Abstract
Acute Graft-Versus-Host Disease (aGVHD) continues to be a frequent and devastating complication of allogeneic hematopoietic stem cell transplantation (HSCT); posing as a significant barrier against the widespread use of HSCTs as a curative modality. Recent studies suggested serum/plasma microRNAs (miR) may predict aGVHD onset. However, little is known about the functional role of circulating miRs in aGVHD. Here, we show in two independent cohorts that miR-29a expression is significantly upregulated in the serum of allo-HSCT patients at aGVHD onset compared to those who did not. Serum miR-29a is also elevated as early as two weeks before time of diagnosis of aGVHD compared to time-matched controls. We demonstrate novel functional significance of serum miR-29a by showing that miR-29a binds and activates dendritic cells via Toll like receptors (TLRs) 7 and 8, resulting in the activation of the NF-κB pathway and secretion of pro-inflammatory cytokines TNF-α and IL-6. Treatment with locked nucleic acid (LNA) anti miR-29a significantly improved survival in a mouse model of aGVHD while retaining graft-versus-leukemia (GVL) effects, unveiling a novel therapeutic target in aGVHD treatment or prevention.
Introduction
Acute Graft-versus-host disease (aGVHD) is a frequent and lethal complication of allogeneic hematopoietic stem cell transplantation (HSCT) in which donor T cells destroy HLA mismatched host tissues by secreting inflammatory cytokines (TNF-α and IFN-γ) and/or inducing direct cytotoxic cellular responses (1-4). Despite recent advances, aGVHD remains a major clinical problem, underscoring the need to further elucidateits mechanisms to then develop novel therapeutic strategies (1, 2, 5, 6). Previous studies indicate that microRNAs (miRs) play critical roles in the pathogenesis of aGVHD, including but not limited to miR-155, miR-146a, miR-181a, miR34a, and miR-100 (refs. 7-10). These miRs were found differentially expressed in effectors cells associated with aGVHD such as T cells subsets and antigen presenting cells (APCs) and modulate aGVHD by affecting mostly T cell function (7-10). Emerging data indicate that miRs can also be found in different body fluids such as serum, plasma, saliva and urine (11-14). Recently, four miRs (miR-423, miR-199a-3p, miR-93*, and miR-377) were found to be over-expressed in the plasma of aGVHD patients 16 days before diagnosis as compared to non-GVHD patients (15). A separate study identified miR-155 to be upregulated in the serum of patients that developed aGVHD versus those who did not (16). While these studies indicate that circulating miRs could be used as biomarkers to predict aGVHD onset, the functional implications of circulating miRs have not been investigated. In this study we examined the serum miR expression in aGVHD patients using a deep sequencing approach and performed functional studies to define the role of deregulated circulating miRs in aGVHD pathogenesis. Our results indicate that miR-29a expression is upregulated in the serum of allogeneic HSCT patients that develop aGVHD compared to those who do not. We further show that miR-29a binds and functionally activates dendritic cells via Toll like receptors (TLRs) 7 and 8, resulting in the activation of the NF-κB pathway and secretion of pro-inflammatory cytokines TNF-α and IL-6. In addition, we show that targeting miR-29a using LNA anti miR-29a increases survival in a mouse model of aGVHD without perturbing graft versus leukemia (GVL) effects.
Materials and Methods
Patients and samples
Patients ≥18 years with hematologic malignancies requiring a myeloablative (MA) or reduced-intensity conditioning (RIC) allogeneic HSCT were eligible for participation in the study Assessment of MicroRNA Expression in Acute Graft-versus-Host Disease- NCT01521039 and approved by the Ohio State University Institutional Review Board. Patients were required to have a Karnofsky performance status > 70, adequate renal function (creatinine clearance of ≥ 40% of normal), bilirubin, AST, and ALT < 2× upper limit of normal, and no other serious organ dysfunction or medical conditions at the time of consent. Donors were related, unrelated, haploidentical and stem cell source were peripheral blood (PB), bone marrow (BM) or cord blood (CB). Patients received appropriate fungal, viral and Penumocystis jiroveci prophylaxis per institution guidelines. Appropriate GVHD prophylactic medications were instituted according to institution guidelines. Peripheral blood samples were collected (one 6 ml EDTA and one 10 ml serum separator tube) prior to start of the conditioning regimen, on day 0 (day of stem cell infusion) and weekly thereafter until day 100 post allogeneic HSCT. aGVHD samples were also collected within 3 days of aGVHD onset. Grading of aGVHD was done using the consensus conference criteria (17). While not required, the diagnosis of aGVHD was histologically confirmed whenever possible. Serum was isolated from all blood samples by a centrifugation protocol (2000 rpm for 10 min). Patient characteristics are summarized in Supplementary Tables 1 and 2. Patients and controls were matched to age, intensity of conditioning regimen (MA vs RIC), donor source (related, unrelated, or haplo), stem cell source (PB, BM or CB) and day of transplant at which GVHD occurred.
RNA isolation from human serum
Serum total RNA was extracted using the miRNeasy Serum/Plasma Kit (Cat. no.217184, QIAGEN, Valencia, CA, USA) with a modified protocol. Prior to RNA extraction, serum samples were thawed completely on ice, centrifuged at 20,000g for 15 min at 4°C to remove remaining cell debris. Following this, 200 μL of serum was carefully transferred to a new 2-mL DNA-LoBind tube (Eppendorf, Hamburg, Germany). Serum was mixed with 1 ml QIAzol Lysis Reagent, then placed on a vortex mixer at 3000 rpm for 30s and left at room temperature for 5 min to allow complete inactivation of serum RNAses. Four microliters of a mixture of serially diluted (1, 0.1 and 0.01 pg/μL) pooled synthetic microRNAs was spiked into the homogenate lysis mixture. From here, the manufacturer's protocols were followed for RNA extraction. RNA was eluted twice with 14μL of RNase-free water and the elution volumes from the same sample were pooled to obtain a final volume of 28μL.
RNA spike-in
Synthetic Caenorhabditis elegans microRNAs (cel-miR-39, cel-miR-54, and cel-miR-238; Integrated DNA Technologies, Coralville, IA, USA) were reconstituted in nuclease-free water (Ambion, Austin, TX, USA). Ten-fold serial dilutions into nuclease-free water were made for titration.
Small RNA Sequencing
RNA was extracted from patient serum as described. Libraries were constructed using the small RNA profiling kit (SOLiD™ Small RNA Expression Kit (P/N 4397682), Applied Biosystems) and sequenced on the Solid analyzer (Applied Biosystems). Sequence alignment was performed using miRBase version 14. Normalization as reads per million was performed using quantiles. We compared miR expression between all patients with aGVHD and non-aGVHD controls using two sample t-test. All the analyses were performed using BRB-Array Tools, Version 4.2.1 (R. Simon and A. P. Lam, National Cancer Institute, Bethesda, MD) and using the R version 2.13.2 (R Foundation for Statistical Computing).
Real-time PCR
Qiagen miScript PCR System was used for reverse transcription and qPCR. Hundred nanograms of RNA was converted into cDNA using the miScript II Reverse Transcription Kit (Cat. N.218161) with HiSpec Buffer according to the manufacturer's protocol. qPCR was performed with the miScript SYBR® Green PCR Kit (Cat. N. 218073) in a total volume of 20μl per reaction containing 2μl diluted cDNA according to the manufacturer's protocol. C-elegans microRNAs spike-in control was used to normalize expression. The miScript universal primer and the miRNA-specific miScript primer assay for Hs_miR-29a (Cat. MS00003262), Ce_miR-238 (Cat. MS00019439), Ce_miR-54 (Cat. MS00019894) and Ce_miR-39 (Cat. 219610) (all from QIAGEN), was use to detected mature miRNA. Individual real-time PCR assays were performed in triplicate wells on an ABI 7500 real-time PCR system (Applied Biosystems).
Mice
C57/BL/6(H2b), B6D2F1, BALB/c (H2d) mice were purchased from Jackson ImmunoResearch laboratories (Ban harbor, ME). 29ab1 knock-out mice were a kind gift from Dr. Carlo Croce (OSU) (18). All mice were bred and maintained in an OSU animal care facility. For all experiments mice were used between 8 and 12 weeks of age. All animal studies were conducted in accordance to the rules and regulations of the Institutional Animal Care and Use Committee at the Ohio State University.
Migration Assay
Day 6 BMDC were treated for 48 hrs with ssRNA/DOTAP conjugated miR-29a/miR-16 to induce maturation. BMDC were washed and cells (5×105) were placed in a Transwell migration chamber (pore size 5um, Costar 3421, Corning) and allowed to migrate for 3 hrs at 37°C. SLC/CCL19 (200 ng/ml) (Peprotech, Rocky Hill, NJ) was placed in the lower chamber to induce CCR7-dependent chemotaxis. Lower chambers with medium only served as a control for spontaneous migration. After 3 h, cells that migrated to the lower chamber were counted, and CCL19-dependent migration was calculated according to the formula: Migration Index (MI) = number of cells CCL-19/number of cells medium only.
Flow cytometry analysis
Human dendritic cells, PBMCs or day 6 BMDC were washed 3 times and treated with DOTAP conjugated miR-29a or miR-16 (1 μg/ml), ssRNA (5 μg/ml) for 48 hrs. The cultured cells were collected and washed and stained with the following monoclonal antibodies. Anti-mouse monoclonal antibodies - eFlour 450 CD11c, PerCP-Cy5.5 CD40, FITC CD80, PE CD86, PE CCR7. Anti-human monoclonal antibodies – APC CD3, FITC CD69, APC conjugated CD11c, PE CD83, FITC CD40. For the LNA anti miR-29a in vivo/ex-vivo experiments the following mAb combinations were used - eFlour 450 CD3, FITC H-2Kd, eVolve 605 CD4, PerCP Cy5.5 CD8, eFlour 660 Ki67, eFlour 660 IFNγ, eFlour 450 CD11c, APC MHC II, PE CD86. All antibodies were purchased from Affymetrix eBioscience. Analysis was performed with a FACS LSRII cytometer; FACSDiva software (BD Pharmingen) data analysis was performed using FlowJo (Treestar).
Cytokine ELISA measurements
Human dendritic cells or day 6 BMDC were washed 3 times and treated with DOTAP conjugated miR-29a or miR-16 (1 μg/ml), ssRNA (5 μg/ml) for 48 hrs. Cytokines levels (TNF-α, IL-6, IL-12) in cell culture supernatants were measured according manufacturer's protocol (BioLegend). Samples were assayed in triplicate and results are shown as the means of three independent experiments.
Cells and cell culture
PBMCs and normal human dendritic cells were purchased from ALLCELLS (Alameda, CA). All cells were cultured in RPMI, 10% FBS and Pen-Strep.
Western Blot
BMDC were stimulated with DOTAP conjugated miR-29a or miR-16 or DOTAP alone for 15 mins. Cells were lysed and western blot was performed according to standard procedure.(19) Primary antibodies against phospho-p65 (Cell Signaling) were used. A separate gel was run to probe against p-65 (Cell Signaling) for normalization.
Immunofluorescent Staining
Day 6 BMDCs were washed and treated with DOTAP-miR29a, miR-16 or DOTAP alone for 4 hrs. Approximately 200,000 cells were cytospinned to a slide, fixed in 3.7% formaldehyde for 15 min followed by permeabilization with 0.25% Triton. The slides were blocked by 1% goat serum for 30 min and incubated with primary antibody, IRF7, (Santa Cruz Biotechnology) for 1 hour at room temperature. After washing, the slides were incubated with fluorochrome conjugated secondary antibody for 1 hr at room temperature. Nucleus was stained by DAPI. Samples were analysed by confocal microscope (OSU Image facility).
In vitro T cell Proliferation assay
B6D2F1 BMDCs were treated with for 48 hrs with ssRNA/DOTAP conjugated miR-29a/miR-16 to induce maturation as described earlier. T cells were isolated from B6 mice using the Pan T cell isolation kit (Miltenyi Biotec) stained with CFSE and incubated with allogeneic B6D2F1 BMDC (stimulator: effector =1:5). Cell division was measured by CFSE dilution after 7 days using LSRII (Becton Dickinson, Mountain View, CA) and FACSDiva software (Becton Dickinson).
Transfection
Cells were transfected with Flag empty vector or Flag-TLR8 plasmids (kind gift provided by Dr. Misako Matsumoto, Hokkaido University Graduate School of Medicine, Japan) using Electroporation followed by the manufacturer's protocol (BioRad) with minor modifications. After a period of 12 hrs plasmid DNA transfections, cells were also transfected with precursors of Scr or miR-29a or miR-155 using DOTAP liposomal transfection reagent (Roche Life Science) for an additional period of 60 hrs. Cells were harvested 72 hours post-transfection and separated the pellets into two parts. Majority of pellets were used for RNA IP and the remaining used for IP-Western blot assays.
Immunoprecipitation of protein-associated RNAs (RNA IP)
RNA-protein complexes immunoprecipitation of was performed as described with minor modifications.(20) Briefly, cells were lysed in polysome lysis buffer (PLB) (100 mM KCl, 5 mM MgCl2, 10 mM HEPES [pH 7.0], 0.5% Nonidet P-40, 1 mM DTT, 100 U of RNase inhibitor/ml) supplemented with 20 mM EDTA and protease inhibitors on ice for 20 min followed by centrifugation. The supernatants were then diluted (1:10 [vol/vol]) in freshly made NT2 buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1 mM MgCl2, 0.05% Nonidet P-40, 1 mM DTT, 100 U of RNase inhibitor/ml) supplemented with 20 mM EDTA and protease inhibitors and incubated with α-Flag beads (Sigma) at 4°C for 6 h. The beads were washed five times with NT2 buffer supplemented with protease inhibitors. The bead-bound protein-RNA complexes were then treated with DNase I and proteinase K and eluted twice with NT2 buffer containing 0.1% SDS. RNAs were extracted from the elution with phenol-chloroform and ethanol precipitation followed by RT-qPCR assay for miRNAs.
Immunoblot (IB) and immunoprecipitation (IP)
Cells were lysed in lysis buffer consisting of 50 mM Tris-HCl (pH 8.0), 0.5% Nonidet P-40, 1 mM EDTA, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM DTT, 1 μg/ml pepstatin A, and 1 mM leupeptin. Equal amounts of clear cell lysate were used for IP analysis. Cell lysates were incubated with α-Flag beads (Sigma) at 4°C for 6 h. Beads were washed with lysis buffer for four times. Bound proteins were detected by IB using α-Flag (Sigma) or α-Actin (Cell Signal Technology) antibodies.
Locked nucleic acid (LNA) in situ hybridization
Locked nucleic acid (LNA) modified probes complementary to mouse mature miR-29a and scrambled negative control digoxigenin-labeled at the 5′ position were purchased from Exiqon (Vedbaek, Denmark). Detection of mature miRNAs by in situ hybridization utilizing oligonucleotide probes was performed as previously described.(8, 21)
Acute GVHD murine models
Mice were transplanted under standard protocols approved by the University committee on Use and Care of Laboratory Animals at OSU. Only age and sex matched mice were used for transplant experiments. Briefly, 8-12 weeks recipient mice (F1) were irradiated with 9 Gy administered in two fractions to minimize toxicity. T-cell depleted BM cells (t-BM, BM only)(5×106) plus 20 × 106 total splenocytes from B6 WT or B6 miR-29a deficient donors were administered via tail vein injection after the irradiation. One group of mice did not receive any splenocytes (BM only group) and a second group did not receive any cells (Radiation only group). A second MHC mismatched aGVHD murine model was performed, where B6 recipients were lethally irradiated with 900cGy and were divided in two groups: group 1 received only BALB/c t-BM cells (5×106) designated as BM only, group 2 received BALB/c WT BM (5×106) + unfractionated splenocytes (20×106) from BALB/c mice designated as BM+ WT spln.
Mouse serum RNA extraction
Lethally irradiated B6D2F1/B6 mice received donor cell infusions and were sacrificed at indicated time-points post transplant. Serum was harvested from blood drawn via cardiac puncture. RNA isolation from serum (75μl) was performed using the mirVana miRNA isolation kit (Ambient Technologies) according to manufacturer's protocol.
Exosome Purification
Exosomes were isolated using ExoQuick™ solution according to the manufacturer's recommendations (System Biosciences). For confirmation of purity, exosome pellets were lysed in RIPA lysis buffer and used for immunoblotting analysis using the western blot sampler kit (Catalog# EXOAB-KIT-1, System Biosciences, SBI) according to manufacturer's protocols.
Nanoparticle Tracking Analysis (NTA)
Pure exosomes were analyzed using a Nano-Sight LM10 instrument equipped with a 405 nm laser (NanoSight) at 25°C. Particle movement was tracked by NTA software (version 2.2, NanoSight) with low refractive index corresponding to cell-derived vesicles. Each track was then analyzed to get the vesicle concentration (in millions) for each size. Three videos of 60–90 seconds were recorded of each sample. Data analysis was performed with NTA 2.3 software (Nanosight). The diffusion coefficient and hydrodynamic radius were determined using the Stokes–Einstein equation, and results were displayed as a particle size distribution.
In vivo treatment with LNA-anti miR-29a oligonucleotides
The LNA-anti miR-29a and scramble control on a phosphothiorate background were purchased from Exiqon. Lethally irradiated recipient F1 mice transplanted with BM (5×106) + B6 WT spleen cells (20×106) were treated with LNA-anti miR-29a (n=10) or scramble LNA oligos (n= 10) starting at day 7, at a dose of 10 mg/kg i.p. twice weekly up to day 50 after infusion of donor B6 splenocytes. Clinical GVHD scores and survival was followed post-transplant as described earlier. One cohort of mice were euthanized day 10 post-transplant to evaluate serum miR-29a expression and another to evaluate DC and T cell activation, proliferation, effector status.
GVL experiments
Firefly luciferase transduced P815 (22-25) (H-2d) cells (6000 to 10,000) were injected intravenously into F1 recipients on day 0 along with t-BM (5×106 cells) and B6 donor splenocytes (20 × 106 cells). Treatment groups included LNA anti-miR-29a or scramble control. BM and P815 cells (leukemia alone) served as the control group. P815-induced leukemic death was defined by the occurrence of either macroscopic tumor nodules in liver and/or spleen or hindleg paralysis. GVHD death was defined by the absence of leukemia and the presence of clinical and histopathological signs of GVHD.
In vivo imaging
Xenogen IVIS imaging system (Caliper Life Sciences) was used for live animal imaging. Mice were anesthetized using 1.5% isofluorane (Piramal Healthcare). XenoLight RediJect D-Luciferin Ultra Bioluminescent Substrate (150 mg/kg body weight; 30 mg/mL in PBS; Perkin Elmer) was injected intraperitoneally and IVIS imaging was performed 10 minutes after substrate injection. Whole body bioluminescent signal intensity was determined weekly using IVIS Living Image software v4.3.1 (Caliper Life Sciences), and pseudocolor images overlaid on conventional photographs are shown. Data were analyzed and presented as photon counts per area.
Statistical Analysis
A paired t-test was used to compare -ΔCT values for serum miR-29a expression between aGVHD patients and non-aGVHD matched controls. This test only controls for the matching variables (i.e., donor relationship, conditioning regimen, and timing of the sample post-transplant) and ensures that any differences observed in miR-29a expression between cases and controls is not attributable to these factors. Conditional logistic regression was used to model the probability of being called the case within a matched pair as a function of miR-29a expression, adjusting for potentially important or confounding variables not accounted for by the matching. Univariable models were initially fit and any variables with p<0.20 were fit in a multivariable model. Variables other than miR-29a expression considered for model inclusion were patient age at time of transplant, patient sex, donor sex, donor/patient sex (agree vs not agree), Karnofsky performance score (60/70 vs 80/90/100), comorbidity index score (0, 1-4, ≥5), HLA match (perfect match vs less than perfect match), donor stem cell source (BM vs PB; 2 pairs with cord blood as the donor source were excluded), ABO mismatch (yes vs no), CD34+ cells infused, and CD3+ cells infused. All tests were two-sided and statistical significance was declared for p<0.05. All analyses were performed using SAS version 9.4 or GraphPad Prism 6.0.
Results
miR-29a is elevated in the serum of patients with aGVHD
To identify serum miRs associated with aGVHD we performed small RNA sequencing of serum samples obtained from allogeneic HSCT patients at the time of clinical suspicion of aGVHD. In this study we included 10 allogeneic HSCT patients with aGVHD and 9 allogeneic HSCT patients with no aGVHD (Discovery Cohort) that were matched for age, disease, conditioning regimen, donor and timing of sample collection (Controls). Patient characteristics are provided in Table 1. We found 6 miRNAs up-regulated (miR-146a-5p, miR-29a-3p (i.e., miR-29a), miR-323b-3p, miR-34c-3p, miR-363-3p, miR-15a-5p) and 4 miRNAs down-regulated (miR-3168, miR-662, miR-550a-5p and miR-181a-5p) (Fold change (FC) >2, p<0.01).
Table 1. Patient Characteristics of Discovery Cohort.
| Median age | 51.9 years |
|
| |
| Conditioning regimen | Non-myeloablative |
|
| |
| aGVHD | |
| Bowel | N=2 |
| Skin | N=5 |
| Skin and Bowel | N=3 |
| Total | N=10 |
|
| |
| Non-aGVHD controls | N=9 |
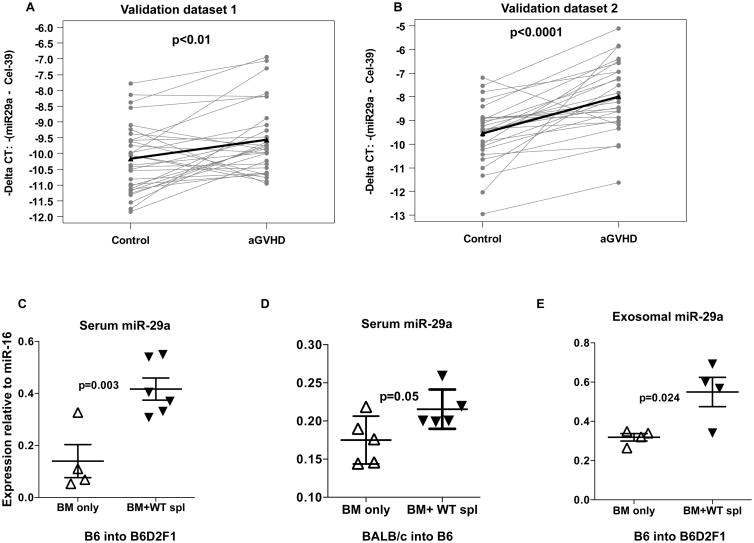
Of all dysregulated serum miRs, we focused on miR-29a since it is a key regulator of adaptive immune function (18, 26). First, we measured serum miR-29a in an independent cohort of allogeneic HSCT patient samples (Validation dataset 1, 30 aGVHD patients and 30 non-GVHD matched controls) obtained at the initial diagnosis of aGVHD using qRT-PCR. Similar to the discovery dataset, we matched each aGVHD sample as described before (patient characteristics are provided in Supplementary Table 1). As shown in Fig. 1A, serum miR-29a was significantly upregulated in aGVHD (grades 2-4) patients compared to their matched controls (p<0.01). Cases had higher miR-29a expression than controls in 22 of 30 pairs (73%), with at least 2-fold higher expression in 11 pairs (50%). miR-29a level did not appear to correlate with grade 2 (n=19) versus grade 3 (n=9) severity. Among the grade 2 cases, the average expression was 1.44-fold higher (95% CI: 0.99-2.76) relative to matched controls. Among the grade 3 cases, the average expression was 1.47-fold higher (95% CI: 0.82-2.64) relative to matched controls. Only 2 cases had grade 4 aGVHD, both with 3-fold higher expression relative to matched controls. To support the association between serum miR-29a and aGVHD diagnosis, a second independent cohort of allogeneic HSCT recipients (Validation dataset 2, 27 aGVHD patients and 27 non-GVHD matched controls, patient characteristics are provided in Supplementary Table 2) showed upregulation of serum miR-29a in aGVHD (grades 1-3) qRT-PCR (Fig. 1B). Here, the cases had higher miR-29a expression than control in 25 of 27 pairs (93%), with at least 2-fold higher expression in 17 pairs (63%). Since miR-29a expression was significantly higher for aGVHD cases relative to controls in each of the datasets, the data were combined for further analyses. In a univariable conditional logistic regression model, the odds of being the case with aGVHD in a matched pair was 3.78 times higher (95% CI: 1.82-7.66, p=0.0002) for every 2-fold increase in miR-29a expression. Among other variables considered, only patient age at time of transplant (p=0.17) and a low Karnofsky performance score were moderately associated with the odds of being the case in a pair (p=0.14). Controlling for these two variables in addition to the matching variables, miR-29a expression remained significant (odds ratio=4.26, 95% CI: 1.96-9.26, p=0.0003) (Table 2).
Figure 1. Serum miR-29a is upregulated in patients with aGVHD and mouse models of aGVHD.
(A) miR-29a expression as measured by real-time PCR in serum of aGVHD (n=30) and non-GVHD (n=30) matched controls (validation dataset 1). Expression is depicted as negative delta CT values, with higher values corresponding with higher expression. Spike-in c. elegans-39 was used as normalizer. P value was determined by paired t-test. (B) miR-29a expression as measured by real-time PCR in validation dataset 2 of aGVHD (n=27) and non-aGVHD (n=27) matched controls. (C-D) Relative miR-29a expression in serum of aGVHD mice, (recipients of BM+ WT splenocytes, with clinical aGVHD score ≥7) and non-GVHD mice (BM only). (E) miR-29a expression in exosomes isolated from serum of aGVHD mice (recipients of BM+ WT splenocytes) and non-GVHD mice (BM only). miR-16 was used as endogenous control in mice serum samples (56).
Table 2. Modeling the Odds of Being Called the Case with aGVHD Relative to a Control without aGVHD within a Matched Pair*.
| Variable | Univariable Models | Multivariable Model | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | Wald P | Odds Ratio | 95% CI | Wald P | |
| Patient age at transplant, 10-year increase | 1.23 | 0.91-1.66 | 0.17 | 1.08 | 0.70-1.66 | 0.74 |
| Patient Sex, male vs female | 0.93 | 0.44-1.98 | 0.85 | --- | --- | --- |
| Donor Sex, male vs female | 1.75 | 0.51-5.98 | 0.37 | --- | --- | --- |
| Donor/Patient Sex, agree vs not agree | 1.60 | 0.73-3.53 | 0.24 | --- | --- | --- |
| Karnofsky performance Score, 60/70 vs 80/90/100** | 5.00 | 0.58-42.78 | 0.14 | 17.76 | 0.95-331.79 | 0.054 |
| Co-morbidity Index score, | 0.67 | --- | --- | --- | ||
| 0 vs 5+ | 1.58 | 0.43-5.83 | ||||
| 1-4 vs 5+ | 1.49 | 0.60-3.69 | ||||
| HLA Match, perfect vs less than perfect | 1.00 | 0.25-4.00 | 1.00 | --- | --- | --- |
| Donor Stem Cell Source, BM vs PB | 0.83 | 0.25-2.73 | 0.76 | --- | --- | --- |
| ABO Mismatch, yes vs no | 0.82 | 0.41-1.67 | 0.59 | --- | --- | --- |
| CD34+ cell × 10ˆ6 infused, 1 unit increase | 0.94 | 0.81-1.09 | 0.39 | --- | --- | --- |
| CD3+ cells × 10ˆ8 infused, 1 unit increase | 0.92 | 0.66-1.29 | 0.63 | --- | --- | --- |
| miR-29a expression, 2-fold increase | 3.78 | 1.86-7.66 | 0.0002 | 4.26 | 1.96-9.26 | 0.0003 |
Cases were individually matched to controls by conditioning regimen type (MA vs RIC) and donor relationship (related vs unrelated); thus, the odds of being called the case with aGVHD within a pair cannot be assessed for these variables.
Although all but one patient with a low Karnofsky performance score was a case with aGVHD, the number of patients with a score of 60/70 was small and the odds ratio estimate for this variable is unstable as exemplified by the wide confidence interval.
To investigate whether miR-29a expression is also up-regulated in the serum mice with aGVHD, two MHC-mismatched HSCT models were used in which spleen cells (20×106) and T cell–depleted BM (5×106) from either C57BL/6 (B6) or BALB/c donors were transferred intravenously into lethally irradiated B6D2F1 (F1) or B6 recipient mice respectively. The mice were monitored and scored as previously described.(8, 27, 28) BM recipients and BM + splenocytes were euthanized when their aGVHD clinical score was ≥ 7and miR-29a was measured from the serum using qRT-PCR. We found that miR-29a was upregulated in mice with aGVHD compared to controls (Fig. 1C-D). Recently, exosome mediated transfer of mRNAs and miRs has emerged as an important and novel mechanism of genetic exchange and communication between cells (29, 30). To investigate whether miR-29a was present in the serum exosomes, we isolated exosomes (Supplementary Figure 1A-B) from the recipients of the B6 into F1 model, and measured miR-29a by qRT-PCR. As shown in Fig. 1E, miR-29a was upregulated in serum exosomes, adding another layer of complexity in aGVHD biology.
Serum miR-29a activates dendritic cells (DC) through TLR7 (mouse) and TLR8 (human)
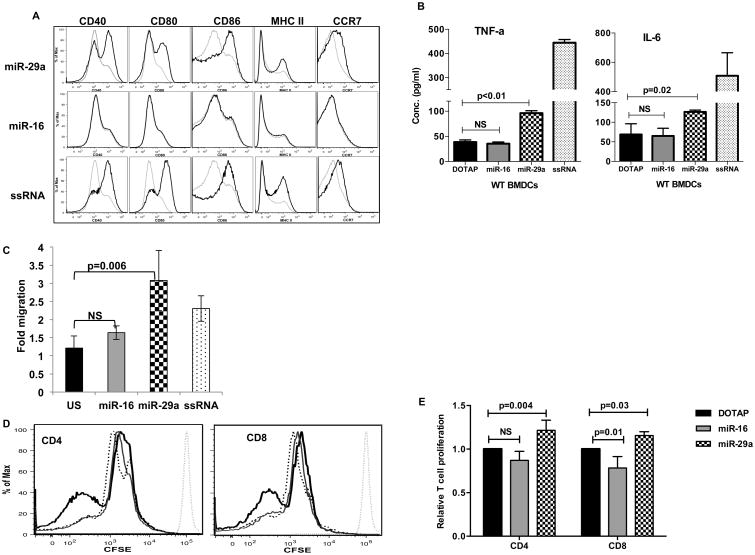
We have previously shown that lung cancer cells secrete exosomal miR-29a that subsequently binds to and activates human TLR8 (or its murine ortholog TLR7) on macrophages within the tumor stroma that sustain tumor growth (19, 31). Thus, we asked whether serum/exosomal miR-29a can bind to and activate TLRs on DC, the predominant antigen-presenting cell (APC) involved in priming/potentiating the alloreactive response in aGVHD. Mouse bone marrow derived DC (BMDC, (32)) were cultured in the presence of liposome conjugated (DOTAP, mimicking exosomes) miR-29a, miR-16 (negative control) or ssRNA (TLR7/8 agonist, positive control) oligonucleotides for 48 hours. Only miR-29a but not miR-16 resulted in strong upregulation of BMDC activation and maturation markers CD40, CD80, CD86, MHC II and CCR7 (Fig. 2A). In addition, miR-29a stimulation of DC released pro-inflammatory cytokines TNF-α and IL-6, cytokines that are critical drivers of aGVHD pathogenesis (Fig. 2B). miR-29a stimulation of BMDCs did not result in the release of IL-12 (data not shown), consistent with previous reports (33) that show that miR-29 directly targets and down-regulates IL-12p40. To further support the miR-29a mediated DC activation we examined the migration properties of BMDC after miR-29a or miR-16 co-culture. Following antigen acquisition, DCs must migrate from the periphery to draining lymph nodes in a CCR7 dependent manner to prime antigen specific alloreactive T cell response (32, 34, 35). miR-29a induced CCR7 upregulation and increased the migratory response of BMDCs to CCL19 as measured by a transwell migration assay (Fig. 2C). Alloreactive T cell activation and proliferation is a hallmark of aGVHD and the strength of the alloreactive response is very critical in determining the severity of the disease (1-3, 36). To study if miR-29a primed DCs could elicit a stronger T cell alloreactive response we stimulated B6D2F1 DC with miR-29a or miR-16 for 48 hours, washed the cells, and then added CFSE labeled B6 T cells to measure proliferation. As expected, miR-29a stimulated DCs elicited a stronger proliferative response from alloreactive CD4+ and CD8+ T cells, (Fig. 2D and E). T cells alone treated with DOTAP-miR29a/miR-16 did not upregulate activation markers such as CD69 (data not shown) showing that the T cell activation by miR-29a requires an APC such as dendritic cells.
Figure 2. miR-29a induces maturation of murine bone marrow derived dendritic cells.
Day 6 BMDCs were washed and treated with DOTAP-miR29a (1μg/ml), miR-16 (1μg/ml), DOTAP alone or ssRNA (ssRNA40/LyoVec™ 5μg/ml, Invivogen) for 48 hrs.
(A) Flow cytometric analysis of maturation markers CD40, CD80, CD86, MHCII and CCR7. Grey histogram, DOTAP alone; Black histogram, indicated treatment. One representative experiment of three is shown. (B) Detection of TNF-α and IL-6 in supernatants by ELISA. Samples were assayed in triplicate and results are shown as the means of three independent experiments. (C) BMDC were washed and cells (5×105) were placed in a Transwell migration chamber and allowed to migrate for 3 hrs at 37°C. SLC/CCL19 (200 ng/ml) was placed in the lower chamber to induce CCR7-dependent chemotaxis. Lower chambers with medium only served as a control for spontaneous migration. CCL19-dependent migration was calculated according to the formula: Migration Index (MI) = number of cells CCL-19/number of cells medium only. The mean ±SEM of two independent experiments performed in triplicate is presented. (D) Day 6 B6D2F1 BMDCs were washed and treated with for 48 hrs with DOTAP-miR-29a/miR-16 or DOTAP alone to induce maturation. B6 T cells were stained with CFSE and incubated with allogeneic BMDC (stimulator: effector =1:5). Cell division was measured by CFSE dilution after 7 days. Dotted grey histogram, undivided T cell peak; dashed black histogram, DOTAP alone; grey histogram, DOTAP-miR-16; black histogram, DOTAP-miR29a. One representative experiment of three is shown. (E) Bar plots showing relative T cell proliferation of experiment described in D. Data (CFSE MFI) was normalized to the DOTAP alone group, and is represented as mean ±S.D. of three independent experiments.
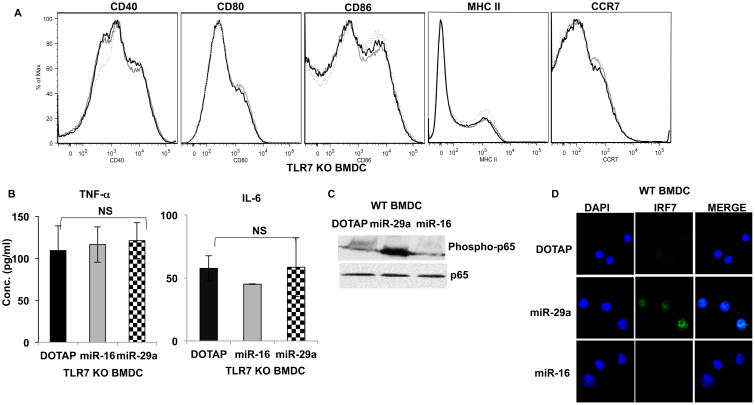
To demonstrate that miR-29a activation of BMDC was TLR dependent, we cultured BMDC obtained from TLR7-/- knock-out or wild type mice. miR-29a failed to activate BMDC from TLR7-/- mice supporting our hypothesis, that in mice, miR-29a mediated activation of DCs is TLR7 dependent (Fig. 3A-B). Stimulation of TLR7-/- BMDCs with LPS, a TLR4 agonist resulted in strong upregulation of DC maturation markers (data not shown) confirming that there was no functional defect in TLR7-/- BMDCs. Since TLR signaling leads to activation of My-D88 and NF-κB pathways (37, 38) we examined nuclear localization of phosphorylated IRF7 and NF-kB-p65 after miR-29a treatment of DC. miR-29a stimulation of WTDC also resulted in the nuclear translocation of phosphorylated IRF7 and phosphorylation of NF-κBp65 (Fig. 3C-D).
Figure 3. miR-29a activation of dendritic cell is dependent on TLR7 in mice.
BMDC were derived from TLR7-/- mice and treated with DOTAP-miR29a (1 μg/ml), miR-16 (1 μg/ml), DOTAP alone or ssRNA (5μg/ml) for 48 hrs as described before.
(A) Flow cytometric analysis of maturation markers CD40, CD80, CD86, MHCII and CCR7 showing no upregulation in the absence of TLR7. Dotted histogram, DOTAP alone; Grey histogram, DOTAP-miR16; black histogram, DOTAP-miR29a. One representative experiment of three is shown. (B) Supernatant cytokine TNF-α and IL-6 detection by ELISA. Samples were assayed in triplicate and results are shown as the means of three independent experiments. miR-29a activates TLR7 signaling pathway in mice. Day 6 WT BMDCs were washed and treated with DOTAP-miR-29a, miR-16 or DOTAP alone for 15 mins (phospho p65) or 4 hrs (IRF7 translocation). (C) Western Blotting showing p65 phosphorylation and (D) Confocal microscopy showing IRF7 nuclear translocation. One representative experiment of two is shown.
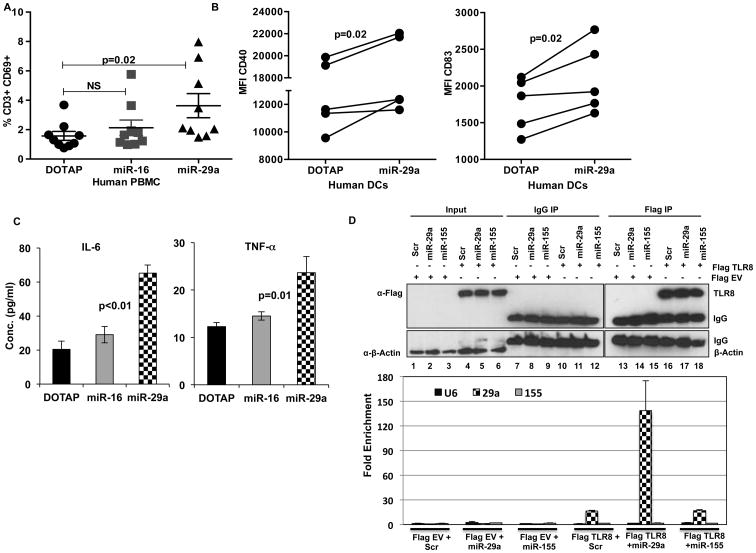
Human PB mononuclear cells (PBMCs) stimulated with miR-29a also show activation of T cells as seen by upregulation of early activation marker CD69 (Fig. 4A). DCs derived from PB monocytes stimulated with miR-29a also showed significant upregulation of maturation/activation markers CD40 and CD83 and secretion of pro-inflammatory cytokines TNF-α and IL-6 (Fig. 4B-C). Finally, to demonstrate that miR-29a binds directly to TLR8 we transfected human DCs with Flag-TLR8 plasmid. The transfected cells were incubated with miR-29a or miR-155 or U6 (negative controls) (39) and immunoprecipitation and RNA-IP was performed 72 hrs post-transfection. As shown in Fig. 4D, immunoprecipitation using Flag Antibody and RNA-IP shows enrichment of only miR-29a in TLR8 transfected cells, demonstrating that miR-29a binds to TLR8 in humans.
Figure 4. miR-29a activates human PBMCs and DCs via TLR8.
(A) Human PBMCs (n=9) from healthy donors were treated with DOTAP-miR29a, DOTAP-miR-16, DOTAP alone for 48 hrs. T cell activation was measured by CD69 upregulation on CD3+ T cells by flow cytometry. (B) CD11c+ CD14- human dendritic cells (n=5) were treated with DOTAP-miR29a, DOTAP-miR-16, DOTAP alone for 48 hrs. DC maturation was assessed by mean fluorescence intensity (MFI) of CD40 and CD83 by FACS and (C) Detection of TNF-a and IL-6 in supernatants by ELISA. (D) Human dendritic cells were transfected with Flag TLR8 or Flag EV plasmids with or without Scr or miR-29a or miR-155. Immunoprecipitation was carried out using IgG or Flag Antibody and RNA-IP showing enrichment of only miR-29a in TLR8 transfected cells. Paired t-test was used to compare differences, p<0.05 was considered significant.
Serum miR-29a expression kinetics and origin
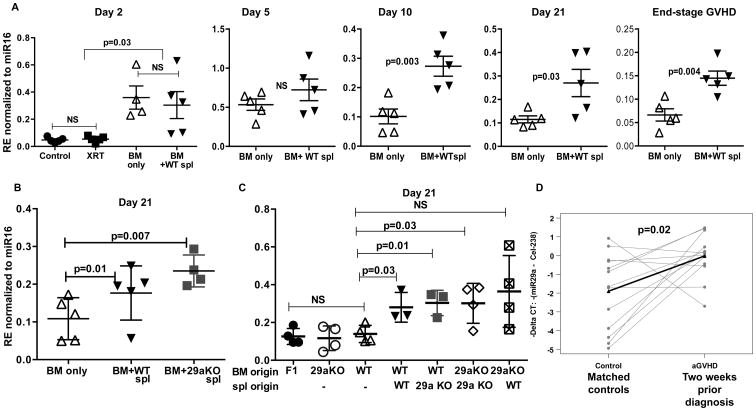
So far, we have shown that serum miR-29a is upregulated in aGVHD and activates DC. However, we examined serum expression only at the time of aGVHD diagnosis in humans and in mice. Thus, it would be important to establish the kinetics of serum miR-29a expression post-transplant to gain a better understanding of the potential mechanisms that leads to miR-29a secretion. To address this question we used the parent to F1 mouse model of aGVHD (B6 into F1) and measured serum miR-29a expression overtime. BM alone and BM plus donor splenocytes recipients were sacrificed in cohorts (n=5) at days 2, 5, 10 and 21 after transplant and when clinically sick with aGVHD (score ≥7, approximately day 35), serum and tissues harvested. Healthy and irradiation only mice (no cell infusion) were also used as controls for the early time-point (day 2). In our model, we observed a significant up-regulation of serum miR-29a two days after transplant in both recipients of BM alone and BM+ splenocytes but not in the irradiated group alone, Fig. 5A. We reasoned these results indicate that the initial upregulation of miR-29a is not caused by irradiation but rather results from the infusion of donor cells, either BM alone or BM + splenocytes. In addition, we saw no difference in serum miR-29a levels at day 2 and day 5 post-transplant between recipients of BM only and BM+ WT splenocytes. However, recipients of BM+ WT splenocytes had significantly elevated serum miR-29a expression compared to BM alone recipients at later time points (days 10, 21 and end-stage aGVHD), Fig. 5A. Our results show that using the B6 into F1 mouse model, Day 10 post-transplant is a reliable early time-point at which elevated serum miR-29a could be detected in animals that go on to develop clinical aGVHD as compared to recipients of BM cells that do not develop aGVHD.
Figure 5. Kinetics and origin of serum miR-29a expression.
(A) Lethally irradiated B6D2F1 recipient mice received either T cell depleted WT bone marrow (BM only) or BM+B6 WT splenocytes (BM+ WT spl). Healthy, non-irradiated mice and irradiated alone mice receiving no cell infusion (XRT) were used as controls for early time-points. Recipients were sacrificed at indicated time-points post-transplant, RNA isolated from serum and miR-29a expression relative to miR-16 analyzed by real-time PCR. 4-5 mice were used per group per time point; one representative experiment of two is shown. (B) Lethally irradiated B6D2F1 recipient mice received either T cell depleted bone marrow (BM only) or BM+B6 WT splenocytes (BM+ WT spl) or BM+ miR29a knockout splenocytes (BM+29a KO spl). Recipients were sacrificed at day 21 post-transplant and relative miR-29a expression determined as described before. (C) Transplant was performed, recipients sacrificed at day 21 post-transplant and relative miR-29a expression determined as described earlier. Donor cell infusions are indicated on the x-axis. P values were determined by unpaired t-test. (D) miR-29a expression as measured by real-time PCR in serum two weeks prior to clinical diagnosis of aGVHD in 13 cases and matched controls. Expression is depicted as negative delta CT values, with higher values corresponding with higher expression. Spike-in c. elegans-238 was used as normalizer. P value was determined by paired t-test.
Only recipients of BM+ WT splenocytes develop aGVHD and given the fact that miR-29a was elevated only in mice that developed aGVHD and that donor splenocytes are critical for the development of this disease, our first hypothesis was that serum miR-29a is secreted by donor T cells. To address this question, we performed a transplant using the following cohorts as donors; a) Wild type B6 (WT) BM + WT splenocytes, b) WT B6 BM only and c) WT B6 BM+ miR-29a-/- knock out (29a KO) splenocytes. The recipients were all sacrificed on day 21 and serum miR-29a levels measured by qRT-PCR. Interestingly, recipients of both WT and miR-29a KO splenocytes had elevated serum miR-29a expression compared to recipients of BM alone, suggesting that while donor splenocytes were required for the secretion of serum miR-29a, they are not the source, Fig. 5B. We conclude that as a result of the interaction between donor T cells and the host, serum miR-29a is secreted by host (recipient) cells.
To further dissect the source of serum miR-29a in animals with aGVHD, we repeated the transplants using the following cohorts; a) WT BM only, b) miR-29a KO BM only, c) WT BM+ WT splenocytes, d) WT BM+ miR-29a KO splenocytes, e) miR-29a KO BM+ miR-29a KO splenocytes and f) miR-29a KO BM+ WT splenocytes. Healthy non-irradiated F1 mice were used as controls. The animals were sacrificed on Day 21 and serum miR-29a levels were determined. As expected, recipients of WT or miR-29a KO BM alone did not have significantly different levels of serum miR-29a as compared to healthy F1. Most remarkably, all recipients of WT BM+splenocytes, irrespective of type of donor splenocytes (WT or miR-29a KO) had significantly elevated serum miR-29a compared to BM alone recipients except for recipients of miR-29a KO BM+ WT splenocytes, where the trend was higher but did not reach significance due to intra-group variation, Fig. 5C. This result is in accordance with our previous experiment that showed that the presence of donor splenocytes was required for the increase expression levels of serum miR-29a. In addition, recipients of miR-29a KO BM+ miR-29aKO splenocytes also had elevated serum miR-29a as compared recipients of BM alone, indicating that host cells were the likely source of serummiR-29a.
Elevated serum miR-29a detected prior to development of aGVHD in humans
Given the kinetics of miR-29a expression in the murine aGVHD models, we were interested in knowing whether miR29a could be detected in humans prior to the clinical manifestations of aGVHD. Of the 30 matched patient pairs studied from validation dataset 1, 13 had serum samples available two weeks before the clinical diagnosis of aGVHD. We found that serum miR-29a expression was significantly higher in patients who later developed clinical aGVHD as compared to the matched controls (p=0.02, Fig. 5D).
miR-29a expression is upregulated in epithelial cells of the gastrointestinal tract in mice with aGVHD
To identify the cells that produce high miR-29a during aGVHD, we performed locked nucleic acid -in situ hybridization (LNA-ISH) for miR-29a in aGVHD end-organ target tissues (liver, small and large bowel) harvested from lethally irradiated B6D2F1 mice that developed aGVHD (recipients of B6 BM+splenocytes) and control mice that did not (recipients of B6 BM only) on day 21 post-transplant. Given the central role that the GI tract plays in the initiation and amplification of aGVHD (40-43) and the importance of miR-29 for the maintenance of the intestinal barrier integrity (44, 45), we speculated that the epithelial cells of the GI tract might strongly express miR-29a. Immunohistochemistry (IHC) with epithelial cell marker PKC-26 shows strong staining in the GI tract of animals with aGVHD and controls (Fig. 6, left panel). The basal epithelial cells of the GI tract in aGVHD mice stain strongly positive for miR-29a, suggesting that GI tract epithelial cells might be the predominant source of serum miR-29a (Fig. 6, middle panel). T cell infiltration of peripheral target tissues (GI tract and liver) is a hallmark of aGVHD pathology (1). CD3 IHC shows strong T cell infiltration in GI tract (Fig. 6, right panel) and liver (Supplementary Figure 2, middle panel) of animals that developed aGVHD and not controls. CD3 T cells in the lamina propria did not stain positively for miR-29a, further corroborating our results that the donor T cells are not the source of serum miR-29a, Fig. 6. There was no signal for miR-29a in the liver (Supplementary Figure 2, left panel) indicating that hepatocytes are not the source of serum miR-29a. LNA-ISH using scramble control did not show any staining (Supplementary Figure 2, right panel).
Figure 6. miR-29a expression is upregulated in the GI tract epithelial cells in mice with aGVHD.
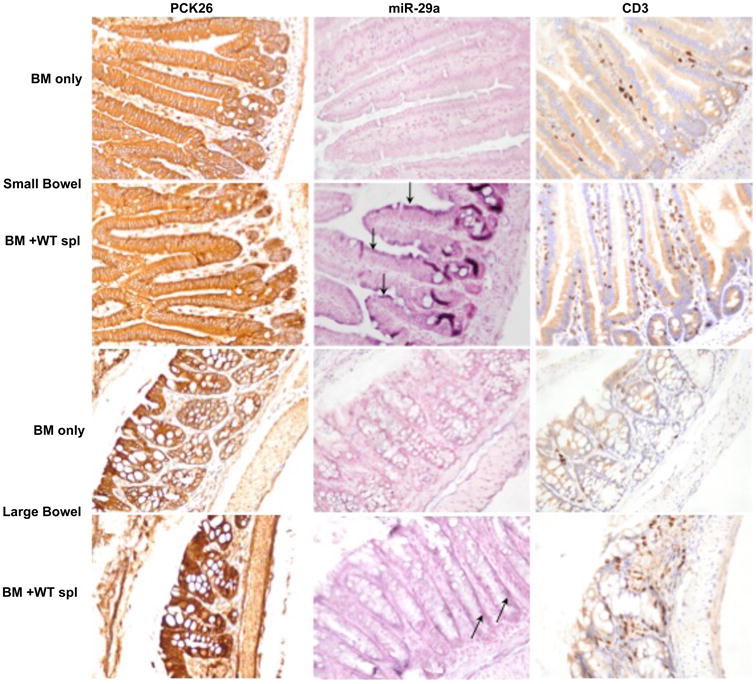
IHC for pancytokeratin (PCK-26, left panel), miR-29a LNA-ISH (middle panel) and CD3 IHC (right panel) in small and large bowel in recipients of BM only and BM+ WT splenocytes. Arrows point to the purple positive staining for miR-29a by LNA-ISH.
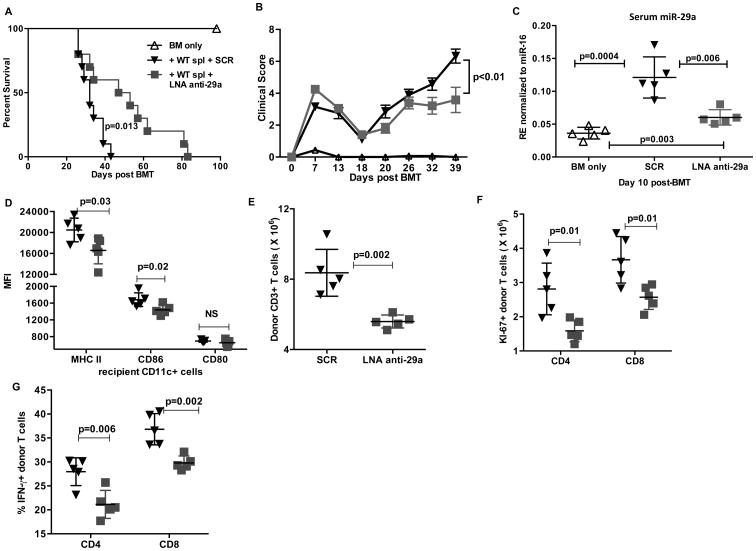
LNA anti miR-29a treatment significantly increases survival in mouse model of aGVHD while retaining Graft versus Leukemia (GVL) after HSCT in recipient mice
To investigate whether pharmacological targeting of miR-29a could decrease aGVHD severity and improve overall survival, we treated lethally irradiated recipient F1 mice with LNA anti miR-29a or scramble control oligonucleotides intraperitoneally (i.p.) twice weekly starting at day 7 up to day 50 after infusion of donor B6 splenocytes. Treatment with LNA anti miR-29a significantly prolonged survival of recipient mice post transplant (mean survival, scramble vs. anti miR-29a, 32 vs. 50 days respectively, p=0.013, Fig. 7A) and decreased severity of clinical aGVHD (Fig. 7B, p<0.01). Down-regulation of serum miR-29a was observed in mice treated with LNA anti miR-29a with respect to controls confirming specificity of targeting (Fig. 7C). Our in-vitro experiments demonstrated a clear role of exosomal miR-29a in promoting DC activation an alloreactive T cell proliferation. Given that the LNA anti-miR29a treatment significantly reduced circulating serum miR-29a in the recipients (Fig. 7C), we wanted to extend our findings and investigate whether in vivo administration of LNA anti miR-29a had any effect on DC activation and/or T cell proliferation and effector function. Lethally irradiated F1 mice that received BM+ WT splenocytes were treated with either SCR or LNA anti miR-29a as described in the survival study. On day 10, the recipients were sacrificed and tissues obtained. Recipients treated with LNA anti miR-29a showed a significant reduction in the expression of MHCII and CD86 on CD11c+ dendritic cells with no significant difference in the expression of CD80 (Fig. 7D). There was a significant decrease in absolute numbers of donor T cells in LNA anti miR-29a treated mice compared to SCR (Fig. 7E). Consistent with this, there was a significant reduction in the number of proliferating donor CD4+ and CD8+ T cells (Fig. 7F). We also observed reduced effector function of donor T cells as seen by reduced secretion of IFN-γ by donor CD4+ and CD8+ T cells (Fig. 7G) in mice received LNA anti miR-29a treatment.
Figure 7. LNA anti-miR-29a treatment significantly increases survival in mouse model of aGVHD.
Lethally irradiated recipient F1 mice transplanted with BM (5×106) + B6 WT spleen cells (20×106) were treated with LNA anti miR-29a (n=10) or scramble control oligonucleotides (n= 10) starting at day 7 at 10 mg/kg I.P. twice weekly up to day 50 after infusion of donor B6 splenocytes. (A) Survival curve of recipient groups. Comparisons between survival curves were performed using log-rank test. (B) Clinical GVHD scores. The p values were obtained by using 2 way ANOVA. (C) A separate cohort of mice (n=5 per group) was euthanized on day 10 post transplant after a single dose of LNA anti miR-29a or scramble control. miR-29a expression in serum was measured by real-time PCR. Lethally irradiated recipient F1 mice transplanted with BM (5×106) + B6 WT spleen cells (20×106) mice were treated with either SCR (inverted triangles, n=5) or LNA anti miR-29a (squares, n=5) and sacrificed on day 10 post transplant. Spleen was harvested, splenocytes isolated and (D) Flow cytometric evaluation of MHC II, CD86 and CD80 expression (mean fluorescence intensity, MFI) on splenic recipient H-2Kd+ CD11c+ dendritic cells. (E) Total H-2Kd- CD3+ donor T cell counts. (F) Intracellular Ki-67+ CD4+ and CD8+ donor H-2Kd- CD3+ T cell counts. (G) Splenocytes were stimulated with cell stimulation cocktail (eBioscience) for 5 hrs. Intracellular cytokine staining was performed to detect the number of donor T cells secreting IFN-γ. Data represent the mean ± SD.
Since aGVHD and GVL are two highly linked immune reactions (1, 3), we sought to determine whether miR-29a down-regulation may interfere with GVL effects. To address this, we performed a transplant using luciferase transduced murine P815 (22-25) leukemic cells. The presence of luciferase allows for tracking of tumor persistence in live animals using whole-body imaging. Separate cohorts were used for imaging and survival. The cohorts were - 1) t-BM + P815; 2) t-BM + P815 +B6 splenocytes treated with LNA anti miR-29a and 3) t-BM + P815 + B6 splenocytes treated with scramble control. This is a very aggressive tumor model, in which the mice receiving leukemic cells in the absence of donor splenocytes succumb to disease by day 14-16. There were no significant differences in the leukemic burden between the LNA anti miR-29a and scramble treated recipients, as measured by bioluminescence assays showing retention of GVL effect (Fig. 8A,B). A single mouse in the scramble-treatment imaging cohort showed focal leukemic infiltration. Histopathological examination of liver (Fig. 8C) and spleen (data not shown) of mice confirmed that cause of death in LNA anti miR-29a or scrambled treatment groups was aGVHD and not leukemia.
Figure 8. LNA anti-miR-29a treatment retains Graft versus Leukemia (GVL) after HSCT in recipient mice.
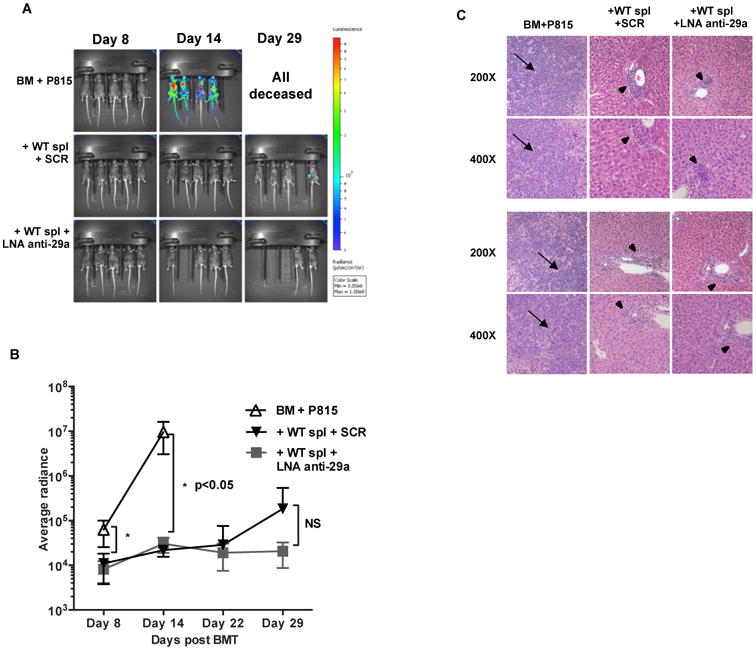
Firefly luciferase transduced P815 (22-25) cells (6-10,000 cells) were injected intravenously into lethally irradiated F1 recipients on day 0 along with t-BM and B6 donor splenocytes. Treatment groups included LNA anti miR-29a or scramble control. BM and P815 cells (leukemia alone) served as the control group. (A) Whole body bioluminescent signal intensity of recipient mice (n= 5 per cohort). Mice were imaged on indicated days. (B) Average radiance expressed as mean ± SD. One representative experiment of two is shown. (C) Liver sections at 200 and 400× magnification of two representative recipients. Note diffuse infiltration of the liver by large anaplastic cells (arrows) in the BM + p815 only group. There were no leukemic cells in either of the groups that received WT splenocytes. There was periportal hepatic lymphocytic infiltration in the mice receiving WT splenocytes (triangles).
Discussion
Recent studies indicate that circulating miRs could be used as biomarkers to predict aGVHD onset. However, the functional implications of circulating miRs have not been investigated. In this study we identified a small number of deregulated serum miRs in patients with aGVHD. These serum miRs did not overlap with the previously reported circulating miR candidates associated with aGVHD (15, 16). This discrepancy could be explained by the use of different sources; serum versus plasma to isolate RNA and perform the profiling. It has been reported that there are differences in the concentration of miRs depending on whether serum or plasma is used even within the same individual (46). Another reason could be the use of different platforms to detect candidate miRs (RT-PCR, microarray or RNA-sequencing). Platform dependent variations in measurements of miRs have been described (47). However, RNA-sequencing is considered a more robust and sensitive method, in particular at detecting low abundance transcripts (47) as is the case for circulating miRs in body fluids. This was the main reason for choosing next generation sequencing as an “unbiased” and more sensitive approach to discover miRs associated with disease.
In our study, we confirmed upregulation of miR-29a in the serum of aGVHD cases relative to matched controls (using similar qRT-PCR technique) in two independent cohorts of allogeneic HSCT samples that were carefully matched for donor relationship, conditioning regimen, and sampling time; these were factors believed to be most important to ensure were not contributing to any differences in expression seen between cases and controls. Other factors moderately associated with case/control designation were patient age at time of transplant and Karnofsky performance score; further adjustment for these factors did not impact the significant association between upregulation of miR-29a and aGVHD. In a smaller subset of patients, we show that levels of serum miR-29a are significantly upregulated two weeks before clinical diagnosis. However, whether serum miR-29a can be used as a biomarker to predict whether or not individual patients develop aGVHD must be investigated in a larger prospective study.
Our results indicate a novel role for miR-29a in the induction of DC maturation and activation through human TLR8 signaling that have a broad impact in the field of immunotherapy. Ex vivo or in vivo treatment of DC with miR-29a may enhance DC maturation and activity that could be important for cancer vaccine treatments or other immunotherapy approaches. It is widely recognized that one of the initiating steps of aGVHD is the activation of TLR4 and 9 by ligands such as lipopolysaccharide (LPS) and bacterial DNA that are released in the gut by the breach of the intestinal mucosa caused by the conditioning regimen (27, 48-51). Upon TLR activation, a wide array of inflammatory cytokines such as TNF-α is released that foster the development of aGVHD (38, 49, 52). TLR 7 and 8 are present in the endosomal compartment of the cell and are important to detect viral and other single-stranded RNA molecules. A role for TLR7 in exacerbating skin GVHD has been reported (50, 51). Here, we have shown that serum miR-29a expression increases after transplant in host with aGVHD and activates DC through TLR pathway. Remarkably, our data indicate that it is the host cells (and not donor cells) that secrete miR-29a into the serum and that these host cells are epithelial cells from the mucosa of the GI tract. Our results are also corroborated by a previous study that showed no difference in miR-29a expression in allo-activated murine T cells (53). It is also possible that a subset of recipient immune cells that are radio-resistant such as regulatory T cells (54, 55) might contribute to the secretion of miR-29a in the serum. The experiments designed to determine if recipient derived miR-29a is one of the major drivers of aGVHD pathogenesis have been hampered by the poor breeding status of miR-29a KO mice. To circumvent this, we are pursuing experiments to generate transgenic mice that harbor a targeted deletion of miR-29a in the intestinal epithelial cells, which can be used as recipients that will allow us to further delineate the importance of miR-29a in aGVHD biology.
Using a murine model of aGVHD, we show that downregulation of miR-29a by using LNA anti miR-29a significantly improved survival and decreased clinical aGVHD severity while maintaining beneficial graft-versus-leukemia effects. We reason that knocking down serum miR-29a does not interfere with the GVL response as this therapeutic strategy will block miR-29a induced DC activation through TLR pathway allowing other mechanisms to activate APCs and contribute to GVL effects.
In this study, we discovered and validated that serum miR-29a is upregulated in aGVHD patients. Furthermore, we showed that serum miR-29a promotes DC maturation, migration and alloreactive T cell proliferation and establish miR-29a as a therapeutic target. This unrecognized function of circulating miRs in aGVHD provides a new paradigm to develop strategies to block the activity of these deleterious circulating miRs and prevent or treat aGVHD more effectively.
Supplementary Material
Acknowledgments
We thank Carlo M Croce at The Ohio State University for providing us with miR29a KO mice. We thank Jordan Smith for assisting in collection and processing of blood samples. Phylogeny Inc., Columbus, Ohio, performed LNA-ISH.
Footnotes
Author Contributions: P.R. designed experiments, performed in-vivo murine aGVHD experiments, serum RNA extraction and real-time PCR analyses, in-vitro experiments with mouse BMDCs and human PBMCs and DCs, analyzed the data, interpreted the results and wrote the manuscript. A.N. performed the human serum RNA isolation and real-time PCR analyses. P.L., N.Z. performed in-vivo murine aGVHD experiments, serum RNA extraction and real-time PCR analyses. X.Y. performed the confocal microscopy and immunoblotting. K.C. performed the RNA-IP experiments. L.C. contributed miR-29a KO mice. P.R., X.Y., S.G., and J.H. collected the blood and serum samples from patients. A.S. and S.V. provided statistical analyses. D.R. performed in-vivo murine aGVHD experiments at the University of Minnesota. M.F., Y.E., S.D., B.B. and R.G. were responsible for supervision of research and manuscript preparation. R.G. designed the study, supervised research, interpreted the data and wrote the manuscript with P.R.
Conflict of Interest: None to declare.
References
- 1.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12:443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrara JLM, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annual review of immunology. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 4.Sun K, Hsiao HH, Li M, Ames E, Bouchlaka M, Welniak LA, Hagino T, Jagdeo J, Pai CC, Chen M, Blazar BR, Abedi M, Murphy WJ. IFN-gamma receptor-deficient donor T cells mediate protection from graft-versus-host disease and preserve graft-versus-tumor responses after allogeneic bone marrow transplantation. Journal of immunology. 2012;189:2033–2042. doi: 10.4049/jimmunol.1102853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holtan SG, Pasquini M, Weisdorf DJ. Acute graft-versus-host disease: a bench-to-bedside update. Blood. 2014;124:363–373. doi: 10.1182/blood-2014-01-514786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paczesny S. Discovery and validation of graft-versus-host disease biomarkers. Blood. 2013;121:585–594. doi: 10.1182/blood-2012-08-355990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonhardt F, Grundmann S, Behe M, Bluhm F, Dumont RA, Braun F, Fani M, Riesner K, Prinz G, Hechinger AK, Gerlach UV, Dierbach H, Penack O, Schmitt-Graff A, Finke J, Weber WA, Zeiser R. Inflammatory neovascularization during graft-versus-host disease is regulated by alphav integrin and miR-100. Blood. 2013;121:3307–3318. doi: 10.1182/blood-2012-07-442665. [DOI] [PubMed] [Google Scholar]
- 8.Ranganathan P, Heaphy CE, Costinean S, Stauffer N, Na C, Hamadani M, Santhanam R, Mao C, Taylor PA, Sandhu S, He G, Shana'ah A, Nuovo GJ, Lagana A, Cascione L, Obad S, Broom O, Kauppinen S, Byrd JC, Caligiuri M, Perrotti D, Hadley GA, Marcucci G, Devine SM, Blazar BR, Croce CM, Garzon R. Regulation of acute graft-versus-host disease by microRNA-155. Blood. 2012;119:4786–4797. doi: 10.1182/blood-2011-10-387522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stickel N, Prinz G, Pfeifer D, Hasselblatt P, Schmitt-Graeff A, Follo M, Thimme R, Finke J, Duyster J, Salzer U, Zeiser R. MiR-146a regulates the TRAF6/TNF-axis in donor T cells during GVHD. Blood. 2014;124:2586–2595. doi: 10.1182/blood-2014-04-569046. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Romero M, Ratajczak P, Leboeuf C, Belhadj S, Peffault de Latour R, Zhao WL, Socie G, Janin A. Increased apoptosis is linked to severe acute GVHD in patients with Fanconi anemia. Bone marrow transplantation. 2013;48:849–853. doi: 10.1038/bmt.2012.237. [DOI] [PubMed] [Google Scholar]
- 11.Alevizos I, Illei GG. MicroRNAs as biomarkers in rheumatic diseases. Nature reviews Rheumatology. 2010;6:391–398. doi: 10.1038/nrrheum.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasemeier B, Christgen M, Kreipe H, Lehmann U. Reliable microRNA profiling in routinely processed formalin-fixed paraffin-embedded breast cancer specimens using fluorescence labelled bead technology. BMC biotechnology. 2008;8:90. doi: 10.1186/1472-6750-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG, Alevizos I. Exosomes from human saliva as a source of microRNA biomarkers. Oral diseases. 2010;16:34–38. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park NJ, Zhou H, Elashoff D, Henson BS, Kastratovic DA, Abemayor E, Wong DT. Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5473–5477. doi: 10.1158/1078-0432.CCR-09-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao B, Wang Y, Li W, Baker M, Guo J, Corbet K, Tsalik EL, Li QJ, Palmer SM, Woods CW, Li Z, Chao NJ, He YW. Plasma microRNA signature as a noninvasive biomarker for acute graft-versus-host disease. Blood. 2013;122:3365–3375. doi: 10.1182/blood-2013-06-510586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie LN, Zhou F, Liu XM, Fang Y, Yu Z, Song NX, Kong FS. Serum microRNA155 is increased in patients with acute graft-versus-host disease. Clin Transplant. 2014;28:314–323. doi: 10.1111/ctr.12314. [DOI] [PubMed] [Google Scholar]
- 17.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 18.Smith KM, Guerau-de-Arellano M, Costinean S, Williams JL, Bottoni A, Mavrikis Cox G, Satoskar AR, Croce CM, Racke MK, Lovett-Racke AE, Whitacre CC. miR-29a b1 deficiency identifies a negative feedback loop controlling Th1 bias that is dysregulated in multiple sclerosis. Journal of immunology. 2012;189:1567–1576. doi: 10.4049/jimmunol.1103171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2110–2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez de Silanes I, Galban S, Martindale JL, Yang X, Mazan-Mamczarz K, Indig FE, Falco G, Zhan M, Gorospe M. Identification and functional outcome of mRNAs associated with RNA-binding protein TIA-1. Molecular and cellular biology. 2005;25:9520–9531. doi: 10.1128/MCB.25.21.9520-9531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nuovo GJ. In situ detection of precursor and mature microRNAs in paraffin embedded, formalin fixed tissues and cell preparations. Methods. 2008;44:39–46. doi: 10.1016/j.ymeth.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Hanash AM, Kappel LW, Yim NL, Nejat RA, Goldberg GL, Smith OM, Rao UK, Dykstra L, Na IK, Holland AM, Dudakov JA, Liu C, Murphy GF, Leonard WJ, Heller G, van den Brink MR. Abrogation of donor T cell IL-21 signaling leads to tissue-specific modulation of immunity and separation of GVHD from GVL. Blood. 2011 doi: 10.1182/blood-2010-07-294785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy P, Teshima T, Hildebrandt G, Duffner U, Maeda Y, Cooke KR, Ferrara JL. Interleukin 18 preserves a perforin-dependent graft-versus-leukemia effect after allogeneic bone marrow transplantation. Blood. 2002;100:3429–3431. doi: 10.1182/blood-2002-04-1252. [DOI] [PubMed] [Google Scholar]
- 24.Teshima T, Hill GR, Pan L, Brinson YS, van den Brink MR, Cooke KR, Ferrara JL. IL-11 separates graft-versus-leukemia effects from graft-versus-host disease after bone marrow transplantation. J Clin Invest. 1999;104:317–325. doi: 10.1172/JCI7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsukada N, Kobata T, Aizawa Y, Yagita H, Okumura K. Graft-versus-leukemia effect and graft-versus-host disease can be differentiated by cytotoxic mechanisms in a murine model of allogeneic bone marrow transplantation. Blood. 1999;93:2738–2747. [PubMed] [Google Scholar]
- 26.Liston A, Papadopoulou AS, Danso-Abeam D, Dooley J. MicroRNA-29 in the adaptive immune system: setting the threshold. Cellular and molecular life sciences : CMLS. 2012;69:3533–3541. doi: 10.1007/s00018-012-1124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooke KR, Gerbitz A, Crawford JM, Teshima T, Hill GR, Tesolin A, Rossignol DP, Ferrara JL. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest. 2001;107:1581–1589. doi: 10.1172/JCI12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J, Jr, Crawford JM, Ferrara JL. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 29.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 30.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 31.Fabbri M, Paone A, Calore F, Galli R, Croce CM. A new role for microRNAs, as ligands of Toll-like receptors. RNA Biol. 2013;10:169–174. doi: 10.4161/rna.23144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fainaru O, Shseyov D, Hantisteanu S, Groner Y. Accelerated chemokine receptor 7-mediated dendritic cell migration in Runx3 knockout mice and the spontaneous development of asthma-like disease. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10598–10603. doi: 10.1073/pnas.0504787102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brain O, Owens BM, Pichulik T, Allan P, Khatamzas E, Leslie A, Steevels T, Sharma S, Mayer A, Catuneanu AM, Morton V, Sun MY, Jewell D, Coccia M, Harrison O, Maloy K, Schonefeldt S, Bornschein S, Liston A, Simmons A. The intracellular sensor NOD2 induces microRNA-29 expression in human dendritic cells to limit IL-23 release. Immunity. 2013;39:521–536. doi: 10.1016/j.immuni.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 34.Coghill JM, Carlson MJ, Panoskaltsis-Mortari A, West ML, Burgents JE, Blazar BR, Serody JS. Separation of graft-versus-host disease from graft-versus-leukemia responses by targeting CC-chemokine receptor 7 on donor T cells. Blood. 2010;115:4914–4922. doi: 10.1182/blood-2009-08-239848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He W, Racine JJ, Johnston HF, Li X, Li N, Cassady K, Liu C, Deng R, Martin P, Forman S, Zeng D. Depletion of host CCR7 (+) dendritic cells prevented donor T cell tissue tropism in anti-CD3-conditioned recipients. Biol Blood Marrow Transplant. 2014;20:920–928. doi: 10.1016/j.bbmt.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koyama M, Hashimoto D, Aoyama K, Matsuoka K, Karube K, Niiro H, Harada M, Tanimoto M, Akashi K, Teshima T. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood. 2009;113:2088–2095. doi: 10.1182/blood-2008-07-168609. [DOI] [PubMed] [Google Scholar]
- 37.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Tudor S, Giza DE, Lin HY, Fabris L, Yoshiaki K, D'Abundo L, Toale KM, Shimizu M, Ferracin M, Challagundla KB, Cortez MA, Fuentes-Mattei E, Tulbure D, Gonzalez C, Henderson J, Row M, Rice TW, Ivan C, Negrini M, Fabbri M, Morris JS, Yeung SC, Vasilescu C, Calin GA. Cellular and Kaposi's sarcoma-associated herpes virus microRNAs in sepsis and surgical trauma. Cell Death Dis. 2014;5:e1559. doi: 10.1038/cddis.2014.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–2759. [PubMed] [Google Scholar]
- 41.Johansson JE, Ekman T. Gut toxicity during hemopoietic stem cell transplantation may predict acute graft-versus-host disease severity in patients. Dig Dis Sci. 2007;52:2340–2345. doi: 10.1007/s10620-006-9404-x. [DOI] [PubMed] [Google Scholar]
- 42.Murphy S, Nguyen VH. Role of gut microbiota in graft-versus-host disease. Leuk Lymphoma. 2011;52:1844–1856. doi: 10.3109/10428194.2011.580476. [DOI] [PubMed] [Google Scholar]
- 43.Penack O, Holler E, van den Brink MR. Graft-versus-host disease: regulation by microbe-associated molecules and innate immune receptors. Blood. 2010;115:1865–1872. doi: 10.1182/blood-2009-09-242784. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Q, Costinean S, Croce CM, Brasier AR, Merwat S, Larson SA, Basra S, Verne GN. MicroRNA 29 targets nuclear factor-kappaB-repressing factor and Claudin 1 to increase intestinal permeability. Gastroenterology. 2015;148:158–169 e158. doi: 10.1053/j.gastro.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lv B, Liu Z, Wang S, Liu F, Yang X, Hou J, Hou Z, Chen B. MiR-29a promotes intestinal epithelial apoptosis in ulcerative colitis by down-regulating Mcl-1. Int J Clin Exp Pathol. 2014;7:8542–8552. [PMC free article] [PubMed] [Google Scholar]
- 46.Wang K, Yuan Y, Cho JH, McClarty S, Baxter D, Galas DJ. Comparing the MicroRNA spectrum between serum and plasma. PLoS One. 2012;7:e41561. doi: 10.1371/journal.pone.0041561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao S, Fung-Leung WP, Bittner A, Ngo K, Liu X. Comparison of RNA-Seq and microarray in transcriptome profiling of activated T cells. PLoS One. 2014;9:e78644. doi: 10.1371/journal.pone.0078644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calcaterra C, Sfondrini L, Rossini A, Sommariva M, Rumio C, Menard S, Balsari A. Critical role of TLR9 in acute graft-versus-host disease. Journal of immunology. 2008;181:6132–6139. doi: 10.4049/jimmunol.181.9.6132. [DOI] [PubMed] [Google Scholar]
- 49.Heidegger S, van den Brink MR, Haas T, Poeck H. The role of pattern-recognition receptors in graft-versus-host disease and graft-versus-leukemia after allogeneic stem cell transplantation. Front Immunol. 2014;5:337. doi: 10.3389/fimmu.2014.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Mellor AL, Munn DH, Blazar BR. Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood. 2009;114:5062–5070. doi: 10.1182/blood-2009-06-227587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor PA, Ehrhardt MJ, Lees CJ, Panoskaltsis-Mortari A, Krieg AM, Sharpe AH, Murphy WJ, Serody JS, Hemmi H, Akira S, Levy RB, Blazar BR. TLR agonists regulate alloresponses and uncover a critical role for donor APCs in allogeneic bone marrow rejection. Blood. 2008;112:3508–3516. doi: 10.1182/blood-2007-09-113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, Steinhoff U, Tchaptchet S, Thiel E, Freudenberg MA, Gobel UB, Uharek L. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010;59:1079–1087. doi: 10.1136/gut.2009.197434. [DOI] [PubMed] [Google Scholar]
- 53.Sun Y, Tawara I, Zhao M, Qin ZS, Toubai T, Mathewson N, Tamaki H, Nieves E, Chinnaiyan AM, Reddy P. Allogeneic T cell responses are regulated by a specific miRNA-mRNA network. J Clin Invest. 2013;123:4739–4754. doi: 10.1172/JCI70013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson BE, McNiff JM, Matte C, Athanasiadis I, Shlomchik WD, Shlomchik MJ. Recipient CD4+ T cells that survive irradiation regulate chronic graft-versus-host disease. Blood. 2004;104:1565–1573. doi: 10.1182/blood-2004-01-0328. [DOI] [PubMed] [Google Scholar]
- 55.Balogh A, Persa E, Bogdandi EN, Benedek A, Hegyesi H, Safrany G, Lumniczky K. The effect of ionizing radiation on the homeostasis and functional integrity of murine splenic regulatory T cells. Inflamm Res. 2013;62:201–212. doi: 10.1007/s00011-012-0567-y. [DOI] [PubMed] [Google Scholar]
- 56.Mi QS, Weiland M, Qi RQ, Gao XH, Poisson LM, Zhou L. Identification of mouse serum miRNA endogenous references by global gene expression profiles. PLoS One. 2012;7:e31278. doi: 10.1371/journal.pone.0031278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.