Abstract
Higher preoperative physical activity (PA) strongly predicts higher postoperative PA in bariatric surgery (BS) patients, providing rationale for preoperative PA interventions (PAIs). However, whether PAI-related increases can be maintained postoperatively has not been examined. This study compared PA changes across pre- (baseline, post-intervention) and postoperative (6-month follow-up) periods in participants randomized to 6 weeks of preoperative PAI or standard care control (SC). Of 75 participants initially randomized, 36 (PAI n=22; SC n=14) underwent BS. Changes in daily bout-related (≥10-minute bouts) moderate-to-vigorous PA (MVPA) and steps were assessed via the SenseWear Armband monitor. PAI received weekly counseling to increase walking exercise. Retention (86%) at postoperative follow-up was similar between groups. Intent-to-treat analyses showed that PAI vs. SC had greater increases across time (baseline, post-intervention, follow-up) in bout-related MVPA minutes/day (4.3±5.1, 26.3±21.3, 28.7±26.3 versus 10.4±22.9, 11.4±16.0, 18.5±28.2; p=.013) and steps/day (5163±2901, 7950±3286, 7870±3936 versus 5163±2901, 5601±3368, 5087±2603; p<.001). PAI differed from SC on bout-related MVPA at post-intervention (p=.016; d=.91), but not follow-up (p=.15; d=.41), and steps at post-intervention (p=.031; d=.78) and follow-up (p=.024; d=.84). PAI participants maintained preoperative PA increases postoperatively. Findings support preoperative PAIs and research to test whether PA changes can be sustained and influence surgical outcomes beyond the initial postoperative period.
Keywords: physical activity, exercise, maintenance, obesity, bariatric surgery
INTRODUCTION
Most bariatric surgery (BS) patients rarely engage in structured or purposeful exercise, reflected by little or no accumulation of daily or weekly moderate-to-vigorous physical activity in bouts lasting ≥10 minutes (i.e. bout-related MVPA).1,2 While research in both non-surgical and BS populations suggests that engagement in bouts of MVPA as short as 10 minutes towards a daily total might have important health and/or weight loss benefits for BS patients2–6, roughly two-thirds do not perform any bout-related MVPA and nearly all (95%–98%) are inactive according to public health recommendations (i.e. performing <150 bout-related MVPA minutes/week3) preoperatively.7–10 Further, patients demonstrate only small bout-related MVPA changes postoperatively, thereby continuing their inactive lifestyle.8–10
Prospective research shows that higher MVPA preoperatively predicts higher MVPA and improved BS outcomes postoperatively9–12, providing rationale for preoperative PA interventions (PAI). We recently reported findings from the Bari-Active trial13, which tested a 6-week PAI versus standard pre-surgical care control (SC) on objectively-measured pre- to post-intervention changes in bout-related MVPA. At post-intervention, PAI (n=40) on average achieved a near five-fold increase in bout-related MVPA (4.4 to 21.0 minutes/day), whereas SC (n=35) showed no change (7.9 to 7.6 minutes/day; PAI versus SC, p=.001). Similarly, daily steps increased by an average of 2028 (4908 to 6935) in PAI compared to only 203 (4629 to 4832) in SC. Although findings show that patients can achieve marked PA increases preoperatively, it is important to determine whether these higher PA levels are maintained postoperatively.
The current study compared changes in bout-related MVPA and steps across pre- (baseline, post-intervention) and postoperative (6-month postoperative follow-up) time points in a sub-sample of Bari-Active trial participants who underwent BS. We hypothesized PAI would demonstrate greater increases in bout-related MVPA and steps across time and higher levels of these types of activity at post-intervention and 6-month postoperative follow-up versus SC.
METHODS
Participants
Thirty-six participants were both randomized to PAI or SC and underwent BS. Initial eligibility criteria included: aged 18–70 years, body mass index (BMI) ≥35 kg/m2, and inactive but able to walk ≥2 blocks (~161 meters) unassisted. Individuals were ineligible if scheduled for BS <10 weeks during screening, participating in another PA/weight loss program, intending to relocate geographically, or had issues (e.g., relocation, substance abuse/severe psychiatric condition, language barriers) that could interfere with protocol adherence.
Design/Procedures
BS-seeking individuals were referred by surgical clinics in Rhode Island, USA between April 2010 and January 2014. During a clinic visit, patients received a study flyer. Those who expressed desire to participate and received surgeon approval to perform walking exercise underwent telephone screening. Eligible patients were invited to an orientation/baseline session where they provided informed consent, had height and weight measured, and were given a SenseWear armband (SWA) monitor to wear for the next 7 days. Participants were then randomly assigned 1:1 to 6 weeks of PAI or SC using a computer-generated random-permuted blocking procedure. Participants wore the activity monitor again at post-intervention and postoperative follow-up. Participants received $100 for completing all assessments. The Miriam Hospital IRB approved study procedures (clinicaltrials.gov registration NCT00962325).
Interventions
PAI
Beginning 1 week after baseline and completion of 7 days of activity monitoring, participants received 6 consecutive weekly individual face-to-face counseling sessions, weekly PA monitoring/planning logs, and a pedometer to increase bout-related, moderate-intensity walking by 30 minutes/day and steps by 5,000/day. Participants recorded bout-related walking minutes and steps at the end of each day. During week 1, participants maintained usual PA patterns to determine baseline values and tailored weekly goal progression. For week 2, 10 additional bout-related walking minutes/day and 1,000 steps/day above baseline were prescribed. For each subsequent week, 5 daily bout-related walking minutes and 1,000 daily steps were added to the previous week’s prescription.
Counseling sessions were conducted by the primary author and focused on: reviewing self-monitoring records and progress towards goals; and problem-solving barriers, teaching behavior change strategies, and developing action plans to achieve goals (see Bond et al.13 for a detailed description). At postoperative follow-up, participants only received additional monitoring/planning logs and a copy of the behavioral contract (including postoperative PA goals) created during the final intervention week prior to 7 days of PA monitoring.
SC
Like PAI, SC participants attended scheduled clinical visits to receive standard surgical care. They were advised to being exercising, but received no PA prescription involving specific activities and performance requirements or strategies to adopt this behavior.
Measures
Objectively-measured PA
The SWA monitor (BodyMedia, Inc., Pittsburgh PA) measured changes in MVPA minutes/day and steps/day. The SWA integrates data from a tri-axial accelerometer and physiologic sensors to estimate energy expenditure (EE) and intensity of activities. The SWA demonstrates acceptable agreement with criterion EE measures and provides MVPA estimates comparable to other objective monitors.14–16 Participants received instructions to wear the SWA during waking hours for 7 consecutive days at all assessments. The SenseWear Professional Software (Version 7.0) calculated wear time. Metabolic equivalent (MET) values determined daily minutes spent in bout-related MVPA (≥3 METs). Allowance of ≥1 minute <3 METs was employed in calculating bout-related MVPA (i.e. accumulated in bouts ≥10-min). Monitor wear time of ≥6 hours/day on ≥4 days for all assessments was required for inclusion in analysis.13 We have previously shown that this criterion maximizes sample size/data inclusion while providing estimates of bout-related MVPA that are similar to those when more stringent wear time criteria are employed.13
Other measures
Demographics information (i.e. age, sex, race/ethnicity, education) was collected via questionnaire at baseline. Weight was measured at each of the assessment points.
Statistical Analysis
Analyses were performed using SPSS Statistics for Windows (version 20.0; SPSS, IBM Corp, Armonk, NY). Means and standard deviations (SD) and frequency counts were calculated to describe participant characteristics. Repeated-measures analysis of variance (ANOVA) compared PAI versus SC on PA across assessments. Planned comparisons of PAI versus SC on MVPA (bout-related) and steps at post-intervention and postoperative follow-up were conducted using independent t-tests. Corrected p values were used when variances were not equal. The intent-to-treat (ITT) principle in which missing data at post-intervention follow-up were substituted with baseline values (assuming no change) was followed. Cohen’s d was calculated to determine effect size between group means at specific assessment points. Significance tests were two-tailed, α=.05. This study involves a secondary analysis of postoperative follow-up changes collected as part of a randomized controlled trial powered to detect a difference of 11 bout-related MVPA minutes/day in PAI versus SC at post-intervention with n=75.
RESULTS
Recruitment/retention
Recruitment/retention data have been reported previously.13 Of 293 patients screened by telephone, 213 were excluded. Eighty participants were randomized, and 5 were inactivated (surgery scheduled prior to study completion [n=4], family emergency [n=1]). Of the remaining 75 participants, 63 completed the post-intervention assessment, and 36 (48%; PAI=22 and SC=14) underwent BS. Reasons for not having BS included: personal decision not to have BS (51.1%), denied insurance/surgeon approval (27.9%), family issues/emergencies (17.1%), and relocation (3.9%). Of the 36 participants who underwent BS, 31 (86.1%) completed postoperative follow-up, with no group differences (p=.96). Study completers and non-completers were similar on all baseline demographic, weight, and PA characteristics (ps>.20).
Participant characteristics
Table 1 shows participants’ baseline characteristics: PAI and SC were similar on all variables (ps>.20). On average, participants were middle-aged with severe obesity (mean BMI=46 kg/m2). Most were female, Non-Hispanic White, and approximately 40% had ≥4-year college degree. Participants had low PA, averaging less than a single ≥10-minute MVPA bout and 5127 steps per day.
Table 1.
Baseline characteristics of participants who were randomized to preoperative Physical Activity Intervention (PAI) or Standard Surgical Care Control (SC) and underwent bariatric surgery
| Full Sample (N = 36) |
PAI Condition (N = 22) |
SC Condition (N = 14) |
|
|---|---|---|---|
| Demographic and anthropometric characteristics | |||
| Sex (%) | |||
| Men | 13.9 | 9.1 | 21.4 |
| Women | 86.1 | 90.9 | 78.6 |
| Age, mean (SD), years | 47.0 (8.2) | 46.4 (9.1) | 47.9 (6.8) |
| Race (%) | |||
| Native Hawaiian/Other Pacific Islander | 2.8 | 4.5 | 0.0 |
| Black | 5.6 | 4.5 | 7.1 |
| White | 86.1 | 90.9 | 78.6 |
| Other | 5.6 | 0.0 | 14.3 |
| Ethnicity (%) | |||
| Hispanic | 0.0 | 0.0 | 0.0 |
| Education (%) | |||
| ≥ 4-year college/university degree | 38.9 | 40.9 | 35.7 |
| Body Mass Index, mean (SD), kg/m2 | 45.8 (7.1) | 46.7 (7.1) | 44.4 (7.1) |
| Physical Activity | |||
| Total MVPA minutes/day, mean (SD) | 34.7 (30.9) | 32.0 (23.1) | 38.8 (41.0) |
| Bout-related MVPA minutes/day, mean (SD) | 6.7 (14.8) | 4.3 (5.1) | 10.4 (22.9) |
| Steps/day, mean (SD) | 5127 (2784) | 5163 (2901) | 5069 (2696) |
Note. No significant differences for PAI vs. SC (ps> .20)
Average time from post-intervention to surgery and from surgery to postoperative follow-up was 84.3±70.9 and 201.3±61.0 days, respectively. Regarding BS procedures, 18 (50%) participants underwent Roux-en-Y gastric bypass, 16 (44%) gastric banding, and 2 (6%) gastric sleeve. Percent weight loss averaged 25.9±9.8% among the 32 (88.9%) participants for whom postoperative weights were available, and did not significantly differ by condition (PAI: 24.5±8.5% vs. SC: 30.1±10.7%, p=.139).
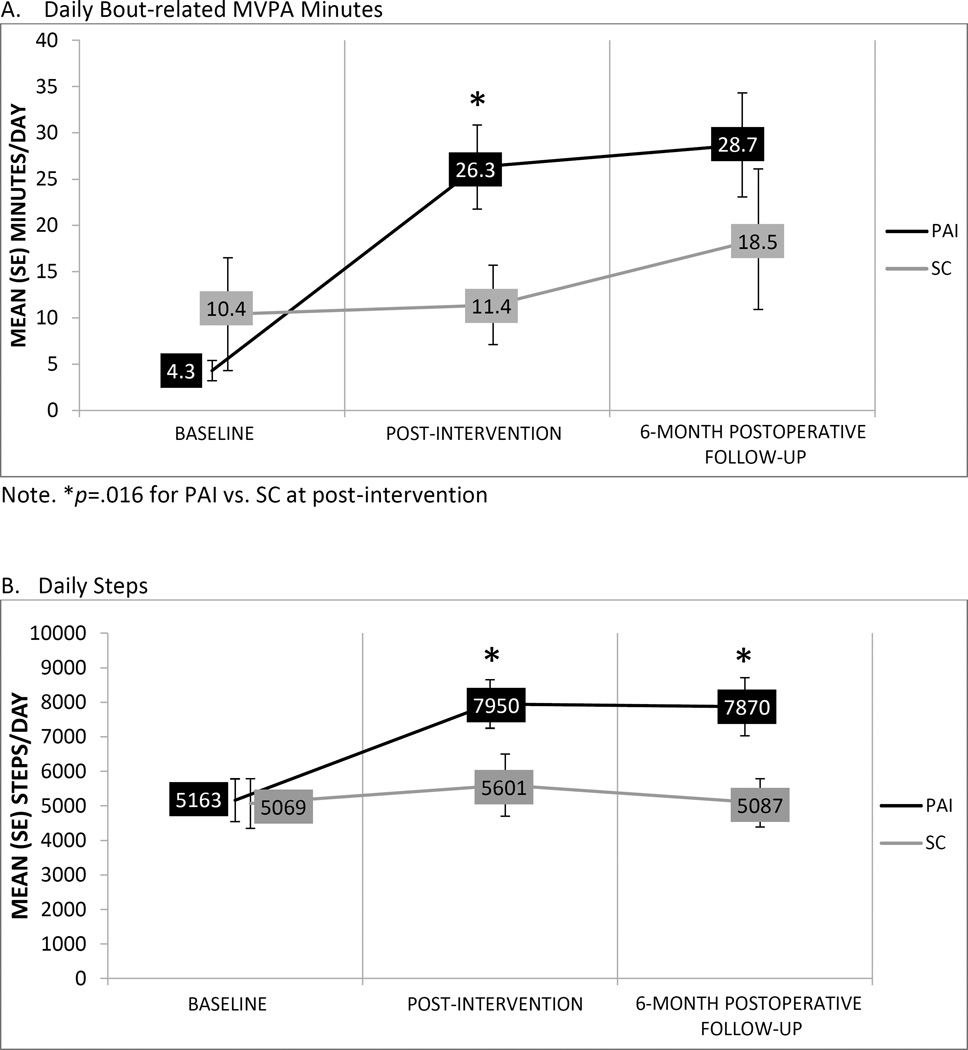
Changes in PA
Figure 1A–B shows changes in daily bout MVPA minutes and steps. PAI vs. SC had greater increases across the study period in bout-related MVPA minutes/day and steps/day. PAI differed from SC on bout-related MVPA at post-intervention (p=.016; d=.91), but not follow-up (p=.15; d=.47), and steps at post-intervention (corrected p=.031; d=.78) and follow-up (p=.024; d=.84).
Figure.
Changes in daily bout-related MVPA and steps across baseline, post-intervention and 6-month postoperative follow-up assessments in the Physical Activity Intervention (PAI) and Standard Surgical Care Control (SC) conditions
DISCUSSION
This study is the first to examine whether intervention-related increases in objectively-measured PA preoperatively can be maintained postoperatively. PAI achieved significantly greater increases in preoperative bout-related MVPA and steps from baseline to post-intervention versus SC, which were maintained at 6-month postoperative follow-up. These findings correspond with observational research showing positive correlations between pre- and postoperative PA9–10 and provide support for behavioral strategies aimed at increasing preoperative PA.
Interestingly, SC increased bout-related MVPA but not overall ambulatory movement (i.e. steps) from post-intervention to postoperative follow-up, whereas PAI maintained post-intervention levels of both types of activity. Consequently, the effect size for the group difference decreased from large to medium for bout-related MVPA and remained large for steps. This suggests that SC increased time spent exercising postoperatively but compensated with less overall movement, whereas PAI maintained a high exercise level and also moved more versus SC.
While PAI were successful in maintaining levels of bout-related MVPA (~200 minutes/week) that were markedly higher than baseline (~30 minutes/week), similar to post-intervention (~184 minutes/week), and above public health guidelines (≥150 bout-related MVPA minutes/week2), even greater amounts may be necessary to enhance weight loss and limit subsequent weight regain after BS.17–18 Thus, prescription of higher PA goals may be required.19 Nonetheless, recent data suggest that the volume of bout-related MVPA performed by PAI can yield clinically-relevant improvements in metabolic health parameters postoperatively.17,20
This study has limitations. Less than half (48%) of participants initially randomized underwent BS, limiting sample size. However, investigators had no influence over participants’ BS decision-making process. Interestingly, 55% of PAI vs. 40% of SC had BS: while not statistically significant (p=.107), this difference warrants additional research to determine whether preoperative interventions contribute to higher BS frequency, possibly through enhanced sense of preparation, motivation, and confidence in ability to adhere to postoperative behavioral recommendations. The small sample, variability in surgical procedures, and differences in number of participants between groups limited ability to test differences in weight loss between conditions. It is unclear whether intervention-related preoperative PA increases can be maintained or enhanced beyond 6-months postoperatively.
In summary, this study shows that BS patients can not only achieve marked increases in PA preoperatively with behavioral intervention, but can sustain these higher PA levels postoperatively. Preoperative PA intervention studies designed and sufficiently powered to test efficacy for enhancing and maintaining BS-related weight, metabolic, and quality of life outcomes are needed.
Acknowledgments
This work was supported by NIH grant DK083438 (PI: Bond)
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Trial Registration: clinicaltrials.gov Identifier: NCT00962325
REFERENCES
- 1.Bond DS, Thomas JG. Measurement and intervention on physical activity and sedentary behaviors in bariatric surgery patient: emphasis on mobile technology. Eur Eat Disord Rev. 2015;23:470–478. doi: 10.1002/erv.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catenacci VA, Grunwald GK, Ingebrigtsen JP, Jakicic JM, McDermott MD, Phelan S, et al. Physical activity patterns using accelerometry in the National Weight Control Registry. Obesity (Silver Spring) 2010;19:1163–1170. doi: 10.1038/oby.2010.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendations for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 4.Strath SJ, Holleman RG, Ronis DL, Swartz AM, Richardson CR. Objective physical activity accumulation in bouts and nonbouts and relation to markers of obesity in US adults. Prev Chronic Dis. 2008;5:A131. [PMC free article] [PubMed] [Google Scholar]
- 5.Jakicic JM, Tate DF, Lang W, Davis KK, Polzien K, Neiberg RH, et al. Objective physical activity and weight loss in adults: the step-up randomized clinical trial. Obesity (Silver Spring) 2014;22:2284–2292. doi: 10.1002/oby.20830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josbeno DA, Kalarchian M, Sparto PJ, Otto AD, Jakicic JM. Physical activity and physical function in individuals post-bariatric surgery. Obes Surg. 2011;21:1243–1249. doi: 10.1007/s11695-010-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bond DS, Jakicic JM, Vithiananthan S, Thomas JG, Leahey TM, Sax HC, et al. Objective quantification of physical activity in bariatric surgery candidates and normal-weight controls. Surg Obes Relat Dis. 2010;6:72–78. doi: 10.1016/j.soard.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bond DS, Jakicic JM, Unick JL, Vithiananthan S, Pohl D, Roye GD, et al. Pre- to postoperative changes in bariatric surgery patients: self-report vs. objective measures. Obesity (Silver Spring) 2010;18:2395–2397. doi: 10.1038/oby.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King WC, Hsu JY, Belle SH, Courcoulas AP, Eid GM, Flum DR, et al. Pre- to postoperative changes in physical activity: report from the longitudinal assessment of bariatric surgery-2 (LABS-2) Surg Obes Relat Dis. 2012;8:48–59. doi: 10.1016/j.soard.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King WC, Chen JY, Bond DS, Belle SH, Courcoulas AP, Patterson EJ, et al. Objective assessment of changes in physical activity and sedentary behavior: pre- through 3 years post-bariatric surgery. Obesity (Silver Spring) 2015;23:1143–1150. doi: 10.1002/oby.21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bond DS, Phelan S, Wolfe LG, Evans RK, Meador JG, Kellum JM, et al. Becoming physically active after bariatric surgery is associated with improved weight loss and health-related quality of life. Obesity (Silver Spring) 2009;17:78–83. doi: 10.1038/oby.2008.501. [DOI] [PubMed] [Google Scholar]
- 12.Browning MG, Baugh NG, Wolfe LG, Kellum JK, Maher JW, Evans RK. Evaluation of pre- and postoperative physical activity participation in laparoscopic adjustable gastric banding patients. Obes Surg. 2014;24:1981–1986. doi: 10.1007/s11695-014-1283-1. [DOI] [PubMed] [Google Scholar]
- 13.Bond DS, Vithiananthan S, Thomas JG, Trautvetter J, Unick JL, Jakicic JM, et al. Bari-Active: a randomized controlled trial of a preoperative intervention to increase physical activity in bariatric surgery patients. Surg Obes Relat Dis. 2015;11:169–177. doi: 10.1016/j.soard.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackey DC, Manini TM, Schoeller DA, Koster A, Glynn NW, Goodpaster BH, et al. Validation of an armband to measure daily energy expenditure in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:1108–1113. doi: 10.1093/gerona/glr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unick JL, Bond DS, Jakicic JM, Vithiananthan S, Pohl D, Roye GD, et al. Comparison of two objective monitors for assessing physical activity and sedentary behaviors in bariatric surgery patients. Obes Surg. 2012;22:347–352. doi: 10.1007/s11695-011-0491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berntsen S, Hageberg R, Aanstad A, Mowinckel P, Anderssen SA, Carlsen KH, et al. Validity of physical activity monitors in adults participating in free-living activities. Br J Sports Med. 2010;44:657–664. doi: 10.1136/bjsm.2008.048868. [DOI] [PubMed] [Google Scholar]
- 17.Woodlief TL, Carnero EA, Standley RA, Distefano G, Anthony SJ, Dubis GS, et al. Dose response of exercise training following roux-en-Y gastric bypass surgery: A randomized trial. Obesity (Silver Spring) 2015;23:2454–2461. doi: 10.1002/oby.21332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 19.Jeffrey RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr. 2003;78:684–689. doi: 10.1093/ajcn/78.4.684. [DOI] [PubMed] [Google Scholar]
- 20.Coen PM, Tanner CJ, Helbling NL, Dubis GS, Hames KC, et al. Clinical trial demonstrates exercise following bariatric surgery improves insulin sensitivity. J Clin Invest. 2015;125:707–715. doi: 10.1172/JCI78016. [DOI] [PMC free article] [PubMed] [Google Scholar]