Abstract
Minimizing donor organ injury during cold preservation (including cold perfusion and storage) is the first step to prevent transplant failure. We recently reported the advantages of hyperbranched polyglycerol (HPG) as a novel substitute for hydroxyethyl starch in UW solution for both cold heart preservation and cold kidney perfusion. This study evaluated the functional recovery of the kidney at reperfusion after cold preservation with HPG solution. The impact of HPG solution compared to conventional UW and HTK solutions on tissue weight and cell survival at 4°C was examined using rat kidney tissues and cultured human umbilical vein endothelial cells (HUVECs), respectively. The kidney protection by HPG solution was tested in a rat model of cold kidney ischemia-reperfusion injury, and was evaluated by histology and kidney function. Here, we showed that preservation with HPG solution prevented cell death in cultured HUVECs and edema formation in kidney tissues at 4°C similar to UW solution, whereas HTK solution was less effective. In rat model of cold ischemia-reperfusion injury, the kidneys perfused and subsequently stored 1-hour with cold HPG solution showed less leukocyte infiltration, less tubular damage and better kidney function (lower levels of serum creatinine and blood urea nitrogen) at 48 h of reperfusion than those treated with UW or HTK solution. In conclusion, our data show the superiority of HPG solution to UW or HTK solution in the cold perfusion and storage of rat kidneys, suggesting that the HPG solution may be a promising candidate for improved donor kidney preservation prior to transplantation.
Keywords: HPG polymer, UW, HTK, donor kidney, cold preservation, transplantation, rodent model
Introduction
The 2014 e-Statistics report of the Canadian Institute for Health Information shows that there were 1350 patients receiving kidney transplants, while 3329 on the waiting list and 244 who withdrew from the waiting list or died while waiting for kidney transplantation, suggesting only 27% of kidney transplant rate. Similar scenario are seen in other places including the United States (https://optn.transplant.hrsa.gov). Therefore, there is an unmet need to expand the donor pool, such as by increasing the use of marginal donor organs or extended criteria donors (ECD) [1,2]. However, it has been documented that the marginal donor kidneys, especially from older donors, are more susceptible to the negative impact of long cold ischemic time (CIT), which leads to an increase in the occurrence of delayed graft function (DGF) [3-5], and represents one of the common reasons quoted for discard or non-recovery [6,7]. In this regard, efforts to minimize the donor kidney injury during cold preservation and consequently to reduce DGF could significantly increase the use of these marginal donors and probably graft survival after transplantation as well.
The simple organ preservation protocol prior to transplantation involves cold storage that comprises flushing the organ with a cold organ preservation solution and storing at 0-4°C. Different types of organ preservation solutions, such as University of Wisconsin (UW) and Histidine Tryptophan Ketoglutarate (HTK) solutions, are currently available for cold preservation of donor organs around the world [8,9]. Although these solutions substantially differ in their composition, their actions are similar - preventing cellular and interstitial edema and cell death and maximizing organ functional recovery after transplantation [8,9]. However, these solutions are often ineffective for the preservation of the marginal donors, indicated by the fact that there is a high percentage (11.25%-56.1%) of DGF in renal allografts from ECD that are flushed and stored with either UW or HTK solution [10-12]. Therefore, an optimal organ preservation solution that can maximally prevent organ damage during cold preservation is needed particularly for those ECD.
Hyperbranched polyglycerol (HPG) is a water-soluble branched, compact polymer that has been investigated for many medical applications, including as an albumin substitute [13] or as a primary osmotic agent in peritoneal dialysis solution [14,15]. Previous studies have demonstrated that this compact polymer has colloidal dimensions as per the standard definition of colloids [15], and has similar intrinsic viscosity to proteins that is approximately 10-fold lower than that of linear polymers, such as polyethylene glycol (PEG), hydroxyethyl starch (HES) and dextran [13,16-19]. Unlike linear HES and PEG that can induce significant red blood cell (RBC) aggregation [20,21], whereas HPG neither aggregate the cells nor precipitate proteins [19,22,23]. All these studies suggest that HPG may be an ideal candidate for the colloid, an important component in organ preservation solutions [24,25]. Indeed, our previous studies have demonstrated that replacing HES in UW solution with HPG (1 kDa, 3%) reduces the relative viscosity to 1.378 at 4°C, more than 2.5-fold lower than that of original UW solution (3.514) [26], and prevents the organ (both hearts and kidneys) damage during cold perfusion and storage and RBC aggregation [26,27]. The objective of the present study was to examine the impact of HPG-based solution as compared to both UW and HTK solutions on kidney injury at reperfusion after cold perfusion and storage in a rat model.
Materials and methods
Reagents, cells and animals
UW solution (SPS-1®) was purchased from Organ Recovery Systems (Itasca, IL, USA), and HTK solution (Custodiol®) from Essential Pharma (Ewing, NJ, USA). Primary human umbilical vein endothelial cells (HUVECs, Lonza, Walkersville, MD, USA) were immortalized with origin-deficient SV40 DNA, and were maintained and grown in Medium 199 as described previously [28].
Fischer 744 (F744) male rats (~300 g bodyweight, 12-14 weeks old) were purchased from the Charles River Laboratories International, Inc. (Wilmington, MA, USA), and maintained in the animal facility of the Jack Bell Research Centre. All the animal experiments were performed in accordance with the Canadian Council on Animal Care guidelines under protocols approved by the Animal Use Subcommittee at the University of British Columbia.
Preparation of HPG preservation solution
HPG (1 kDa) was synthesized by anionic ring opening multi-branching polymerization as described previously [14,15]. HPG-based preservation solution (approximately 305 mOsmol/kg, pH 7.4) was prepared by dissolving HPG (3%, w/v) in a solution containing salts and compounds in the same composition as in UW organ preservation solution without 30 mM raffinose and 5% HES [26].
Trypan blue exclusion assay
Trypan blue exclusion assay was used to determine cell viability or intact cell membrane after cold preservation. In brief, a confluent monolayer of HUVECs (0.2 × 105 cells/well) in 24-well was grown overnight, followed by incubation with HPG, UW or HTK solution at 4°C for 5 h. Cells were detached using Trypsin-EDTA solution (Sigma-Aldrich Canada) and stained with Trypan blue solution (Invitrogen-Gibco). The percentage of viable/surviving cells (trypan blue negative) in total cell count was counted using a TC10TM automated cell counter (Bio-Rad Laboratories Canada, Mississauga, ON, Canada).
Methylthiazoltetrazolium (MTT) assay
MTT assay was used to determine the cellular metabolic activity or mitochondrial NAD(P)H-dependent oxidoreductase activity after cold preservation of cultured cells. Briefly, HUVECs (3 × 103 cells/well) were grown in 96-well plates overnight, followed by incubated with HPG, UW or HTK solution at 4°C for 5 h. After replacing the cold preservation solution with warm culture medium and then incubation at 37°C for 1 h, 10 μL of stock MTT solution (0.5 mg/mL) was added to each well and subsequently incubated at 37°C for another 4 h. The resulting formazan crystals produced by NAD(P)H-dependent oxidoreductase were dissolved in 100 μL DMSO (Sigma-Aldrich Canada), and the optical density (OD) at 562 nm as an index of cell metabolic activity was measured using an ELx808 Ultra Microplate Reader (BioTek, Winooski, VT, USA). The metabolic activity in each cold solution-treated sample was calculated based on the untreated cells in culture medium (Control): % = treated/untreated control × 100%.
Tissue weight determination
The change in total tissue weight (TTW) during cold preservation was used to assess the effect of HPG solution on tissue edema. In brief, a coronal tissue slice of a rat kidney was kept in 2 mL of an organ preservation solution (Saline, HPG, UW or HTK) at 4°C. After 5 or 24 h of cold preservation, the weight change (%) was determined as follows: % = (Wt-W0)/W0 × 100%, where W0 was the tissue weight prior to cold preservation, and Wt the weight after the time period of cold preservation indicated.
Rat model of renal cold ischemia-reperfusion
The preferred method of organ preservation for transplantation is simple cold storage (CS), which involves flushing the organ with a preservation solution and storing at 0-4°C. We established a rat model of kidney cold ischemia-reperfusion for testing the effect of a cold preservation solution on kidney injury at reperfusion after cold perfusion and storage (cold ischemia) (Figure 1). Briefly, a male F744 rat was anesthetized by an intraperitoneal injection of a mixture of ketamine any xylazine, and its anesthesia was maintained by using isoflurane when needed. After the abdominal cavity was exposed via a ventral midline incision, the right kidney was removed after ligation. The aorta was ligated with 11-0 nylon ties (Shanghai Pudong Jinhuan Medical Products Co. Ltd, Shanghai, China) at both the distal and the proximal sites. Renal vein was clamped and subsequently a tiny hole was made at the proximal site near the kidney to allow perfusate to flow freely out of the kidney. At the proximal aorta a needle (22 G) was inserted and was linked to a syringe that contained 4 mL of ice-cold perfusion solution (HPG, UW or HTK solution), and cold kidney perfusion was completed within a period of 3 min. The perfused kidney was then kept under ice-cold temperature for 1 h, followed by blood reperfusion.
Figure 1.
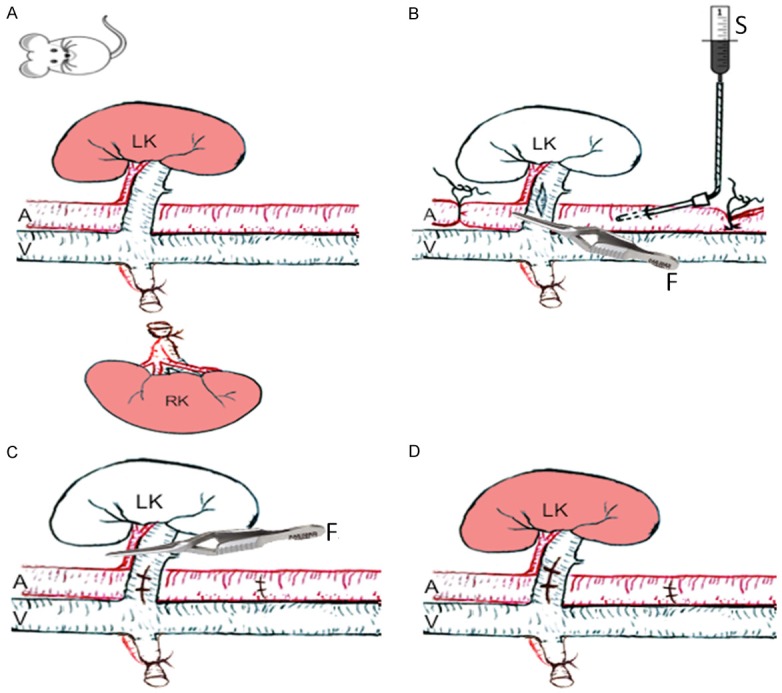
A rat model of renal cold ischemia-reperfusion injury (a simple diagram). Naïve adult Fischer 744 (F744) rats (male) were used in this model. A. Right kidney (RK) was removed. B. Left kidney (LK) was perfused with an ice-cold organ preservation solution (HPG, UW or HTK) that was kept on ice before adding to the syringe. C. The perfused LK was kept under the ice for 1 h. D. The LK was reperfused with the blood after cold perfusion and storage with an organ preservation solution. LK: left kidney, RK: right kidney, A: aorta, V: inferior vena cava, S: Syringe, F: forceps.
Blood chemistry
The function of the remaining left kidneys at 48 h of reperfusion after cold ischemia (3 min of cold perfusion plus 1 h of cold storage) was determined by using serum levels of both creatinine (SCr) or blood urea nitrogen (BUN). The levels of both SCr and BUN in the serum samples were measured in the Chemistry Laboratory at the Vancouver Coastal Health Regional Laboratory Medicine (Vancouver, BC, Canada) by using the Dimension Vista® System with CRE2 and BUN Flex® reagent cartridges (Siemens Healthcare Diagnostics Inc., Newark, DE, USA), respectively.
Histological analyses of tissue injury
After perfusion with saline, a coronal tissue slice was made through the mid-portion of each kidney, followed by fixation in 10% neutral buffered formalin and subsequently embedment in paraffin wax. Sections were cut at 4-μm thickness and stained with hematoxylin and eosin (HE) for the examination of cellular infiltration or periodic acid-Schiff (PAS) for tubular injury. The sections were scanned with Leica SCN400 Slide scanner (Leica Microsystems Inc., Concord, ON, Canada).
The extent of mononuclear cell infiltration in renal cortex of a kidney was assessed in HE-stained sections using a 0 to 4 scale in a blinded fashion, depending on the percentage of cellular infiltrates-occupied area in each microscopic view: 0 (normal or no sign of infiltration), 1 (1-10% of the area affected with cellular infiltration), 2 (11-25%), 3 (26-50%), and 4 (>50%). The average of at least 20 randomly selected views represented the infiltration in each kidney.
The number of injured tubules, including cellular loss (atrophy), intratubular cast formation, tubular cell flattening or vacuolation, was counted in each microscopic view under 400× magnification (high-powered field - hpf) of renal cortex in PAS-stained sectionin a blinded fashion, and the average number of at least 20 randomly selected views represented the injured tubules in each kidney.
Statistical analysis
Student’s t-test with two-tailed distribution or analysis of variance (ANOVA) was performed with GraphPad Prism software (GraphPad, San Diego, CA, USA) as appropriate for analysis of the differences between groups. A P value of ≤0.05 was considered significant.
Results
HPG solution has cellular protection similar to UW at 4°C in vitro for a period of 5 h, but HTK solution is less effective in the protection of cell viability and metabolic activity
The difference of the cellular protection between HPG and UW or HTK solutions was examined in cultured HUVECs after 5 h of cold preservation by different methods. Under microscopic examination (Figure 2A), cultured cells after exposure to all of cold UW, HTK and HPG solutions displayed cell volume loss, indicated by the smaller size as compared to those in culture medium and the presence of finger-like structure around the cell body, whereas, in addition, black dots and vacuolation were noticed inside the cells with HTK solution. Trypan blue exclusion assay showed that HPG solution protected cell viability or plasma cell membrane integrity during cold storage similar to UW solution, indicated by the fact that the numbers of cells negative for trypan blue dye-staining were not significantly different between HPG (86.92 ± 11.29%, n = 10) and untreated medium (89.19 ± 8.27%, n = 9) or UW (89.04 ± 9.89%, n = 10) groups, whereas there was a significant increase in cell death in HTK group (67.46 ± 28.28%, n = 26) (HPG vs. HTK: P = 0.0435, two-tailed t-test) (Figure 2B). We also measured the difference in the metabolic activity of these cells after cold preservation with HPG, UW or HTK solution using MTT assay. As shown in Figure 2C, as compared to untreated control, the cell metabolic activity was reduced to 63.01 ± 7.33% (n = 7) by cold preservation with HPG solution that was similar to 64.82 ± 6.65% (n = 7) with UW solution, but was significantly higher than 44.30 ± 4.37% (n = 7) with HTK solution (HPG vs. HTK: P = 0.0488, two-tailed t-test). Taken together, these data imply that HPG solution has the similar cellular protection in cultured HUVECs compared to UW solution during 5 h of cold preservation, but HTK solution is significantly less effective in the maintenance of the cell viability and metabolic activity as compared with HPG or UW solution.
Figure 2.
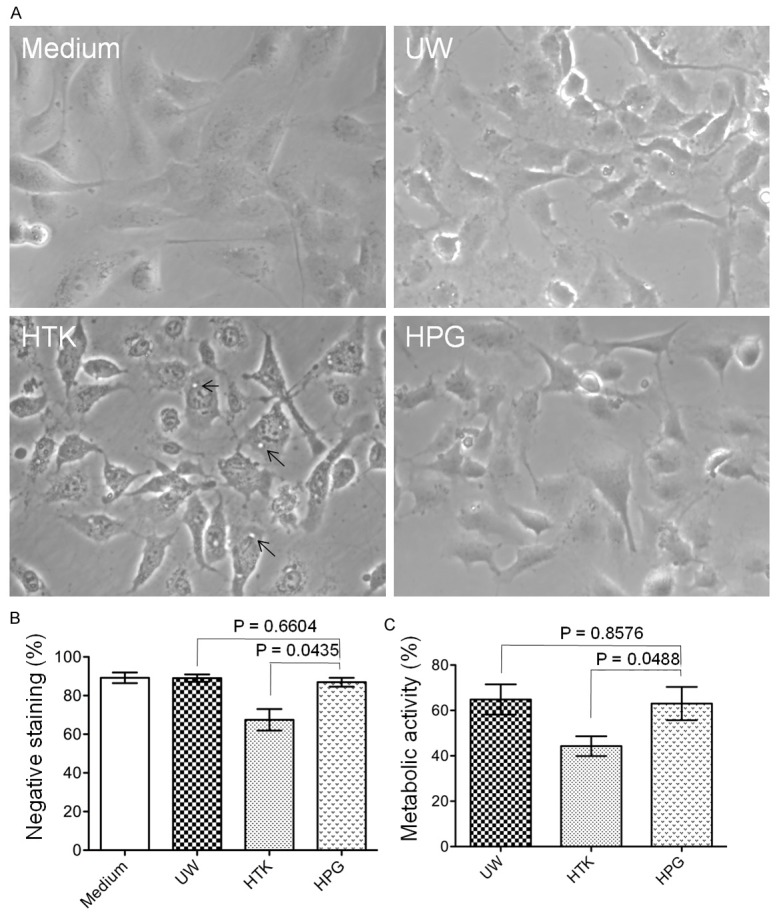
Similar effects of a short period of cold preservation with HPG solution to those with UW solution on cultured HUVECs at 4°C. Monolayers of HUVECs in 24-well plates were incubated with HPG compared to UW or HTK solution at 4°C for 5 h: A. A typical microscopic view of HUVECs after preservation with UW, HTK or HPG solution at 4°C for 5 h. Cells with complete culture medium in a CO2 incubator at 37°C were used as a control. Back arrows: cellular vacuolation. B. Cell survival was determined by a trypan blue exclusion assay. Data are presented as mean ± the standard error of the mean (SEM) of determinants in each group (Medium: n = 9; UW: n = 10; HTK: n = 26; HPG: n = 10) (HPG vs. UW: P = 0.6604; HPG vs. HTK: P = 0.0435, two-tailed t-test). C. The relative metabolic activity, indicated by the intracellular NAD(P)-dependent oxidoreductase activity, was measured by using MTT assay. In each experiment, the OD of medium group was used as a positive control (100% of the metabolic activity) for calculation of relative metabolism in other treatment groups (UW, HTK or HPG). Data are presented as mean ± SEM of seven separate experiments in each group (HPG vs. UW: P = 0.8576; HPG vs. HTK: P = 0.0448, two-tailed t-test).
HPG prevents kidney tissue edema similar to UW solution at 4°C, but HTK solution is less effective
Inclusion of colloidal HES in UW solution prevents interstitial edema of tissues during cold preservation [24,29], which may be important for graft survival after transplantation [30]. To evaluate if HPG polymer is effective as HES in UW solution in the prevention of tissue edema, the change of TTW in kidney tissues after cold preservation with HPG solution was compared to UW or HTK solution. As shown in Figure 3, TTW was markedly gained in saline by 16.90 ± 2.60% (n = 4) after 5 h of cold preservation and further to 35.13 ± 3.81% (n = 6) at the end of 24 h of cold preservation. The hyperosmotic HPG, UW and HTK solutions significantly prevented interstitial edema in comparison to saline but with different capabilities. The TTW in HTK solution remained almost unchanged during a period of 24 h of cold preservation (-0.49 ± 1.17% at 5 h, 4.01 ± 1.49% at 24 h, n = 8-10), and it was significantly reduced by 7.86 ± 2.05% (n = 8) at 5 h or by 10.82 ± 1.54% (n = 10) at 24 h in HPG solution, whereas 11.56 ± 1.63% (n = 8) at 5 h or 19.84 ± 0.91% (n = 10) at 24 h by UW solution (HPG vs. UW: P = 0.0001, two-way ANOVA), suggesting that UW induced the most TTW loss compared to HPG or HTK solution (HTK vs. UW: P<0.0001, two-way ANOVA). However, statistical analyses showed that the effect of UW solution on TTW was not significantly different from that of HPG solution at the earlier time point - 5 h of cold storage (HPG vs. UW at 5 h: P = 0.1384, t-test). These data suggest that just like HES, HPG molecules can prevent interstitial edema of kidney tissue during cold preservation.
Figure 3.
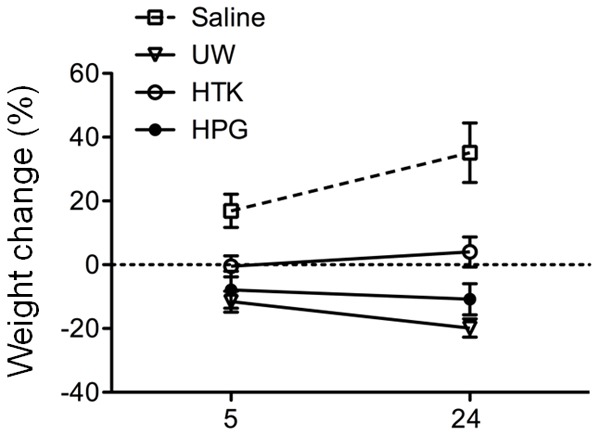
Effective prevention of tissue edema formation at 4°C by preservation with HPG solution. Rat kidney tissues were stored at 4°C in saline, UW, HTK or HPG solution for a period of 24 h. The change of total tissue weight (TTW) as a marker of tissue edema was determined after 5 or 24 h of cold preservation. Data are presented as mean ± standard derivation (SD) of determinants in each group (Saline: n = 4-6; UW: n = 8-10; HTK: n = 8-10; HPG: n = 8-10) (HPG vs. UW: P = 0.0001; HPG vs. HTK: P<0.0001; HTK vs. UW: P<0.0001, two-way ANOVA). No difference was seen between HPG and UW group at 5 h.
HPG solution has less negative impact on kidney functional recovery after cold ischemia-reperfusion
One of common concerns for the use of suboptimal or marginal donors in kidney transplantation is the poor functional recovery or DGF after transplantation [1]. The impact of HPG solution on functional recovery of the kidney at reperfusion after cold ischemia (cold perfusion and storage) was compared with that of UW or HTK solution. As shown in Figure 4, the serum levels of SCr and BUN in rats at 48 h of reperfusion after their kidneys were perfused and stored with cold HPG solution were 0.712 ± 0.042 mg/dL and 80.84 ± 6.138 mg/dL (n = 17), respectively, which were significantly lower than those with UW (SCr: 1.045 ± 0.093 mg/dL; BUN: 97.04 ± 4.164 mg/dL, n = 17) or HTK solution (SCr: 1.192 ± 0.150 mg/dL; BUN: 101.6 ± 5.105 mg/dL, n = 17) (HPG vs. UW or HTK: P<0.05). These data suggest that the kidneys perfused and stored with HPG solution have better function than those with conventional UW or HTK solution at reperfusion.
Figure 4.
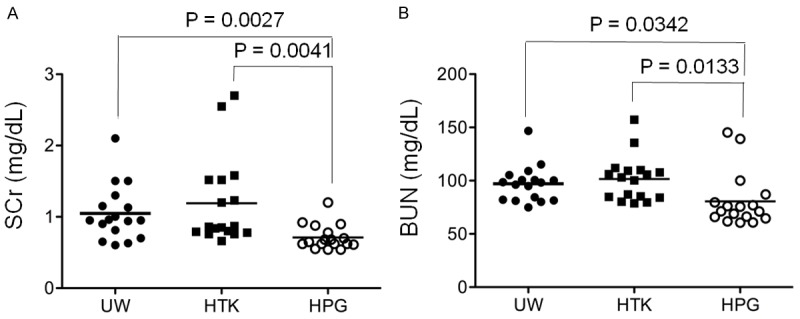
Less negative impact on kidney function after cold perfusion and storage with HPG solution. After 48 h of kidney reperfusion, serum from each rat was collected, and the levels of both serum creatinine (SCr) and blood urea nitrogen (BUN) in each individual were measured as biomarkers of its kidney function. The horizontal line indicated the mean level for each group. A. SCr in HPG group (n = 17) compared to conventional UW or HTK control group (n = 17). HPG vs. UW: P = 0.0027 (two-tailed t-test). HPG vs. HTK: P = 0.0041 (two-tailed t-test). B. BUN in HPG group (n = 17) compared to conventional UW or HTK control group (n = 17). HPG vs. UW: P = 0.0342 (two-tailed t-test). HPG vs. HTK: P = 0.0133 (two-tailed t-test).
HPG solution is associated with less kidney inflammation and injury at reperfusion after cold ischemia
To further confirm the beneficial effect of HPG solution on functional recovery of the kidney compared to UW or HTK solution, the leukocyte infiltration and tubular injury in the kidneys at 48 h of reperfusion were examined by histological analyses. As shown in Figure 5, cellular infiltration in the kidneys in HPG group was significantly less severe than that in UW or HTK group, indicated by the semi-quantitative assay showing that the leukocyte infiltration score was 1.044 ± 0.391 (n = 9) in HPG group, which were significantly lower than 1.840 ± 0.782 (n = 10) in HTK (HPG vs. HTK: P = 0.0136, t-test) or 2.167 ± 0.608 (n = 9) in UW group (HPG vs. UW: P = 0.0003, t-test). In parallel with less leukocyte infiltration, the kidney in HPG group displayed less tubular damage (4.83 ± 1.73 damaged tubules/hpf) than those in HTK (16.48 ± 6.92 damaged/tubules/hpf) (P<0.0001, t-test, n = 10) or UW group (17.63 ± 7.34 damaged/tubules/hpf, n = 10) (P<0.0001, t-test, n = 10) (Figure 6).
Figure 5.
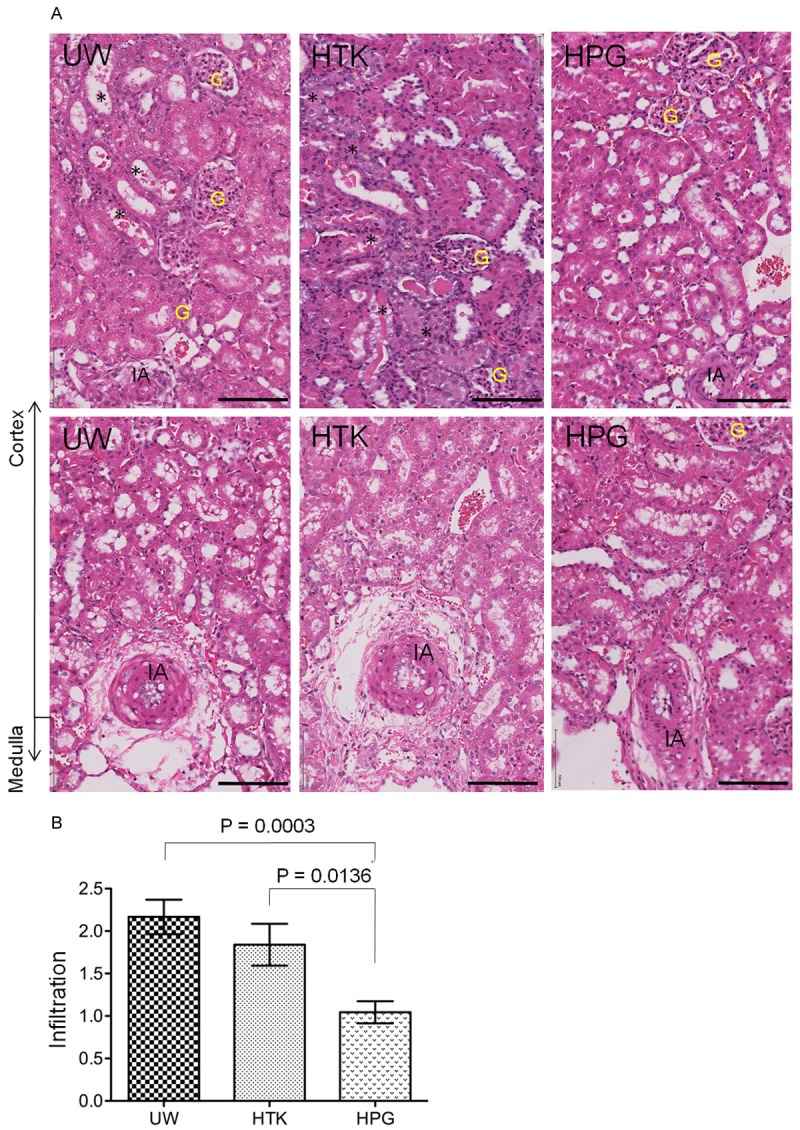
Less cellular infiltration in the kidney after cold perfusion and storage with HPG solution. The sections of the kidney harvested at 48 h after reperfusion were stained with hematoxylin and eosin (HE), and the cellular infiltration in the cortex were scored under the microscope. A. A typical image of the cortex in each group. Black stars (*): damaged tubules (necrotic tubules, tubular dilation, intratubular cast formation and tubular vacuolation). G: glomerulus. IA: interlobular artery. Black bar: 100 μm. B. The infiltration was semi-quantitatively scored in at least 20 randomly selected views in two separate sections of each kidney, and was presented in average per view. Data are presented as mean ± SEM of each group (UW: n = 9; HTK: n = 10; HPG: n = 9) (HPG vs. UW: P = 0.0003; HPG vs. HTK: P = 0.0136, two-tailed t-test).
Figure 6.
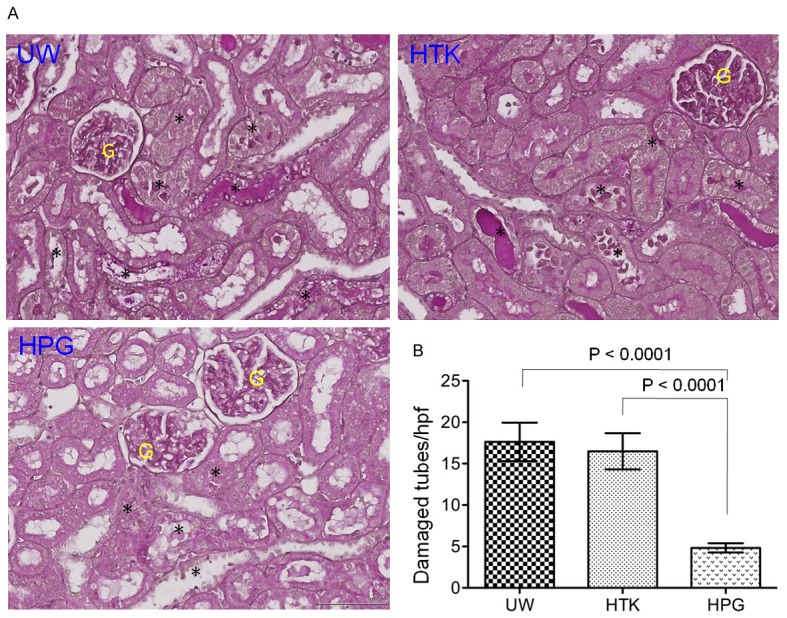
Less tubular injury in the kidney after cold perfusion and storage with HPG solution. The sections of the kidney harvested at 48 h after reperfusion were stained with Periodic acid-Schiff (PAS), and the damaged tubules in the cortex were counted under the microscope. A. A typical image of the cortex in each group. Black stars (*): damaged tubules (necrotic tubules, tubular dilation, intratubular cast formation and tubular vacuolation). G: glomerulus. Black bar: 100 μm. B. The number of damaged tubules in the cortex per view was counted under × 400 magnification (high-powered field - hpf). At least 20 views of the cortex in the sections of each kidney were counted and averaged in a blinded fashion. Data are presented as mean ± SEM of each group (n = 10 each group) (HPG vs. UW: P<0.0001; HPG vs. HTK: P<0.0001, two-tailed t-test).
Discussion
The maintenance of donor organ viability during recovery and preservation with an organ preservation solution is the first step toward the success of a newly transplanted organ. Currently studies have shown that all the conventional organ preservation solutions such as UW or HTK solution, are similarly effective in the preservation of abdominal organs in the case of standard criteria donors for a short period of storage time (<8 h of CIT) [31]. However, due to the shortage of such high-quality donors there is an increasing pressure of using marginal donors (e.g. deceased or ageing donors, and donors with hypertension and diabetes or with CIT of longer than 8 h) for transplantation [32-34]. Such situation may require a novel preservation solution that can be more effective in limiting donor organ damage during cold ischemia-reperfusion for the better preservation of kidney function after transplantation. Previous studies from our group have demonstrated that HPG-based organ preservation is superior to UW solution in the prevention of mouse organ (both hearts and kidneys) damage during cold perfusion and storage as well as of human RBC aggregation in vitro [26,27]. The data from the current study showed that HPG solution was most effective in cold perfusion and preservation of rat kidneys as compared to both conventional UW or HTK solutions in term of reduction in kidney injury or protection of kidney function. The improved function of HPG solution in this rat model may be associated with its prevention of both cell death (Figure 2) and kidney tissue edema at the cold temperature.
During cold preservation of donor organs, hypothermia disrupts normal metabolism and ATP production (ATP depletion), which consequently lead to Na+/K+ ATPase suppression or cellular edema, acidosis and an increase in the production of reactive oxygen species (ROS), and eventually cell death (i.e. necrosis and apoptosis) [8]. Hence, an organ preservation solution must contain active ingredients that can counteract cellular edema, acidosis and the production of ROS, respectively. Different solutions are currently being used for the cold preservation of solid organ around the world [8,31], however UW and HTK solutions are the most two common solutions used in the United States [35]. HTK solution was initially used for open heart surgery, and its use for cold preservation of donor organs may be contributed by the combination of the strong buffer (histidine), osmotic barrier (mannitol), and low-permeable amino acids (tryptophan and ketoglutaric acid), which may stabilize cell membrane at cold temperature and support anaerobic metabolism [8], whereas UW solution contains colloid (i.e. HES) and impermeable substances (lactobionate and raffinose) for edema prevention, adenosine for ATP synthesis and antioxidant defense (allopurinol and reduced glutathione) for reducing ROS cytotoxicity [8]. It has been documented that transplants from donation after brain death (DBD) after a short period (<8 h of CIT) of cold preservation with either HTK or UW solution are found to have equivalent outcomes [31], but liver, kidney, and pancreas national registry reports have found that HTK can limit the graft survival [31,36,37]. Indeed, HTK is an independent risk factor for the development of DGF of transplants from donation after cardiac death [38], and an experimental study has reported that UW solution is superior to HTK solution in prolonged preservation of kidney grafts, indicated by a better renal function and less tissue damage compared with HTK. This observation is possibly due to improved cooling and better cell viability of the graft [39]. Our present study also show that both colloid-based solutions (UW or HPG) are significantly better than HTK solution in the prevention of both hypothermia-induced cell death and - related metabolism decline in cultured human endothelial cells (Figure 1), and as well in the prevention of interstitial edema in cold-preserved kidney tissues (Figure 2). Our data also imply that replacing “sticky” HES and raffinose in UW solution with compact polymer HPG does not affect its function in suppressing tissue edema during cold preservation (Figure 2).
In UW solution, inclusion of colloidal HES is necessary for counteracting the hydrostatic pressure during cold perfusion, and may be beneficial to cold storage of hearts and pancreas [40], but the use of HES in patients increases the risk of coagulopathy, pruritus and acute kidney injury [41-45], and more importantly in kidney transplants from DBD donors it impairs immediate donor kidney function after transplantation [46]. In addition, there are other two limitations of HES for organ preservation: HES increases the viscosity of the solution when it is used for donor organ perfusion [8], resulting in an increase in the vascular resistance of donor organs to the perfusion, and shortening of graft survival [47,48], and HES causes the hyperaggregation of human RBCs [21,27,49], resulting in the complication of donor blood wash-out, such as stasis of blood and/or incomplete removal of donor blood. Our previous studies have shown that replacing HES with HPG polymer significantly improves both effectiveness and efficiency of cold perfusion in the removal of the blood from the kidneys [27], and it also reduces donor tissue injury during cold perfusion [27] or static cold storage [26]. In consistence with these previously reported facts, the kidneys after cold perfusion and storage with HPG solution exhibited less tissue injury or better function after reperfusion than those with HES-based UW solution (Figures 4, 5, and 6).
The mechanism of the beneficial effects of HPG compared to HES on donor organ preservation has not been fully understood. It has been documented that replacing HES with HPG in the preservation solution significantly reduces its intrinsic viscosity and cold perfusion-induced kidney tissue injury in mice [27], and also has beneficial effect on the maintenance of intracellular ATP and cell survival at cold temperature in cultured cells [26], which together may explain the mechanisms underlying the superiority of HPG over HES as a colloid for cold perfusion and storage of donor organs in these experimental models. Further investigation of the mechanism of HPG actions during cold organ perfusion and storage is needed.
In conclusion, our data from this experimental study using the rat model clearly demonstrate that after cold perfusion and storage with HPG solution, less donor kidney injury occurs in comparison to those with conventional UW or HTK solution, suggesting that HPG solution is a promising alternative to UW or HTK solution for the cold perfusion and storage of donor kidneys. The improved cold preservation of kidneys with HPG solution may be translated to the organs collected particularly from marginal donors. Further investigation of HPG-based solution for its effectiveness in the cold preservation of pig kidneys that are similar to humans in size and structure, and or even human subjects are needed to support the superiority of HPG solution compared to UW or HTK solutions.
Acknowledgements
This study was funded by the Canadian Institutes of Health Research (Proof of Principle program) and the Transplant Research Foundation of British Columbia (Vancouver, BC, Canada). SL received scholarship from the China Scholarship Council and funding support from General Hospital of Chengdu Military Command (Grant No.: 2016KC28), Science and Technology Department of Sichuan Province (Grant No.: 2017HH0073) and the Center of Donation After Cardiac Death, Center of Medical Science of Chengdu Military Command. JNK is a recipient of career investigator scholar award from Michael Smith Foundation for Health Research. We also would like to thank Canada Foundation for Innovation for supporting the infrastructure facility at the Centre for Blood Research.
Disclosure of conflict of interest
None.
References
- 1.Bozkurt B, Kilic M. Marginal donors in renal transplantation. Transplant Proc. 2015;47:1273–1281. doi: 10.1016/j.transproceed.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Heilman RL, Mathur A, Smith ML, Kaplan B, Reddy KS. Increasing the use of kidneys from unconventional and high-risk deceased donors. Am J Transplant. 2016;16:3086–3092. doi: 10.1111/ajt.13867. [DOI] [PubMed] [Google Scholar]
- 3.Asderakis A, Dyer P, Augustine T, Worthington J, Campbell B, Johnson RW. Effect of cold ischemic time and HLA matching in kidneys coming from “young” and “old” donors: do not leave for tomorrow what you can do tonight. Transplantation. 2001;72:674–678. doi: 10.1097/00007890-200108270-00020. [DOI] [PubMed] [Google Scholar]
- 4.Frei U, Noeldeke J, Machold-Fabrizii V, Arbogast H, Margreiter R, Fricke L, Voiculescu A, Kliem V, Ebel H, Albert U, Lopau K, Schnuelle P, Nonnast-Daniel B, Pietruck F, Offermann R, Persijn G, Bernasconi C. Prospective age-matching in elderly kidney transplant recipients--a 5-year analysis of the Eurotransplant senior program. Am J Transplant. 2008;8:50–57. doi: 10.1111/j.1600-6143.2007.02014.x. [DOI] [PubMed] [Google Scholar]
- 5.Salahudeen AK, Haider N, May W. Cold ischemia and the reduced long-term survival of cadaveric renal allografts. Kidney Int. 2004;65:713–718. doi: 10.1111/j.1523-1755.2004.00416.x. [DOI] [PubMed] [Google Scholar]
- 6.Hall IE, Schroppel B, Doshi MD, Ficek J, Weng FL, Hasz RD, Thiessen-Philbrook H, Reese PP, Parikh CR. Associations of deceased donor kidney injury with kidney discard and function after transplantation. Am J Transplant. 2015;15:1623–1631. doi: 10.1111/ajt.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanriover B, Mohan S, Cohen DJ, Radhakrishnan J, Nickolas TL, Stone PW, Tsapepas DS, Crew RJ, Dube GK, Sandoval PR, Samstein B, Dogan E, Gaston RS, Tanriover JN, Ratner LE, Hardy MA. Kidneys at higher risk of discard: expanding the role of dual kidney transplantation. Am J Transplant. 2014;14:404–415. doi: 10.1111/ajt.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guibert EE, Petrenko AY, Balaban CL, Somov AY, Rodriguez JV, Fuller BJ. Organ preservation: current concepts and new strategies for the next decade. Transfus Med Hemother. 2011;38:125–142. doi: 10.1159/000327033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bon D, Chatauret N, Giraud S, Thuillier R, Favreau F, Hauet T. New strategies to optimize kidney recovery and preservation in transplantation. Nat Rev Nephrol. 2012;8:339–347. doi: 10.1038/nrneph.2012.83. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal A, Murdock P, Fridell JA. Comparison of histidine-tryptophan ketoglutarate solution and University of Wisconsin solution in prolonged cold preservation of kidney allografts. Transplantation. 2006;81:480–482. doi: 10.1097/01.tp.0000196724.89757.79. [DOI] [PubMed] [Google Scholar]
- 11.Lynch RJ, Kubus J, Chenault RH, Pelletier SJ, Campbell DA, Englesbe MJ. Comparison of histidine-tryptophan-ketoglutarate and University of Wisconsin preservation in renal transplantation. Am J Transplant. 2008;8:567–573. doi: 10.1111/j.1600-6143.2007.02065.x. [DOI] [PubMed] [Google Scholar]
- 12.Stevens RB, Skorupa JY, Rigley TH, Yannam GR, Nielsen KJ, Schriner ME, Skorupa AJ, Murante A, Holdaway E, Wrenshall LE. Increased primary non-function in transplanted deceased-donor kidneys flushed with histidine-tryptophan-ketoglutarate solution. Am J Transplant. 2009;9:1055–1062. doi: 10.1111/j.1600-6143.2009.02624.x. [DOI] [PubMed] [Google Scholar]
- 13.Kainthan RK, Janzen J, Kizhakkedathu JN, Devine DV, Brooks DE. Hydrophobically derivatized hyperbranched polyglycerol as a human serum albumin substitute. Biomaterials. 2008;29:1693–1704. doi: 10.1016/j.biomaterials.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Mendelson AA, Guan Q, Chafeeva I, da Roza GA, Kizhakkedathu JN, Du C. Hyperbranched polyglycerol is an efficacious and biocompatible novel osmotic agent in a rodent model of peritoneal dialysis. Perit Dial Int. 2013;33:15–27. doi: 10.3747/pdi.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du C, Mendelson AA, Guan Q, Chapanian R, Chafeeva I, da Roza G, Kizhakkedathu JN. The size-dependent efficacy and biocompatibility of hyperbranched polyglycerol in peritoneal dialysis. Biomaterials. 2014;35:1378–1389. doi: 10.1016/j.biomaterials.2013.10.076. [DOI] [PubMed] [Google Scholar]
- 16.Kainthan RK, Hester SR, Levin E, Devine DV, Brooks DE. In vitro biological evaluation of high molecular weight hyperbranched polyglycerols. Biomaterials. 2007;28:4581–4590. doi: 10.1016/j.biomaterials.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Kainthan RK, Gnanamani M, Ganguli M, Ghosh T, Brooks DE, Maiti S, Kizhakkedathu JN. Blood compatibility of novel water soluble hyperbranched polyglycerol-based multivalent cationic polymers and their interaction with DNA. Biomaterials. 2006;27:5377–5390. doi: 10.1016/j.biomaterials.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Kainthan RK, Janzen J, Levin E, Devine DV, Brooks DE. Biocompatibility testing of branched and linear polyglycidol. Biomacromolecules. 2006;7:703–709. doi: 10.1021/bm0504882. [DOI] [PubMed] [Google Scholar]
- 19.Imran ul-haq M, Lai BF, Chapanian R, Kizhakkedathu JN. Influence of architecture of high molecular weight linear and branched polyglycerols on their biocompatibility and biodistribution. Biomaterials. 2012;33:9135–9147. doi: 10.1016/j.biomaterials.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Mosbah IB, Franco-Gou R, Abdennebi HB, Hernandez R, Escolar G, Saidane D, Rosello-Catafau J, Peralta C. Effects of polyethylene glycol and hydroxyethyl starch in University of Wisconsin preservation solution on human red blood cell aggregation and viscosity. Transplant Proc. 2006;38:1229–1235. doi: 10.1016/j.transproceed.2006.02.068. [DOI] [PubMed] [Google Scholar]
- 21.Zhao WY, Xiong HY, Yuan Q, Zeng L, Wang LM, Zhu YH. In vitro effects of polyethylene glycol in University of Wisconsin preservation solution on human red blood cell aggregation and hemorheology. Clin Hemorheol Microcirc. 2011;47:177–185. doi: 10.3233/CH-2010-1379. [DOI] [PubMed] [Google Scholar]
- 22.Rossi NA, Constantinescu I, Kainthan RK, Brooks DE, Scott MD, Kizhakkedathu JN. Red blood cell membrane grafting of multi-functional hyperbranched polyglycerols. Biomaterials. 2010;31:4167–4178. doi: 10.1016/j.biomaterials.2010.01.137. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Janzen J, Brooks DE. Adsorption of amphiphilic hyperbranched polyglycerol derivatives onto human red blood cells. Biomaterials. 2010;31:3364–3373. doi: 10.1016/j.biomaterials.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Arrajab A, Ahren B, Sundberg R, Bengmark S. The function of a colloid in liver cold-storage preservation. Transplantation. 1991;52:34–38. doi: 10.1097/00007890-199107000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Southard JH, van Gulik TM, Ametani MS, Vreugdenhil PK, Lindell SL, Pienaar BL, Belzer FO. Important components of the UW solution. Transplantation. 1990;49:251–257. doi: 10.1097/00007890-199002000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Gao S, Guan Q, Chafeeva I, Brooks DE, Nguan CY, Kizhakkedathu JN, Du C. Hyperbranched polyglycerol as a colloid in cold organ preservation solutions. PLoS One. 2015;10:e0116595. doi: 10.1371/journal.pone.0116595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Constantinescu I, Guan Q, Kalathottukaren MT, Brooks DE, Nguan CY, Kizhakkedathu JN, Du C. Advantages of replacing hydroxyethyl starch in University of Wisconsin solution with hyperbranched polyglycerol for cold kidney perfusion. J Surg Res. 2016;205:59–69. doi: 10.1016/j.jss.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Guan Q, Li S, Yip G, Gleave ME, Nguan CY, Du C. Decrease in donor heart injury by recombinant clusterin protein in cold preservation with University of Wisconsin solution. Surgery. 2012;151:364–371. doi: 10.1016/j.surg.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 29.Schlumpf R, Morel P, Loveras JJ, Condie RM, Matas A, Kurle J, Najarian JS, Sutherland DE. Examination of the role of the colloids hydroxyethylstarch, dextran, human albumin, and plasma proteins in a modified UW solution. Transplant Proc. 1991;23:2362–2365. [PubMed] [Google Scholar]
- 30.Ploeg RJ, Boudjema K, Marsh D, Bruijn JA, Gooszen HG, Southard JH, Belzer FO. The importance of a colloid in canine pancreas preservation. Transplantation. 1992;53:735–741. doi: 10.1097/00007890-199204000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Parsons RF, Guarrera JV. Preservation solutions for static cold storage of abdominal allografts: which is best? Curr Opin Organ Transplant. 2014;19:100–107. doi: 10.1097/MOT.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 32.Routh D, Naidu S, Sharma S, Ranjan P, Godara R. Changing pattern of donor selection criteria in deceased donor liver transplant: a review of literature. J Clin Exp Hepatol. 2013;3:337–346. doi: 10.1016/j.jceh.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao PS, Ojo A. The alphabet soup of kidney transplantation: SCD, DCD, ECD--fundamentals for the practicing nephrologist. Clin J Am Soc Nephrol. 2009;4:1827–1831. doi: 10.2215/CJN.02270409. [DOI] [PubMed] [Google Scholar]
- 34.Tuttle-Newhall JE, Krishnan SM, Levy MF, McBride V, Orlowski JP, Sung RS. Organ donation and utilization in the United States: 1998-2007. Am J Transplant. 2009;9:879–893. doi: 10.1111/j.1600-6143.2009.02565.x. [DOI] [PubMed] [Google Scholar]
- 35.Sung RS, Galloway J, Tuttle-Newhall JE, Mone T, Laeng R, Freise CE, Rao PS. Organ donation and utilization in the United States, 1997-2006. Am J Transplant. 2008;8:922–934. doi: 10.1111/j.1600-6143.2008.02171.x. [DOI] [PubMed] [Google Scholar]
- 36.Stewart ZA, Cameron AM, Singer AL, Montgomery RA, Segev DL. Histidine-Tryptophan-Ketoglutarate (HTK) is associated with reduced graft survival in deceased donor livers, especially those donated after cardiac death. Am J Transplant. 2009;9:286–293. doi: 10.1111/j.1600-6143.2008.02478.x. [DOI] [PubMed] [Google Scholar]
- 37.Stewart ZA, Cameron AM, Singer AL, Dagher NN, Montgomery RA, Segev DL. Histidine-tryptophan-ketoglutarate (HTK) is associated with reduced graft survival in pancreas transplantation. Am J Transplant. 2009;9:217–221. doi: 10.1111/j.1600-6143.2008.02449.x. [DOI] [PubMed] [Google Scholar]
- 38.Jochmans I, Darius T, Kuypers D, Monbaliu D, Goffin E, Mourad M, Ledinh H, Weekers L, Peeters P, Randon C, Bosmans JL, Roeyen G, Abramowicz D, Hoang AD, De Pauw L, Rahmel A, Squifflet JP, Pirenne J. Kidney donation after circulatory death in a country with a high number of brain dead donors: 10-year experience in Belgium. Transpl Int. 2012;25:857–866. doi: 10.1111/j.1432-2277.2012.01510.x. [DOI] [PubMed] [Google Scholar]
- 39.Hoeger S, Lueg G, Tsagogiorgas C, Schneider M, Theisinger S, Theisinger B, Fontana J, Waldherr R, Kramer BK, Schnuelle P, Yard B. UW is superior compared with HTK after prolonged preservation of renal grafts. J Surg Res. 2011;170:e149–157. doi: 10.1016/j.jss.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Southard JH, Belzer FO. Organ preservation. Annu Rev Med. 1995;46:235–247. doi: 10.1146/annurev.med.46.1.235. [DOI] [PubMed] [Google Scholar]
- 41.Hartog C, Reinhart K. CONTRA: Hydroxyethyl starch solutions are unsafe in critically ill patients. Intensive Care Med. 2009;35:1337–1342. doi: 10.1007/s00134-009-1521-5. [DOI] [PubMed] [Google Scholar]
- 42.Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, Madsen KR, Moller MH, Elkjaer JM, Poulsen LM, Bendtsen A, Winding R, Steensen M, Berezowicz P, Soe-Jensen P, Bestle M, Strand K, Wiis J, White JO, Thornberg KJ, Quist L, Nielsen J, Andersen LH, Holst LB, Thormar K, Kjaeldgaard AL, Fabritius ML, Mondrup F, Pott FC, Moller TP, Winkel P, Wetterslev J. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367:124–134. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 43.Perner A, Haase N, Wetterslev J, Aneman A, Tenhunen J, Guttormsen AB, Klemenzson G, Pott F, Bodker KD, Badstolokken PM, Bendtsen A, Soe-Jensen P, Tousi H, Bestle M, Pawlowicz M, Winding R, Bulow HH, Kancir C, Steensen M, Nielsen J, Fogh B, Madsen KR, Larsen NH, Carlsson M, Wiis J, Petersen JA, Iversen S, Schoidt O, Leivdal S, Berezowicz P, Pettila V, Ruokonen E, Klepstad P, Karlsson S, Kaukonen M, Rutanen J, Karason S, Kjaeldgaard AL, Holst LB, Wernerman J. Comparing the effect of hydroxyethyl starch 130/0.4 with balanced crystalloid solution on mortality and kidney failure in patients with severe sepsis (6S - Scandinavian Starch for Severe Sepsis/Septic Shock trial): study protocol, design and rationale for a double-blinded, randomised clinical trial. Trials. 2011;12:24. doi: 10.1186/1745-6215-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schortgen F, Lacherade JC, Bruneel F, Cattaneo I, Hemery F, Lemaire F, Brochard L. Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet. 2001;357:911–916. doi: 10.1016/S0140-6736(00)04211-2. [DOI] [PubMed] [Google Scholar]
- 45.Winkelmayer WC, Glynn RJ, Levin R, Avorn J. Hydroxyethyl starch and change in renal function in patients undergoing coronary artery bypass graft surgery. Kidney Int. 2003;64:1046–1049. doi: 10.1046/j.1523-1755.2003.00186.x. [DOI] [PubMed] [Google Scholar]
- 46.Cittanova ML, Leblanc I, Legendre C, Mouquet C, Riou B, Coriat P. Effect of hydroxyethylstarch in brain-dead kidney donors on renal function in kidney-transplant recipients. Lancet. 1996;348:1620–1622. doi: 10.1016/s0140-6736(96)07588-5. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz V, Klawitter J, Bendrick-Peart J, Haschke M, Beckey VE, Laudi S, Neumann U, Schoening W, Neuhaus P, Christians U, Puhl G. Impact of organ preservation using HTK for graft flush and subsequent storage in UW in rat kidney transplantation. Eur Surg Res. 2006;38:388–398. doi: 10.1159/000094600. [DOI] [PubMed] [Google Scholar]
- 48.Tojimbara T, Wicomb WN, Garcia-Kennedy R, Burns W, Hayashi M, Collins G, Esquivel CO. Liver transplantation from non-heart beating donors in rats: influence of viscosity and temperature of initial flushing solutions on graft function. Liver Transpl Surg. 1997;3:39–45. [PubMed] [Google Scholar]
- 49.Morariu AM, Vd Plaats A, V Oeveren W, T Hart NA, Leuvenink HG, Graaff R, Ploeg RJ, Rakhorst G. Hyperaggregating effect of hydroxyethyl starch components and University of Wisconsin solution on human red blood cells: a risk of impaired graft perfusion in organ procurement? Transplantation. 2003;76:37–43. doi: 10.1097/01.TP.0000068044.84652.9F. [DOI] [PubMed] [Google Scholar]


