Abstract
Background
Green tea extract (GTE) may be involved in a favorable postprandial response to high-carbohydrate meals. Catechol-O-methyltransferase (COMT) genotype may modify these effects. We examined the acute effects of GTE supplementation on postprandial response to a high-carbohydrate meal through assessing appetite-associated hormones and glucose homeostasis marker concentrations in women who consumed 843 mg (−)-epigallocatechin-3-gallate [EGCG]) or placebo capsules for 11-12 months.
Methods
Sixty Caucasian postmenopausal women (BMI ≥ 25.0 kg/m2) were included in a randomized, double-blind feeding study. GTE was consumed with a breakfast meal (665.4 kcal; 67.2% carbohydrate). Blood samples were drawn pre-meal, post-meal, and every 30 minutes for 4 h. Participants completed six satiety questionnaires.
Results
Plasma leptin, ghrelin, and adiponectin did not differ between GTE and placebo at any time point; COMT genotype did not modify these results. Participants randomized to GTE with the high-activity form of COMT (GTE-high COMT) had higher insulin concentrations at time 0, 0.5, and 1.0 h post-meal compared to all COMT groups randomized to placebo. Insulin remained higher in the GTE-high COMT group at 1.5, 2.0, and 2.5 h compared to Placebo-low COMT (P < 0.02). GTE-high COMT had higher insulin concentrations at times 0, 0.5, 1.0, 1.5, and 2.0 h compared to the GTE-low COMT (P ≤ 0.04). AUC measurements of satiety did not differ between GTE and placebo.
Conclusions
GTE supplementation and COMT genotype did not alter acute postprandial responses of leptin, ghrelin, adiponectin, or satiety, but may be involved in post-meal insulinemic response of overweight and obese postmenopausal women.
Keywords: green tea, obesity, postprandial response, insulin sensitivity, postmenopausal women, satiety
INTRODUCTION
Overweight and obesity are major public health concerns, with worldwide obesity rates having doubled since 1980 (1). Obesity is associated with several serious health conditions, including cardiovascular disease, diabetes, and select cancers, including postmenopausal breast cancer (2-4). Studies have suggested that green tea and its high content of polyphenolic catechins may reduce risk for these diseases in part via beneficial effects on body weight, as green tea consumption has been shown to modestly reduce body weight and adiposity in many randomized controlled trials (RCTs) and one meta-analysis (5-8). Among the catechins present in green tea, the four most prominent types are epicatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECG), and (−)-epigallocatechin-3-gallate (EGCG). EGCG has been the most widely studied and is thought to be the most bioactive catechin (9). However, these results have not been consistent across varying study designs and populations, as several RCTs and one Cochrane review have shown no clinically meaningful weight loss or weight maintenance effects with green tea supplementation (7,10,11).
Numerous mechanisms have been proposed for the anti-obesity effects of green tea catechins (GTC), including increases in β-oxidation and thermogenesis (12-14) and reductions in adipocyte differentiation and proliferation, lipogenesis, and nutrient absorption (9,15-17), as demonstrated in in vitro, animal, and human experiments. Another mechanism by which GTC may reduce body weight or promote weight maintenance is through modifying several hormones associated with energy balance, the postprandial glycemic response, and satiety. In humans, the gut-derived hormone ghrelin, as well as adipose-derived adiponectin and leptin, have been shown to be involved in appetite cues and satiety after ingestion of a meal, respectively. Insulin has also been associated with energy balance (18), and several RCTs have demonstrated increased insulin sensitivity with consumption of GTC (19-21). However, few human studies examining the immediate postprandial effects of GTC on these hormones have been conducted (22,23). To date, results are inconclusive, with one study finding no differences in area under the curve (AUC) for glucose and insulin after meal consumption, though higher satiety was reported among participants (22), while another single-arm study did not provide a control group for comparison of results (23).
The proposed beneficial effects of GTC on body weight and adiposity may be further modulated by the catechol-O-methyltransferase (COMT) enzyme, one of the main enzymes responsible for catechin degradation as well as metabolism of catecholamines, including norepinephrine. The gene encoding COMT is polymorphic and its alleles correspond to different activity levels of the enzyme: A/A = homozygous low-activity, A/G = heterozygous intermediate-activity, and G/G = homozygous high-activity. An amino acid change from valine to methionine at codon 108/158 reduces the thermostability of the enzyme and lowers its enzymatic activity by 66-75% (24,25). These differences in activity are thought to affect individual variation in metabolism of GTC, thus potentially influencing the biological effects of green tea consumption on weight loss and weight control. In addition, GTC have been shown to inhibit the action of COMT in vitro, which is significant given that COMT is also responsible for the metabolism of norepinephrine, a potent sympathetic nervous system stimulant. Reduced activity of COMT may prolong the effects of norepinephrine on increasing thermogenesis and satiety (26,27).
The primary aim of the present study was to determine the effect of a decaffeinated green tea extract (GTE) containing 1315 mg total catechins/day (843 mg as EGCG) on postprandial concentrations of appetite-related hormones and blood glucose in overweight and obese postmenopausal women. Secondary aims were to assess GTE's effects on satiety and to evaluate the modification of these effects by COMT genotype. We hypothesized that GTE supplementation would cause favorable changes in hormone concentrations and increase postprandial satiety, and that women with the low-activity genotype (A/A) would have a more favorable response to GTE consumption compared to those with the intermediate- (A/G) or high-activity (G/G) COMT genotypes, due to greater exposure to GTC.
MATERIALS AND METHODS
Study Design
This ancillary study was conducted in a subset of overweight and obese women enrolled in the Minnesota Green Tea Trial (MGTT), a phase II, randomized, double blind, placebo-controlled, intervention study described in detail elsewhere (28). In the parent study, healthy postmenopausal women at high-risk of breast cancer due to increased mammographic density were randomized by COMT genotype to consume either four decaffeinated (< 16 mg caffeine/day) GTE capsules containing a total of 1315 ± 116 mg GTC (843 ± 44 mg as EGCG) or placebo capsules daily for 12 months to determine the effects of GTE exposure on several breast cancer biomarkers including mammographic density, reproductive hormones, oxidative stress, and insulin-like growth factor (IGF) axis proteins.
To evaluate the acute postprandial effects of GTE consumption following a standardized meal, 60 subjects (10 from each treatment/genotype group) were invited to participate during month 11 or 12 of the 12-month parent study. This research took place during a half-day clinic visit at the University of Minnesota's Delaware Clinical Research Unit. For the half-day visit, participants were instructed to adhere to their normal energy intake and to refrain from exercise and alcohol the day before the test day. They arrived at the research unit after a 10-hour fast. Baseline satiety questionnaires were completed and fasting blood drawn for assessment of the energy-related hormones. Participants were then instructed to consume two GTE capsules and a standardized high-carbohydrate breakfast meal (consisting of a bagel with cream cheese, orange juice, and low-fat, fruit-flavored yogurt or 2% milk). Blood was drawn immediately post-meal and every 30 minutes over a period of 4 hours for evaluation of change in energy- and obesity-related hormones. In order to minimize external influencers of satiety, each test meal session was conducted independently and the participant and test meal administrator were the only individuals in the room. Participants were allowed to bring in outside materials to read during their session, though instructions were not given to avoid food-related content. Satiety questionnaires were completed before the meal, immediately after consumption of the meal, and every hour thereafter for a total of six questionnaires.
Participant recruitment and eligibility criteria
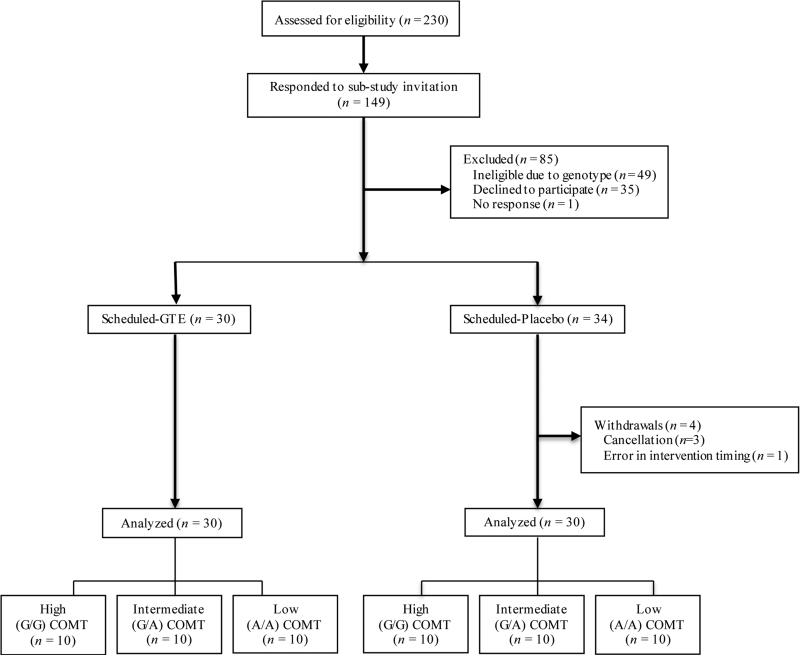
Participant eligibility, screening, recruitment, and randomization followed the same protocol as the parent study (28). Primary inclusion criteria included healthy, non-smoking postmenopausal women aged 50-70 years classified as having “heterogeneously dense” or “extremely dense” breast tissue by a trained radiologist after a routine screening mammogram. Of 1075 women randomized into the parent study, 230 women classified as overweight (BMI = 25.0-29.9 kg/m2) or obese (BMI = 30.0-40.0 kg/m2) at the screening clinic visit were invited to participate in this acute feeding study from 2012 to 2014. Of these, 149 participants responded to the invitation, 50 were excluded due to the need for equal numbers in each COMT genotype group. An additional 38 women did not participate for other reasons (Figure 1). Sixty-one participants were randomized into the study (GTE: n=30; placebo: n=31). One participant in the placebo group was excluded due to not consuming the intervention product with the breakfast meal, so the final sample size was 30 participants per treatment group.
Figure 1. Flow of overweight obese postmenopausal Minnesota Green Tea Trial participants randomized to GTE or Placebo for 12 months.
This figure depicts the flow of Minnesota Green Tea Trial (MGTT) participants through the sub-study of the postprandial response to a high-carbohydrate meal.
Randomization, blinding, and participant consent
Randomization of subjects was performed by the University of Minnesota Medical Center-Fairview's Investigational Drug Service (IDS) pharmacy using a computer-generated permuted block randomization scheme. Both participants and investigators were blinded to the treatment of subjects. Institutional Review Board (IRB) approval was obtained at each clinical center. All participants provided additional written informed consent for this ancillary study. This trial was registered at clinicaltrials.gov as NCT00917735.
Determination of COMT genotype
DNA was purified from buffy coats of peripheral blood samples using a PureGene Blood kit (Gentra Systems, Minneapolis, MN). A TaqMan assay was developed for determining the COMT G/A polymorphism using a TaqMan PCR Core Reagent kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. PCR amplification using ~10 ng of genomic DNA was performed in a thermal cycler (MWG Biotech, High Point, NC) with an initial step of 95°C for 10 min followed by 50 cycles of 95°C for 25 s and 62°C for 1 min. The fluorescence profile of each well was measured in an ABI 7900HT Sequence Detection System and the results analyzed with Sequence Detection Software (Applied Biosystems). Experimental samples were compared with 12 controls to identify the three genotypes at each locus (G/G, G/A, and A/A).
GTE Composition
This study used decaffeinated Green Tea Extract Catechin Complex (referred to as GTE in this publication) in capsule form, provided by Corban Laboratories (Eniva Nutraceutics, Plymouth, MN). Mean daily catechin content was 1315 ± 116 mg/day (843 ± 44 mg as EGCG). Placebo capsules were identical in appearance to GTE and contained 816 mg maltodextrin, 808 mg cellulose, and 8 mg magnesium stearate (flow agent). Participants were required to consume four capsules daily and were advised to ingest 2 capsules in the morning hours and 2 in the evening to maintain circulating catechin concentrations throughout each day, and to take the capsules with meals to reduce any potential gastrointestinal discomfort associated with consuming GTE in the fasted state. For this sub-study, participants were asked to consume the morning dose of GTE with the breakfast meal.
Demographics data collection
All participants completed a health history questionnaire upon entry into the parent study that included comprehensive data of demographics, lifestyle factors (physical activity, smoking history, and alcohol intake), and information about medical history, medication use (current and former), and full reproductive history.
Dietary Intake
To evaluate dietary intake participants were asked to record food intake on two assigned weekdays and one weekend day in the week prior to the half-day visit. Recording errors were minimized by providing the subjects detailed instructions on how to keep accurate diet records. Subjects were encouraged to measure foods eaten using measuring spoons and cups whenever possible. Diet records were then reviewed for accuracy and completeness during the clinic visit by a Registered Dietitian Nutritionist and later analyzed for nutrient content using the Food Processor Diet Analysis and Fitness software, version 10.10 (ESHA Research, Salem, OR). The average of the three days was used to represent typical food and nutrient intake.
Test meal composition
The high-carbohydrate test meal consisted of a plain bagel (300 kcal, 59.4 g carbohydrate, 11.6 g protein, 1.8 g fat), 28.5 g cream cheese spread (84 kcal, 0.1 g carbohydrate, 2 g protein, 8.1 g fat), 8 ounces (240 mL) orange juice (122 kcal, 28.7 g carbohydrate, 1.7 g protein, 0.3 g fat), and choice of 6 ounces (170 g) low-fat fruit yogurt (183 kcal, 34.5 g carbohydrate, 7.4 g protein, 2.1 g fat) or 240 mL 2% milk (138 kcal, 13.5 g carbohydrate, 9.7 g protein, 4.9 g fat). Mean total energy content was 665.4 kcal; macronutrient content was as follows: carbohydrate = 113.3 g (67.2%); protein = 23.9 g (14.3%); and fat = 10.0 g (18.5%). Participants were instructed to take two GTE or placebo capsules with the test meal.
Biological sample analyses
Plasma adiponectin, leptin, and ghrelin were measured using radioimmunoassay kits manufactured by EMD Millipore (Billerica, MA) (inter-assay % coefficient of variation [% CV]: leptin = 9.8%, adiponectin = 8.6%, ghrelin = 6.2%; intra-assay % CV: leptin = 10.1%; adiponectin = 7.7%, ghrelin = 8.1%). Serum insulin was measured using a simultaneous one-step immunoenzymatic, chemiluminescent assay (Access Ultrasensitive Insulin assay, Quest Diagnostics, Wood Dale, IL, intra-assay % CV: 3-5.0%; inter-assay % CV = 3.9%). Serum glucose concentrations were measured using a hexokinase enzymatic reference method (Quest Diagnostics, Wood Dale, IL, monthly % CV = 1.4%).
Assessment of satiety
To test satiety, a set of nine visual analogue scale (VAS) ratings associated with the standardized high-carbohydrate test meal was used. Questionnaires were completed pre-meal, post-meal (time 0), and hourly thereafter and were administered after blood draws at these time points. Participants were asked to answer each separate VAS question relating to hunger, fullness, desire to eat, prospective consumption, perceived satiety, contentedness, irritability, sleepiness, and mental alertness in a continuous linear scale from 0 to 10 centimeters, where 0 represented “not/none at all” and 10 represented “extremely/very”. The VAS is considered to be a valid and reliable assessment tool for monitoring the effects of energy, palatability, and macronutrient manipulations on subjective ratings in appetite studies (29,30).
Statistical analyses
Power calculations were unable to be performed for the half-day postprandial sub-study, since there was no data available on these outcomes at that time. We chose 60 participants as a feasible number that was comparable to similar studies examining energy-related hormones in response to a specific dietary component. We aimed to have equal numbers of participants with each COMT genotype in each treatment group, and while this reduced the power for between-genotype comparisons, it should be noted that these results were intended to be exploratory in nature.
Demographic characteristics of participants at baseline were compared between treatments using a one-sample t-test for continuous variables. Natural logarithmic transformation was considered to normalize the distribution of these variables. Chi-square and Fisher exact tests were used to compare the distribution of categorical variables between treatments. Change in hormones (leptin, ghrelin, adiponectin, and insulin) and blood glucose over time was evaluated using linear regression with repeated measurements. Hormones and blood glucose were transformed using natural logarithms to normalize their distribution. The model included time, treatment, COMT genotype and the 2-way and 3-way interactions as explanatory variables. Interactions were excluded from the model if non-significant at P-value < 0.05, using backward elimination. When statistically significant differences were detected, a post hoc pairwise comparison across treatment and COMT genotype groups was performed. An unstructured working correlation matrix was fitted to model the correlation between time points within participants; this was chosen based on Akaike's information criterion. The area under the curve (AUC) of the VAS at different time points before and after the meal was calculated for each participant and each VAS question using the trapezoid method. The AUC of each question and participant were used as dependent variables in a linear mixed model to evaluate the effect of treatment on the AUC of each question. Baseline VAS (pre-meal) was used as a random effect in the model. Model assumptions were evaluated using residual plots and Cook's distance greater than 0.5 was used to evaluate possible influential outliers for both hormones and VAS measures. Significance was set at P ≤ 0.05 for all comparisons. All analyses were conducted using SAS system version 9.4 (The SAS system for Windows, 2005, Cary NC, SAS Institute, Inc.).
RESULTS
Baseline characteristics
Participants randomized to GTE and placebo were similar with respect to baseline demographics and characteristics (Table 1). Groups differed in the distribution of level of education, in that the GTE group had a greater number of participants who had obtained education beyond high school (P = 0.001). Mean intake of macro- and micronutrients as assessed by 3-day diet records did not differ by treatment group.
Table 1.
Baseline characteristics and mean 3-day dietary intake of study participants, by treatment group.1
| GTE (n=30) | Placebo (n=30) | P-value | |
|---|---|---|---|
| Age, y | 61.0 (59.2, 62.8) | 60.8 (59.0, 62.6) | 0.88 |
| White, non-Hispanic, n (%) | 29 (96.7) | 30 (100) | 1.002 |
| COMT genotype, n (%) | 1.00 | ||
| A/A | 10 (33.3) | 10 (33.3) | |
| G/A | 10 (33.3) | 10 (33.3) | |
| G/G | 10 (33.3) | 10 (33.3) | |
| Weight, kg | 74.2 (71.1, 77.4) | 75.3 (72.1, 78.4) | 0.64 |
| Body mass index, kg/m2 | 28.2 (27.1, 29.4) | 28.3 (27.2, 29.5) | 0.91 |
| Waist-to-hip ratio | 0.87 (0.83, 0.89) | 0.85 (0.82, 0.87) | 0.31 |
| Years postmenopausala | 8.8 (6.3, 12.2) | 7.8 (5.6, 10.8) | 0.60 |
| Type of menopause, n (%) | 0.75 | ||
| Natural | 23 (76.7) | 24 (80.0) | |
| Surgical | 7 (23.3) | 6 (20.0) | |
| Education, n (%) | 0.0012 | ||
| Masters/PhD/Professional | 10 (33.3) | 8 (26.7) | |
| College degree | 8 (26.7) | 15 (50.0) | |
| Some college | 12 (40.0) | 2 (6.7) | |
| High school or below | 0 | 5 (16.7) | |
| Physical activity, MET-hr/week | 39.5 (26.2, 52.7) | 43.8 (30.6, 57.1) | 0.64 |
| Dietary variables | |||
| Total energy, kcal/day | 1760 (1590, 1930) | 1850 (1680, 2020) | 0.48 |
| Protein, g/day | 73.4 (65.7, 81.0) | 78.6 (70.9, 86.2) | 0.35 |
| Total fat, g/day | 67.5 (57.6, 77.4) | 73.9 (64.0, 83.8) | 0.37 |
| Dietary cholesterol, mg | 230 (190, 271) | 238 (197, 278) | 0.79 |
| Saturated fat, g | 23.4 (19.9, 26.9) | 24.5 (21.0, 28.0) | 0.66 |
| MUFAb, g | 11.9 (9.7, 14.6) | 13.4 (10.9, 16.4) | 0.44 |
| PUFA, g | 7.0 (5.0, 9.0) | 8.5 (6.5, 10.5) | 0.31 |
| Omega-3 FAb, g | 0.5 (0.4, 0.7) | 0.6 (0.5, 0.8) | 0.65 |
| Omega-6 FAb, g | 1.2 (1.0, 1.5) | 1.3 (1.1, 1.7) | 0.38 |
| Total carbohydrate, g/day | 217 (192, 241) | 216 (191, 240) | 0.95 |
| Dietary fiber, g/day | 19.0 (16.8, 21.2) | 20.6 (18.4, 22.9) | 0.31 |
| Soluble fiber, g | 1.8 (1.4, 2.2) | 1.6 (1.2, 2.0) | 0.42 |
| Alcohol3, g/day | 0.1 (0.0, 0.3) | 0.1 (0.0, 0.4) | 0.75 |
| Caffeine3, mg/day | 36.8 (13.6, 99.9) | 46.2 (17.0, 125.3) | 0.75 |
Continuous data expressed as arithmetic mean (95% confidence interval) unless otherwise indicated by superscript. Categorical variables compared using Chi-square test and expressed as n (%).
Fisher's exact test used for comparison.
Data expressed as geometric mean (95% confidence interval).
Abbreviations: FA, fatty acid; GTE, green tea extract; MET-hr, metabolic equivalent hours; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
Postprandial hormone and glucose response
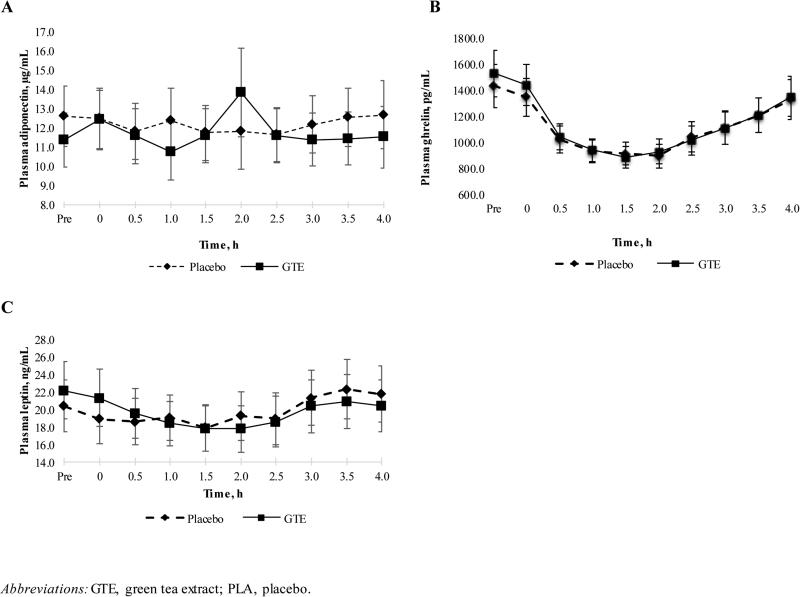
Postprandial concentrations of leptin, ghrelin, and adiponectin are presented in Figure 2. Baseline fasting concentrations of all variables were similar between treatment groups. These hormone concentrations were not different between GTE and placebo at any time point, and COMT genotype did not modify these results.
Figure 2. Mean postprandial adiponectin (A), ghrelin (B), and leptin (C) concentrations in overweight or obese postmenopausal women randomized to GTE or Placebo for 12 months.
The x-axis is labeled in units of hours, with “Pre” indicating the pre-meal (fasting) blood draw. The placebo group (n=30) is indicated by a dashed line and the green tea extract (GTE) group (n=30) is indicated by solid black line.
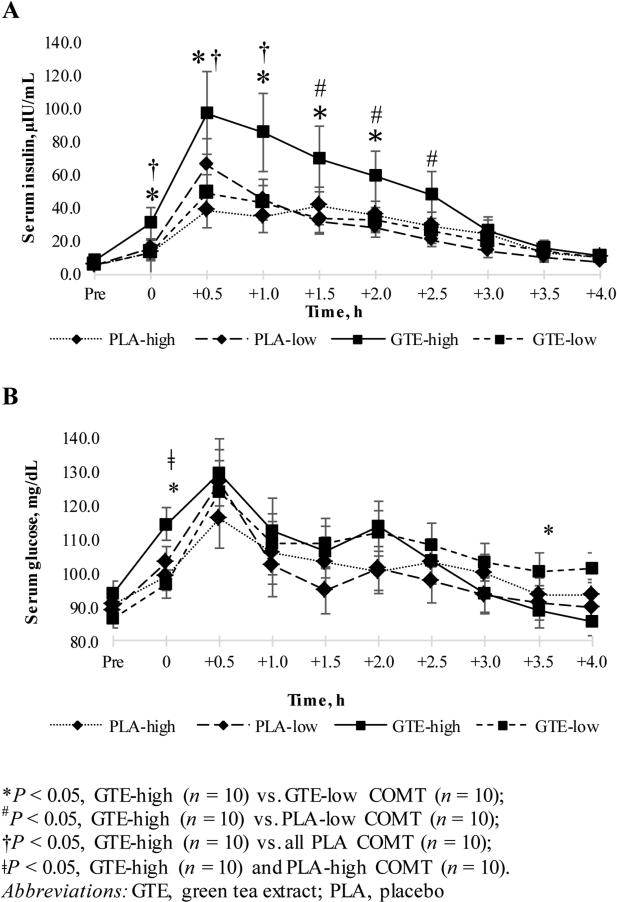
There was a statistically significant interaction between treatment and COMT genotype for insulin and glucose that varied across time (Figure 3). Participants randomized to GTE with the high-activity form of the COMT enzyme (GTE-high COMT) had significantly higher insulin concentrations at time 0 (post-meal), 0.5, and 1.0 h post-meal as compared to all COMT genotype groups randomized to placebo. Insulin concentrations remained significantly higher in the GTE-high COMT group 1.5, 2.0, and 2.5 h as compared to the Placebo-low COMT group (P < 0.02). The GTE-high COMT group also had higher insulin concentrations at time 0, 0.5, 1.0, 1.5, and 2.0 h after the meal as compared to the GTE-low COMT group (P ≤ 0.04). No differences between treatment groups and COMT genotypes existed 3.0, 3.5, or 4.0 h after the meal. With respect to glucose, the GTE-high COMT group had significantly higher glucose concentrations at time 0 as compared to Placebo-high COMT (P = 0.05) and the GTE-low COMT group (P = 0.004). The GTE-low COMT group had higher serum glucose 3.5 h post-meal as compared to the GTE-high COMT and Placebo-low COMT groups (P = 0.01 and P = 0.05, respectively). Insulin AUC comparisons within genotypes between treatment groups are listed in Table 2. The insulin AUC for GTE-high COMT was significantly higher as compared to Placebo-high COMT at all time points, though the mean AUC comparison failed to reach significance (P = 0.06). Mean insulin AUCs between intermediate- and low-COMT groups did not differ at any time point, and glucose AUCs did not differ within any COMT genotype between treatment groups (data not shown).
Figure 3. Mean postprandial serum insulin (A) and glucose (B) concentrations in overweight or obese postmenopausal women randomized to GTE or Placebo for 12 months.
Figure 3 details the mean change in postprandial insulin and glucose concentrations, by treatment group and COMT genotype. Participants in the Placebo group with high (G/G) catechol-O-methyltransferase (COMT) genotype (PLA-high, n=10) are indicated by a small-dashed line and diamond-shaped markers. Participants in the Placebo group with low (A/A) COMT genotype (PLA-low, n=10) are indicated by a wide-dashed line and diamond-shaped markers. In the GTE group, participants with high COMT genotype (GTE-high, n=10) are indicated with a solid black line and squared-shaped marker, while those with the low COMT genotype (GTE-low, n=10) are indicated by a dashed line with a squared-shaped marker. Participants with the intermediate (G/A) COMT genotype (n=10 in both Placebo and GTE groups) have been omitted from this figure for the purposes of simplification, since their postprandial insulin and glucose responses did not significantly differ from participants with high or low COMT genotypes or between treatment groups. An asterisk indicates a significant difference (P < 0.05) between the GTE-high and GTE-low groups. A “#” indicates a significant difference between GTE-high and PLA-low groups and “†” indicates a significant difference between GTE-high and all Placebo-COMT genotype groups. “ǂ” indicates a significant difference between GTE-high and PLA-high groups.
Table 2.
Mean insulin area under the curves in in overweight or obese postmenopausal women after the ingestion of a meal with or without GTE, by COMT genotype (expressed in μIU/(mL/h))1
| Placebo (n=30) | GTE (n=30) | |||||
|---|---|---|---|---|---|---|
| Time (h) | High (G/G) (n = 10) | Intermediate (G/A) (n = 10) | Low (A/A) (n = 10) | High (G/G) (n = 10) | Intermediate (G/A) (n = 10) | Low (A/A) (n = 10) |
| 0-0.52 | 14.0 ± 4.9 | 14.6 ± 4.9 | 21.2 ± 4.9 | 35.4 ± 4.9*3 | 25.4 ± 5.1 | 16.4 ± 4.6 |
| 0-1 | 31.9 ± 11.4 | 37.6 ± 11.4 | 51.1 ± 11.4 | 81.1 ± 11.4* | 55.1 ± 12.0 | 45.0 ± 10.9 |
| 0-1.5 | 48.1 ± 18.5 | 58.9 ±18.5 | 70.5 ± 18.5 | 120 ± 18.5* | 83.9 ± 19.5 | 73.3 ± 17.6 |
| 0-2 | 65.8 ± 25.8 | 79.0 ± 25.8 | 84.1 ± 25.8 | 156 ± 25.8* | 120 ± 27.2 | 99.3 ± 24.6 |
| 0-2.5 | 82.9 ± 32.9 | 95.0 ± 32.9 | 96.1 ± 32.9 | 190 ± 32.9* | 148 ± 34.7 | 124 ± 31.4 |
| 0-3 | 98.7 ± 38.7 | 106 ± 38.7 | 106 ± 38.7 | 216 ± 38.7* | 168 ± 40.8 | 143 ± 36.9 |
| 0-3.5 | 109 ± 42.8 | 114 ± 42.8 | 113 ± 42.8 | 231 ± 42.8* | 184 ± 45.1 | 156 ± 40.8 |
| 0-4 | 115 ± 45.7 | 120 ± 45.7 | 118 ± 45.7 | 241 ± 45.7 | 196 ± 48.2 | 166 ± 43.6 |
Data presented as arithmetic mean ± SEM.
Time 0 indicates post-meal.
Asterisk indicates statistically significant difference between COMT genotypes, GTE vs. placebo (P ≤ 0.05).
Appetite sensations
AUC measurements of satiety-related variables did not show any significant difference between treatments at baseline or over the 4-h time period between GTE and placebo groups (Supplementary Table 1). COMT genotype did not modify these results.
DISCUSSION
The results of the present study indicate that GTE supplementation does not alter the acute postprandial response of leptin, ghrelin, or adiponectin after consumption of a high-carbohydrate meal, and that COMT genotype does not modify this relationship. However, GTE supplementation and COMT genotype may be involved in the post-meal glycemic response, as significant interactions were observed between COMT genotype and glucose and insulin concentrations over the 4-h test period. No effect of GTE or COMT genotype was seen on measures of postprandial satiety.
To our knowledge, this is the first study to examine the effect of GTE supplementation on acute postprandial concentrations of the appetite-associated hormones leptin, ghrelin, and adiponectin. We did not find significant differences between treatment groups in these hormones prior to the meal or at any time point thereafter, indicating that GTE did not modify fasting concentrations of ghrelin, leptin, and adiponectin in these participants. This is consistent with our previous work, which demonstrated that GTE did not modify concentrations of these hormones over the MGTT's 12-month intervention period in a larger sample of overweight and obese study participants (11). We did observe a somewhat unexpected leptin response, in which plasma leptin concentrations decreased moderately from pre-meal to 1.5 h in both groups before increasing to the end of the 4 h period. Yet, these results correlate with other studies that have examined leptin concentrations in overweight and obese women after a high-carbohydrate meal (31,32), affirming that obesity is associated with an impaired postprandial leptin response. When correlated to the AUC analysis of satiety-related endpoints, satiety and prospective food intake were not differentially influenced by the effect of GTE on energy-related hormones. Together, the results of these studies indicate that any effect of GTE supplementation is not mediated through alteration of leptin, ghrelin, or adiponectin concentrations.
We observed a significant interaction between GTE, COMT genotype, and time, in which participants randomized to GTE with the high-activity (G/G) form of the COMT enzyme demonstrated increased postprandial insulin concentrations as compared to the Placebo-high COMT and GTE-low (A/A) COMT groups, despite similar glucose profiles at most time points. The significance of an increased insulin response to a high-carbohydrate meal in participants with the G/G genotype taking GTE is unclear, though it could be indicative of the need to secrete additional insulin to manage blood glucose concentrations over time, as compared to individuals with other forms of the COMT genotype and those not taking GTE; yet, the sample size of this group was small, making it difficult to draw definitive conclusions. Our results are in agreement with those of another study (23) that found greater postprandial insulin concentrations after consumption of GTE (836 mg catechins) in individuals with the G/G genotype as compared to those with the G/A or A/A COMT genotypes. Similarly, Kring, et al. (33), determined that there was an 11.6% increased frequency of the G/G COMT genotype in individuals with impaired glucose tolerance or type 2 diabetes. These studies and our results seem to suggest that the high-activity form of the COMT enzyme is associated with increased risk for glycemia-related health conditions, and that GTE consumption may potentiate an exaggerated insulin response after a meal. These results coincide with our previous findings in a larger sample of postmenopausal overweight and obese women (n = 237) (11), in which participants with the G/G form of COMT showed significantly higher insulin concentrations at month 12 as compared to those with the A/A genotype irrespective of treatment group (GTE and placebo groups combined); yet, among participants with baseline fasting insulin ≥10 μIU/mL, reductions in fasting insulin concentrations were seen in the GTE group over 12 months compared to both the placebo group and all participants with baseline insulin < 10 μIU/mL. It is plausible that GTE consumption may elicit a higher immediate postprandial insulin response, particularly in those with the G/G COMT genotype, while at the same time acting to reduce fasting insulin concentrations over time. Additional research is needed to determine the specific mechanisms of the COMT enzyme on insulin concentrations after administration of GTE and how this may affect short- and long-term glycemic response.
Early animal studies demonstrated reduced food intake with administration of green tea catechins (9,34), indicating that green tea might increase satiety. However, this effect has not been confirmed in human subjects. One study found that inclusion of 167 mg green tea catechins and 100 mg caffeine in a beverage containing 10 g soluble fiber created lower hunger and higher fullness ratings and was associated with the lowest energy intake in the next meal as compared to fiber-only (46 mg caffeine), isocaloric control beverage (no caffeine), and no beverage conditions (35). In contrast, Diepvens, et al. showed that women randomized to receive green tea (1125 mg catechins + 225 mg caffeine/day) for nearly 3 months with a low-energy diet became hungrier over time and showed increased prospective food consumption as compared to placebo (26). The authors suggested that this could be due to down-regulation of the leptin response through stimulation of the sympathetic nervous system by green tea, as leptin is known to reduce appetite. However, leptin concentrations were not measured in their study, so this conclusion could not be confirmed. Several other RCTs examining the effect of GTC on acute measures of satiety (36,37) or long-term changes in energy intake (5,11,38) have shown null results, including the present study, in which we did not observe differences between treatment groups in any of the 9 questions related to hunger, satiation, and prospective food intake in the 4 hours following a high-carbohydrate breakfast meal.
The results of this research contribute depth not only to the growing body of research on the effects of GTC on insulin sensitivity, but also to the association between COMT genotype and risk for glycemia-related health conditions. Our results also indicate that GTE does not induce appetite inhibition or satiety, thus weakening the argument for these effects as mechanisms behind green tea's association with reductions in body weight and adiposity. However, our research has several limitations. We conducted this analysis after just one meal and were unable to compare each participant's response to that of a reference meal. There was no measure of hedonic liking of the meal, which may have influenced satiety and prospective food intake. Standardization of the diets of participants 24 hours prior to the breakfast meal could have increased similarity of the glycemic response. Participants were not required to follow a low-catechin diet for the duration of the study, so ingestion of catechins from foods and beverages other than green tea likely contributed to total catechin intake. However, green tea consumption was restricted throughout the duration of the study, and the amount of GTC given in the intervention was far greater than could be ingested in a typical daily diet. Therefore, any risk of confounding was minimal. Lastly, as this was a small ancillary study with only 10 participants in each treatment/genotype group, we may have been underpowered to detect significant results and cannot rule out the possibility of type 2 error. Despite these limitations, these results indicate that supplementation of 1315 mg GTE per day for 12 months did not influence pre- or postprandial concentrations of appetite-associated hormones or measures of satiety after a high-carbohydrate breakfast meal in overweight or obese postmenopausal women. Yet, GTE and COMT genotype may have specific influences on post-meal insulin and glucose concentrations, and these findings warrant additional research in larger study populations to determine the specific impact of GTE on the postprandial glycemic response.
Transparency Declaration
The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted and that any discrepancies from the study as planned (and registered with) have been explained. The reporting of this work is compliant with CONSORT guidelines.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Institutes of Health/National Cancer Institute grant R01 CA127236, Award Number T32CA132670 from the National Cancer Institute, the University of Minnesota Agricultural Experiment Station Project Number MIN-18-103, and the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The funding sources and study supplement provider did not contribute to the design or conduct of the study, nor to the writing or submission of this manuscript.
Abbreviations
- COMT
catechol-O-methyltransferase
- EGCG
(−)-epigallocatechin-3-gallate
- GTC
green tea catechins
- GTE
green tea extract
- MGTT
Minnesota Green Tea Trial
Footnotes
Authorship: AMD, MSK, and NRS: contributed to the conception, design, and implementation of the project; AMD, SB, AA, and NRS contributed to data collection and analytical procedures; AA and LE conducted the statistical analysis, AMD, AA, LE and MSK interpreted data; AMD and NRS wrote the manuscript and had primary responsibility for final content. All authors read and approved the final version of the manuscript.
Disclosure of Potential Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.World Health Organization. Obesity and overweight fact sheet. . . January. 2015 [Google Scholar]
- 2.Pan SY, Johnson KC, Ugnat AM, Wen SW, Mao Y, Canadian Cancer Registries Epidemiology Research Group Association of obesity and cancer risk in canada. Am J Epidemiol. 2004;159(3):259–268. doi: 10.1093/aje/kwh041. [DOI] [PubMed] [Google Scholar]
- 3.Pichard C, Plu-Bureau G, Neves-E Castro M, Gompel A. Insulin resistance, obesity and breast cancer risk. Maturitas. 2008;60(1):19–30. doi: 10.1016/j.maturitas.2008.03.002. doi: 10.1016/j.maturitas.2008.03.002 [doi] [DOI] [PubMed] [Google Scholar]
- 4.La Vecchia C, Giordano SH, Hortobagyi GN, Chabner B. Overweight, obesity, diabetes, and risk of breast cancer: Interlocking pieces of the puzzle. Oncologist. 2011;16(6):726–729. doi: 10.1634/theoncologist.2011-0050. doi: 10.1634/theoncologist.2011-0050 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auvichayapat P, Prapochanung M, Tunkamnerdthai O, et al. Effectiveness of green tea on weight reduction in obese thais: A randomized, controlled trial. Physiol Behav. 2008;93(3):486–491. doi: 10.1016/j.physbeh.2007.10.009. doi: 10.1016/j.physbeh.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Nagao T, Meguro S, Hase T, et al. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity (Silver Spring) 2009;17(2):310–317. doi: 10.1038/oby.2008.505. doi: 10.1038/oby.2008.505. [DOI] [PubMed] [Google Scholar]
- 7.Phung OJ, Baker WL, Matthews LJ, Lanosa M, Thorne A, Coleman CI. Effect of green tea catechins with or without caffeine on anthropometric measures: A systematic review and meta-analysis. Am J Clin Nutr. 2010;91(1):73–81. doi: 10.3945/ajcn.2009.28157. doi: 10.3945/ajcn.2009.28157. [DOI] [PubMed] [Google Scholar]
- 8.Hursel R, Viechtbauer W, Westerterp-Plantenga MS. The effects of green tea on weight loss and weight maintenance: A meta-analysis. Int J Obes (Lond) 2009;33(9):956–961. doi: 10.1038/ijo.2009.135. doi: 10.1038/ijo.2009.135 [doi] [DOI] [PubMed] [Google Scholar]
- 9.Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000;141(3):980–987. doi: 10.1210/endo.141.3.7368. [DOI] [PubMed] [Google Scholar]
- 10.Jurgens TM, Whelan AM, Killian L, Doucette S, Kirk S, Foy E. Green tea for weight loss and weight maintenance in overweight or obese adults. Cochrane Database Syst Rev. 2012;12:CD008650. doi: 10.1002/14651858.CD008650.pub2. doi: 10.1002/14651858.CD008650.pub2 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dostal AM, Samavat H, Espejo L, Arikawa AY, Stendell-Hollis NR, Kurzer MS. Green tea extract and catechol-O-methyltransferase genotype modify fasting serum insulin and plasma adiponectin concentrations in a randomized controlled trial of overweight and obese postmenopausal women. J Nutr. 2015 doi: 10.3945/jn.115.222414. doi: jn222414 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dulloo AG, Seydoux J, Girardier L, Chantre P, Vandermander J. Green tea and thermogenesis: Interactions between catechin-polyphenols, caffeine and sympathetic activity. Int J Obes Relat Metab Disord. 2000;24(2):252–258. doi: 10.1038/sj.ijo.0801101. [DOI] [PubMed] [Google Scholar]
- 13.Dulloo AG, Duret C, Rohrer D, et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr. 1999;70(6):1040–1045. doi: 10.1093/ajcn/70.6.1040. [DOI] [PubMed] [Google Scholar]
- 14.Hodgson AB, Randell RK, Boon N, et al. Metabolic response to green tea extract during rest and moderate-intensity exercise. J Nutr Biochem. 2013;24(1):325–334. doi: 10.1016/j.jnutbio.2012.06.017. doi: 10.1016/j.jnutbio.2012.06.017 [doi] [DOI] [PubMed] [Google Scholar]
- 15.Yang X, Yin L, Li T, Chen Z. Green tea extracts reduce adipogenesis by decreasing expression of transcription factors C/EBPalpha and PPARgamma. Int J Clin Exp Med. 2014;7(12):4906–4914. [PMC free article] [PubMed] [Google Scholar]
- 16.Rains TM, Agarwal S, Maki KC. Antiobesity effects of green tea catechins: A mechanistic review. J Nutr Biochem. 2011;22(1):1–7. doi: 10.1016/j.jnutbio.2010.06.006. doi: 10.1016/j.jnutbio.2010.06.006 [doi] [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Zhang M, Wu T, Dai S, Xu J, Zhou Z. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015;6(1):297–304. doi: 10.1039/c4fo00970c. doi: 10.1039/c4fo00970c [doi] [DOI] [PubMed] [Google Scholar]
- 18.Schloegl H, Percik R, Horstmann A, Villringer A, Stumvoll M. Peptide hormones regulating appetite--focus on neuroimaging studies in humans. Diabetes Metab Res Rev. 2011;27(2):104–112. doi: 10.1002/dmrr.1154. doi: 10.1002/dmrr.1154; 10.1002/dmrr.1154. [DOI] [PubMed] [Google Scholar]
- 19.Narotzki B, Reznick AZ, Navot-Mintzer D, Dagan B, Levy Y. Green tea and vitamin E enhance exercise-induced benefits in body composition, glucose homeostasis, and antioxidant status in elderly men and women. J Am Coll Nutr. 2013;32(1):31–40. doi: 10.1080/07315724.2013.767661. doi: 10.1080/07315724.2013.767661 [doi] [DOI] [PubMed] [Google Scholar]
- 20.Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res. 2012;32(6):421–427. doi: 10.1016/j.nutres.2012.05.007. doi: 10.1016/j.nutres.2012.05.007 [doi] [DOI] [PubMed] [Google Scholar]
- 21.Hsu CH, Liao YL, Lin SC, Tsai TH, Huang CJ, Chou P. Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? A randomized, double-blind, and placebo-controlled clinical trial. Altern Med Rev. 2011;16(2):157–163. [PubMed] [Google Scholar]
- 22.Josic J, Olsson AT, Wickeberg J, Lindstedt S, Hlebowicz J. Does green tea affect postprandial glucose, insulin and satiety in healthy subjects: A randomized controlled trial. Nutr J. 2010;9:63. doi: 10.1186/1475-2891-9-63. doi: 10.1186/1475-2891-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller RJ, Jackson KG, Dadd T, Mayes AE, Brown AL, Minihane AM. The impact of the catechol-O-methyltransferase genotype on the acute responsiveness of vascular reactivity to a green tea extract. Br J Nutr. 2011;105(8):1138–1144. doi: 10.1017/S0007114510004836. doi: 10.1017/S0007114510004836 [doi] [DOI] [PubMed] [Google Scholar]
- 24.Dawling S, Roodi N, Mernaugh RL, Wang X, Parl FF. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: Comparison of wild-type and variant COMT isoforms. Cancer Res. 2001;61(18):6716–6722. [PubMed] [Google Scholar]
- 25.Syvanen AC, Tilgmann C, Rinne J, Ulmanen I. Genetic polymorphism of catechol-O-methyltransferase (COMT): Correlation of genotype with individual variation of S-COMT activity and comparison of the allele frequencies in the normal population and parkinsonian patients in finland. Pharmacogenetics. 1997;7(1):65–71. doi: 10.1097/00008571-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Diepvens K, Kovacs EM, Nijs IM, Vogels N, Westerterp-Plantenga MS. Effect of green tea on resting energy expenditure and substrate oxidation during weight loss in overweight females. Br J Nutr. 2005;94(6):1026–1034. doi: 10.1079/bjn20051580. [DOI] [PubMed] [Google Scholar]
- 27.Kovacs EM, Mela DJ. Metabolically active functional food ingredients for weight control. Obes Rev. 2006;7(1):59–78. doi: 10.1111/j.1467-789X.2006.00203.x. doi: OBR203 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Samavat H, Dostal AM, Wang R, et al. The minnesota green tea trial (MGTT), a randomized controlled trial of the efficacy of green tea extract on biomarkers of breast cancer risk: Study rationale, design, methods, and participant characteristics. Cancer Causes Control. 2015;26(10):1405–1419. doi: 10.1007/s10552-015-0632-2. doi: 10.1007/s10552-015-0632-2 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 30.Hill AJ, Rogers PJ, Blundell JE. Techniques for the experimental measurement of human eating behaviour and food intake: A practical guide. Int J Obes Relat Metab Disord. 1995;19(6):361–375. [PubMed] [Google Scholar]
- 31.Romon M, Lebel P, Fruchart JC, Dallongeville J. Postprandial leptin response to carbohydrate and fat meals in obese women. J Am Coll Nutr. 2003;22(3):247–251. doi: 10.1080/07315724.2003.10719300. [DOI] [PubMed] [Google Scholar]
- 32.Poretsky L, Lesser M, Brillon D. Lack of postprandial leptin peaks in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2001;3(2):105–111. doi: 10.1046/j.1463-1326.2001.00119.x. doi: dom119 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Kring SI, Werge T, Holst C, et al. Polymorphisms of serotonin receptor 2A and 2C genes and COMT in relation to obesity and type 2 diabetes. PLoS One. 2009;4(8):e6696. doi: 10.1371/journal.pone.0006696. doi: 10.1371/journal.pone.0006696 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sayama K, Lin S, Zheng G, Oguni I. Effects of green tea on growth, food utilization and lipid metabolism in mice. In Vivo. 2000;14(4):481–484. [PubMed] [Google Scholar]
- 35.Carter BE, Drewnowski A. Beverages containing soluble fiber, caffeine, and green tea catechins suppress hunger and lead to less energy consumption at the next meal. Appetite. 2012;59(3):755–761. doi: 10.1016/j.appet.2012.08.015. doi: 10.1016/j.appet.2012.08.015 [doi] [DOI] [PubMed] [Google Scholar]
- 36.Gregersen NT, Bitz C, Krog-Mikkelsen I, et al. Effect of moderate intakes of different tea catechins and caffeine on acute measures of energy metabolism under sedentary conditions. Br J Nutr. 2009;102(8):1187–1194. doi: 10.1017/S0007114509371779. doi: 10.1017/S0007114509371779 [doi] [DOI] [PubMed] [Google Scholar]
- 37.Belza A, Toubro S, Astrup A. The effect of caffeine, green tea and tyrosine on thermogenesis and energy intake. Eur J Clin Nutr. 2009;63(1):57–64. doi: 10.1038/sj.ejcn.1602901. doi: 1602901 [pii] [DOI] [PubMed] [Google Scholar]
- 38.Maki KC, Reeves MS, Farmer M, et al. Green tea catechin consumption enhances exercise-induced abdominal fat loss in overweight and obese adults. J Nutr. 2009;139(2):264–270. doi: 10.3945/jn.108.098293. doi: 10.3945/jn.108.098293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.