Abstract
Although CD31 expression on human thymocytes has been reported, a detailed analysis of CD31 expression at various stages of T cell development in the human thymus is missing. Herein, we provide a global picture of the evolution of CD31 expression from the CD34+ hematopoietic precursor to the CD45RA+ mature CD4+ and CD8+ single positive T cells. Using 9-color flow cytometry, we show that CD31 is highly expressed on CD34+ progenitors and stays high until the early double positive stage (CD3− CD4+ CD8α+β −). After β-selection, CD31 expression levels become low to undetectable. CD31 expression then increases and peaks on CD3high CD4+CD8+ double positive thymocytes. However, following positive selection, CD31 expression differs dramatically between CD4+ and CD8+ lineages: homogeneously high on CD8 SP but lower or negative on CD4 SP cells, including a subset of CD45RA+ CD31− mature CD4+ thymocytes. CD31 expression on TCRγδ thymocytes is very similar to that of CD4 SP cells. Remarkably, there is a substantial subset of semi-mature (CD45RA−) CD4 SP thymocytes that lack CD31 expression. Moreover, FOXP3+ and ICOS+ cells are over-represented in this CD31− subpopulation. Despite this CD31− CD45RA− subpopulation, the majority of egress-capable mature CD45RA+ CD4 SP thymocytes expresses CD31. The variations in CD31 expression appear to coincide with three major selection processes occurring during thymopoiesis: β-selection, positive selecion and negative selection. Considering the ability of CD31 to modulate the TCR’s activation threshold via the recruitment of tyrosine phosphatases, our results suggest a significant role for CD31 during T cell development.
Keywords: CD31, human thymus, regulatory T cells, thymopoiesis, hematopoietic stem cells
Introduction
Although much is known of the development of naïve T cells, the role of CD31 (PECAM-1) on naïve T cells and its expression during differentiation of hematopoietic stem cells into mature T cells in the human thymus is still unresolved. Elucidating CD31 expression in thymopoiesis is important as CD31 has been shown to regulate T cell receptor (TCR) mediated signaling ((1–3) and reviewed in (4)). CD31, also known as PECAM-1 (Platelet Endothelial Cell Adhesion Molecule-1), is a single chain 130 kDa glycoprotein consisting of 6 Ig-like extracellular domains, a short transmembrane segment and a cytoplasmic tail containing two ITIMs (ImmunoTyrosine Inhibitory Motif). CD31, expressed on endothelial cells, platelets and leukocytes (reviewed in (4, 5)), can engage in both homophilic (CD31-CD31) and heterophilic interactions with other cell surface markers including CD38, an ectoenzyme expressed on the majority of thymocytes (6, 7).
Upon TCR activity, the 2 ITIMs located in the CD31 cytoplasmic tail can be phosphorylated (2) and subsequently recruit phosphotyrosine phosphatases (PTP), such as SHP-1, SHP-2 or SHIP, leading to inhibition of TCR signaling (3). Moreover, phosphorylation of ZAP-70, a key upstream mediator of TCR signaling, is partially inhibited following the interaction of CD31 and the TCR (8). Similar observations have been made with respect to B-cells in which CD31 has been shown to inhibit B-cell receptor (BCR) signaling (9). Therefore, CD31 engagement can prevent lymphocyte hyper-reactivity by raising the activation threshold of antigen-receptor signaling.
CD31 has been proposed as a marker to aid in identification of CD4+ recent thymic emigrants (RTE) (10, 11). Thymocytes that successfully navigate the stages of positive and negative selection emigrate to the periphery as CD45RA+CD62L+ CD4+ T cells and can be distinguished from their proliferative offspring by their expression of CD31, while proliferation due to antigenic priming or homeostatic signals eventually results in CD31 down-regulation (12). In contrast, naïve CD8+ T cells egress the thymus expressing CD31 and retain its expression during differentiation in the periphery (13).
While much is known about the patterns of CD31 expression on T cells in the periphery, little is known about CD31 expression on human thymocytes. Stockinger et al. (13) and Tenca et al. (7) reported that CD31 is expressed by a majority of human thymocytes, however they did not provide a detailed analysis of its expression during different stages of T cell development. In this report, we provide a global picture of the expression of CD31 during human T cell development in the thymus and illustrate the strong dichotomy between CD4 and CD8 lineages. We show that CD31 expression is high on CD34+ hematopoietic progenitors and is quickly reduced after T cell lineage commitment around the early double positive stage (EDP, CD3− CD1a+ CD4+CD8α+β − cells), likely during expansion post β-selection. CD31 expression then increases and peaks on CD4+CD8+ DP thymocytes. Following CD4/CD8 lineage commitment the CD31 expression pattern becomes dramatically different on CD8+CD4− (CD8 SP) and CD4+CD8− (CD4 SP) thymocytes. CD31 is high on all CD8 SP thymocytes, whereas CD4 SP thymocytes express lower levels or lack expression of CD31, including on a subset of CD45RA+ mature CD4+ T cells, ready to egress the thymus. Surprisingly the absence of CD31 expression is more frequent on FOXP3-expressing natural regulatory CD4+ T cells (Treg), as compared to conventional FOXP3− CD4+ thymocytes at an equivalent developmental stage, and coincides with a heightened level of activation as shown by increased expression of ICOS, CD25 and CD127.
Material and Methods
Tissue collection and primary thymocyte preparation
Normal human postnatal anonymous thymus specimens were obtained from children undergoing corrective cardiac surgery at the UCLA Mattel Children’s hospital. Thymocytes were prepared and cultured as previously described (14). Briefly, tissues were placed in NH4Cl-Tris lysing buffer to remove the red blood cells while the tissue was cut into small pieces and passed over a cell strainer to generate a single-cell suspension of thymocytes. Cells were washed in serum-free medium consisting of IMDM (Omega Scientific) supplemented with 1100 μg/mL delipidated BSA (Sigma-Aldrich), 85 μg/mL transferrin (Sigma-Aldrich), 2 mM glutamine and 25 U/25 μg/mL penicillin/streptomycin, then resuspended at 4 × 107 cells/ml in serum-free medium.
Postnatal thymus (PNT) tissue for experiments done at the Academic Medical Center was obtained from surgical specimens removed from children up to 3 year of age undergoing open heart surgery with informed consent from patients in accordance with the Declaration of Helsinki and was approved by the Medical Ethical Committee of the Academic Medical Center. The tissue was disrupted by mechanical means and pressed through a stainless steel mesh to obtain a single-cell suspension and thymocytes were isolated from a Ficoll-Hypaque density gradient (Lymphoprep; Axis-Shield) as previously described (15).
Flow cytometry
Flow cytometry data were acquired on LSRII or Fortessa analyzer (Becton Dickinson) and analyzed with FCS Express (De Novo software). Surface and intracellular immunophenotyping of thymocytes with directly conjugated antibodies (see supplemental Table S1) were performed as previously described (16). For detection of intracellular FOXP3, TCR Cβ1 and TCRγδ chains, cells were first stained for cell surface markers, fixed and permeabilized with eBioscience recommended buffers following manufacturer instructions, then incubated with the appropriate antibody.
Cell sorting and quantitative PCR
Prior to separation of thymocyte subsets by flow cytometry, CD27+ cells were enriched by immunomagnetic separation. Briefly CD27+ cells were separated using an EasySep human “DIY” selection kit (StemCell Technologies) associated to a purified monoclonal antibody against CD27 (eBioscience) on a RoboSep magnetic cell separator. The purity of the positively selected fraction was above 90%. For further isolation of various subsets of mature CD4 SP thymocytes, CD27+ thymocytes were stained for CD3, CD4, CD8, CD27, CD31, CD45RA and CD69. Cells were sorted on a FACSAria II cell sorter (Becton Dickinson). The purity of sorted subsets exceeded 95%.
Sorted cells were immediately washed once in phosphate buffered saline (PBS) before DNA and RNA extraction using the AllPrep DNA/RNA Micro Kit (Qiagen). Because of the limited amount of initial material, complementary DNAs were generated with Qiagen’s Whole Transcriptome Amplification kit following manufacturer’s guidelines. Quantitative PCR was performed on an Applied Biosystems 7300 Real-Time PCR System with the QuantiFast Multiplex PCR Kit (Qiagen) and the following primers/probe sets (TaqMan Gene Expression Assay, Applied Biosystems): FOXP3 (FAM), assay ID Hs01085835_m1; eukaryotic 18S ribosomal RNA (VIC, TaqMan Endogenous Controls, Applied Biosystems).
sjTREC quantification
Signal joint (sj) TRECs resulting from the rearrangement of the TCRA locus were detected in genomic DNA extracted from sorted thymocytes subsets. The protocol was adapted from (11). Cell numbers and sjTRECs in each sample were quantified by multiplexed real-time PCR analysis on the Applied Biosystems 7300 system using Custom TaqMan® Copy Number Assays. The sequences of the primers and FAM dye-labeled MGB probe to detect sjTREC sequences were designed with the GeneAssist™ Copy Number Assay Workflow Builder tool, available on the Applied Biosystems website, to be compatible with the RNase P detection kit used to quantify the cells (TaqMan® Copy Number Reference Assays, VIC dye-labeled TAMRA probe). Primers were located on each side of the single joint sequence: sjTREC forward, CCATGCTGACACCTCTGGTTTT; sjTREC reverse, TGCCAGCTGCAGGGTTTAG; sjTREC probe, CAGGTGCCTATGCATCACC.
The 20μL reaction mixture consisted of 8μL of genomic DNA solution, 10μL of QuantiFast Multiplex PCR master mix (Qiagen), and 1μL each of sjTREC and RNase P primers/probe sets. Standards consisted of known concentrations of a mixture of a plasmid containing a sjTREC fragment (7.5 to 6 × 103 TRECs per reaction) and genomic DNA from non-TREC containing HeLa epithelial cells (37.5 to 30,000 cells per reaction). All samples and controls were prepared and performed in triplicate on the same plate to reduce error. The mean value for each triplicate was used for further analysis.
Gene expression quantification
CD31/CD45RA-defined subsets of CD4 SP thymocytes were sorted and total RNA was extracted as described above. Gene expression analysis using the QuantiGene Plex Assay was performed for a number of selected targets.
The QuantiGene Plex Assay from Affymetrix combines Luminex® xMAP® multiplexing technology with branched DNA (bDNA) for signal amplification. The principle of the assay is described in detail at www.ebioscience.com/QGP. In brief, fluorescent magnetic microspheres conjugated with capture probes are incubated overnight with the sample and a target-specific probe set. The probes capture the target sequence and hybridize to the beads, while also permitting formation of the signal amplification tree by bDNA oligos. The bDNA oligos (Pre-amplifier, Amplifier, and a biotinylated Label Probe) hybridize sequentially to the target-specific probes in order to amplify the signal. The addition of streptavidin phycoerythrin (SAPE) produces fluorescence, which is quantified by the Luminex instrument as median fluorescence intensity (MFI). An excel macro was used to normalize the MFI data to the geometric mean of the signals from the housekeeping genes RPS20 and PPIB. The QuantiGene Plex Assay was performed as a service by the Affymetrix Research Services Laboratory.
Statistical analysis
All analyses were conducted with GraphPad Prism 6 (GraphPad Software). Variables were expressed as means with standard error of the mean (SEM). One or two-way ANOVA with appropriate post comparison tests and two-tailed Student’s t-tests were used to analyze differences between populations where appropriate. A p value inferior or equal to 0.05 was considered significant.
Results
CD31 expression fluctuates during thymopoiesis
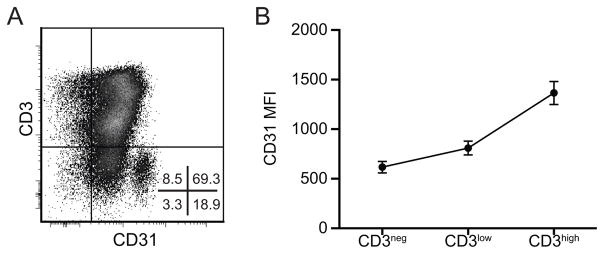
Although CD31 expression on human thymocytes has been reported previously (7, 13), a detailed analysis of CD31 expression levels on the various thymocyte populations during the course of T cell development has not been reported to date. Herein, CD31 expression was analyzed using multicolor flow cytometry combining CD31 with up to 8 markers specific for different thymocyte developmental stages. As shown in Figure 1, CD31 is expressed during thymopoiesis by the majority of the developing thymocytes (87.3 ± 1.3% of CD31+ cells) and the average level of CD31 expression increases during maturation (Figure 1b). However the evolution of CD31 expression during the development of T lymphocytes is not linear. Despite globally lower expression among immature CD3− thymocytes, a cluster of cells within this population expresses a high level of CD31 (19.8 ± 2.7% of CD3− thymocytes, CD31 MFI: 4276 ± 281). In contrast, although CD3high thymocytes globally express the highest level of CD31, this population contains a small subpopulation that doesn’t express detectable CD31 (11.2 ± 1.1%). Thus, levels of CD31 vary during T cell development. This may be due to interactions with CD31 ligands, such as CD38 or CD31 itself (4, 7, 17) expressed on thymocytes or on cortical or medullary thymic epithelial cells (TEC) which mediate positive and negative selection (18). The following sections focus on delineating CD31 expression in the CD3− and CD3high thymocyte populations.
Figure 1. Global CD31 expression in the human thymus.
(A) CD31 expression as a function of CD3 expression by flow cytometry on freshly prepared total thymocytes. (B) Median fluorescence intensity of CD31 on CD3−, CD3low/dim and CD3high populations (n=15, mean ± SEM).
Progenitors and thymocytes prior to β-selection express high levels of CD31
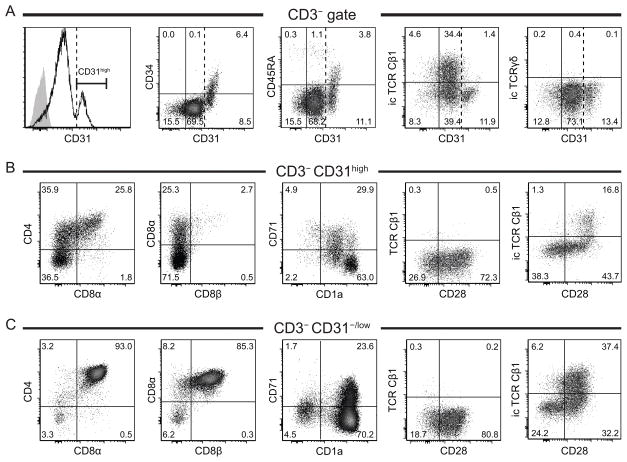
The immature CD3− thymocyte subset consists of several populations ranging from the CD34+ hematopoietic progenitors that populate the thymus to the double positive CD4+CD8+ (DP) thymocytes that do not yet express a TCR (19), as well as NK cells developing in the thymus (20). CD3− thymocytes overall express low levels of CD31. However a cluster of cells within this population expresses high levels of CD31. In-depth immunophenotyping revealed that this CD31high cluster consists of CD34+ hematopoietic progenitors and their immediate CD34− progeny at various stages of differentiation: CD34+ CD45RA+ CD1a− CD7high CD10+ thymic immigrant cells, CD1alow pre-T cells, CD4−CD8− double negative (DN), CD4+CD8α −β − immature single positive (ISP4) and early CD4+CD8α+β − early double positive (EDP) cells (Figures 2 and S1). This is in accordance with previous reports showing that peripheral blood CD34+ hematopoietic stem cells express CD31 (21–23) and that CD31 may contribute to the extravasation of the CD34+ hematopoietic progenitors from the blood stream to the thymus (24). Thus, although CD31 does not allow the isolation of a single homogeneous population, it can be used as a complementary and novel marker to better distinguish, within the CD3− population, the small population made of these hematopoietic progenitor cells (CD34 high CD31high) and their immediate progeny (CD34− CD31high) from the much larger population of more advanced CD3− DP thymocytes (CD34− CD31−/low CD1ahigh CD4+CD8α+β+).
Figure 2. Phenotype of CD3−CD31high and CD31−/low thymocytes.
(A) Expression of CD34, CD45RA, intracellular TCR Cβ1 and TCRγδ chains relative to CD31 on CD3− thymocytes; histogram plot: black line: CD31 staining, gray solid plot: isotype control, dashed vertical line indicates lower limit for CD31high. (B, C) Phenotyping of CD3− CD31high (B) and CD3−CD31−/low thymocytes (C) using early T cell development markers. TCR Cβ1 antibody is known to react with 50–75% of T cells (49). Results shown are representative of at least 3 independent experiments.
Interestingly, our results also show that lower CD31 expression coincides with the start of TCRβ intracellular expression (Figure 2A). Indeed the earliest detection of TCRβ expression was intracellularly in CD31high CD34low CD28+ ISP4 cells and the frequency of icTCRβ+ cells within the CD3− population increased to a maximum at the CD31−/low stage. Unlike TCRβ, we could not detect intracellular expression of TCRγδ chains in CD3− thymocytes (Figure 2A). Also, as shown in Figure 2B and C, no TCR could be detected extracellularly before CD3 expression. This suggests that TCRB locus recombination had been initiated in prior stages (CD31high cells) as proposed previously (19, 25, 26). Additionally, concomitant expression of CD28 and CD71 (Figure 2B–C) may be a sign of proliferation following successful β-selection (25).
TCRβ chain selection begins at the CD3−CD4+CD8− (ISP4) and is followed by proliferation of the cells that successfully signaled through their pre-TCR complex. Our results suggest that CD31 down-regulation on CD3− thymocytes coincides with proliferation post β-selection, in a similar fashion to what is observed on peripheral CD4+ naïve T cells.
Differential CD31 expression patterns on mature CD4 SP and CD8 SP thymocytes
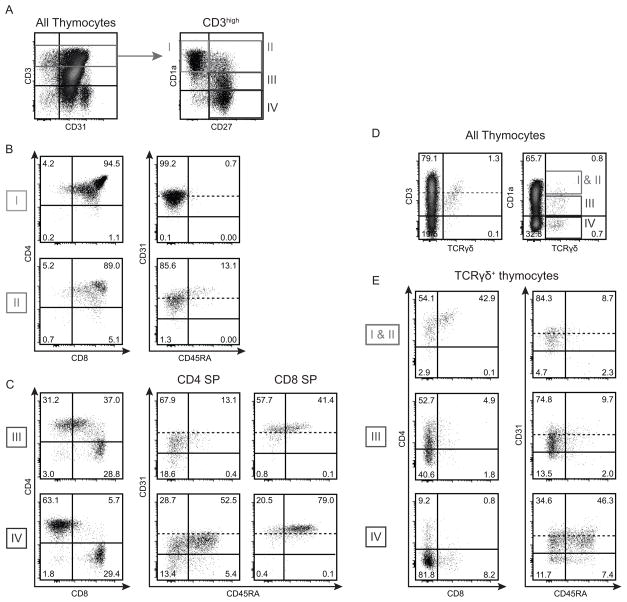
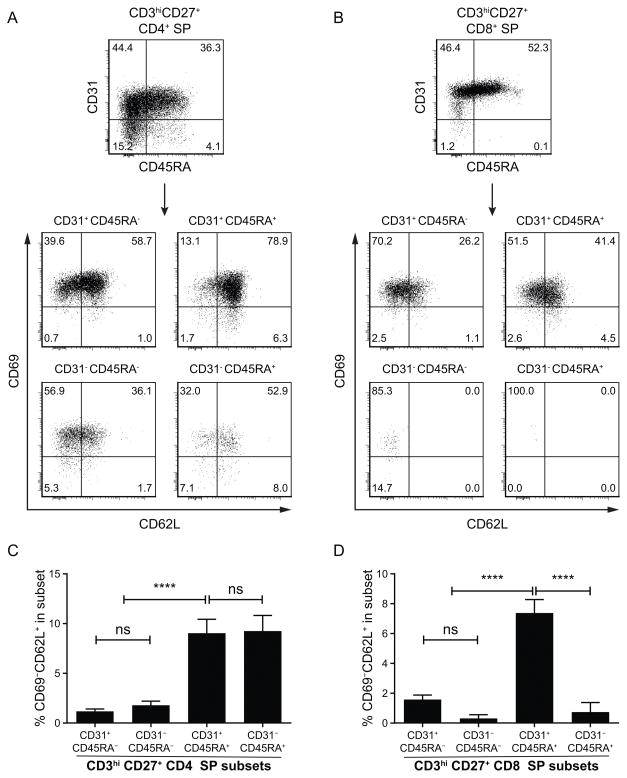
To study CD31 expression at more advanced stages of T cell development, CD3high thymocytes were divided into four subpopulations based on their expression of CD1a and CD27 (Figure 3A): from least mature (CD1a+ CD27−, gate I) to most mature (CD1a− CD27+, gate IV). Stages I (CD1a+ CD27−) and II (CD1a+ CD27+) contain mainly late DP thymocytes and cells that show early commitment to CD4 or CD8 lineage (Figure 3B); those cells express CD31 (99.3 ± 0.1% and 98.1 ± 0.3% of CD31+ cells at stage I and II respectively) (Figure 3B). At stages III (CD1alow CD27+) and IV (CD1a− CD27+) all thymocytes have committed to CD4 or CD8 lineages (Figure 3C, left panels), therefore expression of CD31 and CD45RA is shown for either CD4+ single positive (CD4 SP) or CD8+ single positive (CD8 SP) cells (Figure 3C, middle panels). During stage III, when thymocytes are undergoing negative selection, CD8 SP cells are homogeneously CD31high (MFI: 2782 ± 194) and already acquiring CD45RA (33.5 ± 0.3% of CD45RA+ cells). At stage IV, a majority of CD8 SP cells have acquired CD45RA, a characteristic marker of a mature naïve phenotype (63.5 ± 3.7% of CD45RA+) (Figure 3C, bottom right panel).
Figure 3. Evolution of CD31 expression during the development of CD3high thymocytes.
(A) CD3high thymocytes were divided into 4 subpopulations based on their level of expression of CD1a and CD27, from the least to most advanced maturation stage: CD1a+CD27− (I), CD1a+CD27+ (II), CD1alowCD27+ (III) and CD1a−CD27+ (IV). (B) Expression of CD4 vs. CD8 and CD31 vs. CD45RA on DP thymocytes shortly after positive selection and lineage commitment in subpopulations I and II. (C) Expression of CD4 vs. CD8, and expression of CD31 vs. CD45RA on CD4 and CD8 SP thymocytes, on subpopulations III and IV. The dashed line indicates the threshold between CD31low and CD31high as described in Figure 2. (D) TCRγδ+ thymocytes were divided into 3 subpopulations based on their level of CD1a expression, from the least to most advanced maturation stage: CD1a+ (I & II), CD1alow (III) and CD1a− (IV). (E) Expression of CD4 vs. CD8 and CD31 vs. CD45RA on TCRγδ+ thymocytes in each CD1a-defined subpopulation. Results shown are representative of 16 (A–C) or 3 (D–E) independent experiments.
The evolution of CD31 expression during the development of the CD4 SP thymocytes is dramatically different from that of the CD8 SP cells (Figure 3C, middle panel). At stage III, CD31 expression on CD4 SP cells is reduced (MFI: 937 ± 79) and a subset of CD31− cells appeared within the CD45RA− “semi-mature” population (CD31− CD45RA−: 13.2 ± 1.6%). Also, only a minor fraction of CD31+ cells express CD45RA at this stage (CD31+ CD45RA+: 14.1 ± 1.8%). At stage IV, only 42.7 ± 2.5% of CD4 SP thymocytes express both CD31 and CD45RA, the phenotype of “true naïve” CD4+ recent thymic emigrant (RTE) in the periphery (10). Remarkably, about a quarter of the CD4+ SP cells at stage IV still lacks CD31 expression (22.5 ± 2.0% of CD31− cells), including mature CD45RA+ cells (CD31− CD45RA−: 17.1 ± 1.8%, CD31− CD45RA+: 5.3 ± 0.6%). Thus, in contrast to mature CD8 SP thymocytes, which are a homogeneous population, CD4 SP thymocytes are a heterogeneous population with regard to CD31 expression.
We also investigated CD31 and CD45RA expression on TCRγδ thymocytes (Figure 3D–E). We observed that TCRγδ thymocyte maturation went from CD1a+ CD4+CD8+ DP to CD1alow CD4lowCD8− and eventually to a CD1a− population which consisted mostly of CD4−CD8− DN cells and a small subset of CD8 SP cells that were more mature (CD45RA+, not shown) than the DN cells. The CD31/CD45RA profile of the TCRγδ+ was very similar to the profile of CD4 SP thymocytes: 10–15% CD31−CD45RA− cells, 35–40% CD31+CD45RA− cells, about 50% CD31+CD45RA+ cells and <10% CD31−CD45RA+ cells.
Within the CD4 SP CD3high subset, CD31− cells contain less sjTREC than CD31+ cells
On peripheral naïve CD4+; T cells, CD31 down-regulation is thought to be associated with TCR triggering and homeostatic proliferation (11, 12, 27). Therefore we asked whether CD31− CD4+ thymocytes have proliferated more than CD31+ thymocytes. To estimate the number of replications each subset had undergone, CD4+CD8− CD27+ CD3high thymocytes were sorted into four subsets according to their expression of CD31 and CD45RA. Genomic DNA was extracted and the number of sjTREC per cell was measured by real-time PCR. Signal Joint T cell Receptor Excision Circles (sjTREC) are non-replicating DNA circles produced during the recombination of the TCRA gene locus. As they won’t replicate with the cell, they can be used as a surrogate marker of the proliferative history of a T cell population (28). For instance, they have been found to be highly enriched in peripheral CD45RA+ naïve CD4+ T cells co-expressing CD31 in comparison with their proliferative progeny, the CD31− CD45RA+ naïve CD4+ T cell subset, in which sjTRECs can hardly be detected (10).
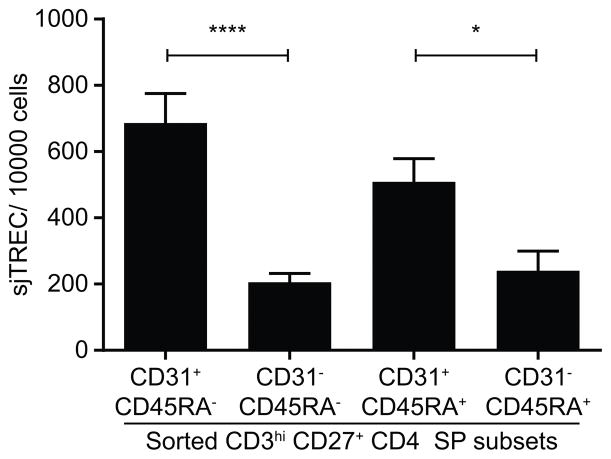
Figure 4 shows that sjTREC contents of the four CD4 SP thymocyte subsets defined by CD45RA and CD31 expression are different (one-way ANOVA, p<10−4). CD31− cells have less than half the quantity of sjTREC per cell compared to CD31+ cells, both among mature CD45RA+ and in “semi-mature” CD45RA− CD4 SP thymocytes (sjTREC/10,000 cells: CD45RA+: 240.5 ± 59.48 vs. 508.5 ± 70.19, CD45RA−: 205.5 ± 26.32 vs. 686 ± 89.32 in CD31− and CD31+ subsets respectively). There is no significant difference between CD45RA− and CD45RA+ subsets. Thus it appears that CD31− CD4 SP have undergone at least one more cell cycle than their CD31+ counterparts.
Figure 4. Quantification of sjTREC in sorted CD3high CD27+ CD4 SP thymocytes subsets.
Freshly prepared thymocytes were enriched for CD27+ cells by positive selection using magnetic beads, then stained with antibodies against CD3, CD4, CD8, CD27, CD31 and CD45RA and sorted by FACS into 4 subsets of CD3high CD27+ CD4+CD8− thymocytes based on their expression of CD31 and CD45RA. Genomic DNA was extracted immediately after sorting and stored at −80°C until sjTREC measurement by qPCR. Values of sjTREC/10,000 cells were compared by one-way ANOVA to determine whether the subsets were globally different (p<10−4), followed by Tukey’s multiple comparisons tests with single pooled variance (*: p<0.05, ****: p<10−4) (n=10, mean ± SEM).
A subset of CD31− CD4 SP thymocytes displays markers of maturity
CD45RA expression is not the only marker of T cell maturity. CD69 expression is up-regulated during positive selection followed by CD62L up-regulation. However CD69 has to be down-regulated for T cells to leave the thymus (29, 30). In order to evaluate the maturation status of CD31− CD4 SP thymocytes, we measured CD69 and CD62L expression on the CD31/CD45RA defined subsets (Figure 5). As expected, in both CD4 SP and CD8 SP thymocytes, there are no mature cells (CD69− CD62L+) in the CD45RA− subsets. Unexpectedly, mature CD69− CD62L+ cells can be found in similar proportions in both CD31+ and CD31− subsets of CD45RA+ CD4 SP cells (9.0 ± 1.4% and 9.2 ± 1.6% respectively, see Figure 5C–D). Although average CD69 mRNA levels for the CD31−CD45RA+ as provided by the gene expression analysis seem inconsistent with the flow cytometry results (Figure S2C), it is not uncommon that RNA levels do not reflect protein expression and, eventually, the expressed proteins provide the functional properties to the cells (e.g. egress abilities).
Figure 5. Expression of CD69 and CD62L as late markers of maturity on thymocyte subsets within subsets expressing CD31 and CD45RA.
Post-natal human thymocytes were stained for CD3, CD4, CD8, CD27, CD31, CD45RA, CD62L and CD69. Cells were gated on CD3high CD27+ CD4 SP (A) or CD8 SP (B) and then on subsets defined by CD31 and CD45RA. CD69 and CD62L expressions are displayed for each subset in lower panel dot-plots (results representative of 11 experiments). (C, D) Comparison of the percentage of mature CD69−CD62L+ cells in each CD31/CD45RA defined subset for CD4 SP (C) and CD8 SP (D) thymocyte populations. Values were compared by one-way ANOVA to determine whether the subsets were globally different (p<10−4), followed by Tukey’s multiple comparisons tests with single pooled variance (****: p<10−4) (n=11, mean ± SEM).
Additionally, gene expression analysis showed that Sphingosine-1-phosphate receptor 1 (S1P1), whose expression is required for mature thymocytes to egress from the thymus (31), as well as Kruppel-Like Factor 2 (KLF2), a transcription factor that drives expression of S1P1 and CD62L (31, 32), are expressed on both CD31+ and CD31− subsets of CD45RA+ CD4 SP subsets at similar levels (Figure S2C). Therefore it is possible that a minor subset of CD4+ T cells leaving the thymus has already lost cell surface expression of CD31 and contributes to the CD31-naïve CD4+ T cells in the periphery and hence would not fit into the “classic” phenotype for CD4+ recent thymic emigrants (RTE).
FOXP3-expressing cells are over-represented within the CD31− CD4 SP subset
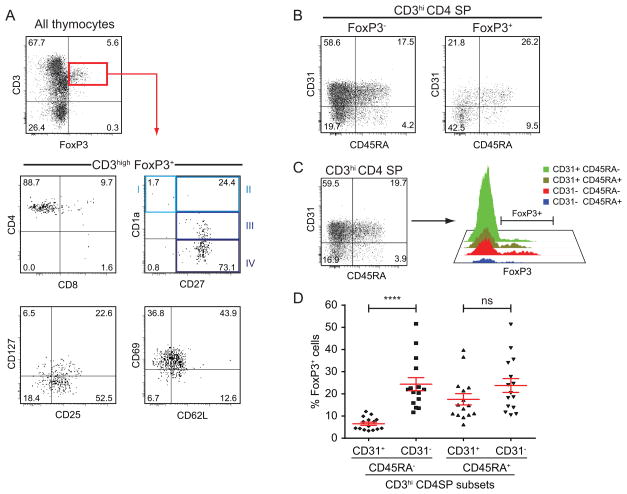
In order to further elucidate specific characteristics of CD31− CD4 SP thymocytes, we tested the expression of FOXP3, the transcription factor specific of regulatory T cells, as natural regulatory T cells arise in the thymus (33, 34). As shown in Figure 6A, FOXP3+ thymocytes are a population of CD4 SP cells bearing a medullary phenotype: CD3high CD27+. Like Treg in the periphery (35), FOXP3+ thymocytes show low to no expression of the IL-7 receptor α-chain (CD127) (Figure 6A). However, unlike Treg in the periphery, they are not homogeneously CD25high and, although the majority of the FOXP3+ cells in the thymus are CD25+, some lack CD25 expression. As for other CD4 SP thymocytes, a small fraction of FOXP3+ thymocytes displays the phenotype of fully mature T cells (CD69− CD62L+) (Figure 6A). Regarding CD31 and CD45RA, the expression pattern of these markers differ significantly between FOXP3− and FOXP3+ CD4 SP thymocytes: about 40% (40.5 ± 3.3%) of the FOXP3+ CD4 SP thymocytes are CD31− CD45RA− whereas only about 19% (19.6 ± 1.9%) of their FOXP3− counterparts display the same phenotype (Figure 6B).
Figure 6. CD31 expression level on FOXP3+ thymocytes.
(A) Phenotype of FOXP3+ thymocytes using the principal thymocyte markers (CD3, CD4, CD8, CD1a, CD27, CD25, CD127, CD69, CD62L). (B) Expression of CD31 and CD45RA on FOXP3− vs. FOXP3+ CD4 SP thymocytes. (C) Gating scheme for measuring the expression of FOXP3 in CD3high CD4 SP thymocytes subsets based on the expression of CD31 and CD45RA. (D) Frequency of FOXP3+ cells within the CD31/CD45RA defined subsets as described above (n=15, in red: mean ± SEM, Student’s t-test, ****: p<10−4).
We measured FOXP3 expression in each of the CD31/CD45RA defined subsets of CD3high CD4 SP thymocytes as shown in Figure 6C and compared the proportion of FOXP3-expressing cells. A one-way ANOVA test confirmed that FOXP3 expression was significantly different across the 4 subsets (p<10−4). Furthermore, among semi-mature (CD45RA−) thymocytes, FOXP3+ CD31− cells were about 4 times more frequent than FOXP3+ CD31+ cells (24.4 ± 2.9% vs. 6.5 ± 0.7% respectively, t-test: p<10−4). However among mature (CD45RA+) CD4 SP thymocytes, the frequency of FOXP3+ cells is similar in CD31− and CD31+ subsets (23.8 ± 3.1% vs. 17.5 ± 2.5%, t-test: p=0.13) and close to the level observed in the CD31− CD45RA− subset. Therefore the CD31− CD4 SP subset is characterized by an over-representation of FOXP3+ cells. Moreover, the overexpression of FOXP3 in the CD31− CD45RA− subset was confirmed at the mRNA level by gene expression analysis (Figure S2E).
CD31−CD45RA− FOXP3+ thymocytes display enhanced levels of activation markers
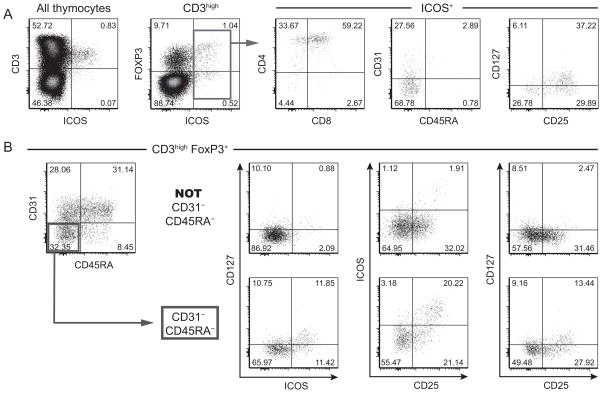
We further analyzed the expression of markers associated with FOXP3: CD25, CD127 and ICOS. ICOS (Induced COStimulator) is a T cell activation marker and member of the CD28 family. In 2008, Ito et al. used ICOS to distinguish two populations of Treg (FOXP3+) in the human thymus: ICOS+ Treg secreted IL-10 and TGF-β as suppressive factors while ICOS− Treg produced only TGF-β (36). We found that ICOS is expressed by CD3high thymocytes committed to the CD4 lineage but still immature with a late DP, early CD4 SP phenotype (Figure 7A). Interestingly ICOS+ thymocytes lacked expression of CD31 and being immature thymocytes they lacked expression of CD45RA as well. Therefore ICOS+ thymocytes contribute exclusively to the CD31− CD45RA− subset. Additionally, ICOS was mostly expressed on FOXP3high cells; the rest of the ICOS+ thymocytes belonged to a group of FOXP3low/− cells. The phenotype of both groups of ICOS+ thymocytes was similar for the markers analyzed in this study (Figure S3).
Figure 7. FOXP3+ thymocytes in the CD45RA− CD31− subset display activation markers ICOS, CD25, CD127.
(A) Phenotype of ICOS+ thymocytes using the following thymocyte markers: CD3, CD4, CD8, FOXP3, CD31, CD45RA, CD25, CD127. (B) Expression of ICOS, CD25 and CD127 on FOXP3+ CD3high thymocytes divided in the 4 subsets based on the expression of CD31 and CD45RA. Results shown are representative of at least 3 independent experiments.
Next we analyzed the expression of ICOS as well as CD25 and CD127 on FOXP3+CD3high thymocytes divided in the 4 subsets defined by the expression CD45RA and CD31 (Figure 7B). As expected, ICOS expression was restricted to the semi-mature CD45RA− CD31− subset, and in addition expression levels of CD25 and CD127 were the highest in this subset. Indeed CD25high FOXP3+ cells were only detected in the CD45RA− CD31− subset whereas CD25 expression levels were lower in the CD45RA− CD31+ subset and even more reduced in the mature CD45RA+ FOXP3+ cells (both CD31+ and CD31−). Regarding CD127, its expression on FOXP3+ CD3high thymocytes only coincided with high levels of CD25; therefore CD127+/low FOXP3+ thymocytes could be found only in that same CD45RA− CD31− subset. Restricted expression of FOXP3, ICOS and CD25 in the CD31−CD45RA− subset was confirmed by gene expression analysis (Figure S2E).
Simonetta et al. (37, 38) have shown the existence of IL-7Rα-expressing Foxp3+ cells in the mouse thymus and that CD127 expression coincides with Icos expression on mouse Treg. They also showed that CD127 is up-regulated on Treg during activation and that IL-7 promotes the survival and proliferation of CD127high Treg. Additionally, Di Caro et al. reported that in freshly isolated mouse splenocytes Foxp3 expression was found to be the highest in CD25+ CD127+ CD4+ T cells and that IL-7 treatment of CD4+ CD25+ T cells induced an increase in Foxp3 expression and up-regulation of CTLA-4 (39). Altogether those observations support the hypothesis that CD31− semi-mature Foxp3+ thymocytes are activated cells. Their fate is unclear but it is possible that IL-7 promotes the survival and proliferation of those cells while they undergo selection in the thymus.
Discussion
The present study addresses the evolution of CD31 expression in the human thymus and shows that it varies extensively from high to undetectable levels during thymopoiesis. First, CD31 expression levels are high on CD34+ thymic progenitors, stay high on CD3− thymocytes after the loss of CD34 all the way through the CD4+ immature single positive (ISP4) phase, and remain high until the early double positive (EDP) CD4+CD8α+β − stage. Next, as TCRβ chain intracellular expression increases and CD4+CD8α+ EDP cells up-regulate expression of CD8β to become CD3−CD4+CD8+ DP (double positive), CD31 expression levels drop. After CD4/CD8 lineage commitment, CD31 expression patterns differ dramatically between CD4 SP and CD8 SP thymocytes. Thus we provide a global picture of the expression of CD31 during human T cell development in the thymus and illustrate the strong dichotomy between CD4 and CD8 lineages.
It has been proposed that TCRβ chain selection can begin in immature CD4 SP (ISP4) and continue in cells that up-regulate expression of CD8α and subsequently CD8β (19, 25, 26). Our results are agreement with these previous reports as TCRβ expression was detected, at its earliest, intracellularly in CD31high CD34−/low CD1alow cells acquiring CD4 expression. This shows that TCRB loci recombination occurred and β-selection had begun at the CD3− CD31high stage. Moreover, since successful β-selection is followed by cell replication, it is likely that CD31 down-regulation is associated with pre-TCR signaling and proliferation, in a similar fashion to what is observed for stimulated CD31+ peripheral naïve CD4+ T cells (10).
At a later stage of development, as DP thymocytes acquire higher levels of CD3 cell surface expression, CD31 expression steadily increases. Remarkably CD31 expression levels and patterns become dramatically different on CD8 SP and CD4 SP CD3high thymocytes after positive selection. CD31 expression is homogeneously high on all CD8 SP cells whereas on CD4 SP it is globally lower and includes a subset of CD31− cells within both CD45RA− “semi-mature” and CD45RA+ mature thymocyte populations. These CD31− CD4 SP thymocytes have fewer sjTREC, thereby showing a longer proliferation history, and are enriched in ICOS+ CD127+/low FOXP3+ cells. In contrast, CD31+ FOXP3+ thymocytes express neither ICOS nor CD127, suggesting that those thymic CD31− natural Treg are activated. The dramatically different CD31 expression patterns between CD4 SP and CD8 SP thymocytes suggest that both lineages may not go through negative selection exactly in the same way or with the same stringency.
CD31 can modulate the TCR signal through recruitment of PTPs (protein-tyrosine phosphatases) such as SHP-1/SHP-2 upon TCR-mediated phosphorylation of its cytoplasmic tail’s ITIMs. Consequently ZAP-70 phosphorylation is inhibited and the activation threshold of the TCR signaling pathway is raised, which makes the cell less responsive to MHC II-peptide complex engagement of its TCR (2, 3, 8). Moreover in the classical affinity model of positive and negative selection, DP thymocytes expressing TCRs with no or low affinity for peptide-MHC complexes die by neglect, whereas very high affinity interactions lead to death through negative selection. Only interactions within the affinity threshold, the narrow range between positive and negative selection, will allow survival (40, 41).
It is therefore interesting to observe that CD31 levels vary for the different selection processes that occur during thymocyte development: 1) CD31 expression is high during the αβ/γδ lineage decision (26), which can be influenced by TCR signal strength in the mouse (42), and when β-selection begins; 2) CD31 expression level is moderate to high during positive selection (CD3+/high CD4+CD8+ DP); 3) CD31 expression is either low during negative selection of CD4 SP thymocytes or high for the negative selection of CD8 SP thymocytes; 4) CD31 expression is absent from a subset of CD45RA− CD4 SP thymocytes in which FOXP3+ cells are over-represented (>20% of the subset), most of them showing markers of activation (ICOS, CD25, CD127). From these observations, we hypothesize that CD31 has a role during selection as a TCR signal modulator. CD31+/high thymocytes will have a higher affinity threshold while CD31− thymocytes will have a lower affinity threshold. Consequently, CD31+ thymocytes will require a stronger affinity interaction of their TCR with the MHC-peptide complexes presented by medullary thymic epithelial cells (mTEC) and dendritic cells (DC) to become activated than CD31− cells. Therefore CD31− thymocytes may be more susceptible to depletion by negative selection than CD31+ thymocytes.
CD31 is known to be down-regulated from CD4+ peripheral T cells upon activation and proliferation following priming (1, 12). We have observed that the CD31 expression level drops around the time of TCRβ chain selection on immature CD4 SP (ISP4) and early DP (CD4+CD8α+β −) CD3− thymocytes, and, again, after positive selection on a subset of CD4 SP thymocytes, including activated ICOS+FOXP3+ cells. It is therefore possible that the down-regulation of CD31 on those cells is a consequence of the encounter with an MHC-peptide complex of high affinity for this cell’s TCR, leading to a strong TCR signal that would trigger activation and proliferation.
Approximately one-fifth of the semi-mature CD45RA− CD31− subset of CD4 SP thymocytes consists of FOXP3+ cells, natural Treg, which are hypothesized to have self-reactive TCRs (43, 44). We aimed to determine whether the other cells in this subset could also belong to a group of known self-reactive T cells such as natural Th17 (45, 46), which can be identified with markers such as ICOS, CCR6, CD200, RORγT (47, 48). While most of the ICOS+ thymocytes co-express FOXP3, we could not find other Th17 associated markers on the FOXP3− ICOS+ thymocytes (data not shown). Natural Treg and natural Th17 share similar developmental features, such as the requirement for TGFβ. Both are thought to arise in the thymus and to recognize self-peptide-MHC class II complexes (45). Altogether these elements suggest that natural Th17, like natural Treg, could also lose CD31 expression during their negative selection in the thymus. However, experiments would be necessary to test this hypothesis.
Finally, the fate of the CD31− CD4 SP thymocytes is unknown. As for the semi-mature CD45RA− CD31− CD4 SP thymocytes, it is possible that they re-express CD31 upon successful completion of their maturation and acquisition of CD45RA expression. Experiments using humanized mouse models or in vitro fetal organ thymic cultures (FTOC) would be necessary to determine whether any CD31− CD45RA− CD4 SP cells survive, acquire CD45RA and up-regulate CD31 again.
We also observed a small fraction of mature CD45RA+ CD69− CD62L+ CD4 SP thymocytes that lacked CD31 expression. Since CD31 is a hallmark of CD4+ recent thymic emigrants (RTE) in peripheral blood lymphocytes, it is therefore expected that all CD4+ naïve T cells egressing from the thymus are CD31+. When comparing the number of TREC in mature CD45RA+ CD31−CD4 SP cells in the thymus with the TREC numbers reported for peripheral CD31−CD45RA+ CD4+ naïve T cells (10, 11), we found that the CD31− CD45RA+ CD4+ thymocytes expressed 3–5 times as many sjTREC/cells as the peripheral CD31− naïve CD4+ T cells. Thus, it is unlikely that they are recirculating cells. In the event that those mature CD31− T cells can egress, they will contribute to a pool of CD31− RTE hardly distinguishable from the naïve CD31− peripheral CD4+ T cells described by Kimmig et al. and Kilpatrick et al. (10, 11). Although gene expression data showed that both CD45RA+ subsets express S1P1, a chemotaxis receptor required for mature naïve T cells to egress from the thymus (31), as well as KLF2, a transcription factor that drives expression of S1P1 and CD62L (31, 32), it is unclear at this point whether those CD31− cells are able to egress, as CD31 might be necessary to cross the endothelium at the cortico-medullary junction. Functional experiments beyond the scope of this study would be necessary to address this and the above-mentioned questions.
Supplementary Material
Acknowledgments
Grant support: This work was supported by Research Grants from the National Institutes of Health (NIH) AI102771, AI 080564 to CU, NIA AG030327 to BDJ, MACS NIH/NIAID AI035040, BDJ co-investigator, NIH T32 (5T32HL086345-08) to RR and an Idea Award (ID08-LA-053 to CU) from the University of California HIV/AIDS Research Program. The “UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility” is supported by the NIH awards CA-16042 and AI-28697, and by the JCCC, the UCLA AIDS Institute, and the David Geffen School of Medicine at UCLA. The UCLA CFAR Virology Core is supported by the NIH award AI-28697.
Flow cytometry was performed in the “UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research (CFAR) Flow Cytometry Core Facility”. Postnatal thymus specimens were obtained from children undergoing corrective cardiac surgery at the UCLA Mattel Children’s hospital and from children undergoing corrective cardiac surgery in The Netherlands.
Footnotes
M.D. designed and performed the experiments, the statistical analyses, and wrote the manuscript; R.S.R. performed experiments and contributed to data analysis; J.C. and M.N. performed experiments; B.D.J. assisted with data analysis and interpretation as well as manuscript review; B.B. assisted with the data analysis and manuscript review, and C.H.U. directed the work, assisted with the data analysis and interpretation, and contributed to the writing of the manuscript. Together with B.D.J., C.H.U. and M.D. conceptualized the original scientific question to be addressed.
References
- 1.Fornasa G, Groyer E, Clement M, Dimitrov J, Compain C, Gaston AT, Varthaman A, Khallou-Laschet J, Newman DK, Graff-Dubois S, Nicoletti A, Caligiuri G. TCR stimulation drives cleavage and shedding of the ITIM receptor CD31. J Immunol. 2010;184:5485–92. doi: 10.4049/jimmunol.0902219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman DK, Hamilton C, Newman PJ. Inhibition of antigen-receptor signaling by Platelet Endothelial Cell Adhesion Molecule-1 (CD31) requires functional ITIMs, SHP-2, and p56(lck) Blood. 2001;97:2351–7. doi: 10.1182/blood.v97.8.2351. [DOI] [PubMed] [Google Scholar]
- 3.Newton-Nash DK, Newman PJ. A new role for platelet-endothelial cell adhesion molecule-1 (CD31): inhibition of TCR-mediated signal transduction. J Immunol. 1999;163:682–8. [PubMed] [Google Scholar]
- 4.Marelli-Berg FM, Clement M, Mauro C, Caligiuri G. An immunologist’s guide to CD31 function in T-cells. J Cell Sci. 2013;126:2343–2352. doi: 10.1242/jcs.124099. [DOI] [PubMed] [Google Scholar]
- 5.Kohler S, Thiel A. Life after the thymus: CD31+ and CD31-human naive CD4+ T-cell subsets. Blood. 2009;113:769–74. doi: 10.1182/blood-2008-02-139154. [DOI] [PubMed] [Google Scholar]
- 6.Vanhecke D, Leclercq G, Plum J, Vandekerckhove B. Characterization of distinct stages during the differentiation of human CD69+CD3+ thymocytes and identification of thymic emigrants. J Immunol. 1995;155:1862–72. [PubMed] [Google Scholar]
- 7.Tenca C, Merlo A, Zarcone D, Saverino D, Bruno S, De Santanna A, Ramarli D, Fabbi M, Pesce C, Deaglio S, Ciccone E, Malavasi F, Grossi CE. Death of T cell precursors in the human thymus: a role for CD38. Int Immunol. 2003;15:1105–16. doi: 10.1093/intimm/dxg111. [DOI] [PubMed] [Google Scholar]
- 8.Ma L, Mauro C, Cornish GH, Chai JG, Coe D, Fu H, Patton D, Okkenhaug K, Franzoso G, Dyson J, Nourshargh S, Marelli-Berg FM. Ig gene-like molecule CD31 plays a nonredundant role in the regulation of T-cell immunity and tolerance. Proc Natl Acad Sci U S A. 2010;107:19461–6. doi: 10.1073/pnas.1011748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henshall TL, Jones KL, Wilkinson R, Jackson DE. Src homology 2 domain-containing protein-tyrosine phosphatases, SHP-1 and SHP-2, are required for platelet endothelial cell adhesion molecule-1/CD31-mediated inhibitory signaling. J Immunol. 2001;166:3098–3106. doi: 10.4049/jimmunol.166.5.3098. [DOI] [PubMed] [Google Scholar]
- 10.Kimmig S, Przybylski GK, Schmidt Ca, Laurisch K, Möwes B, Radbruch A, Thiel A. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195:789–94. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilpatrick RD, Rickabaugh T, Hultin LE, Hultin P, Hausner MA, Detels R, Phair J, Jamieson BD. Homeostasis of the naive CD4+ T cell compartment during aging. J Immunol. 2008;180:1499–507. doi: 10.4049/jimmunol.180.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demeure CE, Byun DG, Yang LP, Vezzio N, Delespesse G. CD31 (PECAM-1) is a differentiation antigen lost during human CD4 T-cell maturation into Th1 or Th2 effector cells. Immunology. 1996;88:110–5. doi: 10.1046/j.1365-2567.1996.d01-652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stockinger H, Schreiber W, Majdic O, Holter W, Maurer D, Knapp W. Phenotype of human T cells expressing CD31, a molecule of the immunoglobulin supergene family. Immunology. 1992;75:53–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Gurney KB, Uittenbogaart CH. Human immunodeficiency virus persistence and production in T-cell development. Clin Vaccine Immunol. 2006;13:1237–1245. doi: 10.1128/CVI.00184-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schotte R, Schmidlin H, Nagasawa M, Dontje W, Karrich JJ, Uittenbogaart C, Spits H, Blom B. Isolation and In Vitro Generation of Gene-Manipulated Human Plasmacytoid and Conventional Dendritic Cells. Methods in molecular biology (Clifton, NJ) 2010;595:67–85. doi: 10.1007/978-1-60761-421-0_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmid I, Uittenbogaart CH, Giorgi JV. Sensitive method for measuring apoptosis and cell surface phenotype in human thymocytes by flow cytometry. Cytometry. 1994;15:12–20. doi: 10.1002/cyto.990150104. [DOI] [PubMed] [Google Scholar]
- 17.Ilan N, Madri JA. PECAM-1: old friend, new partners. Curr Opin Cell Biol. 2003;15:515–24. doi: 10.1016/s0955-0674(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 18.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–35. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 19.Spits H. Development of alphabeta T cells in the human thymus. Nat Rev Immunol. 2002;2:760–772. doi: 10.1038/nri913. [DOI] [PubMed] [Google Scholar]
- 20.Blom B, Spits H. Development of human lymphoid cells. Annu Rev Immunol. 2006;24:287–320. doi: 10.1146/annurev.immunol.24.021605.090612. [DOI] [PubMed] [Google Scholar]
- 21.Shaw J. Hematopoietic stem cells and endothelial cell precursors express Tie-2, CD31 and CD45. Blood Cells, Mol Dis. 2004;32:168–175. doi: 10.1016/j.bcmd.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 22.D’Alessio F, Mirabelli P, Gorrese M, Scalia G, Gemei M, Mariotti E, Di Noto R, Martinelli P, Fortunato G, Paladini D, Del Vecchio L. Polychromatic flow cytometry analysis of CD34+ hematopoietic stem cells in cryopreserved early preterm human cord blood samples. Cytometry A. 2011;79:14–24. doi: 10.1002/cyto.a.20989. [DOI] [PubMed] [Google Scholar]
- 23.Zocchi MR, Poggi A. PECAM-1, Apoptosis and CD34 + Precursors. Leuk Lymphoma. 2004;45:2205–2213. doi: 10.1080/10428190410001724312. [DOI] [PubMed] [Google Scholar]
- 24.Yong KL, Watts M, Shaun Thomas N, Sullivan A, Ings S, Linch DC. Transmigration of CD34+ cells across specialized and nonspecialized endothelium requires prior activation by growth factors and is mediated by PECAM-1 (CD31) Blood. 1998;91:1196–205. [PubMed] [Google Scholar]
- 25.Taghon T, Van de Walle I, De Smet G, De Smedt M, Leclercq G, Vandekerckhove B, Plum J. Notch signaling is required for proliferation but not for differentiation at a well-defined beta-selection checkpoint during human T-cell development. Blood. 2009;113:3254–63. doi: 10.1182/blood-2008-07-168906. [DOI] [PubMed] [Google Scholar]
- 26.Dik WA, Pike-Overzet K, Weerkamp F, de Ridder D, de Haas EFE, Baert MRM, van der Spek P, Koster EEL, Reinders MJT, van Dongen JJM, Langerak AW, Staal FJT. New insights on human T cell development by quantitative T cell receptor gene rearrangement studies and gene expression profiling. J Exp Med. 2005;201:1715–23. doi: 10.1084/jem.20042524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohler S, Wagner U, Pierer M, Kimmig S, Oppmann B, Möwes B, Jülke K, Romagnani C, Thiel A. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur J Immunol. 2005;35:1987–94. doi: 10.1002/eji.200526181. [DOI] [PubMed] [Google Scholar]
- 28.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 29.Weinreich MA, Hogquist KA. Thymic emigration: when and how T cells leave home. J Immunol. 2008;181:2265–70. doi: 10.4049/jimmunol.181.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiow LR, Rosen DB, Brdicková N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–4. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 31.Resop RS, Douaisi M, Craft J, Jachimowski LCM, Blom B, Uittenbogaart CH. Sphingosine-1-phosphate/sphingosine-1-phosphate receptor 1 signaling is required for migration of naive human T??cells from the thymus to the periphery. J Allergy Clin Immunol. 2016;138:551–557.e8. doi: 10.1016/j.jaci.2015.12.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fink PJ. The biology of recent thymic emigrants. Annu Rev Immunol. 2013;31:31–50. doi: 10.1146/annurev-immunol-032712-100010. [DOI] [PubMed] [Google Scholar]
- 33.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of FOXP3 expression during ontogeny. J Exp Med. 2005;202:901–6. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh CS, Lee HM, Lio CWJ. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12:157–67. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 35.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FOXP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, Qin FXF, Gilliet M, Liu YJ. Two Functional Subsets of FOXP3+ Regulatory T Cells in Human Thymus and Periphery. Immunity. 2008;28:870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonetta F, Chiali A, Cordier C, Urrutia A, Girault I, Bloquet S, Tanchot C, Bourgeois C. Increased CD127 expression on activated FOXP3+CD4+ regulatory T cells. Eur J Immunol. 2010;40:2528–38. doi: 10.1002/eji.201040531. [DOI] [PubMed] [Google Scholar]
- 38.Gestermann N, Tissières P, Simonetta F, Boniotto M, Bourgeois C, Seddon B, Martinet KZ. Interleukin-7 Influences FOXP3+CD4+ Regulatory T Cells Peripheral Homeostasis. PLoS One. 2012;7:e36596. doi: 10.1371/journal.pone.0036596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Caro V, D’Anneo A, Phillips B, Engman C, Harnaha J, Lakomy R, Styche A, Trucco M, Giannoukakis N. Interleukin-7 matures suppressive CD127(+) forkhead box P3 (FOXP3)(+) T cells into CD127(−) CD25(high) FOXP3(+) regulatory T cells. Clin Exp Immunol. 2011;165:60–76. doi: 10.1111/j.1365-2249.2011.04334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer E, Naeher D. Affinity threshold for thymic selection through a T-cell receptor-co-receptor zipper. Nat Rev Immunol. 2009;9:207–13. doi: 10.1038/nri2469. [DOI] [PubMed] [Google Scholar]
- 41.Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833–44. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- 42.Taghon T, Rothenberg EV. Molecular mechanisms that control mouse and human TCR-αβ and TCR-γδ T cell development. Semin Immunopathol. 2008;30:383–398. doi: 10.1007/s00281-008-0134-3. [DOI] [PubMed] [Google Scholar]
- 43.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 44.Lee HM, Bautista JL, Scott-Browne J, Mohan JF, Hsieh CS. A Broad Range of Self-Reactivity Drives Thymic Regulatory T Cell Selection to Limit Responses to Self. Immunity. 2012;37:475–486. doi: 10.1016/j.immuni.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annu Rev Immunol. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marks BR, Nowyhed HN, Choi JY, Poholek AC, Odegard JM, Flavell RA, Craft J. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat Immunol. 2009;10:1125–1132. doi: 10.1038/ni.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JS, Smith-Garvin JE, Koretzky GA, Jordan MS. The requirements for natural Th17 cell development are distinct from those of conventional Th17 cells. J Exp Med. 2011;208:2201–7. doi: 10.1084/jem.20110680. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, Caproni M, Tonelli F, Lazzeri E, Parronchi P, Liotta F, Maggi E, Romagnani S, Annunziato F. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–16. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viney JL, Prosser HM, Hewitt CR, Lamb JR, Owen MJ. Generation of monoclonal antibodies against a human T cell receptor beta chain expressed in transgenic mice. Hybridoma. 1992;11:701–13. doi: 10.1089/hyb.1992.11.701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.