Abstract
Old age and sex steroid deficiency are the two most critical factors for the development of osteoporosis. It remains unknown, however, whether the molecular culprits of the two conditions are similar or distinct. We show herein that at 19.5 months of age —a time by which the age-dependent decline of cortical and cancellous bone mass and cortical porosity were fully manifested in C57BL/6J mice—these animals remained functionally estrogen sufficient. Transgenic mice with conditional expression of mitochondria-targeted catalase—a potent H2O2 inactivating enzyme—in cells of the myeloid lineage (mitoCAT;LysM-Cre mice) were protected from the loss of cortical, but not cancellous, bone caused by gonadectomy in either sex. Consistent with these findings, in vitro studies with ERα-deficient Prx1+ cells and gonadectomized young adult mice showed that in both sexes decreased ERα signaling in Prx1+ cells leads to an increase in SDF1, a.k.a. CXCL12, an osteoclastogenic cytokine whose effects were abrogated in macrophages from mitoCAT;LysM-Cre mice. In contrast to sex steroid deficiency, the adverse effects of aging on either cortical or cancellous bone were unaffected in mitoCAT;LysM-Cre mice. On the other hand, attenuation of H2O2 generation in cells of the mesenchymal lineage targeted by Prx1-Cre partially prevented the loss of cortical bone caused by old age. Our results suggest the effects of sex steroid deficiency and aging on the murine skeleton are independent and result from distinct mechanisms. In the former, the prevailing mechanism of the cortical bone loss in both sexes is increased osteoclastogenesis caused by estrogen deficiency; this is likely driven, at least in part, by mesenchymal/stromal cell–derived SDF1. Decreased osteoblastogenesis, owing in part to increased H2O2, combined with increased osteoclastogenesis caused by aging mechanisms independent of estrogen deficiency, are the prevailing mechanisms of the loss of cortical bone with old age.
Keywords: AGING, SEX STEROIDS, OSTEOBLASTS, OSTEOCLASTS, MOLECULAR PATHWAYS-REMODELING
Introduction
A decrease of estrogen levels at menopause or old age in both women and men causes an imbalance between bone resorption and formation, leading to loss of bone mass and strength and increased risk of osteoporotic fractures.(1) Within 5 to 10 years after menopause, the slope of the decline of bone mass in women slows and becomes indistinguishable from the slope of bone loss found in elderly males. This slower phase of bone loss in both sexes later in life affects primarily the cortical compartment and is characterized histologically by a decrease in bone formation by osteoblasts,(2,3) as well as by an increase in cortical porosity.(4,5)
However, it remains unclear whether the cellular and molecular events responsible for the imbalance between resorption and formation in sex steroid deficiency versus old age are similar or distinct and whether and how much sex steroid deficiency contributes to the age-dependent involution of the skeleton. Because of the abrupt decline of ovarian function at menopause in women and a slower decline of both androgen and estrogen levels in men with advancing age, the two conditions inexorably overlap, making it impossible to dissect their independent contribution to the cumulative anatomic deficit. As a result, the optimal treatment choice, including hormone replacement, for an elderly individual with osteoporosis remains empirical.
In difference to humans, mice do not experience menopause but are a faithful model of the adverse effects of estrogen deficiency (caused by gonadectomy) on both the cortical and cancellous bone compartments.(6) We have previously shown that the loss of bone mass caused by either aging or sex steroid deficiency in mice is associated with an increase in reactive oxygen species (ROS) levels in bone cells.(7–11) These findings raised the possibility that an increase in ROS may be a common mechanism of the adverse effects of sex steroid deficiency and old age on bone and that sex steroid deficiency may accelerate the effects of aging.
High ROS levels cause damage to proteins, lipids, and DNA, leading to cell demise.(12,13) In line with this, ROS accumulation adversely affects osteoblastogenesis and bone formation.(11) However, at lower levels, ROS can also promote intracellular signaling for physiological cell functions. H2O2 is the most stable and abundant form of ROS generated in the mitochondria and results from the conversion of superoxide via the action of superoxide dismutase enzymes.(14–16) Receptor activator of NF-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF)—the two critical cytokines for osteoclast generation—promote mitochondria biogenesis and the accumulation of H2O2 in osteoclasts and H2O2 stimulates osteoclastogenesis.(17–21) We have shown earlier in mice that lowering H2O2 generation in osteoclasts, by overexpressing human catalase targeted to the mitochondria, reduces osteoclast numbers and increases bone mass.(21)
In the work presented here, we have examined the contribution of estrogen deficiency to the loss of cortical and cancellous bone mass and the development of cortical porosity caused by old age. In addition, we have investigated the contribution of mitochondria-derived H2O2 to the loss of bone mass caused by sex steroid deficiency versus old age, using transgenic mice in which the gene for human catalase has been targeted to the mitochondria of myeloid or mesenchymal progenitors—the precursors of osteoclasts and osteoblasts, respectively.
Materials and Methods
Animal experimentation
To generate mice expressing human catalase targeted to the mitochondria in the mesenchymal lineage, mice heterozygous for mitoCAT-FS transgene(22) were crossed with hemizygous Prx1-Cre transgenic mice.(23) Transgenic offspring were identified by PCR using the following primer sequences: MitoCat forward 5′CTGAGGATCCTGTTAAACAATGC3′; MitoCat reverse 5′CTATCTGTTCAACCTCAGCA AAG3′, product size 1087 bp. Hemizygous mitoCAT-FS offspring with and without the Cre allele were then intercrossed to generate wild-type mice and mice hemizygous for either the Cre allele or mitoCAT-FS or both. Mice overexpressing the mitoCAT transgene in the myeloid lineage were generated by crossing homozygous LysM-Cre mice with hemizygous mitoCAT-FS mice using the breeding strategy described previously.(21) Offspring from all genotypes were tail-clipped for DNA extraction at the time of weaning (21 days) and then group-housed with same sex littermates. ER−/− mice were generated by intercrossing ER+/− mice.(24) The generation of ERαf/f;Prx1-Cre and ERαf/f;Osx1-Cre has been previously described.(25) All mice were in C57BL/6J background. Mice were maintained with a constant temperature of 23°C, a 12-hour light/dark cycle, and had access to food and water ad libitum.
Twelve-week-old female and 20-week-old male mitoCAT; LysM-Cre mice and LysM-Cre littermates were either gonadectomized or sham-operated after being stratified by their presurgical femoral bone mineral density (BMD) as described before.(26) Sham or gonadectomy operations were performed in the morning under sedation with 2% isoflurane, as previously described.(21) After 6 weeks, animals were euthanized and the tissues dissected for further analyses. BMD measurements were performed 2 days before surgery and before euthanasia. Within each genotype or treatment group, mice were sorted from low to high BMD values and they were then attributed the number 1 and 2, successively. All animals of the same number were assigned to the same group. BMD means and standard deviation for each group were calculated and compared by t test to assure that means were similar. Mice were injected with tetracycline (15 mg/kg body weight) 10 and 3 days before before euthanasia to quantify bone-formation rates. To determine the impact of age on ovariectomized (OVX)-induced bone loss, 4- and 18-month-old C57Bl6/J mice (Charles River Laboratories, Wilmington, MA, USA) were either OVX or sham-operated for 6 weeks as described above. To examine the contribution of sex steroids replacement and antioxidants on bone, 5-month-old C57BL/6 male and female mice (Harlan Sprague-Dawley Inc., Indianapolis, IN, USA) were either sham-operated or gonadectomized for 6 weeks. Males were implanted with osmotic minipumps (model 2006; Durect Corp., Cupertino, CA, USA) delivering either a vehicle (sham and orchidectomized [ORX]) or E2 at a rate of 6 μg/d (ORX+E2) or 60-day slow-release pellets containing DHT (10 mg). A subset of ORXed mice were injected with 100 mg/kg/d N-acetyl cysteine (NAC) twice a day. After surgery, mice were carefully monitored for behavioral changes or any signs of distress. Mice that died because of surgical stress were eliminated from study. For the aging studies, mice that died or had age-associated tumors were not included in analysis. Protocols involving genetically modified mice and their wild-type littermates were approved by the Institutional Animal Care and Use Committees of UAMS and the Central Arkansas Veterans Healthcare System.
Bone imaging
BMD measurements were performed by dual-energy X-ray absorptiometry (DXA) using a PIXImus densitometer (GE Lunar, Madison, WI, USA) in mice sedated with 2% isoflurane, as previously described.(21) Micro-CT analysis (μCT40, Scanco Medical, Bruttisellen, Switzerland) was done on bones dissected, fixed for 24 hours in 10% Millonig’s formalin, and dehydrated to 100% ethanol. Detailed description of the micro-CT methods is provided in Supplemental Materials.
Histology
Description of the methods is provided in Supplemental Materials.
Cell culture
Description of the methods is provided in Supplemental Materials.
RNA isolation and quantitative RT-PCR analysis
Soft tissues from mice were frozen in liquid N2 immediately upon harvest. Total RNA was extracted from cultured cells using Trizol (Invitrogen, Carlsbad, CA, USA) and reverse-transcribed the RNA using the High-Capacity cDNA Archive Kit (Applied Biosystems, Carlsbad, CA, USA). Taqman quantitative PCR was performed as previously described(27) to determine mRNA levels using the following primers: Mm00433148_mH (ERα), Mm00445553_m1 (SDF1), and Mm00475528_m1 (ribosomal protein S2) manufactured by the TaqMan Gene Expression Assays service (Applied Biosystems). The mRNA expression levels were normalized to the house-keeping gene ribosomal protein S2 using the ΔCt method.(28)
Statistical analysis
Group mean values were compared, as appropriate, by Student’s two-tailed t test or two-way ANOVA with Holm-Sidak multiple comparison test after determining that the data were normally distributed and exhibited equivalent variances. When variance was not equivalent, data were log-transformed or the nonparametric Mann-Whitney rank sum test was used. Comparisons of BMD values between experimental groups at individual time points in the longitudinal aging study were based on differences in least squares means from a mixed-effects model in the SAS software package, including genotype, age, and their interaction. Previous studies have shown that 8 to 12 animals per group are needed to observe a statistically significant 25% change in histomorphometric, micro-CT, and BMD measurements. Sample number (n) represents biological replicates. A p value ≤0.05 was considered significant for all statistical comparisons. Most data are presented as box plots with the central box spanning 25th to 75th percentiles and the central line representing the mean. The whiskers represent 10th and 90th percentiles and values outside this range are shown as dots. Data from in vitro studies are plotted as bar graphs that represent the mean and SD.
Results
The effects of aging on bone are independent of estrogen status
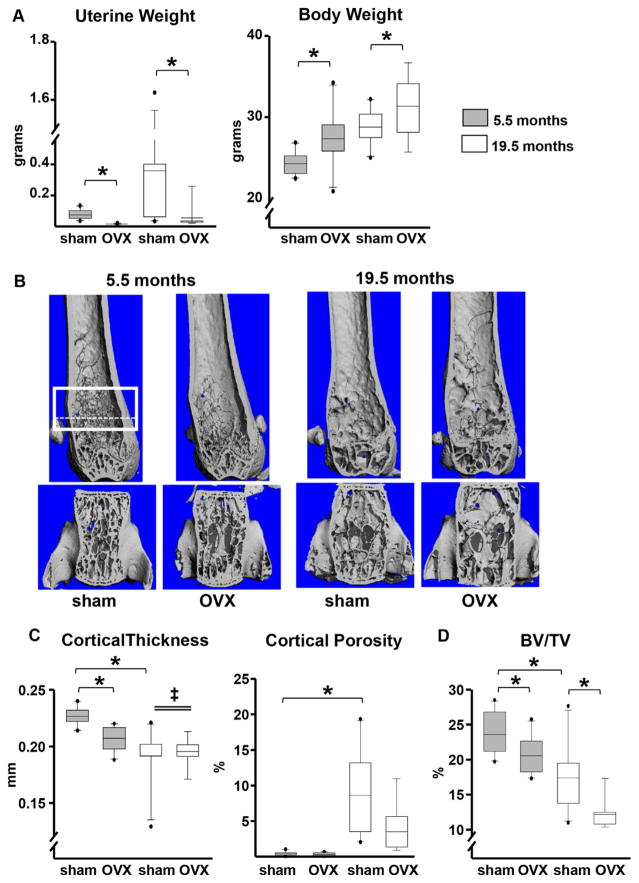
Earlier work of ours has suggested that the decline of bone mass between the ages of 4 and 31 months in C57BL/6 mice is not accompanied by an appreciable decline in sex steroid status.(7,29) Nevertheless, direct measurements of circulating sex steroids in mice have produced conflicting results, with some studies suggesting that there is no change in serum estradiol levels in females or androgen in male with aging,(30–32) whereas others found a decrease in estrogen levels during the pre-ovulatory phases of the estrus cycle in older females(33) and a marked decline in testosterone between 4 and 15 months of age in males.(34) To establish the contribution, or lack thereof, of sex steroid status to the adverse effects of aging on the murine skeleton, we ovariectomized C57BL/6J mice at 4 or 18 months of age. Six weeks after the loss of ovarian function at either age, the expected decrease in uterine weight and increase in body weight were indistinguishable between young and old mice (Fig. 1A). Hence, at least up to the age of 19.5 months, mice remain functionally estrogen sufficient. Be that as it may, we cannot exclude the possibility that the thresholds for estrogen sufficiency are different in bone versus other tissues. Nonetheless, femoral cortical thickness and vertebral cancellous bone volume did decline and cortical porosity increased in the estrogen-intact (sham-operated) mice between ages 5.5 and 19.5 months, demonstrating that the effects of aging are independent of sex steroid status (Fig. 1B–D). Notably, OVX in young mice caused a decline in femoral cortical thickness, but this effect did not occur in old mice. However, loss of ovarian function caused a similar decline in vertebral cancellous bone volume in both young and old mice. OVX at young or old age had no measurable effect on cortical porosity.
Fig. 1.
The effects of aging on cortical bone are independent of estrogen status. Mice were either sham-operated (n =10) or ovariectomized (5.5 months, n =10; 19.5 months, n =9) for 6 weeks. (A) Uterine and body weights. (B) Representative micro-CT images of femurs (top) and L5 vertebrae (bottom). The box depicts a region of 151 consecutive slices (12 μm/slice) analyzed for cancellous bone volume, as detailed in Supplemental Methods. The most proximate 90 slices, contained between the dotted line and the upper box line, were used for the measurements of cortical porosity to completely avoid the growth plate. (C) Femoral cortical (Ct.) thickness by micro-CT in the midshaft region and cortical porosity in the distal metaphysis. (D) Cancellous bone (bone volume/tissue volume [BV/TV]) in the 5th lumbar vertebra. Boxes depict values from the 25th to 75th quartiles, the middle line depicts the mean, and the vertical whiskers show the 10th and 90th percentiles; values outside this range are plotted as dots. Statistical significance calculated by two-way ANOVA, *p <0.05; age × surgery interactions denoted as ‡.
We next investigated whether the adverse effects of aging and sex steroid deficiency on the murine skeleton are mediated via similar or distinct mechanisms. To accomplish this, we generated transgenic mice expressing the human catalase gene targeted to the mitochondria of myeloid or mesenchymal lineage cells. This was done by crossing mitoCAT mice(22) with mice expressing the Cre recombinase under the control of regulatory elements of LysM or Prx1 genes. The LysM gene is expressed in cells of the myeloid lineage and neutrophils.(35) The Prx1 gene is expressed in pluripotent mesenchymal progenitors of the appendicular, but not the axial, skeleton and their progeny.(23)
The OVX- or ORX-induced loss of cortical, but not cancellous, bone is attenuated by restraining H2O2 accumulation in cells of the myeloid lineage
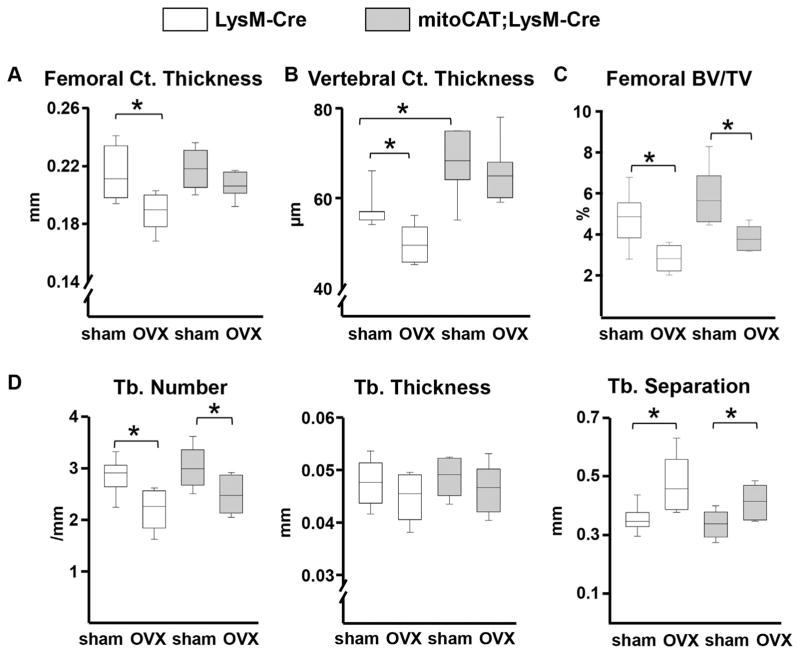
In agreement with our previous work,(7,10) OVX or ORX led to an increase in ROS levels in the bone marrow of the LysM-Cre control mice (Supplemental Fig. S1). This effect was abrogated in the mitoCAT;LysM-Cre mice, strongly suggesting that the source of the increased ROS levels after loss of sex steroids is primarily cells of the myeloid lineage. Using DXA, we had shown in earlier studies that OVX decreases BMD in both the spine and the femur of LysM-Cre controls, but this effect was abrogated in the mitoCAT;LysM-Cre mice.(21) Using micro-CT in the present work, we found that cortical thickness was indistinguishable in the femurs of estrogen-intact control littermates and mito-CAT;LysM-Cre mice (Fig. 2A). Albeit, vertebral cortical thickness (Fig. 2B) was higher in the estrogen-intact mitoCAT;LysM-Cre mice. OVX caused significant loss of cortical bone mass in both the femur and vertebra of the control mice by post hoc statistical analysis, but it had no effect in the mitoCAT;LysM-Cre mice (Fig. 2A, B). However, the surgery × genotype interaction was not significant, perhaps because of insufficient power. OVX also caused loss of cancellous bone mass in the femur (Fig. 2C) and vertebra (Supplemental Table S1) of the control mice; this effect was not influenced by the mitoCAT transgene. Consistent with the cancellous bone changes caused by OVX in both genotypes, trabecular number was decreased and trabecular separation was increased in the femur (Fig. 2D) and the vertebra (Supplemental Table S1). Trabecular thickness was not affected by OVX in either genotype.
Fig. 2.
The OVX-induced loss of cortical bone is prevented by restraining H2O2 accumulation in cells of the myeloid lineage. (A) Cortical thickness in the midshaft region of the femur and (B) 5th lumbar vertebra. (C) Cancellous bone volume (bone volume/tissue volume [BV/TV]) at the distal femur. (D) Cancellous microarchitecture in the femoral distal metaphysis, trabecular (Tb). LysM-Cre mice (sham, n =7; OVX, n =5) and mitoCAT;LysM-Cre mice (n =7 per group). Statistical significance calculated by two-way ANOVA, *p <0.05.
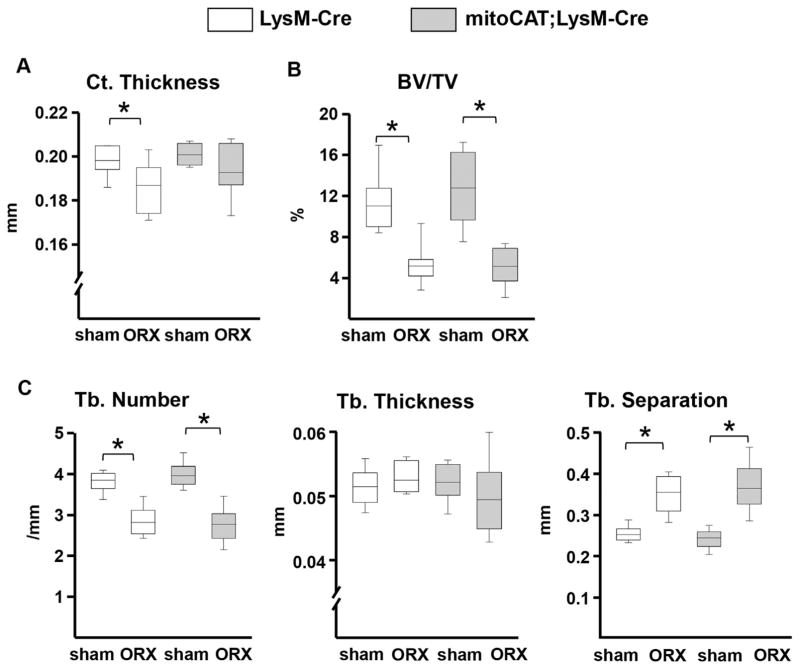
Similar to OVX, ORX caused loss of femoral cortical and cancellous bone mass in the LysM-Cre control mice (Fig. 3A, B). The effect of ORX on femoral cortical thickness, but not on femoral cancellous bone mass, was not found in the mitoCAT;LysM-Cre mice. As in females, the surgery × genotype interaction was not significant. The changes caused by ORX were practically identical to those of the OVX mice (Fig. 3C).
Fig. 3.
The ORX-induced loss of cortical bone is prevented by restraining H2O2 accumulation in cells of the myeloid lineage. (A) Cortical thickness in the midshaft region of the femur. (B) Cancellous bone volume at the distal femur. (C) Cancellous microarchitecture in the femoral distal metaphysis; trabecular (Tb.). LysM-Cre mice (n =7 per group); mitoCAT;LysM-Cre mice (sham, n =10; ORX, n =9). Statistical significance calculated by two-way ANOVA, *p <0.05.
We have previously shown that intraperitoneal injections of the antioxidant N-acetyl cysteine (NAC) to ORX or OVX mice prevents the increase of ROS in the bone marrow and the loss of spinal DXA BMD.(7) In agreement with these earlier findings, we found in the present work that NAC administration attenuated the loss of cortical bone in ORX mice (Supplemental Fig. S2). NAC also caused a small increase in the cancellous bone volume of the ORX mice, but this effect was unrelated to the loss of androgens as evidenced by the fact that NAC had no effect on the ORX-induced decrease of trabecular number and increased trabecular separation. Instead, NAC caused an increase in trabecular thickness—an architectural cancellous parameter that was not affected by ORX—but could readily account for the small increase of cancellous bone volume. Hence, as with the expression of the mitoCAT transgene, NAC administration had a protective effect against the ORX-induced loss of cortical but not cancellous bone mass.
The ORX-induced increase of osteoclast numbers in cortical, but not cancellous, bone is attenuated by restraining H2O2 accumulation in cells of the myeloid lineage
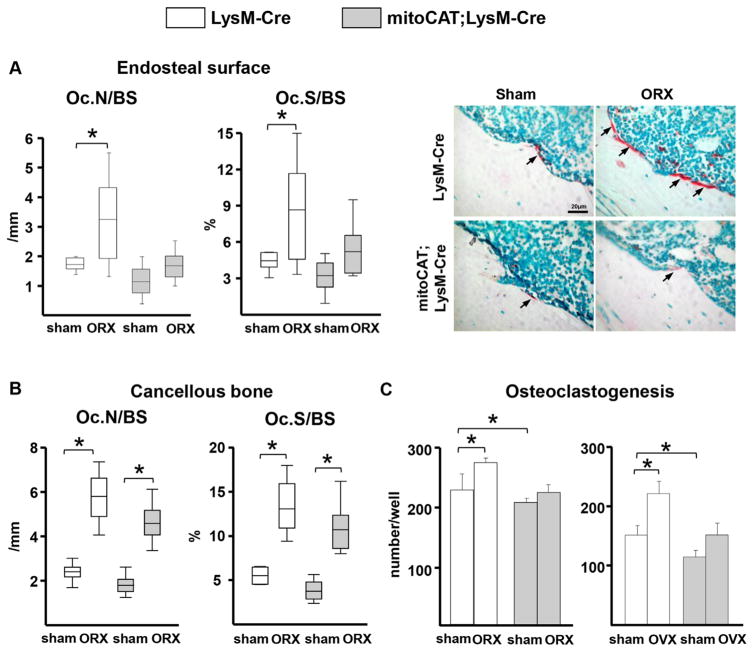
We next sought to determine whether the differential effect on bone mass in the two compartments was associated with differential changes in the number of osteoclasts. Because we had already shown in female mice that OVX increases the number of osteoclasts in the endocortical surface,(25) we performed the present work in ORX mice. ORX increased the number of osteoclasts in the LysM-Cre control mice in both endocortical surface and cancellous bone of the femur (Fig. 4A, B). Nonetheless, in agreement with the protective effect of the mitoCAT transgene on cortical, but not cancellous, bone mass, the ORX-induced increase in endocortical osteoclast numbers was abrogated in the mitoCAT;LysM-Cre mice, but the surgery × genotype interaction was not significant. In contrast, ORX caused an increase in cancellous osteoclast numbers in both control and mitoCAT;LysM-Cre mice. In support of our in vivo findings, the number of osteoclasts formed in ex vivo cultures of bone marrow cells from ORX or OVX LysM-Cre control mice was higher compared with cultures of bone marrow cells from sham-operated controls (Fig. 4C). Moreover, the increase in osteoclastogenesis was abrogated in cells from mitoCAT;LysM-Cre mice.
Fig. 4.
The ORX-induced increase of osteoclast numbers in cortical bone is prevented by restraining H2O2 accumulation in cells of the myeloid lineage. (A) Osteoclast numbers per bone surface (Oc.N/BS) and the ratio of osteoclast surface to bone surface (Oc.S/BS) in the femoral cortex, and representative histological sections stained with tartrate-resistant acid phosphatase (TRAP) (arrows indicate red-stained osteoclasts; scale bar =20 μm). (B) Oc.N/BS and Oc.S/BS in cancellous bone of the distal femur. LysM-Cre mice, n =7 per group; mitoCAT; LysM-Cre mice, n =9 per group. (C) Number of osteoclasts (TRAP-positive cells) generated in ex vivo cultures of bone marrow cells obtained from the femur (triplicate cultures). Bars represent mean and SD. Statistical significance calculated by two-way ANOVA, *p <0.05.
ERα-mediated suppression of SDF1/CXCL12 in mesenchymal cells may protect against endocortical bone resorption in both female and male mice
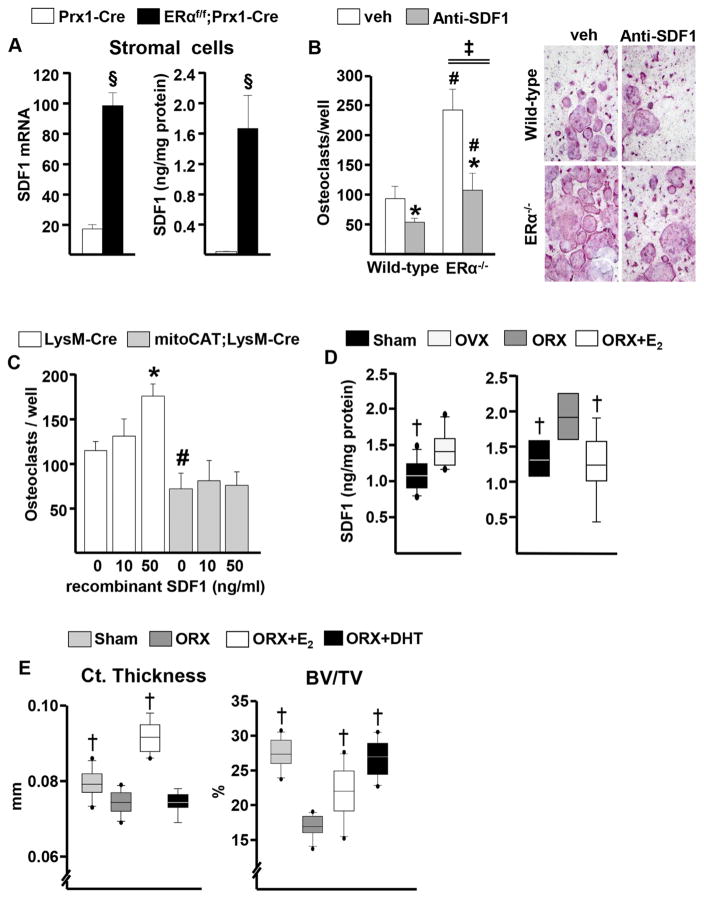
In these studies, we sought a mechanistic explanation for the protective effect of the mitoCAT transgene in LysM-Cre-targeted cells on the cortical bone of OVX and ORX mice. In earlier work, we had elucidated that estrogens attenuate endocortical resorption in females via ERα signaling in mesenchymal cells targeted by Prx1-Cre.(25) On the other hand, the target cells of the effects of androgens on endocortical bone resorption remain unclear. However, these effects are independent of AR signaling in any cell type of the mesenchymal lineage or myeloid lineage.(26) To search for molecular targets of estrogens on mesenchymal cells, we performed microarray analysis in FACS-sorted GFP-positive cells with or without ERα, from cultures of osteoblasts derived from the calvaria of either GFP:Osx1-Cre control mice or ERαf/f;GFP:Osx1-Cre mice, respectively.(25) Among the genes upregulated in the ERα-null cells was stromal cell-derived factor1 (SDF1), a.k.a. CXCL12, a chemotactic cytokine that is abundantly expressed in Prx1- and Osx1-Cre-targeted cells and stimulates osteoclastogenesis in vitro and in vivo.(36,37) The microarray findings were confirmed by RT-PCR (Supplemental Fig. S3A). Furthermore, the mRNA and secreted protein levels of SDF1 were 5- and 100-fold higher, respectively, in bone marrow cell cultures from female ERαf/f;Prx1-Cre mice compared with identical cultures from littermate controls (Fig. 5A).
Fig. 5.
Estrogens protect against endocortical bone resorption in both female and male mice, likely via an ERα-mediated suppression of SDF1 in mesenchymal cells. (A) SDF1 mRNA (left) in stromal cell cultures and protein (right) in culture supernatants (triplicates); §p <0.05 by Student’s t test. (B) (Left) Osteoclasts developed in cocultures of bone marrow macrophages from wild-type mice with wild-type or ERα knockout calvarial osteoblasts, in the absence or presence of SDF1 antibody (triplicates), antibody × genotype interaction by two-way ANOVA denoted as ‡; (right) representative pictures of the osteoclasts. (C) Osteoclasts generated from cultured bone marrow macrophages (triplicates). *p <0.05 effect of treatment within same genotype; #p <0.05 effect of genotype within same treatment by two-way ANOVA. (D) (Left) SDF1 in bone marrow plasma of C57BL/6 females (sham, n =10; OVX, n =11) and males (n =9 per group). (E) Cortical (Ct.) thickness at the femoral midshaft and cancellous bone volume (BV/TV) in L6 by micro-CT. Sham and ORX, n =11 per group; ORX+E2, n = 10; ORX+DHT, n =9. †p <0.05 versus OVX or ORX by one-way ANOVA.
We also cocultured bone marrow-derived macrophages from wild-type mice with bone marrow-derived stromal cells from ERαf/f;Prx1-Cre mice or littermate controls. The number of multinucleated TRAP+ osteoclasts formed in the cocultures with stromal cells lacking ERα was twofold higher (Supplemental Fig. S3B). Similar findings were obtained with cocultures of bone marrow-derived macrophages from wild-type mice with calvaria cells from mice with either global ERα deletion (ERKO)(24) or wild-type mice (Fig. 5B). In addition, a neutralizing SDF1 antibody attenuated the increase in the number of TRAP+ multinucleated osteoclasts in the cocultures with ERKO calvaria. The SDF1 antibody also decreased the number of osteoclasts in the cocultures with calvaria cells from wild-type mice; albeit, the absolute decrease with the antibody was two- to three-fold greater in the cocultures with the calvaria from the ERKO compared with the wild-type mice as determined by two-way ANOVA. In line with the finding that mitoCAT;LysM-Cre mice were protected from the gonadectomy-induced loss of cortical bone, recombinant SDF1 stimulated osteoclast formation in cultures of bone macrophages from LysM-Cre control mice but not from mitoCAT;LysM-Cre (Fig. 5C). These findings are consistent with published evidence on the pro-osteoclastogenic effect of SDF1(38,39) and suggest further that H2O2 accumulation is required for this effect.
Lastly, in this set of experiments, we examined whether estrogen-induced attenuation of SDF1 may also be responsible for the protective effect of sex steroids on cortical bone in male mice. In support of this hypothesis, we found that SDF1 mRNA expression in bone marrow cell cultures from wild-type C57BL/6 mice was decreased by E2, whereas DHT had no effect (Supplemental Fig. S4). Moreover, both OVX or ORX increased the levels of SDF1 in the bone marrow plasma, and E2 administration to ORX mice prevented it (Fig. 5D). Additionally, administration of E2 to ORX C57BL/6 mice prevented the loss of cortical thickness, whereas administration DHT had no effect (Fig. 5E). In contrast, DHT prevented the ORX-induced loss of cancellous bone, whereas E2 had only a modest protective effect in this compartment.
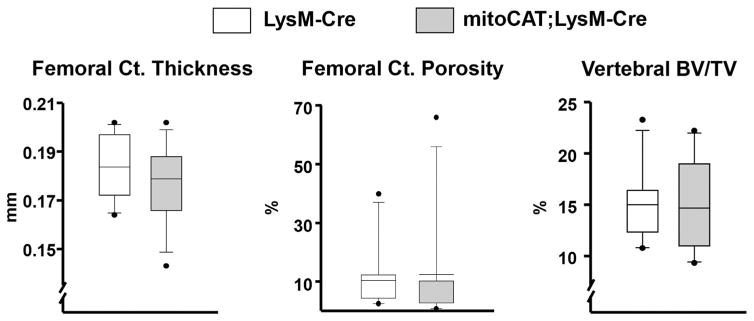
The age-dependent decline in bone mass is not affected by restraining H2O2 accumulation in cells of the myeloid lineage
Next, we examined whether restraining H2O2 in cells of the osteoclast lineage in the mitoCAT;LysM-Cre mice influences the age-dependent loss of bone mass, as it did in the setting of sex steroid deficiency. To do this, we aged a cohort of female mitoCAT;LysM-Cre mice and LysM-Cre littermate controls to 22 months. Femoral cortical thickness and porosity, as well as cancellous bone mass in L5, were indistinguishable between the two genotypes at the age of 22 months (Fig. 6). Likewise, at 22 months, total and medullary area and cortical area fraction of the femur, as well as trabecular number, thickness, and separation in L5 were not different between mitoCAT;LysM-Cre mice and controls (Supplemental Fig. 5A, B). In agreement with our previous findings,(21) the spinal BMD of the mitoCAT; LysM-Cre mice in longitudinal DXA measurements (starting at the age of 3 months and repeated every 3 months thereafter) was higher than the controls up to age 9 months, at which time the difference became statistically significant (Supplemental Fig. 5C). Nonetheless, this difference was no longer present at age 12 months and the rate of decline of bone mass was indistinguishable between the two genotypes, thereafter.
Fig. 6.
The age-dependent decline in bone mass is not affected by restraining H2O2 accumulation in cells of the myeloid lineage. Femoral cortical (Ct.) thickness measured by micro-CT at the midshaft region and cortical porosity measured in the distal metaphysis of 22-month-old female mice. Cancellous bone volume (BV/TV) was measured in the 5th lumbar vertebra. LysM-Cre mice, n =10, and mitoCAT;LysM-Cre mice, n =11.
Restraining H2O2 accumulation in mesenchymal cells partially attenuates the age-dependent cortical thinning, but not cortical porosity
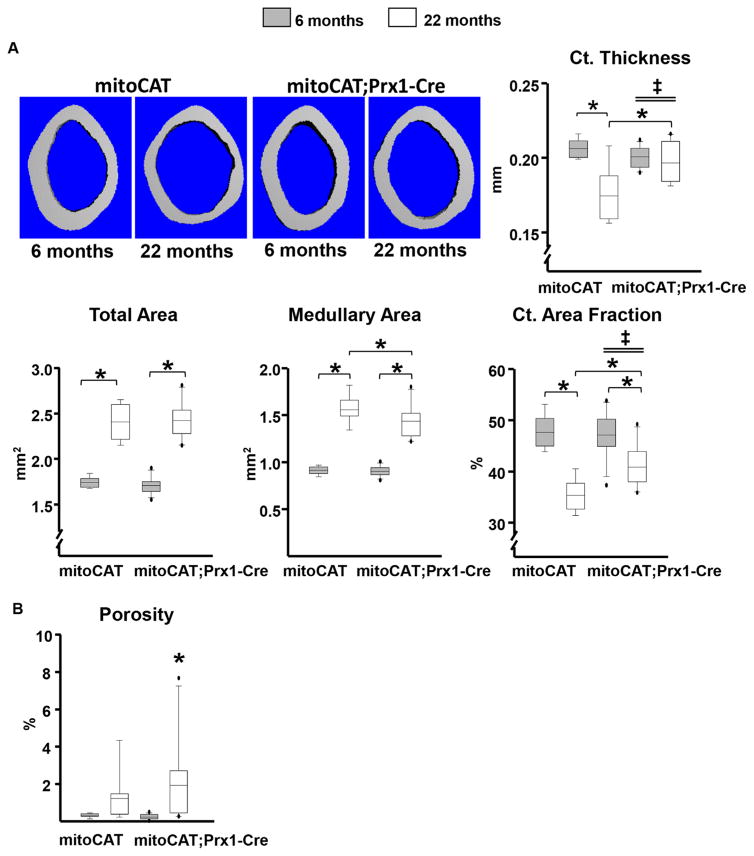
Having shown that the effects of aging are not influenced by restraining H2O2 in cells of the myeloid lineage, we explored the possibility that the adverse effects of aging on bone may be ameliorated by restraining H2O2 in cells of the mesenchymal lineage, by targeting the mitoCAT transgene to Prx1 cells. The effectiveness and specificity of the mitoCAT overexpression in Prx1-Cre cells was demonstrated by the increase in catalase activity in cultured bone marrow-derived osteoblastic cells from mitoCAT;Prx1-Cre mice, whereas catalase activity was unaffected in the kidney, liver, and spleen (Supplemental Fig. 6A). Osteoblastic cells derived from mitoCAT;Prx1-Cre mice were protected from H2O2-induced apoptosis as determined by caspase 3 activity, demonstrating that the mitoCAT transgene was functional in these cells (Supplemental Fig. 6B). At age 3 months, femoral cortical thickness and cancellous bone volume in male mitoCAT;Prx1-Cre mice were indistinguishable from wild-type, Prx1-Cre, and mitoCAT littermate controls (Supplemental Fig. 6C).
The influence of restraining H2O2 accumulation in mesenchymal cells on the effects of old age on cortical bone was determined by comparing the cortical bone mass of 6- and 22-month-old female mitoCAT;Prx1-Cre mice and mitoCAT littermate controls. Similar to the findings in the young males, in 6-month-old female mitoCAT;Prx1-Cre mice, femoral cortical thickness as well as cortical area fraction, total area, and medullary area were indistinguishable from mitoCAT littermate controls (Fig. 7A). At this age, cortical porosity was very low in the two genotypes (Fig. 7B). Compared with 6-month-olds, 22-month-old mitoCAT littermate control mice exhibited the expected decline in cortical thickness (Fig. 7A). This decline, however, was abrogated in the mitoCAT;Prx1-Cre mice. Compared with 6-month-old mice, 22-month-old mice of either genotype exhibited the expected increase in total area and medullary area (due to the lifelong expansion of cortical bone) and a decline in cortical area fraction ([total – medullary]/total area). Post hoc statistical analysis indicated that in 22-month-old mice, medullary area was smaller in mitoCAT;Prx1-Cre compared with control mice, but total area was not different. Nonetheless, the age-dependent increase in medullary area was not affected by the mitoCAT transgene. In line with the decrease in medullary area, cortical area fraction was higher in mitoCAT;Prx1-Cre mice compared with controls at 22 months, and the mitoCAT transgene attenuated the age-related decline in this measurement. Similar findings were obtained in the tibia (Supplemental Fig. S7). However, the protective effect of the mitoCAT transgene against the loss of cortical thickness was less pronounced in tibia compared with the femur. Aging also caused the expected increase in femoral cortical porosity, but the magnitude of this increase was indistinguishable between the two genotypes (Fig. 7B). Because cancellous bone in the distal end of long bones is lost relatively early in life in female mice, as well as the fact that Prx1-Cre does not target the axial skeleton, we were unable to determine whether restraining H2O2 in cells of the mesenchymal lineage alters the loss of cancellous bone with age. These studies were not duplicated in male mice because the requirement of single housing in the case of males (to prevent in-cage flghting and deaths) is cost prohibitive.
Fig. 7.
Restraining H2O2 accumulation in mesenchymal cells attenuates the age-dependent cortical thinning. (A) Representative micro-CT images and femoral cortical thickness of the midshaft region of 6- and 22-month-old female mice. (B) Cortical porosity measured in the distal metaphysis of the femur of mitoCAT (6 months, n =9; 22 months, n =7) and mitoCAT;Prx1-Cre mice (6 months, n =13; 22 months, n =10). Statistical significance calculated by two-way ANOVA, *p <0.05; age × genotype interactions denoted as ‡.
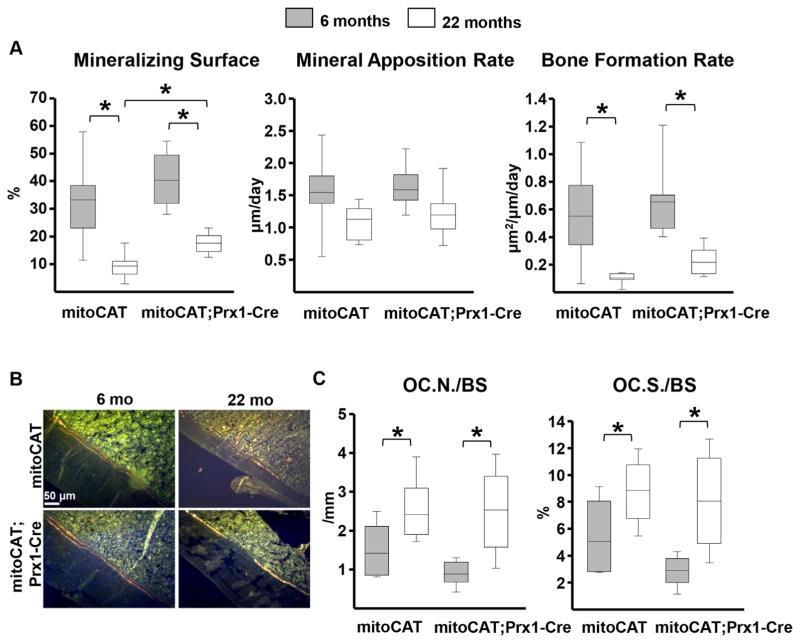
Restraining H2O2 accumulation in mesenchymal cells attenuates the age-dependent decrease in mineralizing surfaces but not the increase in osteoclast numbers at the endocortical surface
Finally, we searched for the cellular mechanisms responsible for the partially protective effects of the mitoCAT transgene in Prx1 cells on cortical bone mass by performing histological analysis at the endocortical surface. In contrast to the ease in determining osteoclast numbers in the endosteal surface (see below), we could not definitively identify osteoblasts and thereby determine osteoblast number in this particular experiment. We have, therefore, relied on tetracycline-labeled mineralizing surfaces—a surrogate of osteoblast number and highly reliable alternative measurement. Aging caused a similar decrease in mineralizing surface in both genotypes, whereas mineral apposition rate was not affected (Fig. 8A–C). Consistent with the protective effect of the mitoCAT transgene on cortical bone mass, 22-month-old female mitoCAT;Prx1-Cre mice had higher mineralizing surface compared with the age-matched mitoCAT controls (Fig. 8A). Bone formation rate (BFR), a measure calculated by multiplying mineralizing surfaces by mineral apposition rate, paralleled the age-dependent decline in mineralizing surface. However, in contrast to mineralized surface, the difference in BFR between the two genotypes at 22 months did not reach statistical significance, most likely because of the large variability of the mineral apposition rate. Hence, attenuation of H2O2 in the mesenchymal lineage is not sufficient to fully prevent the decrease in bone formation with age. The number of osteoclasts at the endocortical surface of the femur and the percent of the surface covered by osteoclasts was increased with age. The magnitude of this increase was indistinguishable between the two genotypes (Fig. 8C).
Fig. 8.
Restraining H2O2 accumulation in mesenchymal cells attenuates the decline in bone formation in aged mice. (A) Dynamic histomorphometry measurements in the femur. (B) Representative images of double tetracycline labeling at the endocortical surface. (C) Osteoclast numbers per bone surface (Oc.N/BS) and the ratio of osteoclast surface to bone surface (Oc.S/BS) at the endocortical surface. MitoCAT mice 6 months, n =8; 22 months, n =7 and mitoCAT;Prx1-Cre mice 6 months, n =7; 22 months, n =8. Statistical significance calculated by two-way ANOVA, *p <0.05.
Discussion
In the work presented in this paper, we have attempted to dissect the contribution of sex steroid deficiency to the age-dependent involution of the mammalian skeleton, using the mouse as a model. Our results show that all major features of skeletal aging, including the decline of cortical and cancellous bone mass as well as the development of cortical porosity, are independent of sex steroid deficiency, as mice are functionally estrogen sufficient—insofar as the reproductive organs and body weight homeostasis are concerned—at the time that all these skeletal changes are already manifested. To the best of our knowledge, these discoveries demonstrate for the first time that the effects of aging and sex steroid deficiency on a mammalian skeleton are independent.
In addition, we have investigated whether the molecular and cellular changes responsible for the adverse effects of estrogen or androgen deficiency and aging are mechanistically similar or distinct. We and others have shown earlier that H2O2 accumulation is required for the generation and survival of osteoclasts(21) and that the loss of bone mass caused by sex steroid deficiency and aging in mice is associated with an increase in ROS.(7) In the present work, we found that attenuation of H2O2 generation in cells of the osteoclast lineage abrogated the increase of osteoclast number in the endocortical surface and the loss of cortical bone caused by OVX or ORX, but it had no influence on the adverse effects of aging on either cortical or cancellous bone. Attenuation of H2O2 generation in cells of the mesenchymal lineage, on the other hand, partially decreased the age-dependent decline in mineralizing surfaces and the loss of cortical bone. These results suggest that the mechanisms responsible for the loss of cortical bone in acute sex steroid deficiency and old age are distinct.
In our mouse model of restrained mitochondria H2O2 accumulation in cells of the myeloid lineage, we observed a striking difference in the effect of sex steroid deficiency on the cortical versus the cancellous bone compartment: complete prevention of the cortical bone loss in both sexes but no effect on the cancellous bone loss. These findings are in full agreement with, and strongly support, earlier observations of ours in mice with conditional deletion of the estrogen receptor (ER) α or the androgen (AR) receptor, elucidating that the antiresorptive effects of estrogens or androgens on bone in the cancellous versus the cortical bone compartment are mediated by different cell types.(6,25,26) Specifically, the protective effect of estrogens on the cancellous, but not cortical, bone compartment are mediated via signaling through the ERα expressed in cells targeted by LysM-Cre.(40,41) On the other hand, ERα signaling in mesenchymal cells targeted by Prx1-Cre is responsible for the protective effect of estrogens against endocortical resorption in females, but it plays no role in their effects on cancellous bone. The effects of androgens on cancellous bone result from AR signaling in mature osteoblasts but not on osteoclasts or via aromatization to estrogens.(26,42–45) Moreover, the effects of androgens on cortical bone do not require AR in any cell of the mesenchymal lineage or ERα signaling in the osteoblast lineage, nor do they require AR or ERα signaling in cells of the myeloid lineage.(26) Together with this earlier evidence, the findings of the present work suggest that the predominant mechanism mediating the effects of estrogens and androgens on cancellous bone osteoclasts are independent of H2O2 generated in the mitochondria of these cells.
In cortical bone, estrogen signaling on mesenchymal cells attenuates endocortical resorption in females.(25) The demonstration of a protective effect of the mitoCAT transgene expression in LysM-Cre-targeted cells against the loss of cortical bone caused by OVX in the present work suggests that paracrine signals originating from mesenchymal cells stimulate osteoclastogenesis in an H2O2-dependent manner. In support of such mechanism, we found that estrogens attenuate the expression of SDF1 in mesenchymal cells. The major source of SDF1 is CXCL12 abundant reticular (CAR) cells, a heterogeneous population closely associated with the perivascular niches in the bone marrow, which can be targeted with Prx1- or Osx1-Cre.(37,46) The receptor for SDF1 is CXCR4,(47) a G-protein-coupled receptor that is expressed in several cell types including monocytes and osteoclasts in humans and rodents,(38,48) indicating that myeloid cells are targets of SDF1 action. Consistent with this, specific antagonists of CXCR4 attenuate bone loss at sites of inflammation in the synovium and bone in collagen-induced arthritis,(49) osteoclastogenesis in multiple myeloma,(50) and also ameliorate the effects of OVX in mice.(51) Notably, in some cell types, the actions of SDF1 require ROS.(52) Accordingly, we found that recombinant SDF1 stimulates osteoclastogenesis, but this effect is abrogated in bone marrow–derived macrophages from mitoCAT;LysM-Cre mice.
The protective effect of mitoCAT gene expression in LysM-Cre-targeted cells against the loss of cortical bone caused by OVX was recapitulated in ORX mice. In line with this, we obtained compelling in vivo and in vitro evidence in support of the working hypothesis that in male mice the protective effect of androgens on cortical bone might be also mediated via ERα signaling (upon aromatization of androgens to estrogens) in cells of the mesenchymal lineage and suppression of SDF1 (Supplemental Fig. S8).(26,53) This working hypothesis can explain the observation that both female and male mitoCAT; LysM-Cre mice are protected from the effects of gonadectomy on cortical bone. Of note, in men, estrogens account for ~70% and testosterone for at most ~30% of the protective effect of sex steroids on bone resorption, remarkably consistent with the fact that the skeleton is ~80% cortical and ~20% cancellous.
Earlier studies by us and others in mice had suggested that the culprits of the age-related loss of bone mass are aging-related mechanisms intrinsic to bone, including oxidative stress and declining autophagy.(7,54,55) Age-related changes in other organs and tissues may also contribute, but the extent of the contribution of sex steroid deficiency to the skeletal involution later in life has remained unclear.(11,56,57) In the present work, we found that attenuation of H2O2 produced in the mitochondria of mesenchymal cells attenuated the cortical thinning and the expansion of the medullary cavity caused by old age; this effect was associated with attenuation of the age-dependent decline in mineralizing surfaces. These observations suggest that part of the mechanisms of aging leading to the decline of cortical bone mass is an increase in H2O2 generation or a decline in the mechanisms that inactivate H2O2 in cells of the mesenchymal lineage. Additional mechanisms, such as osteoprogenitor senescense causing proliferation arrest, may also contribute to the decline of osteoblast numbers.(58) Be that as it may, our findings are consistent with extensive evidence from animals and humans that decreased bone formation is a seminal mechanism of the decreased bone mass in old age.(56) Along with evidence that suppression of mitochondrial ROS prolongs life span and attenuates several age-related pathologies, including cardiovascular disease, insulin resistance, and neurodegeneration,(22,59–61) our findings also support the idea that an increase in ROS is a fundamental mechanism of aging.
Several cell types along the differentiation progression of the osteoblast lineage have been previously implicated in the decline in bone formation with age.(62,63) For example, a decline of bone marrow–derived mesenchymal or osteoblast progenitor function with age has been shown in both human and rodent cells.(64–67) Such decline might be attributable to the accumulation of oxidative damage, as osteoprogenitors from old rats exhibit higher levels of oxidized proteins and lipids compared with cells from young rats.(68) Consistent with this evidence, activation of FoxO transcription factors in osteoblast progenitors, likely resulting from the accumulation of age-associated cellular stressors including oxidative stress, decreases the number of osteoblasts and bone mass in mice by attenuating Wnt signaling.(69,70) The long-lived osteocytes may also play a role in the protective effects of mitoCAT on aging bone observed in the present work. Support for this possibility is provided by the findings that an increase in ROS, owing to targeted deletion of the antioxidant enzyme MnSOD in osteocytes, causes a decrease in bone formation and bone mass in young mice.(71) Because Prx1-Cre causes recombination in mesenchymal progenitors and all their descendants, it remains unclear which cell type is the target of the protective effects of mitoCAT on bone formation. Future work with targeted expression of mitoCAT in other osteoblast lineage cell populations should clarify this issue.
The decline of osteoblast numbers in the endocortical surface aside, we have documented in the present report that with old age the osteoclast numbers are increased in that surface. Moreover, we had demonstrated earlier high osteoclast numbers within cortical pores of aged mice.(55) Attenuation of H2O2 in the mitochondria of cells of the mesenchymal lineage, nonetheless, had no effect on osteoclast numbers in the endocortical surface or cortical porosity. Likewise, attenuation of H2O2 in cells of the myeloid lineage had no effect on the age-dependent decline of cortical or cancellous bone mass. Yet, attenuation of H2O2 in cells of the myeloid lineage attenuated the gonadectomy-induced increase in ROS levels in the bone marrow in either sex and the increase in osteoclast number at the endocortical bone surface. Therefore, the increased osteoclast numbers and resorption in the endocortical surface caused by aging must be the result of distinct mechanisms other than those operating in sex steroid deficiency. An increase in RANKL and decrease in its antagonist osteoprotegerin has been proposed as mechanisms responsible for the increased osteoclast number in old age, but a functional link between these changes and osteoclast number in old age has not been demonstrated.(72)
Attenuation of H2O2 in myeloid cells, likewise, did not alter the increase in cortical porosity with age, indicating that the mechanism responsible for this effect overrides the inhibitory actions of mitoCAT on osteoclast generation. Although the cellular and molecular mechanisms responsible for the increase in cortical porosity with age remain unclear, there is evidence to suggest that osteocytes are the mediators of this effect.(73,74) Indeed, mice lacking the pro-apoptotic proteins Bax and Bak in osteocytes exhibit exacerbated cortical porosity with age,(55) and inhibition of autophagy in osteocytes causes cortical porosity in young mice.(54) These findings, along with the results presented here, suggest that the mechanism underlying the osteocyte-mediated increase in cortical porosity is independent of ROS.
Mice do not normally exhibit osteonal remodeling. Albeit, osteonal organization is likely a consequence of body size and habitual loading related to it, rather than of phylogenetic origin. In fact, histologic evidence supports the contention that rodents do, indeed, have osteonal remodeling but not as well organized as humans.(75) We are, therefore, quick to point out that caution must be exercised in translating the cortical porosity findings of the present study with mice to human cortical bone.
In conclusion, our results show that in the mouse, the decline of cortical and cancellous bone mass and the development of cortical porosity with advancing age are independent of sex steroid deficiency. Moreover, our findings suggest that the mechanisms responsible for the adverse effects of aging and sex steroid deficiency on bone homeostasis are distinct. The effects of sex steroid deficiency result primarily, if not exclusively, from an increase in osteoclast number. This is owing in part to H2O2 accumulation in cell of the osteoclast lineage; albeit, the proximal molecular changes and the cells in which they occur are different in the cancellous versus the cortical bone compartments. The effects of aging, on the other hand, result from a decline of osteoblast progenitors owing in part to mitochondrial dysfunction, ROS accumulation, and DNA damage, as well as increased osteoclastogenesis caused probably by other mechanisms of aging (independent of sex steroid deficiency), such as the senescence-associated secretory phenotype (SASP) and inflammation.(76) The elucidation of distinct pathogenetic mechanisms responsible for the effects of systemic changes (such as aging and sex steroid deficiency) in one compartment versus the other supports the fundamental notion that the progenitors responsible for the supply of the executive cells of bone remodeling are greatly influenced by microenvironmental niches prevailing in the cancellous versus the cortical bone compartment.
Acknowledgments
This work was supported by the National Institutes of Health (P01 AG13918 to SCM), R01 AR56679 to MA); the Biomedical Laboratory Research and Development Service of the Veteran’s Administration Office of Research and Development (I01 BX001405 to SCM); and the University of Arkansas for Medical Sciences Tobacco Funds and Translational Research Institute (1UL1RR029884). RdC is supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health.
We thank S Bartell, A Warren, and J Crawford for technical assistance, and K Poe for help with the preparation of the manuscript.
Authors’ roles: MA and SCM designed the experiments and SU, MA, SI, and SCM analyzed the data. SU, SI, and CR carried out DXA, micro-CT, and histomorphometry analyses. HNK, KA, and LH performed in vitro studies. RdC performed microarray analysis. JT performed statistical analysis. CAO and RLJ provided technical advice. SU, SI, MA, CAO, RLJ, and SCM discussed results. SU, SI, MA, and SCM wrote the paper. All authors revised the manuscript.
Footnotes
Disclosures
SCM serves on the scientific advisory board of Radius Health, Inc. He has ownership of equity in this company and receives $10,000 per annum for his SAB service. All other authors state that they have no conflicts of interest.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21(2):115–37. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 2.Parfitt AM, Villanueva AR, Foldes J, Rao DS. Relations between histologic indices of bone formation: implications for the pathogenesis of spinal osteoporosis. J Bone Miner Res. 1995;10:466–73. doi: 10.1002/jbmr.5650100319. [DOI] [PubMed] [Google Scholar]
- 3.Han ZH, Palnitkar S, Rao DS, Nelson D, Parfitt AM. Effects of ethnicity and age or menopause on the remodeling and turnover of iliac bone: implications for mechanisms of bone loss. J Bone Miner Res. 1997;12(4):498–508. doi: 10.1359/jbmr.1997.12.4.498. [DOI] [PubMed] [Google Scholar]
- 4.Zebaze RM, Ghasem-Zadeh A, Bohte A, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375(9727):1729–36. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 5.Nicks KM, Amin S, Atkinson EJ, Riggs BL, Melton LJ, III, Khosla S. Relationship of age to bone microstructure independent of areal bone mineral density. J Bone Miner Res. 2012;27(3):637–44. doi: 10.1002/jbmr.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manolagas SC, O’Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol. 2013;9(12):699–712. doi: 10.1038/nrendo.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida M, Han L, Martin-Millan M, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282(37):27285–97. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jilka RL, Almeida M, Ambrogini E, et al. Decreased oxidative stress and greater bone anabolism in the aged, as compared to the young, murine skeleton by parathyroid hormone. Aging Cell. 2010;9(5):851–67. doi: 10.1111/j.1474-9726.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ. Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology. 2005;146(2):728–35. doi: 10.1210/en.2004-1021. [DOI] [PubMed] [Google Scholar]
- 10.Lean JM, Davies JT, Fuller K, et al. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest. 2003;112(6):915–23. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31(3):266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 15.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–95. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 16.D’Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8(10):813–24. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 17.Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990;85:632–9. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha H, Kwak HB, Lee SW, et al. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp Cell Res. 2004;301(2):119–27. doi: 10.1016/j.yexcr.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 19.Lee NK, Choi YG, Baik JY, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106(3):852–9. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 20.Ishii KA, Fumoto T, Iwai K, et al. Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med. 2009;15(3):259–66. doi: 10.1038/nm.1910. [DOI] [PubMed] [Google Scholar]
- 21.Bartell SM, Kim HN, Ambrogini E, et al. FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat Commun. 2014;5:3773. doi: 10.1038/ncomms4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai DF, Johnson SC, Villarin JJ, et al. Mitochondrial oxidative stress mediates angiotensin II–induced cardiac hypertrophy and g{alpha}q overexpression-induced heart failure. Circ Res. 2011;108(7):837–46. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33(2):77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 24.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127(19):4277–91. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 25.Almeida M, Iyer S, Martin-Millan M, et al. Estrogen receptor-alpha signaling in osteoblast progenitors stimulates cortical bone accrual. J Clin Invest. 2013;123(1):394–404. doi: 10.1172/JCI65910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ucer S, Iyer S, Bartell SM, et al. The effects of androgens on murine cortical bone do not require AR or ERalpha signaling in osteoblasts and osteoclasts. J Bone Miner Res. 2015;30(7):1138–49. doi: 10.1002/jbmr.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005;280(50):41342–51. doi: 10.1074/jbc.M502168200. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Weinstein RS, Jia D, Powers CC, et al. The skeletal effects of glucocorticoid excess override those of orchidectomy in mice. Endocrinology. 2004;145(4):1980–7. doi: 10.1210/en.2003-1133. [DOI] [PubMed] [Google Scholar]
- 30.Mobbs CV, Cheyney D, Sinha YN, Finch CE. Age-correlated and ovary-dependent changes in relationships between plasma estradiol and luteinizing homrone, prolactin, and growth hormone in female C57BL/6J mice. Endocrinology. 1985;116:813–20. doi: 10.1210/endo-116-2-813. [DOI] [PubMed] [Google Scholar]
- 31.Nelson JF, Latham KR, Finch CE. Plasma testosterone levels in C57BL/6J male mice: effects of age and disease. Acta Endocrinol (Copenh) 1975 Dec;80(4):744–52. doi: 10.1530/acta.0.0800744. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson ME, Vandenput L, Tivesten A, et al. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology. 2015;156(7):2492–502. doi: 10.1210/en.2014-1890. [DOI] [PubMed] [Google Scholar]
- 33.Nelson JF, Felicio LS, Osterburg HH, Finch CE. Differential contributions of ovarian and extraovarian factors to age-related reductions in plasma estradiol and progesterone during the estrous cycle of C57BL/6J mice. Endocrinology. 1992;130:805–10. doi: 10.1210/endo.130.2.1733727. [DOI] [PubMed] [Google Scholar]
- 34.Lacombe A, Lelievre V, Roselli CE, et al. Delayed testicular aging in pituitary adenylate cyclase-activating peptide (PACAP) null mice. Proc Natl Acad Sci U S A. 2006;103(10):3793–8. doi: 10.1073/pnas.0505827103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999 Aug;8(4):265–77. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 36.Kollet O, Dar A, Shivtiel S, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12(6):657–64. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 37.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–30. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu X, Huang Y, Collin-Osdoby P, Osdoby P. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J Bone Miner Res. 2003;18(8):1404–18. doi: 10.1359/jbmr.2003.18.8.1404. [DOI] [PubMed] [Google Scholar]
- 39.Grassi F, Cristino S, Toneguzzi S, Piacentini A, Facchini A, Lisignoli G. CXCL12 chemokine up-regulates bone resorption and MMP-9 release by human osteoclasts: CXCL12 levels are increased in synovial and bone tissue of rheumatoid arthritis patients. J Cell Physiol. 2004;199(2):244–51. doi: 10.1002/jcp.10445. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura T, Imai Y, Matsumoto T, et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130(5):811–23. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 41.Martin-Millan M, Almeida M, Ambrogini E, et al. The estrogen receptor alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol. 2010;24(2):323–34. doi: 10.1210/me.2009-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiang C, Chiu M, Moore AJ, et al. Mineralization and bone resorption are regulated by the androgen receptor in male mice. J Bone Miner Res. 2009;24(4):621–31. doi: 10.1359/jbmr.081217. [DOI] [PubMed] [Google Scholar]
- 43.Notini AJ, McManus JF, Moore A, et al. Osteoblast deletion of exon 3 of the androgen receptor gene results in trabecular bone loss in adult male mice. J Bone Miner Res. 2007;22(3):347–56. doi: 10.1359/jbmr.061117. [DOI] [PubMed] [Google Scholar]
- 44.Sinnesael M, Claessens F, Laurent M, et al. Androgen receptor (AR) in osteocytes is important for the maintenance of male skeletal integrity: evidence from targeted AR disruption in mouse osteocytes. J Bone Miner Res. 2012;27(12):2535–43. doi: 10.1002/jbmr.1713. [DOI] [PubMed] [Google Scholar]
- 45.Maatta JA, Buki KG, Ivaska KK, et al. Inactivation of the androgen receptor in bone-forming cells leads to trabecular bone loss in adult female mice. Bonekey Rep. 2013;2:440. doi: 10.1038/bonekey.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12–CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 48.Wright LM, Maloney W, Yu X, Kindle L, Collin-Osdoby P, Osdoby P. Stromal cell-derived factor-1 binding to its chemokine receptor CXCR4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone. 2005;36(5):840–53. doi: 10.1016/j.bone.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 49.De Klerck B, Geboes L, Hatse S, et al. Pro-inflammatory properties of stromal cell-derived factor-1 (CXCL12) in collagen-induced arthritis. Arthritis Res Ther. 2005;7(6):R1208–20. doi: 10.1186/ar1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandey MK, Kale VP, Song C, et al. Gambogic acid inhibits multiple myeloma mediated osteoclastogenesis through suppression of chemokine receptor CXCR4 signaling pathways. Exp Hematol. 2014;42(10):883–96. doi: 10.1016/j.exphem.2014.07.261. [DOI] [PubMed] [Google Scholar]
- 51.Im JY, Min WK, Park MH, et al. AMD3100 improves ovariectomy-induced osteoporosis in mice by facilitating mobilization of hematopoietic stem/progenitor cells. BMB Rep. 2014;47(8):439–44. doi: 10.5483/BMBRep.2014.47.8.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pi X, Xie L, Portbury AL, et al. NADPH oxidase-generated reactive oxygen species are required for stromal cell-derived factor-1alpha-stimulated angiogenesis. Arterioscler Thromb Vasc Biol. 2014;34(9):2023–32. doi: 10.1161/ATVBAHA.114.303733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iyer S, Ucer S, Kim HN, et al. Estrogens protect against endocortical bone resorption in both female and male mice; likely via an ERa-mediated suppression of SDF1/CXCL12 in uncommitted mesenchymal progenitors. J Bone Miner Res. 2015;30S1:148. [Google Scholar]
- 54.Onal M, Piemontese M, Xiong J, et al. Suppression of autophagy in osteocytes mimics skeletal aging. J Biol Chem. 2013;288(24):17432–40. doi: 10.1074/jbc.M112.444190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jilka RL, O’Brien CA, Roberson PK, Bonewald LF, Weinstein RS, Manolagas SC. Dysapoptosis of osteoblasts and osteocytes increases cancellous bone formation but exaggerates cortical porosity with age. J Bone Miner Res. 2014;29(1):103–17. doi: 10.1002/jbmr.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab. 2010;21(6):369–74. doi: 10.1016/j.tem.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khosla S, Melton LJ, III, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Miner Res. 2011;26(3):441–51. doi: 10.1002/jbmr.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang C, Xu Q, Martin TD, et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349(6255):aaa5612. doi: 10.1126/science.aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schriner SE, Linford NJ, Martin GM, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308(5730):1909–11. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 60.Lee HY, Choi CS, Birkenfeld AL, et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010;12(6):668–74. doi: 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mao P, Manczak M, Calkins MJ, et al. Mitochondria-targeted catalase reduces abnormal APP processing, amyloid beta production and BACE1 in a mouse model of Alzheimer’s disease: implications for neuroprotection and lifespan extension. Hum Mol Genet. 2012;21(13):2973–90. doi: 10.1093/hmg/dds128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kassem M, Marie PJ. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell. 2011;10(2):191–7. doi: 10.1111/j.1474-9726.2011.00669.x. [DOI] [PubMed] [Google Scholar]
- 63.Almeida M, O’Brien CA. Basic biology of skeletal aging: role of stress response pathways. J Gerontol A Biol Sci Med Sci. 2013;68(10):1197–208. doi: 10.1093/gerona/glt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33(6):919–26. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5(1):91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Kasper G, Mao L, Geissler S, et al. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells. 2009;27(6):1288–97. doi: 10.1002/stem.49. [DOI] [PubMed] [Google Scholar]
- 67.Coipeau P, Rosset P, Langonne A, et al. Impaired differentiation potential of human trabecular bone mesenchymal stromal cells from elderly patients. Cytotherapy. 2009;11(5):584–94. doi: 10.1080/14653240903079385. [DOI] [PubMed] [Google Scholar]
- 68.Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006;5(3):213–24. doi: 10.1111/j.1474-9726.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 69.Almeida M, Han L, Martin-Millan M, O’Brien CA, Manolagas SC. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem. 2007;282(37):27298–305. doi: 10.1074/jbc.M702811200. [DOI] [PubMed] [Google Scholar]
- 70.Iyer S, Ambrogini E, Bartell SM, et al. FoxOs attenuate bone formation by suppressing Wnt signaling. J Clin Invest. 2013;123(8):3404–19. doi: 10.1172/JCI68049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kobayashi K, Nojiri H, Saita Y, et al. Mitochondrial superoxide in osteocytes perturbs canalicular networks in the setting of age-related osteoporosis. Sci Rep. 2015;5:9148. doi: 10.1038/srep09148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao J, Venton L, Sakata T, Halloran BP. Expression of RANKL and OPG correlates with age-related bone loss in male C57BL/6 mice. J Bone Miner Res. 2003;18(2):270–7. doi: 10.1359/jbmr.2003.18.2.270. [DOI] [PubMed] [Google Scholar]
- 73.Manolagas SC, Parfitt AM. For whom the bell tolls: distress signals from long-lived osteocytes and the pathogenesis of metabolic bone diseases. Bone. 2013;54(2):272–8. doi: 10.1016/j.bone.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jilka RL, O’Brien CA. The role of osteocytes in age-related bone loss. Curr Osteoporos Rep. 2016;14(1):16–25. doi: 10.1007/s11914-016-0297-0. [DOI] [PubMed] [Google Scholar]
- 75.Vanderschueren D, Laurent MR, Claessens F, et al. Sex steroid actions in male bone. Endocr Rev. 2014;35(6):906–60. doi: 10.1210/er.2014-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]