Abstract
Purpose: This study aimed to determine the function of miR-15a in HCC, and identify cMyb as a target of miR-15a. Methods: RNA expression was evaluated by quantitative real-time PCR (qRT-PCR). The effects of miR-15a or cMyb on HCC cells were evaluated by transwell migration assay and western blot analysis. CMyb, the predicted target, has been frequently verified by luciferase assay. Results: MiR-15a was markedly downregulated in sphere culture HCC cells by qRT-PCR. CMyb was predicted to be a potential target of miR-15a using bioinformatics analysis. This prediction has been frequently verified by luciferase assay and western blot. A positive correlation between cMyb and the migration ability of HCC cells was demonstrated by transwell assays. MiR-15a mimic suppressed cMyb expression to weaken HCC cell migration ability. On the other hand, miR-15a inhibitor upregulated cMyb and induced HCC cell migration. Conclusion: MiR-15a could suppress HCC progression through the repression of cMyb, making miR-15a a potential therapeutic target.
Keywords: MiR-15a, Hepatocarcinoma, cMyb
Introduction
Liver cancer is the fifth most common solid malignant tumor worldwide [1] and the third leading cause of cancer-related deaths in China [2]. Hepatocellular carcinoma (HCC) is the primary pathological liver cancer type, accounting for 70-85% of primary liver cancers globally and 90% in China [3]. Although advances in diagnosis such as regular B-ultrasound examination, computed tomography (CT) and magnetic resonance imaging (MRI), as well as appropriate systemic treatments, have improved prognosis, tumor progression and metastasis usually occur rapidly after primary HCC, causing mortality [4]. To date, the precise mechanism of HCC metastasis remain unclear. Hence, more studies are necessary to clarify the progression of this phenomenon.
Since cancer stem cells (CSCs) possess stem cell properties such as self-renewing capacity, tumor-initiating ability, higher tumorigenicity, metastatic potential and chemoresistance, these cells have been hypothesized to be extremely important for understanding molecular mechanisms underlying tumor biology [5]. Moreover, studies focused on CSCs increase our knowledge regarding HCC characteristics. Recent advances in HCC stem cell biology have shown that cancer stem-like cells play very important roles in HCC initiation, progression, recurrence, metastasis and prognosis [6-9].
In 1992, the sphere culture method was developed by Reynolds and Weiss [10]. Many studies have validated that CSC population can be enriched under serum-free environment with the presence of specific factors such as EGF and basic FGF [10,11]. This serum-free approach has been demonstrated as a reasonable way to enrich these stem-cells like populations [12]. Special culture conditions for HCC sphere formation are helpful in maintaining CSCs in vitro [13,14]. HCC sphere cultured cells were used as a kind of cancer stem-like cell in our study.
MicroRNAs (miRNAs) are non-coding, single stranded RNAs with lengths of 21-23 nucleotides, which are processed from endogenous precursor RNAs with stem-loop structures [15]. MiRNAs can base-pair with the 3’- untranslated region (3’-UTR) of messenger RNA (mRNA), in order as to inhibit translation or destabilize the mRNA, and to frequently and negatively regulate the expression of target genes [16,17]. It has been predicted that miRNAs may regulate protein expression from as many as 10% to 30% of all human genes [18]. Normally, miRNAs play important roles in crucial biological processes such as organ development, cell differentiation and proliferation, apoptosis, and cancer cell invasion [19]. In past few years, the deregulated expression of miRNAs has been widely reported in many diseases including cancer [20,21]. At present, more target genes of miRNAs have been continuously validated in experiments and verified in clinical samples. MiRNAs have been proven to have a new important role of regulating tumorigenesis, and these have been demonstrated to play important roles in various aspects of cancer progression including tumor metastasis [22]. For example, miR-15a is significantly decreased in HCC cells and tissues, especially in metastatic liver cancer cells; resulting in increased FOS and Met expression [23]. Moreover, the expression of miR-15a was significantly reduced in HCC tissues, and this expression was negatively correlated with tumor TNM stage. Furthermore, this downregulation causes lymph node metastasis with a low metastasis-free survival in patients [24]. These studies indicate the importance of conducting thorough investigations on miRNAs that are aberrantly expressed during HCC progression, especially miRNAs associated with HCC metastasis. In the present study, miR-15a was found to be significantly downregulated in HCC MHCC97H and Huh7 sphere cultured cells, compared with their parent cells. By manipulating miR-15a levels in HCC cells, we validated that miR-15a promotes the mobility of HCC cells. cMyb has been predicted to be a target of miR-15a by bioinformatics analysis, which was validated by luciferase assay and western blot. The function of cMyb in HCC metastasis was verified by loss-of-function and gain-of-function assays.
Our study demonstrates that miR-15a plays a role as a metastasis promoter by directly targeting cMyb, suggesting that miR-15a has potential therapeutic value for HCC treatment.
Materials and methods
Cell culture
Human HCC cells (Huh7 and MHCC97H) and HEK293T were obtained from the Cell Bank of Institute of Biochemistry and Cell Biology, China Academy of Sciences (Shanghai, China). Cells were cultured at a density of 10,000 cells/cm2 in a Dulbecco’s Modified Eagle Medium (DMEM; HyClone Laboratories, Logan, UT) high-glucose medium supplement containing 10% inactivated fetal bovine serum (FBS; HyClone Laboratories, Logan, UT) and 0.01% antibiotic-antimycotic (Invitrogen, Carlsbad, CA); and incubated at 37°C with 5% CO2.
Sphere-forming culture
When HCC cell confluence reached 80%, cells were detached using 1× trypsin-EDTA. Serum-free cells were suspended in DMEM/F12 medium supplemented with 1% b27 supplement, 0.01% antibiotic-antimycotic (Invitrogen, Carlsbad, CA), 20 ng/mL of epidermal growth factor (EGF; Invitrogen, Carlsbad, CA) and 20 ng/mL of basic fibroblast growth factor (bFGF; Invitrogen, Carlsbad, CA). Cells were subsequently cultured in ultra-low attachment 24-well plates (24-well plate coated with Ultra-Low Attachment Surface (Corning, NY, USA) at a density of 2,000 cells per well, and were incubated at 37°C with 5% CO2.
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from cultured cells with TRIzol Reagent (Invitrogen, Carlsbad, CA), according to manufacturer’s instructions. MiR-15a quantification was conducted using a 7500 Real-Time PCR System (Applied Biosystems). After real-time PCR reaction was achieved, cycle threshold (Ct) data were determined using fixed threshold settings; and mean Ct was determined from triplicate PCRs. A comparative CT method was used to compare each condition to the controls. GAPDH snRNA was used as an internal control, and the relative amount of miR-15a normalized to GAPDH was calculated using the equation 2-ΔΔCT; where ΔΔCT = (Ct miR-15a - Ct GAPDH) target - (Ct miR-15a - Ct GAPDH) control.
Overexpression or knockdown of cMyb
A full-length cMyb cDNA expression plasmid lacking the 3’-UTR was purchased from GenePharma Co., Ltd (Shanghai, China), and the empty plasmid served as the negative control. The siRNA sequence targeting human cMyb mRNA was designed and synthesized by GenePharma Co., Ltd (Shanghai, China). A scrambled siRNA was used as negative control. Sequences of the cMyb siRNA were as follows: 5’-UGUUAUUGCCAAGCACUUAAA-3’ (sense); 5’-UAAGUGCUUGGCAAUAACAGAA-3’ (antisense).
The cMyb overexpression plasmid or siRNA was transfected into MHCC97H and Huh7 cells using Lipofectamine 2000 (Invitrogen), according to manufacturer’s instructions. The concentration for the transfection of the cMyb cDNA plasmid or siRNA was 0.05 µg/mL or 50 nmol/L, respectively.
Overexpression or knockdown of miR-15a
The overexpression or knockdown of miR-15a was accomplished by transfecting cells with miR-15a mimic or inhibitor purchased from GenePharma Co., Ltd. (Shanghai, China). The transfection concentrations were 50 nmol/L for the miR-15a mimic and 200 nmol/L for the miR-15a inhibitor, which were also adopted when the control mimic or inhibitor was transfected.
Western blot
Total protein extraction from cultured cells was used in electrophoresis and western blot. Briefly, 20 micrograms of total protein were separated by standard SDS-PAGE and transferred onto PVDF membranes. The membranes were washed, blocked and incubated with specific primary antihuman antibodies (1:1,500), followed by incubation with horseradish peroxidase-conjugated secondary antibodies (1:5,000). The reactions were detected by enhanced chemiluminescence assay. The anti-cMyb antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and a concentration of 1:1,500 was used. An antibody against anti-β-actin, as the control, was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA); and a concentration of 1:1,500 was used.
Luciferase reporter assay
The entire 3’-UTR of human cMyb was amplified by PCR using human genomic DNA as the template, and the PCR products were inserted into the p-MIR-reporter plasmid GenePharma Co., Ltd. (Shanghai, China). The insertion was confirmed by sequencing. In order to test the binding specificity, sequences that interact with the miR-15a seed sequence were mutated (from TGCTGCTA to GGTTACG); and the mutant cMyb 3’-UTR was inserted into an equivalent luciferase reporter plasmid. For the luciferase reporter assay, HEK293T cells were seeded in 24-well plates, and each well was transfected with 0.2 µg of luciferase reporter plasmid, 0.4 µg of β-galactosidase (β-gal) expression plasmid and 40 pmol of miR-15a mimic, 120 pmol of miR-15a inhibitor or scrambled negative control RNAs using Lipofectamine 2000 (Invitrogen). The β-gal plasmid was used as the transfection control. At 24 hours after transfection, cells were harvested and analyzed for luciferase activity using luciferase assay kits (Promega, Madison, WI, USA).
Transwell assay
The migration ability of cells was also tested in a Transwell Boyden Chamber (6.5 mm, Costar, USA). Cells were placed in the upper compartment, while the bottom was placed with the polycarbonate membranes (8-μm pore size). At 24 hours after transfection, cells were harvested, counted and suspended in FBS-free DMEM medium. Then, 3×104 cells in 200 µL of DMEM medium were added to each upper chamber, 0.6 mL of DMEM with 10% FBS was added to the lower compartment, and the Transwell-containing plates were incubated at 37°C with 5% CO2 for eight hours. After incubation, cells that entered the lower surface of the membrane were fixed with 4% paraformaldehyde for 20 minutes at room temperature, washed three times with distilled water, and stained with 0.1% crystal violet in 0.1 mol/L of borate and 2% ethanol for 15 minutes at room temperature. Non-migrant cells that remained on the upper surface of the filter membrane were gently scraped off with a cotton swab. The lower surfaces (with migrant cells) were captured using a photomicroscope (five fields per chamber; BX51 Olympus, Japan), and the number of cells were counted.
Statistical analysis
All western blot and Transwell assay images represent at least three independent experiments. Quantitative RT-PCR and luciferase reporter assay were performed in triplicate, and each experiment was repeated at least three times. Results are presented as mean ± SD. Differences between groups were calculated using student’s t-test, and P < 0.05 was considered statistically significant.
Results
MiR-15a is downregulated in HCC sphere cultured cells and is positively correlated to the mobility of HCC cells
First, the levels of miR-15a in HCC sphere cultured cells and their parental cells were individually assessed using qRT-PCR, and revealed that the levels of miR-15a in HCC sphere cultured cells were significantly lower than their relative parental cells (Figure 1A); suggesting that miR-15a is associated with HCC stem cell features including high migration ability. Then, miR-15a levels in two different liver cancer cell lines, MHCC97H and Huh7, were detected; and revealed that the concentration of miR-15a was significantly higher in MHCC97H cells (Figure 1B). MHCC97H cells exhibited a higher migration ability in Transwell assays (Figure 1C and 1D), indicating that miR-15a was positively correlated with HCC cell mobility.
Figure 1.
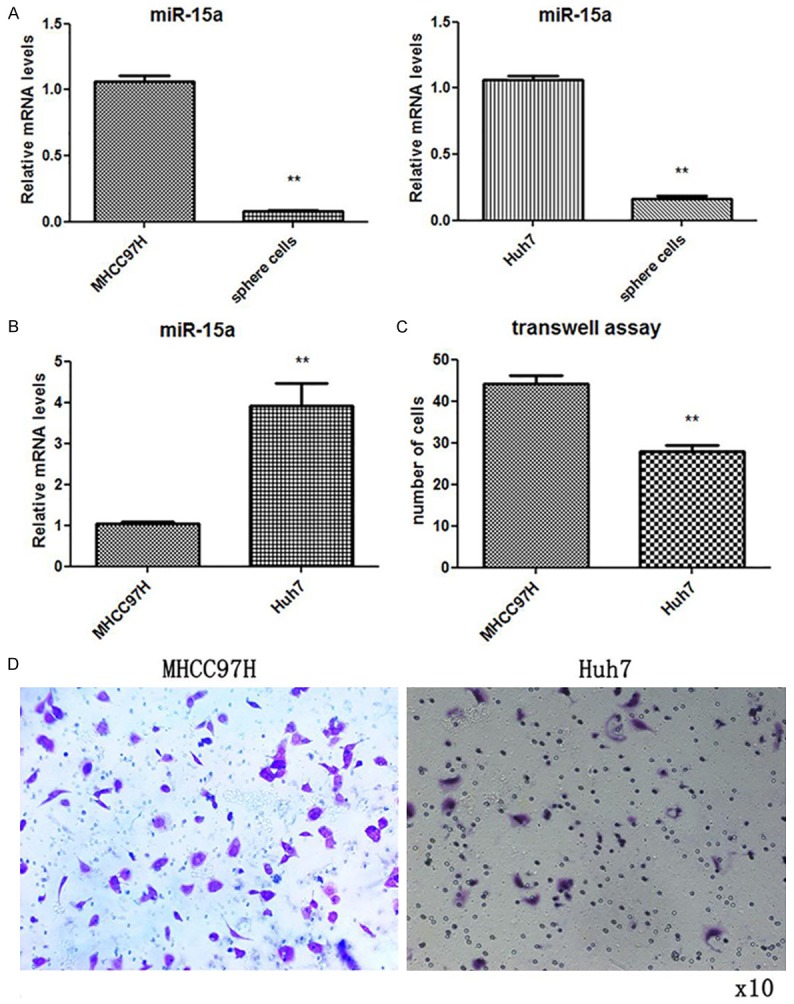
The expression of miR-15a was decreased in HCC sphere culturing cells and was positively correlated with the mobility of breast cancer cells. A: QRT-PCR assay of the relative expression of miR-15a in HCC sphere culturing cells and their relative parental cells. B: QRT-PCR analysis of the relative expression of miR-15a in HCC cell lines MHCC97H and Huh7 cells. C: Quantitative analysis of the migration rates in MHCC97H and Huh7 cells. D: Representative image of Transwell assay. (*P < 0.05; **P < 0.01).
CMyb is a potential target of miR-15a in liver cancer cells: Target genes of miR-15a were predicted in http://www.mirbase.org/ and http://www.targetscan.org/vert_61/, and cMyb was identified as a potential target gene. Putative binding sites for miR-15a in the 3’-UTR of the cMyb mRNA are shown in Figure 2A. The seed region (core sequences that encompass the first 2-8 bases of mature miRNA) of miR-15a was perfectly base-paired with the 3’-UTR of cMyb mRNA. Furthermore, miR-15a binding sequences in the 3’-UTR of cMyb mRNA are highly conserved across species.
Figure 2.
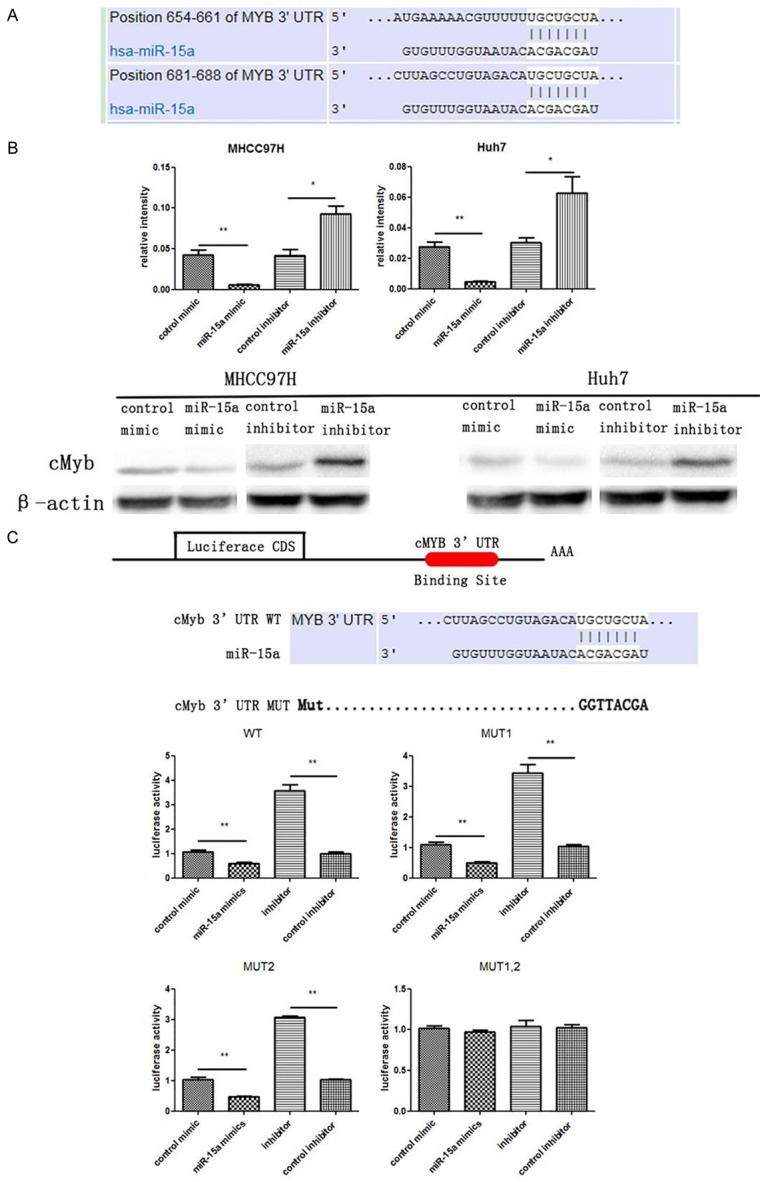
CMyb is a target gene of miR-15a in HCC cells. A: Schematic illustration of the conserved miR-15a binding sites. The cMyb 3’-UTR contains two predicted miR-15a binding sites. The seed regions of miR-15a and the seed-recognizing sites in the cMyb 3’-UTR are indicated in white background, and all nucleotides in seed-recognizing sites are completely conserved across several species. B: Western blotting analysis of cMyb protein levels in MHCC97H and Huh7 cells transfected with miR-15a mimic or inhibitor. C: Direct recognition of the cMyb 3’-UTR by miR-15a. Firefly luciferase reporters individually containing wild-type (WT), mutant (MUT)1, MUT2 or MUT1,2 miR-15a binding sites in the cMyb 3’-UTR were co-transfected into HEK293T cells with the scrambled negative control RNA, miR-15a mimic or inhibitor. At 24 h post-transfection, the cells were treated with a luciferase assay kit. The results were calculated as the ratio of firefly luciferase activity in the miR-15a-transfected cells normalized to the control cells. *P < 0.05; **P < 0.01.
In order to assess whether cMyb could be regulated by miR-15a, the effect of miR-15a on cMyb protein level in MHCC97H and Huh7 cells were investigated. As shown in Figure 2B, cMyb protein level was reduced by the induction of the miR-15a mimic, but significantly increased by transfection with the miR-15a inhibitor in both cell lines.
In order to ascertain whether miR-15a directly regulates cMyb expression by binding with cMyb the 3’-UTR, the full-length 3’-UTR of cMyb was amplified by PCR and fused downstream of the firefly luciferase gene in a reporter plasmid. The reporter plasmid was transfected into HEK293T cells along with a transfection control plasmid (β-gal) and miR-15a mimic or inhibitor. As expected, the overexpression of miR-15a resulted in a reduction of approximately 20% in luciferase reporter activity, while the inhibition of miR-15a resulted in a 1.3-fold increase in reporter activity, compared with cells transfected with the control inhibitor (Figure 2C). Furthermore, point mutations were introduced into the corresponding complementary sites in the cMyb 3’-UTR to eliminate the predicted miR-15a binding sites. This mutated luciferase reporter was unaffected by either the overexpression or knockdown of miR-15a (Figure 2C). In conclusion, these results demonstrate that miR-15a inhibits cMyb expression by binding to cMyb 3’-UTR.
The expression of cMyb increased in HCC sphere cultured cells and is positively correlated with the migration ability of HCC cells: MiRNAs have been generally thought to have an expression pattern that is opposite to that of their targets [25]. As miR-15a expression decreased in HCC sphere cultured cells, it was determined whether cMyb protein level increased. After assessing the protein level of cMyb protein in HCC sphere cultured cells, it was found that cMyb protein level was significantly higher in HCC sphere cultured cells (Figure 3A). Furthermore, protein levels of cMyb were detected in MHCC97H and Huh7 cells; and higher cMyb protein levels were assessed in MHCC97H cells, which revealed a lower level of miR-15a (Figure 3A). These findings further suggest that the level of cMyb protein is negatively correlated with the level of miR-15a, and that cMyb expression is regulated by miR-15a.
Figure 3.
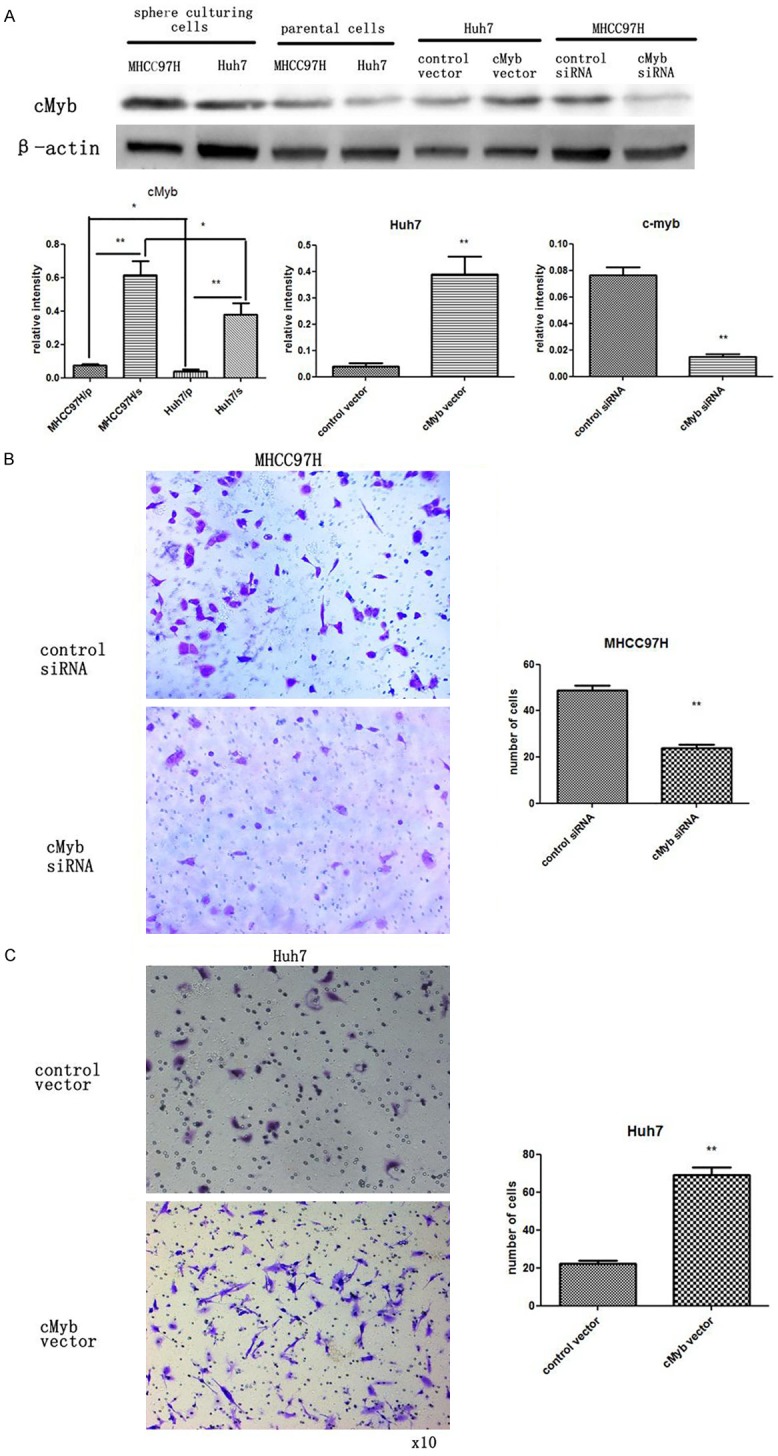
CMyb expression is increased in HCC sphere culturing cells and is positive correlated with the mobility of HCC cells. A: Western blotting analysis and quantification of cMyb protein levels in MHCC97H and Huh7 sphere culturing cells and their relative parental cells. B: Western blotting analysis and quantification of cMyb protein levels in MHCC97H cells transfected with control siRNA or cMyb siRNA. Western blotting analysis and quantification of cMyb protein levels in Huh7 cells transfected with control plasmid or cMyb overexpression plasmid. C: Left panel: Representative image of transwell assay of MHCC97H cells transfected with control siRNA or cMyb siRNA. Right panel: Quantitative analysis of migration rates. C: Left panel: Representative image of Transwell assay of Huh7 cells transfected with control plasmid or cMyb overexpression plasmid. Right panel: Quantitative analysis of the migration rates. *P < 0.05; **P < 0.01 (MHCC97H parental cells = MHCC97H/p, MHCC97H sphere culturing cells = MHCC97H/s, Huh7 parental cells = Huh7/p, Huh7 sphere culturing cells = Huh7/s).
As an adhesion molecule participating in comprising tight junctions, cMyb was reported to be associated with the metastasis of HCC cells. In order to further elucidate the function of cMyb in regulating the mobility of HCC cells, a loss-of-function assay was performed by transfecting a siRNA against cMyb into MHCC97H cells. The left panel in Figure 3B shows that cMyb protein level was knocked-down in MHCC97H cells with cMyb siRNA. Then, Transwell assays were used to investigate MHCC97H cell migration. Within 24 hours, Transwell assays revealed that less MHCC97H cells migrated through the membrane of the lower well when cMyb expression was inhibited by siRNA (Figure 3C).
Gain-of-function assay was also conducted by transfecting cMyb cDNA plasmid into HCC cells. The increase in cMyb protein is shown in the right panel of Figure 3C. As expected, the overexpression of cMyb promoted the migration ability of Huh cells, as demonstrated by Transwell assays (Figure 3C). Altogether, cMyb was found to be upregulated in HCC stem-like cells, and negatively regulate the mobility of HCC cells.
MiR-15a induces HCC cell migration by targeting cMyb: After demonstrating that cMyb is involved in HCC cell migration, the biological significance of miR-15a in HCC was investigated. As shown by transwell assays in Figure 4A, less MHCC97H cells migrated through the membrane when transfected with miR-15a mimic. Consistently, the knockdown of miR-15a by a miRNA inhibitor revealed opposite effects. The same biological function of miR-15a was found in Huh7 cells (Figure 4B). Transwell assays revealed a significant decrease in cells that migrated through the membrane when MHCC97H and Huh7 cells were transfected with miR-15a mimic (Figure 4C and 4D). As anticipated, the mobility of MHCC97H and Huh7 cells was significantly increased by transfection with the miR-15A inhibitor, as individually shown in Figure 4C and 4D.
Figure 4.
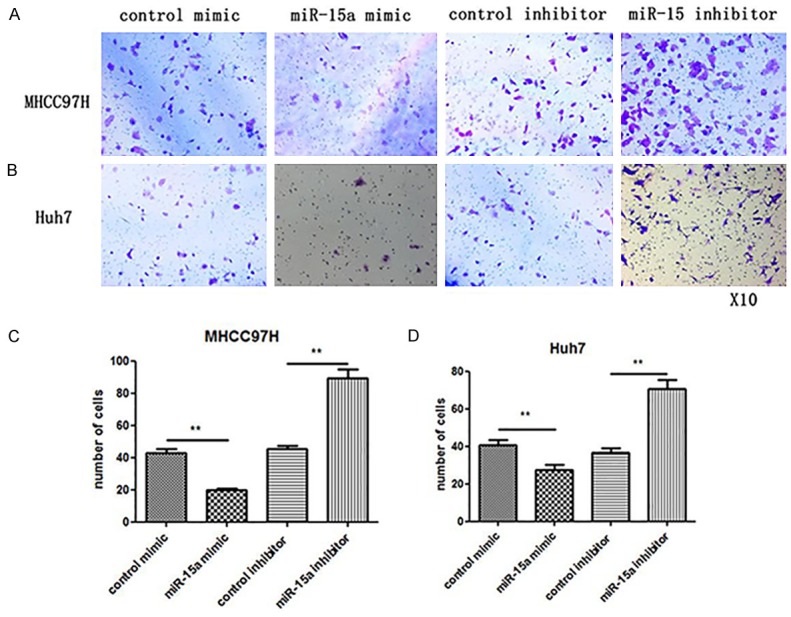
Effects of miR-15a on HCC cell migration. A: Representative image of Transwell assay of MHCC97H cells transfected with control mimic, miR-15a mimic, control inhibitor or miR-15a inhibitor. B: Representative image of Transwell assay of Huh7 cells transfected with control mimic, miR-15a mimic, control inhibitor or miR-15a inhibitor. C, D: Quantitative analysis of the migration rates. *P < 0.05; **P < 0.01.
In order to further confirm that the effects of miR-15a are mediated by the repression of cMyb in HCC cells, knockdown of cMyb in MHCC97H cells by miR-15a mimic was restored by transfecting the cMyb cDNA vector; which is an expression that is not regulated by miR-15a due to its lack of the 3’-UTR (Figure 5A). Figure 5B and 5C show that the inhibition of MHCC97H cell migration by miR-15a mimic can be reversed by the cMyb vector. Furthermore, cMyb siRNA was transfected into Huh7 cells to counteract the increase of cMyb protein caused by the miR-15a inhibitor (Figure 5D). Compared with cells transfected with the miR-15a inhibitor and control siRNA, Huh7 cells transfected with the miR-15a inhibitor and cMyb siRNA exhibited impaired migration ability (Figure 5E and 5F). Taken together, these results demonstrate that miR-15a promotes HCC cell migration by inhibiting cMyb.
Figure 5.
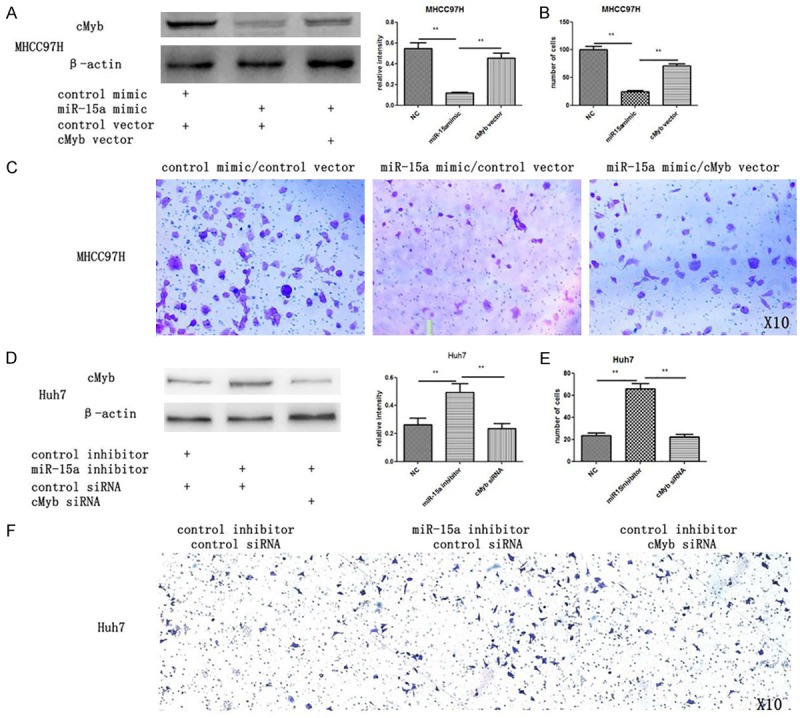
MiR-15a suppresses HCC cells migration by targeting cMyb. A: Western blotting analysis of the protein levels of cMyb in MHCC97H cells transfected with control mimic plus control plasmid, miR-15a mimic plus control plasmid or miR-15a mimic plus cMyb overexpression plasmid. B: Quantitative analysis of the migration rates. C: Representative image of Transwell assay of MHCC97H cells transfected with control mimic plus control plasmid, miR-15a mimic plus control plasmid or miR-15a mimic plus cMyb overexpression plasmid. D: Western blotting analysis of the protein levels of cMyb in Huh7 cells transfected with control inhibitor plus control siRNA, miR-15a inhibitor plus control siRNA or miR-15a inhibitor plus cMyb siRNA. E: Quantitative analysis of the migration rates. F: Representative image of Transwell assay of Huh7 cells transfected with control inhibitor plus control siRNA, miR-15a inhibitor plus control siRNA or miR-15a inhibitor plus cMyb siRNA. *P < 0.05; **P < 0.01.
Discussion
Metastasis remains as one of the major challenges for HCC patients undergoing various therapies including liver resection, local ablation and chemoembolization. Oncogene regulation has been thought to be a therapy strategy for HCC.
MiRNAs are a kind of small, non-coding RNAs that regulate specific mRNAs expression by inhibiting the translation or degradation of mRNA [15,16]. According to Calin’s report, more than 50% of miRNAs are located in cancer-associated genomic points [26]. These miRNAs can function as tumor suppressor actors or promoters, depending on how these target genes are regulated by the miRNA [27]. MiR-15a has been demonstrated to act as a cancer suppress actor in multiple kinds of cancer [28-33]. For example, a study revealed that mir-15a expression decreased in serum of patients who suffered from advanced stage oral squamous cell carcinoma and lymph node metastasis [33]. In addition, the upregulation of miR-15a by recombinant lentiviral vector encoding miR-15a/16-1 contributed to lower bcl2 expression, as well as inhibited the proliferation of CNE-2Z cells, promoted apoptosis and enhanced the sensitivity of CNE-2Z cells to radiotherapy [32]. Furthermore, the upregulation of miR-15a by gossypol acetate (GAA) in pituitary tumor cells induces apoptosis by targeting the bcl2 oncogene [28]. Although the function of miR-15a has been demonstrated in many studies, it remains to be reported in HCC. In our study, we found that the expression of miR-15a was downregulated in HCC sphere cultured cells and MHCC97H cells, indicating that miR-15a may be associated with the progression of HCC.
The HCC line MHCC97H has been classified as a high invasive subtype, which exhibit a characteristic epithelial cobblestone-like morphology with highly expressed cell-cell adhesion molecules such as E-cadherin; and has been identified as a representative HCC cell line with high-invasive ability [34]. By contrast, Huh7 has been classified as a low invasive subtype [35]. In the present study, the highly tumorigenic HCC cell line, MHCC97H, was found to present a lower level of miR-15a, compared with the less tumorigenic cell line, Huh7. These results suggest that miR-15a is negatively associated with the malignant phenotypes of HCC including the migration of breast cancer cells, and may serve as a marker of potential progression in cancer.
According to bioinformatics analysis, cMyb was predicted as a potential target gene of miR-15a. Consistently, the level of cMyb protein increased in HCC sphere cultured cells, which is coincident with the downregulation of miR-15a. Furthermore, MHCC97H cells with lower levels of miR-15a expressed a higher level of cMyb protein, than Huh7 cells. Moreover, western blot analysis revealed that miR-15a inhibits cMyb translation in HCC cells. We also found that luciferase activity of the reporter plasmid containing cMyb 3’-UTR can be remarkably decreased by miR-15a mimic, while knockdown of miR-15a in HCC cells resulted in a decrease in luciferase activity. These results prove that miR-15a can fine-tune the expression of cMyb through binding to the 3’-UTR of cMyb.
Under normal conditions, the transcription factor cMyb has a key role in regulating an exquisite balance among cell division, differentiation and survival; and has now been identified as an oncogene involved in some human leukemia and solid cancers [36-38]. When inappropriately expressed, cMyb appears to activate important gene targets to promote cancer progression and metastasis. These genes include cyclooxygenase-2 (COX-2) [25], Bcl-2, Bcl-X (L) [39] and c-Myc [40]; which influence diverse processes such as angiogenesis, proliferation and apoptosis. As for HCC, cMyb has been recently reported as an oncogene that is involved in the HCC pathogenesis of HCCs [41-43]. Yang et al. [44] validated that the increased expression of cMyb and Sp1 binding to the methionine adenosyltransferase 2A (MAT2A) promoter contribute to the upregulation of MAT2A expression. MAT2A can catalyze the formation of S-adenosylmethionine to promote HCC growth. In the present study, we do demonstrate that c-Myb can positively regulate the mobility of HCC cells.
In our study, loss-of-function assay using MHCC97H cells transfected with c-Myb siRNA revealed that knockdown of c-Myb suppresses the migration of HCC cells, while gain-of-function assay by overexpressing c-Myb in Huh7 cells revealed that c-Myb overexpression promotes migration. These results suggest the role of c-Myb as a positive regulator of migration in HCC cells.
Regarding the mechanisms of the downregulation of c-Myb protein in HCC, a previous report has shown that miR-150 inhibits cMyb gene transcription [45]. Considering that the 3’-UTR of human cMyb is as long as 3.6 kb, we hypothesized that cMyb expression may be downregulated by miRNAs such as miR-15a in HCC. Indeed, a negative correlation between the expression of miR-15a and cMyb was established in the present study. The overexpression of miR-15a decreased the migration ability of HCC cells by downregulating cMyb. Additionally, the upregulation of c-Myb protein can promote migration by expressing the cMyb cDNA vector, although this induced mobility can be reversed by miR-15a mimic. This further confirms that miR-15a regulates the migration of HCC cells by targeting cMyb. Furthermore, in comparing the mobility of Huh7 cells transfected with an miR-15a inhibitor plus control siRNA or miR-15a inhibitor plus cMyb siRNA revealed that the miR-15a inhibitor upregulated the migration of HCC cells by regulating cMyb expression.
In summary, our data reveals a new role for miR-15a as an oncogenic miRNA in HCC. The identification of the miR-15a in targeting the cMyb pathway provides a potential new therapeutic target in the treatment of HCC.
Acknowledgements
This study was supported by a grant from National Natural Science Foundation of China (Grant No. 81202073) and a grant from Natural Science Foundation of Guangdong Province (Grant No. 2015A030313273).
Disclosure of conflict of interest
None.
References
- 1.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuen MF, Hou JL, Chutaputti A Asia Pacific Working Party on Prevention of Hepatocellular. Hepatocellular carcinoma in the Asia pacific region. J Gastroenterol Hepatol. 2009;24:346–353. doi: 10.1111/j.1440-1746.2009.05784.x. [DOI] [PubMed] [Google Scholar]
- 3.Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, Wang L, Zhou J, Qiu SJ, Li Y, Ji XN, Liu H, Xia JL, Wu ZQ, Fan J, Ma ZC, Zhou XD, Lin ZY, Liu KD. A decade’s studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–196. doi: 10.1007/s00432-003-0511-1. [DOI] [PubMed] [Google Scholar]
- 4.Jain S, Singhal S, Lee P, Xu R. Molecular genetics of hepatocellular neoplasia. Am J Transl Res. 2010;2:105–118. [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 6.Mitra A, Satelli A, Xia X, Cutrera J, Mishra L, Li S. Cell-surface Vimentin (csVim): A mislocalized protein for isolating csVimentin CD133 novel stem-like hepatocellular carcinoma cells expressing EMT markers. Int J Cancer. 2015;137:491–496. doi: 10.1002/ijc.29382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Yang X, Ruan B, Dai B, Gao Y, Duan J, Qu S, Tao K, Dou K, Li H. Overexpression of miR-200a suppresses epithelial-mesenchymal transition of liver cancer stem cells. Tumour Biol. 2015;36:2447–2456. doi: 10.1007/s13277-014-2856-2. [DOI] [PubMed] [Google Scholar]
- 8.Hirata H, Sugimachi K, Takahashi Y, Ueda M, Sakimura S, Uchi R, Kurashige J, Takano Y, Nanbara S, Komatsu H, Saito T, Shinden Y, Iguchi T, Eguchi H, Atsumi K, Sakamoto K, Doi T, Hirakawa M, Honda H, Mimori K. Downregulation of PRRX1 Confers Cancer Stem Cell-Like Properties and Predicts Poor Prognosis in Hepatocellular Carcinoma. Ann Surg Oncol. 2015;22:S1402–1409. doi: 10.1245/s10434-014-4242-0. [DOI] [PubMed] [Google Scholar]
- 9.Yoon SK. The biology of cancer stem cells and its clinical implication in hepatocellular carcinoma. Gut Liver. 2012;6:29–40. doi: 10.5009/gnl.2012.6.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 11.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 12.Khan MI, Czarnecka AM, Helbrecht I, Bartnik E, Lian F, Szczylik C. Current approaches in identification and isolation of human renal cell carcinoma cancer stem cells. Stem Cell Res Ther. 2015;6:178. doi: 10.1186/s13287-015-0177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Min SO, Lee SW, Bak SY, Kim KS. Ideal sphere-forming culture conditions to maintain pluripotency in a hepatocellular carcinoma cell lines. Cancer Cell Int. 2015;15:95. doi: 10.1186/s12935-015-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida Y, Tanaka S, Aihara A, Adikrisna R, Yoshitake K, Matsumura S, Mitsunori Y, Murakata A, Noguchi N, Irie T, Kudo A, Nakamura N, Lai PB, Arii S. Analogy between sphere forming ability and stemness of human hepatoma cells. Oncol Rep. 2010;24:1147–1151. doi: 10.3892/or_00000966. [DOI] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 16.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 17.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiemer EA. The role of microRNAs in cancer: no small matter. Eur J Cancer. 2007;43:1529–1544. doi: 10.1016/j.ejca.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Osada H, Takahashi T. MicroRNAs in biological processes and carcinogenesis. Carcinogenesis. 2007;28:2–12. doi: 10.1093/carcin/bgl185. [DOI] [PubMed] [Google Scholar]
- 21.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 22.Lujambio A, Lowe SW. The microcosmos of cancer. Nature. 2012;482:347–355. doi: 10.1038/nature10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen SP, Liu BX, Xu J, Pei XF, Liao YJ, Yuan F, Zheng F. MiR-449a suppresses the epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma by multiple targets. BMC Cancer. 2015;15:706. doi: 10.1186/s12885-015-1738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu K, Liu S, Zhang W, Jia B, Tan L, Jin Z, Liu Y. miR-494 promotes cell proliferation, migration and invasion, and increased sorafenib resistance in hepatocellular carcinoma by targeting PTEN. Oncol Rep. 2015;34:1003–1010. doi: 10.3892/or.2015.4030. [DOI] [PubMed] [Google Scholar]
- 25.Olsen PH, Ambros V. The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev Biol. 1999;216:671–680. doi: 10.1006/dbio.1999.9523. [DOI] [PubMed] [Google Scholar]
- 26.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Croce CM. Causes, consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang J, Wang Z, Chen L, Huang G, Hu X. Gossypol acetate induced apoptosis of pituitary tumor cells by targeting the BCL-2 via the upregulated microRNA miR-15a. Int J Clin Exp Med. 2015;8:9079–9085. [PMC free article] [PubMed] [Google Scholar]
- 29.Macchia G, Lonoce A, Venuto S, Macrí E, Palumbo O, Carella M, Lo Cunsolo C, Iuzzolino P, Hernández-Sánchez M, Hernandez-Rivas JM, Storlazzi CT. A rare but recurrent t(8;13)(q24;q14) translocation in B-cell chronic lymphocytic leukaemia causing MYC up-regulation and concomitant loss of PVT1, miR-15/16 and DLEU7. Br J Haematol. 2016;172:296–299. doi: 10.1111/bjh.13482. [DOI] [PubMed] [Google Scholar]
- 30.Sirotkin AV, Kisová G, Brenaut P, Ovcharenko D, Grossmann R, Mlyncek M. Involvement of microRNA Mir15a in control of human ovarian granulosa cell proliferation, apoptosis, steroidogenesis, and response to FSH. Microrna. 2014;3:29–36. doi: 10.2174/2211536603666140227232824. [DOI] [PubMed] [Google Scholar]
- 31.Lin K, Farahani M, Yang Y, Johnson GG, Oates M, Atherton M, Douglas A, Kalakonda N, Pettitt AR. Loss of MIR15A and MIR16-1 at 13q14 is associated with increased TP53 mRNA, de-repression of BCL2 and adverse outcome in chronic lymphocytic leukaemia. Br J Haematol. 2014;167:346–355. doi: 10.1111/bjh.13043. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Fang X, Li W, Shi Q, Wu L, Chen X, Huang Z, Wu P, Wang Z, Liao Z. [Influence of recombinant lentiviral vector encoding miR-15a/16-1 in biological features of human nasopharyngeal carcinoma CNE-2Z cells] . Cancer Biother Radiopharm. 2014;29:422–427. doi: 10.1089/cbr.2013.1596. [DOI] [PubMed] [Google Scholar]
- 33.Ricieri Brito JA, Gomes CC, Santos Pimenta FJ, Barbosa AA, Prado MA, Prado VF, Gomez MV, Gomez RS. Reduced expression of mir15a in the blood of patients with oral squamous cell carcinoma is associated with tumor staging. Exp Ther Med. 2010;1:217–221. doi: 10.3892/etm_00000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao L, Zhou Y, Zhai B, Liao J, Xu W, Zhang R, Li J, Zhang Y, Chen L, Qian H, Wu M, Yin Z. Sphere-forming cell subpopulations with cancer stem cell properties in human hepatoma cell lines. BMC Gastroenterol. 2011;11:71. doi: 10.1186/1471-230X-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 36.Ramsay RG, Gonda TJ. MYB function in normal and cancer cells. Nat Rev Cancer. 2008;8:523–534. doi: 10.1038/nrc2439. [DOI] [PubMed] [Google Scholar]
- 37.Fang F, Rycyzyn MA, Clevenger CV. Role of c-Myb during prolactin-induced signal transducer and activator of transcription 5a signaling in breast cancer cells. Endocrinology. 2009;150:1597–1606. doi: 10.1210/en.2008-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramsay RG. c-Myb a stem-progenitor cell regulator in multiple tissue compartments. Growth Factors. 2005;23:253–261. doi: 10.1080/08977190500233730. [DOI] [PubMed] [Google Scholar]
- 39.Biroccio A, Benassi B, D’Agnano I, D’Angelo C, Buglioni S, Mottolese M, Ricciotti A, Citro G, Cosimelli M, Ramsay RG, Calabretta B, Zupi G. c-Myb and Bcl-x overexpression predicts poor prognosis in colorectal cancer: clinical and experimental findings. Am J Pathol. 2001;158:1289–1299. doi: 10.1016/S0002-9440(10)64080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greco C, Alvino S, Buglioni S, Assisi D, Lapenta R, Grassi A, Stigliano V, Mottolese M, Casale V. Activation of c-MYC and c-MYB proto-oncogenes is associated with decreased apoptosis in tumor colon progression. Anticancer Res. 2001;21:3185–3192. [PubMed] [Google Scholar]
- 41.Zhang J, Luo N, Luo Y, Peng Z, Zhang T, Li S. microRNA-150 inhibits human CD133-positive liver cancer stem cells through negative regulation of the transcription factor c-Myb. Int J Oncol. 2012;40:747–756. doi: 10.3892/ijo.2011.1242. [DOI] [PubMed] [Google Scholar]
- 42.Chen RX, Xia YH, Xue TC, Ye SL. Transcription factor c-Myb promotes the invasion of hepatocellular carcinoma cells via increasing osteopontin expression. J Exp Clin Cancer Res. 2010;29:172. doi: 10.1186/1756-9966-29-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ladu S, Calvisi DF, Conner EA, Farina M, Factor VM, Thorgeirsson SS. E2F1 inhibits c-Myc-driven apoptosis via PIK3CA/Akt/mTOR and COX-2 in a mouse model of human liver cancer. Gastroenterology. 2008;135:1322–1332. doi: 10.1053/j.gastro.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang H, Huang ZZ, Wang J, Lu SC. The role of c-Myb and Sp1 in the up-regulation of methionine adenosyltransferase 2A gene expression in human hepatocellular carcinoma. FASEB J. 2001;15:1507–1516. doi: 10.1096/fj.01-0040com. [DOI] [PubMed] [Google Scholar]
- 45.Feng J, Yang Y, Zhang P, Wang F, Ma Y, Qin H, Wang Y. miR-150 functions as a tumour suppressor in human colorectal cancer by targeting c-Myb. J Cell Mol Med. 2014;18:2125–2134. doi: 10.1111/jcmm.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]


