Abstract
Background/Objectives
Controlling food portion sizes can help reduce energy intake, but the effect of different portion-control methods on weight management is not known. In a one-year randomized trial, we tested whether the efficacy of a behavioral weight-loss program was improved by incorporating either of two portion-control strategies instead of standard advice about eating less.
Subjects/Methods
The Portion-Control Strategies Trial included 186 women with obesity (81%) or overweight (19%). Participants were randomly assigned to one of three equally intensive behavioral programs consisting of 19 individual sessions over 12 months. The Standard Advice Group was instructed to eat less food while making healthy choices, the Portion Selection Group was instructed to choose portions based on energy density using tools such as food scales, and the Pre-portioned Foods Group was instructed to structure meals around pre-portioned foods such as single-serving main dishes, for which some vouchers were provided. In an intention-to-treat analysis, a mixed-effects model compared weight loss trajectories across 23 measurements; at Month 12, weight was measured for 151 participants (81%).
Results
The trajectories showed that the Pre-portioned Foods Group initially lost weight at a greater rate than the other two groups (P=0.021), but subsequently regained weight at a greater rate (P=0.0005). As a result, weight loss did not differ significantly across groups at Month 6 (mean±SE 5.2±0.4 kg) or Month 12 (4.5±0.5 kg). After one year, measured weight loss averaged 6% of baseline weight. The frequency of using portion-control strategies initially differed across groups, then declined over time and converged at Months 6 and 12.
Conclusions
Incorporating instruction on portion-control strategies within a one-year behavioral program did not lead to greater weight loss than standard advice. Using pre-portioned foods enhanced early weight loss, but this was not sustained over time. Long-term maintenance of behavioral strategies to manage portions remains a challenge.
INTRODUCTION
The rise in obesity prevalence has occurred in parallel with increases in food portion size1–3. In addition, many controlled studies have shown that offering individuals larger portions leads to substantial and sustained increases in energy intake4–9. These data demonstrate that the availability of large portions can override the regulation of energy balance and could have persistent effects that promote obesity. Dietary guidelines10,11 recommend portion control to help limit energy intake, but little is known about the efficacy of different portion-control strategies for weight management12. In this randomized controlled trial, standard advice to eat less was compared with two focused portion-control strategies: one that uses knowledge and tools to select satisfying portions while restricting energy intake, and another that changes the personal eating environment in order to limit exposure to large portions.
Weight-loss recommendations often advise reducing energy intake by simply eating less10. Recent research indicates that such advice should include the influence of energy density (ED) on amounts that can be consumed13–17. When restricting energy intake, individuals can eat their usual amount of food by consuming larger portions of low-energy-dense foods (vegetables, fruits, lean proteins) while limiting portions of higher-energy-dense foods (high-fat, low-water foods). The use of tools such as food scales and household measures could improve the implementation of such recommendations18,19. Surprisingly few studies have examined whether use of portion-control tools is sustained over time and whether they facilitate weight management; however, promising results have been found using plates that indicate appropriate proportions of meal components20–22. We tested whether providing education and tools to limit intake of high-ED foods and promote intake of low-ED foods improved weight loss when incorporated into a standard behavioral program.
Another portion-control strategy is to structure the eating environment to limit exposure to large portions12. This can be achieved using pre-portioned foods that are packaged for a single eating occasion, such as frozen main dishes, single-serving snacks, and liquid meal replacements. Studies show that just as the availability of large portions leads to overconsumption, using pre-portioned foods helps to limit energy intake and promotes weight loss23–25. Several evidence-based reports support the use of pre-portioned meals for weight management, although the evidence for this comes primarily from studies of liquid meal replacements11,26,27. In the few studies that have tested the long-term effects of pre-portioned foods, the items were provided throughout the study24,25,28–30. For example, several studies found that providing portion-controlled main dishes twice a day for the 8- to 12-week duration of the trial led to greater weight loss than a self-selected diet28–30. We examined whether use of pre-portioned foods is sustained over a longer period as provision is reduced, and whether this strategy facilitates long-term weight loss.
There is a need for evidence-based strategies to help individuals limit energy intake for obesity management in an environment characterized by large portions of energy-dense foods. The Portion-Control Strategies Trial helps fill this gap by systematically comparing the effects of different portion-control strategies on weight loss over one year. We tested the hypothesis that participants who were taught to use tools to select portions based on ED or to structure meals with pre-portioned foods would have greater weight loss than those following standard advice to eat less.
SUBJECTS AND METHODS
Subjects
Participants were recruited through local advertisements; eligibility was determined by telephone interview and screening sessions. Eligible participants were women aged 20–65 years with a body mass index (BMI) of 28–45 kg/m2. Potential subjects were excluded if they had blood pressure >160/100 mm Hg, reported a weight change >4.5 kg in the past three months, had a medical condition that precluded participation or that limited physical activity, were following a special diet or weight-loss program, were pregnant or lactating, scored >19 on the Eating Attitudes Test31, or scored >25 on the Beck Depression Inventory32. To be enrolled, potential subjects were required to complete three daily food and activity diaries and a two-week run-in period.
Participants were stratified by BMI and age and randomly assigned to one of three groups using blocks of six sequences from a random number generator. Participants and interventionists were informed of the group assignment for instructional purposes; assignment was masked from those who assessed outcomes including dietary recalls and blood analyses. The sample size was determined by using a mixed-effects model33 to analyze the trajectories of weight loss in exemplary data sets based on trials of behavioral programs in similar populations13,23. Power was determined from the non-centrality parameter and the critical F-value for a two-sided Type 1 error rate of 0.05. It was estimated that differential rates of weight loss (linear and quadratic coefficients) could be detected between any two groups with 80% power by testing 48 participants per group (144 in total). To allow for a 20% non-completion rate, the sample size was set at 180 participants.
The trial protocol was approved by the Office for Research Protections of The Pennsylvania State University and registered at www.clinicaltrials.gov as NCT01474759. Participants provided signed informed consent and were financially compensated for participation. The trial was conducted at the Clinical Research Center on the University Park campus of The Pennsylvania State University from July 2012 to March 2015.
Behavioral programs
In this parallel-group trial, participants met individually with registered dietitians and trained interventionists for 19 sessions over one year. Each interventionist used standard instruction manuals, received regular training to ensure fidelity to program content, and had equal contact with the groups. Thirty-minute sessions were scheduled weekly in Month 1 and biweekly in Months 2–6, and one-hour sessions were scheduled monthly in Months 7–12. In each group, energy balance was explained as the basis for weight loss, but in order to test the influence of different portion-control strategies, instruction focused on following the assigned dietary program and making behavioral changes. The specific principles of each program were reinforced within basic lessons about food categories (fruit, vegetables, fats, whole grains and fiber, lean protein, and dairy foods) as well as meal planning and managing behavior change. All participants received similar instruction on increasing physical activity through walking, with the goal of achieving 10,000 steps daily. At each session, participants were given written lesson plans and supplemental materials to review and add to their personal binders. For purposes of self-monitoring, subjects were encouraged to keep daily records of body weight, pedometer counts, and foods eaten. During each session, the interventionist reviewed these records with the participant and helped set goals for the next session.
Participants in the Standard Advice Group were instructed to follow dietary guidelines10 that emphasized eating less while making healthy choices from all food groups. Educational sessions and materials focused on eating recommended amounts of fruits and vegetables for health purposes; choosing foods containing lean protein, whole grains, and healthy fats; and reducing intake of sodium and added sugar. One session provided instruction on standard serving sizes in each food group and on understanding food labels. Throughout the trial, participants were taught to plan meals with a variety of foods in order to achieve a balance of nutrients and maintain a healthy diet while eating less.
Participants in the Portion Selection Group were instructed to choose food portions based on energy density in order to eat satisfying portions of low-ED foods and to control portions of higher-ED foods13,34. Over several sessions, individuals were taught the concepts of ED and methods for modifying the ED of dishes and meals by altering portions of low- and high-ED foods. To facilitate choosing portions, participants were given a portion-selection tool at each of the first four sessions and instructed in its use: a digital food scale, a set of household measures (cups and spoons), a placemat that illustrated appropriate proportions of meal components, and a card illustrating portion estimation using common objects (such as the hand or a computer mouse). Participants were instructed to continue using the tools that they found helpful; throughout the trial, advice to choose portions based on ED was reinforced.
Participants in the Pre-portioned Foods Group were instructed to structure their meals and to learn appropriate food portions by using pre-portioned foods such as individual servings of main dishes, side dishes, snacks, yogurt, and whole fruits. Participants were instructed to eat pre-portioned main dishes daily for lunch and dinner during Months 1–3 of the trial and were encouraged to continue this practice subsequently. To facilitate this, participants were given prepaid vouchers for single-serving main dishes at the rate of 14 vouchers per week in Month 1, which was gradually reduced to four per week during Months 4–12. Throughout the trial, participants were taught about selecting all types of pre-portioned foods and snacks and how to prepare their own.
Outcome assessments
In addition to the instructional sessions, four assessment sessions were conducted at the end of Months 1, 3, 6, and 12. Blood samples were collected at Months 3, 6, and 12 only.
Anthropometry and blood samples
Weight was measured at the 19 instructional sessions and the four assessment sessions. Subjects were weighed to the nearest 0.1 kg while wearing a lightweight outfit kept for them at the center. Height was measured with a stadiometer; waist circumference was measured at the right iliac crest35.
Fasting blood samples were analyzed by Quest Diagnostics (Pittsburgh, Pennsylvania, USA) for serum lipids, glucose, and insulin. Insulin resistance was calculated from glucose and insulin values using a HOMA calculator36.
Diet and physical activity
Because it was expected that dietary change and the rate of weight loss would be maximal in the first three months of the trial13,23, 24-hour dietary recalls were administered at baseline and Month 3. Recalls were scheduled on two weekdays and one weekend day for a sample of 124 participants (67%) and completed by 123 participants. Recalls were administered by telephone by the Diet Assessment Center of The Pennsylvania State University and analyzed using Nutrition Data System for Research software37. In addition to nutrient intakes, recalls were analyzed for food group servings and number of pre-portioned foods. Reported items were categorized according to ED as Very low (0.0–0.59 kcal/g), Low (0.6–1.49 kcal/g), Medium (1.5–3.99 kcal/g), or High (4.0–9.0 kcal/g)34.
Physical activity was assessed using pedometers provided to participants (Yamax-Digiwalker Model SW-200, Warminster, Pennsylvania, USA). At baseline and prior to each of the four assessment sessions, participants recorded pedometer counts for two weekdays and one weekend day.
Questionnaires
Participants completed questionnaires at the research center on computers using Qualtrics software (Provo, Utah, USA). Questionnaires included the Eating Inventory38 (assessing dietary restraint, disinhibition, and tendency toward hunger) and a 45-item Diet Satisfaction Questionnaire39. Participants also reported the frequency of 34 weight-control strategies, including four practices of eating low-energy-dense fruit and vegetables, four practices of eating low--fat foods, four practices of using portion-selection tools, and two practices of consuming pre-portioned foods.
Statistical analyses
The main outcome was the trajectory of weight change over time, analyzed by a random coefficients model on all measurements from randomized subjects, regardless of the number of sessions attended (an intention-to-treat analysis). Group assignment was treated as a fixed factor in the model and time (trial week) was treated as a continuous covariate. Time was also included as a random factor to account for correlation of repeated measures within subjects; thus the trajectory of weight change was characterized separately for each participant. Polynomial factors of time were tested as both fixed and random factors and included if they improved the fit of the model. Additional baseline characteristics such as restraint score were tested as covariates, and their interactions with polynomial factors of time were included if significant.
Secondary outcomes that were measured at several time points (cardiometabolic factors, dietary intakes, questionnaire responses, and pedometer counts) were analyzed by a linear mixed-effects model with categorical fixed factors of group, time, and their interaction, if significant. Correlation of repeated measures within subjects was accounted for by modeling the covariance structure of the data. All models included the randomization variables of baseline BMI and age. Tests of group means were adjusted for multiple comparisons by the Tukey-Kramer method. Differences across groups in baseline characteristics were tested by a fixed-effects model for continuous variables and by Fisher’s exact test for categorical variables; differences in retention rates in the trial were assessed by the log-rank test. Analyses were performed using SAS software (version 9.4, SAS Institute Inc., Cary, North Carolina, USA). Results were considered significant at P<0.05 and are reported as mean±SD unless otherwise noted.
RESULTS
Participant characteristics
There were 186 participants enrolled in the trial (62 per group); their baseline characteristics did not differ significantly across groups (Table 1). At baseline, participants had mean age of 50±11 years and weight of 91±13 kg; 81% had obesity and 19% had overweight. The majority of participants had some college education and had attempted to lose weight multiple times in the past year. A participant flow diagram is shown in Figure 1. Retention rates in the trial did not differ across groups (P=0.86). For the subset of participants who completed dietary recalls, baseline characteristics did not differ across groups, nor did baseline characteristics differ between those who did and did not complete recalls (data not shown).
Table 1.
Baseline characteristics of women in the three groups of The Portion-Control Strategies Trial
| Characteristic | Standard Advice (n = 62) |
Portion Selection (n = 62) |
Pre-portioned Foods (n = 62) |
Significance of Group effect (P-value)a |
|---|---|---|---|---|
| Age (y) | 49.5 (12.0) | 50.4 (9.6) | 50.1 (10.1) | 0.89 |
| Body weight (kg) | 92.1 (12.3) | 89.9 (13.8) | 91.7 (12.2) | 0.59 |
| Body mass index (kg/m2) | 34.1 (4.3) | 33.6 (4.2) | 34.2 (4.1) | 0.68 |
| Pedometer count (steps/d) | 6446 (3084) | 6531 (3447) | 6729 (3253) | 0.89 |
| Estimated energy expenditure (kcal/d)b | 2121 (192) | 2082 (218) | 2108 (185) | 0.54 |
| Weight loss attempts in past year, n | 2.3 (2.9) | 1.8 (2.0) | 2.3 (2.5) | 0.49 |
| Menopause status, n (%) | 0.66 | |||
| Pre-menopausal | 20 (32%) | 22 (35%) | 25 (40%) | |
| Peri- or post-menopausal | 42 (68%) | 40 (65%) | 37 (60%) | |
| Race, n (%) | 0.77 | |||
| White | 62 (100%) | 61 (98%) | 60 (97%) | |
| African-American | 0 (0%) | 1 (2%) | 1 (2%) | |
| More than one race | 0 (0%) | 0 (0%) | 1 (2%) | |
| Ethnicity, n (%) | 0.99 | |||
| Not Hispanic | 61 (98%) | 61 (98%) | 62 (100%) | |
| Hispanic | 1 (2%) | 1 (2%) | 0 (0%) | |
| Education, n (%) | 0.40 | |||
| High school graduate | 7 (11%) | 8 (13%) | 12 (19%) | |
| Some college education | 21 (34%) | 16 (26%) | 19 (31%) | |
| College degree | 17 (27%) | 21 (34%) | 19 (31%) | |
| Professional or graduate degree | 17 (27%) | 17 (27%) | 12 (19%) | |
| Employment, n (%) | 0.24 | |||
| Employed full-time | 42 (68%) | 31 (50%) | 41 (66%) | |
| Employed part-time | 10 (16%) | 14 (23%) | 8 (13%) | |
| Not employed | 10 (16%) | 17 (27%) | 13 (21%) | |
| Dietary restraint scorec (range 0–21) | 9.3 (3.5) | 8.6 (3.6) | 8.8 (3.8) | 0.60 |
| Disinhibition scorec (range 0–16) | 9.7 (3.1) | 9.8 (3.9) | 9.8 (3.5) | 0.98 |
| Tendency toward hunger scorec (range 0–14) | 6.4 (3.1) | 6.7 (3.6) | 5.5 (3.3) | 0.15 |
Unless otherwise indicated, values are mean (SD).
Differences across groups were tested by a fixed-effects model for continuous variables and by Fisher’s exact test for categorical variables.
Energy expenditure was estimated from height, weight, age, sex, and activity level40.
Scores from the Eating Inventory38.
Figure 1.
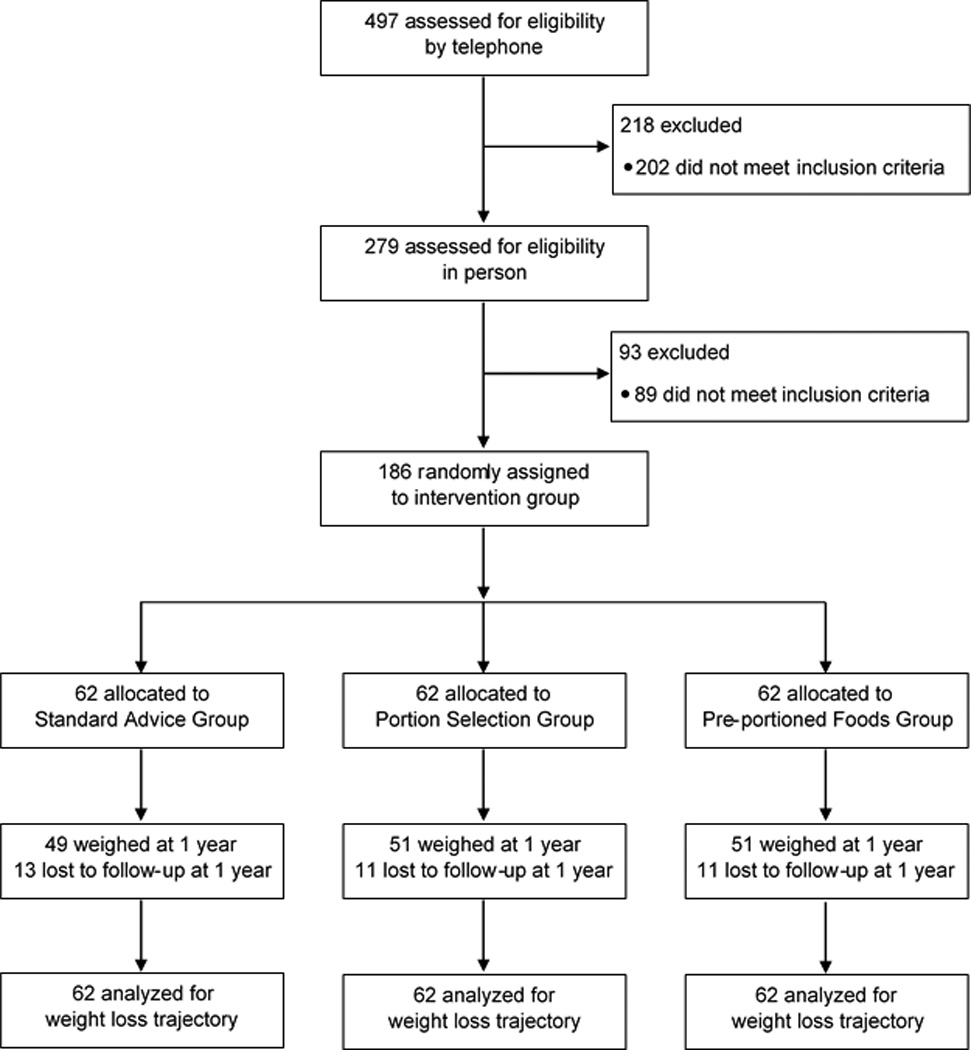
Participant flow chart of The Portion-Control Strategies Trial
Trajectory of weight loss
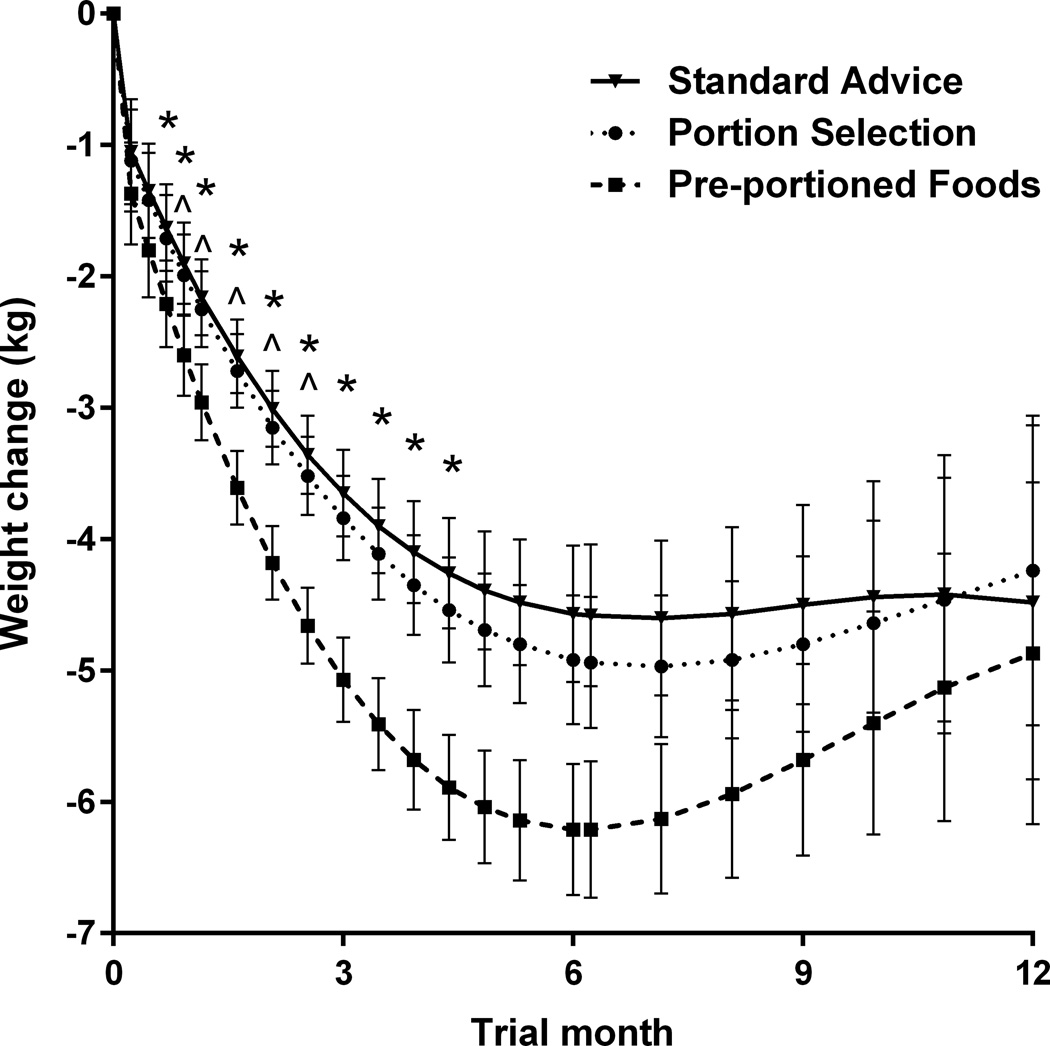
As shown in Figure 2, there were significant differences in trajectories of weight loss across groups. Participants in the Pre-portioned Foods Group had a greater rate of initial weight loss (linear coefficient –0.47 kg/week; P=0.021) than those in the other two groups (both –0.33 kg/week). At Month 3, for example, modeled weight loss (mean±SE) in the Pre-portioned Foods Group was 5.1±0.4 kg compared to 3.7±0.4 kg in the Standard Advice Group and 3.8±0.4 kg in the Portion Selection Group. The Pre-portioned Foods Group also had a greater rate of weight regain (quadratic coefficient 0.013 kg/week2; P=0.0005) than the other two groups (0.008 and 0.009 kg/week2, respectively). As a result, mean weight loss did not differ significantly across groups at Month 6 (mean±SE 5.2±0.4 kg) or Month 12 (4.5±0.5 kg). The trajectories for observed weight loss showed a similar pattern to the intention-to-treat analysis, but the magnitude of weight loss was greater since it did not include estimated trajectories for participants who withdrew from the trial (Supplemental Figure S1).
Figure 2.
Mean trajectories of weight loss for 186 women in three groups. The curves were modeled from all available data for each subject, regardless of the number of sessions attended (an intention-to-treat analysis). The Pre-portioned Foods Group had a greater rate of weight loss (more negative linear coefficient; P=0.021) and a greater rate of regain (more positive quadratic coefficient; P=0.0005) than the other two groups. Symbols indicate time points at which weight loss in the Pre-portioned Foods Group differed significantly (P<0.05, adjusted for multiple comparisons) from that in the Standard Advice Group (*) and the Portion Selection Group (^). Error bars represent the standard error of the mean for the modeled data.
Dietary restraint score was the only baseline characteristic found to influence the trajectory of weight loss. Across all groups, lower baseline restraint score led to a greater rate of weight loss (P=0.006) and a lower rate of weight regain (P=0.03). In addition, participants who completed a greater number of self-monitoring records over the trial had a greater rate of weight loss (P<0.0001) and a lower rate of weight regain (P=0.003).
At the end of the trial, weight was measured for 151 participants (81%); their baseline characteristics did not differ from those participants without a final measure, except for having a higher restraint score (9.3±3.6 versus 7.4±3.4; P=0.006). Mean measured weight loss in these subjects was 5.2±6.5 kg, equivalent to 6% of baseline weight; 45% had lost ≥5% of baseline weight and 20% had lost ≥10%.
Cardiometabolic risk factors
All groups had beneficial changes in cardiometabolic risk factors over the trial, with no significant differences across groups (Table 2). There were decreases in diastolic blood pressure, waist circumference, fasting glucose and insulin, total cholesterol, triglycerides, and insulin resistance from baseline to Month 3. HDL cholesterol increased from baseline to Month 6 and again to Month 12. There were no significant differences in LDL cholesterol over time.
Table 2.
Cardiometabolic outcomes at assessment time points in 186 women
| Outcome measure | Baseline (n = 186) |
Month 3 (n = 170) |
Month 6 (n = 149) |
Month 12 (n = 136) |
Significance of Time effect (P-value) |
|---|---|---|---|---|---|
| Systolic blood pressure, mm Hg | 120.5 (16.7)a | 118.3 (15.1)ab | 117.1 (14.6)b | 119.3 (14.1)ab | 0.0061 |
| Diastolic blood pressure, mm Hg | 82.1 (11.7)a | 79.2 (10.0)b | 78.8 (9.8)b | 79.8 (9.5)ab | 0.0003 |
| Waist circumference, cm | 108.4 (9.8)a | 105.0 (10.7)b | 102.4 (10.7)c | 102.5 (11.2)c | <0.0001 |
| Glucose, mmol/l | 5.35 (1.02)a | 5.16 (0.58)b | 5.12 (0.66)b | 5.07 (0.65)b | 0.0015 |
| Insulin, pmol/l | 77.0 (51.2)a | 55.5 (39.4)c | 60.0 (41.4)b | 60.4 (41.4)b | <0.0001 |
| Insulin resistance, HOMA2 | 1.45 (0.97)a | 1.04 (0.74)c | 1.12 (0.77)b | 1.13 (0.78)b | <0.0001 |
| Total cholesterol, mmol/l | 5.28 (0.98)a | 5.12 (0.92)b | 5.19 (0.96)ab | 5.36 (0.90)a | 0.0007 |
| HDL cholesterol, mmol/l | 1.37 (0.36)a | 1.37 (0.31)a | 1.42 (0.30)b | 1.51 (0.34)c | 0.0001 |
| LDL cholesterol, mmol/l | 3.22 (0.86)a | 3.13 (0.82)a | 3.14 (0.83)a | 3.20 (0.80)a | 0.12 |
| Triglycerides, mmol/l | 1.50 (0.80)a | 1.36 (0.62)b | 1.37 (0.63)ab | 1.41 (0.66)ab | 0.0032 |
Abbreviation: HOMA2, homeostatic model assessment estimation of insulin resistance from fasting glucose and insulin36. Values are means (SD).
aWithin each outcome, means marked with different letters are significantly different (P<0.05, adjusted for multiple comparisons using the Tukey-Kramer method). There were no significant differences across groups in these outcomes.
Diet and physical activity
In the subset of participants with dietary recalls, there were differences across groups in change in reported food consumption from baseline to Month 3 (Table 3). The Standard Advice and Pre-portioned Foods Groups decreased food intake by 116±416 g/d, or 11%; in contrast, the Portion Selection Group maintained food intake (interaction P=0.002). Intake of very low-energy-dense foods (including most fruits and vegetables, low-fat yogurt, broth-based soup) increased in the Portion Selection Group by 128±299 g/d (82%), whereas there was no change in the other two groups (interaction P=0.0007).
Table 3.
Dietary intakes at baseline and Month 3 in the subset of 123 women who were administered 3-day dietary recalls
| Intake | Standard advice (n = 44) |
Portion selection (n = 38) |
Pre-portioned foods (n = 41) |
Significance of Time effect (P-value) |
|||
|---|---|---|---|---|---|---|---|
| Baseline | Month 3 | Baseline | Month 3 | Baseline | Month 3 | ||
| Food weight (g/d) | 1102 (425) | 984 (321) | 998 (290) | 1044 (331) | 1071 (357) | 954 (301) | Group×Timea 0.002 |
| Very low-ED category (g/d) | 189 (233) | 208 (216) | 156 (200) | 283 (270) | 198 (254) | 178 (201) | Group×Timeb 0.0007 |
| Low-ED category (g/d) | 420 (399) | 447 (305) | 415 (289) | 419 (257) | 404 (289) | 488 (301) | Time 0.053 |
| Medium-ED category (g/d) | 437 (280) | 299 (221) | 366 (238) | 308 (204) | 418 (249) | 251 (166) | Timec <0.0001 |
| High-ED category (g/d) | 56 (63) | 30 (39) | 61 (73) | 34 (47) | 52 (54) | 37 (62) | Timec <0.0001 |
| Beverage weight (g/d) | 1013 (567) | 849 (563) | 955 (552) | 909 (652) | 960 (597) | 895 (655) | Timec 0.0004 |
| Food energy (kcal/d) | 1798 (639) | 1394 (477) | 1661 (612) | 1402 (499) | 1744 (578) | 1336 (445) | Timec <0.0001 |
| Beverage energy (kcal/d) | 170 (151) | 124 (129) | 138 (120) | 137 (142) | 143 (142) | 121 (130) | Timec 0.004 |
| Total energy (kcal/d) | 1968 (661) | 1518 (495) | 1799 (635) | 1539 (525) | 1887 (593) | 1458 (487) | Timec <0.0001 |
| Food energy density (kcal/g) | 1.73 (0.53) | 1.48 (0.43) | 1.70 (0.51) | 1.40 (0.46) | 1.71 (0.49) | 1.47 (0.45) | Timec <0.0001 |
| Beverage energy density (kcal/g) |
0.18 (0.14) | 0.16 (0.15) | 0.18 (0.17) | 0.17 (0.16) | 0.16 (0.15) | 0.16 (0.17) | Time 0.51 |
| Fruit intake (servings/d) | 1.45 (1.65) | 1.58 (1.57) | 1.51 (1.59) | 1.87 (1.57) | 1.29 (1.46) | 1.73 (1.66) | Timed 0.0012 |
| Vegetable intake (servings/d) |
3.70 (2.95) | 3.66 (2.57) | 3.47 (2.07) | 3.80 (2.24) | 3.71 (2.65) | 3.11 (1.94) | Time 0.56 |
| Grain intake (servings/d) | 6.59 (3.38) | 5.02 (2.49) | 6.10 (3.77) | 4.73 (2.89) | 6.42 (3.29) | 4.79 (2.79) | Timec <0.0001 |
| Protein foods intake (servings/d) |
5.47 (3.89) | 4.61 (2.74) | 5.25 (3.44) | 4.79 (2.68) | 5.60 (3.73) | 4.30 (2.58) | Timec 0.0001 |
| Dairy foods intake (servings/d) |
3.11 (2.74) | 2.43 (1.92) | 3.00 (2.78) | 2.49 (2.13) | 2.90 (1.95) | 2.17 (1.69) | Timec <0.0001 |
| Fats and oils intake (servings/d) |
2.87 (2.70) | 2.38 (2.28) | 3.32 (2.78) | 2.41 (2.33) | 3.39 (3.28) | 2.21 (1.91) | Timec <0.0001 |
| Sweets intake (servings/d) | 1.57 (2.56) | 1.16 (1.88) | 1.95 (3.09) | 2.24 (3.77) | 1.98 (2.48) | 2.44 (3.12) | Time 0.48 |
| Pre-portioned foods intake (items/d) |
1.28 (1.23) | 1.43 (1.29) | 1.17 (1.22) | 1.33 (1.06) | 0.99 (1.19) | 2.18 (1.42) | Group×Timee <0.0001 |
Abbreviation: ED, energy density. Values are mean (SD). Categories of food energy density are Very low (0.0–0.59kcal/g), Low (0.6–1.49kcal/g), Medium (1.5–3.99kcal/g), and High (4.0–9.0kcal/g).
Decrease in Standard Advice and Pre-portioned Foods Groups from baseline to Month 3; no change in Portion Selection Group.
No change in Standard Advice and Pre-portioned Foods Groups from baseline to Month 3; increase in Portion Selection Group.
Decrease from baseline to Month 3 in all groups.
Increase from baseline to Month 3 in all groups.
Increase in Pre-portioned Foods Group from baseline to Month 3; no change in Standard Advice and Portion Selection Groups.
Changes in energy density and energy content of reported food consumption from baseline to Month 3 did not differ significantly across groups. All groups decreased food ED from 1.72±0.51 to 1.45±0.45 kcal/g (16%; P<0.0001), and energy intake from 1889±633 to 1504±502 kcal/d, a mean decrease of 387±686 kcal/d (20%; P<0.0001). Weight loss at Month 3 was correlated with the decrease in food ED at that time (r=0.23; P<0.0001) but not with the decrease in energy intake (r=0.08; P=0.14). At Month 3, all groups consumed fewer servings of grains, protein foods, dairy foods, and fats and oils; had no change in servings of vegetables and sweets; and consumed more servings of fruit (P=0.001; Table 3). Intake of pre-portioned foods increased in the Pre-portioned Foods Group from 1.0±1.2 to 2.2±1.4 items/d, whereas in the other two groups it did not change significantly (Table 3; interaction P<0.0001).
Pedometer counts increased similarly over time in all groups (P<0.0001). Mean counts increased from 6571±3250 steps/d at baseline to 7855±3204 steps/d at Month 1, increased again to 8516±3213 steps/d at Month 3, and were maintained at Months 6 and 12.
Indicators of adherence and satisfaction
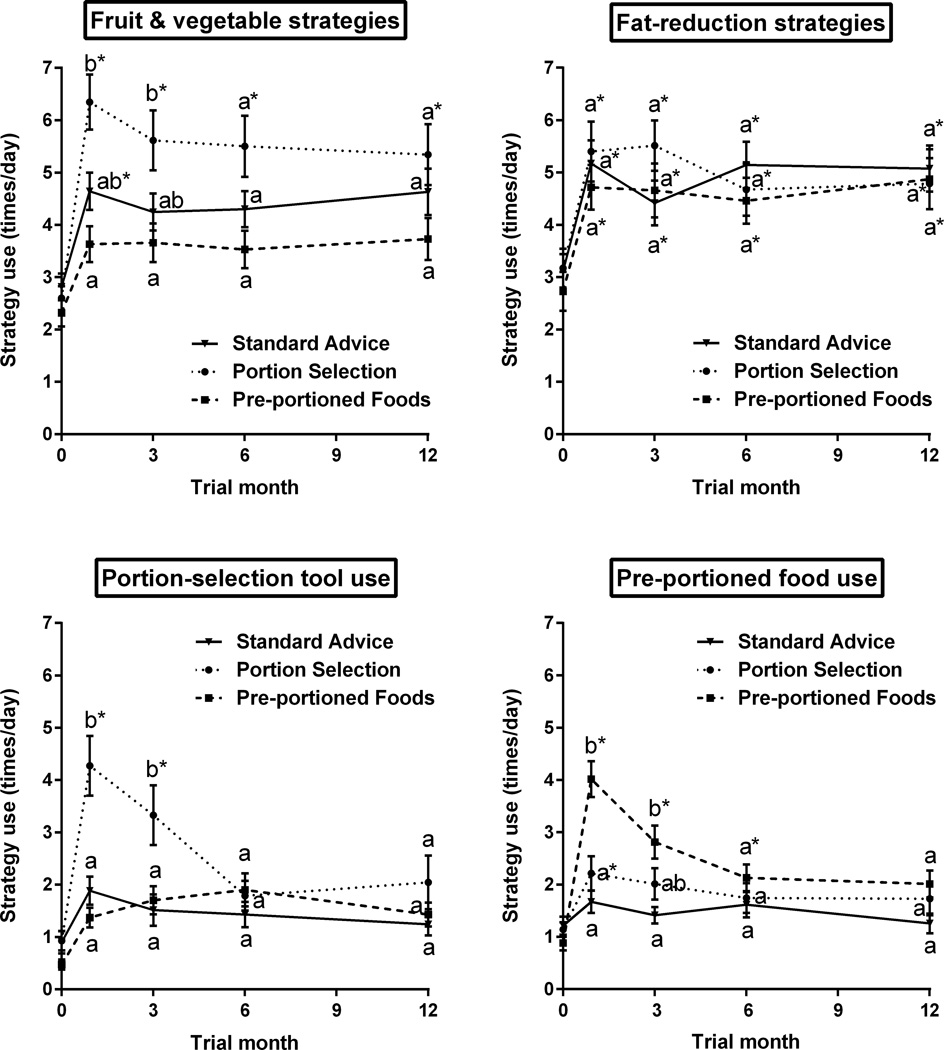
As shown in Figure 3, reported use of the targeted portion-control strategies (fruit and vegetable ED strategies, portion-selection tool use, and pre-portioned food use) differed across groups at Months 1 and 3 (all interactions P<0.0001). Reported use of these strategies then declined over time (P<0.03) and did not differ across groups at Months 6 and 12. In the Portion Selection Group, the most frequently used tool at Month 1 was household measures, which were used more frequently (1.5±1.5 times/day; P<0.014) than common objects (1.1±1.3 times/day), food scales (1.0±1.4 times/day) and the portion placemat (0.7±1.2 times/day). By Month 6, reported use of tools in this group did not differ from baseline (Figure 3). All groups reported similar increases in the use of fat-reduction strategies, which were maintained during the trial (P<0.0001).
Figure 3.
Mean reported daily frequency of use of targeted portion-control strategies by 186 women in three groups. Use of fruit and vegetable strategies and portion-selection tools was taught only to the Portion Selection Group, and use of pre-portioned foods was taught only to the Pre-portioned Foods Group. At a given time point, means with different letters are significantly different from those of other groups (P<0.05, adjusted for multiple comparisons). Within a given group, means with asterisks are significantly different from the baseline value (P<0.03, adjusted for multiple comparisons). Error bars represent the standard error of the mean.
General indicators of adherence were not significantly different across groups: participants attended a mean of 19±4.9 of the 23 sessions, and 50% missed either one or no sessions. Over the year, participants completed a mean of 546±313 daily self-monitoring records of weight, pedometer counts, and diet. The number of self-monitoring records was highly correlated with the number of sessions attended (r=0.72; P<0.0001).
Several ratings of satisfaction with the diet differed across the groups (P=0.001). Compared to baseline, at Month 1 the Pre-portioned Foods Group reported spending less time and effort on meals and feeling more self-conscious and deprived when eating; at Months 1, 3, and 6 they reported spending less money on food (all P<0.0001). The other two groups had no changes over time in ratings of satisfaction with the diet.
DISCUSSION
Although portion control is part of most behavioral weight management programs, little is known about the efficacy or sustainability of different portion-control strategies. The results of The Portion-Control Strategies Trial indicate that within a one-year behavioral program, three strategies led to similar weight loss: the standard message to eat less, using tools to select portions based on energy density, and structuring meals with pre-portioned foods. Across groups, 45% of participants achieved a clinically relevant weight loss of ≥5%41, indicating that all three of the programs had utility for weight management; these results are comparable with those of other behavioral programs in non-clinical populations13,23,42,43. The incorporation of specific portion-control strategies did not improve weight loss or cardiometabolic risk factors compared to standard dietary advice to eat less while making healthy food choices. Initial weight loss was enhanced by provision of pre-portioned foods to limit exposure to large portions, but this was not sustained over time. In all groups, reported use of targeted strategies showed adherence during the first several months, but strategy use then declined and converged over the year. The long-term maintenance of behavioral strategies to manage portion size remains a challenge12, 44–45.
Instruction to use pre-portioned foods at several meals each day, supported by provision of vouchers, resulted in more rapid initial weight loss than the other strategies. This may be related to the structured eating environment provided by pre-portioned foods, which led to fewer decisions about amounts to eat23–25,28–30. The initial success may also have occurred because the use of pre-portioned foods could be adopted immediately with minimal instruction, while the other strategies were taught in sessions extending over several weeks. An important question was whether the use of pre-portioned foods would continue when vouchers were not provided. We found that after the frequency of voucher provision was decreased to several per week, the higher rate of weight loss was not sustained. Weight regain during the second half of the trial was likely related to decreased use of pre-portioned foods, influenced by participant reports of increased cost and feelings of self-consciousness and deprivation. Further work is needed on methods to incorporate portion-controlled options into everyday decisions about what and how much to eat.
One portion-control strategy that is frequently advocated by professional organizations is to use tools such as household measures or common objects to provide guidance on recommended portion sizes46,47. To our knowledge, there are no data on the utility or sustainability of using portion-selection tools for weight management, other than using plates showing appropriate proportions of foods20–22. Most of the research related to such tools has focused on whether they improve the accuracy of portion size estimation18,48. One study, however, assessed the usage of portion-control tools and found that household measures were rated as the easiest to use and reported to be the most likely to be used again19. The current trial found that household measures were initially the most frequently used tool of the four provided, but after three months their reported use decreased substantially. More data are needed to determine whether such short-term adoption of tools improves understanding of appropriate portions, and whether more sustainable tools can be developed49,50.
An unexpected finding of the trial was the similarity in weight loss trajectories of the Portion Selection and Standard Advice groups. In a previous study, we found that instruction on reducing dietary energy density by increasing intake of water-rich foods led to lower ED and greater weight loss than advice to eat less and reduce fat intake13; additionally, other research has found the degree of ED reduction to be associated with the amount of weight loss14,51,52. In the present trial, ED instruction was provided only to the Portion Selection Group, but dietary recalls showed that at three months all groups reported similar decreases in intake of medium-ED and high-ED foods, resulting in substantial reductions in dietary ED. Although the Portion Selection Group additionally reported increased intake of very low-ED foods and maintained the total weight of food eaten, this was insufficient to further reduce dietary ED compared to the other groups. Across the groups, there was also considerable overlap during the later months in the reported use of specific dietary strategies associated with reductions in ED. In this population, the women’s familiarity with various weight-loss strategies probably decreased fidelity to the assigned diet programs53 and contributed to convergence in dietary intake patterns. It is likely that the comparable weight loss across groups was related to these similarities in eating behaviors as well as the common instruction on physical activity and self-monitoring.
In this group of experienced dieters, standard advice to eat less was as successful for weight loss as more specific portion-control strategies. Recently published data suggest that including a rigorous usual-care condition in weight loss trials results in substantial attenuation of treatment effects54, which may have contributed to the similarity of weight change across groups. The convergence of weight change over the trial may relate to participants’ adherence to the program55–56, since frequency of attendance and self-monitoring did not differ across groups and were related to rates of weight loss and regain. It seems likely that the regular individual meetings and the accountability they encouraged were an important driver of weight change; similarly, one commercial program found that participants with >85% attendance had the highest weight loss57. The frequency of instructional sessions was reduced over the trial, and this could account in part for declining rates of weight loss. The trajectories of weight change were also influenced by participant characteristics. Across the programs, weight loss was greater for those with lower baseline restraint scores, which may indicate greater opportunities for beneficial behavior changes.
The Portion-Control Strategies Trial is the first longitudinal comparison of the efficacy of different portion-control strategies for weight management. A strength of the trial was evaluating specific approaches to portion management, accompanied by frequent weight measurement that allowed modeling of trajectories of weight change using an intention-to-treat analysis; however, the generalizability of the findings is limited by the lack of diversity in the sample. The results showed that within a behavioral program, a variety of portion-control approaches can be effective in promoting weight loss, but that over a year strategies such as use of portion-control tools and pre-portioned meals were not well maintained. A goal of future studies should be to find improved techniques for maintaining adherence to dietary programs that incorporate portion-control strategies into sustainable lifestyle changes. Modifications to the eating environment that make it easier to manage large portions of energy-dense foods are needed to support such programs16,51,58.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK059853). The use of the Clinical Research Center was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (UL1TR000127). Food vouchers were provided by unrestricted gifts from ConAgra Foods, Inc. and Nestlé USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding bodies. We thank the participants in the Portion-Control Strategies Trial and the research team at Penn State, particularly interventionists Jennifer Meengs, Kitti Halverson, Cara Meehan, and Amy Ciccarella.
BJR receives royalties from the sale of the Volumetrics books.
Footnotes
POTENTIAL CONFLICT OF INTEREST
LSR, BLJ, and CES declare no conflict of interest.
SUPPLEMENTARY INFORMATION
Supplementary information is available at International Journal of Obesity’s website.
REFERENCES
- 1.Nielsen SJ, Popkin BM. Patterns and trends in food portion sizes, 1977–1998. J Am Med Assoc. 2003;289:450–453. doi: 10.1001/jama.289.4.450. [DOI] [PubMed] [Google Scholar]
- 2.Young LR, Nestle M. The contribution of expanding portion sizes to the U.S. obesity epidemic. Am J Public Health. 2002;92:246–249. doi: 10.2105/ajph.92.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smiciklas-Wright H, Mitchell DC, Mickle SJ, Goldman JD, Cook A. Foods commonly eaten in the United States, 1989–1991 and 1994–1996: are the portion sizes changing? J Am Diet Assoc. 2003;103:41–47. doi: 10.1053/jada.2003.50000. [DOI] [PubMed] [Google Scholar]
- 4.Rolls BJ, Morris EL, Roe LS. Portion size of food affects energy intake in normal-weight and overweight men and women. Am J Clin Nutr. 2002;76:1207–1213. doi: 10.1093/ajcn/76.6.1207. [DOI] [PubMed] [Google Scholar]
- 5.Rolls BJ, Roe LS, Meengs JS. The effect of large portion sizes on energy intake is sustained for 11 days. Obesity. 2007;15:1535–1543. doi: 10.1038/oby.2007.182. [DOI] [PubMed] [Google Scholar]
- 6.Kral TVE, Roe LS, Rolls BJ. Combined effects of energy density and portion size on energy intake in women. Am J Clin Nutr. 2004;79:962–968. doi: 10.1093/ajcn/79.6.962. [DOI] [PubMed] [Google Scholar]
- 7.English L, Lasschuijt M, Keller KL. Mechanisms of the portion size effect. What is known and where do we go from here? Appetite. 2015;88:39–49. doi: 10.1016/j.appet.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Herman CP, Polivy J, Pliner P, Vartanian LR. Mechanisms underlying the portion-size effect. Physiol Behav. 2015;144:129–136. doi: 10.1016/j.physbeh.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Livingstone MBE, Pourshahidi LK. Portion size and obesity. Adv Nutr. 2014;5:829–834. doi: 10.3945/an.114.007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th. Washington, DC: US Government Printing Office; 2010. [Google Scholar]
- 11.Raynor HA, Champagne CM. Position of the Academy of Nutrition and Dietetics: interventions for the treatment of overweight and obesity in adults. J Acad Nutr Diet. 2016;116:129–147. doi: 10.1016/j.jand.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Rolls BJ. What is the role of portion control in weight management? Int J Obes. 2014;38:S1–S8. doi: 10.1038/ijo.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ello-Martin JA, Roe LS, Ledikwe JH, Beach AM, Rolls BJ. Dietary energy density in the treatment of obesity: a year-long trial comparing 2 weight-loss diets. Am J Clin Nutr. 2007;85:1465–1477. doi: 10.1093/ajcn/85.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ledikwe JH, Rolls BJ, Smiciklas-Wright H, Mitchell DC, Ard JD, Champagne C, et al. Reductions in dietary energy density are associated with weight loss in overweight and obese participants in the PREMIER trial. Am J Clin Nutr. 2007;85:1212–1221. doi: 10.1093/ajcn/85.5.1212. [DOI] [PubMed] [Google Scholar]
- 15.Raynor HA, Van Walleghen EL, Bachman JL, Looney SM, Phelan S, Wing RR. Dietary energy density and successful weight loss maintenance. Eat Behav. 2011;12:119–125. doi: 10.1016/j.eatbeh.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolls BJ. The relationship between dietary energy density and energy intake. Physiol Behav. 2009;97:609–615. doi: 10.1016/j.physbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez-Escamilla R, Obbagy JE, Altman JM, Essery EV, McGrane MM, Wong YP, et al. Dietary energy density and body weight in adults and children: A systematic review. J Acad Nutr Diet. 2012;112:671–684. doi: 10.1016/j.jand.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Byrd-Bredbenner C, Schwartz J. The effect of practical portion size measurement aids on the accuracy of portion size estimates made by young adults. J Hum Nutr Diet. 2004;17:351–357. doi: 10.1111/j.1365-277X.2004.00534.x. [DOI] [PubMed] [Google Scholar]
- 19.Faulkner GP, Livingstone MB, Pourshahidi LK, Spence M, Dean M, O'Brien S, et al. An evaluation of portion size estimation aids: precision, ease of use and likelihood of future use. Public Health Nutr. 2016;19:2377–2387. doi: 10.1017/S1368980016000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen SD, Kang J, Kline GA. Portion control plate for weight loss in obese patients with type 2 diabetes mellitus: a controlled clinical trial. Arch Intern Med. 2007;167:1277–1283. doi: 10.1001/archinte.167.12.1277. [DOI] [PubMed] [Google Scholar]
- 21.Kesman RL, Ebbert JO, Harris KI, Schroeder DR. Portion control for the treatment of obesity in the primary care setting. BMC Res Notes. 2011;4:346. doi: 10.1186/1756-0500-4-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber JM, Shapiro JS, Wieland ML, Croghan IT, Vickers Douglas KS, Schroeder DR, et al. Telecoaching plus a portion control plate for weight care management: a randomized trial. Trials. 2015;16:323. doi: 10.1186/s13063-015-0880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rolls BJ, Roe LS, Beach AM, Kris-Etherton PM. Provision of foods differing in energy density affects long-term weight loss. Obes Res. 2005;13:1052–1060. doi: 10.1038/oby.2005.123. [DOI] [PubMed] [Google Scholar]
- 24.Wing RR, Jeffery RW, Burton LR, Thorson C, Nissinoff KS, Baxter JE. Food provision vs structured meal plans in the behavioral treatment of obesity. Int J Obes Relat Metab Disord. 1996;20:56–62. [PubMed] [Google Scholar]
- 25.Wing RR, Jeffery RW. Food provision as a strategy to promote weight loss. Obes Res. 2001;9:271S–275S. doi: 10.1038/oby.2001.130. [DOI] [PubMed] [Google Scholar]
- 26.Heymsfield SB. Meal replacements and energy balance. Physiol Behav. 2010;100:90–94. doi: 10.1016/j.physbeh.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 27.European Food Safety Authority, Panel on Dietetic Products, Nutrition and Allergies, (NDA) Scientific opinion on the substantiation of health claims related to meal replacements for weight control (as defined in Directive 96/8/EC on energy restricted diets for weight loss) and reduction in body weight (ID 1417), and maintenance of body weight after weight loss (ID 1418) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2010;8 [Google Scholar]
- 28.Hannum SM, Carson LA, Evans EM, Canene KA, Petr EL, Bui L, et al. Use of portion-controlled entrees enhances weight loss in women. Obes Res. 2004;12:538–546. doi: 10.1038/oby.2004.61. [DOI] [PubMed] [Google Scholar]
- 29.Hannum SM, Carson LA, Evans EM, Petr EL, Wharton CM, Bui L, et al. Use of packaged entrees as part of a weight-loss diet in overweight men: an 8-week randomized clinical trial. Diabetes Obes Metab. 2006;8:146–155. doi: 10.1111/j.1463-1326.2005.00493.x. [DOI] [PubMed] [Google Scholar]
- 30.Rock CL, Flatt SW, Pakiz B, Barkai HS, Heath DD, Krumhar KC. Randomized clinical trial of portion-controlled prepackaged foods to promote weight loss. Obesity. 2016;24:1230–1237. doi: 10.1002/oby.21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The Eating Attitudes Test: psychometric features and clinical correlates. Psychol Med. 1982;12:871–878. doi: 10.1017/s0033291700049163. [DOI] [PubMed] [Google Scholar]
- 32.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 33.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models, Second Edition. Cary, NC: SAS Institute, Inc.; 2006. [Google Scholar]
- 34.Rolls BJ. The Ultimate Volumetrics Diet: Smart, Simple, Science-Based Strategies for Losing Weight and Keeping It Off. New York, NY: William Morrow; 2012. [Google Scholar]
- 35.National Center for Health Statistics, Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES): Anthropometry Procedures Manual. Hyattsville, MD: US Department of Health and Human Services; 2011. [Google Scholar]
- 36.Hill NR, Levy JC, Matthews DR. Expansion of the homeostatic model assessment of β-cell function and insulin resistance to enable clinical trial outcome modeling through the interactive adjustment of physiology and treatment effects: iHOMA2. Diabetes Care. 2013;36:2324–2330. doi: 10.2337/dc12-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.University of Minnesota (USA) Nutrition Data System for Research - Nutritional Analysis Software [Internet] [cited 14 June 2016];2016 Available from: http://license.umn.edu/technologies/ndsr87072_nutrition-data-system-for-research-nutritional-analysis-software. [Google Scholar]
- 38.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 39.Ello-Martin JA. Reducing dietary energy density for the treatment of obesity: The long-term effects on weight loss, hunger, and diet satisfaction [dissertation] University Park, PA: The Pennsylvania State University; 2006. [Google Scholar]
- 40.Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrates, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: The National Academies Press; 2002. [DOI] [PubMed] [Google Scholar]
- 41.Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591–601. doi: 10.1016/j.cmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. J Am Med Assoc. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 43.Wing RR, Hamman RF, Bray GA, Delahanty L, Edelstein SL, Hill JO, et al. Achieving weight and activity goals among Diabetes Prevention Program lifestyle participants. Obes Res. 2004;12:1426–1434. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poelman PP, de Vet E, Velema E, de Boer MR, Seidell JC, Steenhuis IHM. PortionControl@HOME: Results of a randomized controlled trial evaluating the effect of a multi-component portion size intervention on portion control behavior and body mass index. Ann Behav Med. 2015;49:18–28. doi: 10.1007/s12160-014-9637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marteau TM, Hollands GJ, Shemilt I, Jebb SA. Downsizing: Policy options to reduce portion sizes to help tackle obesity. BMJ. 2015;351:h5863. doi: 10.1136/bmj.h5863. [DOI] [PubMed] [Google Scholar]
- 46.US Department of Health and Human Services, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Just Enough for You. About Food Portions. Washington, DC: US Government Printing Office; 2012. [Google Scholar]
- 47.US Department of Agriculture. ChooseMyPlate.gov website. Portion distortion. [cited 26 September 2016]; Available from: https://www.choosemyplate.gov/tools-portion-distortion.
- 48.Gibson AA, Hsu MS, Rangan AM, Seimon RV, Lee CM, Das A, et al. Accuracy of hands v. household measures as portion size estimation aids. J Nutr Sci. 2016;5:e29. doi: 10.1017/jns.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wharton CM, Johnston CS, Cunningham BK, Sterner D. Dietary self-monitoring, but not dietary quality, improves with use of smartphone app technology in an 8-week weight loss trial. J Nutr Educ Behav. 2014;46:440–444. doi: 10.1016/j.jneb.2014.04.291. [DOI] [PubMed] [Google Scholar]
- 50.Martin CK, Nicklas T, Gunturk B, Correa JB, Allen HR, Champagne C. Measuring food intake with digital photography. J Hum Nutr Diet. 2014;27(Suppl. 1):72–81. doi: 10.1111/jhn.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rouhani MH, Haghighatdoost F, Surkan PJ, Azadbakht L. Associations between dietary energy density and obesity: A systematic review and meta-analysis of observational studies. Nutrition. 2016;32:1037–1047. doi: 10.1016/j.nut.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 52.Stelmach-Mardas M, Rodacki T, Dobrowolska-Iwanek J, Brzozowska A, Walkowiak J, Wojtanowska-Krosniak A, et al. Link between food energy density and body weight changes in obese adults. Nutrients. 2016;8:229. doi: 10.3390/nu8040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lemon SC, Wang ML, Haughton CF, Estabrook DP, Frisard CF, Pagoto SL. Methodological quality of behavioural weight loss studies: a systematic review. Obes Rev. 2016;7:636–644. doi: 10.1111/obr.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dawson JA, Kaiser KA, Affuso O, Cutter GR, Allison DB. Rigorous control conditions diminish treatment effects in weight loss-randomized controlled trials. Int J Obes. 2015;40:895–898. doi: 10.1038/ijo.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heymsfield SB, Harp JB, Reitman ML, Beetsch JW, Schoeller DA, Erondu N, et al. Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. Am J Clin Nutr. 2007;85:346–354. doi: 10.1093/ajcn/85.2.346. [DOI] [PubMed] [Google Scholar]
- 56.Thomas DM, Martin CK, Redman LM, Heymsfield SB, Lettieri S, Levine JA, et al. Effect of dietary adherence on the body weight plateau: a mathematical model incorporating intermittent compliance with energy intake prescription. Am J Clin Nutr. 2014;100:787–795. doi: 10.3945/ajcn.113.079822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Finley CE, Barlow CE, LaMonte MJ, Greenway FL, Rock CL, Rolls BJ, et al. Retention rates and weight loss in a commercial weight management program. Int J Obesity. 2007;31:292–298. doi: 10.1038/sj.ijo.0803395. [DOI] [PubMed] [Google Scholar]
- 58.Crino M, Sacks G, Vandevijvere S, Swinburn B, Neal B. The influence on population weight gain and obesity of the macronutrient composition and energy density of the food supply. Curr Obes Rep. 2015;4:1–10. doi: 10.1007/s13679-014-0134-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.