Abstract
Background
The contribution of women’s mobility to the HIV/AIDS epidemic in Africa is poorly understood, despite women’s high mobility and evidence that it is associated with higher-risk sexual behavior. We sought to measure levels of mobility, HIV prevalence, and related risk behaviors among female traders in Kisumu, Kenya.
Methods
We used GPS mapping to develop a probability-based sample and recruited 305 female market traders for participation in a survey and voluntary HIV counseling and testing in 2014. We estimated HIV prevalence and fitted logistic regression models to measure associations between mobility, risk behaviors, and HIV infection.
Results
HIV prevalence was 25.6% (95% CI 21.0–30.8); 11.5% had migrated (changed residence, over county or national boundary) in past year and 39.3% in past five years. Over one-third (38.3%) spent nights away from main residence in past month, with 11.4% spending more than a week away. Multiple partners were reported by 13.1% of women in the last year; 16% of married women reported a concurrent partnership. Mobility was not significantly associated with HIV prevalence, although recent short-term mobility was significantly correlated with higher numbers of sexual partners in the past year.
Conclusions
Female market traders were highly mobile, and HIV prevalence among traders was higher than in the general population of women of reproductive age in Kisumu (15.3% in 2013), and Nyanza Province, Kenya (16.1% in 2012). High HIV prevalence and risk behavior among women in this study warrant accelerated attention to HIV prevention and care needs of mobile women, including market traders.
Keywords: HIV, migration, mobility, female migrants, East Africa, Kenya
Introduction
The contribution of population mobility to the HIV epidemic in sub-Saharan Africa is well documented; the spread of HIV followed the corridors of population movement1–10. However, mobility and HIV research has tended to focus on risks to male labor migrants and their partners, or migrants overall, often failing to measure the risks to women via their direct participation in migration3,11–17. Yet across the region, women’s levels of participation in internal migration (i.e., changes in residence within national boundaries) and short-term mobility (frequent travel without a permanent change in residence) have now met or exceeded those of men18–22. Moreover, the few studies which have examined HIV risks to female migrants found higher risk behavior and HIV prevalence among migrant compared to non-migrant women23–30, and suggest differential HIV risks associated with mobility for women versus men30.
The literature on migration has highlighted the centrality of gender to understanding migration decision-making, determinants and consequences31–33. Indeed, in Southern and East Africa, mobility patterns differ for men and women, and have important implications for HIV risk; women have tended to migrate shorter distances to informal settlements or regional towns, retaining ties to rural homes and returning frequently, whereas men have migrated over longer distances to urban areas, and have been less likely to return to households of origin18,34. Engagement in different corridors of mobility and destinations likely exposes female migrants to sexual networks and geographic areas of different HIV prevalence compared to males, resulting in different probabilities of infection for each given sexual act35. Population-based studies report HIV prevalence in informal settlements and peri-urban areas to be twice as high compared to urban and rural areas5,28,36. Thus, high female mobility may contribute to the sustained, high prevalence of HIV in Southern and East Africa, enabling greater inter-connectedness of sexual networks beyond those created by male migrants alone.
Despite high levels of participation in mobility combined with a generalized HIV epidemic and large sex disparities in HIV infection37,38, the relationship between women’s migration and HIV risk in East Africa is not well-understood. One Kenyan study assessed risk behaviors by migration status, and identified internal migrants as having higher sexual risk behaviors than non-migrants in 199924. However, current population-representative data on women’s participation in mobility in Kenya, as elsewhere in the region, are limited, and hampered by inconsistency in measures used. The paucity of data to clarify and quantify women’s levels of participation and their unique patterns of mobility hampers a full understanding of the role that women’s mobility plays in sustaining HIV epidemics in the region.
In this study, we sought to measure levels and forms of mobility, HIV prevalence, and related risk behaviors among a representative sample of a population of highly mobile women: informal sector market traders. Our prior qualitative research identified typologies of female migrants and highly mobile women in the Kisumu area of western Kenya39. Key typologies included “house-helps”, often orphaned or destitute young women, who migrated or were sent to work in urban households to work as domestic servants; widows, who upon disinheritance often migrated to Lake Victoria beaches to work in the fishing industry; and female market traders who work in Kibuye market, reputed to be one of the largest open-air markets in East and Central Africa, and who circulated between rural or urban homes, Kibuye, and other regional trading centers. This study explored the feasibility of obtaining a probability-based sample of female market traders using a novel geographic sampling approach, in order to better understand the levels and types of mobility of female market traders as well as their burden of HIV prevalence.
This large urban market sits in the heart of Kisumu, Kenya’s third largest city, in a region burdened with high HIV prevalence. The most recent estimates showed 15.1% HIV prevalence among women of reproductive age in Kisumu in 201340 and 16.1% in 2012 among women of reproductive age in Nyanza Province overall, the highest regional prevalence in the country41. Improving knowledge of women’s mobility and its associations with prevalent HIV infection is important for informing targeted HIV prevention and care efforts among this high-risk yet understudied population.
Methods
A target sample size of 306 was calculated to estimate HIV prevalence within 5% precision with 95% confidence, using exact Clopper-Pearson two-sided confidence intervals for proportions generated with NCSS PASS software [40]. We obtained a probability-based sample of female market traders (n=305) for a survey of mobility, HIV prevalence, and related risk behaviors in Kibuye market in Kisumu, Kenya, in 2014. The market attracts hundreds of informal sector wholesale and retail traders on Sundays, the main market day, with fewer wholesalers operating in the market on weekdays. While some traders are locally based, others travel over long distances (from areas including Nairobi and towns in Uganda and Tanzania) and medium distances (from the beaches at Lake Victoria or rural areas in Nyanza, Western and Rift Valley Provinces) to sell their wares at the market. The market has both open-air spaces where traders display and sell their goods and stalls that are either privately owned or owned by the Kisumu County government. We developed a sampling frame through enumerating and mapping each market stall using global positioning system (GPS) coordinates, on a series of Sundays, the day in which the market is maximally populated. For each stall, fieldworkers recorded the seller’s gender, and the type and origin of wares. A systematic sample of female traders was selected from this sampling frame and was drawn in “replicates”. In this approach, each replicate is a systematic random sample of the whole market place gauged to be a fraction of the whole sample size. The survey is done by undertaking one replicate at a time, completing the replicate, and moving to the next until the minimum number of replicates completes the whole sample. Our process of developing the sampling frame and selecting a systematic sample using replicates to maintain sampling integrity is described in detail elsewhere (Leidich et al., Manuscript in Progress)42. Sample size was calculated based on the assumption of a 25% or higher HIV prevalence among female traders at Kibuye market, given prior studies in the region showing higher HIV prevalence among female migrants23–30, and women in Kisumu43. The GPS locations of 6,390 vendor stalls were collected and mapped on a series of Sundays between September and December, 2013. Of the 6,390 vendor stalls at Kibuye, 4,064 (64%) were occupied by women. These were used to draw 4 replicates of 128 vendors each, and a fifth replicate of 15 pre-selected random alternates for a total of 527 female vendors assigned to one of five replicates. Staff visited 323 stalls from the first three replicates and from these, 305 female vendors were successfully recruited into the study, for a consent rate of 94%.
Fieldworkers recruited 305 selected participants at market stalls and invited them to participate in a short survey and HIV testing at a private mobile HIV-VCT site adjacent to the market (on weekdays designated as a local Health Center and on weekends designated as a nearby primary school). Survey data were collected from January to June 2014. Participants underwent an informed consent process with a study interviewer using materials in their preferred language (English, Kiswahili or Dholuo), and provided written informed consent separately for participation in the survey and HIV testing. A survey interview was conducted to collect information on demographics, mobility patterns, and sexual relationships. After interviewing, participants would move to a second room where they underwent a separate consent for HIV Voluntary Counseling and Testing. Those who consented were tested with the Alere Determine™ HIV 1/2 test (Waltham, MA, USA) and provided results on the spot. Those found to be HIV positive were given a confirmatory rapid test (SD Bioline, Yongin-si, Gyeonggi-do, Republic of Korea) during the same session. If results from the Determine and Bioline tests conflicted, then Unigold® (Bray, Co Wicklow, Ireland) was used as a confirmatory test. If results were confirmed to be HIV-positive, and women were not already in care, participants were counseled and referred for HIV care and treatment at one of the Family AIDS Care & Education Services (FACES)-supported clinics in Kisumu [35]. The entire process from arrival to the study site, consent, interview and testing took less than one hour on average. Participants received a total of Ksh. 500 ($6.20) in exchange for the opportunity costs associated with leaving their market stalls on busy trading days. The study protocol was approved by the KEMRI Ethical Review Committee (#2361) and the Committee on Human Research at the University of California at San Francisco (#10-02936).
Measures
The fifteen-item demographic and behavioral survey captured data on age, marital status, educational attainment, and sexual behavior (i.e., number of male partners over the participants’ lifetime and within the past year). We asked women what wares they sold, where they purchased them, and in which other markets they sold them. We collected data on five measures of mobility: past year migration; past five-year migration; lifetime migration status; duration of current residency; and recent short-term mobility (using response categories informed by our preliminary research). Among participants who indicated migration (past year, past five-year or lifetime), we captured data on reasons for migration, using eight close-ended response categories informed by our preliminary research (business/earning income, marriage, accompanying husband or other family member, sent by family, to care for or be cared for by a family member, flee from violence, education, or other).
Data Analyses
We conducted univariate analyses on participant demographics, behavioral and work characteristics to describe our sample participants. HIV prevalence and 95% confidence intervals were calculated for the overall sample, representing the total proportion testing HIV positive, and for subgroups defined by demographic, mobility and sexual risk behavior characteristics. We estimated logistic regression models to quantify the relationships between mobility and sexual risk behaviors and HIV seropositivity, and between mobility and sexual risk behaviors. Linear regression models were estimated to assess the relationships between past year migration and mobility variables and total number of past year sexual partners; a robust variance adjustment was applied to adjust for heteroskedasticity.44 Regression analyses were conducted both unadjusted and adjusted per five-year age group. All analyses were conducted using estimation commands for probability survey data in Stata analytical software, version 14 (StataCorp, College Station, TX USA). Differences were considered statistically significant at p<0.05.
Results
Demographics
Participants were largely between ages 25 and 44 (Table 1). Over half had completed some secondary education or greater (51.4%), and most were either married (55.1%) or currently in a non-marital sexual relationship (25.6%). Four-fifths of the sample was born in Nyanza province (78.8%), many in Kisumu itself (40.7%), although birth provinces varied across the formerly-named Coast, Nairobi, Rift Valley, Western, Central and Eastern regions. The vast majority were current residents of Kisumu (92.8%; not shown). Overall HIV prevalence among the sample of women recruited in 2014 was 25.6% (95% CI 21.0–30.8). HIV prevalence varied substantially by age group, educational attainment, and marital status. Prevalence estimates across counties of birth are imprecise due to low numbers in most counties. Participants were involved in selling a range of wares, including food or cereals (52.1%), used clothing or accessories (26.6%), and new clothing or accessories (11.2%) (data not shown). A smaller number sold beauty supplies or cosmetics, technological wares or services, housewares or cleaning products, hardware, firewood or charcoal, utensils or cookware, or herbs or traditional medicinal products at the market.
Table 1.
HIV Prevalence by Demographic Characteristics, Female Market Traders, Kisumu, Kenya, 2014 (N=305)
| Characteristic | Overall | HIV Prevalence | |||
|---|---|---|---|---|---|
| n | % | % | 95% CI | ||
| Total | 305 | 100.0 | 25.6 | 21.0–30.8 | |
|
| |||||
| Age Group | |||||
| 18–24 Years | 43 | 14.1 | 11.6 | 4.9–25.3 | |
| 25–34 Years | 98 | 32.1 | 28.6 | 20.4–38.4 | |
| 35–44 Years | 80 | 26.2 | 31.3 | 22.0–42.3 | |
| 45–54 Years | 60 | 19.7 | 28.3 | 18.3–41.1 | |
| 55 or more years | 24 | 7.9 | 12.5 | 4.0–33.1 | |
| Educational Attainment* | |||||
| Some Primary | 69 | 22.9 | 29.0 | 19.4–40.9 | |
| Completed Primary | 78 | 25.8 | 30.8 | 21.5–41.9 | |
| Some Secondary | 67 | 22.2 | 25.4 | 16.3–37.2 | |
| Completed Secondary | 70 | 23.2 | 18.6 | 11.0–29.5 | |
| Post-Secondary | 18 | 6.0 | 11.1 | 2.7–36.3 | |
| Marital Status | |||||
| Married | 168 | 55.1 | 17.3 | 12.2–23.8 | |
| In Relationship | 78 | 25.6 | 35.9 | 26.0–47.2 | |
| Widowed | 38 | 12.5 | 42.1 | 27.4–58.3 | |
| Divorced | 11 | 3.6 | 35.9 | 26.0–47.3 | |
| Single | 10 | 3.3 | 36.4 | 13.5–67.6 | |
| Birth County (by region): | |||||
| Nyanza: | Kisumu | 124 | 40.7 | 27.4 | 20.2–36.0 |
| Siaya | 56 | 18.4 | 41.1 | 28.9–54.4 | |
| Homa Bay | 35 | 11.5 | 22.3 | 11.7–39.8 | |
| Migori | 11 | 3.6 | 27.3 | 8.5–60.3 | |
| Nairobi | Nairobi | 14 | 4.6 | 7.1 | 0.9–39.0 |
| Coast: | Kisii | 21 | 6.9 | 9.5 | 2.3–32.0 |
| Mombasa | 3 | 1.0 | 33.3 | 2.5–90.5 | |
| Western: | Vihiga | 11 | 3.6 | 18.2 | 4.2–52.7 |
| Kakamega | 5 | 1.6 | 0.0 | – | |
| Busia | 4 | 1.3 | 25.0 | 2.4–82.1 | |
| Bungoma | 3 | 1.0 | 0.0 | – | |
| Rift Valley: | Nakuru | 5 | 1.6 | 0.0 | – |
| Uasin Gishu | 2 | 0.7 | 50.0 | 0.2–98.1 | |
| Other: | Other County | 6 | 2.0 | 16.7 | 2.9–68.0 |
| Tanzania | 3 | 1.0 | 0.0 | – | |
| Uganda | 2 | 0.7 | 50.0 | 1.9–98.1 | |
n=3 missing data on educational attainment, excluded.
Mobility
As shown in Table 2, the sample was highly mobile, with 11.5% reporting past year migration (including internal migration, defined as change of residence across a county border, or international migration, change of residence across national boundary). Some 39.3% reported at least one migration in the past five years, and most participants (90.2%) had undertaken at least one migration as an adult (not shown). Participation in recent mobility was also quite high: 38.3% indicated having spent any nights away from their household of main residence in the past one month, with 11.4% spending greater than one week away. Nearly half (46%) had spent at least some nights away from their main residence in the past three months; 11.8% had spent from half of all nights to every night away in the past three months. The duration at current main residence varied (data not shown): many individuals (39.0%) indicated having lived in their current residence for over ten years, while others reported more recent establishment of current residence: 14.5% within the past year, and 29.2% within the past 2–5 years. The most common reasons given for migration (not shown) across all time frames were for purposes of business or income-generating activities (i.e. labor), followed by migration for marriage: for those that undertook any past-year migration, reasons given were labor (45.8%), marriage (8.3%), and caring for or being care for by family (8.3%); for past-five year migration, reasons were labor (39.4%), marriage (28.2%) and accompanying husband or other family (9.9%); for those who hadn’t migrated in past five years but had migrated as an adult, the main reason was marriage (63.7%), followed by labor (16.4%).
Table 2.
Selected Measures of Mobility, Sexual Risk Behavior and HIV Prevalence, Female Market Traders, Kisumu, Kenya, 2014 (N=305)
| Behavioral Prevalence | HIV Prevalence | |||
|---|---|---|---|---|
| n | % | % | 95% CI | |
| Mobility | ||||
|
| ||||
| Past Year Migration | ||||
| Yes | 35 | 11.5 | 17.1 | 7.8–33.6 |
| No | 270 | 88.5 | 26.7 | 21.7–32.3 |
| Duration of Current Residency | ||||
| >10 Years | 119 | 39.0 | 23.5 | 16.7–32.0 |
| 5–10 Years | 53 | 17.4 | 28.3 | 17.7–42.0 |
| 2–5 Years | 63 | 20.7 | 30.2 | 20.0–42.7 |
| 1–2 Years | 26 | 8.5 | 23.1 | 10.5–42.3 |
| ≤ 1 Year | 44 | 14.5 | 22.7 | 12.6–37.6 |
| Nights Away from Residence, Past 1 Month | ||||
| None | 188 | 61.6 | 25.5 | 19.8–32.3 |
| 1 Week or Less | 82 | 26.9 | 29.3 | 20.4–40.1 |
| 1–2 Weeks | 30 | 9.8 | 16.7 | 7.0–34.8 |
| More than 2 Weeks | 5 | 1.6 | 20.0 | 2.1–74.5 |
| Nights Away from Residence, Past 3 Months | ||||
| None | 163 | 53.6 | 24.5 | 18.5–31.8 |
| Few | 104 | 34.2 | 25.0 | 17.6–34.3 |
| Half | 21 | 6.9 | 23.8 | 10.0–46.8 |
| Every | 15 | 4.9 | 46.7 | 23.4–71.5 |
|
| ||||
| Sexual Risk Behaviors | ||||
|
| ||||
| Number Sex Partners, Past Year | ||||
| 0 | 85 | 27.9 | 29.4 | 20.6–40.0 |
| 1 | 180 | 59.0 | 21.1 | 15.7–27.7 |
| 2 or 3 | 40 | 13.1 | 37.5 | 23.8–53.5 |
| Concurrent Partnershipsa | ||||
| Yes | 27 | 16.1 | 33.3 | 18.0–53.2 |
| No | 141 | 83.9 | 14.2 | 9.3–21.1 |
Concurrency was defined as being married (n=168) and having another partner.
Sexual behavior
The median (interquartile range) of male sex partners was 1 (0–1) over the past year and 3 (2–6) over the respondent’s lifetime (not shown). Thirteen percent (13.1%) of participants reported more than one sex partner in the past year (Table 2). Over sixteen percent of married individuals (16.1%) indicated that they were in a concurrent partnership. Lifetime numbers of partners were also reported but not shown in Table 2: 91.1% reported more than one sex partner, and just over one-third (36.3) reported five or more sex partners in their lifetime. HIV prevalence estimates within each mobility and behavioral sub-group are also shown in Table 2. HIV prevalence was strikingly high among those who had spent most or every night away from home in the past three months (46.7%, 23.4–71.5 [95% CI]). However, statistical tests of association between these characteristics and HIV prevalence revealed no statistically significant relationships between six measures of mobility and HIV seropositivity in unadjusted and adjusted models (Table 3 shows results for three key measures).
Table 3.
Migration, HIV Seropositive Status and Sexual Risk Behaviors, Female Market Traders, Kisumu, Kenya, 2014 (N=305)
| Measure of Mobility | HIV-positive status | Two or More Sex Partners, Past Year | Concurrent Partnership a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age-adjusted models | Age-adjusted models | Age-adjusted models | |||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Past Year Migration | 0.60 | 0.24–1.54 | 0.292 | 2.09 | 0.84–5.19 | 0.112 | 1.24 | 0.23–6.60 | 0.801 |
| Nights Away from Residence, Past Month | |||||||||
| None | REF | – | – | REF | – | – | REF | – | – |
| 1 Week or Less | 1.15 | 0.64–2.07 | 0.645 | 2.30 | 1.06–4.99 | 0.036 | 2.13 | 0.79–5.76 | 0.134 |
| 1–2 Weeks | 0.56 | 0.20–1.58 | 0.274 | 3.90 | 1.37–11.09 | 0.011 | 2.20 | 0.56–8.56 | 0.256 |
| > 2 Weeks | 0.58 | 0.06–5.36 | 0.631 | 11.06 | 1.23–99.23 | 0.032 | 23.91 | 1.08–530.91 | 0.045 |
| Nights Away from Residence, Past 3 Months | |||||||||
| None | REF | – | – | REF | – | – | REF | – | – |
| Few | 0.96 | 0.54–1.72 | 0.89 | 1.66 | 0.71–3.87 | 0.239 | 1.43 | 0.52–3.96 | 0.487 |
| Half | 0.91 | 0.31–2.67 | 0.858 | 6.60 | 2.13–20.43 | 0.001 | 7.29 | 1.58–33.60 | 0.011 |
| Most/Every | 2.28 | 0.78–6.72 | 0.133 | 12.23 | 3.64–41.40 | <0.001 | 3.59 | 0.69–18.60 | 0.127 |
Concurrency was defined as being married (n=168) and having another partner.
However, there were statistically significant associations between HIV prevalence and recent higher risk sexual behavior. As shown in Table 2, HIV prevalence levels increased in tandem with number of sexual partners in the past year and concurrent partnerships. Logistic regression models (Table 3) indicated that the odds of having two or more sexual partners in the past year increased significantly with each increase in level of duration of time spent away from the household in the past month. In adjusted models, relative to participants who reported no nights away from their primary residence within the past month, odds of two or more partners ranged from 2.3 (95% CI 1.1–5.0) among those who spent one week or less, to 3.9 (95% CI 1.4–11.1) among those who spent one to two weeks away, to 11.1 (95% CI 1.2–99.2) who spent two or more weeks away.
A similar increasing relationship was observed in number of past year sexual partners across nights away from main residence in the past three months. Compared to those reporting no nights away in the past three months, participants reporting half of the nights away had a six-fold odds of two or more partners (OR 6.6, 95% CI 2.1–20.4); those away most or every night had over twelve-fold odds of two or more partners (OR 12.2, 95% CI 3.6–41.4). Longer duration away from home in the past month and past three months was also associated with partnership concurrency among married participants. We found in additional analyses (not shown) that higher risk sexual behavior was in turn associated with prevalent infection, as expected; for example, among married participants, those who indicated having a concurrent partner to their husband had a three-fold increased odds of HIV infection (OR 3.1, 95% CI 1.1–8.9).
Positive linear associations between levels of mobility and the number of sexual partners in the past year were also observed (Table 4). The mean number of partners increased in tandem with increasing levels of recent short-term mobility; beta coefficients for associations were statistically significant in age-adjusted linear regression models. Our adjusted analyses show a statistically significant increasing mean number of sexual partners in the past year within increasing nights away in the past one and three months.
Table 4.
Past Year Mobility and Number of Sexual Partners, Female Market Traders, Kisumu, Western Kenya, 2014 (N=305)
| Number of Sex Partners in Past Year | |||||||
|---|---|---|---|---|---|---|---|
| Number of Sex Partners in Past Year | Unadjusted Models | Age–adjusted Models | |||||
| Mean (SD) | β | 95% CI | p | β | 95% CI | p | |
| Past Year Migration | |||||||
| Yes | 0.88 (0.71) | 0.12 | −0.18, 0.43 | 0.432 | 0.04 | −0.24, 0.32 | 0.758 |
| No | 1.00 (0.84) | REF | – | – | REF | – | – |
| Nights Away from Residence, Past 1 Month | |||||||
| None | 0.76 (0.61) | REF | – | – | REF | – | – |
| 1 Week or Less | 1.09 (0.76) | 0.32 | 0.14, 0.51 | 0.001 | 0.30 | 0.12, 0.48 | 0.001 |
| 1–2 Weeks | 1.13 (0.94) | 0.37 | 0.19, 0.73 | 0.039 | 0.43 | 0.10, 0.75 | 0.010 |
| More than 2 Weeks | 1.40 (1.52) | 0.64 | -0.86, 2.13 | 0.401 | 0.67 | -0.77, 2.11 | 0.357 |
| Nights Away from Residence, Past 3 Months | |||||||
| None | 0.76 (0.61) | REF | – | – | REF | – | – |
| Few | 0.93 (0.67) | 0.17 | 0.01, 0.33 | 0.036 | 0.19 | 0.03, 0.35 | 0.019 |
| Half | 1.38 (1.07) | 0.62 | 0.14, 1.10 | 0.012 | 0.64 | 0.20, 1.07 | 0.005 |
| Most or Every | 1.44 (1.09) | 0.68 | 0.11, 1.24 | 0.019 | 0.66 | 0.13, 1.18 | 0.014 |
β = unstandardized linear regression coefficient. Robust variance adjustment applied to estimates.
Discussion
Among a population-based sample of female traders in a large open-air market in East Africa, we estimated the HIV prevalence at 25.6% (95% CI 21.0–30.8%). This is substantially higher than among other recent population-based samples within the region. Westercamp et al. found a HIV prevalence of 15.1% among women of reproductive age in Kisumu overall for 2013 (Manuscript in Progress)40, and the Kenya AIDS Indicator Survey (KAIS) conducted in 2012 reported HIV prevalence among adult and adolescent women at 16.1% within Nyanza Province overall, the highest regional prevalence in the country41. Our study is the first to develop a truly population-based estimate of HIV prevalence among female market traders, and such high prevalence, relative to the general population of women, substantiates the need to focus HIV prevention and treatment efforts among this group at particularly high risk of HIV.
Our study participants were highly mobile. Over ten percent had changed their primary residence within the past twelve months, and nearly forty percent had done so within the past five years. Furthermore, more than one-third indicated having spent nights away from their main residence in the past month. Data on localized mobility in the region are rare and definitions of mobility vary across data sources, but the levels of mobility observed in our study are consistent with those seen among highly mobile subpopulations in other research in the region. Internal migration is common in eastern Africa, especially among young women45. Over 10% of Kenyan men and women between the ages of 15 and 24 move across district boundaries each year (National Research Council and Institute of Medicine 2005)46. Rates of in-and out-migration of an urban population living within a demographic surveillance system (DSS) in two Nairobi slum areas showed that about 25.8% of people in-migrated to the areas every year and about 22.5% out-migrated47. The Nairobi surveillance data demonstrated a higher intensity of female compared to male migration, finding that females are increasingly contributing to the growth of Nairobi slum populations through in-migration. These findings add to the growing evidence for a “feminization of internal migration” in Kenya as elsewhere sub-Saharan Africa39, and match data from other DSS sites in the region in the past two decades showing that rates of localized movements in and out of DSS areas peak among women in their twenties.48,49 In DSS populations, women’s migrations have been found to be driven by their search for better livelihood opportunities; in contrast, women engaged in informal sector market trading, the population for the present study, can be seen to be actively engaged in a livelihood that entails a high level of mobility.
Female market traders reported higher HIV-risk behaviors than compared to other women in the general population. Compared to the Kenyan female population as a whole, our sample reported a higher number of sexual partners in both the past twelve months and over their lifetimes. In our sample, 86.9% reported fewer than two partners in the past twelve months, compared to 97.6% of women in Kenya overall41 and 95.2% in Kisumu40. Just over half (51.6%) reported three or fewer lifetime sexual partners compared to 85.9% among all Kenyan women of reproductive age41. The higher risk sexual behavior in our sample was associated with higher levels of mobility: in both categorical and continuous analyses, increasing short-term mobility in the past month or three months was associated with significantly increased odds of higher number of sexual partners or mean number of sexual partners. These findings are consistent with previous research that has identified increased risk behaviors among migrant compared to non-migrant women23–29.
There are potential explanations for these findings from our prior qualitative research documenting high mobility and HIV risk behavior among female market traders in Kisumu.39 Market trading as a livelihood for women involved work in and transit through HIV transmission “hotspots” including lodgings and temporary residences in trading centers along the corridors of major population movement. Moreover, the social contexts of traders facilitated high risks of HIV acquisition and transmission: many women engaged in transactional sex, of varying regularity, from clandestine to overt, to supplement earnings from informal sector trading. Many market traders participated not only in transactional sex in commercial sex areas, referred to locally as “parking”, on weekends or aftermarket days, but were also vulnerable to transactional sexual exchanges with “businessmen” at lodges in regional market towns and trading centers while traveling. In addition, women with dual residences and a circulatory mobility pattern often maintained relationships with more than one main partner (e.g. both a husband and at least one non-marital partner).39
In the present study, however, despite overall high levels of mobility, high HIV prevalence, and significant associations between mobility and risk behavior, there were no statistically significant associations between measures of mobility and HIV infection. We believe this null finding reflects limitations of the study design: the study was not powered to explore subgroup differences; moreover, data were cross-sectional, and neither timing of past mobility nor timing of infection could be measured, thus we caution that the study provides limited evidence of causality and its direction. For example, our study identified a trend toward higher HIV prevalence among those individuals who did not indicate a primary residence change within the past year; however, HIV infection may reduce mobility where individuals are symptomatic. An additional limitation of the study is that we collected a limited number of measures of sexual behavior; for instance we did not directly survey respondents about condom use, which we knew from prior research to be low in the population; nor did we ask women about their engagement in transactional or commercial sex, because we felt that the brief survey modality, which we felt was necessary in order to capture information from busy traders during their work day, was not conducive to the collection of sensitive data. Finally, it was beyond the scope of this study to examine sex differences in mobility patterns and HIV prevalence among market traders. More research is needed to better understand sex differences in patterns and levels of localized mobility in African populations, as well as the impact of these forms of mobility on the continued spread of HIV, and on the ability of HIV-infected individuals to successfully navigate HIV care and treatment systems.
Despite these limitations, this study offers the first known estimates of mobility and HIV prevalence in a population-representative sample of female market traders in East Africa. This study demonstrates the usefulness of innovative sampling methods for reaching and measuring risk behaviors and HIV infection in highly mobile populations, including women who are informal sector traders in market areas. We also have offered evidence that providing HIV voluntary counseling and testing and referral services can be done in busy open air market settings: this contribution can provide impetus for an expansion of mobile testing and outreach services for mobile populations in the spaces through which they circulate. Ultimately, our study findings of substantially higher HIV prevalence among female market traders, and of significant relationships between their recent short-term mobility and higher risk sexual behavior, substantiate the need for focusing HIV prevention and treatment efforts on this high-risk yet underserved population.
Figure 1. HIV Prevalence by Age Group Among Female Market Traders Compared with Population of Women of Reproductive Age in Kisumu, Western Kenya (2013–2014).
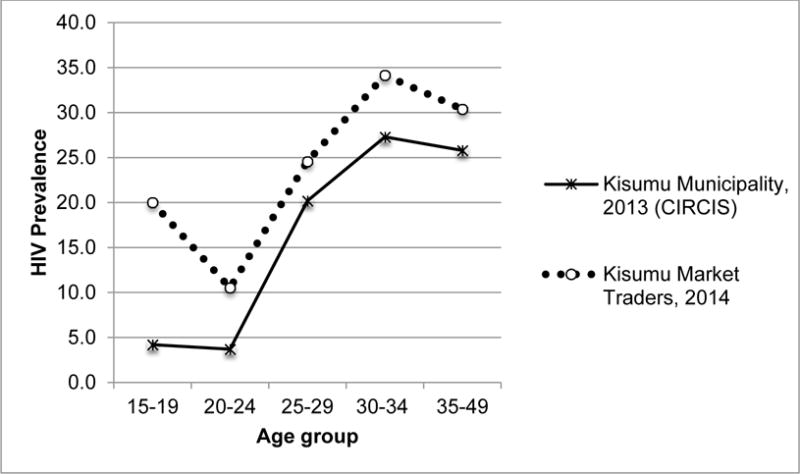
Data for Kisumu Municipality, 2013: Courtesy of M. Westercamp et al. (Manuscript in Progress)40
Acknowledgments
We thank everyone who participated in this research, and acknowledge the contributions of study coordinator Lillian Achiro and team members David Ang’awa, Phoebe Olugo, Catherine Makhoka, Moses Okombo, and Sarah Ambunya.
Funding sources: This research was supported by a grant from the National Institutes of Health, University of California, San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research (UCSF-CFAR), P30-AI027763. Dr. Camlin was supported by a Research Scientist Development Award from the National Institute of Mental Health (NIMH), K01MH093205. Dr. Johnson was supported by grant number K24DA037034.
References
- 1.Barongo LR, Borgdorff MW, Mosha FF, et al. The epidemiology of HIV-1 infection in urban areas, roadside settlements and rural villages in Mwanza Region, Tanzania. Aids. 1992;6(12):1521–1528. doi: 10.1097/00002030-199212000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Glynn JR, Ponnighaus J, Crampin AC, et al. The development of the HIV epidemic in Karonga District, Malawi. Aids. 2001;15(15):2025–2029. doi: 10.1097/00002030-200110190-00016. [DOI] [PubMed] [Google Scholar]
- 3.Jochelson K, Mothibeli M, Leger JP. Human immunodeficiency virus and migrant labor in South Africa. International Journal of Health Services. 1991;21(1):157–173. doi: 10.2190/11UE-L88J-46HN-HR0K. [DOI] [PubMed] [Google Scholar]
- 4.Garin B, Jeannel D, Kazadi K, Combe P, Singa L, De The G. Introduction of HIV-1 in a rural city of Zaire. Ann Soc Belg Med Trop. 1993;73(2):143–147. [PubMed] [Google Scholar]
- 5.Coffee MP, Garnett GP, Mlilo M, Voeten HA, Chandiwana S, Gregson S. Patterns of movement and risk of HIV infection in rural Zimbabwe. Journal of Infectious Diseases. 2005;191(Suppl 1):S159–167. doi: 10.1086/425270. [DOI] [PubMed] [Google Scholar]
- 6.Wawer MJ, Serwadda D, Musgrave SD, Konde-Lule JK, Musagara M, Sewankambo NK. Dynamics of spread of HIV-I infection in a rural district of Uganda. Bmj. 1991;303(6813):1303–1306. doi: 10.1136/bmj.303.6813.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanser F, Lesueur D, Solarsh G, Wilkinson D. HIV heterogeneity and proximity of homestead to roads in rural South Africa: an exploration using a geographical information system. Trop Med Int Health. 2000;5(1):40–46. doi: 10.1046/j.1365-3156.2000.00513.x. [DOI] [PubMed] [Google Scholar]
- 8.Bwayo J, Plummer F, Omari M, et al. Human immunodeficiency virus infection in long-distance truck drivers in east Africa. Archives of internal medicine. 1994;154(12):1391–1396. [PubMed] [Google Scholar]
- 9.Mbugua GG, Muthami LN, Mutura CW, et al. Epidemiology of HIV infection among long distance truck drivers in Kenya. East African medical journal. 1995;72(8):515–518. [PubMed] [Google Scholar]
- 10.Ramjee G, Gouws a EE. Prevalence of HIV among truck drivers visiting sex workers in KwaZulu-Natal, South Africa. Sex Transm Dis. 2002;29(1):44–49. doi: 10.1097/00007435-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Barnighausen T, Hosegood V, Timaeus IM, Newell ML. The socioeconomic determinants of HIV incidence: evidence from a longitudinal, population-based study in rural South Africa. AIDS. 2007;21(Suppl 7):S29–38. doi: 10.1097/01.aids.0000300533.59483.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hope KR., Sr Mobile workers and HIV/AIDS in Botswana. AIDS Analysis Africa. 2000;10(4):6–7. [PubMed] [Google Scholar]
- 13.Nunn AJ, Wagner HU, Kamali A, Kengeya-Kayondo JF, Mulder DW. Migration and HIV-1 seroprevalence in a rural Ugandan population. AIDS. 1995;9(5):503–506. [PubMed] [Google Scholar]
- 14.Lurie M, Harrison A, Wilkinson D, Karim SA. Circular migration and sexual networking in rural KwaZulu/Natal: implications for the spread of HIV and other sexually transmitted diseases. Health Transition Review. 1997;7:17–27. Supplement 3. [PubMed] [Google Scholar]
- 15.Hope KR. Population mobility and multi-partner sex in Botswana: implications for the spread of HIV/AIDS. Afr J Reprod Health. 2001;5(3):73–83. [PubMed] [Google Scholar]
- 16.Lurie MN, Williams BG, Zuma K, et al. Who infects whom? HIV-1 concordance and discordance among migrant and non-migrant couples in South Africa. Aids. 2003;17(15):2245–2252. doi: 10.1097/00002030-200310170-00013. [DOI] [PubMed] [Google Scholar]
- 17.Zuma K, Lurie MN, Williams BG, Mkaya-Mwamburi D, Garnett GP, Sturm AW. Risk factors of sexually transmitted infections among migrant and non-migrant sexual partnerships from rural South Africa. Epidemiology & Infection. 2005;133(3):421–428. doi: 10.1017/s0950268804003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collinson M, Tollman S, Kahn K, Clark S, Garenne M. Highly prevalent circular migration: households, mobility and economic status in rural South Africa. In: Tienda M, Findley S, Tollman S, Preston-Whyte E, editors. Africa On the Move: African Migration and Urbanisation in Comparative Perspective. Johannesburg: Wits University Press; 2006. pp. 194–216. [Google Scholar]
- 19.Posel D. Have Migration Patterns in Post-apartheid South Africa Changed? Journal of Interdisciplinary Economics. 2004;15(3–4):277–292. Special Issue. [Google Scholar]
- 20.Dodson B. Women on the move: Gender and cross-border migration to South Africa from Lesotho, Mozambique and Zimbabwe. In: McDonald DA, editor. On Borders: Perspectives on International Migration in Southern Africa. New York: St. Martin’s Press; 2000. pp. 119–150. [Google Scholar]
- 21.Zlotnick H. The Global Dimensions of Female Migration. Migration Information Source. 2003 http://www.migrationinformation.org/Feature/display.cfm?ID=109.
- 22.Zlotnick H. The Dimensions of Migration in Africa. In: Tienda M, Findley S, Tollman S, Preston-Whyte E, editors. Africa on the Move: African Migration and Urbanisation in Comparative Perspective. Johannesburg: Wits University Press; 2006. [Google Scholar]
- 23.Pison G, Le Guenno B, Lagarde E, Enel C, Seck C. Seasonal migration: a risk factor for HIV infection in rural Senegal. Journal of Acquired Immune Deficiency Syndromes. 1993;6(2):196–200. [PubMed] [Google Scholar]
- 24.Brockerhoff M, Biddlecom AE. Migration, Sexual Behavior and the Risk of HIV in Kenya.(Statistical Data Included) International Migration Review. 1999;33(4):833–856. [Google Scholar]
- 25.Abdool Karim Q, Abdool Karim SS, Singh B, Short R, Ngxongo S. Seroprevalence of HIV infection in rural South Africa. AIDS. 1992;6(12):1535–1539. doi: 10.1097/00002030-199212000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Zuma K, Gouws E, Williams B, Lurie M. Risk factors for HIV infection among women in Carletonville, South Africa: migration, demography and sexually transmitted diseases. International Journal of STD & AIDS. 2003;14(12):814–817. doi: 10.1258/095646203322556147. [DOI] [PubMed] [Google Scholar]
- 27.Kishamawe C, Vissers DC, Urassa M, et al. Mobility and HIV in Tanzanian couples: both mobile persons and their partners show increased risk. AIDS. 2006;20(4):601–608. doi: 10.1097/01.aids.0000210615.83330.b2. [DOI] [PubMed] [Google Scholar]
- 28.Boerma JT, Urassa M, Nnko S, et al. Sociodemographic context of the AIDS epidemic in a rural area in Tanzania with a focus on people’s mobility and marriage. Sexually Transmitted Infections. 2002;78(Suppl 1):i97–105. doi: 10.1136/sti.78.suppl_1.i97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lydie N, Robinson NJ, Ferry B, Akam E, De Loenzien M, Abega S. Mobility, sexual behavior, and HIV infection in an urban population in Cameroon. Journal of Acquired Immune Deficiency Syndromes. 2004;35(1):67–74. doi: 10.1097/00126334-200401010-00010. [DOI] [PubMed] [Google Scholar]
- 30.Camlin CS, Hosegood V, Newell ML, McGrath N, Barnighausen T, Snow RC. Gender, migration and HIV in rural KwaZulu-Natal, South Africa. PLoS One. 2010;5(7):e11539. doi: 10.1371/journal.pone.0011539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Jong GF. Expectations, gender, and norms in migration decision-making. Popul Stud-J Demogr. 2000;54(3):307–319. doi: 10.1080/713779089. [DOI] [PubMed] [Google Scholar]
- 32.Curran SR, Shafer S, Donato KM, Garip F. Mapping Gender and Migration in Sociological Scholarship: Is It Segregation or Integration? International Migration Review. 2006;40(1):199–223. doi: 10.1111/j.1747-7379.2006.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gubhaju B, De Jong GF. Individual versus Household Migration Decision Rules: Gender and Marital Status Differences in Intentions to Migrate in South Africa. International Migration. 2009;47(1):31–61. doi: 10.1111/j.1468-2435.2008.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camlin CS, Hosegood V, Snow RC. Gendered patterns of migration in South Africa. Population, Space and Place. 2012 doi: 10.1002/psp.1794. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. Aids. 1997;11(5):641–648. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Shisana O, Rehle T, Simbayi LC, et al. South African National HIV Prevalence, HIV Incidence, Behaviour and Communication Survey. Cape Town: Human Science Research Council; 2005. [Google Scholar]
- 37.Brockerhoff M. Fertility and family planning in African cities: the impact of female migration. Journal of biosocial science. 1995;27:347–358. doi: 10.1017/s0021932000022872. [DOI] [PubMed] [Google Scholar]
- 38.Thadani VN. Social Relations and Geographic Mobility: Male and Female Migration in Kenya. New York: Center for Policy Studies, The Population Council; 1982. [Google Scholar]
- 39.Camlin CS, Kwena ZA, Dworkin SL, Cohen CR, Bukusi EA. “She mixes her business”: HIV transmission and acquisition risks among female migrants in western Kenya. Social Science & Medicine. 2014;102(0):146–156. doi: 10.1016/j.socscimed.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westercamp M, Bailey RC, Jaoko W, et al. Changes in male circumcision prevalence and risk compensation in the Kisumu, Kenya population. :2008–2013. doi: 10.1097/QAI.0000000000001180. [Manuscript In Progress] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National AIDS and STI Control Programme (NASCOP) K. Kenya AIDS Indicator Survey 2012: Final Report. Nairobi: NASCOP; 2014. [Google Scholar]
- 42.Leidich A, Achiro L, Kwena ZA, et al. Methods for sampling geographically mobile female traders in an East African market setting. doi: 10.1371/journal.pone.0190395. [Manuscript In Progress] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen CR, Montandon M, Carrico AW, et al. Association of attitudes and beliefs towards antiretroviral therapy with HIV-seroprevalence in the general population of Kisumu, Kenya. PLoS One. 2009;4(3):e4573. doi: 10.1371/journal.pone.0004573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidson R, MacKinnon JG. Estimation and Inference in Econometrics. New York: Oxford University Press; 1993. [Google Scholar]
- 45.Clark S, Cotton C. Transitions to adulthood in urban Kenya: A focus on adolescent migrants. Demogr Res. 2013;28(37):1053–1092. [Google Scholar]
- 46.Medicine NRCaIo. The transition to work. In: Lloyd CB, editor. Growing up Global: The changing transitions to adulthood in developing countries. Washington, D.C: The National Academies Press; 2005. pp. 265–345. [Google Scholar]
- 47.Beguy D, Elung’ata P, Mberu B, et al. HDSS Profile: The Nairobi Urban Health and Demographic Surveillance System (NUHDSS) International journal of epidemiology. 2015 doi: 10.1093/ije/dyu251. [DOI] [PubMed] [Google Scholar]
- 48.Camlin CS, Snow RC, Hosegood V. Gendered Patterns of Migration in Rural South Africa. Population, Space and Place. 2014;20(6):528–551. doi: 10.1002/psp.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collinson M. Age-Sex Proflies of Migration: Who ia a Migrant? In: Collinson MA, Adazu K, White M, Findley S, editors. The Dynamics of Migration, Health and Livelihoods: INDEPTH Network Perspectives. Surrey, England: Ashgate; 2009. [Google Scholar]


