Abstract
With enhanced concerns of terrorist attacks, dual exposure to radiation and thermal combined injury (RCI) has become a real threat with devastating immunosuppression. NLRP12, a member of the NOD-like receptor family, is expressed in myeloid and bone marrow cells and has been implicated as a checkpoint regulator of inflammatory cytokines as well as an inflammasome activator. We show that NLRP12 has a profound impact on hematopoietic recovery during RCI by serving as a checkpoint of TNF signaling and preventing hematopoietic apoptosis. Using a mouse model of RCI, increased NLRP12 expression was detected in target tissues. Nlrp12−/− mice exhibited significantly greater mortality, inability to fight bacterial infection, heightened levels of pro-inflammatory cytokines, overt granulocyte/monocyte progenitor cell apoptosis and failure to reconstitute peripheral myeloid populations. Anti-TNF antibody administration improved peripheral immune recovery. These data suggest that NLRP12 is essential for survival after RCI by regulating myelopoiesis and immune reconstitution.
Keywords: Apoptosis, TNF, Stem Cells, Innate Immunity, Immune Regulation
Introduction
The hematopoietic system is capable of rapidly increasing myeloid cell production in response to tissue damage and is critical for wound healing and infection clearance (1–10). While the factors that initiate emergency myelopoiesis are not fully elucidated, it is generally accepted that emergency myelopoiesis is tightly coupled with cytokine and growth factor production, namely TNF and IL-6, and is mediated by NF-κB and other immune regulatory transcription factors.
Rare mutations in Nlrp12, a nucleotide-binding leucine rich repeat and pyrin domain-containing receptor (NLR, also known as NOD-like receptor) 12, have been associated with periodic fevers in humans although the association needs to be further studied. Nonsense and splice mutations within human-Nlrp12 have been shown to diminish suppression of NF-κB signaling (11), however some variants do not exhibit such activity but are associated with modestly enhanced or more rapid inflammasome activation (12). The different functions observed with NLRP12 may be consistent with NLRP12 exhibiting an inflammasome function in certain infections (13, 14) but not other infections or inflammatory conditions (15). While the pyrin-domain containing members of the NLR family have largely been studied in the context of the inflammasome (16), there is growing evidence that a few play an important role in regulating inflammatory signaling. Some NLR proteins have been shown to be positive regulators of NF-κB, while NLRP12 has been implicated as a negative regulator of both the canonical and non-canonical pathways of NF-κB (17–21). NLRP12-mediated NF-κB suppression has been implicated in colonic inflammation and tumorigenesis (17) and osteoclast differentiation (22).
The cytokines that regulate hematopoietic stem cell (HSC) function, such as IFNα/β, IFNγ, IL-12, and TNF, are tightly controlled elements of cell expansion. Type I IFNs and TNF, induced by TLR signaling, can act upon myeloid progenitors to promote the expansion of granulocyte/monocyte progenitors (GMP), leading to systemic myeloid expansion (23). Alternatively, excessive TNF signaling reduces myelopoiesis by inducing caspase-3/caspase-8-dependent progenitor cell apoptosis (24). Excessive TNF, TLR signaling, and deficiencies in negative regulation of NF-κB lead to apoptosis of HSCs and defects in myeloid progenitor function (23, 25).
We and others have shown that burn and radiation injuries lead to increased susceptibility to infection within survivors (4, 6, 26). This is a pressing clinical problem in the face of nuclear accidents and possible incorporation of nuclear materials within explosives. This susceptibility has been attributed to a loss of inflammatory regulation, incomplete immune restoration and a systemic anti-inflammatory response following sepsis and shock (27–29). Following a radiation-thermal combined injury (RCI), an immature monocyte population (iMo) rapidly expands and predominates the periphery (26). Using this model, we observed that TNF is significantly increased in RCI compared to burn, radiation, and sham alone (26).
Given that NLRP12, which is known to suppress a number of cytokines, is present in bone marrow and myeloid cells (1, 25), we tested NLRP12-mediated regulation of TNF signaling within the context of emergency myelopoiesis. Unexpectedly, we demonstrate that NLRP12-deficient mice are vulnerable to RCI due to decreased myelopoiesis.
Methods
Mice and Combined Irradiation and Burn Injury procedure
The Nlrp12−/−, Caspase1/11−/−, Asc−/− and IL-1Ra−/− mouse strains have been described (30–33). All experiments were conducted with female mice housed under SPF conditions that were age-matched and backcrossed for at least nine generations onto the C57BL/6 background. All studies were conducted in accordance with the IACUC guidelines of the University of North Carolina at Chapel Hill and NIH Guidelines for the Care and Use of Laboratory Animals. Our model of RCI has been previously described (26); briefly, mice received a subcutaneous injection of morphine (3mg/kg body weight) for pain control immediately before burn injury. A full-thickness contact burn of 20% total body surface area (TBSA) was produced and within 1 hour, mice received a 5Gy (dose rate of 0.98 Gy/min) whole-body dose of ionizing radiation and were maintained on oral morphine (0.4mg/ml) for the duration of the experiment. Sham controls with 0% TBSA underwent all described interventions except for the burn and γ-irradiation exposure.
Quantitative RT-PCR
RNA was extracted from organ homogenates, suspended in TRIzol and isolated according to the manufacturer’s protocol (Life Technologies, Carlsbad, CA). qPCR was performed using the Verso 1-step RT-qPCR SYBR Green Fluorescein Kit (Thermo Fisher, San Jose, CA). The expression of mouse mRNA encoding NLRP12 and GAPDH was assessed using the SYBR kit and analyzed on an Applied Biosystems machine; results were normalized to expression of the gene encoding GAPDH and were quantified by the change-in-threshold method (ΔΔCT) using primers previously described (18).
Histology
Mouse femurs were extracted and muscle and connect tissue were removed and initially preserved in 10% formalin. Femurs were then decalcified with Immunocal, waterwashed, and paraffin infused. Following sectioning and processing, sections were then stained by hematoxylin and eosin. Samples were processed using ImageJ to determine area of cell loss within each femur.
Pseudomonas aeruginosa infection
A wildtype strain (PAK) of P. aeruginosa was obtained from M. Wolfgang (University of North Carolina, Chapel Hill, NC). 106 bacteria were then aerosolized intratracheally as described previously (26).
Serum Collection and Cytokine ELISA
Animals underwent a submandibular bleed and systemic cytokines were measured by single-plex ELISA (eBioscience, CA, USA or Biolegend, CA, USA) according to the manufacturer’s instructions or by Cytokine Mouse 20-Plex Panel (Life Technologies, Carlsbad, CA) on Luminex Bead Array technology.
Flow Cytometry
All fluorescence-conjugated FACS antibodies were purchased from BD Biosciences or Biolegend. Antibody panel used to identify neutrophils and macrophages are described in the figures. The antibody panel for monocyte and neutrophil analysis was comprised of CD11c-PerCPCy5.5, CD11b-PECy7, Ly6G-APC, Ly6G-PE, and F4/80-FITC. The antibody panel for progenitor analysis was comprised of CD3, CD8, NK1.1, CD19, CD45RA, TER-119 (Ly-76) as a lineage negative gate with all antibodies conjugated to FITC, CD127-PE/CF594, Sca1-APC, cKit-BUV395, FcγR-BV605, CD34-Alexa647, and Annexin V-Pacific Blue. In each case, a million cells per organ were used for flow cytometric analysis.
Intracellular Staining and Phospho-flow cytometry
Intracellular staining was performed using a BD Bioscience Cytofix/Cytoperm kit. Antibodies used were TNF-PE (BD Biosciences), phosphor-p65 S528 (BD Biosciences), phospho-IκBa S32/536-eFlour 660 (eBiosciences), phospho-p38 ST180/Y182-PE and phospho-IKKα/β S176/180-PE (Cell Signaling Technologies). In each case, a million cells per organ were used for flow cytometric analysis.
TNF-Depletion
Immediately following combined irradiation and burn injury, mice were given 25mg/kg of rat IgG1, kappa anti-mouse TNF, clone MP6-XT3 or ratIgG1 isotype control (eBioscience, CA, USA) intraperitoneally dissolved in PBS (Sigma, CA, USA).
Statistical Analysis
Analysis was carried out with Prism 7.0 for Windows. All data are presented as the mean +/− standard error of the mean (SEM). Complex data sets were analyzed by analysis of variance (ANOVA) with a Tukey-Kramer post-test HSD for multiple comparisons. Single data points were assessed by the Student’s two-tailed t test. For the CFU assays, we set the CFU at 100 if they fell below the theoretical limit of detection (101) for the assay; i.e treated as “0” regardless of their absolute values and did not include in the statistical analysis. The product limit method of Kaplan-Meier was utilized for generating the survival curves, which were compared using the log rank test. A p value less than 0.05 was considered statistically significant for all data sets.
Results
NLRP12 limits morbidity and mortality following RCI
Previous work has implicated NLRP12 in suppression of canonical and non-canonical NF-κB, a key driver of inflammatory cytokine signaling (14, 15, 17, 18, 21, 34). We therefore investigated whether NLRP12 was acting to limit excessive inflammatory signaling and consequently promote peripheral immune reconstitution in our model of emergency myelopoiesis.
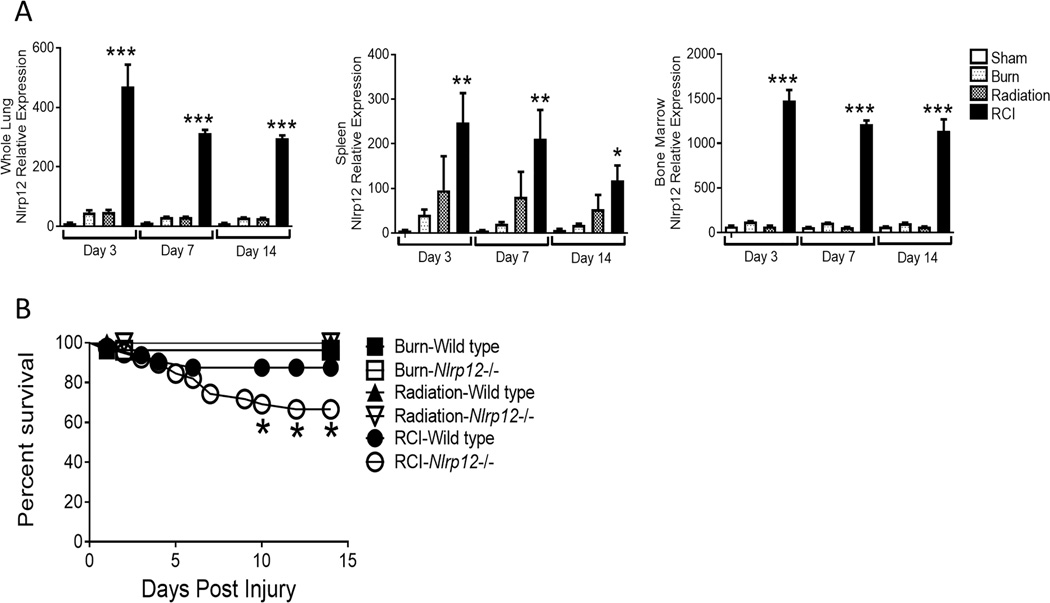
Wild type and Nlrp12−/− mice received a 20% TBSA burn and were irradiated with 5-Gy of γ-irradiation within an hour of burn injury. In wild type mice, we observed elevated NLRP12 expression in spleen, bone marrow and lung tissues early (3, 7, and 14 days post-injury) after RCI (Figure 1A) compared to burn or radiation alone and sham controls. Mortality among NLRP12-deficient mice was significantly elevated following RCI, but not following burn or radiation alone (Figure 1B). While RCI-wild type animals lost weight initially, they were able to return to a baseline weight by seven days after injury and exceed their baseline weight by 14 days post injury; RCI-Nrlp12−/− animals lost more weight and did not fully recover weight in comparison to wild type animals (Figure 1C). These data suggest that NLRP12 protected against morbidity after RCI.
Figure 1. NLRP12 expression is increased after combined injury, and acts to limits mortality and weight loss.
Wildtype C57BL/6 mice were subjected to sham, 5Gy of γ-irradiation, a 20% total body surface area burn or a combined injury (RCI). (A) mRNA was isolated from spleen, bone marrow, and whole lung at 3, 7, and 14 days post injury. Relative Nlrp12 - expression was determined by qRT-PCR. (n=6/timepoint). Wildtype C57BL/6 or Nlrp12−/− mice were subjected to sham, 5Gy of γ-irradiation, a 20% total body surface area burn or RCI. (B) Survival and (C) weight loss were quantified. Data represented as mean ± SEM, with statistical significance compared to sham defined as *, p<0.05, **, p<0.005 and ***, p<0.001 by Student’s t test, with experiments performed in triplicate.
Splenic and pulmonary immune repopulation is impaired following RCI in Nlrp12−/− mice
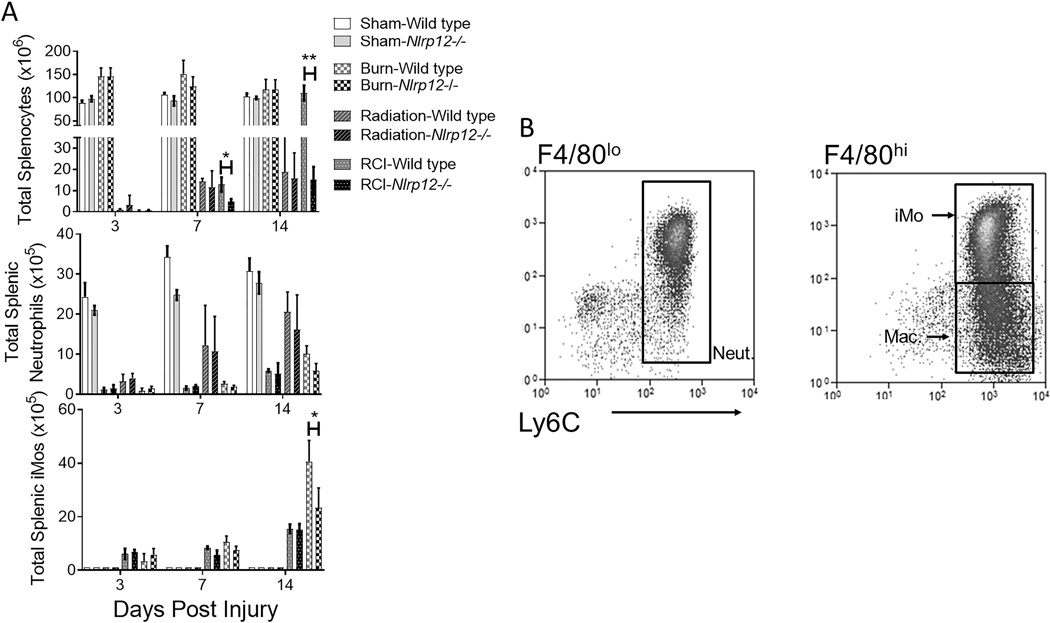
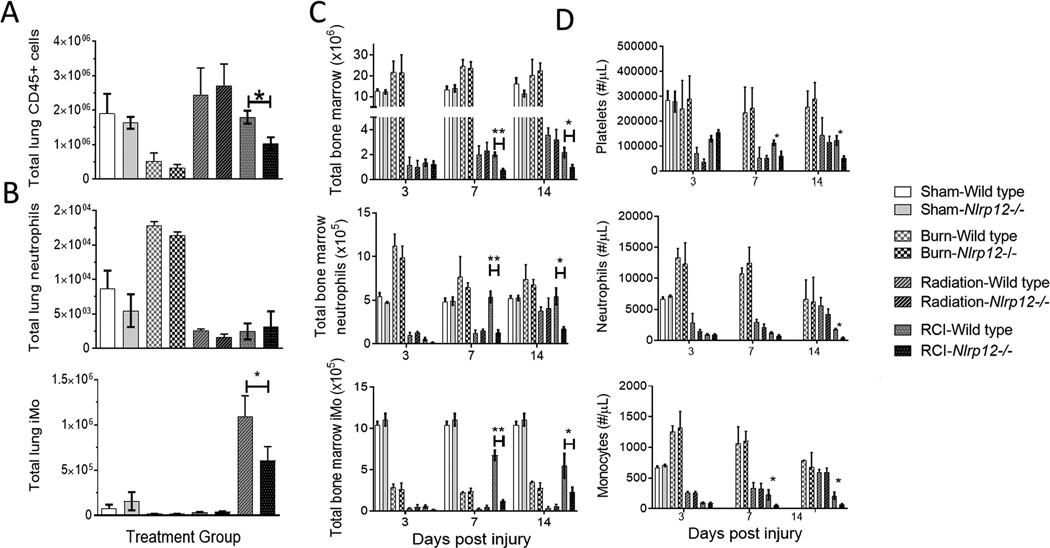
During events that induce enhanced myelopoiesis and inflammation, specifically RCI, we have shown that immature monocytes with high granularity comprise the majority of the peripheral immune system (26). We examined the splenic compartment in the Nlrp12−/− mice after RCI. NLRP12 deficiency resulted in a significant decrease in the total number of splenocytes by 14 days post-injury (Figure 2A). Using flow cytometry, with representative staining in Figure 2B, we observed a decreased number of splenic neutrophils (CD11b+ Ly6Cint Ly6G+ F4/80−) and immature monocytes (iMOs; CD11b+ Ly6C+ Ly6GhiF4/80hi) post-injury in Nlrp12−/− mice (Figure 2C). We also investigated the contribution of NLRP12 to the repopulation of lung immune cells (common sites of opportunistic infection in burn patients) after RCI. Nlrp12−/− mice displayed a reduced ability to repopulate the lung after RCI. This inability was characterized by a decrease in total CD45+ leukocytes and by the absence of the immature monocyte accumulation normally observed following RCI at two weeks post-injury (Figure 3A–B). There were no differences in macrophage (CD11b+ Ly6C+Ly6GloF4/80hi) accumulation in Nlrp12−/− mice when compared to wild type (Figure S1A). The total number of pulmonary macrophages, B and T cells were similar in Nlrp12−/− and wild type mice (Figure S1B–D). These data implicate a role for NLRP12 in regulating emergency hematopoiesis following RCI.
Figure 2. NLRP12 regulates peripheral immune repopulation after combined injury.
Wildtype C57BL/6 or Nlrp12−/− mice were subjected to sham or combined radiation and burn injury (RCI). Spleens were harvested 3, 7 and 14 days post injury and the total number of (A) splenocytes, (C)neutrophils (CD11b+ Ly6Cint Ly6G+ F4/80−) and immature monocytes (iMOs; CD11b+ Ly6C+ Ly6GhiF4/80hi) were quantified by flow cytometry analysis, (B) representative flow cytometric gating from an RCI mouse after gating on CD45+ and F4/80 expression level). Data represented as mean ± SEM, with statistical significance defined as *, p<0.05 and **, p<0.005 by Student’s t test with n=10 mice per group, with experiments performed in triplicate.
Figure 3. NLRP12 regulates pulmonary immune repopulation and bone marrow cell numbers after combined injury.
Wildtype C57BL/6 or Nlrp12−/− mice were subjected to sham or combined radiation and burn injury (RCI). Lungs were harvested 14 days post injury and the total number of (A) CD45+ cells, (B) neutrophils (CD11b+ Ly6Cint Ly6G+ F4/80−) and immature monocytes (iMOs; CD11b+ Ly6C+ Ly6GhiF4/80hi) were quantified by flow cytometry analysis. Wildtype C57BL/6 or Nlrp12−/− mice were subjected to sham or combined radiation and burn injury (RCI). Bone marrow from femurs and tibias and blood from a cheek bleed were harvested 3, 7 and 14 days post injury and the total number of (C) bone marrow cells, immature monocytes (iMOs; CD11b+ Ly6C+ Ly6GhiF4/80hi), neutrophils (CD11b+ Ly6Cint Ly6G+ F4/80−) from the bone marrow and platelets (CD62P+TER119−), monocyte and neutrophils from blood were quantified by flow cytometry analysis. Data represented as mean ± SEM, with statistical significance defined *, p<0.05, **, p<0.005 and ***, p<0.001 by Student’s t test with n=6 mice per group, with experiments performed in triplicate.
Nlrp12−/− mice show decreased bone marrow and peripheral cell numbers following RCI
NLRP12 has been shown to be expressed constitutively in bone marrow cells (15, 18, 19, 35). We hypothesized that reduced immune repopulation in the periphery of injured Nlrp12−/− mice was due to reduced cell generation and output by the bone marrow. To test this, we investigated the impact of NLRP12 deficiency on bone marrow populations after RCI. Nlrp12−/− mice had reduced total bone marrow cells compared to wild type mice after RCI. We also observed a decrease in total iMO and neutrophils (Figure 3C) within the bone marrow of Nlrp12−/− mice as early as seven days post injury compared to wild type mice. Additionally, we observe decreases in the total numbers of monocytes and neutrophils in the peripheral blood (Figure 3D). These data suggest that peripheral immune repopulation defects after RCI are likely attributed to decreased bone marrow cell numbers, which appear to be regulated by NLRP12.
Defects in myelopoiesis following RCI are not observed in inflammasome-deficient animals
NLRP12 is also found to form an inflammasome complex or regulate caspase-1 activity (14, 17, 20) and regulates IL-1β processing by complexing with ASC during infection with Yersinia or malaria (14). To examine whether NLRP12 is important for inflammasome activation following RCI, we assessed IL-1β levels after RCI in wild type or Nlrp12−/− mice. There were no detectable levels of IL-1β at any time point measured (Figure S2A). Due to inability to capture IL-1β levels in serum because of its high turnover, we examined the role of the inflammasome in RCI. We applied the RCI model to various mice strains lacking key components of genes encoding proteins that encode common shared components of the inflammasome. These include Caspase1/11−/− which lacks both canonical and noncanonical inflammasome caspases, Asc−/− which lacks the common adaptor shared by multiple inflammasome NLRs and AIM2, or Il1r−/− which lacks the IL-1 receptor protein. Following RCI, Caspase1/11−/−, Asc−/− and Il1r−/− mice had a similar immune repopulation in the lung and spleen as wild type mice (Figure S2B–C). We also saw no significant differences in bone marrow populations in Caspase-1/11−/−, Asc−/− or Il1r−/− mice following RCI (Figure S2D). Additionally, injured Caspase-1/11−/−, Asc−/− or Il1r−/− animals did not show an increase in mortality compared to wild type (Figure S2E). Together, these results suggest that NLRP12 controls myelopoiesis in an inflammasome-independent pathway.
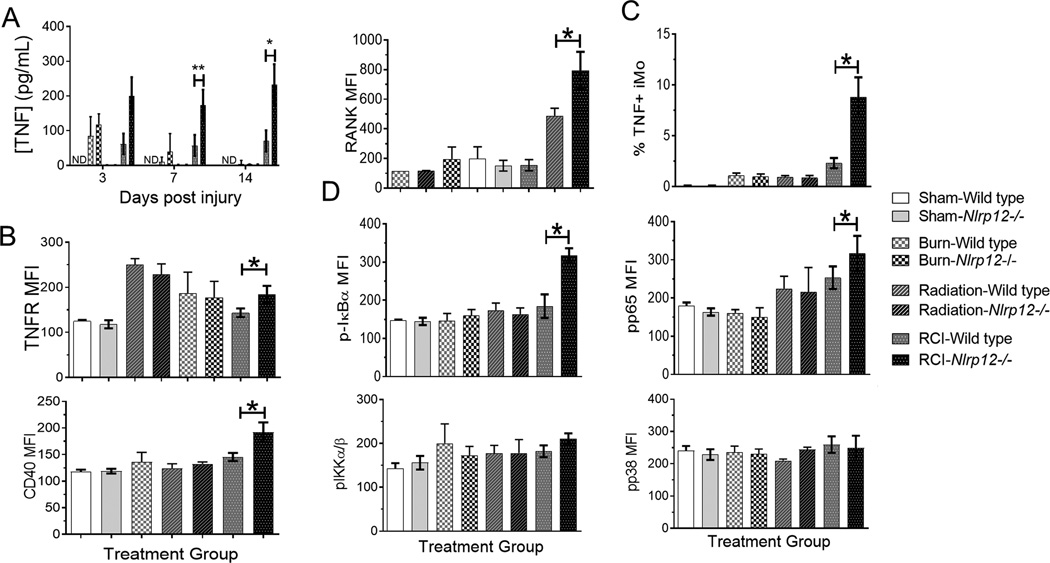
Nlrp12−/− mice display increased serum TNF, IL-6 and IL-12 cytokine and bone marrow TNF- receptor expression
Cytokines that are attenuated by NLRP12, in particular TNF, have been shown to enhance hematopoietic stem cell (HSC) expansion (2, 5, 10, 25). We therefore examined NLRP12-dependent production of selected cytokines and their receptors after RCI. In wild type animals, serum TNF expression increased early following injury and declined over time (26). In sham-treated Nlrp12−/− animals, the TNF level was similar to sham wild type controls. However, during RCI in Nlrp12−/− animals, the TNF increased initially and was maintained over time – a significant elevation when compared to wild type mice (Figure 4A). In addition, Nlrp12−/− bone marrow cells displayed increased TNFR expression (Figure 4B) as well as CD40 and RANK (Figure 4C). Using intracellular straining, we observed that monocyte production of TNF after RCI is increased when compared to burn and radiation controls; however, in the absence of NLRP12, monocyte production of TNF is significantly elevated compared to wild type controls (Figure 4D). As well as TNF, other cytokines such as IL-6, IL-12, IFNα and IFNγ were increased in Nlrp12−/− mice compared to wild type mice but less so than TNF (Figure S3A). This elevation is potentially derived from the initial shock and the selective apoptotic environment induced by the absence of NLRP12 and necessary myelopoiesis. Heightened levels of IL-6 following trauma have been shown to be the major predictor of poor outcome (bacterial infection) following a traumatic injury (36). Other cytokines and growth factors (IL-4, IL-10, and GM-CSF) were measured but showed no significant differences between wild type and Nlrp12−/− animals (Figure S3B).
Figure 4. Nlrp12−/− animals have increased serum cytokine and bone marrow receptor expression following combined injury.
Wildtype C57BL/6 or Nlrp12−/− mice were subjected to sham or combined radiation and burn injury (RCI). The concentration of (A) TNF was quantified using ELISA in serum 3, 7, and 14 days post injury. We also analyzed mean fluorescent intensity of (B) TNFR, CD40, and (C) RANK on bone marrow cells harvested at 14 days post injury using flow cytometry. (D) The percentage of TNF producing iMos was determined using intracellular staining and flow cytometry. (E) The level of phopso-IκBα, phospo-IKKα/β, phosphor-p65, and phospho-p38 was quantified using intracellular staining and flow cytometry. Data represented as mean ± SEM, with statistical significance defined as ** p<0.005 by Student’s t test with n=5 mice per group, with experiments performed in triplicate.
IκBa activity is increased in CD34+ cells Nlrp12−/− animals after RCI
Both the canonical and non-canonical pathways of NFκB have been shown to be negatively regulated by NLRP12 (13, 17, 22). We therefore examined NLRP12-dependent activation of key regulators of each pathway. No changes were seen in phosphorylation levels in sham, burn, or radiation controls; however, RCI NLRP12-deficient animals showed greater levels of pIκBα as well as pp65 (Figure 4D). Increased phosphorylation is indicative of increased canonical NFκB signaling in the absence of NLRP12. However, pIKKα/β and the downstream p38/MAPK showed no changes in activity when comparing wild type to Nlrp12−/− injured animals. Taken with the increased TNFR expression on bone marrow cells, these results suggest that NLRP12 is negatively regulating the canonical NFκB signaling cascade.
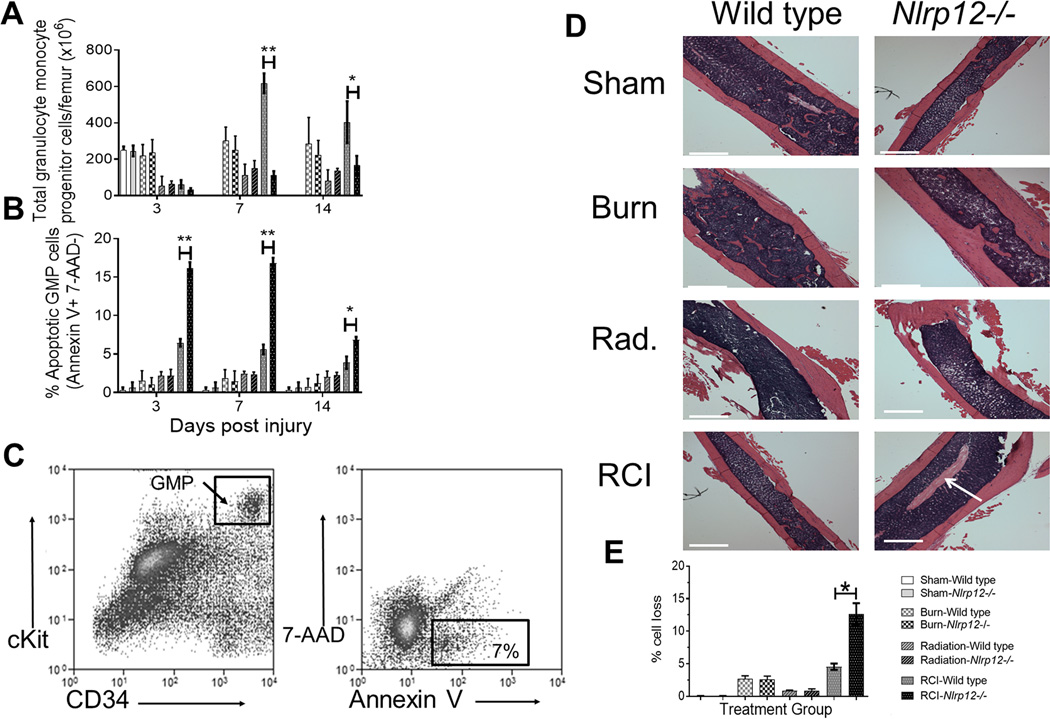
RCI of Nlrp12−/− animals leads to increased granulocyte/monocyte progenitor apoptosis
We observed that NLRP12 regulates reconstitution of granulocytic and monocytic bone marrow and peripheral cells in Nlrp12−/− mice following RCI. We therefore hypothesized that NLRP12 regulates bone marrow granulocyte/monocyte progenitors (GMP), the source of granulocytes and immature monocytes. To test this, we utilized flow cytometric analysis to evaluate the number of GMP (Lin− IL7R− Sca1− cKit+ FcγRhi CD34+) in Nlrp12−/− mice following RCI.
We detected a similar number of bone marrow GMP in Nlrp12−/− and wild type mice at 3 days after injury. However, at 7 and 14 days after injury, wild type GMP expanded and increased in numbers while Nlrp12−/− GMP expansion was attenuated (Figure 5A). There were no measured differences in lymphoid lineage progenitors (Figure S4). We tested the hypothesis that the significant decrease in GMP in Nlrp12−/− mice is due to increased apoptosis. To distinguish apoptotic cells from necrotic cells, 7-AAD and Annexin V staining was performed. While sham control revealed no difference in WT and Nlrp12−/− mice, a significant percent of Nlrp12−/− GMP underwent apoptosis compared to wild type GMP during RCI. This increase in apoptosis was detected as early as 3-days post injury (Figure 5B). Representative flow gating is shown in Figure 5C.
Figure 5. Nlrp12−/− animals have increased granulocyte/monocyte progenitor apoptosis after combined injury.
Wildtype C57BL/6 or Nlrp12−/− mice were subjected to sham or combined radiation and burn injury (RCI). Bone marrow was collected from wild type and Nlrp12−/− mice at 3, 7, and 14 days post RCI or sham treatment (n= 6/group). Using flow cytometric analysis, (A) the total number of bone marrow Granulocyte/Monocyte Progenitors (GMP, Lin− IL7R− Sca1− ckit+ FcγRhi CD34+) and (B) the percentage of GMP cells undergoing apoptosis was determined by positive Annexin V staining in the absence of 7-AAD− staining cells; representative flow staining from Nlrp12−/− mice is shown is shown in (C). Data represented as mean ± SEM, with statistical significance defined *, p<0.05, **, p<0.005 and ***, p<0.001 by Student’s t test with n=5 mice per group. In separate experiments, wildtype C57BL/6 or Nlrp12−/− mice were subjected to sham, irradiation, burn or RCI. Femurs were collected 14 days post injury and H&E staining performed for histological analysis. (D) shows representative sections from each group, with areas of cell death marked by white arrow (white bar represents 25um;), (E) quantification of cell death area was performed using ImageJ, with experiments performed in triplicate.
Increased apoptosis and decreased bone marrow cellularity was confirmed by histological staining. H&E femur sections were obtained at 14 days post injury. There were no histological changes from wild type to Nlrp12−/− mice in sham, burn, or radiation alone animals. However, RCI-Nlrp12−/− mice displayed medial patches of cell loss within the femurs, which was not present in RCI-wild type femurs (Figure 5D), and quantified by imaging analysis in Figure 5E. Collectively, our findings imply that NLRP12 prevents progenitor cell apoptosis, thus allowing myelopoiesis and peripheral immune cell reconstitution to occur in wild type animals.
Leukopenia can have complex etiologies in both inflammatory and non-inflammatory conditions, many of which involve alterations in HSC steady-state hematopoiesis (9, 10, 37). Our data show that NLRP12 limits TNF following RCI, resulting in expansion of myeloid precursors and monocyte populations throughout the periphery. Previous studies showed increased hematopoiesis following total body irradiation; however, our results are novel because we have shown that NLRP12 promotes hematopoiesis of specific lineages during RCI (1, 26).
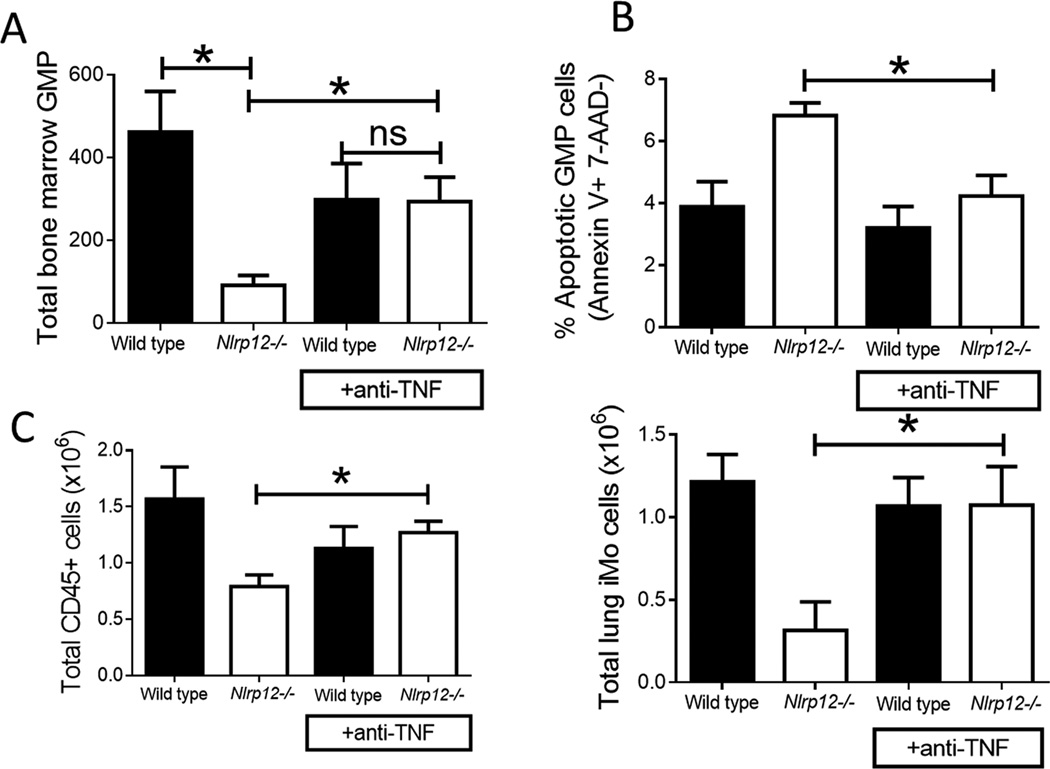
Anti-TNF antibody administration prevents NLRP12-associated GMP apoptosis after combined injury
After observing significantly elevated levels of TNF and reduced myelopoiesis in injured Nlrp12−/− mice, we hypothesized that increased levels of TNF were leading to pathology through TNF-mediated apoptosis of immune progenitor cells as seen in other models of excessive TNF(38). Specifically, we hypothesized that GMP were undergoing TNF-mediated apoptosis with reduced peripheral neutrophil and inflammatory monocyte accumulation. To test this, wild type and Nlrp12−/− mice received a single administration of anti-TNF or isotype control antibody immediately following RCI.
We observed significantly fewer GMP in the Nlrp12−/− mice given the isotype control compared to wild type mice. However, Nlrp12−/− mice given the anti-TNF antibody had similar numbers of GMP compared to isotype and anti-TNF treated wild type mice (Figure 6A). Additionally, the proportion of GMP actively undergoing apoptosis was higher in the Nlrp12−/− isotype treated animals compared to Nlrp12−/− mice treated with anti-TNF (Figure 6B). This is correlated with a decrease in the total CD45+ pulmonary cells as well as pulmonary iMO (Figure 6C). We also observed no differences in overall mortality/morbidity compared to wild type mice and a reversal of the RCI-dependent bone marrow apoptosis by histology (data not shown). These data indicate that in the absence of NLRP12, TNF mediates the enhanced bone marrow death during RCI and resultant incomplete restoration of the peripheral immune system.
Figure 6. Anti-TNF antibody administration prevents NLRP12-associated GMP apoptosis after combined injury.
Wild type and Nlrp12−/− C57/BL6 mice received a single administration of anti-TNF or isotype control antibody immediately following combined radiation and burn injury (RCI). We harvested bone marrow and lung from these mice 14 days after injury. We quantified (A) the total number of bone marrow Granulocyte/Monocyte Progenitors (GMP, Lin− IL7R− Sca1− ckit+ FcγRhi CD34+) and (B) the percentage of GMP cells undergoing apoptosis by 7-AAD− Annexin V+ staining by flow cytometry. We measured (C) the total number of pulmonary CD45+ cells and immature monocytes (iMOs; CD11b+ Ly6C+ Ly6GhiF4/80hi) by flow cytometry analysis. Data represented as mean ± SEM, with statistical significance defined as *, p<0.05 by Student’s t test with n=5 mice per group, with experiments performed in triplicate.
Nlrp12−/− mice lack control of pulmonary infection following radiation-thermal combined injury
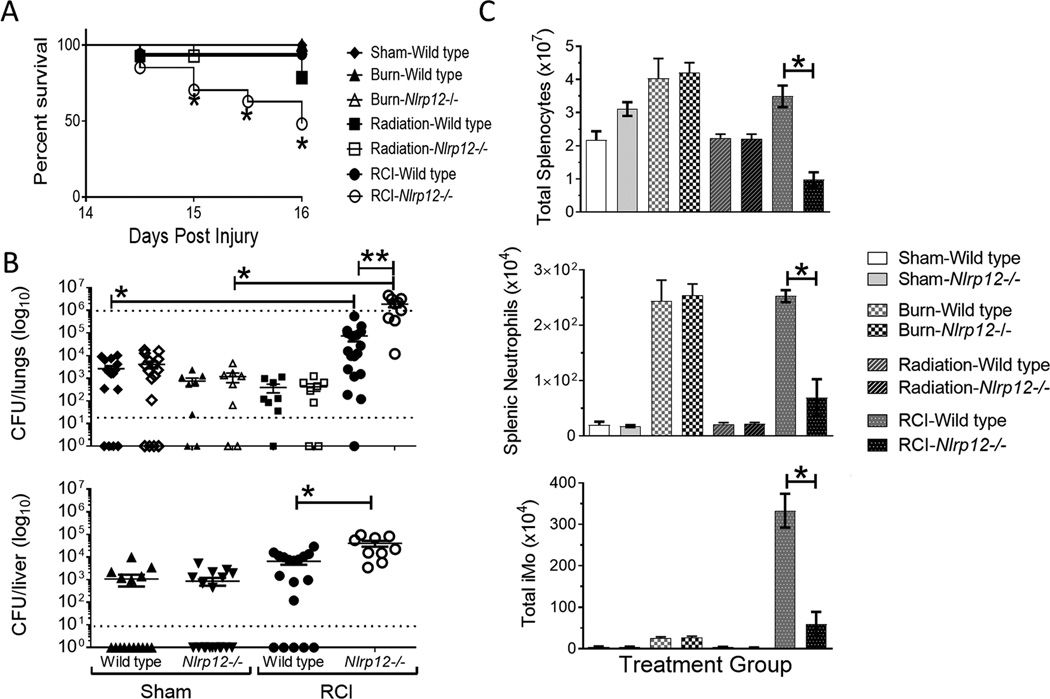
In a clinical setting, patients that are able to survive initial shock from a burn or radiation-thermal-combined injury will often succumb to a pulmonary infection associated with the prolonged hospital stay (34). We sought to evaluate the role NLRP12 deficiency plays in a clinically relevant model of a lung infection following injury. Wild type and Nlrp12−/− animals were subjected to either sham, burn, or radiation only, and RCI. Mice were then sustained for two weeks in individual housing wherein they were infected intratracheally with 1×106 CFU of Pseudomonas aeruginosa (PAK).
RCI-Nlrp12−/− infected animals displayed a significant increase in mortality, with animals starting to succumb to infection after as few as 12 hours (Figure 7A), significantly earlier than all other control groups, even radiation injured controls. Lung and liver from infected animals that survived until 48 hours post infection were collected and plated to enumerate bacterial load locally and systemically. RCI-Nlrp12−/− mice lungs and liver showed a 10-fold increase in bacteria compared to injured, wild type animals (Figure 7B). We consistently observed that every mouse became infected within the RCI injured Nlrp12−/− groups, unlike every other group that frequently contained mice that cleared the infection. These data suggest that NLRP12 plays a vital role in response to an infection insult following a traumatic injury.
Figure 7. Nlrp12−/− mice lack control of pulmonary infection following combined injury.
Wildtype C57BL/6 or Nlrp12−/− mice were subjected to sham or combined radiation and burn injury (RCI). Mice were inoculated 14 days post-injury intratracheally with 1×106 CFU of Pseudomonas aeruginosa (PAK). We quantified (A) survival, (B) bacterial load within lungs and liver by culture, and (C) number of splenic CD45+ cells, neutrophils (CD11b+ Ly6Cint Ly6G+ F4/80−) and immature monocytes (iMOs; CD11b+ Ly6C+ Ly6GhiF4/80hi) harvested two days after inoculation. Data represented as mean ± SEM, with statistical significance defined as *, p<0.05; **, p<0.05 by Student’s t test with n=6 mice per group (3 for burn and radiation only), with experiments performed in triplicate.
We next sought to determine the immune response to infection following RCI. Nlrp12−/− mice showed a decrease in innate, pulmonary immune cell populations following RCI and infection (Figure 7C), leading us to conclude that NLRP12 results in increased hematopoietic recovery which is likely crucial to the effective control of infection after traumatic injury.
Discussion
Our study demonstrates that NLRP12 suppresses TNF signaling in vivo during inflammation-induced emergency myelopoiesis. Most importantly, our research indicates a role for NLRP12 in hematopoietic progenitor cells by limiting TNF-induced apoptosis of these cells. TNF inflammation initiated by RCI without NLRP12 leads to the apoptosis of progenitor cells and a defective peripheral immune reconstitution, associated with increased mortality and inability to control an infectious challenge.
In addition to inhibiting inflammation, defects in NF-κB signaling lead to weakened hematopoiesis (25). There is no single mechanism that has been defined for immune suppression in the response to traumatic injury, but hematopoietic stem cell (HSC) expansion and immune repopulation have been shown to be important factors (2, 5, 10, 25, 37).
We propose that NLRP12 suppression of immune signaling pathways leading to attenuated cytokines contributes to homeostatic proliferation of granulocytes and monocytes following induction of severe leukopenia. Moreover, this NLRP12-mediated suppression limits overt TNF-induced inflammation that could lead to HSC apoptosis by limiting canonical NFκB signaling. Our findings add to the studies that suggest that NLRP12 acts as a cellular rheostat to limit inflammation, and is emerging as a “checkpoint” or inhibitor (17, 22) of canonical NFκB signaling (17, 32, 39, 40). Although we demonstrate increased p-IkB and pp65 levels in CD34+ cells from NLRP12 deficient mice undergoing RCI, suggestive of increased canonical NFkB signaling, it does not rule out a role for the non-canonical pathway, which could be evaluated by measuring either NIK or p100 processing. NLRP12-mediated NFκB suppression likely limits TNF and cellular death during inflammation and hematopoiesis. Our data in NLRP12-deficient mice shows compromised hematopoiesis due to enhanced TNF production, leading to flagrant HSC/GMP apoptosis. This lack of HSC function leads to global leukopenia and correlates with increased mortality compared to wild type mice.
We have evaluated reconstitution as best we can; for the clinical situation that we are most interested in, namely infection control after combined injury, we were interested in functional reconstitution which we demonstrate by control of Pseudomonas. The cellular reconstitution is not optimal, rather, we have uncovered a “last ditch” attempt by the organism to reconstitute albeit with immature monocytes and a gene which appears to control this response. These studies would be applicable in instances of increased myelopoiesis, TNF-driven inflammation, and induced apoptosis.
Supplementary Material
Acknowledgments
We would like to acknowledge the UNC Flow Cytometry Core Facility, UNC Microscopy Services Laboratory, Lineberger Comprehensive Cancer Center’s Animal Histopathology Core Lab, Danier Moore, Wesley Stepp, Rebecca Drapp, Sha’Leema Miller, Lindsey Glenn, Lucas Sjeklocha and Steven Mouro for their technical support and advice.
Sources of support: NIH DUMC/NIAID-RADDCORE #203-2315 and NIH NIGMS 5R01GM076250.
References
- 1.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32:57–65. doi: 10.1016/j.it.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14:302–314. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Nguyen-Jackson H, Panopoulos AD, Li HS, Murray PJ, Watowich SS. STAT3 controls myeloid progenitor growth during emergency granulopoiesis. Blood. 2010;116:2462–2471. doi: 10.1182/blood-2009-12-259630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dugan AL, Thellin O, Buckley DJ, Buckley AR, Ogle CK, Horseman ND. Effects of prolactin deficiency on myelopoiesis and splenic T lymphocyte proliferation in thermally injured mice. Endocrinology. 2002;143:4147–4151. doi: 10.1210/en.2002-220515. [DOI] [PubMed] [Google Scholar]
- 5.Gardner JC, Noel JG, Nikolaidis NM, Karns R, Aronow BJ, Ogle CK, McCormack FX. G-CSF drives a posttraumatic immune program that protects the host from infection. J Immunol. 2014;192:2405–2417. doi: 10.4049/jimmunol.1302752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noel JG, Guo X, Wells-Byrum D, Schwemberger S, Caldwell CC, Ogle CK. Effect of thermal injury on splenic myelopoiesis. Shock (Augusta, Ga.) 2005;23:115–122. doi: 10.1097/01.shk.0000154239.00887.18. [DOI] [PubMed] [Google Scholar]
- 7.Noel JG, Valente JF, Ogle JD, Cornelius J, Custer DA, Li BG, Alexander JW, Ogle CK. Changes in bone marrow-derived myeloid cells from thermally injured rats reflect changes in the progenitor cell population. J Burn Care Rehabil. 2002;23:75–86. doi: 10.1097/00004630-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Santangelo S, Shoup M, Gamelli RL, Shankar R. Prostaglandin E2 receptor antagonist (SC-19220) treatment restores the balance to bone marrow myelopoiesis after burn sepsis. J Trauma. 2000;48:826–830. doi: 10.1097/00005373-200005000-00005. discussion 830-821. [DOI] [PubMed] [Google Scholar]
- 9.Serafini M, Dylla SJ, Oki M, Heremans Y, Tolar J, Jiang Y, Buckley SM, Pelacho B, Burns TC, Frommer S, Rossi DJ, Bryder D, Panoskaltsis-Mortari A, O'Shaughnessy MJ, Nelson-Holte M, Fine GC, Weissman IL, Blazar BR, Verfaillie CM. Hematopoietic reconstitution by multipotent adult progenitor cells: precursors to long-term hematopoietic stem cells. J Exp Med. 2007;204:129–139. doi: 10.1084/jem.20061115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toliver-Kinsky TE, Lin CY, Herndon DN, Sherwood ER. Stimulation of hematopoiesis by the Fms-like tyrosine kinase 3 ligand restores bacterial induction of Th1 cytokines in thermally injured mice. Infect Immun. 2003;71:3058–3067. doi: 10.1128/IAI.71.6.3058-3067.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeru I, Duquesnoy P, Fernandes-Alnemri T, Cochet E, Yu JW, Lackmy-Port-Lis M, Grimprel E, Landman-Parker J, Hentgen V, Marlin S, McElreavey K, Sarkisian T, Grateau G, Alnemri ES, Amselem S. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc Natl Acad Sci U S A. 2008;105:1614–1619. doi: 10.1073/pnas.0708616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borghini S, Tassi S, Chiesa S, Caroli F, Carta S, Caorsi R, Fiore M, Delfino L, Lasiglie D, Ferraris C, Traggiai E, Di Duca M, Santamaria G, D'Osualdo A, Tosca M, Martini A, Ceccherini I, Rubartelli A, Gattorno M. Clinical presentation and pathogenesis of cold-induced autoinflammatory disease in a family with recurrence of an NLRP12 mutation. Arthritis and rheumatism. 2011;63:830–839. doi: 10.1002/art.30170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ataide MA, Andrade WA, Zamboni DS, Wang D, Souza Mdo C, Franklin BS, Elian S, Martins FS, Pereira D, Reed G, Fitzgerald KA, Golenbock DT, Gazzinelli RT. Malaria-induced NLRP12/NLRP3-dependent caspase-1 activation mediates inflammation and hypersensitivity to bacterial superinfection. PLoS Pathog. 2014;10:e1003885. doi: 10.1371/journal.ppat.1003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vladimer GI, Weng D, Paquette SW, Vanaja SK, Rathinam VA, Aune MH, Conlon JE, Burbage JJ, Proulx MK, Liu Q, Reed G, Mecsas JC, Iwakura Y, Bertin J, Goguen JD, Fitzgerald KA, Lien E. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity. 2012;37:96–107. doi: 10.1016/j.immuni.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaki MH, Man SM, Vogel P, Lamkanfi M, Kanneganti TD. Salmonella exploits NLRP12-dependent innate immune signaling to suppress host defenses during infection. Proc Natl Acad Sci U S A. 2014;111:385–390. doi: 10.1073/pnas.1317643111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen H, Miao EA, Ting JP. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39:432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, Arthur JC, Woodford RM, Davis BK, Uronis JM, Herfarth HH, Jobin C, Rogers AB, Ting JP. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity. 2012;36:742–754. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arthur JC, Lich JD, Ye Z, Allen IC, Gris D, Wilson JE, Schneider M, Roney KE, O'Connor BP, Moore CB, Morrison A, Sutterwala FS, Bertin J, Koller BH, Liu Z, Ting JP. Cutting edge: NLRP12 controls dendritic and myeloid cell migration to affect contact hypersensitivity. J Immunol. 2010;185:4515–4519. doi: 10.4049/jimmunol.1002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savic S, Dickie LJ, Battellino M, McDermott MF. Familial Mediterranean fever and related periodic fever syndromes/autoinflammatory diseases. Curr Opin Rheumatol. 2012;24:103–112. doi: 10.1097/BOR.0b013e32834dd2d5. [DOI] [PubMed] [Google Scholar]
- 20.Ye Z, Lich JD, Moore CB, Duncan JA, Williams KL, Ting JP. ATP binding by monarch-1/NLRP12 is critical for its inhibitory function. Mol Cell Biol. 2008;28:1841–1850. doi: 10.1128/MCB.01468-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaki MH, Vogel P, Malireddi RK, Body-Malapel M, Anand PK, Bertin J, Green DR, Lamkanfi M, Kanneganti TD. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 2011;20:649–660. doi: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krauss JL, Zeng R, Hickman-Brecks CL, Wilson JE, Ting JP, Novack DV. NLRP12 provides a critical checkpoint for osteoclast differentiation. Proc Natl Acad Sci U S A. 2015;112:10455–10460. doi: 10.1073/pnas.1500196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buechler MB, Teal TH, Elkon KB, Hamerman JA. Cutting edge: Type I IFN drives emergency myelopoiesis and peripheral myeloid expansion during chronic TLR7 signaling. J Immunol. 2013;190:886–891. doi: 10.4049/jimmunol.1202739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei B, Yue P, Qingwei L. The molecular mechanism of necroptosis. Yi Chuan. 2014;36:519–524. doi: 10.3724/SP.J.1005.2014.0519. [DOI] [PubMed] [Google Scholar]
- 25.Stein SJ, Baldwin AS. Deletion of the NF-kappaB subunit p65/RelA in the hematopoietic compartment leads to defects in hematopoietic stem cell function. Blood. 2013;121:5015–5024. doi: 10.1182/blood-2013-02-486142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendoza AE, Neely CJ, Charles AG, Kartchner LB, Brickey WJ, Khoury AL, Sempowski GD, Ting JP, Cairns BA, Maile R. Radiation combined with thermal injury induces immature myeloid cells. Shock (Augusta, Ga.) 2012;38:532–542. doi: 10.1097/SHK.0b013e31826c5b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neely CJ, Kartchner LB, Mendoza AE, Linz BM, Frelinger JA, Wolfgang MC, Maile R, Cairns BA. Flagellin treatment prevents increased susceptibility to systemic bacterial infection after injury by inhibiting anti-inflammatory IL-10+ IL-12− neutrophil polarization. PLoS One. 2014;9:e85623. doi: 10.1371/journal.pone.0085623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neely CJ, Maile R, Wang MJ, Vadlamudi S, Meyer AA, Cairns BA. Th17 (IFNgamma- IL17+) CD4+ T cells generated after burn injury may be a novel cellular mechanism for postburn immunosuppression. J Trauma. 2011;70:681–690. doi: 10.1097/TA.0b013e31820d18a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones SW, Zhou H, Ortiz-Pujols SM, Maile R, Herbst M, Joyner BL, Jr, Zhang H, Kesic M, Jaspers I, Short KA, Meyer AA, Peden DB, Cairns BA, Noah TL. Bronchoscopy-derived correlates of lung injury following inhalational injuries: a prospective observational study. PLoS One. 2013;8:e64250. doi: 10.1371/journal.pone.0064250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirsch E, Irikura VM, Paul SM, Hirsh D. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc Natl Acad Sci U S A. 1996;93:11008–11013. doi: 10.1073/pnas.93.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 32.Arthur JC, Lich JD, Aziz RK, Kotb M, Ting JP. Heat shock protein 90 associates with monarch-1 and regulates its ability to promote degradation of NF-kappaB-inducing kinase. J Immunol. 2007;179:6291–6296. doi: 10.4049/jimmunol.179.9.6291. [DOI] [PubMed] [Google Scholar]
- 33.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 34.Moore CB, Medina MA, van Deventer HW, O'Connor BP, Cameron S, Taxman DJ, Maile R, Ting JP, Cairns BA. Downregulation of immune signaling genes in patients with large surface burn injury. J Burn Care Res. 2007;28:879–887. doi: 10.1097/BCR.0b013e318159a41e. [DOI] [PubMed] [Google Scholar]
- 35.Vitale A, Rigante D, Maggio MC, Emmi G, Romano M, Silvestri E, Lucherini OM, Emmi L, Gerloni V, Cantarini L. Rare NLRP12 variants associated with the NLRP12-autoinflammatory disorder phenotype: an Italian case series. Clin Exp Rheumatol. 2013;31:155–156. [PubMed] [Google Scholar]
- 36.Gebhard, Pfetsch, Steinbach, Strecker Is Interleukin 6 an Early Marker of Injury Severity Following Major Trauma in Humans? JAMA Surgery. 2000;135 doi: 10.1001/archsurg.135.3.291. [DOI] [PubMed] [Google Scholar]
- 37.Jones TD, Morris MD, Young RW, Kehlet RA. A cell-kinetics model for radiation-induced myelopoiesis. Exp Hematol. 1993;21:816–822. [PubMed] [Google Scholar]
- 38.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 39.Lich JD, Ting JP. Monarch-1/PYPAF7 and other CATERPILLER (CLR, NOD, NLR) proteins with negative regulatory functions. Microbes Infect. 2007;9:672–676. doi: 10.1016/j.micinf.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lich JD, Williams KL, Moore CB, Arthur JC, Davis BK, Taxman DJ, Ting JP. Monarch-1 suppresses non-canonical NF-kappaB activation and p52-dependent chemokine expression in monocytes. J Immunol. 2007;178:1256–1260. doi: 10.4049/jimmunol.178.3.1256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.