Abstract
Limited head-to-head comparative safety and effectiveness data exist between denosumab and zoledronic acid in real-world healthcare. We aimed to examine the safety and effectiveness of denosumab compared to zoledronic acid with regard to risk of serious infection and cardiovascular disease (CVD) and osteoporotic fracture. We conducted a cohort study using claims data (2009-2013) from a US commercial insurance. We included patients aged ≥50 years who newly initiated denosumab or zoledronic acid. The primary outcomes were 1) hospitalization for serious infection, 2) composite CVD endpoint including myocardial infarction, stroke, coronary revascularization, and heart failure and 3) non-vertebral osteoporotic fracture including hip, wrist, forearm and pelvic fracture. To control for potential confounders, we used 1:1 propensity score matching. Cox proportional hazards models compared the risk of serious infection, CVD and osteoporotic fracture within 365 days after initiation of denosumab versus zoledronic acid. After PS matching, a total of 2,467 pairs of denosumab and zoledronic acid initiators were selected with a mean age of 63 years and 96% were female. When compared with zoledronic acid, denosumab was not associated with an increased risk of serious infection (HR 0.81, 95% confidence interval [CI] 0.55-1.21) or CVD (HR 1.11, 95% CI 0.60-2.03). Similar results were obtained for each component of CVD. The risk of osteoporotic fracture was also similar between groups (HR 1.21, 95% CI 0.84-1.73). This large population-based cohort study shows that denosumab and zoledronic acid have comparable clinical safety and effectiveness with regard to the risk of serious infection, CVD and osteoporosis fracture within 365 days after initiation of medications.
Keywords: Denosumab, Zoledronic Acid, Infection, Cardiovascular Disease, Osteoporotic Fracture
Background
Osteoporosis continues to pose major health threats to the ageing population. Denosumab is the first biologic agent approved for men or postmenopausal women with osteoporosis who are at high risk for fractures. It is a fully human monoclonal antibody and inhibits bone resorption by binding to the receptor activator of nuclear factor- kappa B ligand (RANKL), thereby decreasing the differentiation of osteoclasts. Denosumab has shown sustained efficacy in increasing bone mineral density and decreasing fracture risk, with data now through 8 years of use (1, 2).
While the benefits of denosumab have been documented, several potential safety issues have been raised. Given RANKL inhibition of non-skeletal immune cells potentially causing immunosuppression (3), there is a theoretical concern for infection with denosumab. In the pivotal trial of denosumab, the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial, serious infections were more frequently noted in the denosumab group than placebo (1). Moreover, osteoporosis and atherosclerosis share common risk factors and pathophysiologic mechanisms. While it is not fully understood whether use of denosumab leads to increased risk of vascular calcification and cardiovascular disease (CVD) (4-6), many previous epidemiologic studies support a positive association between serum osteoprotegerin (OPG), which has essentially the same mechanism of action as denosumab, and atherosclerosis, vascular calcification, or CV disease in human (7-9). In contrast, beneficial anti-atherosclerotic effects of denosumab have been also reported in animal studies (10, 11). Nevertheless, relevant data in humans are still insufficient and inconclusive.
In terms of effectiveness, denosumab is effective in reducing the risk of vertebral, non-vertebral, and hip fractures in the FREEDOM trial (1). However, despite the availability of alternative therapeutic compounds to reduce fracture risk, this trial was placebo-controlled rather than comparing different drugs head-to-head. In addition, there are a growing number of reports of atypical femur fractures in denosumab users (12-15).
Zoledronic acid is an intravenous bisphosphonate administered annually and thought to have similar efficacy compared to denosumab, which is given biannually. In a large randomized placebo-controlled trial, zoledronic acid resulted in significantly lower rates of vertebral fractures, hip fractures, and non-vertebral fractures (16). Zoledronic acid may cause acute flu-like symptoms for up to 3 days after the first infusion (17) and has been associated with the possibility of an increased risk of atrial fibrillation (16, 18).
To date, limited head-to-head comparative safety or effectiveness data exists between denosumab and zoledronic acid in real-world settings. Aggregate evidence on both risks and benefits is a key component in improving clinical decision-making in the management of osteoporosis. To fill this evidence gap, we examined the safety and effectiveness of denosumab compared to zoledronic acid for serious infection, CVD and osteoporotic fracture in patients with osteoporosis.
Methods
Data Source
We conducted a cohort study using the claims data from United HealthCare, a large commercial U.S. health plan, for the period January 1, 2009 to December 31, 2013. This database contains longitudinal claims information including medical diagnoses, procedures, hospitalizations, physician visits, and pharmacy dispensing on more than 13 million fully-insured subscribers with medical and pharmacy coverage at any particular time point across the United States. Personal identifiers were removed from the dataset before the analysis to protect subject confidentiality. Patient informed consent was therefore not required. The study protocol was approved by the Institutional Review Board of the Brigham and Women's Hospital.
Study Cohort
We included patients aged 50 years or older who newly initiated on denosumab or zoledronic acid. The index date was defined as the first date of study medication denosumab or zoledronic acid was prescribed. Because zoledronic acid is given yearly, we defined the new users based on a minimum of 455 days of continuous insurance eligibility without record of denosumab or zoledronic acid dispensing before the index date. To eliminate patients who might receive osteoporosis medications for other indications, we excluded patients who had a diagnosis of malignancy or received chemotherapy or oncology radiation services in the 455-day period prior to the index date. Patients who received a diagnosis of human immunodeficiency virus infection or underwent bone marrow or solid organ transplantation, renal dialysis during the 455-day baseline period were excluded. Because the study database has no data from nursing home stay, we further excluded nursing home residents at baseline. Follow-up starts 1 day after the index date and continued through the earliest of the following dates: occurrence of the study outcomes, switch to another regimen (denosumab to zoledronic acid or zoledronic acid to denosumab), 365th day of follow-up, insurance disenrollment, end of study period (December 31, 2013), or death.
Outcome Definition
The outcomes of interest were 1) serious infection, 2) composite CVD endpoint and 3) nonvertebral osteoporotic fracture. Serious infection was defined as infections that required hospitalization based on principal or secondary discharge International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnoses codes (Appendix Table 1) (19). The positive predictive value (PPV) for this algorithm was reported to be at least 80% (20, 21). Patients were at risk only for their first hospitalized infection in the analysis. Patients could have experienced more than one infection in a single hospitalization (e.g., sepsis and pneumonia). However, all infections occurring during each hospitalization were treated as one serious infection. We defined a composite CVD endpoint as the first occurrence of nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or heart failure after the index date, according to inpatient principal or secondary diagnosis codes and/or procedure codes (Appendix Table 1) (22). In addition, we assessed each component of the composite CVD endpoint separately. In prior studies, the positive predictive values of these claims-based algorithms for cardiovascular events were at least 80% (23-26). We also defined any non-vertebral osteoporotic fracture including hip, wrist, forearm and pelvic fracture by diagnosis or procedure codes (Appendix Table 1). These claims-based algorithms were found to have a PPV over 93% (27, 28). Hospital admission, diagnosis, or procedure dates were used as the date of outcome occurrence according to each outcome definitions. Patients were at risk only for their first serious infection, CVD endpoint, or non-vertebral osteoporotic fracture in the analysis. Each outcome was indentified separately, so a patient could contribute one or more outcome incidents in different models.
Covariates
Patients' baseline variables potentially related to initiation of denosumab or zoledronic acid and development of infection, CVD, and osteoporotic fracture were examined using data from the 455 days before the index date. These variables (listed in Table 1) included demographic factors, markers of health care utilization intensity, vaccination for flu and pneumonia, comorbidities, use of other osteoporosis medications (bisphosphonates except zoledronic acid, calcitonin, raloxifene, and parathyroid hormone), and use of other drugs. To quantify patients' comorbidities further, we also calculated a comorbidity score that combined conditions included in both the Charlson Index and the Elixhauser system based on the ICD-9-CM (29).
Table 1.
Baseline characteristics of propensity score-matched cohorts in the 455 days before the index date
| Characteristic | Denosumab N=2,467 | Zoledronic acid N=2,467 | Standard difference |
|---|---|---|---|
| Demographic | |||
| Age (years) | 63.2±8.4 | 63.2±8.6 | <0.01 |
| Male sex | 3.9 | 4.4 | −0.03 |
| Regions | 0.03 | ||
| Midwest | 21.5 | 21.6 | |
| Northeast | 6.8 | 7.2 | |
| South | 55.2 | 55.7 | |
| West | 16.5 | 15.5 | |
| History of healthcare utilizations | |||
| Number of hospitalizations | 0.3±1.1 | 0.3±1.2 | −0.02 |
| Number of hospitalizations for infections | 0.03±0.2 | 0.04±0.2 | −0.04 |
| Number of outpatient infection diagnoses | 1.8±3.6 | 1.9±4.7 | −0.04 |
| Number of outpatient visits | 14.6±13.2 | 15.4±14.7 | −0.06 |
| Number of emergency room visits | 0.3±1.0 | 0.3±0.9 | <0.01 |
| Number of cardiology visits | 0.9±2.3 | 0.7±2.3 | 0.07 |
| Number of endocrinology visits | 0.6±1.5 | 0.7±1.6 | −0.01 |
| Number of rheumatology visits | 1.3±3.3 | 1.6±3.3 | −0.07 |
| Bone density mineral test | 84.4 | 84.5 | 0.00 |
| Flu vaccination | 39.2 | 38.9 | 0.01 |
| Pneumonia vaccination | 6.0 | 6.0 | <0.01 |
| History of comorbidities | |||
| Combined comorbidity score | 0.5±1.4 | 0.6±1.4 | −0.04 |
| Osteoporosis | 95.5 | 95.2 | 0.02 |
| Fracture | 15.4 | 15.8 | −0.01 |
| Inflammatory arthritis | 19.2 | 22.5 | −0.08 |
| Chronic obstructive pulmonary disease | 5.3 | 5.5 | −0.01 |
| Diabetes mellitus | 10.6 | 10.5 | 0.00 |
| Chronic kidney disease | 4.1 | 4.3 | −0.01 |
| Hypertension | 43.3 | 44.0 | −0.01 |
| Prior acute myocardial infarction | 0.5 | 0.5 | 0.01 |
| Other ischemic heart disease | 1.3 | 1.4 | −0.01 |
| Other heart disease | 2.0 | 2.7 | −0.05 |
| Stroke | 1.7 | 1.6 | 0.00 |
| Heart failure | 9.0 | 8.4 | 0.02 |
| Coronary revascularization | 20.3 | 21.0 | −0.02 |
| Smoking | 12.0 | 12.8 | −0.02 |
| Obesity | 4.3 | 4.0 | 0.02 |
| History of medication use | |||
| Other osteoporosis medication use | 30.0 | 30.2 | <0.01 |
| Other bisphosphonates except zoledronic acid | 18.7 | 18.4 | 0.01 |
| Calcitonin | 1.7 | 1.8 | −0.01 |
| Raloxifene | 4.5 | 4.6 | −0.01 |
| Parathyroid hormone | 6.6 | 6.9 | −0.01 |
| Antibiotics | 67.7 | 69.0 | −0.03 |
| Anticoagulants | 5.2 | 4.5 | 0.03 |
| Antidepressants | 30.9 | 31.1 | −0.01 |
| Antihypertensives | 41.1 | 41.9 | −0.02 |
| Antiplatelets/antithrombotics | 3.6 | 5.0 | −0.07 |
| Biologic DMARDs | 3.9 | 6.1 | −0.10 |
| Lipid lowerings | 35.6 | 35.6 | 0.00 |
| Non-biological non-specific immunosuppressive agents | 11.3 | 13.1 | −0.05 |
| NSAIDs and Coxibs | 30.0 | 32.3 | −0.05 |
| Opioids | 40.6 | 41.4 | −0.02 |
| Proton pump inhibitors | 24.3 | 27.8 | −0.08 |
| Steroids, oral | 26.6 | 27.8 | −0.03 |
| Oral steroid use in 90 day period | 11.0 | 11.4 | −0.01 |
| Cumulative prednisone equivalent dose in mg | 237±943 | 298±1,117 | −0.06 |
| Steroids, injectable | 22.6 | 24.2 | −0.04 |
Values are percentage or mean ±SD. NSAIDs = nonsteroidal anti-inflammatory drugs; Coxibs = selective cyclooxygenase-2 inhibitors, DMARD= disease-modifying antirheumatic drug
Statistical Analyses
To control for potential confounders, we used propensity score (PS) matching. We used a single, generic-outcome model which includes all measured covariates affecting any of the outcomes (30). Multivariable logistic regression models including all the aforementioned baseline covariates estimated the PS, defined as the predicted probability of a patient starting denosumab compared with zoledronic acid. We then used a 5-to-1 digit greedy matching algorithm with 1:1 ratio. After assembling the matched cohorts, baseline characteristics were compared between denosumab and zoledronic acid users by calculating standardized mean difference. We defined imbalance as an absolute value greater than 0.1 (31).
For each outcome, we estimated the incidence rate (IR) with 95% CIs for denosumab and zoledronic acid users. The incidence rate ratio (IRR) with 95% CIs was calculated as the IR among denosumab users divided by the IR among zoledronic acid users.
Cox proportional hazards model compared the risk of serious infection, CVD and osteoporotic fracture within 365 days after initiation of denosumab versus zoledronic acid. The proportional hazard assumptions were tested by adding an interaction term of exposure and follow-up time to the model for each outcome and were not violated in any models.
We conducted sensitivity analyses to assess the robustness of the findings. First, we stratified patients by use of biologic disease-modifying antirheumatic drugs (DMARDs) at baseline. Subgroups analyses were performed after 1:1 ratio PS matching for both of users and non-users of biologic DMARDs. Second, due to different risk profiles in women and men, stratified analysis by sex were performed. Third, to avoid including reactions which were not related to the exposure, we excluded the outcomes that occurred on the first day of followup. Fourth, we applied 60, 90, 180, or 270 days of follow-up time periods in order to see whether results would change substantially according to the different follow-up days. All analyses were run using SAS (Cary, North Carolina, USA), V.9.4.
Results
There were 2,760 denosumab and 5,210 zoledronic acid users in the study database (Figure 1). After 1:1 PS matching, a total of 2,467 pairs of denosumab and zoledronic acid initiators were identified. All the baseline characteristics were well-balanced between the denosumab and zoledronic acid initiators in the PS-matched cohort. Both groups had a mean age of 63 years and majority (96%) of the patients were female (Table 1). Nearly all of the patients had a diagnosis of osteoporosis (95%) at baseline and more than 84% of patients had a bone mineral density test ordered. At baseline, use of any antibiotics (68%), antihypertensives (42%), opioids (41%), and lipid-lowering medications (36%) was common in both groups.
Figure 1.
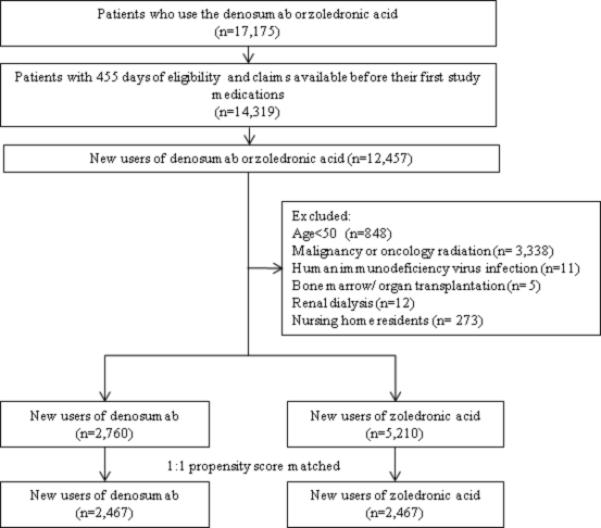
Study cohort selection. The final study cohort included a total of 2,467 propensity score-matched pairs of denosumab and zoledronic acid initiators.
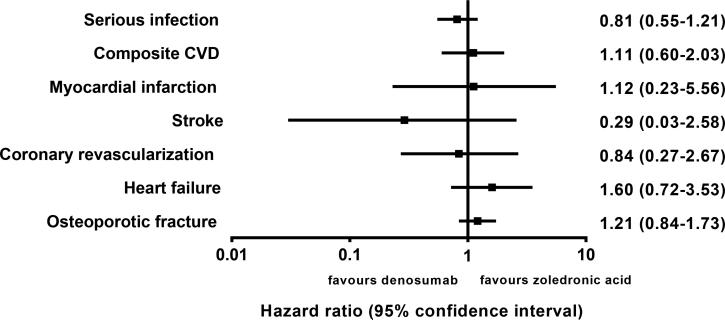
The IRs for all of the outcomes including serious infection, composite CVD, non-vertebral osteoporotic fracture were similar within 365 days after initiation of denosumab and zoledronic acid (Table 2). When compared with zoledronic acid, denosumab was not associated with an increased risk of serious infection (HR 0.81, 95% confidence interval [CI] 0.55-1.21) or CVD (HR 1.11, 95% CI 0.60-2.03) (Figure 2). Similar results were obtained for each component of the composite CVD endpoint (Figure 2).
Table 2.
Incidence rates of serious infection, composite CVD, and non-vertebral osteoporotic fracture within 365 days after initiation denosumab and zoledronic acid in the propensity score matched cohort
| Denosumab N=2,467 |
Zoledronic acid N=2,467 |
||||||
|---|---|---|---|---|---|---|---|
| Cases | Person years | IR*(95% CI) | Cases | Person years | IR*(95% CI) | IRR† (95% CI) | |
| Serious infection | 42 | 1,618 | 25.96 (18.97-34.73) | 62 | 1,994 | 31.1 (24.06-39.58) | 0.83 (0.56-1.23) |
| Composite CVD | 20 | 1,627 | 12.29 (7.74-18.61) | 22 | 2,012 | 10.93 (7.05-16.25) | 1.12 (0.61-2.05) |
| Myocardial infarction | 3 | 1,635 | 1.83 (0.51-4.90) | 3 | 2,021 | 1.48 (0.41-3.96) | 1.24 (0.25-6.14) |
| Stroke | 1 | 1,636 | 0.61 (0.06-2.85) | 4 | 2,020 | 1.98 (0.66-4.71) | 0.31 (0.03-2.77) |
| Coronary revascularization | 5 | 1,634 | 3.06 (1.16-6.71) | 7 | 2,019 | 3.47 (1.55-6.81) | 0.88 (0.28-2.77) |
| Heart failure | 14 | 1,629 | 8.59 (4.92-14.03) | 11 | 2,018 | 5.45 (2.89-9.43) | 1.58 (0.72-3.48) |
| Non-vertebral osteoporotic fracture | 60 | 1,606 | 37.36 (28.78-47.74) | 58 | 1,988 | 29.17 (22.37-37.43) | 1.28 (0.89-1.84) |
CI=confidence interval; IR=incidence rate; IRR=incidence rate ratio
Per 1,000 person-years
Zoledronic acid users were the reference group
Figure 2.
Forest plot demonstrating hazard ratios (95% confidence interval) comparing denosumab with zoledronic acid. Composite cardiovascular disease (CVD) endpoint included of myocardial infarction, stroke, coronary revascularization, and heart failure.
Subgroup analyses in 69 pairs of the matched biologic DMARD users and in 2,379 pairs of matched biologic DMARD non-users confirmed that denosumab was not associated with an increased risk of the each outcome of interest (Appendix Figure 1). However, among the biologic DMARD non-users denosumab use was associated with an increased the risk of heart failure (HR 2.51, 95% CI 1.01-6.24) compared with zoledronic acid. Subgroup analyses in 2,367 pairs of the matched women and in 94 pairs of matched men confirmed that denosumab was not associated with an increased risk of the each outcome of interest (data not shown).
A series of sensitivity analyses with excluding the outcomes that occurred on the first follow-up day as well as those with varied the duration of follow-up from 60 days to 365 days showed similar results (Appendix Table 4).
Discussion
Using a cohort which is representative of the privately insured population in the U.S., we found that the use of denosumab was not associated with any appreciable excess risk of serious infections or composite CVDs including nonfatal myocardial infarction, coronary revascularization, nonfatal stroke, and heart failure compared with use of zoledronic acid within 365 days after initiation in patients aged 50 years or older with osteoporosis. We also did not observe any differences in effectiveness evaluated by the risk of incident non-vertebral osteoporotic fracture in denosumab versus zoledronic acid users.
Our results add interesting and clinically important evidence to the existing body of literature in the pharmacologic management of osteoporosis. To date, few studies have conducted a comprehensive evaluation of effectiveness and safety of denosumab compared with zoledronic acid head-to-head. There is a previous meta-analysis of nine randomized controlled trials reporting that the safety and efficacy of denosumab for reducing fracture risk was not significantly different from bisphosphonates after 12 to 24 months despite higher gains in bone mineral density (32). The studies included in this meta-analysis used various bisphosphonates as their comparator group. One of the strengths of our study is that we performed a direct comparison of biannual subcutaneous denosumab initiators versus annual intravenous zoledronic acid initiators. Zoledronic acid among all bisphosphonates has clinical efficacy and infrequent parental dosing schedule most similar to denosumab. Because the, adherence to bisphosphonates is known to be generally poor, regardless of whether daily, weekly, or monthly dosing strategies are used, with approximately 50% of patients discontinuing therapy within the first year (33, 34) and the anti-fracture effectiveness is associated with high adherence to bisphosphonates (35, 36), it is critical to utilize a comparator drug that has similar route and frequency of administration to minimize the risk of informative or differential censoring. Because we followed up the patients upto 1-year after initiating of drugs, the adherence to zoledronic acid (which is given annually) was not an issue in this study. For adherence to denosumab, there were 1,500 denosumab users with more than 180 days of follow up. Among them 1,177 (78.5%) patients had a record of the 2nd denosumab dose. Based on these numbers, adherence to denosumab could estimate about 87% in the whole matched cohort. This high adherence of denosumab could increase the comparability of the groups.
Furthermore, we evaluated the risk of a number of different safety events as well as the risk of osteoporotic fracture as an effectiveness measure within 1-year among new users of denosumab versus zoledronic acid in a large cohort representative of a population enrolled in a nation-wide commercial health plan in the U.S. Such collective information on risks and benefits as well as the generalizability of the data is practical and valuable in clinical care, because aggregate risks need to be weighed against benefits upon choosing a pharmacologic treatment for patients with osteoporosis.
In this study, about 95% of patients had diagnosis of osteoporosis in the baseline period. That was because osteoporosis is known to be under-recorded in administrative databases in which diagnosis codes are the sole source of case ascertainment (37, 38). Thus, instead of the diagnosis of osteoporosis, use of osteoporosis drugs has been proposed for case ascertainment in pharmacy databases (37-40). In this study, as we excluded all the patients who used denosumab or zoledronic acid for potentially other indications, we believe that the rest of (about 5%) patients who had denosumab or zoledronic acid but no osteoporosis diagnosis in the 455-day baseline period most likely had osteoporosis.
We explored a potential heterogeneity of treatment effects stratified by sex and use of biologic DMARDs and found the consistent results with the main analysis. A previous cohort study of patients with rheumatoid arthritis showed a similar rate of hospitalized infection among rheumatoid arthritis patients receiving denosumab concurrently with biologic DMARDs compared to those receiving zoledronic acid and a biologic DMARDs for rheumatoid arthritis (19). Our stratified analyses showed a similar result that there was no association with an increased risk of infection between denosumab and zoledronic acid regardless of biologic DMARD use although our subgroup analysis results should be interpreted with a caution due to potentially inadequate statistical power in the subgroups. In one of the subgroup analyses, we observed an association between the use of denosumab and risk of heart failure among the biologic DMARD non-users compared with zoledronic acid. While this association could be a chance finding, it may be also biologically plausible. Denosumab and bisphosphonates including zoledronic acid are known to cause hypocalcaemia (41),(42). It is possible that hypocalcemia leads to development or exacerbation of heart failure (43). Severe hypocalcemia and subsequent QT prolongation has been also reported in patients treated with denosumab (44, 45). Nonetheless, this finding needs to be confirmed in other populations.
Our study has several limitations. First, even though we controlled for a large number of potential confounders using a propensity score matching method, there is a potential for unmeasured or residual confounding by the severity of osteoporosis and other clinical risk factors such as obesity, physical activity or frailty. However, the degree of unmeasured or residual confounding would be minimal as we used the new user design with active comparator (46). Second, our study did not examine the safety or effectiveness of denosumab versus zoledronic acid in patients with a diagnosis of malignancy or a receipt of chemotherapy or oncology services. Third, as we followed up to 365 days from the initial drug use, this study was unable to determine the long-term safety and effectiveness. Fourth, our study may not have adequate powers to detect a significant different between the treatments for some of the individual endpoints and subgroup analyses. Lastly, although we compared safety and effectiveness comprehensively, this study was not designed to examine other potential safety events such as atypical fractures, hypocalcemia and osteonecrosis of the jaw.
In conclusion, denosumab and zoledronic acid have comparable clinical safety and effectiveness with regard to the risk of serious infection, CVD and non-vertebral osteoporosis fracture in this large population-based cohort within 365 days after initiation of medications. Future research may need to examine comparative effectiveness and safety of these medications in patients with osteoporosis and other comorbidities such as immunosuppressive diseases, organ transplantation, or chronic kidney disease.
Supplementary Material
Acknowledgments
Dr. Choi has received research support from the National Research Foundation of Korea (NRF-2014R1A1A2058601 and NRF-2015K2A1A2070210). Dr. Solomon has received research support through grants from Amgen, Lilly, Pfizer, Genentech, and AstraZeneca to Brigham and Women's Hospital. Dr. Kim has received research support through grants from Pfizer, AstraZeneca, Lilly, Bristol-Myers Squibb, and Genentech to Brigham and Women's Hospital.
Authors’ roles: Study design: NKC and SCK. Study conduct: NKC. Data analysis: TNT and JEL. Data interpretation: NKC, DHS, HJS and SCK. Drafting manuscript: NKC. Revising manuscript content: DHS and SCK. Approving final version of manuscript: NKC, DHS, TNT, JEL, HJS and SCK. NKC takes responsibility for the integrity of the data analysis.
Footnotes
Potential COI Disclosures: This study had no specific funding.
References
- 1.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 2.Papapoulos S, Lippuner K, Roux C, Lin CJ, Kendler DL, Lewiecki EM, et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos Int. 2015;26(12):2773–83. doi: 10.1007/s00198-015-3234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 4.Anastasilakis AD, Toulis KA, Polyzos SA, Anastasilakis CD, Makras P. Long-term treatment of osteoporosis: safety and efficacy appraisal of denosumab. Ther Clin Risk Manag. 2012;8:295–306. doi: 10.2147/TCRM.S24239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewiecki EM. Safety and tolerability of denosumab for the treatment of postmenopausal osteoporosis. Drug Healthc Patient Saf. 2011;3:79–91. doi: 10.2147/DHPS.S7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samelson EJ, Miller PD, Christiansen C, Daizadeh NS, Grazette L, Anthony MS, et al. RANKL inhibition with denosumab does not influence 3-year progression of aortic calcification or incidence of adverse cardiovascular events in postmenopausal women with osteoporosis and high cardiovascular risk. J Bone Miner Res. 2014;29(2):450–7. doi: 10.1002/jbmr.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, et al. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109(18):2175–80. doi: 10.1161/01.CIR.0000127957.43874.BB. [DOI] [PubMed] [Google Scholar]
- 8.Jono S, Ikari Y, Shioi A, Mori K, Miki T, Hara K, et al. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation. 2002;106(10):1192–4. doi: 10.1161/01.cir.0000031524.49139.29. [DOI] [PubMed] [Google Scholar]
- 9.Knudsen ST, Foss CH, Poulsen PL, Andersen NH, Mogensen CE, Rasmussen LM. Increased plasma concentrations of osteoprotegerin in type 2 diabetic patients with microvascular complications. Eur J Endocrinol. 2003;149(1):39–42. doi: 10.1530/eje.0.1490039. [DOI] [PubMed] [Google Scholar]
- 10.Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, et al. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(-/-) mice. Circulation. 2008;117(3):411–20. doi: 10.1161/CIRCULATIONAHA.107.707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helas S, Goettsch C, Schoppet M, Zeitz U, Hempel U, Morawietz H, et al. Inhibition of receptor activator of NF-kappaB ligand by denosumab attenuates vascular calcium deposition in mice. Am J Pathol. 2009;175(2):473–8. doi: 10.2353/ajpath.2009.080957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khow KS, Yong TY. Atypical femoral fracture in a patient treated with denosumab. J Bone Miner Metab. 2015;33(3):355–8. doi: 10.1007/s00774-014-0606-6. [DOI] [PubMed] [Google Scholar]
- 13.Thompson RN, Armstrong CL, Heyburn G. Bilateral atypical femoral fractures in a patient prescribed denosumab - a case report. Bone. 2014;61:44–7. doi: 10.1016/j.bone.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 14.Farrier AJ, L CSF, Shoaib A, Gulati V, Johnson N, Uzoigwe CE, et al. New anti-resorptives and antibody mediated anti-resorptive therapy. Bone Joint J. 2016;98-B(2):160–5. doi: 10.1302/0301-620X.98B2.36161. [DOI] [PubMed] [Google Scholar]
- 15.Drampalos E, Skarpas G, Barbounakis N, Michos I. Atypical femoral fractures bilaterally in a patient receiving denosumab. Acta Orthop. 2014;85(1):3–5. doi: 10.3109/17453674.2013.854668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 17.Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab. 2010;95(9):4380–7. doi: 10.1210/jc.2010-0597. [DOI] [PubMed] [Google Scholar]
- 18.Cummings SR, Schwartz AV, Black DM. Alendronate and atrial fibrillation. N Engl J Med. 2007;356(18):1895–6. doi: 10.1056/NEJMc076132. [DOI] [PubMed] [Google Scholar]
- 19.Curtis JR, Xie F, Yun H, Saag KG, Chen L, Delzell E. Risk of hospitalized infection among rheumatoid arthritis patients concurrently treated with a biologic agent and denosumab. Arthritis Rheumatol. 2015;67(6):1456–64. doi: 10.1002/art.39075. [DOI] [PubMed] [Google Scholar]
- 20.Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH. Veteran's affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol. 2007;60(4):397–409. doi: 10.1016/j.jclinepi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Patkar NM, Curtis JR, Teng GG, Allison JJ, Saag M, Martin C, et al. Administrative codes combined with medical records based criteria accurately identified bacterial infections among rheumatoid arthritis patients. J Clin Epidemiol. 2009;62(3):321–7. 7, e1–7. doi: 10.1016/j.jclinepi.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SC, Schneeweiss S, Choudhry N, Liu J, Glynn RJ, Solomon DH. Effects of xanthine oxidase inhibitors on cardiovascular disease in patients with gout: a cohort study. Am J Med. 2015;128(6):653, e7–e16. doi: 10.1016/j.amjmed.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Choma NN, Griffin MR, Huang RL, Mitchel EF, Jr., Kaltenbach LA, Gideon P, et al. An algorithm to identify incident myocardial infarction using Medicaid data. Pharmacoepidemiol Drug Saf. 2009;18(11):1064–71. doi: 10.1002/pds.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrade SE, Harrold LR, Tjia J, Cutrona SL, Saczynski JS, Dodd KS, et al. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):100–28. doi: 10.1002/pds.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saczynski JS, Andrade SE, Harrold LR, Tjia J, Cutrona SL, Dodd KS, et al. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):129–40. doi: 10.1002/pds.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudson M, Avina-Zubieta A, Lacaille D, Bernatsky S, Lix L, Jean S. The validity of administrative data to identify hip fractures is high--a systematic review. J Clin Epidemiol. 2013;66(3):278–85. doi: 10.1016/j.jclinepi.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45(7):703–14. doi: 10.1016/0895-4356(92)90047-q. [DOI] [PubMed] [Google Scholar]
- 29.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–59. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wyss R, Girman CJ, LoCasale RJ, Brookhart AM, Sturmer T. Variable selection for propensity score models when estimating treatment effects on multiple outcomes: a simulation study. Pharmacoepidemiol Drug Saf. 2013;22(1):77–85. doi: 10.1002/pds.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Communications in Statistics-Simulation and Computation. 2009;38(6):1228–34. [Google Scholar]
- 32.Beaudoin C, Jean S, Bessette L, Ste-Marie LG, Moore L, Brown JP. Denosumab compared to other treatments to prevent or treat osteoporosis in individuals at risk of fracture: a systematic review and meta-analysis. Osteoporos Int. 2016 doi: 10.1007/s00198-016-3607-6. [DOI] [PubMed] [Google Scholar]
- 33.Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81(8):1013–22. doi: 10.4065/81.8.1013. [DOI] [PubMed] [Google Scholar]
- 34.Huas D, Debiais F, Blotman F, Cortet B, Mercier F, Rousseaux C, et al. Compliance and treatment satisfaction of post menopausal women treated for osteoporosis. Compliance with osteoporosis treatment. BMC Womens Health. 2010;10:26. doi: 10.1186/1472-6874-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curtis JR, Westfall AO, Cheng H, Lyles K, Saag KG, Delzell E. Benefit of adherence with bisphosphonates depends on age and fracture type: results from an analysis of 101,038 new bisphosphonate users. J Bone Miner Res. 2008;23(9):1435–41. doi: 10.1359/JBMR.080418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siris ES, Selby PL, Saag KG, Borgstrom F, Herings RM, Silverman SL. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med. 2009;122(2 Suppl):S3–13. doi: 10.1016/j.amjmed.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Leslie WD, Lix LM, Yogendran MS. Validation of a case definition for osteoporosis disease surveillance. Osteoporos Int. 2011;22(1):37–46. doi: 10.1007/s00198-010-1225-2. [DOI] [PubMed] [Google Scholar]
- 38.Lix LM, Yogendran MS, Leslie WD, Shaw SY, Baumgartner R, Bowman C, et al. Using multiple data features improved the validity of osteoporosis case ascertainment from administrative databases. J Clin Epidemiol. 2008;61(12):1250–60. doi: 10.1016/j.jclinepi.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Goettsch WG, de Jong RB, Kramarz P, Herings RM. Developments of the incidence of osteoporosis in The Netherlands: a PHARMO study. Pharmacoepidemiol Drug Saf. 2007;16(2):166–72. doi: 10.1002/pds.1245. [DOI] [PubMed] [Google Scholar]
- 40.Maio V, Yuen E, Rabinowitz C, Louis D, Jimbo M, Donatini A, et al. Using pharmacy data to identify those with chronic conditions in Emilia Romagna, Italy. J Health Serv Res Policy. 2005;10(4):232–8. doi: 10.1258/135581905774414259. [DOI] [PubMed] [Google Scholar]
- 41.Laskowski LK, Goldfarb DS, Howland MA, Kavcsak K, Lugassy DM, Smith SW. A RANKL Wrinkle: Denosumab-Induced Hypocalcemia. J Med Toxicol. 2016 doi: 10.1007/s13181-016-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreutle V, Blum C, Meier C, Past M, Muller B, Schutz P, et al. Bisphosphonate induced hypocalcaemia - report of six cases and review of the literature. Swiss Med Wkly. 2014;144:w13979. doi: 10.4414/smw.2014.13979. [DOI] [PubMed] [Google Scholar]
- 43.Solzbach U, Kitterer HR, Haas H. Reversible congestive heart failure in severe hypocalcemia. Herz. 2010;35(7):507–10. doi: 10.1007/s00059-010-3374-7. [DOI] [PubMed] [Google Scholar]
- 44.Jackson T, Kondic J, Seebeck J. QT-prolonging effects of monoclonal antibody drugs in humans: a systematic review of two literature and a public adverse event database. Int J Clin Pharmacol Ther. 2015;53(9):705–11. doi: 10.5414/CP202337. [DOI] [PubMed] [Google Scholar]
- 45.Oiwa H, Mokuda S. Severe hypocalcemia and prolonged QT interval due to denosumab in an elderly woman with rheumatoid arthritis and chronic kidney disease. Eur J Rheumatol. 2016 doi: 10.5152/eurjrheum.2015.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol. 2015;11(7):437–41. doi: 10.1038/nrrheum.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.