Abstract
Hypothermia may attenuate reperfusion injury and thereby improve acute myocardial infarction therapy. Systemic cooling trials failed to reduce infarct size, perhaps because the target temperature was not reached fast enough. The use of selective intracoronary hypothermia combined with intracoronary temperature monitoring allows for titrating to target temperature and optimizing the cooling rate. We aimed to the test the feasibility of intracoronary cooling for controlled, selective myocardial hypothermia in an isolated beating pig heart. In five porcine hearts the left anterior descending artery (LAD) was occluded by an over-the-wire balloon (OTWB). After occlusion, saline at 22°C was infused through the OTWB lumen for 5 minutes into the infarct area at a rate of 30 ml/min. Thereafter the balloon was deflated but infusion continued with saline at 4°C for 5 minutes. Distal coronary temperature was continuously monitored by a pressure/temperature guidewire. Myocardial temperature at several locations in the infarct and control areas was recorded using needle thermistors. In the occlusion phase, coronary temperature decreased by 11.4°C (range 9.4-12.5°C). Myocardial temperature throughout the infarct area decreased by 5.1°C (range 1.8-8.1°C) within three minutes. During the reperfusion phase, coronary temperature decreased by 6.2°C (range 4.1-10.3°C) and myocardial temperature decreased by 4.5°C (range 1.5-7.4°C). Myocardial temperature outside the infarct area was not affected. In the isolated beating pig heart with acute occlusion of the LAD, we were able to rapidly “induce, maintain, and control” a stable intracoronary and myocardial target temperature of at least 4°C below body temperature without side effects and using standard PCI equipment, justifying further studies of this technique in humans.
Keywords: Intracoronary hypothermia, acute myocardial infarction, reperfusion injury
Introduction
Early reperfusion by primary percutaneous coronary intervention (PCI) remains the cornerstone of treatment for acute myocardial infarction (AMI) [1-3]. Nevertheless, despite timely reperfusion 7-10% of AMI patients die during the index hospitalization and 23% of survivors develop heart failure within one year [4,5].
Besides ischemia-induced myocardial necrosis, considerable further damage to the myocardium may occur due to the reperfusion process itself [6]. This reperfusion injury contributes substantially to the final infarct size [7]. Despite many advances made in revascularization therapies, the prevention and treatment of reperfusion injury in AMI has not proven successful in clinical practice.
Mild hypothermia, i.e. cooling of endangered myocardium to 33°C (91.4°F), may attenuate reperfusion injury [8]. Animal studies in a variety of species have shown that induction of hypothermia prior to reperfusion reduces infarct size [9-23]. However, if hypothermia is initiated after the onset of reperfusion, this benefit is not observed [12,24-26]. Human studies in patients with AMI have not demonstrated a reduction in infarct size using different methods of systemic hypothermia [27-36]. This is likely related to the fact that, in the majority of patients, sufficient hypothermia was not reached before the onset of reperfusion [27-31]. In most of these studies, whole body hypothermia was induced by an endovascular cooling system in combination with administration of cold saline or noninvasive surface cooling [29]. This mode of delivery hampered rapid and sufficient cooling of the endangered myocardium and led to adverse systemic reactions such as shivering, enhanced adrenergic state, or volume overload [31,37].
Selective intracoronary induction of hypothermia preceding primary PCI may greatly reduce the time needed to achieve therapeutic hypothermia while simultaneously preventing adverse systemic effects. The intracoronary application of hypothermia has already been investigated in two animal studies demonstrating safety, feasibility, and a significant reduction in myocardial necrosis [9,38].
In those studies, however, achievement of sufficient hypothermia required up to 60 minutes-too much delay for clinical application in humans with AMI. Furthermore, only myocardial temperature was monitored by external thermistors implanted in the myocardium, which is fundamentally not possible during primary PCI in humans.
In the present study we aimed to: (1) evaluate the feasibility and reproducibility of selective intracoronary hypothermia in isolated beating pig hearts during occlusion and subsequent reperfusion of the left anterior descending coronary artery using regular PCI equipment; and (2) study the relationship between intracoronary temperature distal to the occlusion, measured by a pressure/temperature guidewire (PTW), and distribution of hypothermia across the endangered myocardium, measured by thermistors. Knowledge of such distribution is mandatory to apply hypothermia safely in humans, where only intracoronary temperature can be measured.
This study was performed to test only the methodology of rapid selective intracoronary cooling using regular PCI equipment but did not aim to say anything about outcome. That should be the goal of further studies.
Methods
In humans a target temperature of 33°C (4°C below normal blood temperature) has been suggested to protect against reperfusion injury and has proven to be safe [39-41]. By analogy our present study sought to lower the myocardial temperature throughout the infarct area by 4°C. Such a decrease of temperature should be obtained quickly during occlusion and maintained after reperfusion. Moreover, the temperature decrease should be limited to the infarct area.
Therefore, prior to the present study, we performed 2 dose-finding experiments to search for the most appropriate saline temperature and infusion rate. Our goals included both induction and maintenance: to reach the target temperature rapidly but without the risk of decreasing temperature too much; and to keep this temperature stable in the infarct-related artery and its dependent myocardium throughout the occlusion and reperfusion phases. The infusion rate and temperature of saline in this study are based upon those dose-finding experiments.
Isolated beating pig heart model
In this study hearts were obtained from Dutch landrace hybrid pigs slaughtered for human consumption. Following regular slaughterhouse protocols, the chest was opened to extract the heart. The isolated heart was immediately cooled and cannulated to administer 1 L of cold cardioplegic solution (4°C modified St. Thomas 2 added with 5000 IU of heparin) via the coronary arteries, such that warm ischemic time never exceeded 5 min. Further preparation was carried out according to the standards of our isolated beating heart pig model as previously described [42,43].
After preparation the isolated pig heart was fixed in a supine position and mounted on an external circulation platform [42]. The circulation loop consisted of a centrifugal pump (Sarns 9000 Perfusion System, 3 M, St. Paul, MN, USA) that drew blood from a venous reservoir through an arterial filter (AFFINITY Arterial 38 mm blood filter, Medtronic, Minneapolis, MN, USA) and a combined oxygenator-heat exchanger (AFFINITY NT Oxygenator, Medtronic) retrogradely into the aortic cannula. Blood was kept at 38°C and oxygenated with a 20% O2, 5% CO2, 75% N2 carbogen gas mixture. The heart started to have spontaneous contractions and gradually regained sinus rhythm after coronary perfusion was reinstated. In some cases, defibrillation was required with 10-30 J to restore sinus rhythm. An external pacemaker (Medtronic Model 5388) was connected to the left atrium to maintain a stable heart rate as necessary.
Blood gas values and electrolytes were monitored using a VetScan i-STAT 1 (Abaxis, Union City, CA, USA). Coronary blood flow was measured by an ultrasonic flow probe (em-tec Sonno TT 1/2“×3/32”, em-tec GmbH, Finning, Germany) with a flow amplifier (HFM-10-1, LifeTec Group, Eindhoven, The Netherlands).
Catheterization and thermistors
The following specific instrumentation was applied in this study: A 6 F guiding catheter was advanced to the ostium of the left coronary artery and affixed to the aortic wall. The pressure/temperature wire (Pressurewire Certus®, St Jude Medical, Minneapolis, MN) was advanced through this guiding catheter and positioned in the distal LAD. Next, a regular guide wire was also advanced into the distal LAD. Next an over the wire balloon (OTWB, Apex, Boston Scientific, 12×2.5-3.5 mm) was advanced over this second wire and positioned in the mid LAD, whereafter that second wire was removed. In this way the OTWB allowed us both to create a mid-LAD occlusion, and to infuse saline through its lumen selectively into the infarct area. The pressure/temperature guidewire was kept in place in the distal coronary artery for continuous temperature recording. Saline was infused using an infusion pump (Medrad® Mark V ProVis® Angiographic system) with an adjustable infusion rate.
A thermal imaging camera (FLIR ThermaCam Researcher Professional S65HS) was used to delineate the cooled infarct area after a short infusion of cold saline (Figure 1). Based upon those images, three intramyocardial thermistors (HYP-1 connected to TC-08 thermocouple data logger, OMEGA Engineering INC, Connecticut, USA) were placed within the infarct area: proximal (close to the occlusion), mid (center of infarct area) and distal (close to the apex), respectively (Figure 2). A fourth thermistor was placed in the non-infarcted myocardium as a control and a fifth in the proximal great cardiac vein to determine coronary venous blood temperature (Figure 2).
Figure 1.
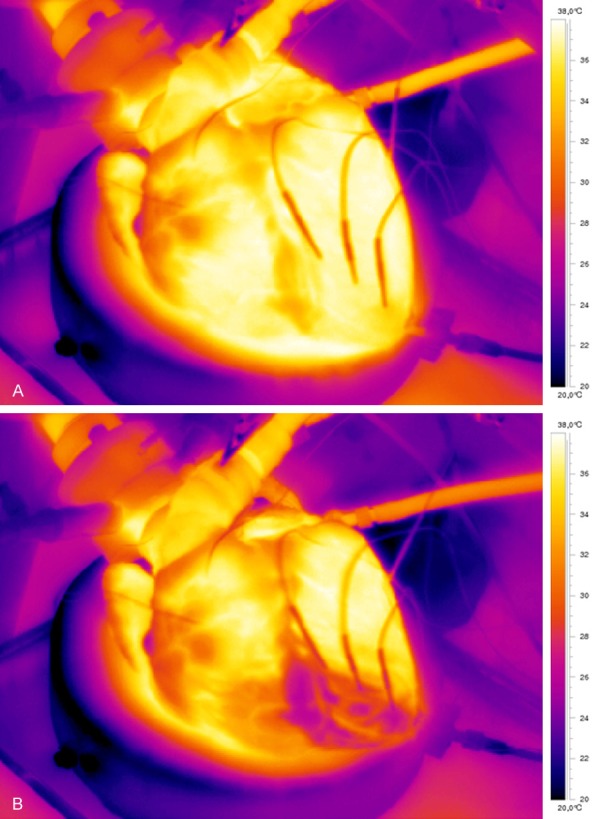
Visualization of the infarct area by infrared camera before (A) and during (B) selective infusion of cold saline through the balloon catheter in the occluded coronary artery. Moving images of the isolated beating heart and recordings of the cooled infarct area by the infrared camera are presented in the supplemental appendix online.
Figure 2.
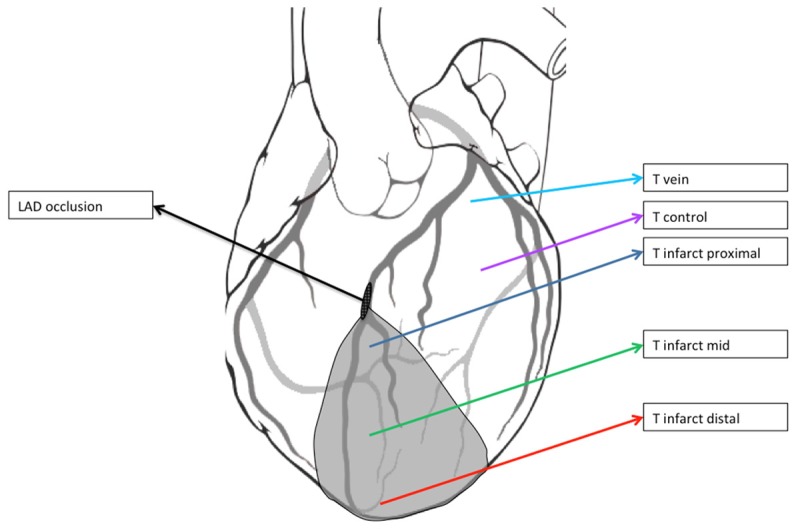
Overview of the thermistor position. The grey zone indicates the infarct area. The black oval indicates the site of occlusion in the Left Anterior Descending Artery (LAD). T infarct proximal: thermistor close to the site of the LAD occlusion; T infarct mid: thermistor at the mid infarct area; T infarct distal: thermistor at the distal infarct area; T control: thermistor in the non-infarcted myocardium; T vein: thermistor in the proximal great cardiac vein. In addition, the guidewire with temperature sensor is present within the distal lumen of the coronary artery (Not shown).
After having completed these preparations, the beating heart was completely submerged in a 38°C (100°F) water bath to simulate the surrounding body temperature. Finally, 100 mg of amiodarone was added to the circulating blood to prevent ischemia-related rhythm disturbances as these frequently occur in pigs during occlusion of a coronary artery.
Using this setup, temperature was continuously monitored at 6 locations: distal coronary artery, 3 myocardial positions throughout the infarct area, reference myocardium, and the great cardiac vein.
Experimental protocol
At first the balloon in the LAD was inflated to 4 atmospheres to create coronary occlusion. Immediately thereafter, infusion of 22°C saline (room temperature) at a rate of 30 ml/min was started and maintained during the next 5 minutes of balloon occlusion. Next, the balloon was deflated, and anterograde blood flow was restored while the infusion of saline was continued at a temperature of 4°C at a rate of 30 ml/min for another 5 minutes (Figure 3). After an interval of 20 minutes, the whole sequence was repeated.
Figure 3.
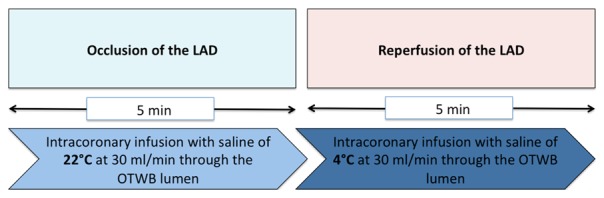
Intracoronary hypothermia protocol.
At the end of the experiment after the heart had died, methylene blue staining was administered through the inflated balloon to delineate the infarct area post-mortem and to determine the mass of the infarct related territory.
Statistics
Data are expressed as mean with range. Repeatability within an experiment was assessed as the relative difference between two consecutive measurements. In this study, reproducibility was defined as the capability to replicate the same temperature courses among the five experiments using the predefined protocol.
Results
Baseline characteristics and procedural results
Baseline characteristics of the five hearts are presented in Table 1. The isolation, instrumentation, resuscitation, and catheterization was uncomplicated and the experimental protocol could be performed in all 5 hearts.
Table 1.
Baseline characteristics of the five isolated pig heart models
| Heart | 1 | 2 | 3 | 4 | 5 |
| Heart rate (bpm.) | 126 | 91 | 77 | 102 | 105 |
| Blood temp (°C) | 38.3 | 38.5 | 37.7 | 37.2 | 38 |
| Area at risk (g, %) | 42 (17%) | 38 (15%) | 55 (22%) | 60 (24%) | 45 (18%) |
The area at risk was determined at the end of the experiments by methylene blue staining of the infarct area and is expressed as a percentage of the mean left ventricular mass of a domestic pig. Bpm = beats per minute.
In all hearts, infrared camera recordings allowed excellent delineation of the infarct area (Figure 1, Supplementary Video).
Coronary and myocardial temperature during saline infusion
The target myocardial temperature of 4°C below blood temperature was obtained in all hearts with the exception of the first one, in which the mean temperature decrease was 3.5°C. The mean time intervals to target temperature in the coronary artery and the different parts of the infarct area are listed in Table 2.
Table 2.
Top, time to induce the target temperature in the distal coronary artery and at 3 sites of the infarct area
| Occlusion mean [range] | Reperfusion mean [range] | |||
|---|---|---|---|---|
| Time to achieve 34°C (s) | ||||
| Coronary artery | 4.6 | [3.6-5.1] | - | - |
| Infarct area | ||||
| Proximal | 56 | [37.5-89.5] | - | - |
| Mid | 77.1 | [42.3-130.6] | - | - |
| Distal | 110.1 | [62.9-176.4] | - | - |
| Temperature in °C | ||||
| Coronary artery | 26.5 | [25.4-28.5] | 31.7 | [27.6-33.8] |
| Infarct area | ||||
| Proximal | 32.6 | [29.9-36.4] | 33.8 | [30.0-37.7] |
| Mid | 33.0 | [29.3-35.6] | 33.7 | [30.5-37.7] |
| Distal | 32.7 | [30.3-36.4] | 32.8 | [30.9-33.7] |
| Great cardiac vein | 34.4 | [33.5-36.0] | 34.7 | [33.6-35.5] |
Bottom, blood and myocardial temperatures during the occlusion and reperfusion phases. Temperature in the reference myocardium remains unchanged and normal during all experiments.
An example of the temperature recordings at all relevant sites during occlusion and reperfusion is illustrated in Figure 4 and a mean graph of all hearts is shown in Figure 5.
Figure 4.
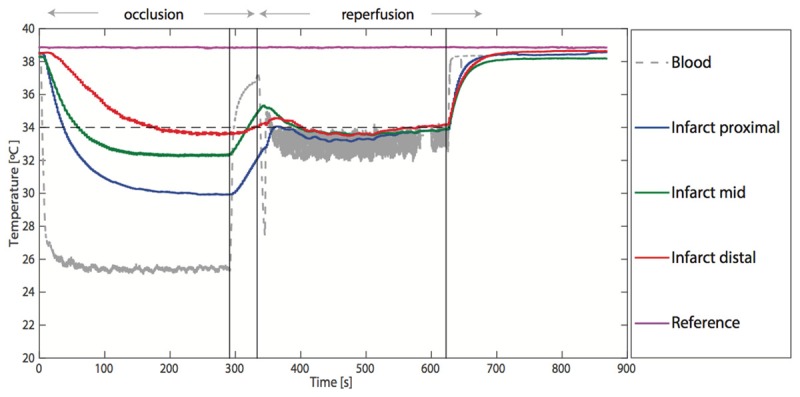
Continuous registration of the temperatures in the coronary arterial blood, the proximal infarct area, the mid infarct area, the distal infarct area, and the reference myocardium during one experiment. After the coronary artery is occluded, there is a rapid induction of the target temperature in the infarcted myocardium during an infusion of 30 ml/min saline at 22°C. The temperature remains stable during the occlusion phase. In the reperfusion phase (middle vertical line), the infusion rate is maintained at 30 ml/min, but the temperature is lowered to 4°C. This results in a stable myocardial temperature in the infarct area. In the reperfusion phase, the temperatures at the different sites within the infarct area are closer to the temperature in the coronary artery. This is explained in the discussion. During the very short time needed to change the temperature of the infused saline (between the left and middle vertical line), only coronary temperature increases rapidly, but myocardial temperature remains at the target temperature. Temperatures normalize rapidly after stopping saline infusion during the reperfusion phase (right vertical line).
Figure 5.
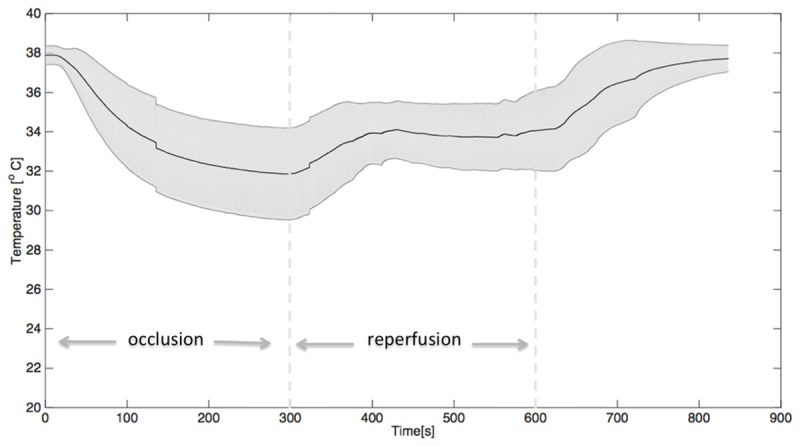
Graph demonstrating the mean ± SEM of the temperature in the infarct area recorded in the five experiments. At 300 seconds, the first dotted line indicates the start of reperfusion when changing the infusion temperature from 22°C to 4°C. After 600 seconds (the second dotted line) the infusion of cold saline is stopped, whereafter temperatures normalize within minutes.
During the occlusion phase, temperature in the distal coronary artery decreased by 11.4°C (range 9.4-12.5°C) and myocardial temperature by 5.3°C (range 1.5-8.0°C), 4.9°C (range 2.3-8.6°C) and 5.2°C (range 1.5-7.6°C) in the proximal, mid, and distal infarct areas, respectively. Temperature in the non-infarct reference area was not affected and remained unchanged during occlusion. Temperature in the great cardiac vein decreased by 2.9°C (range 1.0-4.2°C).
During the reperfusion phase, temperature in the distal coronary artery decreased by 6.2°C (range 4.1-10.3°C) and myocardial temperature decreased by 4.1°C (range 0.2-7.9°C), 4.2°C (range 0.2-7.4°C) and 5.1°C (range 4.2-7.0°C) in the proximal, mid, and distal infarct areas, respectively. Temperature in the non-infarct reference area was not affected and remained unchanged during reperfusion. Temperature in the great cardiac vein decreased by 2.6°C (range 1.5-3.4°C) (Table 2).
During the very short time needed to change the infusion of saline from room temperature to 4°C, only coronary temperature increased, but myocardial temperature remained close to target temperature (Figures 4, 5).
After stopping the infusion of saline, all temperatures returned to normal within 60 seconds.
The relation between distal coronary temperature and myocardial temperature-important for safe application of this technique in humans with AMI-was reproducible in all hearts, but differed between the occlusion and reperfusion phase (Figure 4), which will further be explained in the discussion.
Repeatability and reproducibility
With the exception of the first heart, the cooling protocol was performed twice in each heart with an interval of 20 minutes as described in the methods. Relative differences between the two measurements of myocardial temperatures were small and are shown in Supplementary Table 1. The temperature courses of the 5 experiments were similar and reproducible.
Discussion
In this beating pig heart model we demonstrated the feasibility of selective intracoronary infusion of cold saline as a method to achieve myocardial hypothermia for potentially limiting reperfusion injury. Using routine equipment of an over-the-wire balloon infusion catheter and an intracoronary pressure/temperature wire, we were able to cool the endangered myocardium selectively, rapidly, and stability without side effects. At an infusion rate of 30 ml/min saline at a temperature of 22°C in the occlusion phase and 4°C in the reperfusion phase, the target temperature of 34°C was rapidly achieved and maintained. Repeatability was excellent.
In addition, and in contrast to earlier studies in this field, the saline infusion rate was primarily determined and safe-guarded by intracoronary temperature measurement using routine PCI equipment. Our experimental setup allowed the relationship between coronary temperature distal to the occlusion and myocardial temperature to be carefully assessed.
Currently there is no effective therapy for preventing reperfusion injury in patients with acute myocardial infarction. Factors responsible for this complex cascade of reperfusion-induced myocardial necrosis include oxidative stress, mitochondrial dysfunction, calcium overload, depletion of high-energy phosphates, infiltration of leucocyte aggregates with activation of cytokines and vasoconstrictors, and intramyocardial edema with no-reflow [6,7,44-47]. Therefore, the pathophysiology of reperfusion injury offers numerous therapeutic windows of opportunity, but all clinical studies focusing on a single part of the cascade (such as the use of anti-oxidants, calcium-channel blockers, anti-inflammatory agents and cyclosporine) have not been able to prove a clear benefit.
This absence of effect may be due to several factors including treating an epiphenomenon instead of a true pathophysiologic part of the cascade, intolerable delay in door-to-balloon time, or maybe because the therapeutic agents only reach the target tissue (i.e. infarcted myocardium) after reperfusion has occurred.
Hypothermia has proven to affect almost every pathway involved in reperfusion injury and has proven to be effective in a number of experimental studies, but is limited by the time needed to reach the target temperature and undesirable side-effects (such as shivering, enhanced adrenergic state, and iatrogenic volume overload) when using whole body cooling. Moreover, it is unclear to what extent the endangered myocardium behind an occluded coronary artery is truly cooled.
Our study is the first to overcome these limitations, enabling fast and selective cooling, and reaching specifically the target tissue before onset of reperfusion, while being able to monitor the temperature in the distal coronary artery.
Unique strengths of the present study
The methodology used in the present study has some unique advantages compared to earlier studies in this field. For safe and effective application of selective hypothermia to protect endangered myocardium from reperfusion injury in humans, three conditions are paramount. First, the endangered myocardium should be cooled to the target temperature quickly enough to avoid delay in PCI and opening of the vessel. With our protocol, a decrease by 4°C below body temperature was achieved within minutes (Figures 4 and 5).
Second, the cooling must be limited to the infarct area and not affect the adjacent healthy myocardium. Using the infrared camera and thermistors in the control area, we confirmed that hypothermia was limited to the perfusion territory of the infarct-related artery (as expected because cold saline is administered only distal to the occlusion).
Third, and maybe most importantly, to apply such a technique safely in humans it is paramount to monitor and control the temperature in the distal coronary artery and the myocardium. This was achieved in our study by the presence of a commercially available pressure/temperature sensor-tipped guidewire in the distal coronary artery and by showing a reproducible relationship between distal coronary artery temperature and temperature in the endangered myocardium.
Thus in contrast to earlier studies, the paramount conditions for clinical application of intracoronary hypothermia were fulfilled in the present study.
In addition, the total volume of saline is limited in our protocol to 300-600 ml, thereby avoiding volume overload.
Relation between coronary and myocardial temperature during cooling
Of special interest is the relationship between distal coronary temperature and myocardial temperature during saline infusion throughout the occlusion and the reperfusion phases, as demonstrated in Figures 4 and 5.
During the occlusion phase, coronary blood flow is interrupted and cooling of the myocardium occurs mainly by the physical phenomenon of heat-conduction. Consequently, there is a temperature gradient between the coronary artery and the myocardium.
During the reperfusion phase, distal coronary blood flow is present again and cooling of the dependent myocardium occurs mainly by heat-convection. Consequently, only a minimal temperature gradient is present between the coronary artery and the surrounding myocardium. These phenomena also explain why the adjacent, non-infarcted myocardium does not cool down, as it is the convection heat from the non-infarcted coronary artery that maintains normal temperature in the reference area. Understanding of these phenomena and a reproducible relation between distal coronary and myocardial temperature is important for safe application in humans, when the rate of cold saline infusion needs to be guided by the achieved coronary temperature.
Study limitations
Our study has several limitations.
First, although the isolated beating pig heart is an elegant way of replacing classical in vivo animal studies of simulating acute myocardial infarction, ex vivo testing is not equivalent to in vivo testing. Not all physiologic feedback mechanisms remain intact in the isolated heart and unknown differences may be present [48].
Second, there is a difference between an artificial LAD occlusion in an isolated beating pig heart and a myocardial infarction in humans. The absence of noticeable side effects in the pig heart, which is usually more vulnerable to ischemic arrhythmia than a human heart, is reassuring.
Third, it has not been firmly established that a myocardial temperature of 4°C below body temperature, is the most effective temperature for preventing reperfusion injury. The choice of 4°C is therefore somewhat arbitrary. Nevertheless, because of other studies with hypothermia both in animal and in human organs, like the brain, and based upon data from cardiac surgery, we selected a decrease of 4°C as the target for this study [39,49].
Fourth, it is unclear when hypothermia should be started and how long it should be continued. From a theoretical point of view, it seems plausible to assume that cooling time before opening the occluded coronary artery can be short and that the major condition here is that the temperature has decreased sufficiently at the moment when reperfusion occurs. It is unknown how long after reperfusion hypothermia should be continued. The post-reperfusion hypothermia period of 5 minutes in our study was chosen because of practical considerations and is arbitrary.
Fifth, it should be emphasized that this study focuses only on the method of applying selective intracoronary hypothermia and did not evaluate outcome effects on infarct size compared to normothermia or standard therapy. The logical next step is assessing of safety and feasibility of this method in human subjects with acute myocardial infarction. Such study is currently performed, using exactly the same interventional methodology as in the present study (ClinicalTrials.gov. Identifier NCT02753478). Only thereafter, a larger randomized controlled outcome study comes into perspective as proof-of-principle. The present study is a first but mandatory step in such promising development and justifies further investigations.
Conclusion
In the isolated beating pig heart model of acute myocardial infarction, controlled and selective myocardial hypothermia can be achieved rapidly, safely, stable, and effectively. Our technique adds the advantage of minimal volume load and uses standard PCI equipment.
This study justifies further investigation on the use of intracoronary hypothermia in patients with acute myocardial infarction to reduce reperfusion injury and infarct size.
Acknowledgements
We would like to thank Dr. Nils Johnson for his critical appraisal of the manuscript. This study was supported in part by a grant of the “Stichting Vrienden Van Het Hart” (Friends of The Heart Foundation) in Eindhoven, The Netherlands.
Disclosure of conflict of interest
None.
Supplementary Table 1
Supplementary Video
References
- 1.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 2.Berger PB, Ellis SG, Holmes DR Jr, Granger CB, Criger DA, Betriu A, Topol EJ, Califf RM. Relationship between delay in performing direct coronary angioplasty and early clinical outcome in patients with acute myocardial infarction: results from the global use of strategies to open occluded arteries in acute coronary syndromes (GUSTO-IIb) trial. Circulation. 1999;100:14–20. doi: 10.1161/01.cir.100.1.14. [DOI] [PubMed] [Google Scholar]
- 3.O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation. 2013;127:e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 4.Kaul P, Ezekowitz JA, Armstrong PW, Leung BK, Savu A, Welsh RC, Quan H, Knudtson ML, McAlister FA. Incidence of heart failure and mortality after acute coronary syndromes. Am Heart J. 2013;165:379–385. e372. doi: 10.1016/j.ahj.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 5.McManus DD, Gore J, Yarzebski J, Spencer F, Lessard D, Goldberg RJ. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med. 2011;124:40–47. doi: 10.1016/j.amjmed.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 7.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simkhovich BZ, Hale SL, Kloner RA. Metabolic mechanism by which mild regional hypothermia preserves ischemic tissue. J Cardiovasc Pharmacol Ther. 2004;9:83–90. doi: 10.1177/107424840400900203. [DOI] [PubMed] [Google Scholar]
- 9.Otake H, Shite J, Paredes OL, Shinke T, Yoshikawa R, Tanino Y, Watanabe S, Ozawa T, Matsumoto D, Ogasawara D, Yokoyama M. Catheter-based transcoronary myocardial hypothermia attenuates arrhythmia and myocardial necrosis in pigs with acute myocardial infarction. J Am Coll Cardiol. 2007;49:250–260. doi: 10.1016/j.jacc.2006.06.080. [DOI] [PubMed] [Google Scholar]
- 10.Miki T, Liu GS, Cohen MV, Downey JM. Mild hypothermia reduces infarct size in the beating rabbit heart: a practical intervention for acute myocardial infarction? Basic Res Cardiol. 1998;93:372–383. doi: 10.1007/s003950050105. [DOI] [PubMed] [Google Scholar]
- 11.Chien GL, Wolff RA, Davis RF, van Winkle DM. “Normothermic range” temperature affects myocardial infarct size. Cardiovasc Res. 1994;28:1014–1017. doi: 10.1093/cvr/28.7.1014. [DOI] [PubMed] [Google Scholar]
- 12.Maeng M, Mortensen UM, Kristensen J, Kristiansen SB, Andersen HR. Hypothermia during reperfusion does not reduce myocardial infarct size in pigs. Basic Res Cardiol. 2006;101:61–68. doi: 10.1007/s00395-005-0550-7. [DOI] [PubMed] [Google Scholar]
- 13.Dae MW, Gao DW, Sessler DI, Chair K, Stillson CA. Effect of endovascular cooling on myocardial temperature, infarct size, and cardiac output in human-sized pigs. Am J Physiol Heart Circ Physiol. 2002;282:H1584–1591. doi: 10.1152/ajpheart.00980.2001. [DOI] [PubMed] [Google Scholar]
- 14.Duncker DJ, Klassen CL, Ishibashi Y, Herrlinger SH, Pavek TJ, Bache RJ. Effect of temperature on myocardial infarction in swine. Am J Physiol. 1996;270:H1189–1199. doi: 10.1152/ajpheart.1996.270.4.H1189. [DOI] [PubMed] [Google Scholar]
- 15.Hale SL, Dave RH, Kloner RA. Regional hypothermia reduces myocardial necrosis even when instituted after the onset of ischemia. Basic Res Cardiol. 1997;92:351–357. doi: 10.1007/BF00788947. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz LM, Verbinski SG, Vander Heide RS, Reimer KA. Epicardial temperature is a major predictor of myocardial infarct size in dogs. J Mol Cell Cardiol. 1997;29:1577–1583. doi: 10.1006/jmcc.1997.0391. [DOI] [PubMed] [Google Scholar]
- 17.Dai W, Herring MJ, Hale SL, Kloner RA. Rapid surface cooling by ThermoSuit system dramatically reduces scar size, prevents post-infarction adverse left ventricular remodeling, and improves cardiac function in rats. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herring MJ, Dai W, Hale SL, Kloner RA. Rapid induction of hypothermia by the ThermoSuit system profoundly reduces infarct size and anatomic zone of no reflow following ischemia-reperfusion in rabbit and rat hearts. J Cardiovasc Pharmacol Ther. 2015;20:193–202. doi: 10.1177/1074248414535664. [DOI] [PubMed] [Google Scholar]
- 19.Kanemoto S, Matsubara M, Noma M, Leshnower BG, Parish LM, Jackson BM, Hinmon R, Hamamoto H, Gorman JH 3rd, Gorman RC. Mild hypothermia to limit myocardial ischemia-reperfusion injury: importance of timing. Ann Thorac Surg. 2009;87:157–163. doi: 10.1016/j.athoracsur.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voorhees WD 3rd, Abendschein DR, Tacker WA Jr. Effect of whole-body hypothermia on myocardial blood flow and infarct salvage during coronary artery occlusion in dogs. Am Heart J. 1984;107:945–949. doi: 10.1016/0002-8703(84)90833-0. [DOI] [PubMed] [Google Scholar]
- 21.Haendchen RV, Corday E, Meerbaum S, Povzhitkov M, Rit J, Fishbein MC. Prevention of ischemic injury and early reperfusion derangements by hypothermic retroperfusion. J Am Coll Cardiol. 1983;1:1067–1080. doi: 10.1016/s0735-1097(83)80109-0. [DOI] [PubMed] [Google Scholar]
- 22.Meerbaum S, Haendchen RV, Corday E, Povzhitkov M, Fishbein MC, Y-Rit J, Lang TW, Uchiyama T, Aosaki N, Broffman J. Hypothermic coronary venous phased retroperfusion: a closed-chest treatment of acute regional myocardial ischemia. Circulation. 1982;65:1435–1445. doi: 10.1161/01.cir.65.7.1435. [DOI] [PubMed] [Google Scholar]
- 23.Wakida Y, Haendchen RV, Kobayashi S, Nordlander R, Corday E. Percutaneous cooling of ischemic myocardium by hypothermic retroperfusion of autologous arterial blood: effects on regional myocardial temperature distribution and infarct size. J Am Coll Cardiol. 1991;18:293–300. doi: 10.1016/s0735-1097(10)80251-7. [DOI] [PubMed] [Google Scholar]
- 24.Gotberg M, Olivecrona GK, Engblom H, Ugander M, van der Pals J, Heiberg E, Arheden H, Erlinge D. Rapid short-duration hypothermia with cold saline and endovascular cooling before reperfusion reduces microvascular obstruction and myocardial infarct size. BMC Cardiovasc Disord. 2008;8:7. doi: 10.1186/1471-2261-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamamoto H, Sakamoto H, Leshnower BG, Parish LM, Kanemoto S, Hinmon R, Plappert T, Miyamoto S, St John-Sutton MG, Gorman JH 3rd, Gorman RC. Very mild hypothermia during ischemia and reperfusion improves postinfarction ventricular remodeling. Ann Thorac Surg. 2009;87:172–177. doi: 10.1016/j.athoracsur.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erlinge D. A review of mild hypothermia as an adjunctive treatment for ST-elevation myocardial infarction. Ther Hypothermia Temp Manag. 2011;1:129–141. doi: 10.1089/ther.2011.0008. [DOI] [PubMed] [Google Scholar]
- 27.Dixon SR, Whitbourn RJ, Dae MW, Grube E, Sherman W, Schaer GL, Jenkins JS, Baim DS, Gibbons RJ, Kuntz RE, Popma JJ, Nguyen TT, O’Neill WW. Induction of mild systemic hypothermia with endovascular cooling during primary percutaneous coronary intervention for acute myocardial infarction. J Am Coll Cardiol. 2002;40:1928–1934. doi: 10.1016/s0735-1097(02)02567-6. [DOI] [PubMed] [Google Scholar]
- 28.Kandzari DE, Chu A, Brodie BR, Stuckey TA, Hermiller JB, Vetrovec GW, Hannan KL, Krucoff MW, Christenson RH, Gibbons RJ, Sigmon KN, Garg J, Hasselblad V, Collins K, Harrington RA, Berger PB, Chronos NA, Hochman JS, Califf RM. Feasibility of endovascular cooling as an adjunct to primary percutaneous coronary intervention (results of the LOWTEMP pilot study) Am J Cardiol. 2004;93:636–639. doi: 10.1016/j.amjcard.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 29.Ly HQ, Denault A, Dupuis J, Vadeboncoeur A, Harel F, Arsenault A, Gibson CM, Bonan R. A pilot study: the noninvasive surface cooling thermoregulatory system for mild hypothermia induction in acute myocardial infarction (the NICAMI Study) Am Heart J. 2005;150:933. doi: 10.1016/j.ahj.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 30.Gotberg M, Olivecrona GK, Koul S, Carlsson M, Engblom H, Ugander M, van der Pals J, Algotsson L, Arheden H, Erlinge D. A pilot study of rapid cooling by cold saline and endovascular cooling before reperfusion in patients with ST-elevation myocardial infarction. Circ Cardiovasc Interv. 2010;3:400–407. doi: 10.1161/CIRCINTERVENTIONS.110.957902. [DOI] [PubMed] [Google Scholar]
- 31.Erlinge D, Gotberg M, Lang I, Holzer M, Noc M, Clemmensen P, Jensen U, Metzler B, James S, Botker HE, Omerovic E, Engblom H, Carlsson M, Arheden H, Ostlund O, Wallentin L, Harnek J, Olivecrona GK. Rapid endovascular catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. The CHILL-MI trial: a randomized controlled study of the use of central venous catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. J Am Coll Cardiol. 2014;63:1857–1865. doi: 10.1016/j.jacc.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 32.Grines CL. Intravascular cooling adjunctive to percutaneous coronary intervention for acute myocardial infarction. Presented at the 16th annual transcatheter cardiovascular therapeutics, Washington DC, USA, september 2004. O’Neill WW, Dixon SR. The year in interventional cardiology. J Am Coll Cardiol. 2004;43:875–90. [Google Scholar]
- 33.O’Neill WW, Dixon SR. The year in interventional cardiology. J Am Coll Cardiol. 2004;43:875–90. doi: 10.1016/j.jacc.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 34.Erlinge D, Gotberg M, Grines C, Dixon S, Baran K, Kandzari D, Olivecrona GK. A pooled analysis of the effect of endovascular cooling on infarct size in patients with ST-elevation myocardial infarction. EuroIntervention. 2013;8:1435–1440. doi: 10.4244/EIJV8I12A217. [DOI] [PubMed] [Google Scholar]
- 35.Koreny M, Sterz F, Uray T, Schreiber W, Holzer M, Laggner A, Herkner H. Effect of cooling after human cardiac arrest on myocardial infarct size. Resuscitation. 2009;80:56–60. doi: 10.1016/j.resuscitation.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Nichol G, Strickland W, Shavelle D, Maehara A, Ben-Yehuda O, Genereux P, Dressler O, Parvataneni R, Nichols M, McPherson J, Barbeau G, Laddu A, Elrod JA, Tully GW, Ivanhoe R, Stone GW VELOCITY Investigators. Prospective, multicenter, randomized, controlled pilot trial of peritoneal hypothermia in patients with ST-segment- elevation myocardial infarction. Circ Cardiovasc Interv. 2015;8:e001965. doi: 10.1161/CIRCINTERVENTIONS.114.001965. [DOI] [PubMed] [Google Scholar]
- 37.Kim F, Nichol G, Maynard C, Hallstrom A, Kudenchuk PJ, Rea T, Copass MK, Carlbom D, Deem S, Longstreth WT Jr, Olsufka M, Cobb LA. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. JAMA. 2014;311:45–52. doi: 10.1001/jama.2013.282173. [DOI] [PubMed] [Google Scholar]
- 38.Kim H, Lee J, Song W, Shin J, Oh D, Harrison K, Jakkula M, Wong SC, Hong MK. Feasibility and safety of regional myocardial hypothermia during myocardial ischemia and infarction in pigs. Coron Artery Dis. 2005;16:125–129. doi: 10.1097/00019501-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37:S186–202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 40.Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–1969. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- 41.Otterspoor LC, Van’t Veer M, van Nunen LX, Wijnbergen I, Tonino PA, Pijls NH. Safety and feasibility of local myocardial hypothermia. Catheter Cardiovasc Interv. 2016;87:877–83. doi: 10.1002/ccd.26139. [DOI] [PubMed] [Google Scholar]
- 42.de Hart J, de Weger A, van Tuijl S, Stijnen JM, van den Broek CN, Rutten MC, de Mol BA. An ex vivo platform to simulate cardiac physiology: a new dimension for therapy development and assessment. Int J Artif Organs. 2011;34:495–505. doi: 10.5301/IJAO.2011.8456. [DOI] [PubMed] [Google Scholar]
- 43.Schampaert S, van Nunen LX, Pijls NH, Rutten MC, van Tuijl S, van de Vosse FN, van’t Veer M. Intra-aortic balloon pump support in the isolated beating porcine heart in nonischemic and ischemic pump failure. Artif Organs. 2015;39:931–938. doi: 10.1111/aor.12470. [DOI] [PubMed] [Google Scholar]
- 44.Kloner RA, Przyklenk K, Whittaker P. Deleterious effects of oxygen radicals in ischemia/reperfusion. Resolved and unresolved issues. Circulation. 1989;80:1115–1127. doi: 10.1161/01.cir.80.5.1115. [DOI] [PubMed] [Google Scholar]
- 45.Ito H, Maruyama A, Iwakura K, Takiuchi S, Masuyama T, Hori M, Higashino Y, Fujii K, Minamino T. Clinical implications of the ‘no reflow’ phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93:223–228. doi: 10.1161/01.cir.93.2.223. [DOI] [PubMed] [Google Scholar]
- 46.Herskowitz A, Choi S, Ansari AA, Wesselingh S. Cytokine mRNA expression in postischemic/reperfused myocardium. Am J Pathol. 1995;146:419–428. [PMC free article] [PubMed] [Google Scholar]
- 47.Frohlich GM, Meier P, White SK, Yellon DM, Hausenloy DJ. Myocardial reperfusion injury: looking beyond primary PCI. Eur Heart J. 2013;34:1714–1722. doi: 10.1093/eurheartj/eht090. [DOI] [PubMed] [Google Scholar]
- 48.Schampaert S, van’t Veer M, Rutten MC, van Tuijl S, de Hart J, van de Vosse FN, Pijls NH. Autoregulation of coronary blood flow in the isolated beating pig heart. Artif Organs. 2013;37:724–730. doi: 10.1111/aor.12065. [DOI] [PubMed] [Google Scholar]
- 49.Tissier R, Chenoune M, Ghaleh B, Cohen MV, Downey JM, Berdeaux A. The small chill: mild hypothermia for cardioprotection? Cardiovasc Res. 2010;88:406–414. doi: 10.1093/cvr/cvq227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


