinsky M, Nugent P, Lafferty C, Greene RM. Effects of dexamethasone on the expression of transforming growth factor-beta in mouse embryonic palatal mesenchymal cells. J Cell Physiol. 1996;166:380–386. doi: 10.1002/(SICI)1097-4652(199602)166:2<380::AID-JCP16>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Meisler N, Keefer KA, Ehrlich HP, Yager DR, Myers-Parrelli J, Cutroneo KR. Dexamethasone abrogates the fibrogenic effect of transforming growth factor-beta in rat granuloma and granulation tissue fibroblasts. J Invest Dermatol. 1997;108:285–289. doi: 10.1111/1523-1747.ep12286461. [DOI] [PubMed] [Google Scholar]
- 27.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 28.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Syed DN, Afaq F, Maddodi N, Johnson JJ, Sarfaraz S, Ahmad A, Setaluri V, Mukhtar H. Inhibition of human melanoma cell growth by the dietary flavonoid fisetin is associated with disruption of Wnt/beta-catenin signaling and decreased Mitf levels. J Invest Dermatol. 2011;131:1291–1299. doi: 10.1038/jid.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Callahan JF, Burgess JL, Fornwald JA, Gaster LM, Harling JD, Harrington FP, Heer J, Kwon C, Lehr R, Mathur A, Olson BA, Weinstock J, Laping NJ. Identification of novel inhibitors of the transforming growth factor beta1 (TGF-beta1) type 1 receptor (ALK5) J Med Chem. 2002;45:999–1001. doi: 10.1021/jm010493y. [DOI] [PubMed] [Google Scholar]
- 31.Bryja V, Schulte G, Arenas E. Wnt-3a utilizes a novel low dose and rapid pathway that does not require casein kinase 1-mediated phosphorylation of Dvl to activate beta-catenin. Cell Signal. 2007;19:610–616. doi: 10.1016/j.cellsig.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Mosimann C, Hausmann G, Basler K. Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol. 2009;10:276–286. doi: 10.1038/nrm2654. [DOI] [PubMed] [Google Scholar]
- 33.Waghabi MC, de Souza EM, de Oliveira GM, Keramidas M, Feige JJ, Araujo-Jorge TC, Bailly S. Pharmacological inhibition of transforming growth factor beta signaling decreases infection and prevents heart damage in acute Chagas’ disease. Antimicrob Agents Chemother. 2009;53:4694–4701. doi: 10.1128/AAC.00580-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Distler A, Deloch L, Huang J, Dees C, Lin NY, Palumbo-Zerr K, Beyer C, Weidemann A, Distler O, Schett G, Distler JH. Inactivation of tankyrases reduces experimental fibrosis by inhibiting canonical Wnt signalling. Ann Rheum Dis. 2013;72:1575–1580. doi: 10.1136/annrheumdis-2012-202275. [DOI] [PubMed] [Google Scholar]
- 35.Fu JD, Stone NR, Liu L, Spencer CI, Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau BG, Srivastava D. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Reports. 2013;1:235–247. doi: 10.1016/j.stemcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamakawa H, Muraoka N, Miyamoto K, Sadahiro T, Isomi M, Haginiwa S, Kojima H, Umei T, Akiyama M, Kuishi Y, Kurokawa J, Furukawa T, Fukuda K, Ieda M. Fibroblast Growth Factors and Vascular Endothelial Growth Factor Promote Cardiac Reprogramming under Defined Conditions. Stem Cell Reports. 2015;5:1128–1142. doi: 10.1016/j.stemcr.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lian X, Zhang J, Zhu K, Kamp TJ, Palecek SP. Insulin Inhibits Cardiac Mesoderm, not Mesendoderm, formation during Cardiac Differentiation of Human Pluripotent Stem Cells and Modulation of Canonical Wnt Signaling Can Rescue this Inhibition. Stem Cells. 2013;31(3):447–457. doi: 10.1002/stem.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nature protocols. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao N, Huang Y, Zheng J, Spencer CI, Zhang Y, Fu JD, Nie B, Xie M, Zhang M, Wang H, Ma T, Xu T, Shi G, Srivastava D, Ding S. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science. 2016;352(6290):1216–1220. doi: 10.1126/science.aaf1502. [DOI] [PubMed] [Google Scholar]
- 40.Kahn M. Can we safely target the WNT pathway? Nature reviews. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buijs JT, Stayrook KR, Guise TA. The role of TGF-beta in bone metastasis: novel therapeutic perspectives. Bonekey Rep. 2012;1:96. doi: 10.1038/bonekey.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.