Abstract
Studies have shown that exposure to psychological stressors leads to inflammation throughout the body. This has been widely studied using social disruption stress (SDR), a social stressor, which involves repeated social defeat in subordinate mice. Exposure to SDR increases serum cytokine levels, results in accumulation of spleen CD11b+ myeloid cells, and primes macrophages for increased cytokine and microbicidal activity. Our previous studies showed that intestinal microbes are necessary for SDR-enhancement of innate immunity. Here, we show that SDR increases spleen CD11b+Ly6CintermLy6G+ neutrophil and CD11b+Ly6ChiLy6G− monocyte numbers compared to control mice. Further, we found that neutrophils and monocytes from stressor-exposed mice expressed higher levels of IL-1β mRNA. To determine whether bacterial translocation may contribute to these effects, bacterial 16S rRNA was quantified using qRT-PCR with bacterial group-specific primers. Exposure to the SDR stressor specifically increased Lactobacillus RNA in the spleen, which localized in spleen monocytes. The increased spleen levels of Lactobacillus 16S rRNA in SDR mice positively correlated with increased levels of IL-1β and IL-23 mRNA. Our findings indicate that during stressor exposure, Lactobacillus spp. can translocate to the spleen and prime the innate immune system for enhanced reactivity.
Keywords: Psychosocial stress, Psychoneuroimmunology, Immunopotentiation, Lactobacillus, Microbiota gut-brain axis, Cytokine, microbiota, bacterial translocation
Introduction
Chronic, low-grade inflammation has been linked to a myriad of diseases, including obesity, diabetes, anxiety, and depression. Stressor exposure significantly increases inflammation throughout the body, and may help to explain why these diseases can be exacerbated or precipitated by exposure to stressful situations. For example, depression is often preceded by major stressful events, with 50–80% of depressed patients experiencing a major life event in the 3–6 months prior to onset of symptoms (1–3). Studies involving laboratory animals demonstrate that stress-induced increases in inflammatory cytokines (such as IL-1β or IL-6) contribute to the development of anxiety-like and depressive-like behaviors. In humans, a subset of depressed patients have many of the cardinal features of an inflammatory response, including increased expression of pro-inflammatory cytokines (such as IL-1β, IL-6 and TNF-α), increases in chemokines, and increases in classical monocytes (reviewed in (4)). Thus, in both human patients and laboratory animals there exists a link between stressor exposure, inflammatory cytokines, and disease (e.g., anxiety or depression). While a growing number of studies have begun to define mechanisms by which inflammation can impact the body, it is not completely understood how the physiological stress response leads to increased inflammatory responses. It is our contention that commensal microbes play an essential role in stress-induced increases in inflammatory cytokines.
Recent studies have shown that the commensal microbiota have extensive bidirectional interactions with the central nervous system and the immune system (5, 6). Every surface of the body is colonized by commensal microbes, collectively called the microbiota, with the vast majority of these microbes residing within the gastrointestinal tract. Although it has been known for decades that these microbes can have beneficial effects on the body, it has only been over the past few years that the extent of their impact on host physiology has become realized. For example, studies in germfree mouse models, as well as conventionally housed mice treated with probiotics and antibotics, demonstrate that gut microbes can impact brain activity and behavioral responses (reviewed in (7)). Similarly, the commensal gut microbiota can impact inflammatory responses within the intestines themselves, as well as at distant sites, such as the bone marrow, lungs, and spleen (8). Studies from our laboratory, as well as others, have demonstrated that stressor exposure changes the composition of the gut microbiota and that these changes in the microbiota impact immune system activity and behavioral responses (9–12). How commensal gut microbes contribute to stressor-induced inflammatory responses are not yet clearly delineated, but could involve the gut-brain axis and dysregulation of the intestinal epithelial layer, often referred to as the leaky gut. Ultimately, the leaky gut allows microbes, and their products, to cross the intestinal epithelium and enter into the interior of the body where they can trigger an immune response (13). In the current study, a well characterized social stressor was used to study whether stress leads to the translocation of intestinal microbes and increased inflammatory responses in the spleen.
The social disruption stressor (SDR) is a social stressor that involves repeated social defeat in subordinate mice (14). Stressor-exposed mice exhibit many behavioral features of humans during stressful periods, including depressive and anxiety-like behaviors (15–17), as well as increased serum levels of pro-inflammatory cytokines, such as IL-1β and IL-6 (18–20). The mice also show increases in spleen mass due to an accumulation of CD11b+ myeloid cells. These myeloid cells show increased inflammatory activity upon exposure to LPS, including increased pro-inflammatory cytokine production and increased production of reactive oxygen and nitrogen species (9, 10, 17, 18, 21–23). These cells are also resistant to the suppressive effects of glucocorticoids, which could help to explain why they have increased activity, even in the presence of stressor-induced increases in glucocorticoids (15, 20, 24). However, it is not yet known how stressor exposure enhances immune system activity. Although data from this laboratory, as well as others, suggest that the commensal microbiota are involved in stressor-induced increases in immune system activity (9, 25), whether bacteria need to translocate from the lumen of the intestines to the interior of the body in order for them to impact systemic immune compartments is not clear. Thus, this study assessed cytokine levels, macrophage subsets, and bacterial RNA within the spleens of stressor-exposed and non-stressed control mice.
Microbes that translocate from their natural niche into the circulation are filtered from the blood by macrophages in the spleen (26). Blood travels through the marginal zone (MZ) in the spleen, into the red pulp (RP), and then the venous sinuses. The MZ and RP contain distinct macrophage and monocyte populations that filter microbes and microbial products from the blood. The MZ contains marginal zone macrophages, which are highly phagocytic, but represent a small fraction of the total macrophage/monocyte population in the spleen (27). The F4/80+ RP macrophage population can also phagocytose blood-borne microbes, particulate matter and aged erythrocytes, and are known to produce cytokines, including IL-1β, IL-6, IL-10, IL-12 and TGFβ (26, 28, 29). Exposure to the SDR stressor is known to increase monocyte levels, particularly CD11b+Ly6Chi monocytes, in the blood and in the spleen (10, 15, 18, 23). These Ly6Chi monocytes are the classical monocytes that during infection and inflammation, migrate from the spleen and bone marrow to sites of inflammation where they are activated and differentiate into macrophages and dendritic cells (30–32).They are prolific cytokine producers and are known to mediate progression of inflammatory and autoimmune diseases, such as experimental autoimmune encephalomyelitis (33) and atherosclerosis (34). These cells traffick to the brain, through microbiota-mediated mechanisms (35), and play a role in the development of anxiety-like and depressive-like behaviors (36, 37). Exposure to the SDR stressor also increases neutrophil numbers in the bone marrow, blood, lungs, and spleen (19, 38, 39). Although neutrophils have a relatively short half life in vivo, it has long been recognized that peripheral pools of neutrophils exist. In the absence of overt inflammation and infection, neutrophils are commonly found in the bone marrow, spleen, liver, and lungs (40–42). However, the exact reasons why neutrophils accumulate, and what their physiological role is, within these organs are not completely understood. This study determined whether stressor-induced translocation of microbes from the lumen of the intestines to the spleen was associated with differences in MZ and RP macrophage populations, CD11b+Ly6G+ neutrophils, CD11b+Ly6Chi monocytes, as well as cytokine production. To detect translocated bacteria in the spleen, qRT-PCR of spleen RNA was used with bacterial group specific primers to 16S rRNA. To determine a more direct link between commensal bacteria, splenic myeloid cells, and cytokine production, a RNA Flow cytometry assay was used to detect monocyte and neutrophil populations that express IL-1β mRNA and qRT-PCR was used to detect bacterial 16S rRNA in isolated monocyte and neutrophils.
Materials and Methods
Mice
Male C57BL/6 mice between the ages of 6 and 8 weeks were purchased from Charles Rivers Laboratories (Wilmington, MA). The mice were housed in groups of 3 per cage and allowed to acclimate to the animal facility for 1 week before experimentation. They were maintained on a 12h light/dark schedule with lights on at 6:00 am and food and water available ad libitum.
Social Disruption
The social disruption (SDR) stressor was performed as previously described (15, 21, 43, 44). The SDR stressor involves repeated social defeat from interactions between an aggressive intruder male mouse and resident male mice for six consecutive nights. The mice were randomly divided into SDR or home cage control (HC) conditions. A retired breeder CD-1 male intruder mouse was placed in a cage of three mice, starting at 17:00. If the intruder mouse did not initiate an attack within 10 min and defeat all three resident mice, the mouse was removed and replaced with a new intruder mouse. After 2 hrs, the intruder mouse was removed and residents left undisturbed until the following day when the SDR protocol was repeated. The morning after the sixth cycle of SDR, the mice were sacrificed. Mice with wounds penetrating the cutaneous layer were not used in the study. Home cage control mice were left undisturbed throughout the experiment.
Splenocyte and MLN Isolation
Mice were euthanized via CO2 asphyxiation and spleens and mesenteric lymph nodes (MLN) were removed. A single-cell suspension of splenocytes was obtained by dicing each spleen into 1–2 mm pieces and gently pressing the pieces through a 70 µm cell strainer. Erythrocytes were lysed with Gey’s lysis buffer (8mM NH4Cl, 5 mM KHCO3) and splenocytes suspended in RPMI 1640 media.
Isolation of spleen monocytes and Neutrophils
Spleen monocytes and neutrophils were isolated by negative selection using EasySep® mouse monocyte and neutrophil enrichment kits (STEMCELL Technologies). Splenocytes were incubated with the EasyStep® antibody cocktails to non-moncytes and non-neutrophils followed by biotin selection cocktail and magnetic beads. Labeled non-monocytes and non-neutrophills were removed using EasySep magnets. Monocyte purity determined by flow cytometry was 90% (CD11b+Ly6G−). Neutrophil purity was 83% (CD11b+Ly6G+).
Flow cytometry analysis of splenocytes
Splenocytes were aliquoted into FACS tubes (6 × 105 /tube) and centrifuged at 200 × g for 10 min. Cell pellets were resuspended in 80 µl FACs buffer (PBS buffer with 2% BSA and 0.10% sodium azide) and 20 µl of mouse serum. After 10 min incubation at RT, the cells were stained with antibodies for 30 min in the dark at 4°C. The cells were washed twice with FACS buffer and analyzed on a LSRII cytometer using Flow-Jo software (Tree Star). Antibodies used were Alexa-488 anti-NK1.1 (clone P136), Alexa-488 anti-CD45R (B220) (clone RA3–6B2), Alexa-448 anti-CD4 (clone GK1.5), Alexa-488 anti-CD8α (clone 53–6.7), Bv421 anti-CD11b (clone M1/70), BV605 anti-Ly6C (clone HK1.4), APC anti-Ly6G (clone1A8), PE anti-Ly6G (clone 1A8), Alexa 700 anti-F4/80 ( clone BM8), BV421 Rat IgGb, BV605 Rat IG2b, APC Rat IgG2a, and PE Rat IgG2a, Alexa700 Rat IgG2a (obtained from BioLegends); APC anti-SIGNR1 (clone 22D1) and APC hamster IgG (obtained from eBioscience). Gating strategy for identification of myeloid spleen populations is outlined in Suppl. Fig. 1. The number of each myeloid population/spleen was calculated from the % of the population determined by flow cytometry and the total spleen cell counts.
RNA Flow Cytometry
RNA flow cytometry (PrimeFlow RNA Assay, eBioscience) was carried out according to manufacturer instructions. Prior to fixation and permeablization, splenocytes (5 ×106 cells/ sample) were labeled with antibodies listed above to detect spleen myeloid populations as outlined in Suppl. Fig. 1. Splenocytes were hybridized with a Alexa-647-labeled gene-specific probe set specific for mouse IL-1β (VB1–11873) and followed by signal amplification. The cells were analyzed on the LSRII flow cytometer.
Real-time RT-PCR
Splenocytes, isolated spleen monocytes and neutrophils, and mesenteric lymph nodes (MLN) were homogenized in 1 ml TRIzol reagent (Invitrogen). RNA was extracted with chloroform and precipitated with isopropanol. The RNA pellet was washed once with 75% ethanol and the RNA reconstituted with DNase/RNase-free and bacterial DNA- free water (Qiagen). Residual DNA was removed using the TURBO-DNA-free Kit (Ambion, Life Technologies) according to manufacturer instructions. RNA was reversed-transcribed using random primers by the Promega Reverse Transcription System. Cytokine mRNA and bacterial group-specific 16S RNA expression was analyzed by qRT-PCR using IQ SYBR Green Supermix (Roche). The amplification conditions were 95° for 10 minutes followed by 45 cycles of 95° for 15 s, 58° for 30s, and 72° for 30s. Each measurement was carried out in duplicate. Primers for mouse genes were as follows: Actb (forward, 5-TACAGCTTCACCACCACAGC-3, reverse, 5-AAGGAAGGCTGGTAAAAGAGC), Gapdh (forward, 5-GTGTGAACGGATTTGGCC-GTATTGGGCG-3, reverse, 5-TCGCTCCTGGAAGATGGTGATGGGC-3), Il1b (forward, 5-TTCAGGCAGGCAGTATCACTC-3, reverse, 5-CCACGGGAAAGACACAGGTAG-3), Il6 (forward, 5-CACAAGTCCGG-AGAGGAGAC-3, reverse, 5-CAGAATTGCCATTGCACAAC-3), Tnfa (forward, 5-CATCTTCTC-AAAATTCGAGTGACAA, reverse, 5-TGGGAGTAGACAAG-GTACAACC-3), Il23a (forward, 5-ATGCTGGATTGCAGAGCAGTA-3, reverse, 5-ACGGGG-CACATTATTTTTAGTCT-3). Group-specific primers to bacterial 16S RNA (42, 43) were as follows: Lactobacillus (forward, 5-AGCAGTAGGGAATCTTCCA-3, reverse, 5-CGCCA-CTGGTGTTCYTCCATATA-3), Bacteroides (forward, 5-CCTWCGATGGATAGGGGTT-3, reverse, 5-CACGCT-ACTTGGCTGGTTCAG-3), Bifidobacterium (forward, 5-CGGGTG-AGTAATGCGTGACC-3, reverse 5-TGATAGGACGCGACCCCA-3), C.leptum (forward, 5-CCTTCCGTGCCGSAGTTA-3, reverse, 5-GAATTAAACCACATACTCCACTGCTT-3), C. coccoides (forward, 5-CGCCGCGTGAAGGA-3, reverse, 5-AGCCCAGCCTTTCACATC-3), Enterococus (forward, 5-CCCTTATTGTTAGTTGCCATCATT-3, reverse, 5- ACTCGTTG-TACTTCCCATTGT-3), Candidatus Arthromitus (segmented filamentous bacteria) (forward, 5-TGTGGGTTGTGAATAAACAAT-3, reverse, 5-GCGGGCTTCCCTCATTACAAGG-3). Relative expression was calculated by the ΔCT method using mouse GAPDH or Actb RNA as the normalizer (80). Expression levels of individual home cage and SDR mice were determined relative to the mean ΔCT of the home cage mice. To confirm the specificity of the bacterial 16S RNA amplifications and to identify the bacterial species, the PCR products were cloned into PGEM-T easy vector (Promega) and individual colonies sequenced. Bacterial species were identified by searching the 16S bacterial ribosomal RNA database using the nucleotide Basic Local Alignment Search Tool (BLAST). (www.ncbi.nlm.nih.gov/BLAST).
Bacteria
Lactobacillus animalis strain 35046 (American Type Culture Collection [ATCC]) was grown overnight in Difco Lactobacillus MRS media at 37°C with 5% CO2. The bacteria were heat-killed by incubation at 80°C for 30 minutes. The bacteria were washed three times in PBS and stored at −80° C in RPMI 1640 media. To identify mice with live lactobacilli in the spleen, spleens from home cage control mice and SDR stressed mice were homogenized in PBS and serially diluted. Each dilution was grown on Difco Lactobacillus MRS agar at 37°C with 5% CO2.
In vitro activation of spleen monocytes and neutrophils with L.animalis
Spleen monocytes and neutrophils isolated by negative selection as described above were cultured for 6 hrs in RPMI 1640 media containing 2 mM glutamine, 10% fetal bovine serum, and either heat killed or live L.animalis. RNA was isolated and cytokine mRNA determined as described above.
Statistical analysis
Statistical differences were analyzed by unpaired Student t tests. Welch’s correction was used when the variances were unequal as indicated in figure legends. Bivariate correlation analysis was used to analyze correlations between Lactobacillus 16S RNA levels and cytokine mRNA levels. The p values <0.05 were considered to indicate significant differences between groups.
Study Approval
Mice use and procedures was approved by The Ohio State University’s Animal Care and Use Committee.
Results
SDR increases classical monocytes and neutrophils in the spleen
Previous research by our laboratories, as well as others (9, 10, 15, 18, 45), showed that exposure to the SDR stressor increases the levels of CD11b+ cells in the spleen. To define the splenic myeloid populations increased by the SDR stressor, splenocytes from mice exposed to 6 cycles of the SDR stressor were examined by flow cytometry using the gating strategy outlined in Suppl. Fig. 1. Since SDR increases spleen size, the absolute numbers of each myeloid population in the spleen was determined. Classical monocytes (CD11b+Ly6ChiLy6G−) and neutrophils (CD11b+Ly6CintermLy6G+) were significantly increased in the SDR mice compared to home cage control mice (p<.05) (Fig. 1). Non-classical monocytes (CD11b+Ly6ClowLy6G−) (Fig. 1) and red pulp macrophages (CD11b− F4/80+) (Fig. 2) were not increased by the SDR stressor. The SDR stressor tended to decrease marginal zone macrophages (CD11b−SIGNR1+) but the difference did not quite reach statistical significance (p=.059) (Fig. 2).These results are consistent with previous studies indicating that classical monocytes and neutrophils are the CD11b+ splenocytes increased by the stressor (10, 15, 18, 23).
Figure 1. Exposure to the SDR stressor increases the numbers of classical monocytes and neutrophils in the spleen.
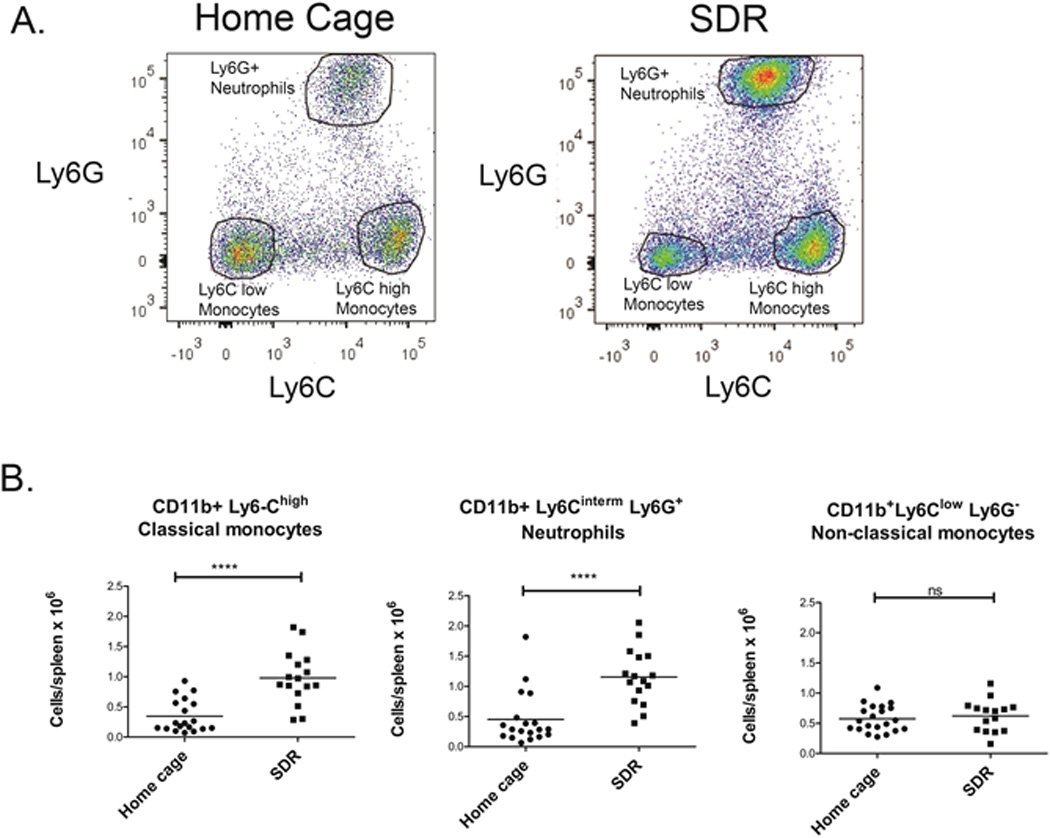
Spleens were harvested from SDR stressor-exposed mice and home cage control mice and single cell suspensions analyzed by flow cytometry using the gating strategy outlined in supplemental Fig. 1. (A) Representative flow cytometry graphs of CD11b+ cells from SDR and home cage mice that were stained with Ly6C and Ly6G antibodies to detect Ly6Chi Ly6G classical monocytes, Ly6Cintermediate Ly6Ghi neutrophils, and non-classical Ly6Clow Ly6G macrophages. (B). Since the SDR-stressor increases spleen size, total cell numbers of each population in the spleen of individual SDR stressed mice and home cage control mice was determined by flow cytometry. Statistical analysis was performed by Student t test. **** p<0.0001 N=15–21 mice per group
Figure 2. Analysis of red pulp macrophages and marginal zone macrophages in the spleen of SDR stressed mice.
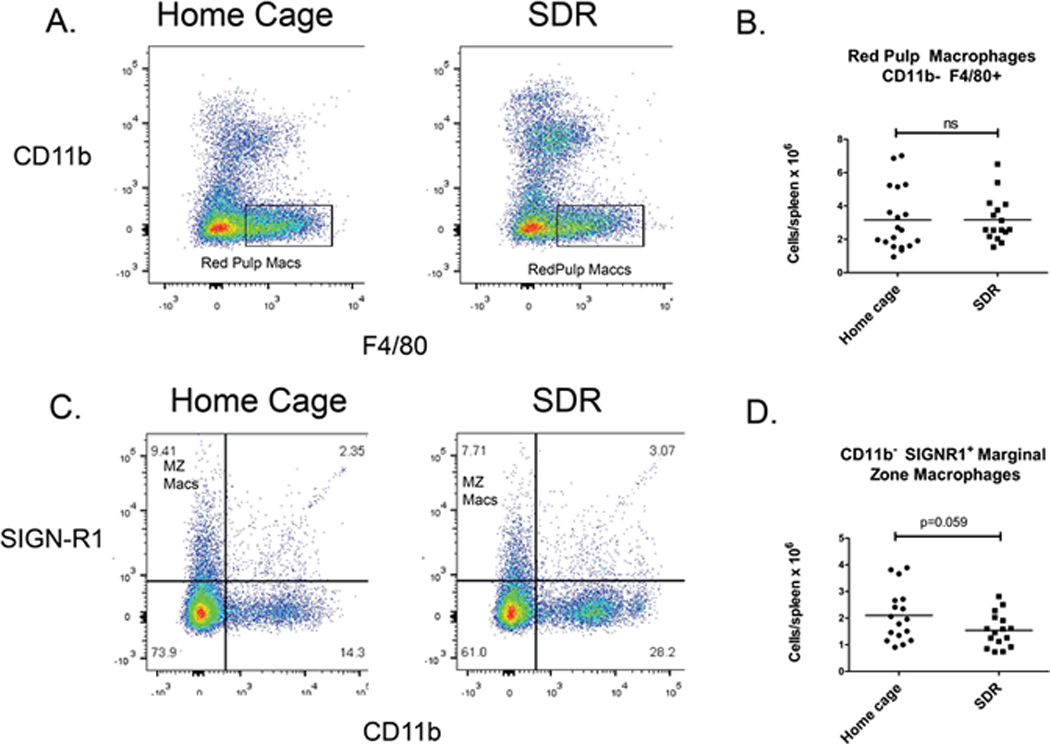
Spleen cells from mice exposed to the SDR stressor and non-stressed home cage control mice were analyzed by flow cytometry. Spleen cells were gated to remove lymphocytes and red pulp macrophages (CD11b F4/80+) (A) and marginal zone macrophages (CD11b SIGNR1+) (C) populations analyzed by flow cytometry. (B) and (D) Since the SDR-stressor increases spleen size, total cell numbers of red pulp macrophages and marginal zone marcrophages in the spleen of individual SDR stressed mice and home cage control mice as determined by flow cytometry. Statistical analysis was performed by Student t test. N=16–19 mice per group.
Classical monocytes and neutrophils have increased expression of IL-1β and IL-23p19 mRNA in response to SDR
Cytokine mRNA levels in the spleen and mesenteric lymph nodes were examined by qRT-PCR. Splenic levels of IL-1β and IL-23p19 were increased in SDR stressor-exposed mice (p<.05)(Fig. 3). IL-6, IL-10 and TGFβ mRNA levels were unchanged between SDR stressor-exposed and non-stressed home cage control mice, whereas TNFα mRNA was significantly decreased in stressor-exposed mice (p<.05)(Fig. 3). Mesenteric lymph node levels of IL-1β and IL-23p19 mRNA were the same in stressed and non-stressed mice (Suppl. Fig. 2A), suggesting the stressor specifically up-regulated IL-1β and IL-23p19 in the spleen. TNFα mRNA in the mesenteric lymph nodes was also significantly decreased, suggesting that the stressor–induced decrease in TNFα mRNA is not specific to the spleen.
Figure 3. IL-1β and IL-23p19 mRNA levels are increased in spleens of SDR stressed mice.
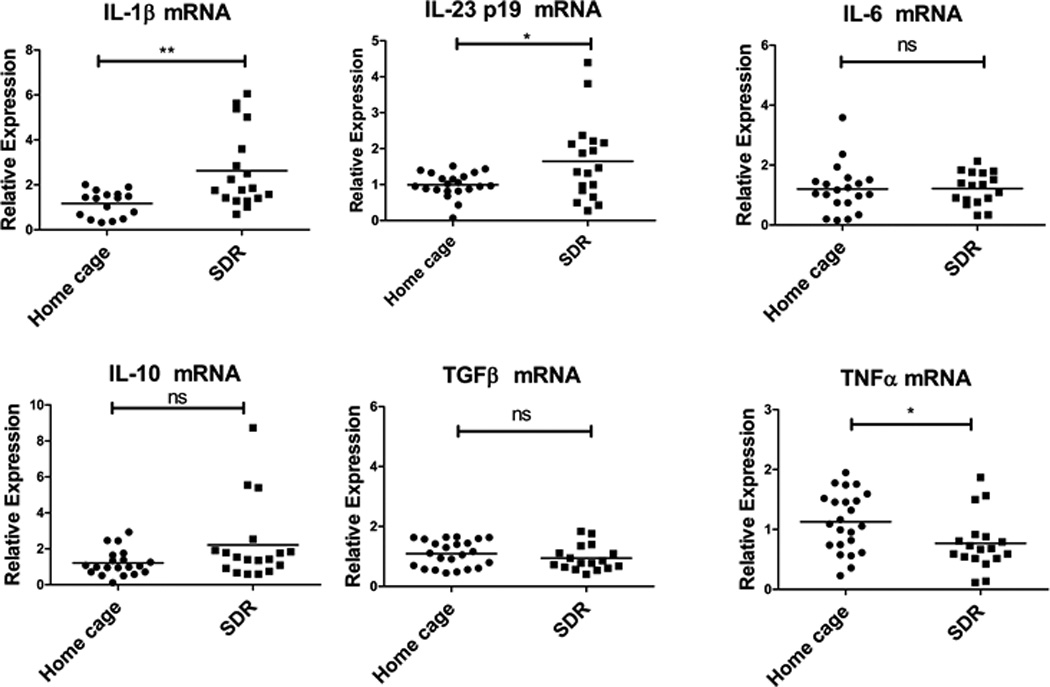
RNA was isolated from spleens of SD stressed and home cage control mice and cytokine mRNA levels in the spleens of individual mice were analyzed by qRT-PCR. Relative mRNA expression was determined using GAPDH mRNA as the normalizer. Expression levels of individual home cage and SDR mice were determined relative to the mean ΔCT of the home cage mice. Statistical analysis was performed by Student t test with Welch’s correction for unequal variance. qRT-PCR. Relative mRNA expression was determined using GAPDH mRNA as the normalizer. Expression levels of individual home cage and SDR mice were determined relative to the mean ΔCT of the home cage mice. Statistical analysis was performed by Student t test with Welch’s correction for unequal variance. N=16–23 mice per group. * p<0.05, ** p<0.01
To determine which myeloid cell population was producing IL-1β mRNA, expression was examined by RNA flow cytometry which combines detection of mRNA by oligonucleotide probe hybridization and branched DNA signal amplification with flow cytometry analysis of cell populations (gating strategy and representative plots outlined in Suppl. Fig. 3). We chose this method over intracellular cytokine staining in which cytokine release is inhibited by Berfeldin A, because IL-1β secretion is not blocked by Berfeldin A. Interleukin-1β is processed in the cytoplasm by cleavage of Pro-IL-1β and secretion of mature IL-1β occurs by a number of different mechanisms (reviewed in(46)). Thus, blocking IL-1β is problematic and accuracy of IL-1β intracellular cytokine staining without blocking secretion is uncertain. The percentages of neutrophils, classical monocytes, and non-classical monocytes positive for IL-1β mRNA were significantly higher in the SDR mice compared to home cage control mice (p<.05)(Fig. 4). The percentage of neutrophils positive for IL-1β mRNA was substantially higher than the percentage of classical and non-classical monocytes positive for IL-1β mRNA (65% vs. 7% respectively), suggesting that neutrophils are a major source for IL-1β in the spleen.
Figure 4. Exposure to the SDR stressor increased the percentage of IL-1β producing myeloid cells.
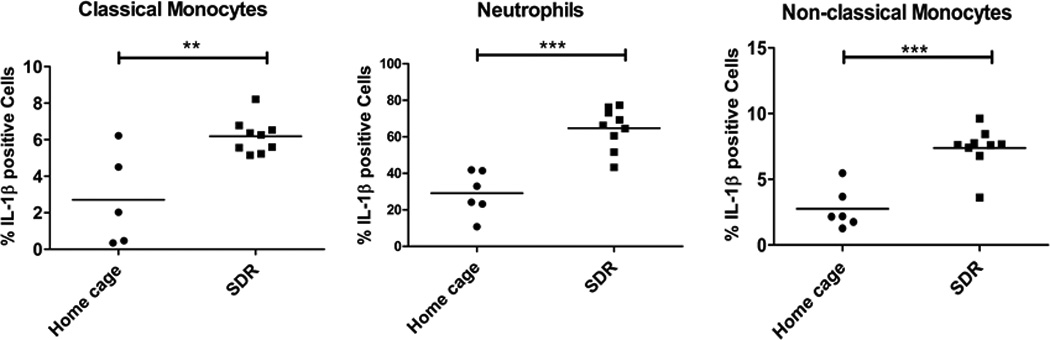
Spleens were harvested from SDR stressed mice and home cage control mice and single cell suspensions analyzed by RNA flow cytometry using the gating strategy outlined in supplemental Fig. 1 and a RNA probe set specific for IL-1β mRNA. The percentage of IL-1β mRNA positive cells was determined for individual home cage and SDR mice. Statistical analysis was performed by Student t test with Welch’s correction for unequal variance. N=5–9 mice per group ** p<0.01, *** p<0.001
SDR induces translocation of Lactobacillus to the spleen
Our previous research using germ-free mice and antibiotic-treated mice showed that the intestinal microbiota are required for stressor-induced increases in spleen macrophage cytokine production and microbicidal activity (10). This led us to hypothesize that bacterial translocation from the gut to the spleen activates macrophages/monocytes in the spleen. To determine if SDR induces bacterial translocation to the spleen, we examined bacterial 16S rRNA in spleen cells by rRNA-targeted qRT-PCR using previously described bacterial group-specific primers to seven different bacterial groups (47, 48). This qRT-PCR approach has been previously used to detect commensal and pathogenic bacteria in the human intestine and is more sensitive than qPCR due to the high copy number of 16S rRNA in bacteria (49, 50). Bacterial 16S rRNA from C. leptum, C. coccoides, Enterococcus and Candidatus Arthromitus (segmented filamentous bacterium) were either not-detected or present in very low levels (data not shown). However, bacterial 16S rRNA from Lactobacillus, Bacteroides, and Bifidobacterium were detected in the spleen by qRT-PCR (Fig. 5). Only Lactobacillus showed a statistically significant increase in mice exposed to the SDR stressor compared to non-stressed home cage control mice (p<.05). SDR stressor-exposed mice had variable levels of Bacteroides 16S rRNA with several of the stressed mice having high levels. However, there was no statistically significant difference between the stressor-exposed and the non-stressed control mice. Also, there was no correlation between the levels of Bacteroides 16S rRNA and Lactobacillus rRNA in the stressor-exposed mice. These results suggest that bacterial translocation from the gut to the spleen is selective, and Lactobacillus is one bacterial type capable of translocating during stressor exposure. We were also able to culture lactobacilli from spleens of SDR stressed mice, with 3/6 having culturable lactobacilli, while only 1/6 home cage controls had culturable lactobacilli. To confirm that qRT-PCR is detecting Lactobacillus and Bacteroides 16S rRNA and to identify Lactobacillus and Bacteroides species, PCR products from the qRT-PCR reactions were cloned and individual clones were sequenced. Clones were identified by searching the 16S Ribosomal RNA sequence data base. Lactic acid bacteria detected were L.murnius/ L animalis (7/18 clones), L. crispatus/ L. gallinarium (4/18 clones), and Dolosigranulum pigrum (7/18). Bacteroides species detected were B. faecis/ B.uniformis (4/17 clones), B. fragilis (2/17 clones), B. salyersiae (1/17 clones), B.chinchillae/B. sartoriii (1/17 clones). There were 4 unknown Bacteroides sequences detected (total of 9/17 clones). These were clearly Bacteroides sequences as they were 96–98% identical to identical to B. luti and the sequences were not present in the mouse nucleotide database.
Figure 5. The SDR stressor induces translocation of Lactobacillus to the spleen.
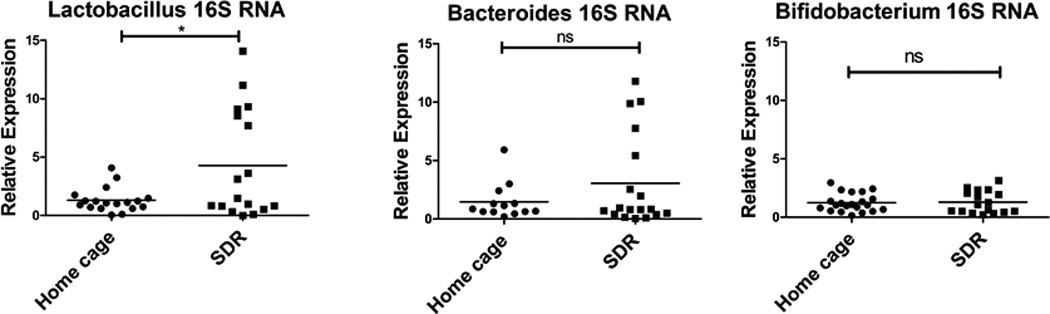
The presence of translocated bacteria was analyzed by qRT-PCR using primers specific for 16S bacterial rRNA. RNA was isolated from the spleens of SDR stressed and home cage control mice and real-time RT-PCR performed using bacterial group-specific primers to 16S RNA. Relative expression of bacterial 16S rRNA was determined using GAPDH mRNA as the normalizer. Expression levels of individual home cage and SDR mice were determined relative to the mean ΔCT of the home cage mice. Statistical analysis was performed by Student t test with Welch’s correction for unequal variance. N=13–21 mice per group * p<0.05
We also examined the levels of bacterial 16S rRNA in mesenteric lymph nodes (Suppl. Fig. 2B). The levels of Lactobacillus 16S rRNA were not increased by exposure to SDR, while levels of Bacteroides and Bifidobacterium 16S rRNA actually decreased with SDR exposure. This suggests that the SDR stressor-induced translocation of Lactobacillus is specific to the spleen.
Lactobacillus 16S rRNA positively correlates with IL-1β and IL-23p19 in SDR stressed mice
We hypothesized that translocation of Lactobacillus to the spleen induces IL-1β and IL-23 mRNA. Thus, we determined if the levels of Lactobacilllus 16S rRNA in the spleen of SDR mice correlated with 1L-1β and IL-23p19 mRNA. Bivariate correlation analysis (Fig. 6) showed strong positive correlations between Lactobacillus 16S rRNA with both IL-1β (p<.001) and IL-23p19 mRNA (p<.001). In contrast, there was no correlation between Bacteriodes 16S rRNA and IL-1β and IL-23p19 mRNA levels (data not shown).
Figure 6. IL-1β and IL-23p19 mRNA levels positively correlate with levels of Lactobacillus 16S RNA in SDR stressed mice.
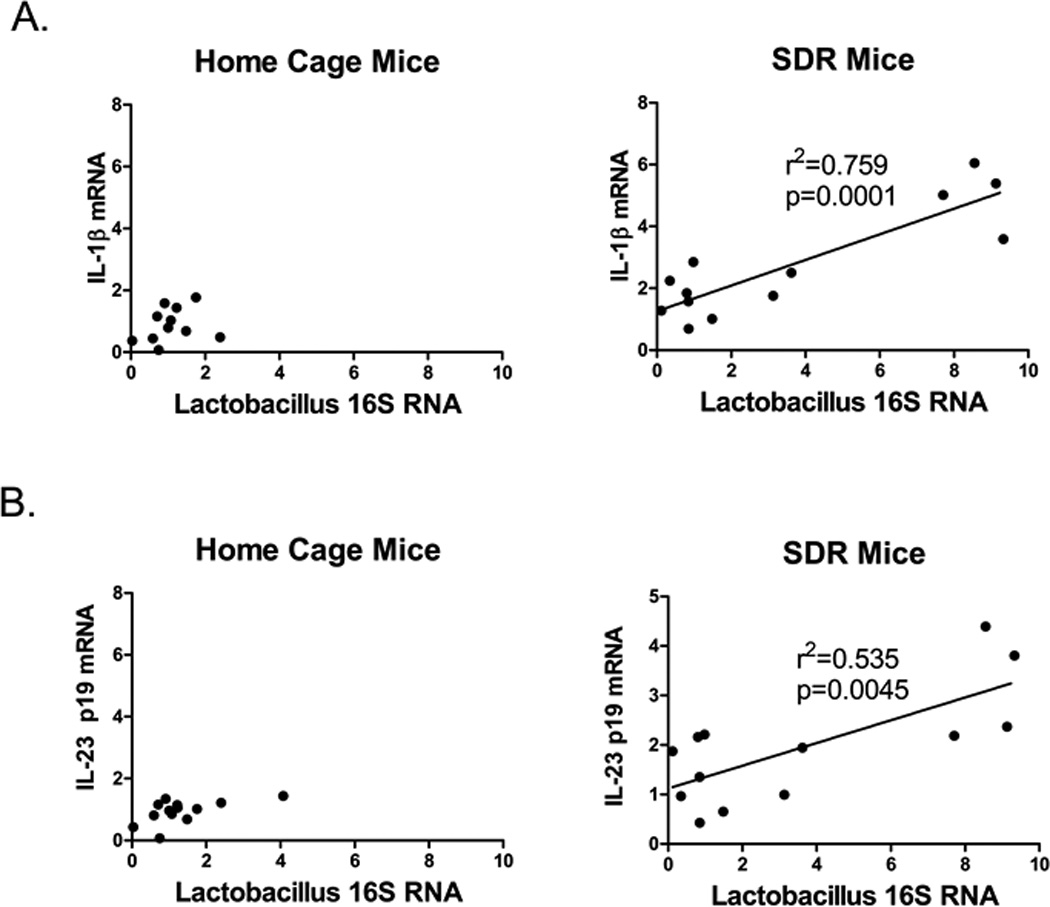
Bivariate correlation analysis was used to compare IL-1β and IL-23p19 mRNA levels with Lactobacillus 16S RNA levels in individual mice from home cage control and SDR mice. (A) IL-1β mRNA levels in SDR stressed mice positively correlated with Lactobacillus 16S RNA levels. (B) IL-23p19 mRNA levels in SDR stressed mice positively correlated with Lactobacillus 16S RNA levels.
Bacterial 16S rRNA is present in monocytes of SDR stressed mice
To determine if Lactobacillus and Bacteroides16S rRNA is present in the monocyte and neutrophil populations, we isolated spleen monocytes and neutrophils from SDR stressed mice and home cage control mice. Lactobacillus 16S rRNA levels were determined by qRT-PCR using RNA from the isolated monocytes and neutrophils. The monocytes from the SDR stressed mice contained increased levels of Lactobacillus 16S rRNA compared to the home cage control mice (p<.05) There was no increase in Bacteriodes 16S RNA with exposure to the SDR-stressor. (Fig. 7A). In contrast the neutrophils from both the SDR stressed mice and home cage control mice only contained low levels of Lactobacillus and Bacteriodes 16S rRNA (Fig. 7B). This is likely due to the higher antimicrobial activity and production of reactive molecules in neutrophils compared to monocytes and macrophages (reviewed in (51)), leading to increased bacterial destruction.
Figure 7. Monocytes contain increased levels of Lactobacillus 16S rRNA.
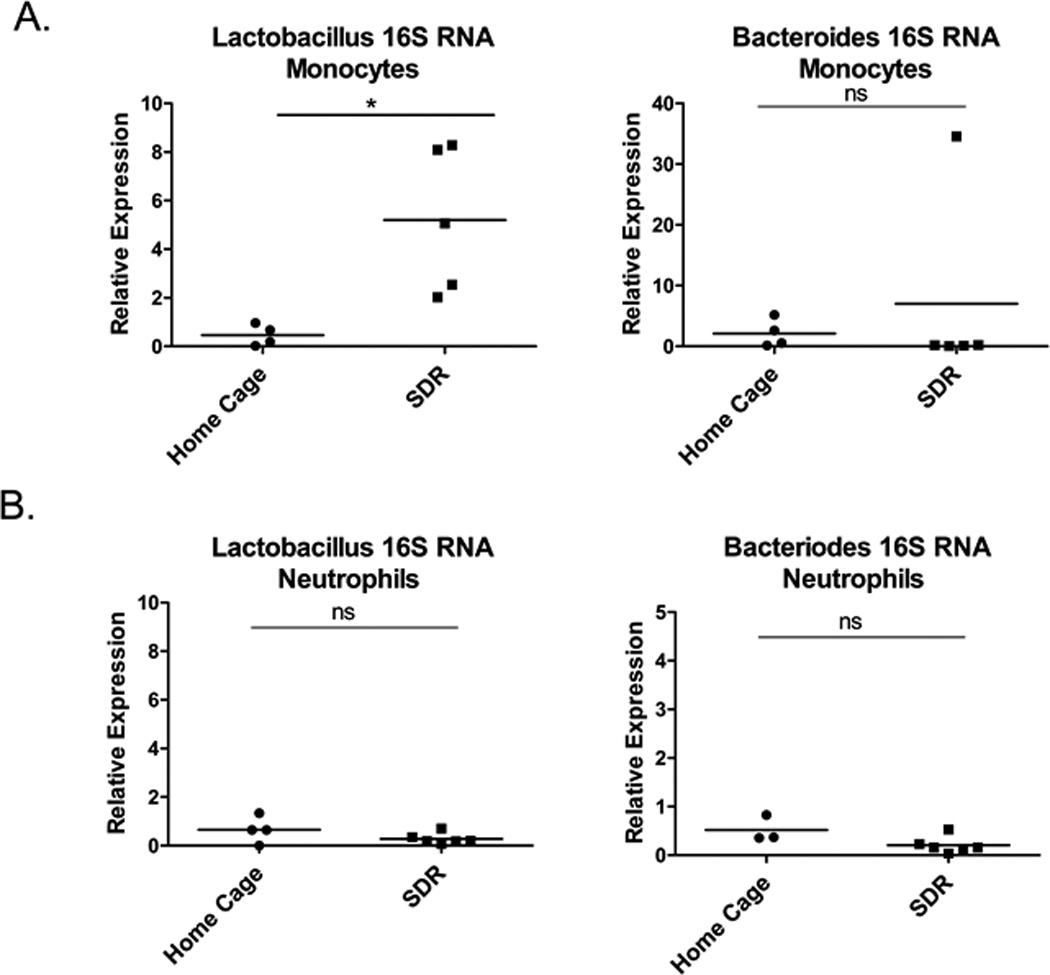
Spleen monocytes (A) and neutrophils (B) were isolated from the spleens of individual home cage control mice and SDR stressed mice. RNA was isolated and real-time RT-PCR performed using primers to Lactobacillus and Bacteroides 16S rRNA. Relative expression of bacterial 16S rRNA was determined using GAPDH mRNA as the normalizer. Expression levels of individual home cage and SDR mice were determined relative to the mean ΔCT of the home cage mice. Statistical analysis was performed by Student t test with Welch’s correction for unequal variance. N=4–6 mice per group * p<0.05
In vitro infection of spleen monocytes and neutrophils with Lactobacillus induces pro-inflammatory cytokine mRNA
To confirm that Lactobacillus is able to induce IL-1β and IL-23, we isolated spleen monocytes and neutrophils from non-stressed mice and stimulated them with heat-killed or live L. animalis (the most predominant Lactobacillus species found in the spleen of stressor-exposed mice). L. animalis induced IL-1β mRNA in both monocytes and neutrophils (Fig. 8). Interestingly, IL-23p19 mRNA was induced in neutrophils but not in monocytes. The ability of L. animalis to induce cytokines was not limited to IL-1β and IL-23p19, however, since IL-6 (p<.05) and TNFα (p<.05) mRNA were also induced in both monocytes and neutrophils (Fig. 8). Interestingly, mRNA for the anti-inflammatory cytokine, TGFβ, was not induced by L.animalis in monocytes or neutrophils (data not shown). These results contrasts with the observed in vivo SDR stressor-induced decrease in spleen and MLN TNFα mRNA and no change in IL-6 mRNA expression (Fig. 3). Thus, we determined whether live, rather than heat-killed, L. animalis would increase cytokine production by monocytes and neutrophils. As with heat-killed bacteria, live L. animalis led to increases in IL-1β and IL-23p19. However, live L. animalis also increased TNF-α, while having no effect on IL-6 (Suppl. Fig. 4). The results suggest that although translocated lactobacilli are capable of inducing TNFα and IL-6, the stressor may negatively modulate expression of these cytokines, such as through stressor-induced release of norepinephrine (NE). To test this hypothesis, we stimulated isolated monocytes in vitro with L.animalis in the presence of NE. NE reduced basal levels of TNFα mRNA and decreased the induction of TNFα by L. animalis (Suppl. Fig. 4B). Since previous studies have shown that NE can inhibit TNFα by β-adrenergic stimulation (52, 53), we treated mice with propranolol, a β-adrenergic receptor antagonist, 30 min prior to each SDR cycle. Blocking β-adrenergic receptors with propranolol, increased TNFα mRNA expression in the SDR-stressed mice (Suppl. Fig. 4B).
Figure 8. In vitro stimulation with heat-killed Lactobacillus animalis induces pro-inflammatory cytokine mRNA in monocytes and neutrophils.
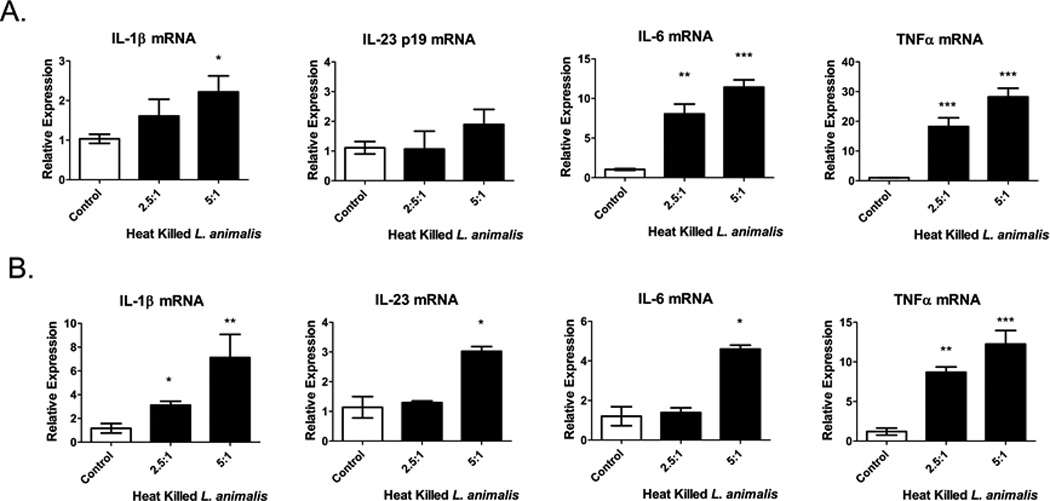
Spleen monocytes (A) and neutrophils (B) were isolated by negative selection from non-stressed C57BL/6 mice and stimulated with heat-killed Lactobacillus animalis at 2.5 and 5 bacteria/cell for 6 hrs. Cytokine mRNA levels were analyzed by qRT-PCR and the relative mRNA expression was determined using β-actin mRNA as the normalizer. Data represents means ±SEM of 3–7 replicate wells from two experiments. Statistical analysis was performed by Student t test with Welch’s correction for unequal variance *p<0.05, **p<0.01, ***p<0.001 compared to control wells.
Discussion
Social disruption is a murine stressor that encompasses both physical components (involving physical wrestling/fighting) and psychological components (involving repeated social defeat, loss of social status, and the development of anxiety-like behaviors) (9, 15, 17, 18, 22). The behavioral sequelae of the SDR stressor, most notably anxiety-like behavior, are common in humans during stressful periods, and anxiety is often associated with enhanced immune system activity (54). Studies in laboratory mice exposed to the SDR stressor, as well as other stressor paradigms, clearly demonstrate that this enhanced immune system activity impacts the behavioral response to stressor exposure. For example, the development of anxiety-like behavior in mice exposed to the SDR stressor has been found to involve trafficking of classical monocytes from the bone marrow and spleen to the brain (14, 37, 55, 56), through mechanisms that involve signaling through the IL-1 type 1 receptor (IL-1R1) (55). These studies illustrate the important effects that stressor-induced immune system activity can have on the body. However, the complete set of factors that contribute to stressor-induced immune activity have not been thoroughly studied. Our study confirms and extends previous results indicating that the commensal gut microbiota are important contributors to stressor-induced immunoenhancement.
Multiple studies have demonstrated that exposure to the SDR stressor increases the number of CD11b+ cells in the spleen (9, 16). This increase in splenic CD11b+ cells is dependent upon sympathetic nervous system (SNS)-induced β-adrenergic signaling (16), and is found in both conventional and germfree mice (9), indicating that the microbiota are not involved in the accumulation of these cells in the spleen. In the present study, we extended these earlier observations by further defining the CD11b+ cell populations that are increased in the spleens of stressor exposed mice. Consistent with previous studies, we report that exposure to the SDR stressor increases the levels of classical monocytes (CD11b+Ly6ChiLy6G−) and neutrophils (CD11b+Ly6CintermLy6G+) in the spleen. There was a trend for the SDR stressor to decrease marginal zone macrophages, however red pulp macrophages and non-classical monocytes were not affected by stressor exposure. Only the classical monocytes and neutrophils were significantly increased by stressor exposure
In addition to inducing an increase in classical monocytes and neutrophils in the spleen, the SDR stressor also increases cytokines, including IL-1β (15, 18). Our previous research (9) showed that the stressor-induced increases in cytokines are dependent upon the commensal microbiota. Exposing germfree mice or mice given oral antibiotics to the SDR stressor did not result in increased cytokine production (9, 43). We also showed that exposure to the SDR stressor primed CD11b+ macrophages/monocytes for increased bacterial killing and increased pro-inflammatory cytokine production (10). This priming effect was shown to be dependent upon signaling through the IL-1 receptor and upon the commensal microbiota (9, 10), supporting previous studies that the microbiota are needed for stressor-induced increases in IL-1β that signals through the IL-1R1 to enhance innate immune responses and induce anxiety-like behavior (10, 55). However, the mechanisms by which the commensal microbiota are linked to stressor-induced increases in IL-1β have not been clearly defined.
Previous studies indicate that stressor exposure increases the ability of gut microbes to translocate from the lumen of the intestines to the interior of the body (8, 57). To determine whether the increase in IL-1β might be due to translocation of commensal microbiota, we first tested whether exposure to SDR induced the translocation of microbiota by utilizing a qRT-PCR assay to detect bacterial 16S rRNA in the spleen and mesenteric lymph nodes. Exposure to the SDR stressor specifically increased Lactobacillus group 16S rRNA present in spleen cells. Bacteroides 16S rRNA was also detected at high levels in some of the SDR-exposed mice, but the increase in Bacteroides 16S rRNA was not statistically significant. It is not yet clear why Lactobacillus, but not Bacteroides, was evident within the spleen, but there are likely multiple factors that contribute. For example, several species of Lactobacillus, including species with probiotic properties, have been shown to stimulate an increase in phagocytosis and to increase bacterial translocation to the mesenteric lymph nodes and Peyer’s patches (58–60). At the same time, it may be more challenging for the innate myeloid cells to break down the Gram-positive cell wall, and subsequently the rRNA, of lactobacilli than it is to break down the Gram-negative cell envelope of Bacteroides. More work is needed to understand why highr levels of Lactobacillus, but not Bacteroides, 16s rRNA were found in spleens of stressor-exposed mice.
It was beyond the scope of this study to assess individual differences in the response to the SDR stressor, but it is interesting to note that the increase in Lactobacillus rRNA was not found in all of the stressor-exposed animals. Variability in behavioral and physiological responses to the SDR stressor is common, and there are multiple factors that can contribute to this variability. For example, we have previously found that mice that are more submissive within their home cage and during the SDR stressor have stronger immunological responses to the repeated social defeat (61). Further studies are needed to completely understand individual differences in bacterial translaction in response to SDR stressor exposure.
The use of bacterial 16S qRT-PCR assay allowed us to detect both bacterial 16S rRNA and cytokine mRNA in the same sample. Thus, we were able to show that increases in IL-1β, as well as IL-23p19, mRNA in mice exposed to the SDR stressor were positively correlated with increased levels of Lactobacillus 16S rRNA. This was followed by an RNA flow assay that demonstrated that neutrophils, classical monocytes, and non-classical monocytes expressed IL-1β mRNA. There were significantly higher levels of IL-1β mRNA in these cells from stressor-exposed mice compared to cells from non-stressed home cage control mice. The high percentage of neutrophils expressing IL-1β mRNA in mice exposed to the SDR stressor indicates that splenic neutrophils are a major source of IL-1β during stressor exposure. This is consistent with studies involving bacterial infection, such as cutaneous Staphylococcus aureus (62) or corneal Streptococcus pneumonia infection (63), that demonstrate that neutrophils are the major source of IL-1β . It is interesting to note that neutrophils, while producing high levels of IL-1β, were not found to contain bacterial 16s rRNA, whereas monocytes contained high levels of Lactobacillus 16s rRNA. This effect is likely due to the greater abilities of neutrophils to phagocytose, kill, and degrade microbes compared to monocytes and macrophages (51, 64, 65). Further work is needed to determine when/if neutrophils are affected by translocating microbes in vivo.
To further address whether the translocation of L. animalis could be responsible for stressor-induced increases in cytokines, splenic monocytes and neutrophils were stimulated with heat-killed L. animalis (which was the primary species of Lactobacillus found in the spleen). Stimulation with heat-killed or live L. animalis led to the production of IL-1β, confirming that lactobacilli are capable of inducing IL-1β. IL-23p19 mRNA was also induced, but only in neutrophils. This was somewhat surprising, but a low level of IL-23p19 mRNA expression has been reported in bone marrow derived neutrophils in response to parasites (66). In vitro stimulation of spleen monocytes and neutrophils with L. animalis also induced expression of TNFα and IL-6 mRNA. This contrasts with the observed SDR stressor-induced decrease in spleen TNFα mRNA and no change in IL-6 mRNA expression. The results suggest that although translocated lactobacilli are capable of inducing TNFα and IL-6, the stressor may negatively modulate expression of these cytokines, such as through stressor-induced release of norepinephrine (NE) by the splenic nerve. Treating spleen cells with norepinephrine prevented the ability of L. animalis to induce TNFα expression. Moreover, treating stressor exposed mice with an antagonist for the β-adrenergic receptor resulted in higher TNFα expression in the spleen of stressor-exposed mice, indicating that splenic norepinephrine has an inhibitory effect on inflammatory cytokine expression. This is consistent with previous studies demonstrating that splenic nerve-induced catecholamine release attenuates TNFα production by red pulp and marginal zone macrophages (67) and that activation of the β1-adrenergic (53) and the β2-adrenergic (52) receptors inhibit TNFα and IL-6 in the liver and the spleen.
Surprisingly, we did not find any increases in cytokine production or bacterial translocation in the mesenteric lymph nodes of stressor-exposed mice. Stressor exposure is well recognized to increase the permeability of the epithelial barrier to allow bacterial translocation to occur (68–71). While there are multiple possible mechanisms, corticotrophin releasing hormone (CRH) as well as other neuroendocrine mediators have been shown to lead to mast cell degranulation to enhance epithelial permeability (69–71). Indeed, the ability of the SDR stressor to prime splenic macrophages can be attenuated through the use of the mast cell stabilizer cromolyn (57). Stressor-induced increases in epithelial barrier permeability would eliminate the need for bacterial uptake by M cells, transport to regional mesenteric lymph nodes, and subsequent dissemination throughout the body through the lymphatics. This is consistent with our inability to detect bacteria in mesenteric lymph nodes or stressor-induced increases in cytokine production. Additional studies are needed to determine how stressor exposure leads to increased bacterial translocation in the current model.
Interleukin-1 signaling through the IL-1R1 is a key component of stressor-enhanced immunopotentiation and the development of emotional disorders, such as anxiety and depression (13). As such, our results contribute to a growing number of studies that have linked the microbiota to behavioral disorders during stressful periods (7, 72, 73), and indicates that the immune system plays a crucial role in the microbiota-gut-brain axis. IL-1α/β is increased in serum, spleen, brain, and liver in stressor-exposed animals, even in the absence of pathogen challenge (18), but the cellular source of the IL-1 has not been thoroughly studied. Although the microbiota, as well as danger-associated molecular patterns (DAMPs) have been shown to be important triggers for IL-1 production (9, 18, 25, 43), how the microbiota could impact immune cells in sites such as the spleen has not been clearly defined. Our results indicate that commensal bacteria translocate from the intestines to the interior of the body where they prime the innate immune system for enhanced reactivity to antigen challenge. This supports the notion that commensal microbes are key players in the physiological response to stress.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge editorial assistance of Ms. Carolyn Moore.
Funding Source
This project was supported by NIH grant R21AI107238.
Footnotes
Conflict of Interest Statement
The authors have declared that no conflict of interest exists.
Author Contributions
WPL and MTB designed research studies. WPL, RG, SF, CN, AM conducted experiments and acquired data. WPL and MTB analyzed data and wrote the paper.
References
- 1.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1985–1987. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 2.Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: Implications for depressive disorders. Pschol. Bull. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- 3.Van Praag Hm DKE, Van Os J. Stress, the Brain and Depression. Cambridge, England: Cambridge University Press; 2004. [Google Scholar]
- 4.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Kaspar LH. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014;30:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yarandi SS, Peterson Da T, Moran GJTH, Paricha PJ. Modulatory effects of gut microbiota on the central nervous system: How gut could play a role in neuropsychiatric health and diseases. J. Neurogastroenterol. Motil. 2016;22:201–212. doi: 10.5056/jnm15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster JA, Mcvey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Bailey MT. Psychological stress, immunity, and the effects on the indigenous microflora. In: Lyte MFPP, editor. Microbial Endocrinology. New York: Springer; 2010. pp. 191–212. [Google Scholar]
- 9.Allen RG, Lafuse WP, Galley JD, Ali MM, Ahmer BM, Bailey MT. The intestinal microbiota are necessary for stressor-induced enhancement of splenic macrophage microbicidal activity. Brain Behav. Immun. 2012;26:371–382. doi: 10.1016/j.bbi.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen RG, Lafuse WP, Powell ND, Webster Marketon JI, Stiner-Jones LM, Sheridan JF, Bailey MT. Stressor-induced increase in microbicidal activity of splenic macrophages is dependent upon peroxynitrite production. Infect. Immun. 2012;80:3429–3437. doi: 10.1128/IAI.00714-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galley JD, Nelson MC, Yu Z, Dowd SE, Walter J, Kumar PS, Lyte M, Bailey MT. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. B.M.C. Microbiol. 2014;14:189. doi: 10.1186/1471-2180-14-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galley JD, Yu Z, Kumar P, Dowd SE, Lyte M, Bailey MT. The structures of the colonic mucosa-associated and luminal microbial communities are distinct and differentially affected by a prolonged murine stressor. Gut Microbes. 2014;5:748–760. doi: 10.4161/19490976.2014.972241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller AHRCL. The role of inflammation in depsression: from evolutionary imperative to modern treatment target. Nat Rev. Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reader BF, Jarret BL, Mckim DB, Woleb ES, Godbout JP, Sheridan JF. Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience. 2015;289:429–442. doi: 10.1016/j.neuroscience.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey MT, Kinsey SG, Padgett DA, Sheridan JF, Leblebicioglu B. Social stress enhances IL-1beta and TNF-alpha production by Porphyromonas gingivalis lipopolysaccharide-stimulated CD11b+ cells. Physiol. Behav. 2009;98:351–358. doi: 10.1016/j.physbeh.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav. Immun. 2012;26:1150–1159. doi: 10.1016/j.bbi.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R. Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain Behav. Immun. 2007;21:458–466. doi: 10.1016/j.bbi.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engler H, Bailey MT, Engler A, Stiner-Jones LM, Quan N, Sheridan JF. Interleukin-1 receptor type 1-deficient mice fail to develop social stress-associated glucocorticoid resistance in the spleen. Psychoneuroendocrinology. 2008;33:108–117. doi: 10.1016/j.psyneuen.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinsey SG, Bailey MT, Sheridan JF, Padgett DA. The inflammatory response to social defeat is increased in older mice. Physiol. Behav. 2008;93:628–646. doi: 10.1016/j.physbeh.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stark JL, Avitsur R, Hunzeker J, Padgett DA, Sheridan JF. Interleukin-6 and the development of social disruption-induced glucocorticoid resistance. J. Neuroimmunol. 2002;124:9–15. doi: 10.1016/s0165-5728(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 21.Bailey MT, Kierstein S, Sharma S, Spaits M, Kinsey SG, Tliba O, Amrani Y, Sheridan JF, Panettieri RA, Haczku A. Social stress enhances allergen-induced airway inflammation in mice and inhibits corticosteroid responsiveness of cytokine production. J. Immunol. 2009;182:7888–7896. doi: 10.4049/jimmunol.0800891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Horm. Behav. 2001;39:247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- 23.Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc. Natl. Acad. Sci. U. S. A. 2013;110:16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avitsur R, Stark JL, Dhabhar FS, Padgett DA, Sheridan JF. Social disruption-induced glucocorticoid resistance: kinetics and site specificity. J. Neuroimmunol. 2002;124:54–61. doi: 10.1016/s0165-5728(02)00010-3. [DOI] [PubMed] [Google Scholar]
- 25.Maslanik T, Tannura K, Mahaffey L, Loughridge AB, Beninson L, Ursell L, Greenwood BN, Knight R, Fleshner M. Commensal bacteria and MAMPs are necessary for stress-induced increases in IL-1β and IL-18 but not IL-6, IL-10 or MCP-1. P.L.o.S. One. 2012;7:e50636. doi: 10.1371/journal.pone.0050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mebius RE, Kraal G. Structure and function of the spleen. Nat. Rev. Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 27.Kraal G, Mebius R. New insights into the cell biology of the marginal zone of the spleen. Int. Rev. Cytol. 2006;250:175–215. doi: 10.1016/S0074-7696(06)50005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirby AC, Beattie L, Maroof A, Van Rooijen N, Kaye PM. SIGNR1-negative red pulp macrophages protect against acute streptococcal sepsis after Leishmania donovani-induced loss of marginal zone macrophages. Am. J. Pathol. 2009;175:1107–1115. doi: 10.2353/ajpath.2009.090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurotaki D, Kon S, Bae K, Ito K, Matsui Y, Nakayama Y, Kanayama M, Kimura C, Narita Y, Nishimura T, Iwabuchi K, Mack M, Van Rooijen N, Sakaguchi S, Uede T, Morimoto J. CSF-1-dependent red pulp macrophages regulate CD4 T cell responses. J. Immunol. 2011;186:2229–2237. doi: 10.4049/jimmunol.1001345. [DOI] [PubMed] [Google Scholar]
- 30.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 31.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 32.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 33.Ajami B, Bennett JL, Krieger C, Mcnagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 34.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, French T, Hambardzumyan D, Matzinger P, Dunay IR, Wolf SA. Ly6C(hi) Monocytes Provide a Link between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. Cell Rep. 2016;15:1945–1956. doi: 10.1016/j.celrep.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 36.Mckim DB, Patterson JM, Wohleb ES, Jarrett BL, Reader BF, Godbout JP, Sheridan JF. Sympathetic Release of Splenic Monocytes Promotes Recurring Anxiety Following Repeated Social Defeat. Biol. Psychiatry. 2016;79:803–813. doi: 10.1016/j.biopsych.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J. Neurosci. 2013;33:13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curry JM, Hanke ML, Piper MG, Bailey MT, Bringardner BD, Sheridan JF, Marsh CB. Social disruption induces lung inflammation. Brain Behav. Immun. 2010;24:394–402. doi: 10.1016/j.bbi.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J. Neuroimmunol. 2004;148:106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 41.Peters AM. Just how big is the pulmonary granulocyte pool? Clin. Sci. (Lond) 1998;94:7–19. doi: 10.1042/cs0940007. [DOI] [PubMed] [Google Scholar]
- 42.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailey MT, Engler H, Powell ND, Padgett DA, Sheridan JF. Repeated social defeat increases the bactericidal activity of splenic macrophages through a Toll-like receptor-dependent pathway. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1180–R1190. doi: 10.1152/ajpregu.00307.2007. [DOI] [PubMed] [Google Scholar]
- 45.Merlot E, Moze E, Dantzer R, Neveu PJ. Cytokine production by spleen cells after social defeat in mice: activation of T cells and reduced inhibition by glucocorticoids. Stress. 2004;7:55–61. doi: 10.1080/1025389042000208150. [DOI] [PubMed] [Google Scholar]
- 46.Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1beta secretion. Cytokine Growth. Factor. Rev. 2011;22:189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furet JP, Firmesse O, Gourmelon M, Bridonneau C, Tap J, Mondot S, Dore J, Corthier G. Comparative assessment of human and farm animal faecal microbiota using real-time quantitative PCR. F.E.M.S. Microbiol. Ecol. 2009;68:351–362. doi: 10.1111/j.1574-6941.2009.00671.x. [DOI] [PubMed] [Google Scholar]
- 48.Yin YEA. Comparative analysis of the distribution of segmented fialamentous bacteria in humans, mice, and chickens. Int. Soci. Microbial Ecol. 2013;7:615–621. doi: 10.1038/ismej.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurakawa T, Kubota H, Tsuji H, Matsuda K, Asahara T, Takahashi T, Ramamurthy T, Hamabata T, Takahashi E, Miyoshi S, Okamoto K, Mukhopadhyay AK, Takeda Y, Nomoto K. Development of a sensitive rRNA-targeted reverse transcription-quantitative polymerase chain reaction for detection of Vibrio cholerae/mimicus, V. parahaemolyticus/alginolyticus and Campylobacter jejuni/coli. Microbiol. Immunol. 2012;56:10–20. doi: 10.1111/j.1348-0421.2011.00405.x. [DOI] [PubMed] [Google Scholar]
- 50.Matsuda K, Tsuji H, Asahara T, Kado Y, Nomoto K. Sensitive quantitative detection of commensal bacteria by rRNA-targeted reverse transcription-PCR. Appl. Environ. Microbiol. 2007;73:32–39. doi: 10.1128/AEM.01224-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silva MT, Correia-Neves M. Neutrophils and macrophages: the main partners of phagocyte cell systems. Front. Immunol. 2012;3:174. doi: 10.3389/fimmu.2012.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deng J, Muthu K, Gamelli R, Shankar R, Jones SB. Adrenergic modulation of splenic macrophage cytokine release in polymicrobial sepsis. Am. J. Physiol. Cell Physiol. 2004;287:C730–C736. doi: 10.1152/ajpcell.00562.2003. [DOI] [PubMed] [Google Scholar]
- 53.Zapater P, Gomez-Hurtado I, Peiro G, Gonzalez-Navajas JM, Garcia I, Gimenez P, Moratalla A, Such J, Frances R. Beta-adrenergic receptor 1 selective antagonism inhibits norepinephrine-mediated TNF-alpha downregulation in experimental liver cirrhosis. P.L.o.S. One. 2012;7:e43371. doi: 10.1371/journal.pone.0043371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav. Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 55.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J. Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wohleb ES, Mckim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, Godbout JP. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol. Psychiatry. 2014;75:970–981. doi: 10.1016/j.biopsych.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bailey MT. The contributing role of the intestinal microbiota in stressor-induced increases in susceptibility to enteric infection and systemic immunomodulation. Horm. Behav. 2012;62:286–294. doi: 10.1016/j.yhbeh.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 58.Ishida-Fujii K, Sato R, Goto S, Yang XP, Kuboki H, Hirano S, Sato M. Prevention of pathogenic Escherichia coli infection in mice and stimulation of macrophage activation in rats by an oral administration of probiotic Lactobacillus casei I-5. Biosci. Biotechnol. Biochem. 2007;71:866–873. doi: 10.1271/bbb.60464. [DOI] [PubMed] [Google Scholar]
- 59.Paturi G, Phillips M, Kailasapathy K. Effect of probiotic strains Lactobacillus acidophilus LAFTI L10 and Lactobacillus paracasei LAFTI L26 on systemic immune functions and bacterial translocation in mice. J. Food Prot. 2008;71:796–801. doi: 10.4315/0362-028x-71.4.796. [DOI] [PubMed] [Google Scholar]
- 60.Ren D, Li C, Qin Y, Yin R, Du S, Liu H, Zhang Y, Wang C, Rong F, Jin N. Evaluation of immunomodulatory activity of two potential probiotic Lactobacillus strains by in vivo tests. Anaerobe. 2015;35:22–27. doi: 10.1016/j.anaerobe.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 61.Avitsur R, Kinsey SG, Bidor K, Bailey MT, Padgett DA, Sheridan JF. Subordinate social status modulates the vulnerability to the immunological effects of social stress. Psychoneuroendocrinology. 2007;32:1097–1105. doi: 10.1016/j.psyneuen.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cho JS, Guo Y, Ramos RI, Hebroni F, Plaisier SB, Xuan C, Granick JL, Matsushima H, Takashima A, Iwakura Y, Cheung AL, Cheng G, Lee DJ, Simon SI, Miller LS. Neutrophil-derived IL-1beta is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. P.L.o.S. Pathog. 2012;8:e1003047. doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karmakar M, Katsnelson M, Malak HA, Greene NG, Howell SJ, Hise AG, Camilli A, Kadioglu A, Dubyak GR, Pearlman E. Neutrophil IL-1beta processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J. Immunol. 2015;194:1763–1775. doi: 10.4049/jimmunol.1401624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levy O. Antimicrobial proteins and peptides: anti-infective molecules of mammalian leukocytes. J. Leukoc. Biol. 2004;76:909–925. doi: 10.1189/jlb.0604320. [DOI] [PubMed] [Google Scholar]
- 65.Segal AW. How neutrophils kill microbes. Annu. Rev. Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Charmoy M, Megnekou R, Allenbach C, Zweifel C, Perez C, Monnat K, Breton M, Ronet C, Launois P, Tacchini-Cottier F. Leishmania major induces distinct neutrophil phenotypes in mice that are resistant or susceptible to infection. J. Leukoc. Biol. 2007;82:288–299. doi: 10.1189/jlb.0706440. [DOI] [PubMed] [Google Scholar]
- 67.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hill LT, Kidson SH, Michell WL. Corticotropin-releasing factor: a possible key to gut dysfunction in the critically ill. Nutrition. 2013;29:948–952. doi: 10.1016/j.nut.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 69.Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. P.L.o.S. One. 2012;7:e39935. doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vanuytsel T, Van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, Salim Rasoel S, Tomicronth J, Holvoet L, Farre R, Van Oudenhove L, Boeckxstaens G, Verbeke K, Tack J. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63:1293–1299. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- 71.Zheng G, Wu SP, Hu Y, Smith DE, Wiley JW, Hong S. Corticosterone mediates stress-related increased intestinal permeability in a region-specific manner. Neurogastroenterol. Motil. 2013;25:e127–e139. doi: 10.1111/nmo.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diaz Heijtz R, Wang S, Anuar F, Qian J, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011;23:255–264. doi: 10.1111/j.1365-2982.2010.01620.x. e119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


