Abstract
Decitabine treatment improves immunological recognition that increases expression of cancer-testis antigens (CTAs) against solid tumors. The mechanisms of decitabine enhancement of immunogenicity when used for patients with myelodysplastic syndromes (MDS) remain unclear. In the present study, we found relatively low baseline expression of MAGE-A1, MAGE-A3, and SP17 in MDS-derived cell lines. Decitabine treatment significantly improved MAGE-A1, MAGE-A3, and SP17 expression in these cell lines and in MDS patients. Decitabine-treated K562 and SKM-1 target cells with incrementally induced MAGE-A1, MAGE-A3, or SP17 levels up-regulated T lymphocyte function. Decitabine treatment improved CTA-specific cytotoxic T lymphocyte (CTL) recognition of MDS cells via the up-regulation of CTAs. This response was accompanied by enhanced T lymphocyte function and HLA class antigen expression, and increased ICAM-1. These findings suggested that decitabine may have a broad range of therapeutic applications when it is used in association with active adaptive immunity responses against up-regulated CTAs.
Keywords: Myelodysplastic syndromes, decitabine, T cytotoxicity, cancer-testis antigens
Introduction
Myelodysplastic syndrome (MDS) refers to a group of pathophysiologically diverse premalignant hematopoietic diseases characterized by inflammation-associated cytopenias, co-existent autoimmunity, myeloid dysplasia, and variable risk acute myeloid leukemia (AML) progression [1]. Different MDS subgroups share various treatment strategies, including hematopoietic cytokines, immunosuppressive drugs, hypomethylating agents (HMAs; e.g., decitabine (DAC) and 5-azacitidine), and allogeneic hematopoietic stem cell transplantation [2].
DAC is a typical HMA. It is a cytosine analog and potent DNA methyltransferase inhibitor that induces DNA demethylation. DAC treatment is used for patients with higher-risk MDS [3]; it is also used for subgroups of AML and chronic myelomonocytic leukemia (CMML) patients [4,5]. DAC treatment induces a late clinical response in some patients. Because immune modulatory interventions often have a slower onset of efficacy than direct cytotoxic drugs, this late response suggests that immunoregulatory mechanisms are involved [6]. DAC upregulates cancer-testis antigen (CTA) expression in solid tumors as a result of CTA gene promoter component demethylation [7,8]. CTAs are well-known targets for immune recognition during cancer treatment. This up-regulation may increase immune recognition of tumor cells.
Known immunogenic CTAs (e.g., MAGE, SSX gene families, PRAME, NY-ESO-1, and SP17) are expressed in solid tumors of different histotypes, but not in normal tissues, and are expressed primarily in immune-privileged sites such as the testis, the placenta, and during fetal development [9]. Due to their unique tissue distribution, and recognition by cytotoxic T lymphocytes (CTLs) or B lymphocytes, or both, CTAs are useful therapeutic targets for treatment of solid malignancies [10]. However, the effects of DAC treatment on CTAs and T-cells in MDS patients have not been thoroughly investigated.
The objectives of this study were to investigate whether DAC treatment increased expression of CTAs on tumor cells and in MDS patients. We evaluated whether up-regulation of CTAs could facilitate tumor cell lysis by MAGE-A1, MAGE-A3, and SP17 peptide-stimulated CTLs. We analyzed the alterations in T lymphocytes after DAC therapy in MDS patients and examined the expression of surface molecules, including MHC Class I, MHC Class-II, and ICAM-1. These molecules have been implicated in enhancement of anti-tumor T cell responses through adaptive immunity mechanisms. The results of this study provide a basis for the development of new immunotherapy strategies against MDS.
Materials and methods
Cell culture
MDS-L cells were donated by Prof. Tohyama [11]. MDS-derived leukemia cell line SKM-1 cells were donated by Prof. Nakagawa [12]. The leukemia cell line (K562) was obtained from the ATCC. Cell lines were maintained in complete medium (RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 1% glutamine, and 1% sodium pyruvate). IL-3 (100 U/ml) was added to the MDS-L cell culture. The cell lines were maintained at 37°C in a humidified, 5% CO2 atmosphere.
Treatment of cell lines with DAC
The cell lines were treated with 1 μM DAC (Selleck Chemicals LLC, Houston TX, USA) for 5 days. The samples that were used for the molecular and functional assays were removed from these cultures. We chose 1 μM DAC to treat the above cell lines to obtain a DAC concentration comparable to that achieved pharmacologically (i.e., plasma concentrations of DAC of 1.3 μM at a DAC dose of 15 mg/m2/day) [13]. In drug concentration experiment, the cell lines were treated with three consecutive 24 h incubations of DAC (5, 10, and 20 μM) for 5 days.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted from cell lines or from bone marrow mononuclear cells (BMMCs) of MDS patients using the RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. cDNA was prepared using the RevertAidTM First Strand cDNA Synthesis Kit (Fermentas, Burlington, ON, Canada) following the manufacturer’s protocol. Each 20 µL RT-PCR mixture contained 10 µL RealMasterMix (Takara, Dalian, China), 0.8 µL of each primer, 2 µL cDNA, and distilled water. PCR was performed using an ABI 7500 Real-Time PCR machine (Applied Biosystems, Foster City, CA, USA). The PCR conditions consisted of a hold stage of 95°C for 30 s and a cycling sequence of 40 cycles at 95°C for 5 s, 60°C for 30 s, and 72°C for 30 s. MAGE-A1, MAGE-A3, and Sp17 primer sequences were used (Table 1). The threshold cycle (Ct) value was determined, and the relative quantification of mRNA expression was calculated using the comparative Ct method. The relative quantification value of the target was normalized to that of an endogenous control (GAPDH gene) and relative to that of a calibrator (the mean expression level of normal controls). It was expressed as 2-ΔΔCt (fold difference), where ΔCt = Ct of the target gene -Ct of the endogenous control gene (GAPDH). ΔΔCt = ΔCt of the samples for the target gene -ΔCt of the calibrator for the target gene.
Table 1.
qPCR primer sequences
| qPCR primer sequence | ||
|---|---|---|
| MAGE A1 | Forward | 5’-GGGAGCCTCCGCCTTT-3’ |
| Reverse | 5’-CCTCACTGGGTTGCCTCTG-3’ | |
| MAGE A3 | Forward | 5’-CCAGCCTCCCCACTACCA-3’ |
| Reverse | 5’-GGTTGCTGGAGTCCTCATAGG-3’ | |
| Sp17 | Forward | 5’-GGAGGAAGAGACATCAGTCACC-3’ |
| Reverse | 5’-GCC TCC TCT CTG GCT ATG TG-3’ | |
| GAPDH | Forward | 5’-AACTTTGGCATTGTGGAAGG-3’ |
| Reverse | 5’-ACACATTGGGGGTAGGAACA-3’ |
Western blot analysis
Whole cell lysates were obtained from MDS-derived cell lines. Equal quantities of protein were separated using 12% SDS-PAGE and were blotted onto polyvinylidene difluoride (PVDF) membranes. The PVDF membranes were blocked for 1 husing tris-buffered saline (TBS) containing 5% skimmed milk powder, and were incubated with rabbit p53 (1:1000; Abcam Inc., Cambridge, MA, USA), rabbit Sp17 (1:00; Abcam Inc.), and mouse GAPDH (1:1000; Abcam Inc.) as loading controls. The membranes were incubated with anti-mouse or anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences, Piscataway, NJ, USA). ECL Western Blotting Detection Reagents (Amersham Biosciences) were used to visualize the specific bands.
Generation of dendritic cells (DCs) for CTL stimulation
Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated using Ficoll-Hypaque (Sigma, St. Louis, MO, USA) density gradient centrifugation. The dendritic cells (DCs) were generated as previously described [14]. The PBMCs were seeded into 6-well culture plates containing 3 ml RPMI 1640 and 10% fetal calf serum at 5 to 10×106 cells/well. They were incubated for 2 h to allow adherence and to separate monocytes from non-adherent cells. The non-adherent cells were cryopreserved at -80°C for later use. The adherent monocytes were cultured in CellGenixTM DC medium supplemented with human recombinant granulocyte-macrophage colony-stimulating factor (1,000 U/mL, Bayer, Seattle, WA, USA) and interleukin 4 (IL-4; 10 ng/mL; R&D Systems, Minneapolis, MN, USA). Immature DCs were matured for 48 h in 10 ng/ml TNF-α, 10 ng/ml IL-1β, 10 ng/ml IL-6 (R&D Systems), and 1 μg/ml PGE2 (Sigma). All investigational protocols were approved by the ethics committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital.
DC pulsing
Peripheral blood lymphocytes from healthy donors were stimulated three times (once per week) with autologous DC pulsed with MAGE-A1, MAGE-A3, and SP17 overlapping peptide mixes. These mixtures included pooled, 11 amino acid overlapping 15 mers derived from the full-length protein (JPT Peptide Technologies, Berlin, Germany). IL-7 and IL-15 (10 ng/ml; R&D Systems) were added when the cultures were first prepared and were replaced weekly. IL-2 (50 units/ml, Novartis, East Hanover, NJ, USA) was first added at day 3 and then every 3 days.
In vitro generation of CT antigen-specific T cell cultures
Antigen-specific T cells were selected and expanded as previously described [15]. Briefly, 24 h after the third stimulation, antigen-specific cells expressing CD137 were selected using a CD137 isolation kit (human CD137 MicroBead Kit, MiltenyiBiotec Inc. Auburn, CA, USA). The selected cells were expanded using anti-CD3 monoclonal antibody OKT3 (30 ng/ml; Ortho Biotech, Raritan, NJ, USA) and IL-2 (50 units/ml, Novartis, East Hanover, NJ, “USA) in the presence of irradiated (3 Gy) allogeneic feeder PBMCs (1×106 cells/ml), and irradiated (10 Gy) BLCL (0.1×106 cells/ml). After 5 to 6 days, the OKT3 was removed, and 50% of the culture medium (RPMI 1640 with 10% FBS) was replaced every 3 days, or as needed, with fresh medium and IL-2. The functions of the expanded CTLs were characterized using chromium release assays.
Cytotoxicity assays
Cytotoxicity was determined using 51Cr release assays and the target cells autologous B cell blasts (BB, used as a negative control), BB pulsed with the MAGE-A1, MAGEA3, or Sp17 peptide mixes, and cell lines before and after DAC treatment. The targets were labeled using overnight exposure to 51Cr (100 μCi/106 cells; PerkinElmer Life and Analytical Sciences, Monza, Italy), washed in PBS, and dispensed in triplicate into 96-well V bottom plates (ICN) at 4×103 cells/well [16]. The CTLs were added at a 20:1 responder: target ratio for the healthy donor cells and at 50:1 for the CTLs generated from patient blood. After a 4-h incubation, the supernatant was analyzed using a gamma counter. The values for spontaneous and total release obtained for each target were used to calculate percent-specific release: (% specific release = (experimental cpm-spontaneous cpm)/(total cpm-spontaneous cpm)).
Patients and treatment
Seventeen patients treated at our medical center were included in this study. Minimum diagnostic criteria were used to diagnosis MDS (Vienna, 2006). MDS classification and prognostic risk scoring were performed using WHO criteria and the revised International Prognostic Scoring System. A summary of the patients’ characteristics is presented in Table 2. DAC (product name: Dacogen, DAC, Xi’an Janssen Pharmaceutical Ltd, Xi’an, China) was administered (15 mg/M2, I.V.) over a period of 1 h once per day for 5 consecutive days. This course of treatment was repeated every 4 weeks. Unless disease progression, unacceptable adverse events, or voluntary withdrawal from the study occurred, each patient received at least four courses of continued DAC treatment. A routine blood examination was performed two times per week. To evaluate response to treatment, bone marrow (BM) was examined using routine aspirate smears and G-banding analysis every 2 to 4 treatment courses. All subjects provided written informed consent, in accordance with Declaration of Helsinki guidelines. The research protocol was approved by the ethics committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital.
Table 2.
Characteristics of MDS patients treated using decitabine (DAC) (n = 17)
| No. | Sex | Age | Blasts (%) | Karyotype | Diagnosis* | IPSS | DAC response |
|---|---|---|---|---|---|---|---|
| P1 | M | 85 | 6.2 | Normal | RAEB1 | 1 | CR |
| P2 | F | 40 | 11.1 | Normal | RAEB2 | 2 | CR |
| P3 | M | 83 | 8.6 | Normal | RAEB1 | 1 | CR |
| P4 | F | 66 | 12.6 | Normal | RAEB2 | 2 | CR |
| P5 | F | 61 | 11.2 | 46,XX,del(5)(q13q31),del(12)(p11.2)der19[3] | RAEB2 | 3 | CR |
| P6 | M | 67 | 5.8 | 45,XY,del(2;11)(p24;q25),del(7)(q22),inc[12] | RAEB2 | 3.5 | mCR+Hi |
| P7 | F | 67 | 23 | 50,XX,+8,+9,+10,+18,del(20)(q12),inc[4]/46,XX[2] | RAEBt | 3.5 | Cri+CCR |
| P8 | F | 70 | 24.2 | Normal | RAEBt | 3 | CR |
| P9 | M | 76 | 9.3 | 46,XY,-5,der (7),+mar[15] | RAEB1 | 2 | CR+CCR |
| P10 | M | 61 | 30.5 | 46,XY,+8,del(20)(q12)[22]/47,idem,add(1)(p36)[3] | RAEBt | 3.5 | mCR+Hi |
| P11 | M | 79 | 12.7 | 44,xy,+der(1),del(3)(p21),-5,+8,-16,-18,-20,-21, +1 mar[3] | RAEB2 | 3 | CR+CCR |
| P12 | M | 54 | 6.4 | 46,XY,del(12)(p12)[3] | RAEB1 | 1.5 | CR |
| P13 | M | 59 | 8.4 | 45,XX,-7[15]/46,XX[3] | CMML1 | 2 | mCR+Hi |
| P14 | F | 62 | 14 | 48,X,-X,der(7)t(7;11)(q11q11),+3mar,inc[2]/46,XX[8] | RAEB2 | 3 | CR+CCR |
| P15 | F | 54 | 11 | Normal | RAEB2 | 2 | NR |
| P16 | F | 60 | 7 | Normal | RAEB1 | 1 | NR |
| P17 | F | 64 | 3.6 | 45,XX,-7[22]/46,XX[3] | RCMD | 1.5 | NR |
Morphologic diagnosis at presentation in MDS according to WHO classification.
Note: RCMD, refractory cytopenia withmultilineage dysplasia; RAEB-1/2, refractory anemia with excess blasts 1/2. F, female; M, male. IPSS, International PrognosticScoring System. DAC response was assessed by IWG 2006 criteria: complete response (CR), partial response (PR), marrow CR(mCR), hematological improvement (HI), and cytogenetic response (including cytogenetic CR [cCR] defined as no detectablecytogenetic abnormality and cPR defined as at least 50% reduction in abnormal metaphases). Responders included CR, PR,mCR, and HI. Non-responders included stable disease (SD) and progressive disease (PD).
Detection of BM lymphocytes, MHC, and ICAM-1
Flow cytometry was performed using the FACSC alibur platform (BD Biosciences, San Jose, CA, USA). The results were analyzed using CellQuest software (BD Biosciences). For each tube, 10,000 events were collected in a gate created around the viable lymphocyte population using FSC/SSC. Anti-CD3 conjugated with phycoerythrin-cyanin 5 (PC5), anti-CD4 conjugated with fluorescein isothiocyanate (FITC), and anti-CD8 antibody conjugated with Phycoerythrin-Texas Red were used to label nucleated BM cells. Flow cytometry was also performed to examine MHC Class I and Class II expression on cell surfaces. MHC Class I, Class II, and ICAM-1 molecule expression levels were determined using staining with directly conjugated monoclonal antibodies (mAb). PE-conjugated anti-human HLA-ABC mAb was used to detect MHC Class I and FITC-conjugated anti-human HLA-DR, DP, DQ mAb was used to detect MHC Class II and PE-conjugated ICAM-1 (CD54) (BD Biosciences).
Subset and polarization of T lymphocytes
BMMCs were incubated with phorbol 12-myristate 13-acetate and ionomycin (Sigma, St. Louis, MO, USA) at for 4 h at 37°C [17]. The cells were then stained using a 15-min exposure to anti-CD3-PerCP and anti-CD8-APC (Becton Dickinson, Franklin Lakes, NJ, USA). After treatment with IntraPreP permeabilization reagent B (Becton Dickinson), the cells were stained with anti-human IFNγ-FITC and IL-4-PE (Becton Dickinson) for 15 min. All antibodies were purchased from BD Biosciences. Flow cytometry was performed using the FACS Calibur system. The results were analyzed using Cell Quest software. The T-helper (Th) and T-cytotoxic (Tc) subsets were classified as Th1 (CD8-INF-γ+), Th2 (CD8-IL-4+), Tc1 (CD8+ INF-γ+), or Tc2 (CD8+ IL-4+).
Statistical analysis
Comparisons of mRNA levels of the CTAs between the control and treated cell line groups were performed using independent-sample T tests. Comparisons of the mRNA levels of the CTAs, BM lymphocytes, MHC, ICAM-1, and subset and polarization of T lymphocytes in MDS patients before and after DAC treatment were performed using paired-sample T tests. All statistical analyses were performed using SPSS v20.0 statistical analysis software. A P-value <0.05 was considered to indicate a statistically significant result.
Results
DAC-induced increase in CTAs expression in cell lines and MDS patients
To study the effects of DAC on CTAs expression, cell lines were treated with 1 μM DAC for 5 days and analyzed for the expression of MAGE-A1, MAGE-A3, and SP17 using quantitative real-time PCR (RT-qPCR). Expression of MAGE-A1, MAGE-A3, and SP17 was relatively low before DAC treatment. After exposure to DAC, expression of the MAGE-A1, MAGE-A3, and SP17 antigens was up-regulated on the three cell lines (Figure 1A-C). The 17 MDS patients were also analyzed for the expression of these CTAs after DAC treatment. The RT-qPCR results indicated that the expression of MAGE-A1, MAGE-A3, and SP17 were increased after treatment (Figure 1D). We also compared the expression of CTAs between DAC responders and non-responders. The CTAs showed continuous stable high expression in the DAC responders, and decreased expression in the DAC non-responders (Figure 2). The expression of MAGE-A1 and SP17 in patient 2 was sustained at a high level until a disease relapse occurred. CTAs expression declined when patient 2’s disease progressed and transformed into leukemia (Figure 2A, 2C).
Figure 1.
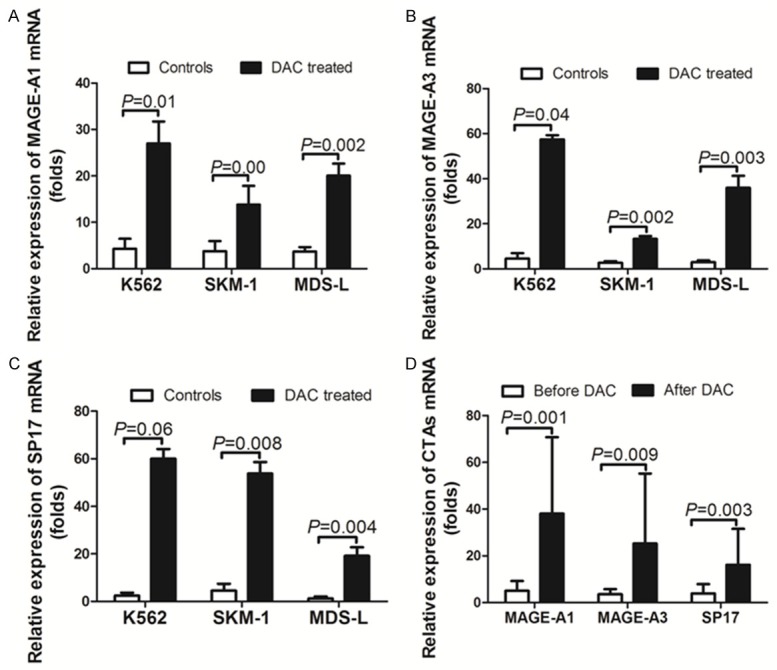
Decitabine (DAC) treatment induced the expression of MAGE-A1, MAGE-A3, and SP17 on MDS-derived cell lines and in MDS patients. MAGE-A1, MAGE-A3, and SP17 mRNA expression on K562, SKM-1, and MDS-L cell lines was analyzed using quantitative real-time PCR. Each cell line was exposed to 1 µM DAC for 5 days. The fold increase in the expression levels of each CTA in the treated cell lines was compared with the untreated control response (A-C). The expression of MAGE-A1, MAGE-A3, and SP17 increased in the MDS patients after DAC treatment (D). Individual error bars indicate the value for the standard deviation of the mean response. Abbreviations: DAC, Dacogen, product name of decitabine, Xi’an Janssen Pharmaceutical Ltd.
Figure 2.
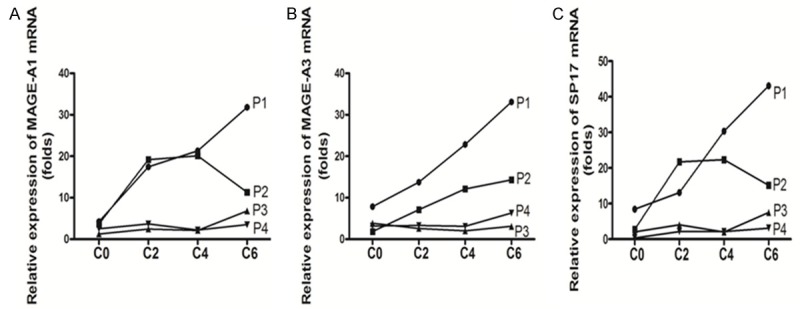
The expression of MAGE-A1, MAGE-A3, and SP17 was monitored in MDS patients receiving DAC treatment. Four patients were treated using DAC, and expression of CTAs was measured during different DAC treatment courses. P1 experienced a complete response after two courses, but disease progressed after four courses of DAC treatment. P2 experienced a persistent complete response (CR). While P3 and P4 had no treatment response, the expression of CTAs in P1 and P2 showed continuous, stable high expression. P2 experienced a disease progression after four courses of DAC treatment, which transformed into leukemia, and the expression of MAGE-A1 and SP17 declined. During DAC treatment, the CTAs in P3 and P4 showed relatively low expression. Abbreviation: P = patient, C = DAC treatment course. The fold expression of CTAs presented using the mean value of triple determination.
Western blot analysis was performed on K562, SKM-1, and MDS-L cells to further determine whether DAC treatment induced up-regulation of CTA protein (e.g., SP17 expression) (Figure 3A). The results indicated that when they were exposed to DAC, all of the tested cell lines responded with up-regulation of SP17 antigen. Along with incremental doses of DAC, the expression of SP17 showed a continuously high expression in the K562 and SKM-1 cells (Figure 3B).
Figure 3.
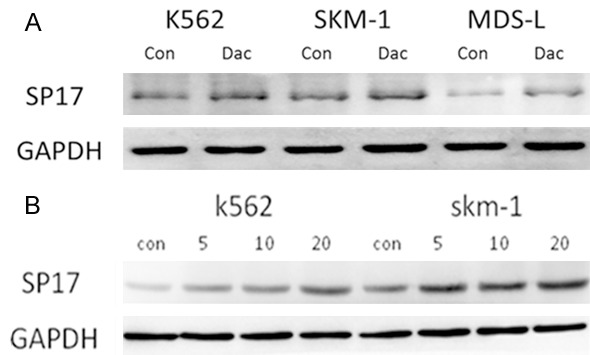
DAC treatment induced up-regulation of SP17 protein expression. Western blot analysis for SP17 expression on K562, SKM-1, and SP17 cell lines following treatment with 1 μM decitabine (DAC). DAC treatment induced up-regulation of SP17 protein expression, compared with the control cells (A). Along with incremental dose DAC treatment, expression of SP17 protein was continuously increased in the K562 and SKM-1 cell lines (B).
DAC treatment enhances tumor cell recognition by CT antigen-specific CTLs
We used a combination of MAGE-A1, MAGE-A3, and SP17 peptide mixes to generate CT antigen-specific CTLs [14]. The CTLs were generated from two healthy donors (donors 1 and 2) and from two MDS patients (patients 1 and 2). Cytotoxicity assays were performed on partially HLA-matched SKM-1 and K562 cells, with or without DAC treatment. The results for the antigen-specific cytotoxicity of these peptide-mix-stimulated CTLs are presented in Figure 4. The resulting CTLs from different donors (Figure 4A, 4B) and patients (Figure 4C, 4D) exhibited heterogeneity in antigen-specific cytotoxicity against different CTAs. CTLs from donor 1 had specific cytotoxicity against only SP17 (Figure 4A). CTLs from donor 2 had specific cytotoxicity against MAGE-A1 and SP17 (Figure 4B). CTLs from patient 1 had specific cytotoxicity against MAGE-A3, SP17, and not against MAGE-A1 (Figure 4C). CTLs from patient 2 had specific cytotoxicity against MAGE-A1, MAGE-A3, and SP17 (Figure 4D). Low-resolution HLA-typing was performed on cells from healthy donors, patients, and cell lines. CTLs from healthy donors and patients had significant cytotoxicity against partially HLA-matched SKM-1 and K562 cells following treatment with DAC, compared with the untreated controls. These results indicated that DAC enhances the recognition of tumor cells by antigen-specific CTLs.
Figure 4.
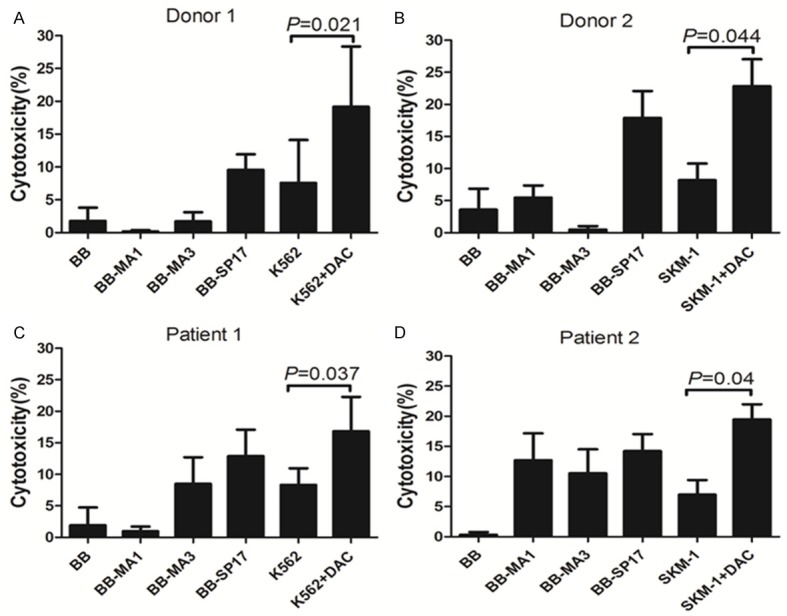
Killing of DAC-treated MDS-derived lines by CTA-specific CTLs. Peripheral blood lymphocytes from healthy adult donors and MDS patients were stimulated three times weekly using MAGE-A1, MAGE-A3, and SP17 peptide mix pulsed autologous DCs. The activated antigen-specific cells expressing CD137+ were selected and expanded using OKT3 and IL-2. The resulting CTLs were assessed for their ability to kill partially HLA-matched K562 and SKM-1 cell lines, with or without treatment with DAC. Error bars represent the values for the standard deviation of the mean value.
DAC treatment induces up-regulation of BM lymphocytes, MHC molecules, and ICAM-1 in MDS patients
The results indicated that DAC treatment increased the CD3 and CD8T lymphocyte counts (Figure 5A). Before DAC treatment, the expression levels of MHC Class I and Class II molecules, and ICAM-1 were generally low. Treatment with DAC induced increased expression of MHC Class I and Class II molecules, and ICAM-1, in MDS patients (Figure 5B-E).
Figure 5.
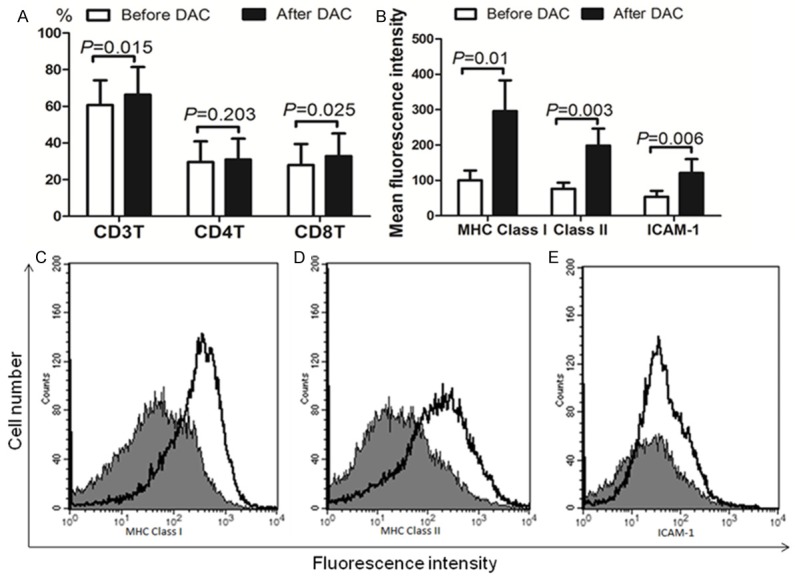
Comparison of lymphocytes, MHC molecules, and ICAM-1 before and after DAC therapy. The CD3 and CD8 T lymphocyte percentage values showed a significant increase after DAC treatment (A). The expression levels of MHC Class I, Class II, and ICAM-1 increased significantly after DAC treatment (B). Cells were analyzed using flow cytometry. Columns: mean values for mean fluorescence intensity results. (C-E) Histogram profile of each investigated antigen, stained with the specific mAb as described in (B). One representative experiment is shown before and after DAC-treated MDS patient cells. Mark 1 Before DAC treatment; □ After DAC treatment. Abbreviations: DAC, Dacogen, the product name of decitabine from Xian Janssen Pharmaceutical Ltd.
DAC treatment strengthened Th1 and Tc1 polarization in MDS patients
Subset and polarization of T lymphocytes from 17 patients were analyzed. After the DAC therapy, there was stronger polarization toward Th1 in the CD4+ subset and toward Tc1 in the CD8+ subset (P<0.05). CD3 toward I polarization was also strengthened (Figure 6).
Figure 6.
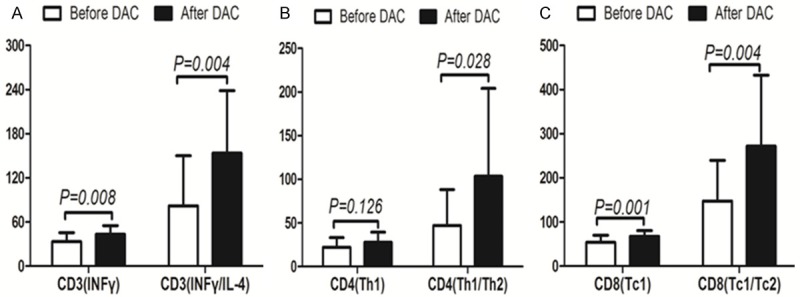
Comparison of T lymphocyte polarization status, before and after DAC therapy. The results indicated that there was stronger Th1 polarization in the CD4+ subset and Tc1 polarization in the CD8+ subset (P<0.05). CD3 toward I polarization was also strengthened.
Discussion
The success of cancer immunotherapy depends on identifying methods to increase tumor-specific antigen expression, T lymphocyte function, MHC molecules, adhesion molecules that facilitate tumor cell recognition, and addressing factors that promote development of an immune response against tumor antigens [18]. Previous studies have revealed the up-regulation of CTAs on tumors and in hematologic tumor cells after treatment with DAC [8,19,20]. A recent study found that there was complete remission following treatment with DAC and with a CT antigen-based DC vaccine in a patient with a relapsed solid tumor. After vaccination, the patient had an increased number of MAGE-A3-specific T cells, which indicated that there was cancer protein up-regulation and recognition of tumor cells by the immune system [21]. A better understanding of MDS and CTAs expression in individuals is essential for the study of these types of therapeutic options. Only a few publications describe the expression of CT antigens in hematological neoplasms [22,23].
The before-treatment level of CTAs expression in tumors, and expression in normal tissues, is an important consideration for the use of DAC in a therapeutic setting. MAGE-A1 and NY-ESO-1 are expressed on malignant tissue, but not on normal tissues (except primarily immune-privileged sites) [9,24,25]. DAC has negligible effects on the expression of CT antigens in normal tissues [25]. Before the DAC treatment, we found that MDS derived cell lines expressed low levels of CTAs. After exposure to DAC, the expression of MAGE-A1, MAGE-A3, and SP17 antigens was up-regulated on these three cell lines. Consistent with our results, MaikaAlmstedta [23] found that CTAs (e.g., NY-ESO-1, MAGEA1, MAGEA3, and MAGEB2) have increased expression in AML cell lines after exposure to DAC. An increase in the CTAs at the mRNA and protein levels in DAC-treated AML cell lines confirms that exposure to DAC is associated with up-regulation of CTAs in AML cell lines. Goodyear et al. also found that HMAs can significantly up-regulate CTAs expression [22]. Consistent with our MDS-derived cell line results, the expression levels of CTAs were also increased in MDS patients post-treatment with DAC.
Gang et al. found that HMA treatment sensitizes tumor cells to T-cell-mediated cytotoxicity and modulates NK cells in patients with myeloid malignancies [18]. They measured a transient increase in CTA-specific T cells, followed by stabilization or declining levels in peripheral blood at later cycles. No significant differences in the CD4 or CD8 T cell or the CD56+CD16+/- NK-cell populations were found between the first and late cycles in absolute counts or in the frequency of CD107a-expressing cells, upon SEB or K562 stimulation, respectively [18]. We found that peptide mixes could stimulate CD4 and CD8 T cells, and previous studies have revealed individual and synergistic roles for tumor-specific CD4 and CD8 T cells during mediation of anti-tumor immune responses [26,27]. These peptide mixes might also stimulate polarization toward Th1 of CD4-positive and TC1 of CD8-positive cells, which induces the secretion of inflammatory cytokines such as INF-γ, TNF-α, and IL-17 [8,17,28]. Above-active T-lymphocytic function strengthened the adaptive immunity to control MDS progression. These responses are some of the limitations of the use of peptide mixes; further studies are required to determine the in vivo and in vitro mechanisms of antigen-specific effector CTL-mediated killing of target cells [29].
MHC molecules and ICAM-1 have important roles in adaptive immunity [30]. ICAM-1 is a cell adhesion molecule and a co-stimulatory molecule. Up-regulation of ICAM-1 expression on tumor cells correlates with increased binding of T cells and tumor cell lysis [31]. Fonsatti et al. correlated the up-regulation of MHC Class I and ICAM-1 expression and improved CTL recognition of DAC-treated melanoma cells [32]. Here, we provide the first evidence that treatment with DAC can lead to up-regulation of MHC molecules and ICAM-1 expression on MDS patient cells. DAC enhances the expression of CTAs, T function, MHC Class I/II, and ICAM-1 molecules. It therefore has the potential to increase the susceptibility of MDS cells to CTL-mediated lysis.
To examine this function, MAGE-A1, MAGE-A3, and SP17-specific CTLs were generated using overlapping peptide mixes and tested against partially matched K562 and SKM-1 cells. The preferential killing of K562 and SKM-1 cells after exposure to DAC indicated that DAC treatment improved tumor cell recognition and suggested it could be used in combination with immunotherapy for MDS treatment. The precursor frequencies of CT antigen-specific cells are too low in healthy individuals. These cells require multiple rounds of stimulation, followed by selection and expansion, to be able to detect an effect in vitro. MDS patients are presumed to have higher numbers of CTA-specific cells in the peripheral blood, but several rounds of chemotherapy and irradiation challenge immune system efficiency. These characteristics could contribute to the low levels of killing that occurred in our cytotoxicity assays.
This is the first study to reveal the killing of MDS cells by in vitro generated tumor-specific CTLs, after exposure to DAC. We exploited peptide mixes derived from full-length MAGE-A1, MAGE-A3, and SP17 proteins to generate multi-antigen-specific CTLs with reactivity against multiple CTA epitopes, in the context of multiple HLA alleles. Depending upon a patient’s HLA background, a mixture of antigenic peptides may contain epitopes relevant to multiple or even no HLA alleles for that individual patient. Therefore, although a CTL matches with its target at a particular allele, it might not represent the immune-dominant epitope for that particular donor. The alleles also might not be an exact match if low-resolution HLA typing is an issue. These factors make the partial matching of HLA haplotypes less significant for the interpretation of our cytotoxicity assay results. Further studies are required to determine the mechanisms of CTL-mediated killing of target cells.
In conclusion, our study revealed that DAC treatment up-regulated the expression of MAGE-A1, MAGE-A3, and SP17 on MDS-derived cell lines and in MDS patients. These CTAs maintained high expression levels, which contributed to activated adaptive immunity after cessation of treatment. The up-regulation of MHC class I/II and ICAM-1 molecules together with enhanced T function killing of DAC-treated cells suggests that DAC combined with immunotherapy could be used for the treatment of MDS.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 81400091).
Disclosure of conflict of interest
None.
Authors’ contribution
ZZ, CKC and XL conceived and designed the experiments. QH, YT, JG, and FX performed the experiments. LYW and YSZ analyzed the data. DW, LYZ, JYS, LXS, and CX contributed reagents, materials, and analysis tools. ZZ and CKC wrote the paper.
References
- 1.Gangat N, Patnaik MM, Tefferi A. Myelodysplastic syndromes: contemporary review and how we treat. Am J Hematol. 2016;91:76–89. doi: 10.1002/ajh.24253. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg PL, Stone RM, Bejar R, Bennett JM, Bloomfield CD, Borate U, De Castro CM, Deeg HJ, DeZern AE, Fathi AT, Frankfurt O, Gaensler K, Garcia-Manero G, Griffiths EA, Head D, Klimek V, Komrokji R, Kujawski LA, Maness LJ, O’Donnell MR, Pollyea DA, Scott B, Shami PJ, Stein BL, Westervelt P, Wheeler B, Shead DA, Smith C National comprehensive cancer network. Myelodysplastic syndromes, version 2.2015. J Natl Compr Canc Netw. 2015;13:261–272. doi: 10.6004/jnccn.2015.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverman LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach CL, Larson RA Cancer and Leukemia Group B. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the cancer and leukemia Group B. J. Clin. Oncol. 2006;24:3895–3903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- 4.Fenaux P, Mufti GJ, Hellström-Lindberg E, Santini V, Gattermann N, Germing U, Sanz G, List AF, Gore S, Seymour JF, Dombret H, Backstrom J, Zimmerman L, McKenzie D, Beach CL, Silverman LR. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J. Clin. Oncol. 2010;28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 5.Adès L, Sekeres MA, Wolfromm A, Teichman ML, Tiu RV, Itzykson R, Maciejewski JP, Dreyfus F, List AF, Fenaux P, Komrokji RS. Predictive factors of response and survival among chronic myelomonocytic leukemia patients treated with azacitidine. Leuk Res. 2013;37:609–613. doi: 10.1016/j.leukres.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Mei Q, Nie J, Fu X, Han W. Decitabine: a promising epi-immunotherapeutic agent in solid tumors. Expert Rev Clin Immunol. 2015;11:363–375. doi: 10.1586/1744666X.2015.1002397. [DOI] [PubMed] [Google Scholar]
- 8.Chou J, Voong LN, Mortales CL, Towlerton AM, Pollack SM, Chen X, Yee C, Robbins PF, Warren EH. Epigenetic modulation to enable antigen-specific T-cell therapy of colorectal cancer. J Immunother. 2012;35:131–141. doi: 10.1097/CJI.0b013e31824300c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinnasamy N, Wargo JA, Yu Z, Rao M, Frankel TL, Riley JP, Hong JJ, Parkhurst MR, Feldman SA, Schrump DS, Restifo NP, Robbins PF, Rosenberg SA, Morgan RA. A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J Immunol. 2011;186:685–696. doi: 10.4049/jimmunol.1001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pellat-Deceunynck C, Mellerin MP, Labarrière N, Jego G, Moreau-Aubry A, Harousseau JL, Jotereau F, Bataille R. The cancer germ-line genes MAGE-1, MAGE-3 and PRAME are commonly expressed by human myeloma cells. Eur J Immunol. 2000;30:803–809. doi: 10.1002/1521-4141(200003)30:3<803::AID-IMMU803>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 11.Tohyama K, Tohyama Y, Nakayama T, Ueda T, Nakamura T, Yoshida Y. A novel factor-dependent human myelodysplastic cell line, MDS92, contains haemopoietic cells of several lineages. Br J Haematol. 1995;91:795–799. doi: 10.1111/j.1365-2141.1995.tb05391.x. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa T, Matozaki S. The SKM-1 leukemic cell line established from a patient with progression to myelomonocytic leukemia in myelodysplastic syndrome (MDS)-contribution to better understanding of MDS. Leuk Lymphoma. 1995;17:335–339. doi: 10.3109/10428199509056841. [DOI] [PubMed] [Google Scholar]
- 13.Blum W, Klisovic RB, Hackanson B, Liu Z, Liu S, Devine H, Vukosavljevic T, Huynh L, Lozanski G, Kefauver C, Plass C, Devine SM, Heerema NA, Murgo A, Chan KK, Grever MR, Byrd JC, Marcucci G. Phase I study of decitabine alone or in combination with valproic acid in acute myeloid leukemia. J. Clin. Oncol. 2007;25:3884–3891. doi: 10.1200/JCO.2006.09.4169. [DOI] [PubMed] [Google Scholar]
- 14.Bao L, Dunham K, Lucas K. MAGE-A1, MAGE-A3, and NY-ESO-1 can be upregulated on neuroblastoma cells to facilitate cytotoxic T lymphocyte-mediated tumor cell killing. Cancer Immunol Immunother. 2011;60:1299–1307. doi: 10.1007/s00262-011-1037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnadas DK, Stamer MM, Dunham K, Bao L, Lucas KG. Wilms’tumor 1-specific cytotoxic T lymphocytes can be expanded from adult donors and cord blood. Leuk Res. 2011;35:1520–1526. doi: 10.1016/j.leukres.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 16.Schrump DS, Fischette MR, Nguyen DM, Zhao M, Li X, Kunst TF, Hancox A, Hong JA, Chen GA, Pishchik V, Figg WD, Murgo AJ, Steinberg SM. Phase I study of decitabine-mediated gene expression in patients with cancers involving the lungs, esophagus, or pleura. Clin Cancer Res. 2006;12:5777–5785. doi: 10.1158/1078-0432.CCR-06-0669. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Xiao ZJ, Chang CK, Xu F, Wu LY, He Q, Xu ZF, Song LX, Zhang Z, Zhou LY, Su JY, Zhang X, Guo J. Distinct clinical and experimental characteristics in the patients younger than 60 years old with myelodysplastic syndromes. PLoS One. 2013;8:e57392. doi: 10.1371/journal.pone.0057392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gang AO, Frøsig TM, Brimnes MK, Lyngaa R, Treppendahl MB, Grønbæk K, Dufva IH, Straten PT, Hadrup SR. 5-Azacytidine treatment sensitizes tumor cells to T-cell mediated cytotoxicity and modulates NK cells in patients with myeloid malignancies. Blood Cancer J. 2014;4:e197. doi: 10.1038/bcj.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srivastava P, Paluch BE, Matsuzaki J, James SR, Collamat-Lai G, Blagitko-Dorfs N, Ford LA, Naqash R, Lübbert M, Karpf AR, Nemeth MJ, Griffiths EA. Induction of cancer testis antigen expression in circulating acute myeloid leukemia blasts following hypomethylating agent monotherapy. Oncotarget. 2016;7:12840–12856. doi: 10.18632/oncotarget.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li B, Zhu X, Sun L, Yuan L, Zhang J, Li H, Ye Z. Induction of a specific CD8+ T-cell response to cancer/testis antigens by demethylating pre-treatment against osteosarcoma. Oncotarget. 2014;5:10791–10802. doi: 10.18632/oncotarget.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnadas DK, Shapiro T, Lucas K. Complete remission following decitabine/dendritic cell vaccine for relapsed neuroblastoma. Pediatrics. 2013;131:e336–341. doi: 10.1542/peds.2012-0376. [DOI] [PubMed] [Google Scholar]
- 22.Goodyear O, Agathanggelou A, Novitzky-Basso I, Siddique S, McSkeane T, Ryan G, Vyas P, Cavenagh J, Stankovic T, Moss P, Craddock C. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood. 2010;116:1908–1918. doi: 10.1182/blood-2009-11-249474. [DOI] [PubMed] [Google Scholar]
- 23.Almstedt M, Blagitko-Dorfs N, Duque-Afonso J, Karbach J, Pfeifer D, Jäger E, Lübbert M. The DNA demethylating agent 5-aza-2’-deoxycytidine induces expression of NY-ESO-1 and other cancer/testis antigens in myeloid leukemia cells. Leuk Res. 2010;34:899–905. doi: 10.1016/j.leukres.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Jungbluth AA, Chen YT, Stockert E, Busam KJ, Kolb D, Iversen K, Coplan K, Williamson B, Altorki N, Old LJ. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 25.Ries J, Schultze-Mosgau S, Neukam F, Diebel E, Wiltfang J. Investigation of the expression of melanoma antigen-encoding genes (MAGE-A1 to -A6) in oral squamous cell carcinomas to determine potential targets for gene-based cancer immunotherapy. Int J Oncol. 2005;26:817–824. [PubMed] [Google Scholar]
- 26.Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Liénard D, Lejeune F, Rimoldi D, Guillaume P, Meidenbauer N, Mackensen A, Rufer N, Lubenow N, Speiser D, Cerottini JC, Romero P, Pittet MJ. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64:2865–2873. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- 27.Huang H, Li F, Gordon JR, Xiang J. Synergistic enhancement of antitumor immunity with adoptively transferred tumor-specific CD4+ and CD8+ T cells and intratumoral lymphotactin transgene expression. Cancer Res. 2002;62:2043–2051. [PubMed] [Google Scholar]
- 28.Zhang Z, Li X, Guo J, Xu F, He Q, Zhao Y, Yang Y, Gu S, Zhang Y, Wu L, Chang C. Interleukin-17 enhances the production of interferon-γ and tumour necrosis factor-α by bone marrow T lymphocytes from patients with lower risk myelodysplastic syndromes. Eur J Haematol. 2013;90:375–384. doi: 10.1111/ejh.12074. [DOI] [PubMed] [Google Scholar]
- 29.Xiao L, Qi Z, Qiusheng C, Li X, Luxi S, Lingyun W. The use of selective immunosuppressive therapy on myelodysplastic syndromes in targeted populations results in good response rates and avoids treatment-related disease progression. Am J Hematol. 2012;87:26–31. doi: 10.1002/ajh.22184. [DOI] [PubMed] [Google Scholar]
- 30.Krishnadas DK, Bao L, Bai F, Chencheri SC, Lucas K. Decitabine facilitates immune recognition of sarcoma cells by upregulating CT antigens, MHC molecules, and ICAM-1. Tumour Biol. 2014;35:5753–5762. doi: 10.1007/s13277-014-1764-9. [DOI] [PubMed] [Google Scholar]
- 31.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–7994. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 32.Fonsatti E, Nicolay HJ, Sigalotti L, Calabrò L, Pezzani L, Colizzi F, Altomonte M, Guidoboni M, Marincola FM, Maio M. Functional up-regulation of human leukocyte antigen class I antigens expression by 5-aza-2’-deoxycytidine in cutaneous melanoma: immunotherapeutic implications. Clin Cancer Res. 2007;13:3333–3338. doi: 10.1158/1078-0432.CCR-06-3091. [DOI] [PubMed] [Google Scholar]


