Abstract
Background: To examine the possible regulatory mechanisms of osteopontin (OPN) and the nuclear factor-κB (NF-κB) signaling pathway in the temporomandibular joint (TMJ) of rats subjected to chronic sleep deprivation (CSD). Methods: Rats were subjected to CSD using the modified multiple platform method. The histomorphology of the TMJ was observed by hematoxylin-eosin staining. OPN and NF-κB/p65 expression were detected by immunohistochemical and immunofluorescence staining together with western blotting. The condylar chondrocytes were isolated from the rat TMJ and treated with recombinant OPN (r-OPN) before detection for the expression of NF-κB/p65 and matrix metalloproteinases (MMPs). Western blotting and reverse transcription-polymerase chain reaction were performed to determine the expression of MMP-1, MMP-3, MMP-9, and MMP-13 in the TMJ and chondrocytes respectively. Results: There was a statistically significant difference in OPN and NF-κB/p65 expression between the CSD group and control (CON) group. OPN and NF-κB/p65 expression was increased in the CSD group as compared with in the CON group. NF-κB/p65 expression was significantly increased by r-OPN treatment in the chondrocytes. Furthermore, MMP-1, MMP-3, MMP-9, and MMP-13 production was also remarkably elevated in the CSD group as well as in the chondrocytes. Treatment with 1 μg/ml r-OPN for 48 h led to the highest production of inflammatory cytokines in chondrocytes. Conclusions: CSD causes pathological alterations in the TMJ. OPN treatment activates the NF-κB signaling pathway and stimulates MMPs in the TMJ and condylar chondrocytes through NF-κB signaling pathway. Chondrocytes treated with 1 μg/ml r-OPN for 48 h produced the highest level of inflammatory cytokines.
Keywords: Chronic sleep deprivation, temporomandibular joint, condylar chondrocytes, osteopontin, nuclear factor-κB, matrix metalloproteinases
Introduction
Temporomandibular joint disorders (TMD) are characterized by involvement of the articular cartilage and the masticatory muscle system that leads to chronic pain and functional restrictions [1]. It consists of masticatory muscle disorders, structural disorders, inflammatory diseases, and osteoarthrosis [1]. The clinical features of TMD include joint pain, joint movement with a ring or murmur, and joint movement disorders, seriously affecting life quality of patients [2]. In North America, about 9%-15% of adults are suffering with symptomatic TMD. However, the etiology of TMD remains poorly elucidated [3], with occlusal factors and psychological factors accounting for the main proportion of causes [4]. It has been proved that, sleep deprivation, an important component of psychological factors, may induce TMD [5,6].
Osteopontin (OPN) is a 44-75 kD matricellular protein that plays a pivotal role in regulating tissue repair and remodeling [7-9]. OPN is involved in the pathogenesis of a variety of disease, including glomerulonephritis, cancer, atherosclerosis, and several chronic inflammatory diseases [10-12]. The nuclear factor-κB (NF-κB) pathway regulates the expression of multiple chemokines, cytokines, growth factors, and adhesion molecules, and participates in the inflammatory response [13,14]. Moreover, OPN and the NF-κB signaling pathway are very closely related, for OPN can induce the activation of NF-κB signaling in various diseases [15]. Some studies have shown that OPN may be involved in the molecular pathogenesis of osteoarthritis (OA) through the NF-κB pathway, contributing to progressive degeneration of articular cartilage [16,17]. It has also been reported that OPN and NF-κB/p65 expression is increased in cartilage tissue with rheumatoid arthritis and OA [18]. Preliminary research has also demonstrated that OPN gene silencing impedes the process of OA and decreases the expression of some inflammatory cytokines [19]. Moreover, the production of inflammatory cytokines in knee chondrocytes can be increased after stimulation with recombinant OPN (r-OPN) [20]. MMPs are important cofactors or disease mediators in OA [21]. MMP-1 and MMP-13 can cleave type II collagen, MMP-3 is active to decomposition other extracellular matrix components, and MMP-9 is a gelatinase that activates the expression of pro-MMP-13 [22,23].
Although OPN, NF-κB, and MMP expression has been investigated in patients with knee OA, there are few studies on the effect of OPN on NF-κB and MMP expression in TMJ. Here, we developed a rat model of TMD caused by chronic sleep deprivation (CSD). Then, we examined the expression levels of the above cytokines as well as their correlation, in the TMJ and the condylar chondrocytes treated with r-OPN, in order to elucidate the role of these factors in the pathogenesis of TMD.
Materials and methods
Ethics statement
Prior approval from the Animal Care and Use Committee of Jinan Military General Hospital was obtained in accordance with international guidelines for care in animal research. The protocol (Permit Number: IACUC-2013-001) was approved by the Committee on the Ethics of Animal Experiments of Jinan Military General Hospital. All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize rats suffering.
Experimental design
One hundred and eighty male Wistar rats (8 weeks old, 200±20 g) were purchased from the Laboratory Animal Center of Shandong University (Jinan, China). The rats were randomly divided into two groups (n = 90 per group): control (CON) and CSD. Then, each group was equally divided into three subgroups (n = 30 per group) based on the observation time points (4, 6, and 8 weeks). Under the modified multiple platform method (MMPM) model, the rats in the CSD group were placed on small ledges [24], while their counterparts in the CON group were placed on a grid under the same conditions. Then, the rats were sacrificed at 4, 6, and 8 weeks respectively. The specific surgical method was as follows: rats were anesthetized by injecting 10% pentobarbital sodium (50 mg/kg) into the abdominal cavity. The bilateral TMJ was removed; one was stored in a -80°C for western blotting and reverse transcription-PCR detection(RT-PCR), the other was fixed in 4% paraformaldehyde immediately, decalcified for 1 months, and then paraffin-embedded before sagittal paraffin sections were obtained.
Histological staining
Some sections were stained with hematoxylin-eosin (HE). After deparaffinization, rehydration, and rinsing with distilled water, the sections were stained with hematoxylin for 5 min, differentiated by hydrochloric acid and ethanol, and then placed in eosin staining solution for 2 min. Stained section of 5 different portions at random were examined under a light microscope (DM 2500, Leica, Germany). Image acquisition was performed using a Leica DFC490 system (Leica).
Immunohistochemistry
After deparaffinization and rehydration, sections were treated with citrate buffer (pH 6.0) to unmask the epitopes, and then blocked in normal goat serum for 20 min. The sections were then incubated overnight with antibodies against OPN (1:60, Abcam, Cambridge, MA, USA) and NF-κB/p65 (1:70, Cell Signaling Technology, Danvers, MA, USA) at 4°C. After washing, horseradish peroxidase/Fab polymer conjugate (S-A/HRP) was applied to the sections for 20 min at 37°C. The sections were then stained using diaminobenzidene (DAB) chromogen and Hoechst for 3 min, observed microscopically, and photographed. Positive OPN immunostaining was defined as detectable immunoreactivity in the perinuclear and/or other cytoplasmic regions in the chondrocytes. Positive NF-κB/p65 immunostaining was defined as detectable immunoreactivity in the endonuclear region. The relative OPN and NF-κB/p65 distribution in cartilage tissue was visualized and quantified as the integrated optical density (IOD). Semi-quantitative assessment of the mean IOD of OPN and NF-κB/p65 expression was performed on scanned autoradiograms using Image-Pro Plus (Media Cybernetics, USA).
Immunofluorescence histochemistry
After deparaffinization, rehydration, and disposal of the 3% hydrogen peroxide, the pressure cooking method was used for antigen retrieval. Sections were blocked with normal goat serum for 20 min at room temperature in case of nonspecific protein staining. Then, they were incubated with anti-NF-κB/p65 (1:70, Cell Signaling Technology) antibody overnight at 4°C. After extensive washing with phosphate-buffered saline (PBS), the sections were incubated with secondary antibody A568 (Hebei Bio-high technology, China) with fluorescence and Hoechst nuclear staining at room temperature away from light. After washing with PBS, the sections were covered with 2% Mowiol fluorescence mounting medium cover slips (Hebei Bio-high technology, China). Confocal microscopic images were acquired using a Leica scanning microscope (TCS-SP5), and the images were processed using Image-Pro Plus.
Isolation and culture of condylar chondrocytes
Articular chondrocytes were isolated from the condyle heads and condyles of male Wistar rats (200±20 g, Experimental Animal Center of Shandong University) under aseptic conditions. Condyle cartilage specimens were minced and digested with 0.15% type II collagenase (Abcam) for 6 h at 37°C with 5% CO2. The digested cartilage tissue was transferred to clean 15 ml tubes and centrifuged at 1000 rpm for 8 min. The pellet was resuspended in Dulbecco’s modified Eagle’s medium (DMEM)/high glucose (HyClone, Logan, UT, USA) supplemented with 20% fetal bovine serum (FBS)(ScienceII, USA) to obtain a condylar chondrocyte suspension. Cells were seeded into 25 T culture bottles in DMEM containing 20% FBS and 1% penicillin-streptomycin, and cultured under normal growth conditions.
r-OPN intervention of condylar chondrocytes
Experiments were performed using the third passage cells. In each experiment, monolayer cells were starved for 24 h in serum-free DMEM and then incubated in DMEM containing 2% FBS with different concentrations (0.5 μg/ml, 1.0 μg/ml, 1.5 μg/ml, 2.0 μg/ml) of r-OPN (Cloud-Clone Corp, USA) for different durations (24 h, 48 h, 72 h), after which they were processed for subsequent analyses.
Immunohistochemistry identification of condylar cartilage cells
Cells were inoculated on 24-well plates, fixed in 4% paraformaldehyde for 20 min, and rinsed with PBS before incubation with blocking buffer (400 μl/well; 10 ml: 8.8 ml PBS + 1 ml normal goat serum [NGS] + 0.2 ml 10% Triton) for 1 h at room temperature. Then, the cells were rinsed with PBS and incubated with anti-type II collagen antibody (1:500; Beyotime, USA) for 3-4 h, rinsed with PBS for three times, and incubated with secondary antibody (1:1000; Abcam, USA) for 1-2 h in the dark. Subsequently, the cells were stained using DAB and Hoechst for 3 min, observed microscopically, and photographed.
Western blot analysis of protein expression
Total proteins were extracted from the TMJ tissues and the cultured chondrocytes by mixed cold lysis buffer (Beyotime, China) and 1:100 volume of phenylmethanesulfonyl fluoride. Protein concentrations were determined by the bicinchoninic acid method (Beyotime, China). Protein samples (50 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride (PVDF) membranes (Solarbio, China). The PVDF membranes were blocked with 5% non-fat dried milk at room temperature for 1 h and were subsequently incubated overnight at 4°C with the following primary antibodies: polyclonal anti-NF-κB/p65 (1:1000; Cell Signaling Technology), anti-MMP-1 (1:400; Bioss, China), anti-MMP-3 (1:500; Bioworld, China), anti-MMP-13 (1:500; Bioss, China), and monoclonal anti-MMP-9 (1:1000; Abcam, USA) and anti-β-actin (1:1000; Beyotime, China). The blots were developed with secondary antibody (Beyotime, China) and enhanced chemiluminescence using an ECL chemiluminescence kit (Beyotime, China), then exposed to autoradiographic film for 1-2 min for detection.
Reverse transcription and real-time quantitative PCR (RT-qPCR) analysis
Total RNA was extracted from the TMJ tissue and cultured chondrocytes using TRIzol reagent (Sangon Biotech, China) according to the manufacturer’s instructions. A PrimeScript RT Reagent kit (Perfect Real Time, TaKaRa, Japan) and SYBR Premix Ex Taq II (TaKaRa, Japan) were used for reverse transcription and RT-qPCR analysis. Sequence of primers used in RT-PCR is listed in Table 1. RT-qPCR was carried out in an Eppendorf realplex 4 (Eppendorf AG, Germany) with the following settings: pre-incubation at 95°C for 10 min followed by 40 cycles at 95°C for 20 s and 55°C for 60 s. Melting curve analysis was carried out using the default program. After each reaction, the cycle threshold (Ct) was recorded when the amplification curve reflected the exponential kinetic measurements. The 2-ΔΔCt method was adopted using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the reference gene [25].
Table 1.
Sequence of primers used in RT-PCR
| Primers | Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|
| GAPDH | CAGTGCCAGCCTCGTCTCAT | AGGGGCCATCCACAGTCTTC |
| MMP-1 | CTCCCTTGGACTCACTCATTCTA | AGAACATCACCTCTCCCCTAAAC |
| MMP-3 | ATGATGAACGATGGACAGATGA | CATTGGCTGAGTGAAAGAGACC |
| MMP-9 | CCTGCGTATTTCCATTCATC | GCCTTGGGTCAGGTTTAGAG |
| MMP-13 | GCGGTTCACTTTGAGGACAC | TATGAGGCGGGGATAGTCTTT |
Statistical analysis
All data were analyzed using SPSS13.0 software (SPSS, Chicago, IL, USA) and demonstrated as the mean ± standard error. Experimental data of the number of cartilage damage were analyzed by factorial experiment design. The experimental data of the same point time from the CON group and CSD group were analyzed by t-test. In the cytological experiments, the experimental data of different groups were analyzed by one-way analysis of variance (ANOVA). A P-value of <0.05 was considered statistically significant. Bars represent the mean and SD of each group.
Results
Histological observation of TMJ
The normal condylar cartilage surface was covered with the fibrocartilage, starting from the surface and deep into the fibrous articular surface, multicellular belt (proliferation), fibrocartilage zone (mast), of which four are calcified cartilage zones. The fibrous articular surfaces of the condylar cartilage were smooth and integrated (Figure 1A). Meanwhile, the condyles displayed characteristic regional cellular arrangements and no obvious histological changes in all CON groups. In the CSD at 4 weeks (CSD4w) group, histopathological changes including the tough fibrous articular surfaces, some distorted collagen fibers, and disorganized cellular arrangements could be observed. Compared with the CSD4w group, the condylar cartilage of the CSD6w group displayed more obvious histopathological changes such as detachment of the fibrous layer and disordered cellular arrangements. The above symptoms were most prominent in the CSD8w group. In addition, the number of damaged cartilage increased in the CSD group over time compared to the CON group (P<0.01) (Figure 1B).
Figure 1.

CSD induced pathological changes in the temporomandibular joint. A. HE-staining of condylar cartilage from the control group rats at 4, 6 and 8 weeks and the CSD group rats at 4, 6 and 8 weeks of sleep deprivation (bar = 50 μm, n = 10). B. The changes of the number of cartilage damage at different time of the CON group and the CSD group. **P<0.01 vs the control group.
According to the variance results of factorial design analysis, the number of damaged cartilage in the CON and CSD group was statistically different (F = 306.62, P = 0.000). At the same time, the degree of cartilage damage was changed at different time points (F = 24.59, P = 0.000), the interaction existed between the different groups and different time points (F = 24.39, P = 0.000).
OPN expression in rat TMJ condylar cartilage
In all CON groups, there was little or no OPN expression in all nucleated cells of the condylar cartilage (Figure 2A). In the CSD4w group, faint staining was visible in the territorial matrix surrounding the deep zone chondrocytes. With the extension of the sleep deprivation, the staining became more obvious in the deep zone chondrocytes and their surrounding matrix. Thus, the strongest expression of OPN, observed as brown staining of chondrocyte clusters and chondrocytes from the deep cartilage zone, was detected in the CSD8w group. Based on the immunohistochemical staining results, the brown staining was converted into the IOD to evaluate the OPN expression semi-quantitatively. Figure 2B depicted the OPN expression in condylar cartilage from the CON and CSD groups. The CSD4w group showed higher OPN expression in the articular cartilage as compared to the CON group, but was not statistically different (P>0.05). Moreover, OPN expression was significantly higher in the CSD6w and CSD8w groups as compared to that in the corresponding CON groups (P<0.05, P<0.01, respectively).
Figure 2.
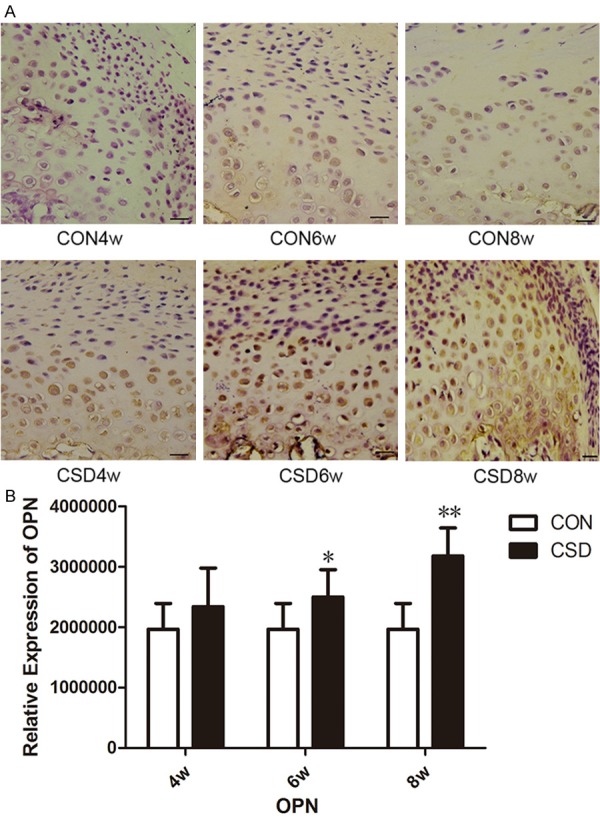
CSD induced the up-regulation of OPN expression in the condylar cartilage of rat TMJs. A. DAB staining of OPN in rat temporomandibular joint at the different groups. (bar = 10 μm, n = 10). B. The changes of OPN expression in the TMJ at different time of the CON group and the CSD group. (n = 10). *P<0.05 vs the control group, **P<0.01 vs the control group.
NF-κB pathway activation in rat TMJ condylar cartilage
To evaluate the activation of NF-κB pathway in the condylar cartilage of inflamed rat TMJ, NF-κB/p65 expression was examined by immunohistochemical and immunofluorescence staining and western blotting. NF-κB/p65 expression in the CON group was detectable in the cytoplasm of the chondrocytes (Figure 3A). In the CSD4w group, there was light staining in the nuclear of the territorial matrix surrounding the deep zone chondrocytes. With the extension of the sleep deprivation, the nuclear staining became more obvious in the deep zone chondrocytes. The strongest NF-κB/p65 expression was detected in the CSD8w group. The result of NF-κB activation in the rat TMJ condylar cartilage showed the same trend as the immunohistochemical and immunofluorescence findings. The fluorescence signal of p65 in the CON groups was located in the cytoplasm of the chondrocytes (Figure 3B). As for the CSD groups, the signal was mainly restricted in the nuclei. When the CSD was extended, the fluorescence signal became more pronounced in the deep zone chondrocytes.
Figure 3.
CSD induced and activated the NF-κB signing pathway in the condylar cartilage of rat TMJs. A. DAB staining of NF-κB p65 in rat TMJ at the different groups. (bar = 10 μm, n = 10). B. Immunofluorescent staining of NF-κB p65 in rat TMJ at the different groups. (bar = 50 μm, n = 10). C. Representative immunoblots of NF-κB p65 at the different groups. D. The positive cell number of NF-κB activation in the TMJ at the different groups. E. Mean relative protein levels of NF-κB p65 at the different groups (n = 10). *P<0.05 vs the control group, **P<0.01 vs the control group. CON: control group, CSD: chronic sleep deprivation group.
Semi-quantification of NF-κB activation was evaluated by counting the positive cells in immunohistochemical and immunofluorescence assays. The protein expression of NF-κB/p65 was detected by western blotting. After 4, 6, and 8 weeks of sleep deprivation, NF-κB signaling was significantly activated in the CSD group, with no activation in the CON group. Figure 3D shows the NF-κB activation in the condylar cartilage from the CON and CSD groups. There was higher NF-κB activation in TMJ articular cartilage from the CSD groups than in the CON groups. In the CSD6w and CSD8w groups in particular, NF-κB activation was significantly higher than that in the corresponding CON groups, (P<0.05, P<0.01, respectively). Figure 3E shows the expression of NF-κB/p65. As the duration of sleep deprivation increased, NF-κB/p65 expression was remarkably elevated in the CSD6w and CSD8w groups (P<0.05, P<0.01, respectively) as compared with the respective CON groups.
MMP expression in TMJ tissue
We observed significantly upregulated MMP-1, MMP-3, MMP-9, and MMP-13 production in the CSD group as compared with the CON group. MMP-1, MMP-3, MMP-9, and MMP-13 protein levels were increased in all CSD groups as compared with their respective CON groups (Figure 4). MMP-1 protein levels were significantly increased in CSD8w groups (P<0.05), MMP-3 and MMP-13 protein levels were significantly increased in the CSD6w and CSD8w group (P<0.05, P<0.01, respectively), and MMP-9 protein levels were significantly increased in the CSD4w and CSD6w group (P<0.01, P<0.05, respectively). RT-qPCR revealed significantly higher MMP-1, MMP-3, MMP-9, and MMP-13 mRNA expression in the CSD group as compared with the CON group (Table 2). MMP-1 mRNA was significantly increased in the CSD6w and CSD8w groups (P<0.05, P<0.05, respectively), as was MMP-3 mRNA (P<0.05, P<0.05, respectively) and MMP-13 mRNA (P<0.05, P<0.05, respectively). MMP-9 mRNA was significantly increased in the CSD4w group (P<0.05). Interestingly, only MMP-1, MMP-3, and MMP-13 expression was up-regulated as CSD increased, and not MMP-9.
Figure 4.
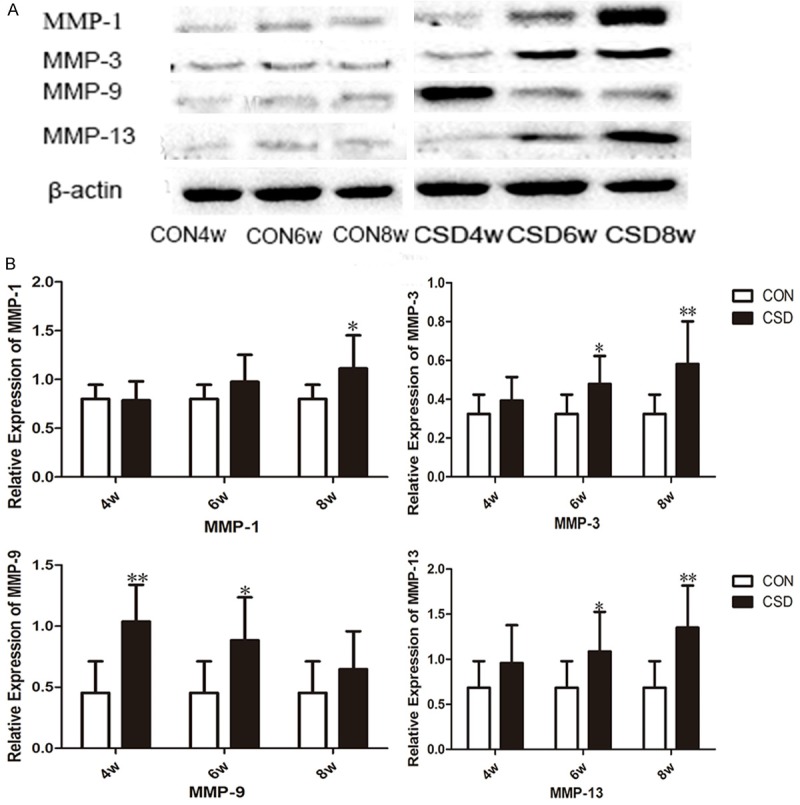
CSD changed inflammatory cytokine levels in TMJ. A. Representative immunoblots of MMP-1, MMP-3, MMP-9 and MMP-13 subjected to CSD in the TMJ tissue at the different groups. B. Mean relative protein levels of MMP-1, MMP-3, MMP-9 and MMP-13 at different groups (n = 10). *P<0.05 vs the control group, **P<0.01 vs the control group. CON: control group, CSD: chronic sleep deprivation group.
Table 2.
The expression of MMP mRNA in TMJ tissue
| CON | CSD | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 4 w | 6 w | 8 w | 4 w | 6 w | 8 w | |
| MMP-1 | 0.76±0.14 | 0.89±0.21 | 1.05±0.36 | 1.31±0.22 | 1.97±0.25* | 2.89±0.20** |
| MMP-3 | 1.05±0.24 | 1.27±0.38 | 1.35±0.28 | 1.47±0.31 | 2.55±0.30* | 3.51±0.36** |
| MMP-9 | 0.96±0.13 | 1.18±0.20 | 1.39±0.35 | 3.30±0.67** | 2.72±0.38* | 1.94±0.62 |
| MMP-13 | 1.08±0.28 | 1.93±0.35 | 2.21±0.41 | 1.72±0.49 | 1.98±0.61* | 3.55±0.76** |
P<0.05 vs the control group;
P<0.01 vs the control group.
Characterization and r-OPN intervention of condylar chondrocytes
Type II collagen expression is one of the major characterization of condylar chondrocyte. Obvious type II collagen staining was detected in the cytoplasm of the condylar chondrocytes (Figure 5A). The primary condylar chondrocytes grew in irregular triangular or polygonal filamentous shapes. The second passage showed good homogeneity and shared the same morphology with the primary chondrocytes. After r-OPN intervention, the the third passage chondrocytes became elongated (Figure 5B).
Figure 5.
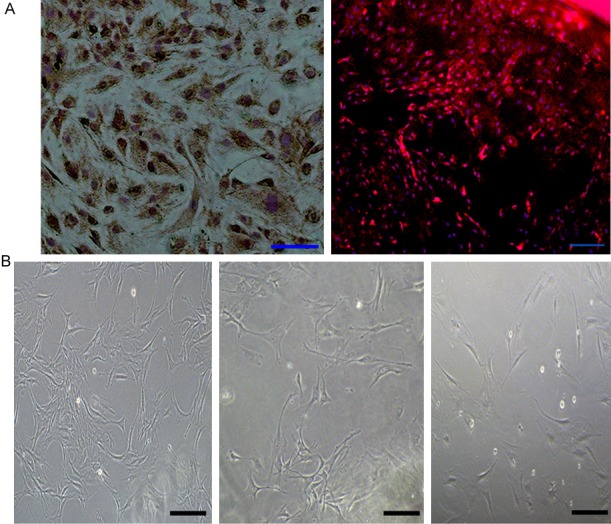
The shape and identification of condyle chondrocytes. A: Immunohistochemical staining and immunofluorescent staining of Type II collagen identified condyle chondrocytes. B. The shape of condyle chondrocytes at the different generations (bar = 100 μm).
NF-κB expression in condylar chondrocytes
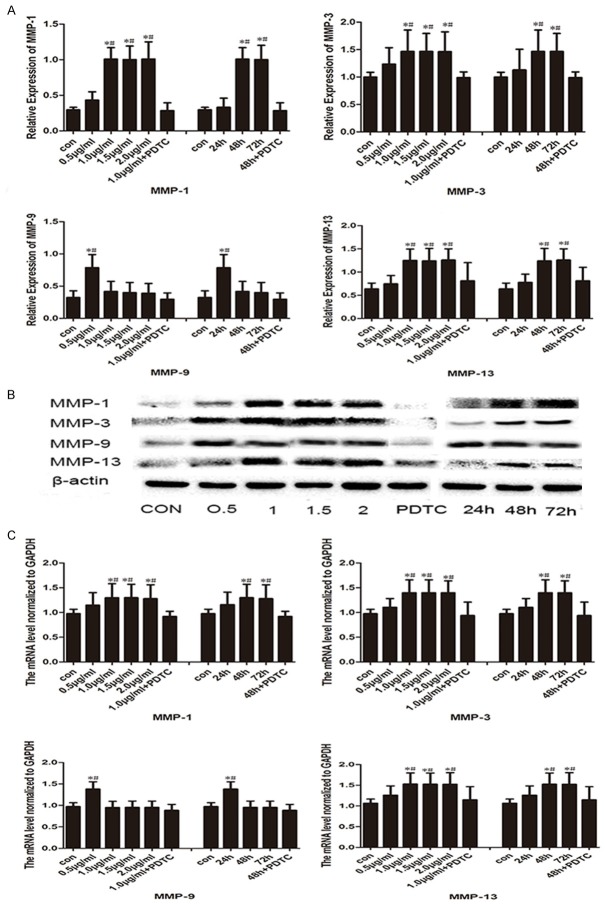
Figure 6 depicts the different chondrocyte treatment designs. To determine the relationship between OPN and NF-κB, western blotting was employed to detect the NF-κB/p65 expression in all groups of chondrocytes. Chondrocytes treated with r-OPN expressed more NF-κB/p65 as compared with the CON group (P<0.05) and the pyrrolidine dithiocarbamate (PDTC) group (P<0.05). Figure 7B shows the relative expression of NF-κB/p65 in the chondrocytes. NF-κB expression was highest when the chondrocytes had been treated with 1 μg/ml r-OPN for 48 h, then remained stable when the concentration and duration of intervention increased. Moreover, the administration of PDTC, an NF-κB inhibitor, significantly decreased the NF-κB/p65 level as compared with the r-OPN group (1 μg/ml, 48 h).
Figure 6.
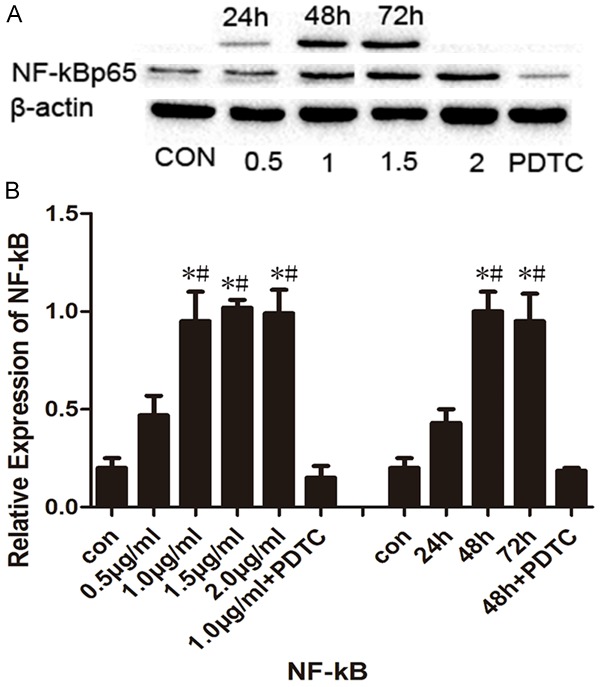
Effect of r-OPN on the expression of NF-κB p65 in the condyle chondrocytes. A. Representative immunoblots of the NF-κB p65 expression in the different groups as determined by Western blot. B. Mean relative expression of NF-κB p65 protein in different groups. *P<0.05 vs the control group; #P<0.05 vs the PDTC group.
Figure 7.
Effect of r-OPN on the expression of MMP-1, MMP-3, MMP-9 and MMP-13 in the condyle chondrocytes. A. Mean relative protein levels of MMP-1, MMP-3, MMP-9 and MMP-13 in different groups. B. Comparison of the MMP-1, MMP-3, MMP-9 and MMP-13 expression in the different groups as determined by western blot. C. Mean relative mRNA levels of MMP-1, MMP-3, MMP-9 and MMP-13 in different groups. *P<0.05 vs the control group; #P<0.05 vs the PDTC group.
MMP expression in condylar chondrocytes
To investigate whether OPN induced MMP upregulation in chondrocytes, we performed western blotting and RT-PCR to detect MMP protein and mRNA expression, respectively. As expected, all groups of chondrocytes treated with r-OPN showed increased MMP expression. Specifically, quantification showed that chondrocytes treated with 1 μg/ml r-OPN for 48 h expressed the highest level of MMP-1, MMP-3, and MMP-13 as compared with the CON group (P<0.05, respectively) and the PDTC group (P<0.05, respectively). Furthermore, we observed increased MMP-9 expression following 24 h intervention with 0.5 μg/ml r-OPN as compared with the CON group (P<0.05) and PDTC group (P<0.05), but it was not significantly different from the other groups (Figure 7).
Discussion
TMD is a common oral and maxillofacial disease and the most prevalent in all TMJ diseases. The incidence of TMD are higher in the young and middle-aged people, especially in the 20-30 year old group. Preliminary research has confirmed that MMPM is a successful simulation model of sleep deprivation in rats which can reduce the interference of other factors by using the small platforms [26,27]. Our study reinforced the idea that CSD plays an important role in inducing TMJ [27]. Our data showed that CSD might cause pathological alterations in rat TMJ. In the present experiment, histopathological changes such as tough fibrous articular surfaces, distorted collagen fibers, and detachment of the fibrous layer were observed in the CSD group, which are consistent with our previous findings [26,27].
OPN is related to OA [8]. As a common inflammatory cytokine, OPN promotes cartilage tissue destruction and accelerates the progression of knee OA [10,11]. As an extracellular cytokine, OPN combines with cell membrane molecules to participate in the intracellular production of some inflammatory cytokines [11]. As the TMJ isolated in our experimental were small, we were unable to extract the synovial fluid for analysis. Gao SG [28] reported that OPN expression were consistent in articular cartilage and in synovial fluid, both being closely related to the severity of OA. The present study revealed dramatically increased OPN expression in the condylar articular cartilage of rats suffering CSD as compared with the controls. Interestingly, OPN expression in the CSD group was significantly increased when the TMJ damage became worse, which showed that OPN expression level and TMD severity are positively correlated, thus OPN may play a significant role in the progress of TMD. Qin et al. showed that OPN and NF-κB pathway are involved in the occurrence and development of knee OA [17]. A previous study had proved that OPN triggers the NF-κB pathway and leads to elevation of MMP expression [17]. In our data, OPN activated the NF-κB signaling pathway, by which promoted MMP-1, MMP-3, MMP-9, and MMP-13 expression in the TMJ. We demonstrate that CSD leads to pathological changes in the TMJ by upregulating OPN secretion, activating the NF-κB signaling pathway, and then increasing MMP expression in the TMJ condyles of rats. These findings provide important and solid evidence indicating that abnormally secreted OPN resulting from CSD activates the NF-κB signaling pathway and may contribute to TMJ destruction by stimulating MMP production.
Xu M found that chondrocytes treated with OPN increased MMP gene and protein expression [29]. Jiang W [30] demonstrated that OPN treatment upregulated MMP-13 expression in knee chondrocytes in a time- and dose-dependent manner. The optimal time and concentration for OPN treatment were 48 h and 1 μg/ml, respectively [30]. To further dissect the mechanism of TMJ cartilage damage and the role of OPN in this process, we isolated rat condylar chondrocytes, cultured them in vitro, then administered OPN and detected the expression of the related factors. We observed NF-κB activation and MMP upregulation in the r-OPN-treated condylar chondrocytes. Forty-eight-hour intervention using 1 μg/ml r-OPN yielded the highest levels of inflammatory cytokines and the greatest damage to the chondrocytes. Our results are consistent with the findings of previous in vivo and in vitro studies [29,30].
NF-κB is a factor with multi-regulatory function that plays a critical role in cytokine-induced gene expression. It has been proved that NF-κB over expression is associated with the occurrence of many diseases. Bondeson J [31] reported that NF-κB expression levels and MMP-9 transcription levels in the knee articular organization of OA were significantly higher than the normal control group and that there was a positive correlation between interleukin-1 (IL-1) and MMP-9 expression levels and NF-κB activation. Bondeson J [32] and Amos N [33] showed that NF-κB activation accounted for the regulation of MMP-1, MMP-3, and MMP-13 expression levels. Synovial cell co-cultures showed that the expression MMP-1, MMP-3, MMP-9, and MMP-13 are all NF-κB-dependent [32,33]. In our experiments, the production of MMP-1, MMP-3, MMP-9, and MMP-13 protein and mRNA were all altered. Interestingly, MMP-9 expression was the highest in the initial stage of TMD. and was slightly increased in the chondrocytes treated with lower concentrations of r-OPN. Moreover, MMP-1, MMP-3, and MMP-13 expression increased as NF-κB activation. It is believed that the secretion of MMP-9 in macrophages depends largely on cell-to-cell and cell-to-matrix interactions mediated by integrins rather than inflammatory mediators [34,35]. Sarén P and Galt SW found that MMP-9 might be involved in the activation of pro-MMP-13 [34,35]. Hence, in our experiment, that MMP-9 expression was independent from NF-κB activation and contrary to MMP-13 expression was not surprising.
However, recent studies have proved that OPN in arthritis upregulated some anti-inflammatory cytokines and inhibited some pro-inflammatory factors to suppress the development of inflammation. The specific mechanism remains to be further studied [20].
Conclusions
In summary, we demonstrated that the pathological alterations of the TMJ are determined by sleep deprivation via modulating some molecular mechanisms. We also revealed for the first time that OPN induces MMP expression by activating the NF-κB signaling pathway in vivo and in vitro. Moreover, increased OPN expression correlates with NF-κB activation. Inflammatory cytokine production was the highest and chondrocyte damage was the greatest under the condition of 48-h treatment with 1 μg/ml r-OPN. We provided evidence showing that OPN increases the pathological alterations by inducing MMP expression through the NF-κB signaling pathway.
Acknowledgements
This study was supported by Natural Science Foundation of China No. 61471384 and 81400573, Science and Technology Development Plans of Shandong province No. 2014GSF118101, Army Health Breeding Programme No. 15QNP019 and Youth Science and Technology Star Plant No. 2013032. Meanwhile, the study was carried out at Shandong provincial key laboratory of oral tissue regeneration lab; We are indebted to its participants for their help and advice.
Disclosure of conflict of interest
None.
Authors’ contribution
Ding Feng: Conception and design, analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content, final approval of the article, collection and assembly of data. Wang Jing: Conception and design, analysis and interpretation of the data, critical revision of the article for important intellectual content, final approval of the article, collection and assembly of data. Zhu Guoxiong, Zhao Huaqiang: Critical revision of the article for important intellectual content, final approval of the article. Wu Gaoyi, Chen Lei: Conception and design, analysis and interpretation of the data, critical revision of the article for important intellectual content, final approval of the article, obtaining of funding.
Abbreviations
- CSD
Chronic sleep deprivation
- TMJ
Temporomandibular joint
- OPN
Osteopontin
- NF-κB
Nuclear factor kappa B
References
- 1.Mello VV, Barbosa AC, Morais MP, Gomes SG, Vasconcelos MM, Caldas Junior Ade F. Temporomandibular disorders in a sample population of the Brazilian northeast. Braz Dent J. 2014;25:442–446. doi: 10.1590/0103-6440201302250. [DOI] [PubMed] [Google Scholar]
- 2.Fernandes G, van Selms MK, Goncalves DA, Lobbezoo F, Camparis CM. Factors associated with temporomandibular disorders pain in adolescents. J Oral Rehabil. 2015;42:113–119. doi: 10.1111/joor.12238. [DOI] [PubMed] [Google Scholar]
- 3.de Leeuw R, Eisenlohr-Moul T, Bertrand P. The association of smoking status with sleep disturbance, psychological functioning, and pain severity in patients with temporomandibular disorders. J Orofac Pain. 2013;27:32–41. doi: 10.11607/jop.1040. [DOI] [PubMed] [Google Scholar]
- 4.Stockstill JW, Bowley JF, Dunning D, Spalding P, Stafford K, Erickson L. Prevalence of temporomandibular disorders (TMD) in children based on physical signs. ASDC J Dent Child. 1998;65:459–467. 438. [PubMed] [Google Scholar]
- 5.Smith MT, Wickwire EM, Grace EG, Edwards RR, Buenaver LF, Peterson S, Klick B, Haythornthwaite JA. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 2009;32:779–790. doi: 10.1093/sleep/32.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitter M, Kares-Vrincianu A, Kares H, Bermejo JL, Schindler HJ. Sleep-associated aspects of myofascial pain in the orofacial area among Temporomandibular Disorder patients and controls. Sleep Med. 2015;16:1056–1061. doi: 10.1016/j.sleep.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Giachelli CM, Steitz S. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol. 2000;19:615–622. doi: 10.1016/s0945-053x(00)00108-6. [DOI] [PubMed] [Google Scholar]
- 8.Kahles F, Findeisen HM, Bruemmer D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metab. 2014;3:384–393. doi: 10.1016/j.molmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao SG, Zeng C, Song Y, Tian J, Cheng C, Yang T, Li H, Zhang FJ, Lei GH. Effect of osteopontin on the mRNA expression of ADAMTS4 and ADAMTS5 in chondrocytes from patients with knee osteoarthritis. Exp Ther Med. 2015;9:1979–1983. doi: 10.3892/etm.2015.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichikawa H, Imano M, Takeyama Y, Shiozaki H, Ohyanagi H. Involvement of osteopontin as a core protein in cholesterol gallstone formation. J Hepatobiliary Pancreat Surg. 2009;16:197–203. doi: 10.1007/s00534-009-0043-4. [DOI] [PubMed] [Google Scholar]
- 11.Atalar E, Ozturk E, Ozer N, Haznedaroglu IC, Kepez A, Coskun S, Aksoyek S, Ovunc K, Kes S, Kirazli S, Ozmen F. Plasma soluble osteopontin concentrations are increased in patients with rheumatic mitral stenosis and associated with the severity of mitral valve calcium. Am J Cardiol. 2006;98:817–820. doi: 10.1016/j.amjcard.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Lund SA, Giachelli CM, Scatena M. The role of osteopontin in inflammatory processes. J Cell Commun Signal. 2009;3:311–322. doi: 10.1007/s12079-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu X, Song Y, Huo R, Zhang J, Sun S, He Y, Gao H, Zhang M, Sun X, Zhai T, Li H, Sun Y, Zhou Z, Shen B, Xiao L, Li N. Cyr61 participates in the pathogenesis of rheumatoid arthritis by promoting proIL-1beta production by fibroblast-like synoviocytes through an AKT-dependent NF-kappaB signaling pathway. Clin Immunol. 2015;157:187–197. doi: 10.1016/j.clim.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Yan M, Wang Z, Jing S, Li Y, Liu G, Yu J, Fan Z. Effects of canonical NF-kappaB signaling pathway on the proliferation and odonto/osteogenic differentiation of human stem cells from apical papilla. Biomed Res Int. 2014;2014:319651. doi: 10.1155/2014/319651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Liu Q, Wan Y, Zhao Z, Yu H, Luo H, Tang Z. Osteopontin promotes the progression of gastric cancer through the NF-kappaB pathway regulated by the MAPK and PI3K. Int J Oncol. 2014;45:282–290. doi: 10.3892/ijo.2014.2393. [DOI] [PubMed] [Google Scholar]
- 16.Cao L, Fan X, Jing W, Liang Y, Chen R, Liu Y, Zhu M, Jia R, Wang H, Zhang X, Zhang Y, Zhou X, Zhao J, Guo Y. Osteopontin promotes a cancer stem cell-like phenotype in hepatocellular carcinoma cells via an integrin-NF-kappaB-HIF-1alpha pathway. Oncotarget. 2015;6:6627–6640. doi: 10.18632/oncotarget.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin LF, Wang WC, Fang H, Mao XZ, Huang GL, Chen Y, Zhou HD, Shen Y, Qin LH, Peng D. Expression of NF-kappaB and osteopontin of synovial fluid of patients with knee osteoarthritis. Asian Pac J Trop Med. 2013;6:379–382. doi: 10.1016/S1995-7645(13)60042-5. [DOI] [PubMed] [Google Scholar]
- 18.Zheng W, Li R, Pan H, He D, Xu R, Guo TB, Guo Y, Zhang JZ. Role of osteopontin in induction of monocyte chemoattractant protein 1 and macrophage inflammatory protein 1beta through the NF-kappaB and MAPK pathways in rheumatoid arthritis. Arthritis Rheum. 2009;60:1957–1965. doi: 10.1002/art.24625. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Liu F, Zhu G, Wang Z. Recombinant osteopontin attenuates hyperoxia-induced acute lung injury through inhibiting nuclear factor kappa B and matrix metalloproteinases 2 and 9. Chin Med J (Engl) 2014;127:4025–4030. [PubMed] [Google Scholar]
- 20.Cheng C, Zhang FJ, Tian J, Tu M, Xiong YL, Luo W, Li YS, Song BB, Gao SG, Lei GH. Osteopontin inhibits HIF-2alpha mRNA expression in osteoarthritic chondrocytes. Exp Ther Med. 2015;9:2415–2419. doi: 10.3892/etm.2015.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue H, Arai Y, Kishida T, Terauchi R, Honjo K, Nakagawa S, Tsuchida S, Matsuki T, Ueshima K, Fujiwara H, Mazda O, Kubo T. Hydrostatic pressure influences HIF-2 alpha expression in chondrocytes. Int J Mol Sci. 2015;16:1043–1050. doi: 10.3390/ijms16011043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KS, Lee YA, Choi HM, Yoo MC, Yang HI. Implication of MMP-9 and urokinase plasminogen activator (uPA) in the activation of pro-matrix metalloproteinase (MMP)-13. Rheumatol Int. 2012;32:3069–3075. doi: 10.1007/s00296-011-2095-4. [DOI] [PubMed] [Google Scholar]
- 23.Dahiya S, Givvimani S, Bhatnagar S, Qipshidze N, Tyagi SC, Kumar A. Osteopontin-stimulated expression of matrix metalloproteinase-9 causes cardiomyopathy in the mdx model of Duchenne muscular dystrophy. J Immunol. 2011;187:2723–2731. doi: 10.4049/jimmunol.1101342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma C, Wu G, Wang Z, Wang P, Wu L, Zhu G, Zhao H. Effects of chronic sleep deprivation on the extracellular signal-regulated kinase pathway in the temporomandibular joint of rats. PLoS One. 2014;9:e107544. doi: 10.1371/journal.pone.0107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Cao R, Huang F, Wang P, Chen C, Zhu G, Chen L, Wu G. Chronic sleep deprivation alters the myosin heavy chain isoforms in the masseter muscle in rats. Br J Oral Maxillofac Surg. 2015;53:430–435. doi: 10.1016/j.bjoms.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Geng W, Wu G, Huang F, Zhu Y, Nie J, He Y, Chen L. Sleep deprivation induces abnormal bone metabolism in temporomandibular joint. Int J Clin Exp Med. 2015;8:395–403. [PMC free article] [PubMed] [Google Scholar]
- 28.Gao SG, Li KH, Zeng KB, Tu M, Xu M, Lei GH. Elevated osteopontin level of synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis patients. Osteoarthritis Cartilage. 2010;18:82–87. doi: 10.1016/j.joca.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Xu M, Zhang L, Zhao L, Gao S, Han R, Su D, Lei G. Phosphorylation of osteopontin in osteoarthritis degenerative cartilage and its effect on matrix metalloprotease 13. Rheumatol Int. 2013;33:1313–1319. doi: 10.1007/s00296-012-2548-4. [DOI] [PubMed] [Google Scholar]
- 30.Jiang W, Lei G, Lin B, Wang H, Lu M, Gao S, Zhang F, Zeng C. [Effect of osteopontin on expression of matrix metalloproteinase 13 in human knee osteoarthritic chondrocytes] . Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2014;28:1342–1345. [PubMed] [Google Scholar]
- 31.Hunter C, Bond J, Kuo PC, Selim MA, Levinson H. The role of osteopontin and osteopontin aptamer (OPN-R3) in fibroblast activity. J Surg Res. 2012;176:348–358. doi: 10.1016/j.jss.2011.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bondeson J, Foxwell B, Brennan F, Feldmann M. Defining therapeutic targets by using adenovirus: blocking NF-kappaB inhibits both inflammatory and destructive mechanisms in rheumatoid synovium but spares anti-inflammatory mediators. Proc Natl Acad Sci U S A. 1999;96:5668–5673. doi: 10.1073/pnas.96.10.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amos N, Lauder S, Evans A, Feldmann M, Bondeson J. Adenoviral gene transfer into osteoarthritis synovial cells using the endogenous inhibitor IkappaBalpha reveals that most, but not all, inflammatory and destructive mediators are NFkappaB dependent. Rheumatology (Oxford) 2006;45:1201–1209. doi: 10.1093/rheumatology/kel078. [DOI] [PubMed] [Google Scholar]
- 34.Saren P, Welgus HG, Kovanen PT. TNF-alpha and IL-1beta selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol. 1996;157:4159–4165. [PubMed] [Google Scholar]
- 35.Galt SW, Lindemann S, Medd D, Allen LL, Kraiss LW, Harris ES, Prescott SM, McIntyre TM, Weyrich AS, Zimmerman GA. Differential regulation of matrix metalloproteinase-9 by monocytes adherent to collagen and platelets. Circ Res. 2001;89:509–516. doi: 10.1161/hh1801.096339. [DOI] [PubMed] [Google Scholar]