Abstract
Neural Wiskott-Aldrich syndrome protein (N-WASP) is an important member of the WASP family involved in the actin cytoskeleton reorganization. Recent evidence suggests that N-WASP may play important roles in tumor progression and metastasis. However, the contribution of N-WASP to cervical cancer is still unknown. The present study focused on elucidating the role of N-WASP in the malignant behavior of cervical cancer cells. We found that N-WASP overexpressed in cervical cancer tissues compared with paired paracancerous tissues and normal tissues, and similar results were observed in several cervical cancer cell lines. Furthermore, we demonstrated that overexpression of N-WASP facilitated migration and invasion of cervical cancer cells, while downregulation of N-WASP resulted in decreased cell migration and invasion. In addition, the data showed that N-WASP might promote invasion and migration of cervical cancer cells via regulating the activity of p38 MAPKs pathway. Altogether, the study suggested that N-WASP might serve as an oncogene in cervical cancer, and provided novel insights into the mechanism that how N-WASP promoted invasion and migration of cervical cancer cells.
Keywords: N-WASP, cervical cancer, migration, invasion
Introduction
Cervical cancer is the sixth main type of cancer and the third leading cause of cancer in women worldwide. Recent progress in cervical cancer screening tests has successfully decreased incidence and mortality rates in developed countries. However, there is still a high incidence rate in the developing countries, accounting for 85% of all cervical cancer cases [1]. For cervical cancer in the early stages (Stages I-II) and Stage III, the cure rate is up to 80-90% and 60%, respectively. However, for cancer progression to an advanced stage or recurrence, the prognosis is still poor [2]. Therefore, exploring the molecular mechanisms in the progression and metastasis of cervical cancer is essential for development of novel targeted and effective therapeutic treatments.
Neural Wiskott-Aldrich syndrome protein (N-WASP) is an important member of the WASP family involved in the actin cytoskeleton reorganization [3]. It is also well established that WASP family proteins are key regulators of actin polymerization during cell motility and invasion [4]. N-WASP could activate Arp2/3-complex-mediated actin polymerization, under the regulation of Cdc42, Nck, Grb2, Src and phosphoinositides [5]. Moreover, N-WASP was previously demonstrated as an essential component of invadopodia, but not lamellipodia or filopodia in metastatic cancer cells [6-8]. Increased expression of N-WASP was detected in tumor tissues of esophageal squamous cell carcinoma (ESCC), suggested that the N-WASP staining patterns might be useful for the diagnosis of ESCC [9]. Conversely, Martin et al [10,11] found that the expression of N-WASP was significantly decreased in cell lines and tumor tissues of breast cancer and colon cancer, and the prognosis of patients with high expression of N-WASP was better than those with low expression, which indicated that N-WASP might serve as a tumor suppressor. Taken together, N-WASP may play dual and cancer type specific effects on tumor development and progression. However, expression pattern and function of N-WASP in cervical cancer is still unclear.
In the present study, we observed increased expression of N-WASP in cervical cancer samples and cell lines. Furthermore, gain-of-function and loss-of-function studies by overexpression and knockdown of N-WASP in cervical cancer cells were performed to determine its role in cell survival, proliferation, invasion and migration in vitro.
Methods and materials
Specimens, cell culture and reagents
Cervical cancer tissue samples, paracancerous tissue samples and matched normal tissue samples from 26 patients were collected between June 2014 and October 2014 at Zhongnan Hospital of Wuhan University (Wuhan, China). The fresh tissues were immediately snap-frozen in liquid nitrogen and kept in -80°C. The study protocol was approved by the Ethics Committee of Wuhan University.
The human cervical cancer cell lines (Hela, Siha and C33a) and a normal cervical epithelial cell line (H8) were obtained from the Cancer Research Center of Wuhan University (Wuhan, China). C33a-R cell line was established by receiving multiple radiations from the parent cell line C33a in our lab. Cells were cultured in Dulbercco’s modified Eagle’s medium (DMEM, Gibico, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA), and incubated in a humidified atmosphere at 37°C with 5% CO2. SB203580, a selective inhibitor for p38, was purchased from MerckMillipore (Billerica, MA, USA).
Quantitative real-time polymerase chain reaction assay (qRT-PCR)
Total RNA processing and qRT-PCR was performed as described previously [12]. The primers were as follows: N-WASP sense, 5’-GAACGAGTCCCTCTTCACTTTC-3’, antisense, 5’-GTTCCGATCTGCTGCATATAACT-3’; GAPDH sense, 5’-TGACTTCAACAGCGACACCCA-3’, antisense, 5’-CACCCTGTTGCTGTAGCCAAA-3’. The amplification was performed on MX3000p real-time PCR detection system (Agilent Co., USA). The data were normalized to GAPDH, and the relative fold change was calculated using the 2-ΔΔCt method.
Western blot
Western blot analyses were performed as described previously [13]. Briefly, cells were first washed with PBS and lysed in 2 × sodium dodecyl sulphate sample buffer (100 mM Tris-HCl pH6.8, 200 mM DTT, 4% SDS, 20% glycerol and 0.2% bromoplenol blue). Cell lysates were separated by 10% SDS-PAGE. Proteins were transferred to PVDF membranes (Immobilon 0.2 µm, Millipore, USA), which was then immersed in a blocking solution containing 5% non-fat milk and 0.1% tween-20 for 1 h. Afterwards, the membranes were washed and incubated with primary antibody for 2 h and then with secondary antibody for 1 h at room temperature. Primary antibodies used were N-WASP antibody (Abcam, Cambrige, USA), phosphorylated (p)-p38, p38, p-HSP27, HSP27 (Cell Signaling Technology, Beverly, MA, USA), GAPDH and β-actin (Santa Cruz Biotechnology, Santa Cruz, USA). Appropriate secondary antibodies were used. Enhanced chemiluminescence (Beyotime, Shanghai, China) was used to visualize the immuno-reactive bands.
Construction of N-WASP overexpression and knockdown cell lines
The lentivirus containing N-WASP ORF sequence (NM_003941) was constructed by Genomics Co. (Guangzhou, China), which utilized the lentiviral expression vector pLVX-EF1a-IRES-Puro. Small hairpin RNA (shRNA) of N-WASP lentivirus gene transfer vector encoding enhanced green fluorescent protein (EGFP) sequence was constructed by Genechem Co., Ltd (Shanghai, China). The shRNA targeting sequences of N-WASP were: KD1, 5’-ACAACTTAAAGACAGAGAA-3’; KD2, 5’-GCAAGAAATGTGTGACTAT-3’; KD3, 5’-CCCAAATGGTCCTAATCTA-3’; and negative control (NC) was 5’-TTCTCCGAACGTGTCACGT-3’. The N-WASP overexpression and knockdown cell lines were established by infecting cells with lentivirus according to the manufacturer’s instructions.
Cell proliferation assay
The proliferation of cervical cancer cells and the normal epithelial cell was detected by the cell counting kit-8 reagent (CCK-8, Dojindo, Japan) as described previously [14]. In brief, 4 × 103 cells were seeded in 96-well culture plates. 6 h later, CCK-8 assay buffer (100 μl per well) was added at the time point of 0, 24, 48 and 72 h. The optical density (OD) (490 nm values) was determined with a Bio-TEK microplate reader (MD, USA). Each experiment was done in triplicate and the mean value was calculated.
Apoptosis assay
The cells were labeled with annexin V and the apoptosis assay was performed with the annexin V apoptosis detection kit APC (ebioscience, USA) according to the manufacturer’s instruction. The prepared samples were measured by flow cytometry (Millipore, USA). Each experiment was done in triplicate and the mean value was calculated.
Wound healing assay
The wound healing assay was performed using a 96 wounding replicator (VP scientific, CA, USA). In briefly, 3 × 104 cells were seeded in 96-well culture plates and incubated at 37°C in 5% CO2 overnight. After the cells attached, the monolayer was gently scratched using the sterile wounding replicator. The culture medium was replaced, and the migration of the cells was monitored at 0 and 24 h by a microscope.
Transwell migration and invasion assay
For invasion assay, Transwell with an 8 µm diameter pore membrane (Costar, NY, USA) was coated with 200 µL Matrigel (BD Bioscience, Bedford, USA) at 200 µg/ml and incubated overnight. 2 × 104 cells were seeded into the upper chamber of the Transwell and the lower chamber was filled with 500 µL medium supplemented with 10% FBS. After 24 h of incubation at 37°C, migration cells on the bottom surface were stained with crystal violet and counted under a microscope. The migration assay was performed in a similar mode, except that the cells were seeded into the uncoated filter and the medium filled in the lower chamber was supplemented with 5% FBS.
Intracellular signaling array
To explore the molecule mechanism under the biological behavior, we further performed intracellular signaling array using PathScan® Intracellular Signaling Array Kit (Cell Signaling Technology, Beverly, MA). Each experiment was done in triplicate according to the manufacturer’s instruction.
Statistical analysis
All experiments were performed a minimum of three times. All data were analyzed using GraphPad Prism software version 5.0 for windows (CA, USA), and the values were expressed as mean ± sd. For statistical comparisons between two groups of data sets, the Student’s t test (two-tailed) was used; and for more than two groups, one-way ANOVA test was employed. P values less than 0.05 were considered as statistically significant.
Results
N-WASP was significantly overexpressed in human cervical cancer tissues and cell lines
Quantitative RT-PCR was used to detect the mRNA level of N-WASP in 26 human cervical cancer tissues, and matched paracancerous tissues and normal tissues. As shown in Figure 1A, mRNA levels of N-WASP in human cervical cancer tissues were significantly higher than that in matched controls. Moreover, the mRNA levels of N-WASP were determined in four human cervical cancer cell lines (Hela, Siha, C33a and C33a-R) and a normal epithelial cell line H8. The mRNA levels in cancer cell lines were significantly higher compared to that in H8 (Figure 1B). Furthermore, protein expression of N-WASP also indicated that N-WASP is highly expressed in cancer cell lines than the normal epithelial cells (Figure 1C and 1D). Considering the data together, high expression of N-WASP in cancer tissues and cancer cell lines may correlate with the malignancy of cervical cancer.
Figure 1.
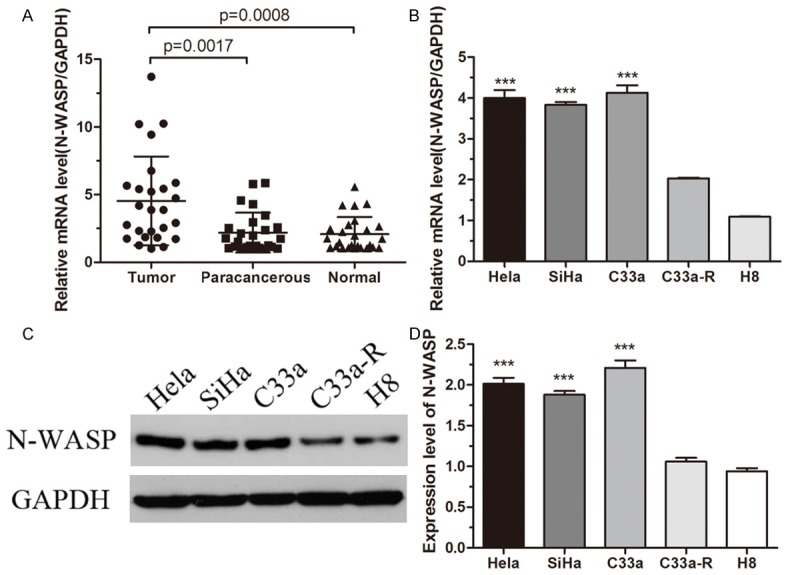
N-WASP expression is increased in cervical cancer tissues and cell lines. (A) N-WASP expression levels in 26 pairs of cervical cancer tissues, the matched paracancerous tissues and normal tissues were measured by quantitative real-time PCR (qRT-PCR). GAPDH was used as an internal control. (B) The expression levels of N-WASP in four cervical cell lines (Hela, SiHa, C33a and C33a-R) and a normal cervical epithelial cell line (H8) were measured by qRT-PCR. (C and D) The protein levels of N-WASP were detected by Western blot (C) and quantified by Image J software (D). ***p < 0.001.
Overexpression of N-WASP promoted migration and invasion of C33a-R cells in vitro
To investigate the role of N-WASP in cervical cancer cell proliferation, apoptosis, migration and invasion, we overexpressed N-WASP in C33a-R cells which exhibits relatively low expression of N-WASP (Figure 1A). C33a-R cells were infected with lentivirus that stably expressed N-WASP (C33a-R OE) and negative control vector (C33a-R NC), respectively. Overexpression efficiency of N-WASP was confirmed using qRT-PCR and western blot. Both mRNA level and protein level of N-WASP were significantly increased by more than 2-fold in C33a-R OE cells than those in C33a-R NC cells and original cells (Figure 2A and 2B). CCK8 assay showed that overexpression of N-WASP had no effect on proliferation of C33a-R cells (Figure 2C). Furthermore, the effect of N-WASP on apoptosis was measured by flow cytometry. Similarly, the effect of N-WASP upregulation on apoptosis was not significantly different (Figure 2D). Moreover, wound healing assay and transwell assay were performed to evaluate the effect of N-WASP on the motility and invasion of C33a-R cells. The results showed that overexpression of N-WASP significantly promoted migration of C33a-R cells in vitro (Figure 2E and 2F), and enhanced the invasive ability of C33a-R cells (Figure 2G). Altogether, our data suggested an oncogenic role of N-WASP. Overexpression of N-WASP decreased cell apoptosis and promoted migration as well as invasion of C33a-R cells.
Figure 2.
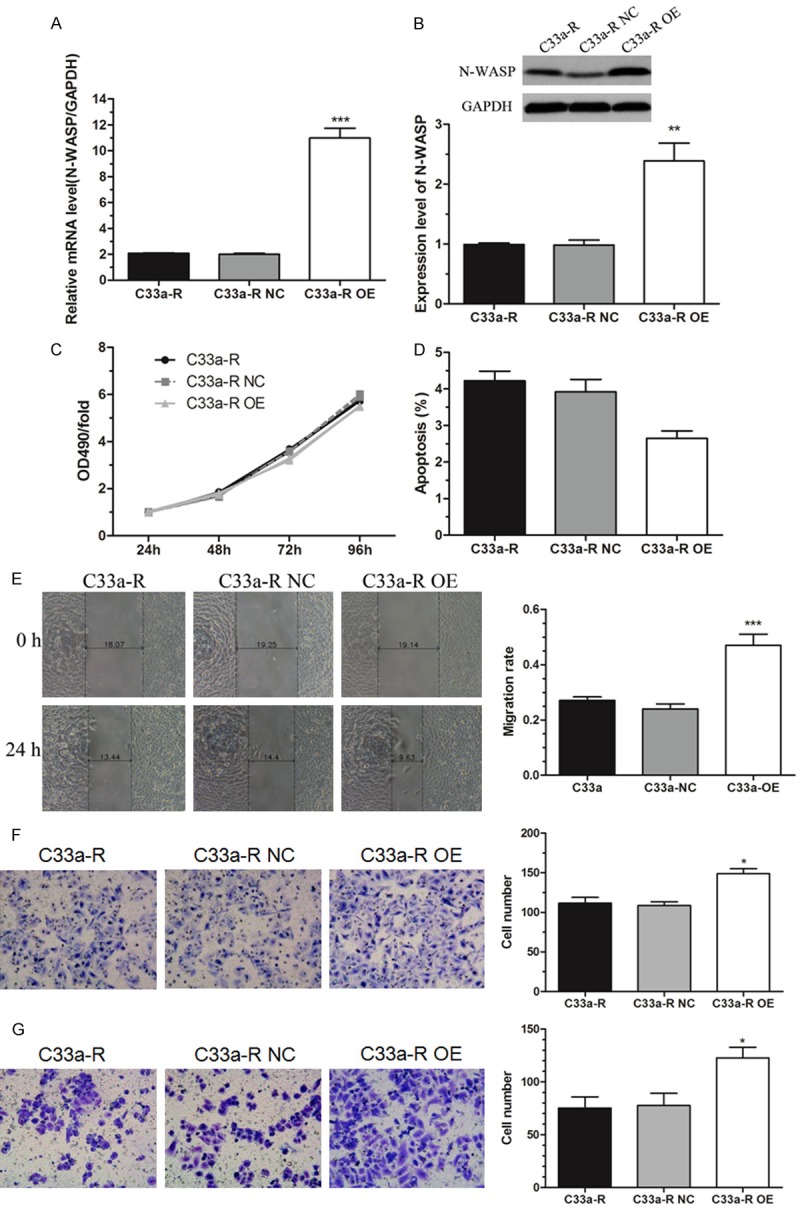
Overexpression of N-WASP promoted migration and invasion of C33a-R cells in vitro. A. The mRNA level of N-WASP was significantly increased after infection with recombinant lentivirus in C33a-R cells (C33a-R OE vs C33a-R NC and C33a-R), which was measured by qRT-PCR analysis. B. Western bolt analysis validated that the protein level of N-WASP was remarkably increased in C33a-R OE cells. C. CCK8 analysis showed that overexpression of N-WASP had no effect on proliferation of C33a-R cells. D. Overexpression of N-WASP did not influence the apoptosis of C33a-R cells which was measured by flow cytometry. E. Wound healing assay showed that overexpression of N-WASP significantly promoted migration of C33a-R cells. F. Transwell migration assay confirmed that overexpression of N-WASP significantly promoted motility of C33a-R cells. G. Transwell invasion assay showed that overexpression of N-WASP significantly facilitated the invasion of C33a-R cells. *P < 0.05, **P < 0.01, ***P < 0.001.
N-WASP suppression induced cell death of C33a cells in vitro
We further explored the role of N-WASP in C33a cells, which have high expression of N-WASP, by using shRNA-mediated gene silencing (Figure 3A). Surprisingly, we found that C33a cells are extremely sensitive to letivirus infection. Almost all cells died after infection with lentvirus with either N-WASP shRNAs or non-target control (NC) shRNA (Figure 3B). Therefore, another cervical cancer cell line Hela with high N-WASP expression was chosen to continuously investigate the effect of N-WASP silencing (Figure 3C). The knockdown efficiency of various lentivirus was evaluated by qRT-PCR, and the results showed that KD1 shRNA has the highest knockdown efficiency (Figure 3D).
Figure 3.
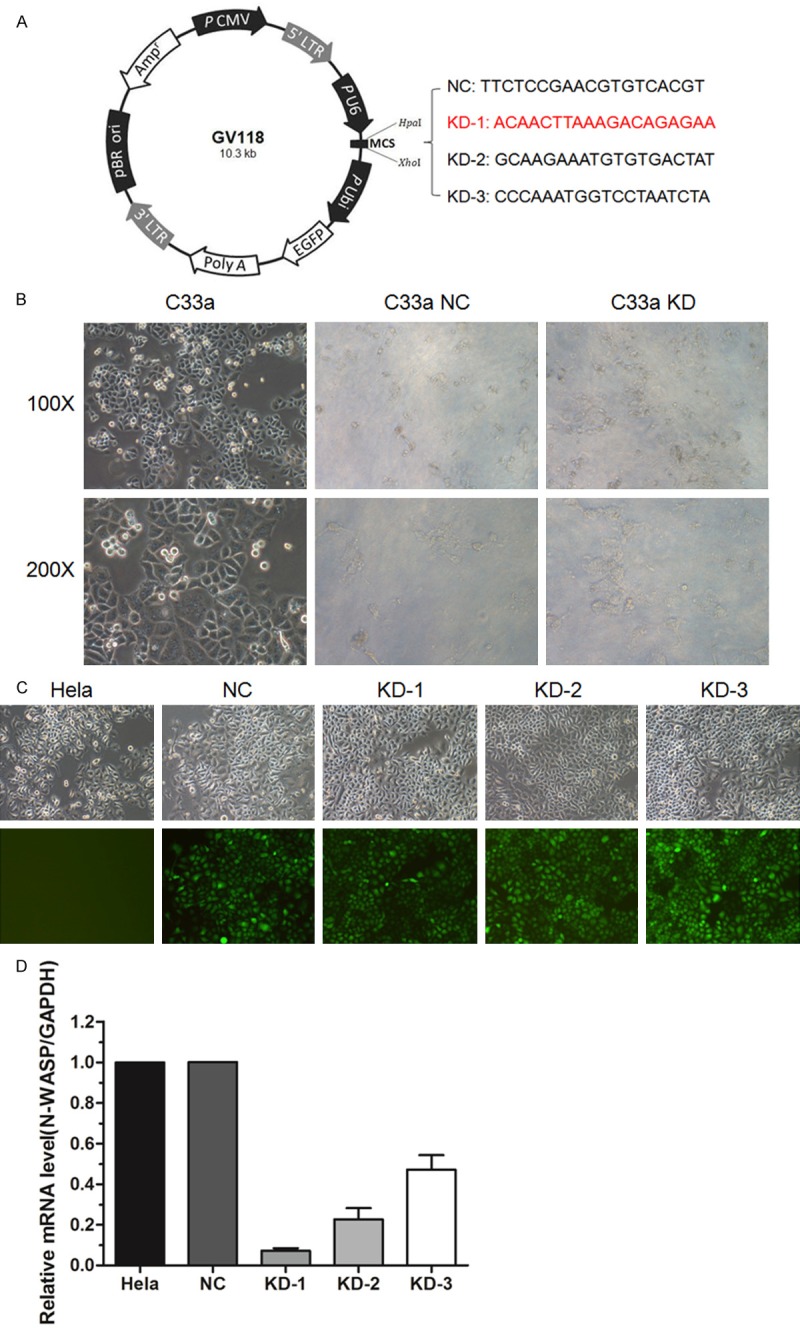
Establishment of N-WASP silenced cervical cell lines. A. Design of lentivirus mediated siRNA. B. Representative images of C33a cells infected with lentivruses as described previously. Almost all the cells were dead after infection, which could not be used for the subsequent studies. C. Representative images of Hela cells infected with the same lentivruses. D. Western blot analysis showed that the protein level of N-WASP was knocked down by infection with lentiviruses mediated siRNA. KD-1 was picked up for the following studies.
N-WASP knockdown inhibited migration and invasion of hela cells in vitro
The efficiency of N-WASP knockdown in Hela cells was confirmed by western blot as shown in Figure 4A. CCK8 assay showed that N-WASP suppression had no effect on Hela cell proliferation (Figure 4B). Moreover, apoptosis assay by flow cytometry supported that N-WASP inhibition did not influence cell apoptosis (Figure 4C). Importantly, the data of wound healing assay and transwell assay indicated that knockdown of N-WASP significantly inhibited cell migration and invasion (Figure 4D-F). These results were consistent with N-WASP overexpression in C33a-R cells, which suggested that N-WASP might play an important oncogenic role in progression of cervical cancer cells.
Figure 4.
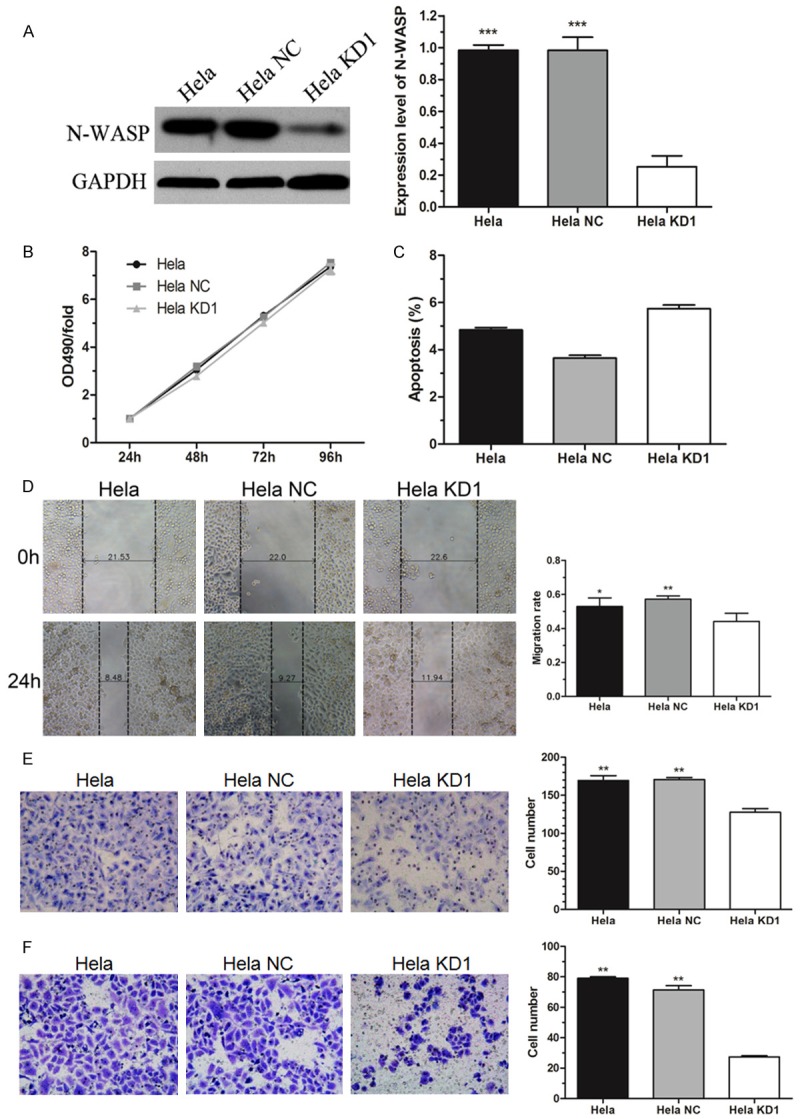
N-WASP knockdown inhibited migration and invasion of Hela cells in vitro. A. Western bolt analysis verified that the protein level of N-WASP was significantly knocked down by infection with lentivirus mediated siRNA against N-WASP. B. CCK8 analysis showed that knockdown of N-WASP had no effect on proliferation of Hela cells. C. Silence of N-WASP did not induce obvious apoptosis of Hela cells which was measured by flow cytometry. D. Wound healing assay showed that knockdown of N-WASP significantly inhibited migration of Hela cells. E. Transwell migration assay confirmed that silence of N-WASP significantly inhibited motility of C33a-R cells. F. Transwell invasion assay showed that knockdown of N-WASP significantly inhibited Hela cell invasion. *P < 0.05, **P < 0.01, ***P < 0.001.
N-WASP knockdown inhibited malignant behaviors of Hela cells through p38 MAPKs signaling pathway
To better understand the detailed regulation mechanism of N-WASP in cervical cancer progression, we determined the activated intracellular signaling pathways upon N-WASP suppression using PathScan® Intracellular Signaling Array Kit. As shown in Figure 5, the phosphorylation levels of S6 Ribosomal Protein, mTOR, HSP27, Bad, p70 S6 Kinase, PRAS40, p38, SAPK/JNK and GSK-3β were significantly decreased in Hela KD1 cells than that in Hela NC cells. First, S6 Ribosomal Protein, mTOR and p70 S6 Kinase are involved in mTOR signaling pathway which plays an important role in cell growth and homeostasis [15]. Second, PRAS40, Bad and GSK-3β are downstream molecules of AKT signaling pathway which transmits growth and survival signals [16]. Third, HSP27, p38 and SAPK/JNK are core components of p38 MAPKs signaling pathway which participates in invasion and metastasis [17]. Therefore, the data suggested that N-WASP might promote invasion and migration through p38 MAPKs signaling pathway in cervical cancer.
Figure 5.
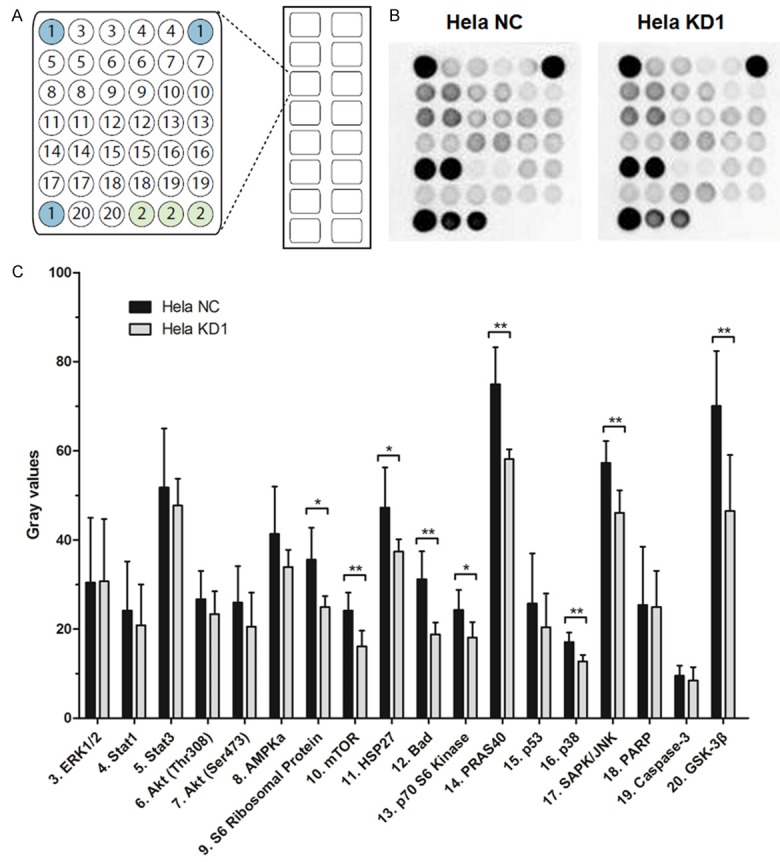
Intracellular signaling array of Hela cells without and with N-WASP silencing. A. Target map of the PathScan® Intracellular Signaling Array Kit. 1, positive control; 2, negative control. B. Hela cells without and with N-WASP silencing were analyzed for the signal activation. C. The phosphorylation levels of signaling component were compared in Hela NC cells and Hela KD1 cells using semi-quantitative analysis. *P < 0.05, **P < 0.01.
Furthermore, a selectivep 38 kinase inhibitor, SB203580 [18], was employed to explore the role of p38 phosphorylation on invasion and migration in cervical cancer cells with and without N-WASP overexpression. Interestingly, the pharmacological inhibition of p38 induced significantly decreased migration (Figure 6A and 6B) and invasiveness (Figure 6C) in cervical cancer cells, which is consistent with the effect of N-WASP knockdown described previously (Figure 4). Moreover, the inhibition effect in N-WASP overexpression cells was stronger than that in negative control cells. The phosphorylated levels of p38 and its downstream molecule HSP27 were detected in N-WASP overexpression cervical cancer cells with or without the presence of SB203580. The results showed that overexpression of N-WASP increased the phosphorylated level of p38, which could be abolished by treatment with SB203580 (Figure 6D). Taken together, the data suggested that N-WASP might be a critical upstream molecule of p38, and both of them might serve as therapeutic targets for inhibition of cervical cancer progression.
Figure 6.
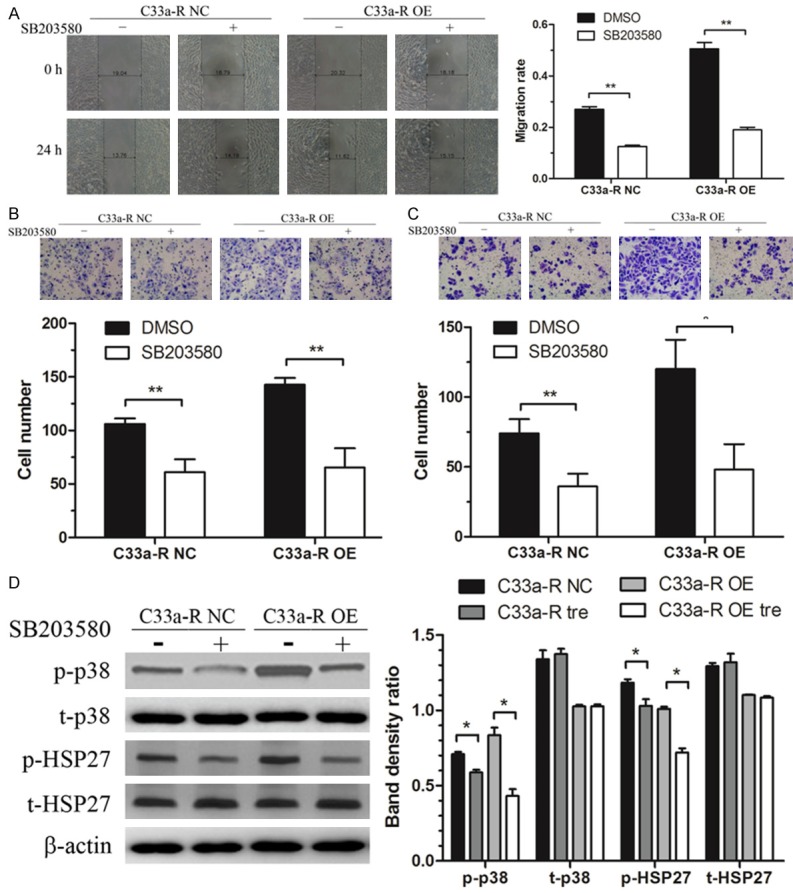
The invasion and migration induced by N-WASP overexpression was abolished by the p38 inhibitor, SB203580, in cervical cancer. Starved cells were treated with 10 uM SB203580 or DMSO for 24 h and subjected to wound healing assay and transwell assay. Wound healing assay (A) and transwell migration assay (B) showed that the migration of C33a-R cells induced by overexpression of N-WASP was significantly decreased by SB203580. (C) Transwell invasion assay showed that overexpression of N-WASP induced invasion of C33a-R cells was significantly reduced after SB203580 treatment. (D) The phosphorylated levels of p38 and its downstream molecule HSP27 were increased by overexpression of N-WASP, which could be abolished by treatment with SB203580. *P < 0.05, **P < 0.01.
Discussion
Although considerable achievements have been made in cancer screening and vaccines, cervical cancer remains a major health threat in women and the prognosis at the advanced stages is still very poor. Metastasis is a multistage biological process and responsible for majority of cancer-related deaths, which is still a big challenge to understand what kind of tumor cells will migrate and invade in cancer progression [19]. In the present study, we focused on a potential biomarker, N-WASP, and its association with malignant behaviors of cervical cancer cells. We observed a significant increase of N-WASP expression in tissue specimens as well as cell lines of cervical cancer (Figure 1). Furthermore, cell migration and invasion of cervical cancer was promoted by upregulation of N-WASP (Figure 2), while inhibited by suppression of N-WASP (Figures 3 and 4). Intracellular signaling array showed N-WASP promoted invasion and migration of cervical cancer cells via regulating the activity of p38 MAPKs pathway (Figure 5), which was further validated using a selective p38 kinase inhibitor (Figure 6). These data suggested that N-WASP may function as a driver in the progression of cervical cancer.
Previously, N-WASP was confirmed to contribute to migration and invasion in several kinds of malignant tumor cells. Bourguignon et al claimed that N-WASP promoted cell migration and transcriptional activation in ovarian cancer by interaction with CD44 and ErbB2 [20]. Gligorijevic et al demonstrated that tumor cells with parental N-WASP levels have an increased ability to form invadopodia, migrate, invade and metastasis compared with those with suppressed N-WASP levels [21]. N-WASP was confirmed to play key roles in mediating the molecular pathogenesis of low oxygen-induced accelerated brain invasion by gliomas [22]. N-WASP and WASP family verprolin-homologous protein 2 (WAVE2) were observed to be co-localized with intensive F-actin accumulations at the infiltration site in a three-dimensional (3D) invasion system. Further knockdown of N-WASP and WAVE2 remarkably reduced cell invasion and F-actin accumulation [23]. It is also known that microfilament rearrangement and cell migration was induced by the activation of integrin receptors recruiting FAK signaling proteins and N-WASP [24].
However, there are some other studies showed that N-WASP appears to inhibit rather than promote tumor progression. Martin et al [10] showed that N-WASP was significantly downregulated in cell lines and specimens of breast cancer, especially in patients with aggressive and node positive tumors as well as poor prognosis. They further observed that overexpression of N-WASP significantly reduced cell motility and invasion of breast cancer in vitro and in vivo, indicating that N-WASP as a tumor suppressor for inhibition of motility and invasion in human breast cancer. Similar results were observed in human colon cancer [11]. Interestingly, Yu et al [25] showed a totally opposite data that N-WASP was overexpressed in breast cancer and played a pivotal role in matrix degradation by mediating the assembly of elongated pseudopodia. They employed 3D matrices to demonstrate that N-WASP was a key regulator for invasive pseudopods extension. Although these studies exhibited an opposing role of N-WASP in cell motility of breast cancer, the molecular mechanisms responsible for the changes are still being explored [26].
In addition, since invadopodium formation correlates with the invasive capacity of cancer cells, it is reported that N-WASP is required for formation of invadopodia by highly invasive and metastatic cancer cells using RNA interference methods [7]. A further study used the N-WASP inhibitor wiskostatin to treat breast cancer cells, resulting in a significant decrease of cell motility. It also found that N-WASP might be involved in the motility of cancer cells by interaction with the tight junction component, claudin-5 [27]. Therefore, N-WASP can be used as a therapeutic target of cancer metastasis, and development of pharmacological agents against N-WASP might arrest tumor progression.
To the best of our knowledge, this is the first study to demonstrate the biological function of N-WASP in cervical cancer progression. The results showed that N-WASP promoted tumor cell migration and invasion possibly through regulating the activity of p38 MAPKs pathway, supported that N-WASP might serve as an oncogene in cervical cancer. The potential of N-WASP as a target for novel treatment modalities against invasion and metastasis should be further investigated.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81302365 and 81302132) and Doctoral Fund of Ministry of Education of China (No. 20130141120059).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Lee M, Kim HJ, Kim SW, Park SA, Chun KH, Cho NH, Song YS, Kim YT. The long non-coding RNA HOTAIR increases tumour growth and invasion in cervical cancer by targeting the Notch pathway. Oncotarget. 2016;7:44558–44571. doi: 10.18632/oncotarget.10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stradal TE, Rottner K, Disanza A, Confalonieri S, Innocenti M, Scita G. Regulation of actin dynamics by WASP and WAVE family proteins. Trends Cell Biol. 2004;14:303–311. doi: 10.1016/j.tcb.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 5.Prehoda KE, Scott JA, Mullins RD, Lim WA. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 2000;290:801–806. doi: 10.1126/science.290.5492.801. [DOI] [PubMed] [Google Scholar]
- 6.Lorenz M, Yamaguchi H, Wang Y, Singer RH, Condeelis J. Imaging sites of N-wasp activity in lamellipodia and invadopodia of carcinoma cells. Curr Biol. 2004;14:697–703. doi: 10.1016/j.cub.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, Condeelis J. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desmarais V, Yamaguchi H, Oser M, Soon L, Mouneimne G, Sarmiento C, Eddy R, Condeelis J. N-WASP and cortactin are involved in invadopodium-dependent chemotaxis to EGF in breast tumor cells. Cell Motil Cytoskeleton. 2009;66:303–316. doi: 10.1002/cm.20361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang WS, Zhong HJ, Xiao DW, Huang X, Liao LD, Xie ZF, Xu XE, Shen ZY, Xu LY, Li EM. The expression of CFL1 and N-WASP in esophageal squamous cell carcinoma and its correlation with clinicopathological features. Dis Esophagus. 2010;23:512–521. doi: 10.1111/j.1442-2050.2009.01035.x. [DOI] [PubMed] [Google Scholar]
- 10.Martin TA, Pereira G, Watkins G, Mansel RE, Jiang WG. N-WASP is a putative tumour suppressor in breast cancer cells, in vitro and in vivo, and is associated with clinical outcome in patients with breast cancer. Clin Exp Metastasis. 2008;25:97–108. doi: 10.1007/s10585-007-9120-8. [DOI] [PubMed] [Google Scholar]
- 11.Martin TA, Toms AM, Davies LM, Cheng S, Jiang WG. The clinical and biological implications of N-WASP expression in human colorectal cancer. Transl Gastrointest Cancer. 2012;1:10–20. [Google Scholar]
- 12.Xu Y, Leng XH, Wu L, Hou JX, Li Y. Association of macrophage migration inhibitory factor (MIF) with metastasis of hepatocellular carcinoma. Int J Clin Exp Pathol. 2016;9:4329–4338. [Google Scholar]
- 13.Hou J, Dong J, Sun L, Geng L, Wang J, Zheng J, Li Y, Bridge J, Hinrichs SH, Ding SJ. Inhibition of phosphorylated c-Met in rhabdomyosarcoma cell lines by a small molecule inhibitor SU11274. J Transl Med. 2011;9:64. doi: 10.1186/1479-5876-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu CL, Wang JZ, Xia XP, Pan CW, Shao XX, Xia SL, Yang SX, Zheng B. Rab11-FIP2 promotes colorectal cancer migration and invasion by regulating PI3K/AKT/MMP7 signaling pathway. Biochem Biophys Res Commun. 2016;470:397–404. doi: 10.1016/j.bbrc.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 15.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–1527. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 17.del Barco Barrantes I, Nebreda AR. Roles of p38 MAPKs in invasion and metastasis. Biochem Soc Trans. 2012;40:79–84. doi: 10.1042/BST20110676. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Zheng Y, Zhang H, Sun H. Targeting cancer cell metabolism: The combination of metformin and 2-deoxyglucose regulates apoptosis in ovarian cancer cells via p38 MAPK/JNK signaling pathway. Am J Transl Res. 2016;8:4812–4821. [PMC free article] [PubMed] [Google Scholar]
- 19.Buchheit CL, Weigel KJ, Schafer ZT. Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression. Nat Rev Cancer. 2014;14:632–641. doi: 10.1038/nrc3789. [DOI] [PubMed] [Google Scholar]
- 20.Bourguignon LY, Peyrollier K, Gilad E, Brightman A. Hyaluronan-CD44 interaction with neural Wiskott-Aldrich syndrome protein (N-WASP) promotes actin polymerization and ErbB2 activation leading to beta-catenin nuclear translocation, transcriptional up-regulation, and cell migration in ovarian tumor cells. J Biol Chem. 2007;282:1265–1280. doi: 10.1074/jbc.M604672200. [DOI] [PubMed] [Google Scholar]
- 21.Gligorijevic B, Wyckoff J, Yamaguchi H, Wang Y, Roussos ET, Condeelis J. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. J Cell Sci. 2012;125:724–734. doi: 10.1242/jcs.092726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Z, Araysi LM, Fathallah-Shaykh HM. c-Src and neural Wiskott-Aldrich syndrome protein (N-WASP) promote low oxygen-induced accelerated brain invasion by gliomas. PLoS One. 2013;8:e75436. doi: 10.1371/journal.pone.0075436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi K, Suzuki K. WAVE2, N-WASP, and Mena facilitate cell invasion via phosphatidylinositol 3-kinase-dependent local accumulation of actin filaments. J Cell Biochem. 2011;112:3421–3429. doi: 10.1002/jcb.23276. [DOI] [PubMed] [Google Scholar]
- 24.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X, Zech T, McDonald L, Gonzalez EG, Li A, Macpherson I, Schwarz JP, Spence H, Futo K, Timpson P, Nixon C, Ma Y, Anton IM, Visegrady B, Insall RH, Oien K, Blyth K, Norman JC, Machesky LM. N-WASP coordinates the delivery and F-actin-mediated capture of MT1-MMP at invasive pseudopods. J Cell Biol. 2012;199:527–544. doi: 10.1083/jcb.201203025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frugtniet B, Jiang WG, Martin TA. Role of the WASP and WAVE family proteins in breast cancer invasion and metastasis. Breast Cancer (Dove Med Press) 2015;7:99–109. doi: 10.2147/BCTT.S59006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escudero-Esparza A, Jiang WG, Martin TA. Claudin-5 is involved in breast cancer cell motility through the N-WASP and ROCK signalling pathways. J Exp Clin Cancer Res. 2012;31:43. doi: 10.1186/1756-9966-31-43. [DOI] [PMC free article] [PubMed] [Google Scholar]


