Abstract
Salivary adenoid cystic carcinoma (AdCC) is a common head and neck cancer with the propensity for local spread and distant metastasis. In our previous study, elevated expression of Anterior gradient 2 (AGR2) was detected in head and neck squamous cell carcinoma (HNSCC), associated with epithelial-mesenchymal transition (EMT) and cancer stemness. However, to date, the expression and function of AGR2 in AdCC has yet to be elucidated. In the present study, human AdCC tissue microarrays including 18 cases of normal salivary gland (NSG), 12 cases of pleomorphic adenoma (PMA) and 72 cases of AdCC were employed for immunohistochemical staining analysis. Results indicated that AGR2, which was remarkably correlated with Ki-67, transforming growth factor beta-1 (TGF-β1) and CD147, was significantly elevated in human salivary AdCC tissues. Knockdown of AGR2 significantly repressed the proliferation and migration of human SACC-83 and SACC-LM cell lines. Additionally, AGR2 silencing obviously reversed the EMT phenomena induced by TGF-β1. Taken together, our present study revealed the potential pro-metastasis role of AGR2 in AdCC, indicating that AGR2 might be a novel therapeutic target of AdCC with distant metastasis.
Keywords: Anterior gradient 2, Adenoid cystic carcinoma, proliferation, migration, epithelial-mesenchymal transition
Introduction
Salivary adenoid cystic carcinoma (AdCC) is a common malignancy arises in the major and minor salivary glands [1], with the propensity of insidious local spread, neural invasion, abundant angiogenesis and distant metastasis [1]. Morphologically, AdCC are distinguished in three major histology types: tubular, cribriform, and solid. Even though variations in clinical presentation of the three types have been widely reported, all three patterns are neuroinvasive, and show an extremely high tendency of recurrence. Current conventional treatment options for AdCC are limited to surgery with or without radiotherapy, ignoring an insight into the underlying molecular drivers [2]. Therefore, a better understanding of the mechanisms of initiation and progression of AdCC is urgently needed.
Anterior gradient 2 (AGR2), the human homolog of Xenopus laevis-secreted protein XAG-2, belongs to the protein disulfide isomerase (PDI) family [3]. Generally, AGR2 is physiologically localized in endoplasmic reticulum of the mucus secreting cells and functions as a molecular chaperone in protein folding [4]. Notably, emerging evidences suggested that AGR2 expression was closely associated to hormone-depended cancers. Clinical studies have shown that elevated expression of AGR2 predicted an unfavorable prognosis in breast cancer patients and mediated tamoxifen drug resistance as an estrogen agonist [5,6]. Analogous to breast cancer, AGR2 expression in prostate cancers has been reported to be induced by androgens, promoting the propensity of metastasis [7,8]. In addition to hormone-depended cancers, overexpression of AGR2 also has been identified in variety of hormone-independent cancers including esophagus cancer, liver cancer, colorectal cancer, lung cancer, as reviewed by Chevet E et al. in Ref [9]. Abnormal expression of AGR2 protein was identified by a proteomics screen aiming to access factors that is able to suppress the tumor suppressive p53 activity in Barrett’s esophagus, a pre-neoplastic condition of esophagus cancer [10]. Additionally, AGR2 was also reported exerting functions of supporting aggressive growth and metastasis of numerous cancers in distinct mechanism [11-13]. These findings highlighted the critical functions of AGR2 in carcinogenesis and progression of these cancers. However, to date, the expression and functions of AGR2 in salivary AdCC is remaining to be investigated.
Epithelial-mesenchymal transition (EMT) is a common event during cancer progression [14]. In this process, epithelial cells rapidly lose epithelial characteristics and gain mesenchymal characteristics, transforming to a more invasive phenotype with stronger motility. EMT provides cancer cells with the ability to escape from the primary tumor and cause lymph node or distant metastasis [15]. Although the full spectrum of signaling pathways that contribute to EMT remained unclear, transforming growth factor-beta 1 (TGF-β1) was suggested as a master regulator for the induction of EMT by multiple studies [16]. In the case of many cancers, TGF-β1 appeared to be responsible for a series of EMT-inducing transcription factors, notably Slug, Snail, ZEB1 and Twist [17-20]. In our previous study, overexpression of AGR2 was found in human head and neck squamous cell carcinoma (HNSCC) patient samples and significantly correlated with EMT markers [21]. This finding suggested the potential role of AGR2 in regulating EMT process during cancer progression. Nevertheless, the relationship between AGR2 and EMT in salivary AdCC is still unclear and need to be further determined.
In the present study, AGR2 expression, for the first time, was identified via immunohistochemical staining on human salivary AdCC tissues. The relationship between AGR2 and TGF-β1 in salivary AdCC tissues was elucidated. Knock-down of AGR2 by specific shRNA significantly repressed the proliferation and migration of human salivary AdCC cell lines. Furthermore, TGF-β1 induced EMT was significantly inhibited by AGR2 silencing. In conclusion, these data revealed the potential therapeutic role of AGR2 in salivary AdCC with the tendency of distant metastasis.
Material and methods
Ethics statement and AdCC tissue microarrays
The present study was performed under the permission of the Medical Ethics of Hospital of Stomatology Wuhan University (PI: Zhi-Jun Sun). Each patient was informed consent before surgery. The specimens, which contain 18 cases of normal salivary gland (NSG), 12 cases of pleomorphic adenoma (PMA) and 72 cases of salivary adenoid cystic carcinoma (AdCC, 28 cribriform pattern, 24 tubular pattern, 20 solid pattern), were diagnosed by two expert pathologists. The custom made human AdCC tissue microarrays used in this study were described previously [22].
Histology and immunohistochemistry
Antibodies against human AGR2 (CST, 1:100), Ki-67 (Abcam, 1:50), TGF-β1 (Proteintech, 1:50), CD147 (Abcam, 1:200), were used as primary antibodies. The AdCC tissue microarrays were stained with the antibodies by immunohistochemistry (IHC) using an appropriate biotin-conjugated, secondary antibody and a Maxin SP kit (Vector Laboratories), as previously reported [23]. Slides were scanned by an Aperio ScanScope CS scanner. The scanned image data was then processed with background subtraction and white balance. IHC staining of the membrane, nuclei and pixel was quantified by Aperio quantitative software Version 12.1. Histoscores were calculated as previously described [24]. Briefly, scanned high power field of each sample, membrane and nuclear immunostaining were quantified by the algorithm: (1 × the percentage of weakly positive staining) + (2 × the percentage of moderately positive staining) + (3 × the percentage of strongly positive staining). Quantification of pixel intensity was calculated as total intensity/total cell number. The threshold for scanning of different positive cells was set by a pathologist according to the standard controls provided by Aperio.
Cell culture
Human salivary gland AdCC cell lines SACC-83 and SACC-LM were obtained from the School and Hospital of Stomatology, Peking University with material transfer agreement. Cells were maintained in PRMI-1640 (Hyclone) with 10% FBS (fetal bovine serum, Hyclone) under the condition of 37°C in a humidified atmosphere with 5% CO2.
Establishment of AGR2-silenced cell line
Two shRNA sequences (shAGR2#1 and shAGR2#2) targeting AGR2 and negative shRNA were designed and synthesized by Genepharma (Shanghai, China). In order to construct human AGR2 shRNA plasmids, the sequences were inserted into the BamHI/EcoRI restriction sites of pGLVU6/Puro lentivectors. Then, lentiviral expression vectors and packaging plasmids were co-transduced into 293T cells. Viral particles were collected to infected SACC-83 and SACC-LM cells. Puromycin (2 μg/ml, R&D system) was used to select the infected cells.
Cell proliferation assay
The proliferation ability of SACC-83 and SACC-LM cells transfected with negative control shRNA or AGR2 shRNA was assessed by using CCK-8 kit (Dojindo Molecular Technologies) as previously described [22]. Briefly, 5 × 103 cells were seeded into 96-well plates cultured for appropriate time. Then 10 μl CCK-8 solution was added to each well and incubated for 1 h. Absorbance at 450 nm was measured using a microplate reader. All experiments were repeated in triplicate.
Anchorage-dependent colony formation assay
Appropriate number (300 cells/well) of cells which were transfected with negative control shRNA or AGR2 shRNA were re-suspended in PRMI-1640 with 10% FBS and then seeded in flat-bottomed 12-well culture plates. After 3 weeks, the colonies were fixed by 4% paraformaldehyde and stained by crystal violet (Sigma-Aldrich). The numbers of colonies were calculated. Each assay was performed in triplicate.
AdCC xenograft study
Animal study in this study was conducted in accordance with NIH guidelines for the Care and Use of Laboratory Animals and approved by Animal Care and Use Committee of Wuhan University. Female nude mice (6-8 weeks of age) were purchased from SLAC Laboratory Animal Center (Hunan, China). Mice were housed in sterile filter-capped cages with an inverse 12 h day-12 h night cycle at 22 ± 1°C and 55 ± 5% humidity. For AdCC xenograft, the nude mice were divided into two groups (n = 5, each group) randomly. About 4 × 106 SACC-LM cells transfected with negative shRNA (NC) or AGR2 shRNA (shAGR2) in 0.2 ml of serum-free medium were injected subcutaneously into the right flank of nude mice. Tumor growth was determined by measuring the volume of the tumor with a caliper every other day followed the formula (width2 × length)/2. The mice were euthanized using CO2 at the experimental endpoint.
Cell migration assays
Cell migration assays were performed with 0.8 μm pore size transwell inserts (Corning Life Sciences). Briefly, certain amount (1 × 105) cells transfected with negative shRNA (NC) or AGR2 shRNA were seeded in the upper chamber and cultured in serum-free culture medium. 500 μl culture medium supplemented with 10% FBS was added to lower wells as chemoattractant. After 24 hours’ incubation, cells were fixed by 4% paraformaldehyde and stained by crystal violet. Non-migrating cells on the upper surface of the membrane were scrubbed off. Migratory cells were photographed at a constant magnification (20 ×) and cell numbers were counted with Image-Pro Plus. Each assay was performed in triplicate.
Western blot analysis
Harvested cells were lysed in RIPA (Beyotime) containing a complete mini-protease inhibitor cocktail and phosphate inhibitors (Roche, Branchburg, NJ). Antibodies against human AGR2 (CST, 1:1000), Survivin (CST, 1:1000), Cyclin D1 (Abcam, 1:1000), CD147 (Abcam, 1:1000), E-cadherin (CST, 1:1000), N-cadherin (CST, 1:1000), Slug (CST, 1:1000) were used as primary antibodies. β-actin was used as loading control. Detailed procedures for immunoblotting performed were as described previously [21]. Each assay was performed in triplicate.
Real time qPCR analysis
Total RNA was extracted using Trizol (Invitrogen, Life Technologies). Reverse transcription was primed with Oligo (dT) using a PrimeScriptTM RT reagent Kit with gDNA Eraser kit (Takara Bio). Real-time quantitative PCR were performed with SYBR Green I (Takara Bio) on a QuantStudio 6 Flex System (Thermo Fisher Scientific). Relative expressions were normalized for GAPDH. Melting curves were examined to ensure specific amplification. The sequences of primers for AGR2, CD147, E-cadherin, N-cadherin, Slug, Snail, Survivin and Cyclin D1 were shown in Supplementary Table 1.
Immunofluorescence and confocal microscopy
Cells were cultured on 10 mm glass-bottom dish (Nest Biotechnology), fixed with 4% paraformaldehyde and permeabilized in 0.1% Triton X-100. After that cells were washed with PBS and blocked with 5% bovine serum albumin (BSA). The cells were incubated with primary antibody (E-cadherin and N-cadherin, 1:200, CST) and secondary antibody. Coverslips were mounted with mounting medium (Vector Laboratories) containing diamidino-2-phenylindole (DAPI) and photographed under a laser scanning confocal microscope (Olympus).
Statistical analysis
All statistical analyses, including unpaired t test and one-way ANOVA followed by the post-Tukey test, were carried out using Graph-Pad Prism version 5.00 for windows (Graph-Pad Software Inc.). Unpaired t test was used to compare the differences of colony numbers, tumor volumes and migrated cells between the negative control group and shAGR2 group. The One-way ANOVA followed by the post-Tukey test was used to analyze the differences in immunohistochemical staining. The data were presented as the Mean ± SEM, and statistical significance was determined as P<0.05.
Results
Overexpression of AGR2 in human salivary adenoid cystic carcinoma tissue microarray
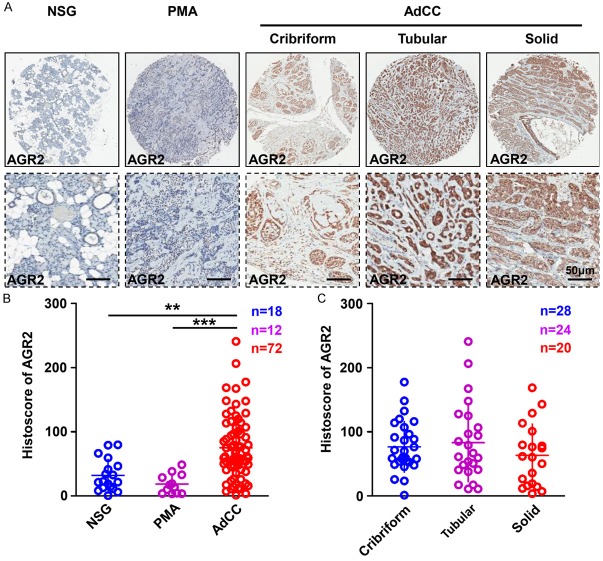
Overexpression of AGR2 was reported in variety of human cancers [9]. In this study, to explore the expression pattern of AGR2 in human salivary AdCC tissues, human AdCC tissue microarray, which was constructed by 18 cases of normal salivary glands (NSG), 12 cases of pleomorphic adenoma (PMA) as well as 72 cases of AdCC was employed for immunohistochemical (IHC) staining. Representative AGR2 IHC staining photos of NSG, PMA and three distinct histology types (tubular, cribriform, and solid) of AdCC were shown in Figure 1A. AGR2 was mostly located in the cytoplasm of the tumor cells. The expression of AGR2 (Histoscore) was significantly up-regulated in AdCC tissues as compared with NSG or PMA (Figure 1B). Additionally, there was no significant difference in the histoscore of AGR2 in three different histology types of AdCC (Figure 1C). These results emphasized the potential oncogenic role of AGR2 in AdCC.
Figure 1.
Overexpression of AGR2 in human salivary adenoid cystic carcinoma tissue microarray. A. Representative AGR2 IHC staining images of normal salivary gland (NSG), pleomorphic adenoma (PMA) and salivary adenoid cystic carcinoma (AdCC), scale bar = 50 μm. B. Quantification of AGR2 histoscore in NSG, PMA and AdCC, AGR2 was significantly increased in AdCC (Mean ± SEM, **P<0.01, ***P<0.001, one way ANOVA post tukey test). C. Quantification of AGR2 histoscore in cribriform, tubular and solid AdCC, There is no significant difference in the histoscore of AGR2 among the three types of AdCC (Mean ± SEM, one way ANOVA post tukey test).
Overexpression of AGR2 is correlated with Ki-67, TGF-β1 and CD147 in human salivary AdCC tissue microarray
Considering the pro-metastasis and pro-survival functions of AGR2 in variety of human solid tumors, herein, we discussed the correlation of AGR2, Ki-67 (proliferation marker), TGF-β1 (EMT inducer) and CD147 (invasion marker) in AdCC tissues. Representative IHC photos of AGR2, TGF-β1, and Ki-67 in NSG or AdCC were displayed respectively in Figure 2A. Ki-67 was mainly located at the nuclears of AdCC tumor cells. TGF-β1 was mostly located at the cytoplasm of both AdCC tumor cells and stroma cells. Meanwhile, CD147 was mostly located at the membrane of AdCC tumor cells. Pearson’s statistics results indicated that overexpression of AGR2 was significantly correlated with Ki-67 (P<0.01, r = 0.2660), TGF-β1 (P<0.01, r = 0.3058) and CD147 (P<0.05, r = 0.2055) in NSG, PMA, and AdCC (Figure 2B). A further hierarchical clustering analysis was used to determine the relationship of AGR2, Ki-67, TGF-β1 and CD147 (Figure 2C). On the basis of these results, we hypothesized that AGR2 was probably involved in AdCC tumor cell survival, and conferred to EMT progression through combination with TGF-β1, leading a further metastasis.
Figure 2.
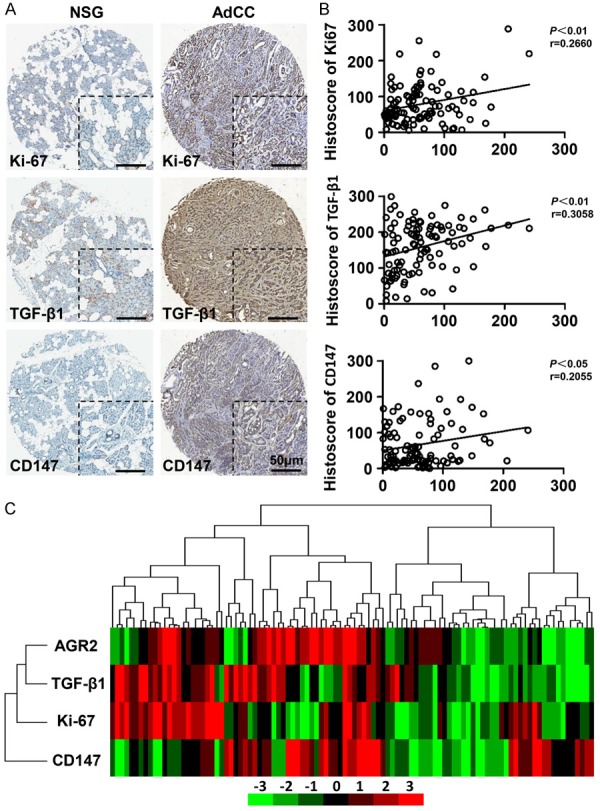
Overexpression of AGR2 is correlated with Ki-67, TGF-β1 and CD147 in human salivary AdCC tissue microarray. A. Representative photos of Ki-67, TGF-β1 and CD147 of NSG or AdCC tissue (scale bar = 50 μm). B. Two-tailed Pearson’s statistics showed the significant correlation of AGR2 with Ki-67, TGF-β1 and CD147. C. Hierarchical clustering of AGR2, Ki-67, TGF-β1 and CD147 histoscore results in human AdCC tissue microarrays.
Knockdown of AGR2 represses cell proliferation of AdCC cell lines in vitro and in vivo
In order to assess the functions of AGR2 in AdCC, we selected two AdCC cell lines (SACC-83 and SACC-LM) for functional study. Through employing specific shRNA targeting AGR2 (shAGR2#1 and shAGR2#2), the mRNA level and protein level of AGR2 were significantly reduced (Supplementary Figure 1A and 1B). A followed cell viability assay showed that knockdown of AGR2 significantly repressed the proliferation of both SACC-83 and SACC-LM cell lines (Figure 3A). Furthermore, inhibition of AGR2 significantly reduced the colony numbers of SACC-83 cells and SACC-LM cells (Figure 3B). Based on these in vitro findings, we investigated whether or not AGR2 silencing can repress the cell proliferation in vivo. Xenograft tumor model was established by the subcutaneous injection of SACC-LM cells transfected with negative control shRNA (NC) or AGR2 shRNA (shAGR2) into nude mice (n = 5 each group). AGR2 silencing caused a significant repression on the proliferation of SACC-LM cells in vivo at Day 17 (Figure 3C and 3D). In addition, AGR2 knockdown caused an obvious reduction of Survivin and Cyclin D1 on both protein and mRNA level (Figure 3E and 3F). These results indicated the pro-survival role of AGR2 in AdCC cell lines.
Figure 3.
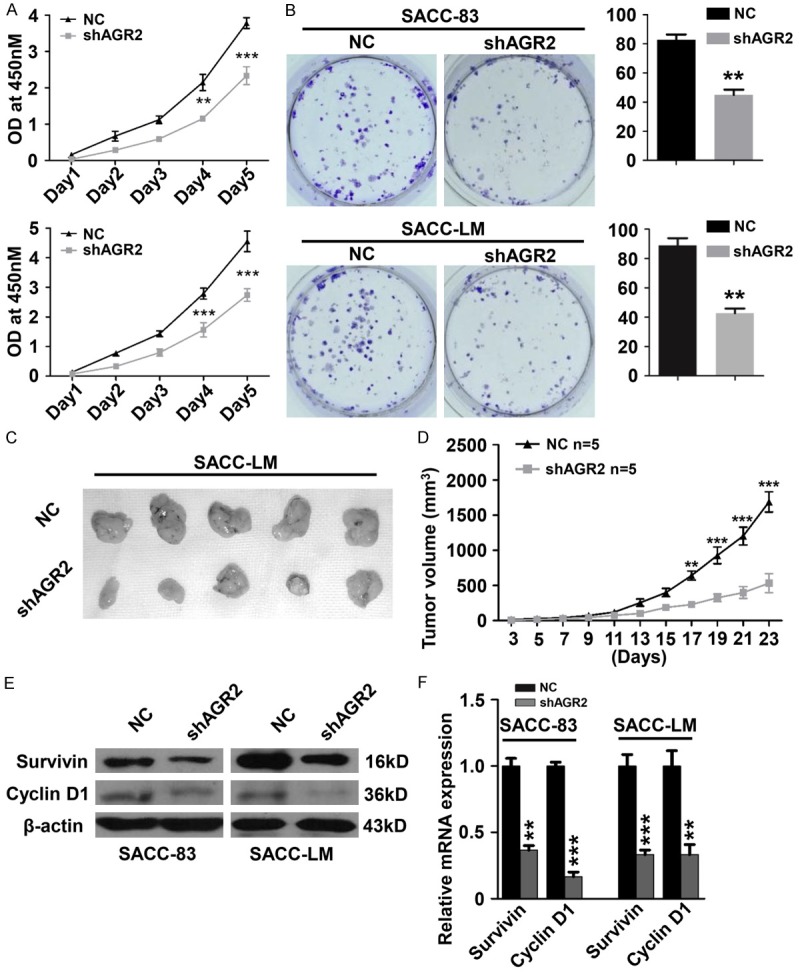
Knockdown of AGR2 represses cell proliferation of AdCC cell lines in vitro and in vivo. A. Cell viability assays on SACC-83 and SACC-LM cells. Knockdown of AGR2 significantly repressed the proliferation of SACC-83 and SACC-LM cells on Day4 and 5 (Mean ± SEM, **P<0.01, ***P<0.001). B. Anchorage-dependent colony formation assay of SACC-83 and SACC-LM cells. Knockdown of AGR2 significantly attenuated the colony formation ability of both SACC-83 cells and SACC-LM cells (Mean ± SEM, **P<0.01, unpaired t test). C. Representative images of the tumors of negative control group (NC, n = 5) and AGR2 shRNA group (shAGR2, n = 5). D. Tumor size of NC group and shAGR2 group was assessed (Mean ± SEM, **P<0.01, ***P<0.001 unpaired t test). E. Western blotting indicated that knockdown of AGR2 reduced the protein level of Survivin and Cyclin D1 in SACC-83 cells and SACC-LM cells. The β-actin was used as loading control. F. Knockdown of AGR2 significantly reduced the mRNA levels of Survivin and Cyclin D1 in SACC-83 cells and SACC-LM cells (Mean ± SEM, **P<0.01, ***P<0.001, unpaired t test).
Knockdown of AGR2 inhibits cell migration of AdCC cell lines
AGR2 was previously identified as a pro-metastasis protein in variety kinds of cancers [11-13]. Here in, we detected both protein level and mRNA level of AGR2 in SACC-83 cells and SACC-LM cells. SACC-LM, with a high potential of metastasis, is derived from the lung metastatic focus of SACC-83 cells in nude mice [25]. Obviously, the protein and mRNA expression of AGR2 and CD147 were significantly elevated in SACC-LM cells as compared with the parental cells SACC-83 (Figure 4A and 4B). These findings indicated that AGR2 was probably involved in the process of metastasis of AdCC cells. To further determine the function of AGR2 in cell migration of AdCC cells, transwell migration assays were performed. As shown in Figure 4C, knockdown of AGR2 by shRNA caused a significant decrease of migration of both SACC-83 cells and SACC-LM cells. Based on the findings above, we suggested that AGR2 expression is necessary during the process of migration of AdCC cells.
Figure 4.
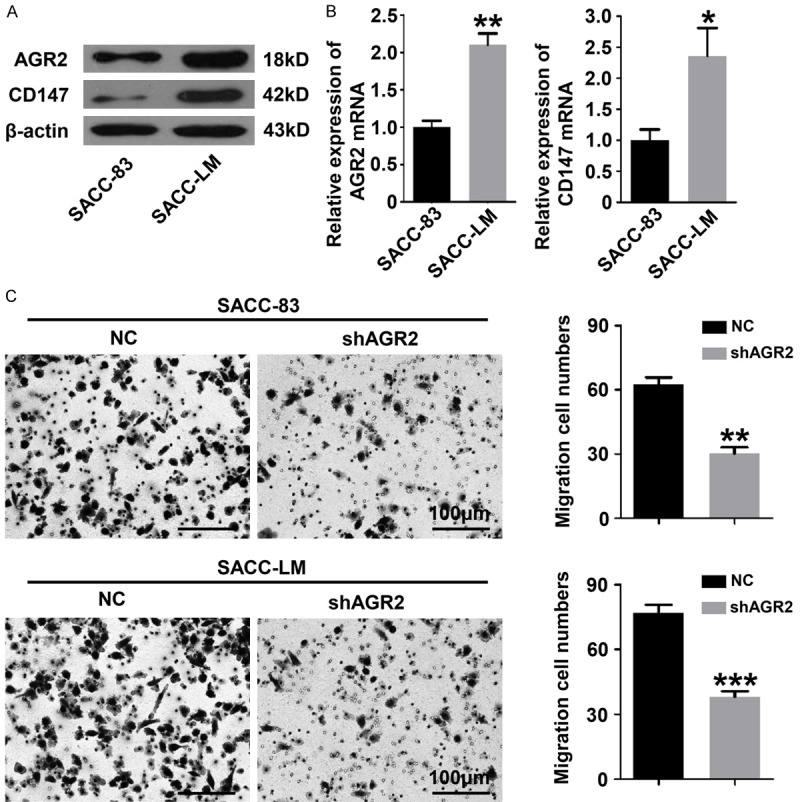
Knockdown of AGR2 inhibits cell migration of AdCC cell lines. A. Western blotting showed that the protein levels of AGR2 and CD147 in SACC-LM cells were higher. The β-actin was used as loading control. B. The mRNA levels of AGR2 and CD147 were significantly increased in SACC-LM cells (Mean ± SEM, *P<0.05, **P<0.01, unpaired t test). C. Transwell migration assays revealed that knockdown of AGR2 significantly inhibited the migration ability of SACC-83 and SACC-LM cells (Mean ± SEM, **P<0.01, ***P< 0.001, unpaired t test, scale bar = 100 μm).
Knockdown of AGR2 reverses the EMT phenomenon induced by TGF-β1
TGF-β1 was suggested as a potent regulator of EMT during cancer progression [16]. Additionally, exogenous addition of TGF-β1 significantly promoted the invasion and migration of AdCC cell lines [25]. Herein, we discussed the role of AGR2 during the process of TGF-β1 induced EMT. Representative immunofluorescence photos of SACC-LM cells were shown in Figure 5A. Knockdown of AGR2 by shRNA increased the activity of E-cadherin, which was reduced in the presence of exogenous TGF-β1. Meanwhile, the increased expression of N-cadherin caused by TGF-β1 was reversed by AGR2 knockdown. Additionally, in SACC-83 cells and SACC-LM cells, the protein levels of E-cadherin, N-cadherin, Slug and CD147 were significantly reduced by AGR2 knockdown in the presence of TGF-β1 (Figure 5B). The followed qRT-PCR assays also identified that knockdown of AGR2 significantly reduced the mRNA level of Slug, Snail and CD147 in the presence of TGF-β1 (Figure 5C). Furthermore, inhibition of AGR2 significantly repressed the enhanced migration of SACC-83 cells and SACC-LM cells which was caused by TGF-β1 (Figure 5D). Taken together, these results above revealed that inhibition of AGR2 attenuated the metastasis of AdCC cells by reducing the EMT status induced by TGF-β1.
Figure 5.
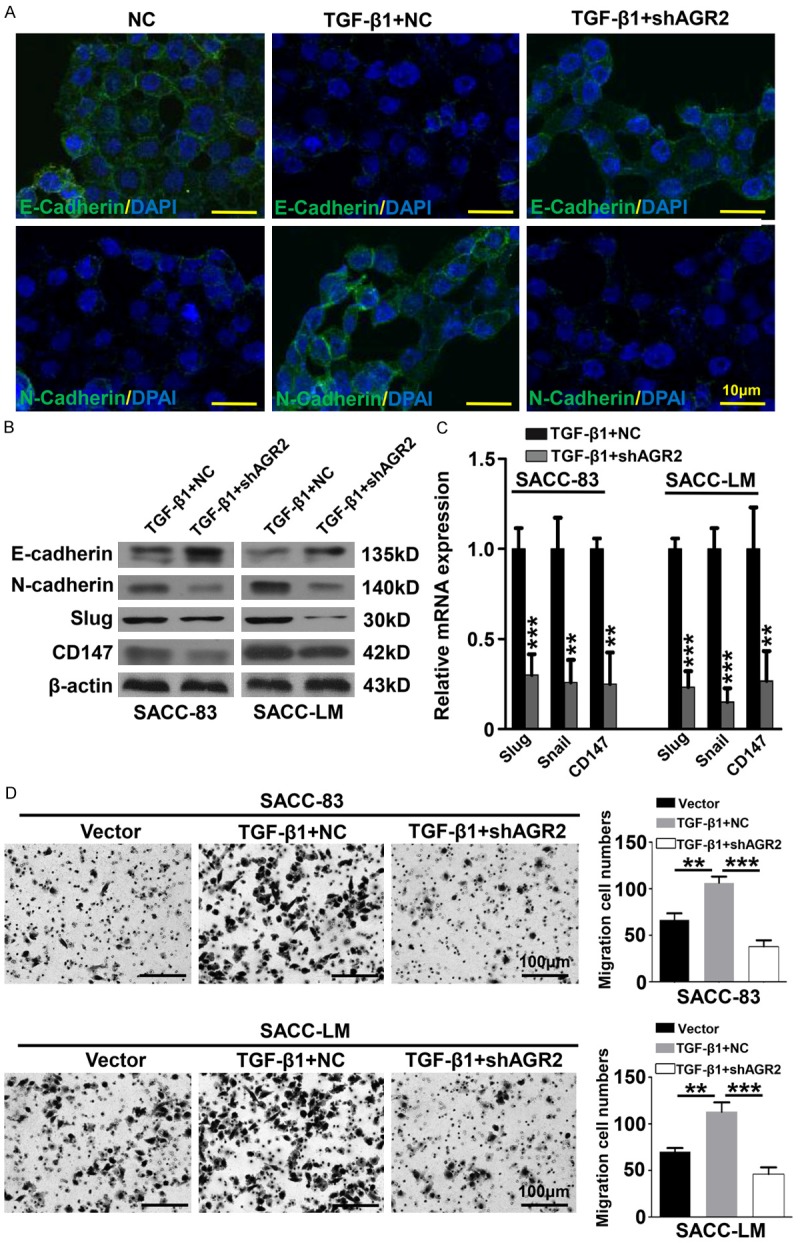
Knockdown of AGR2 reverses the EMT phenomenon induced by TGF-β1. A. Representative Immunofluorescence photos of SACC-LM treated with negative control shRNA (NC), TGF-β1 with or without AGR2 shRNA (shAGR2) were displayed. The expression of E-cadherin was reduced by TGF-β1 but reversed by AGR2 knockdown. The expression of N-cadherin was up-regulated by TGF-β1 but recovered by AGR2 knockdown (scale bar = 10 μm). B. Western blotting showed the expression of E-cadherin, N-cadherin, Slug and CD147 in SACC-83 and SACC-LM cells tranfected with negative control shRNA (NC) or with AGR2 shRNA (shAGR2) in the presence of TGF-β1. The β-actin was used as loading control. C. The mRNA levels of Slug, Snail and CD147 in SACC-83 and SACC-LM cells were significantly reduced by AGR2 silencing in the presence of TGF-β1 (Mean ± SEM, **P<0.01, ***P<0.001, unpaired t test). D. Knockdown of AGR2 reversed the enhanced ability of migration which induced by TGF-β1 in SACC-83 cells and SACC-LM cells (Mean ± SEM, **P<0.01, ***P<0.001, one way ANOVA post tukey test, scale bar = 100 μm).
Discussion
AdCCs are characterized with neural invasion and high potential of distant metastasis [1]. Although AdCC has a tendency for a relative prolonged clinical course, the prognosis become rather poor once the distant metastasis occurred [26]. Therefore, a better understanding of the mechanism of the metastasis of AdCC is urgently needed. Accumulating reports have confirmed the critical role of EMT in cancer invasion and metastasis [14]. In addition, the overexpression of pro-metastasis protein AGR2 was associated with the EMT process in our previous study focused on HNSCC [21]. However, the expression of AGR2, and its relationship with EMT remained unclear in AdCC.
Overexpression of AGR2 was frequently detected in various cancers, promoting the growth and metastasis of malignance cells, and exerting negative impact on the clinical outcome [5,10,12,27]. In the present study, we identified a significant increase of AGR2 protein level by IHC staining in AdCC tissues as compared with the normal salivary glands and benign salivary gland tumors. This result, which was in accordance with the studies on many other cancers, probably emphasized the oncogenic role of AGR2 in AdCC. Recent studies showed that abnormal AGR2 expression contributed to the survival of cancer cells. In breast cancer, elevated AGR2 expression helped malignant cells to survive under serum-depleted conditions or hypoxic conditions [28]. It also reported that AGR2 modulated the growth of breast cancers via Survivin, c-Myc as well as Cyclin D1 [29]. In accordance with these evidences above, AGR2 expression was positively correlated with Ki-67 in the present study. Meanwhile, knockdown of AGR2 by shRNA caused significant repression of proliferation in AdCC cell lines in vitro and in vivo and reduced the protein level of Survivin and Cyclin D1. Migration and invasion are crucial processes for tumor cell circulation and establishment of distant metastasis [30]. Several investigations into tumor progression have indicated the prometastasis role of AGR2 [11,31-33]. In the present study, we found a significant higher AGR2 expression in high-metastatic AdCC cell lines as compared with the parental cell lines. Furthermore, inhibition of AGR2 significantly attenuated the migration ability of AdCC cells. These findings implied the indispensable role of AGR2 during the metastatic process of AdCC cells. However, the underlying complex mechanism remained to be elucidated. Taken together, our findings indicated that AGR2 might promote tumor progression by enhancing the cell proliferation and metastasis in AdCC.
The EMT process is initiated by signaling pathways that respond to extracellular cues, among which TGF-β1 plays a predominant role [16]. In breast cancer, TGF-β1 was found up-regulated to promote metastasis and interfere the clinical outcome [34]. For liver cancer, TGF-β1, combined with CXCR4, enhanced the EMT and contributed to cancer dissemination [35]. Additionally, recent report indicated CD147, a membrane glycoprotein that regulates cell adhesion, participated in EMT program in cancer [36]. Activation of TGF-β1/CD147 axis was significantly associated with poor survival of patients with hepatocellular carcinoma [37]. These studies emphasized the predominate role of TGF-β1 in inducing and maintaining EMT. To assess whether or not AGR2 was involved in EMT progression, we discussed the relationship of AGR2 and TGF-β1/CD147 by Pearson correlation analysis. Notably, AGR2 was positively correlated with TGF-β1 and CD147 in the serial cutting human AdCC tissue microarrays. These results, at least partially, indicated the potential association between AGR2 and EMT. Accumulating studies demonstrated tumor cells that undergo EMT acquire better survival and stronger metastatic capabilities. It has been reported that TGF-β1 potently contributed to the migration and invasion of AdCC cells [25]. Indeed, enhanced migration ability of AdCC cells was induced by TGF-β1 in our present study, but was significantly reversed by AGR2 knockdown. AGR2 silencing reduced the N-cadherin, Slug, Snail and CD147, but up-regulated the E-cadherin in AdCC cells, which were exposure in exogenous TGF-β1. These observations were partially in accordance with Chen’s research on HNSCC [38]. In summary, our data suggested that AGR2 might partially disrupt the metastasis of AdCC cells via decreasing EMT.
In conclusion, the expression pattern of pro-metastasis protein AGR2 was detected in human AdCC tissue microarray. Aberrant AGR2 expression was firmly correlated with Ki-67, TGF-β1 and CD147 in human AdCC tissues. Knockdown of AGR2 by specific shRNA could remarkably repress the proliferation and migration of AdCC cells. In addition, inhibition of AGR2 repressed the enhanced metastatic ability of AdCC cells induced by EMT. According to these finds, we suggest AGR2 is a potential therapeutic target for AdCC, especially the AdCC with the tendency of distant metastasis.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81272963, 81472528) to Z.J. Sun and (81272946, 81472529) to W.F. Zhang. This work was also supported by program for new century excellent talents in university (NCET-13-0439), Ministry of Education of China to Z.J. Sun.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ellington CL, Goodman M, Kono SA, Grist W, Wadsworth T, Chen AY, Owonikoko T, Ramalingam S, Shin DM, Khuri FR, Beitler JJ, Saba NF. Adenoid cystic carcinoma of the head and neck: incidence and survival trends based on 1973-2007 surveillance, epidemiology, and end results data. Cancer. 2012;118:4444–4451. doi: 10.1002/cncr.27408. [DOI] [PubMed] [Google Scholar]
- 2.Dodd RL, Slevin NJ. Salivary gland adenoid cystic carcinoma: a review of chemotherapy and molecular therapies. Oral Oncol. 2006;42:759–769. doi: 10.1016/j.oraloncology.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Brychtova V, Vojtesek B, Hrstka R. Anterior gradient 2: a novel player in tumor cell biology. Cancer Lett. 2011;304:1–7. doi: 10.1016/j.canlet.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 4.Patel P, Clarke C, Barraclough DL, Jowitt TA, Rudland PS, Barraclough R, Lian LY. Metastasis-promoting anterior gradient 2 protein has a dimeric thioredoxin fold structure and a role in cell adhesion. J Mol Biol. 2013;425:929–943. doi: 10.1016/j.jmb.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Hrstka R, Nenutil R, Fourtouna A, Maslon MM, Naughton C, Langdon S, Murray E, Larionov A, Petrakova K, Muller P, Dixon MJ, Hupp TR, Vojtesek B. The pro-metastatic protein anterior gradient-2 predicts poor prognosis in tamoxifen-treated breast cancers. Oncogene. 2010;29:4838–4847. doi: 10.1038/onc.2010.228. [DOI] [PubMed] [Google Scholar]
- 6.Hengel SM, Murray E, Langdon S, Hayward L, O’Donoghue J, Panchaud A, Hupp T, Goodlett DR. Data-independent proteomic screen identifies novel tamoxifen agonist that mediates drug resistance. J Proteome Res. 2011;10:4567–4578. doi: 10.1021/pr2004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bu H, Schweiger MR, Manke T, Wunderlich A, Timmermann B, Kerick M, Pasqualini L, Shehu E, Fuchsberger C, Cato AC, Klocker H. Anterior gradient 2 and 3-two prototype androgen-responsive genes transcriptionally upregulated by androgens and by oestrogens in prostate cancer cells. FEBS J. 2013;280:1249–1266. doi: 10.1111/febs.12118. [DOI] [PubMed] [Google Scholar]
- 8.Kani K, Malihi PD, Jiang Y, Wang H, Wang Y, Ruderman DL, Agus DB, Mallick P, Gross ME. Anterior gradient 2 (AGR2): blood-based biomarker elevated in metastatic prostate cancer associated with the neuroendocrine phenotype. Prostate. 2013;73:306–315. doi: 10.1002/pros.22569. [DOI] [PubMed] [Google Scholar]
- 9.Chevet E, Fessart D, Delom F, Mulot A, Vojtesek B, Hrstka R, Murray E, Gray T, Hupp T. Emerging roles for the pro-oncogenic anterior gradient-2 in cancer development. Oncogene. 2013;32:2499–2509. doi: 10.1038/onc.2012.346. [DOI] [PubMed] [Google Scholar]
- 10.Pohler E, Craig AL, Cotton J, Lawrie L, Dillon JF, Ross P, Kernohan N, Hupp TR. The Barrett’s antigen anterior gradient-2 silences the p53 transcriptional response to DNA damage. Mol Cell Proteomics. 2004;3:534–547. doi: 10.1074/mcp.M300089-MCP200. [DOI] [PubMed] [Google Scholar]
- 11.Liu D, Rudland PS, Sibson DR, Platt-Higgins A, Barraclough R. Human homologue of cement gland protein, a novel metastasis inducer associated with breast carcinomas. Cancer Res. 2005;65:3796–3805. doi: 10.1158/0008-5472.CAN-04-3823. [DOI] [PubMed] [Google Scholar]
- 12.Innes HE, Liu D, Barraclough R, Davies MP, O’Neill PA, Platt-Higgins A, de Silva Rudland S, Sibson DR, Rudland PS. Significance of the metastasis-inducing protein AGR2 for outcome in hormonally treated breast cancer patients. Br J Cancer. 2006;94:1057–1065. doi: 10.1038/sj.bjc.6603065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barraclough DL, Platt-Higgins A, de Silva Rudland S, Barraclough R, Winstanley J, West CR, Rudland PS. The metastasis-associated anterior gradient 2 protein is correlated with poor survival of breast cancer patients. Am J Pathol. 2009;175:1848–1857. doi: 10.2353/ajpath.2009.090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nawshad A, Lagamba D, Polad A, Hay ED. Transforming growth factor-beta signaling during epithelial-mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tissues Organs. 2005;179:11–23. doi: 10.1159/000084505. [DOI] [PubMed] [Google Scholar]
- 17.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 18.Jechlinger M, Grunert S, Beug H. Mechanisms in epithelial plasticity and metastasis: insights from 3D cultures and expression profiling. J Mammary Gland Biol Neoplasia. 2002;7:415–432. doi: 10.1023/a:1024090116451. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 20.Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell. 2008;19:4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma SR, Wang WM, Huang CF, Zhang WF, Sun ZJ. Anterior gradient protein 2 expression in high grade head and neck squamous cell carcinoma correlated with cancer stem cell and epithelial mesenchymal transition. Oncotarget. 2015;6:8807–8821. doi: 10.18632/oncotarget.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang WM, Zhao ZL, Zhang WF, Zhao YF, Zhang L, Sun ZJ. Role of hypoxia-inducible factor-1alpha and CD146 in epidermal growth factor receptor-mediated angiogenesis in salivary gland adenoid cystic carcinoma. Mol Med Rep. 2015;12:3432–3438. doi: 10.3892/mmr.2015.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao ZL, Ma SR, Wang WM, Huang CF, Yu GT, Wu TF, Bu LL, Wang YF, Zhao YF, Zhang WF, Sun ZJ. Notch signaling induces epithelial-mesenchymal transition to promote invasion and metastasis in adenoid cystic carcinoma. Am J Transl Res. 2015;7:162–174. [PMC free article] [PubMed] [Google Scholar]
- 24.Sun ZJ, Zhang L, Hall B, Bian Y, Gutkind JS, Kulkarni AB. Chemopreventive and chemotherapeutic actions of mTOR inhibitor in genetically defined head and neck squamous cell carcinoma mouse model. Clin Cancer Res. 2012;18:5304–5313. doi: 10.1158/1078-0432.CCR-12-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong L, Wang YX, Li SL, Yu GY, Gan YH, Li D, Wang CY. TGF-beta1 promotes migration and invasion of salivary adenoid cystic carcinoma. J Dent Res. 2011;90:804–809. doi: 10.1177/0022034511401407. [DOI] [PubMed] [Google Scholar]
- 26.van der Wal JE, Becking AG, Snow GB, van der Waal I. Distant metastases of adenoid cystic carcinoma of the salivary glands and the value of diagnostic examinations during follow-up. Head Neck. 2002;24:779–783. doi: 10.1002/hed.10126. [DOI] [PubMed] [Google Scholar]
- 27.Dumartin L, Whiteman HJ, Weeks ME, Hariharan D, Dmitrovic B, Iacobuzio-Donahue CA, Brentnall TA, Bronner MP, Feakins RM, Timms JF, Brennan C, Lemoine NR, Crnogorac-Jurcevic T. AGR2 is a novel surface antigen that promotes the dissemination of pancreatic cancer cells through regulation of cathepsins B and D. Cancer Res. 2011;71:7091–7102. doi: 10.1158/0008-5472.CAN-11-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zweitzig DR, Smirnov DA, Connelly MC, Terstappen LW, O’Hara SM, Moran E. Physiological stress induces the metastasis marker AGR2 in breast cancer cells. Mol Cell Biochem. 2007;306:255–260. doi: 10.1007/s11010-007-9562-y. [DOI] [PubMed] [Google Scholar]
- 29.Vanderlaag KE, Hudak S, Bald L, Fayadat-Dilman L, Sathe M, Grein J, Janatpour MJ. Anterior gradient-2 plays a critical role in breast cancer cell growth and survival by modulating cyclin D1, estrogen receptor-alpha and survivin. Breast Cancer Res. 2010;12:R32. doi: 10.1186/bcr2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JY, Tang YA, Huang SM, Juan HF, Wu LW, Sun YC, Wang SC, Wu KW, Balraj G, Chang TT, Li WS, Cheng HC, Wang YC. A novel sialyltransferase inhibitor suppresses FAK/paxillin signaling and cancer angiogenesis and metastasis pathways. Cancer Res. 2011;71:473–483. doi: 10.1158/0008-5472.CAN-10-1303. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Hao Y, Lowe AW. The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer Res. 2008;68:492–497. doi: 10.1158/0008-5472.CAN-07-2930. [DOI] [PubMed] [Google Scholar]
- 32.Maresh EL, Mah V, Alavi M, Horvath S, Bagryanova L, Liebeskind ES, Knutzen LA, Zhou Y, Chia D, Liu AY, Goodglick L. Differential expression of anterior gradient gene AGR2 in prostate cancer. BMC Cancer. 2010;10:680. doi: 10.1186/1471-2407-10-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Ali TZ, Zhou H, D’Souza DR, Lu Y, Jaffe J, Liu Z, Passaniti A, Hamburger AW. ErbB3 binding protein 1 represses metastasis-promoting gene anterior gradient protein 2 in prostate cancer. Cancer Res. 2010;70:240–248. doi: 10.1158/0008-5472.CAN-09-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 35.Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, Liu F, Pan W, Wang TT, Zhou CC, Wang SB, Wang YZ, Yang Y, Yang N, Zhou WP, Yang GS, Sun SH. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–681. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Teja M, Gronau JH, Minamidate A, Darby S, Gaughan L, Robson C, Mauri F, Waxman J, Sturge J. Survival outcome and EMT suppression mediated by a lectin domain interaction of Endo180 and CD147. Mol Cancer Res. 2015;13:538–547. doi: 10.1158/1541-7786.MCR-14-0344-T. [DOI] [PubMed] [Google Scholar]
- 37.Wu J, Lu M, Li Y, Shang YK, Wang SJ, Meng Y, Wang Z, Li ZS, Chen H, Chen ZN, Bian H. Regulation of a TGF-beta1-CD147 self-sustaining network in the differentiation plasticity of hepatocellular carcinoma cells. Oncogene. 2016;35:5468–5479. doi: 10.1038/onc.2016.89. [DOI] [PubMed] [Google Scholar]
- 38.Chen YT, Ho CL, Chen PK, Chen YL, Chang CF. Anterior gradient 2: a novel sensitive tumor marker for metastatic oral cancer. Cancer Lett. 2013;339:270–278. doi: 10.1016/j.canlet.2013.06.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.