Abstract
Early reperfusion of myocardial infarction area is the most effective and important therapy to acute myocardial infarction, but could induce reperfusion injury. Geranylgeranylacetone (GGA), an acyclic polyisoprenoid used as an oral anti-ulcer medication, has been reported to have protective effects on reperfusion injury. In the present study, we explored the protective effect of GGA against MIRI and the underlying mechanism. We pretreated rats with four daily GGA, and then observed its effects on heart function parameters following in situ ischemia/reperfusion. GGA exhibited dramatic improvement in cardiac functions, as manifested by increased LVSP and ± (dP/dt) max and decreased LVDP. Oxidative injury and inflammatory response were also relieved by GGA. Western blot showed that the HSP70 protein expression and the Akt/GSK-3β/eNOS pathway were activated. The inhibition of HSP70 and the Akt/GSK-3β/eNOS pathway significantly reversed the protective effects of GGA on MIRI, indicating the involvements of HSP70 and the Akt/GSK-3β/eNOS pathway.
Keywords: Myocardium ischemic/reperfusion injury, geranylgeranylacetone, HSP70, Akt/GSK-3β/eNOS
Introduction
Despite the rapid developments in medical care, acute myocardial infarction (AMI) remains a major cause of morbidity and mortality throughout the modern world. It was reported that AMI-related complications cost $2 billion in American healthcare resources every year [1]. At present, early reperfusion after myocardial infarction by thrombolysis and percutaneous coronary intervention are two of the most effective and important therapies for AMI [2]. However, these treatments could induce reperfusion injury. Reperfusion injury is initiated when blood flow returns to the ischemic tissue [3], and involves many cell injury pathways, such as membrane destabilization, intracellular calcium dysregulation, free radical production, mitochondrial injury, and pro-apoptotic pathway activation [4]. The cytotoxic cascade, which results in generation of reactive oxygen species (ROS), is thought to be the main factor that leads to contractile dysfunction, cell death, and inflammation [5]. These injuries may cause acute consequences such as low cardiac output and death or chronic diseases such as heart failure [6]. Therefore, there is a desperate need to protect the heart against myocardial infarction reperfusion injury (MIRI)-related complications, although presently, there is no effective treatment available for MIRI.
Geranylgeranylacetone (GGA) is an acyclic polyisoprenoid, which has been used as an oral anti-ulcer medication in Japan and China for more than 20 years with no major adverse effects. Previous studies have shown that GGA is cytoprotective against various stressors in a variety of cells and tissues. By inducing heat shock proteins (HSPs), GGA can protect many organs from ischemia/reperfusion (I/R) injury, including the brain [7], kidney [8], and liver [9]. HSPs are responsible for the maintenance of cellular homeostasis during regular cell growth as well as survival after detrimental environmental stress [10]. Many studies have revealed that HSPs can protect cells from stress and apoptosis [11]. Among the large family of HSPs, HSP70 is the most studied that is involved in protection against injury and apoptosis under various pathological conditions [12]. It has been shown that over-expression of HSP70 can inhibit the release of caspase activators from mitochondria and prevent activation of caspase-9 and caspase-3 [13]. Because cardiomyocytes injury and apoptosis are the main consequences of MIRI, the effect of GGA on MIRI and its underlying mechanism needs to be explored.
Endothelial nitric oxide synthase (eNOS) belongs to the large NOS family that includes two other isoforms, neuronal NOS (nNOS) and in-ducible NOS (iNOS). It has been revealed that eNOS participates in the protection against ischemia/reperfusion injury [14-16]. There are many explanations for the protective action of eNOS that include regulation of cerebral blood flow, mediation of the vascular response to the oxidative stress, or inhibition of platelet aggregation, and platelet and polymorphonuclear neutrophil adhesion to the vascular endothelium [17]. However, how GGA induces eNOS and its effects on myocardial ischemia/reperfusion injury have not been well studied. As a direct substrate of Akt, GSK-3β mediates many cellular processes such as cardiac development, growth, protein synthesis, and gene transcription. The activation of GSK-3β induces cellular defense mechanisms by maintaining the cytoskeletal architecture, preserving redox homeostasis, and shielding cells from apoptosis [18]. The involvement of the Akt/GSK-3β/β-catenin pathway in the protection of GGA against myocardial ischemia/reperfusion injury is worthy of examination.
Hence, the present work was performed to explore the protective effect of GGA against MIRI and its underlying mechanism. We pretreated rats with GGA four times per day, and then observed its effects on heart function parameters following in situ ischemia/reperfusion. Multiple oxidative products in the myocardium were measured to evaluate the anti-oxidative effect of GGA. Myeloperoxidase (MPO) activity, and levels of the proinflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) also were examined. Next, we investigated the role of eNOS and the Akt/GSK-3β/β-catenin pathway in the protective effects of GGA to further explore its underlying mechanism.
Materials and methods
Animals and reagents
Male Wistar rats (150-170 g) were purchased from the Shanghai Laboratory Animal Center (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China) and used in this study. The study was approved by the institutional animal care and use committee at the Shanghai Jiaotong University School of Medicine animal center and performed in accordance with the Guide for the Care and Use of Laboratory Animals, 8th Edition. The animals were housed in the Shanghai Jiaotong University School of Medicine animal center. Housing conditions were 22°C temperature, 41% relative humidity, and 12-/12-hour light/dark cycles. Rats were allowed access to water and food ad libitum. Quercetin (HSP70 inhibitor), triciribine (Akt inhibitor), SB-216763 (GSK-3β inhibitor), and L-NIO (eNOS inhibitor) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Experimental protocol
Rats were randomly assigned to four groups: Sham, Control, 50 mg/kg GGA, and 100 mg/kg GGA (20 animals per group). Rats in the Sham group received 10 mL 5% gum arabic orally for 7 days (four times per day), followed by sham myocardial ischemia/reperfusion (I/R) surgery. Rats in the MIRI group received 10 mL 5% gum arabic orally for 7 days (four times per day), followed by myocardial I/R surgery. Rats in the 50 mg/kg GGA and 100 mg/kg GGA groups re-ceived 10 mL 5% gum arabic with either 50 mg/kg or 100 mg/kg weight of GGA for 7 days (four times per day), followed by myocardial I/R surgery 24 hours later. In each group, 10 rats were used for functional studies, and 10 rats were used for biomarker measurements. Quercetin (3 mg/kg body weight), triciribine (2 mg/kg), SB-216763 (0.6 mg/kg), or L-NIO (10 mg/kg) was administered intraperitoneally once daily during GGA treatment as indicated. All the compounds were purchased from Sigma-Aldrich company located in Shanghai, China.
Myocardial I/R procedure
Rats were anesthetized by intraperitoneal administration of pentobarbital sodium (100 mg/kg, Abbott Laboratories, Chicago, IL). The plane of anesthesia was confirmed by lack of foot-withdrawal reflex. Body temperature was monitored and maintained at 37°C with a heat lamp. Next, a middle cervical incision was made, and a section of tubing was passed through the exposed trachea until the tip was 3 mm below the larynx. A left parasternotomy was performed by dividing three ribs in a cephalocaudal direction parallel to the sternum. After the pericardium was opened, the left coronary artery was located and a 3-0 silk black braided suture was inserted around the artery near its origin. A snare was created by passing both ends of the suture through the tip of an angiocatheter that could then be tightened and released by sliding a Voss clip down the angiocatheter. After 60 minutes of ischemia, the occlusive snare was released for reperfusion. Sham rats underwent the same surgical procedures except that the suture was not snared.
Surgical procedures for evaluating hemodynamic parameters
Rats were anesthetized by intraperitoneal administration of chloral hydrate (300 mg/kg). A small incision was made on the right side of the neck. The external right carotid artery was exposed and a microtip pressure transducer catheter (Millar Instruments, Houston, USA) was inserted into the artery. The proximal end of the catheter was connected to an electrostatic chart recorder (Gould, Cleveland, USA). The inserted tip of this catheter was then advanced until it reached the left ventricular lumen. The left ventricular systolic pressure (LVSP), left ventricular developed pressure (LVDP), and ± ventricular contractility (dP/dt max) measurements were obtained from the left ventricular pressure (LVP) signal.
Measurement of malondialdehyde (MDA), protein carbonyl, and 8-hydroxy-2-deoxyguanosine (8-OHdG) in myocardial tissues
Transmural tissue from the area at risk (AAR; 100 mg, wet weight) was homogenized in 2 mL of 10 mM phosphate buffer (pH 7.4). After centrifugation at 10,000 g for 30 min, MDA and protein carbonyl content were measured using the corresponding kits (Nanjing Jiancheng, Nanjing, China). The protein concentration was determined using a standard BCA protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China). Results were expressed as nmol/mg protein. For the 8-OHdG assay, DNA was extracted from the tissue using a DNA Extraction Kit (DNA Extractor Wb Kit; Wako Chemical, Osaka, Japan), then added to plate wells pre-coated with anti-8-OHdG antibody (Nikken SEIL Co., Fukuroi, Japan) and incubated for 45 min at 37°C. The wells were washed three times then sequentially treated with IgG, Streptavidin-Horseradish Peroxidase, and 3, 3’, 5, 5’-tetramethylbenzidine. After incubation for 15 min, the reaction was terminated by sulfuric acid and the absorbance was read at 450 nm. Results were expressed as pg/g protein.
Measurement of MPO, TNF-α, and IL-1β levels
Transmural tissue from the AAR was harvested, homogenized in saline on ice, and centrifuged at 3000×g at 4°C for 15 min. MPO, TNF-α, and IL-1β levels were then measured using enzyme-linked immunosorbent assay (ELISA) kits, according to the manufacturer’s instructions (Sigma, St. Louis, USA). Absorbance was measured with a microplate reader to obtain the MPO, TNF-α, and IL-1β levels. The protein concentration was determined using a standard BCA protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China). MPO was expressed as nanograms per microgram of protein. TNF-α, and IL-1β were expressed as picograms per microgram of protein.
Western blot
Cardiac tissues were harvested and washed in ice-cold saline, and then homogenized in RIPA lysis buffer (25 mg/mL) with 1 mM phenylmethylsulfonyl fluoride (PMSF) on ice. All the samples were centrifuged at 3000×g at 4°C for 15 min and the supernatants were collected. The protein concentration was determined using a standard BCA protein assay kit (Beyotime Institute of Biotechnology). Fifty mg of protein sample was loaded per lane, separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to nitrocellulose membranes electrophoretically. Membranes were blocked in 5% nonfat milk in TBST (10 mM Tris, 150 mM NaCl, 0.05% Tween-20) for 1 hour at room temperature and then blocked with rat primary antibodies β-actin, Bcl2, and Bax (1:2500); eNOS, P-eNOS (Ser1177), Akt, and p-Akt (Ser473) (1:2000); and GSK-3β and p-GSK-3b (Ser9) (1:1500, Sigma-Aldrich, USA). The primary antibody was detected with horseradish peroxidase-conjugated secondary antibody goat anti-rabbit/goat anti-mouse (1:1000, Sigma-Aldrich, USA). The blots were then scanned and densitometry was performed to quantify the expression using Bio-Rad Quantity One 4.4.0 software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
The statistical analysis was performed using SPSS 17.0 with one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc test. A P value of less than 0.05 was considered significant.
Results
Changes in cardiac function by GGA
As shown in Figure 1A-D, there were significant changes caused by MIRI and GGA on all hemodynamic parameters. MIRI treatment significantly decreased ± (dP/dt) max and LVSP, and increased LVDP (P<0.05 compared to the Sham group). The ± (dP/dt) max and LVSP values in the 50 mg/kg and 100 mg/kg GGA groups were higher than the MIRI group (P<0.05); the LVDP values in the 50 mg/kg and 100 mg/kg GGA groups were lower than the MIRI group (P<0.05).
Figure 1.
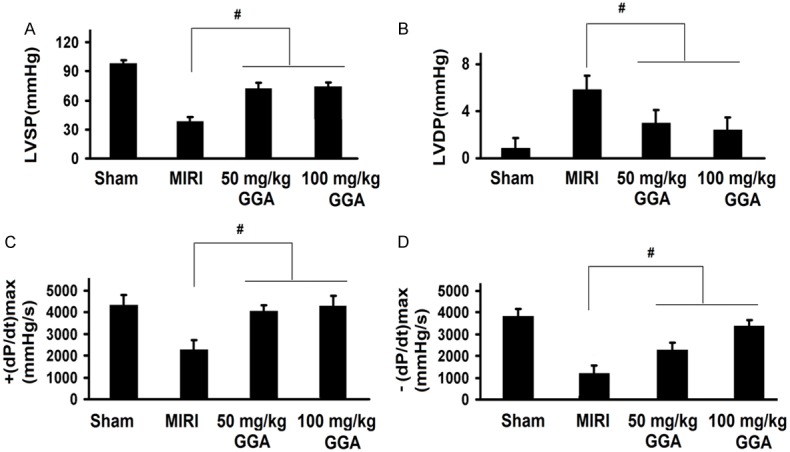
Changes in cardiac function by GGA. MIRI and GGA both caused significant changes on all the hemodynamic parameters. MIRI decreased ± (dP/dt) max and LVSP but increased LVDP, which was reversed by GGA. MIRI: myocardium ischemic/reperfusion injury; GGA: Geranylgeranylacetone. Values are expressed as Mean ± SEM. #: P<0.05 compared to MIRI.
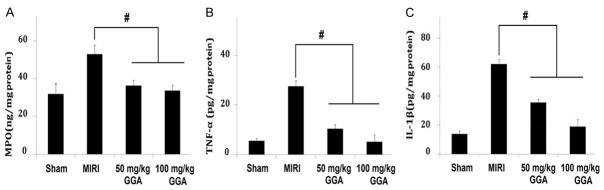
Changes in MPO, TNF-a, and IL-1β levels
As shown in Figure 2, MIRI caused significant increases in myocardial MPO, TNF-α, and IL-1β levels compared to the Sham group (P<0.05). These changes were significantly inhibited by GGA treatment, as shown by the dramatic decreases in the MPO, TNF-α, and IL-1β levels compared to the MIRI group (P<0.05).
Figure 2.
Changes in MPO, TNF-a, and IL-1β levels. It shows a significant increase in myocardial MPO, TNF-a, and IL-1β by MIRI and a decrease of them by GGA. MIRI: myocardium ischemic/reperfusion injury; GGA: Geranylgeranylacetone. Values are expressed as Mean ± SEM. #: P<0.05 compared to MIRI.
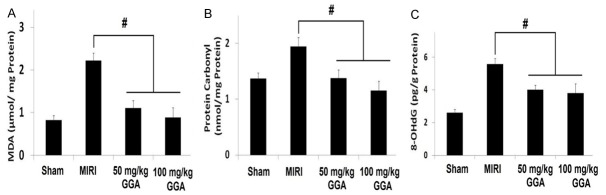
Changes in protein carbonyl, 8-OHdG, and MDA levels
As shown in Figure 3, myocardial MDA, protein carbonyl, and 8-OHdG assays demonstrated that MIRI caused significant oxidative stress (Sham group versus MIRI group, P<0.05). Treatment with 50 mg/kg and 100 mg/kg GGA, however, could significantly mitigate oxidative stress, as shown by the dramatic decreases in the MDA, protein carbonyl, and 8-OHdG levels compared to the MIRI group (P<0.05).
Figure 3.
Changes in the protein carbonyl, 8-OHdG and MDA levels. The increase of myocardial MDA, protein carbonyl and 8-OHdG demonstrates that MIRI caused significant oxidative stress. 50 mg/kg and 100 mg/kg GGA treatments could significantly mitigate the oxidative stress, as shown by the decreases in these parameters. MIRI: myocardium ischemic/reperfusion injury; GGA: Geranylgeranylacetone. Values are expressed as Mean ± SEM. #: P<0.05 compared to MIRI.
Up-regulation of HSP70 protein and activation of the Akt/GSK-3β/eNOS pathway by GGA
HSP70 protein expression and phosphorylation of the Akt/GSK-3β/eNOS pathway by Western blot are shown in Figure 4. As shown in Figure 4A, HSP70 expression was significantly increased at both dose levels of GGA. Figure 4B-D show that the Akt/GSK-3β/eNOS pathway was activated because the values of p-Akt/Akt, p-GSK-3β/GSK-3β, and p-eNOS/eNOS were all increased by GGA.
Figure 4.
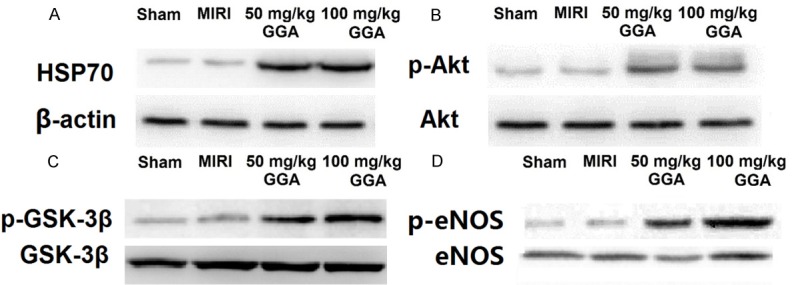
Up-regulation of HSP70 protein and activation of the Akt/GSK-3β/eNOS pathway by GGA. As shown in A, HSP70 expression was significantly increased by both dosages of GGA. As shown in B-D, the values of p-Akt/Akt, p-GSK-3β/GSK-3β and p-eNOS/eNOS were all increased by both dosages of GGA.
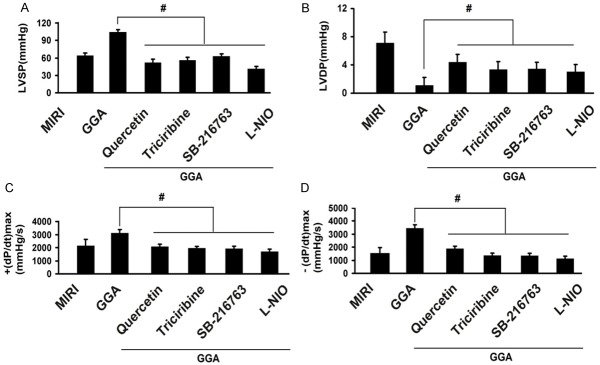
Changes in cardiac function by inhibitors of HSP70 and the Akt/GSK-3β/eNOS pathway
To evaluate the roles of HSP70 and the Akt/GSK-3β/eNOS pathway in the protection mediated by GGA, we treated rats with inhibitors of HSP70 and the Akt/GSK-3β/eNOS pathway and then examined the changes in cardiac function. As shown in Figure 5, LVSP, +(dP/dt) max and -(dP/dt) max were all decreased by the HSP70 inhibitor quercetin, the Akt inhibitor triciribine, the GSK-3β inhibitor SB-216763, and the eNOS inhibitor L-NIO compared to GGA (P<0.05). By contrast, the LVDP was increased by quercetin, triciribine, SB-216763, and L-NIO compared to the GGA groups (P<0.05).
Figure 5.
Changes of cardiac function by inhibitors of HSP70 and Akt/GSK-3β/eNOS pathway. LVSP, +(dP/dt) max and -(dP/dt) max were all decreased by HSP70 inhibitor Quercetin, Akt inhibitor triciribine, GSK-3β inhibitor SB-216763 and eNOS inhibitor L-NIO, while LVDP was increased by those treatments. MIRI: myocardium ischemic/reperfusion injury; GGA: Geranylgeranylacetone. Values are expressed as Mean ± SEM. #: P<0.05 compared to MIRI; L-NIO: N5-(1-Iminoethyl)-L-ornithine. Values are expressed as Mean ± SEM. #: P<0.05 compared to GGA.
Changes in levels of MPO, TNF-α, and IL-1β levels by inhibitors of HSP70 and the Akt/GSK-3β/eNOS pathway
As shown in Figure 6, GGA caused a significant decrease in myocardial MPO, TNF-α, and IL-1β levels compared to the Sham group (P<0.05). These changes were significantly reversed by inhibitors of HSP70 and the Akt/GSK-3β/eNOS pathway, as shown by the increases in the MPO, TNF-α, and IL-1β levels compared to the MIRI group (P<0.05).
Figure 6.
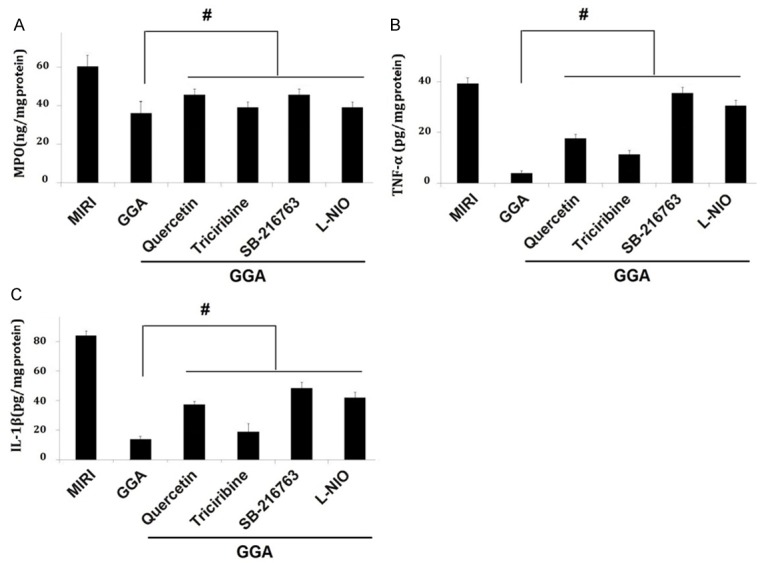
Changes in the MPO, TNF-a, and IL-1β levels by inhibitors of HSP70 and Akt/GSK-3β/eNOS pathway. The effects of GGA on MPO, TNF-a, and IL-1β were significantly inhibited by inhibitors of HSP70 and Akt/GSK-3β/eNOS pathway. MIRI: myocardium ischemic/reperfusion injury: GGA: Geranylgeranylacetone. Values are expressed as Mean ± SEM. #: P<0.05 compared to MIRI; L-NIO: N5-(1-Iminoethyl)-L-ornithine. Values are expressed as Mean ± SEM. #: P<0.05 compared to GGA.
Changes in protein carbonyl, 8-OHdG, and MDA levels by inhibitors of HSP70 and the Akt/GSK-3β/eNOS pathway
As shown in Figure 7, treatment with inhibitors of HSP70 and the Akt/GSK-3β/eNOS pathway could significantly reverse the effects of GGA on protein carbonyl, 8-OHdG, and MDA levels in the myocardial tissues (P<0.05).
Figure 7.
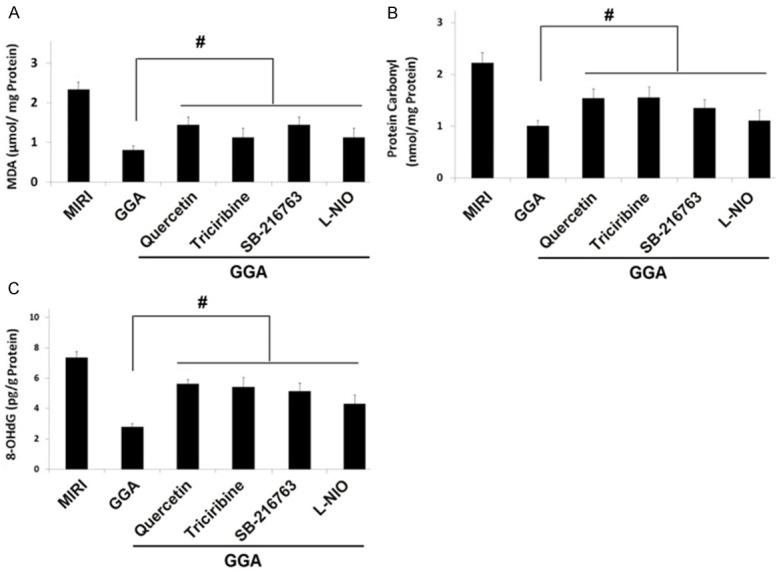
Changes in the protein carbonyl, 8-OHdG and MDA levels by inhibitors of HSP70 and Akt/GSK-3β/eNOS pathway. The inhibitors of HSP70 and Akt/GSK-3β/eNOS pathway could significantly reverse the effects of GGA on protein carbonyl, 8-OHdG and MDA levels in the myocardium tissues. MIRI: myocardium ischemic/reperfusion injury: GGA: Geranylgeranylacetone. Values are expressed as Mean ± SEM. #: P<0.05 compared to MIRI; L-NIO: N5-(1-Iminoethyl)-L-ornithine. Values are expressed as Mean ± SEM. #: P<0.05 compared to GGA.
Discussion
Our study confirmed that MIRI could significantly decrease cardiac function and increase oxidative products including protein carbonyl, 8-OHdG, and MDA in the myocardium. MIRI also led to inflammation, as shown by activation of MPO, TNF-α, and IL-1β. However, rats in the GGA groups exhibited dramatic improvement in cardiac function, as manifested by increased LVSP and ± (dP/dt) max, and decreased LVDP. Western blot showed that HSP70 protein expression and the Akt/GSK-3β/eNOS pathway were activated. The inhibition of HSP70 and the Akt/GSK-3β/eNOS pathway significantly reversed the protective effects of GGA on MIRI, which indicated the involvement of HSP70 and the Akt/GSK-3β/eNOS pathway.
Some investigators have explored the effects of GGA on MIRI, but the mechanism remains controversial. Wang et al. revealed that GGA protected against MIRI by inhibiting high-mobility group box 1 protein in rats and considerably suppressed the expression of HMGB1 induced by I/R [19]. Tsutsumi et al., on the other hand, suggested that GGA protected the heart from ischemia/reperfusion injury via caveolae and caveolin-3 [20,21]. The study by Zhu et al. revealed that oral administration of GGA blunted the endothelial dysfunction induced by ischemia and reperfusion in the rat heart [22]. GGA attenuated ischemia/reperfusion-induced coronary endothelial dysfunction, which may have contributed to its cardioprotective effect; the PI3 kinase and/or Rho kinase pathways also were involved. Ooie et al. found that a single oral dose of GGA induced HSP72, which rendered protection against ischemia/reperfusion injury in the rat heart [23]. Furthermore, Shinohara et al. suggested that mitochondria were targets for GGA-induced cardioprotection against ischemia-reperfusion in the rat heart [24]. These processes may involve opening of the mitoKATP channel. A study by Yamanaka demonstrated the role of protein kinase C in GGA-induced expression of HSP72 and cardioprotection in the rat heart [25].
The increase in oxidative products after MIRI indicated that ROS were produced by the myocardium I/R procedure and contributed to the lethal MIRI. The inflammatory response and cytokines produced by the myocardium I/R procedure play an important role in the pathophysiological process of MIRI [26]. Other studies indicated that an acute inflammatory reaction regulated the IRI and showed that a reduction in inflammatory cytokines or infiltration of leukocytes might attenuate MIRI [27-29]. Consistent with these studies, our results showed that the decrease in MPO, TNF-α, and IL-1β could be one mechanism to explain the protective effects of GGA.
The present study, for the first time, revealed that the Akt/GSK-3β/eNOS pathway played an important role in the process. Akt/GSK-3β is a crucial switch that can subsequently activate various other cytoprotective genes to arrest the progression of cardiomyocyte insult [30]. It was demostrated that phosphorylation of Akt/GSK-3b can improve calcium homeostasis by attenuating intracellular calcium overload in the myocardium [31,32]. Furthermore, pAkt/GSK-3b can preserve actin-myosin cross-bridge cycling and improve contractility and the hemodynamic status of the recovered myocardium [31]. In our study, we showed that the Akt/GSK-3β/eNOS pathway was activated because the values of p-Akt/Akt, p-GSK-3β/GSK-3β, and p-eNOS/eNOS were all increased. The LVSP, +(dP/dt) max, and -(dP/dt) max were all decreased by the Akt inhibitor triciribine, the GSK-3β inhibitor SB-216763, and the eNOS inhibitor L-NIO, while the LVDP was increased by triciribine, SB-216763, and L-NIO. This evidence suggested that the Akt/GSK-3β/eNOS pathway played an important role in the protective effect of GGA against MIRI. Taken together with the results from the inhibitor studies, these data reveal a novel signaling mechanism by which GGA protected against MIRI via the HSP70 and Akt/GSK-3β/eNOS antioxidant defense system.
To conclude, GGA protected against impaired cardiac function resulting from MIRI, and decreased oxidative injury and inflammation. A novel signaling mechanism was revealed through which GGA protected against MIRI via the Akt/GSK-3β/eNOS pathway. This discovery may help us identify new targets for protection against MIRI.
Disclosure of conflict of interest
None.
References
- 1.Mangano DT. Cardiovascular morbidity and CABG surgery-a perspective: Epidemiology, costs, and potential therapeutic solutions. J Card Surg. 1995;10:366–368. doi: 10.1111/j.1540-8191.1995.tb00663.x. [DOI] [PubMed] [Google Scholar]
- 2.Aversano T, Aversano LT, Passamani E, Knatterud GL, Terrin ML, Williams DO, Forman SA. Atlantic cardiovascular patient outcomes research team (C-PORT). Thrombolytic therapy vs primary percutaneous coronary intervention for myocardial infarction in patients presenting to hospitals without on-site cardiac surgery: a randomized controlled trial. JAMA. 2002;287:1943–1951. doi: 10.1001/jama.287.15.1943. [DOI] [PubMed] [Google Scholar]
- 3.Ibáñez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65:1454–1471. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 4.Bartos JA, Debaty G, Matsuura T, Yannopoulos D. Post-conditioning to improve cardiopulmonary resuscitation. Curr Opin Crit Care. 2014;20:242–249. doi: 10.1097/MCC.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 5.Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolli R, Becker L, Gross G, Mentzer R Jr, Balshaw D, Lathrop DA NHLBI Working Group on the Translation of Therapies for Protecting the Heart from Ischemia. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res. 2004;95:125–134. doi: 10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 7.He D, Song X, Li L. Geranylgeranylacetone protects against cerebral ischemia and reperfusion injury: HSP90 and eNOS phosphorylation involved. Brain Res. 2015;1599:150–7. doi: 10.1016/j.brainres.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Kim MG, Jung Cho E, Won Lee J, Sook Ko Y, Young Lee H, Jo SK, Cho WY, Kim HK. The heat-shock protein-70-induced renoprotective effect is partially mediated by CD4+ CD25+ Foxp3+ regulatory T cells in ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2014;85:62–71. doi: 10.1038/ki.2013.277. [DOI] [PubMed] [Google Scholar]
- 9.Fan N, Yang GS, Lu JH, Yang N, Zhang HB. Oral administration of geranylgeranylacetone plus local somatothermal stimulation: a simple, effective, safe and operable preconditioning combination for conferring tolerance against ischemia-reperfusion injury in rat livers. World J Gastroenterol. 2005;11:5725–5731. doi: 10.3748/wjg.v11.i36.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 11.Joyeux M, Faure P, Godin-Ribuot D, Halimi S, Patel A, Yellon DM, Demenge P, Ribuot C. The chaperone function of Hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;19:7146–7159. doi: 10.1128/mcb.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seemampillai B, Germack R, Felkin LE, Mc-Cormack A, Rose ML. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci. 2005;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]
- 13.Jiang B, Wang K, Liang P, Xiao W, Wang H, Xiao X. ATP-binding domain of heat shock protein 70 is essential for its effects on the inhibition of the release of the second mitochondria-derived activator of caspase and apoptosis in C2C12 cells. FEBS J. 2009;276:2615–2624. doi: 10.1111/j.1742-4658.2009.06989.x. [DOI] [PubMed] [Google Scholar]
- 14.He F, Xu BL, Chen C, Jia HJ, Wu JX, Wang XC, Sheng JL, Huang L, Cheng J. Methylophiopo-gonanone A suppresses ischemia/reperfusion-induced myocardial apoptosis in rats via activating PI3K/Akt/eNOS signaling pathway. Acta Pharmacol Sin. 2016;37:763–71. doi: 10.1038/aps.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Y, Li S, Zhang P, Zhu J, Meng G, Xie L, Yu Y, Ji Y, Han Y. Soy isoflavone protects myocardial ischemia/reperfusion injury through increasing endothelial nitric oxide synthase and decreasing oxidative stress in ovariectomized rats. Oxid Med Cell Longev. 2016;2016:5057405. doi: 10.1155/2016/5057405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riquelme JA, Westermeier F, Hall AR, Vicencio JM, Pedrozo Z, Ibacache M, Fuenzalida B, Sobrevia L, Davidson SM, Yellon DM, Sánchez G, Lavandero S. Dexmedetomidine protects the heart against ischemia-reperfusion injury by an endothelial eNOS/NO dependent mechanism. Pharmacol Res. 2016;103:318–27. doi: 10.1016/j.phrs.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, Liu X, Wei X, Chen L, Xiang Y, Yi F, Zhang X. Losartan, an angiotensin II type 1 receptor blocker, ameliorates cerebral ischemia-reperfusion injury via PI3K/Akt-mediated eNOS phosphorylation. Brain Res Bull. 2012;89:65–70. doi: 10.1016/j.brainresbull.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang N, Min X, Li D, He P, Zhao L. GGA protected against MIRI by inhibiting high-mobility group box 1 protein in rats. Mol Med Rep. 2012;5:521–524. doi: 10.3892/mmr.2011.666. [DOI] [PubMed] [Google Scholar]
- 20.Tsutsumi YM, Tsutsumi R, Horikawa YT, Sakai Y, Hamaguchi E, Kitahata H, Kasai A, Kambe N, Tanaka K. Geranylgeranylacetone and volatile anesthetic-induced cardiac protection synergism is dependent on caveolae and caveolin-3. J Anesth. 2014;28:733–739. doi: 10.1007/s00540-014-1816-8. [DOI] [PubMed] [Google Scholar]
- 21.Tsutsumi YM, Tsutsumi R, Horikawa YT, Sakai Y, Hamaguchi E, Ishikawa Y, Yokoyama U, Kasai A, Kambe N, Tanaka K. Geranylgeranylacetone protects the heart via caveolae and caveolin-3. Life Sci. 2014;101:43–48. doi: 10.1016/j.lfs.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Z, Takahashi N, Ooie T, Shinohara T, Yamanaka K, Saikawa T. Oral administration of GGA blunts the endothelial dysfunction induced by ischemia and reperfusion in the rat heart. J Cardiovasc Pharmacol. 2005;45:555–62. doi: 10.1097/01.fjc.0000159879.04444.22. [DOI] [PubMed] [Google Scholar]
- 23.Ooie T, Takahashi N, Saikawa T, Nawata T, Arikawa M, Yamanaka K, Hara M, Shimada T, Sakata T. Single oral dose of geranylgeranylacetone induces heat-shock protein 72 and renders protection against ischemia/reperfusion injury in rat heart. Circulation. 2001;104:1837–1843. doi: 10.1161/hc3901.095771. [DOI] [PubMed] [Google Scholar]
- 24.Shinohara T, Takahashi N, Kohno H, Yamanaka K, Ooie T, Wakisaka O, Murozono Y, Taniguchi Y, Torigoe Y, Hara M, Shimada T, Saikawa T, Yoshimatsu H. Mitochondria are targets for geranylgeranylacetone-induced cardioprotection against ischemia-reperfusion in the rat heart. Am J Physiol Heart Circ Physiol. 2007;293:1892–1899. doi: 10.1152/ajpheart.00493.2007. [DOI] [PubMed] [Google Scholar]
- 25.Yamanaka K, Takahashi N, Ooie T, Kaneda K, Yoshimatsu H, Saikawa T. Role of protein kinase C in geranylgeranylacetone-induced expression of heat-shock protein 72 and cardioprotection in the rat heart. J Mol Cell Cardiol. 2003;35:785–794. doi: 10.1016/s0022-2828(03)00133-0. [DOI] [PubMed] [Google Scholar]
- 26.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO 3rd, Kelm M, Wink DA, Espey MG, Oldfield EH, Pluta RM, Freeman BA, Lancaster JR Jr, Feelisch M, Lundberg JO. The emerging biology of the nitrite anion. Nat Chem Biol. 2005;1:308–14. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 28.Penna C, Rastaldo R, Mancardi D, Raimondo S, Cappello S, Gattullo D, Losano G, Pagliaro P. Post-conditioning induced cardioprotection requires signaling through a redox-sensitive mechanism, mitochondrial ATP-sensitive K+ channel and protein kinase C activation. Basic Res Cardiol. 2006;101:180–189. doi: 10.1007/s00395-006-0584-5. [DOI] [PubMed] [Google Scholar]
- 29.Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 30.Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugden PH. Ras, Akt, and mechanotransduction in the cardiac myocyte. Circ Res. 2003;93:1179–1192. doi: 10.1161/01.RES.0000106132.04301.F5. [DOI] [PubMed] [Google Scholar]
- 32.Michael A, Haq S, Chen X, Hsich E, Cui L, Walters B, Shao Z, Bhattacharya K, Kilter H, Huggins G, Andreucci M, Periasamy M, Solomon RN, Liao R, Patten R, Molkentin JD, Force T. Glycogen synthase kinase-3beta regulates growth, calcium homeostasis, and diastolic function in the heart. J Biol Chem. 2004;279:21383–21393. doi: 10.1074/jbc.M401413200. [DOI] [PubMed] [Google Scholar]