Abstract
The abnormal proliferation and migration of vascular smooth muscle cells (VSMCs) are crucial pathological processes that are involved in atherosclerosis. Growing evidence suggests that microRNAs (miRNAs) play critical roles in VSMCs functions. Here, we analyzed the expression of four atherosclerosis-related miRNAs and found that hsa-miR-148b was significantly down-regulated in plaques from atherosclerotic patients compared to a healthy control group. The restoration of hsa-miR-148b function in cells transfected with a hsa-miR-148b mimicmarkedly inhibited VSMCs proliferation and migration compared to a hsa-miR-148b mimic control. Furthermore, we discovered that heat shock protein 90 (HSP90) was a direct target of hsa-miR-148b in VSMCs. Hsa-miR-148b suppressed HSP90 expression by directly binding its 3’-untranslated region (UTR). In addition, the expression of hsa-miR-148b was negatively correlated with the HSP90 mRNA levels in plaques of atherosclerotic patients. Interestingly, the overexpression of HSP90 partly abrogated the hsa-miR-148b-mediated inhibition of VSMCs proliferation and migration. Our study provides the first evidence that hsa-miR-148b has anti-proliferative and migratory functions by targeting HSP90 in VSMCs and may aidin the development of new biomarkers and potential therapeutic targets for atherosclerosis.
Keywords: VSMCs, atherosclerosis, hsa-miR-148b, HSP90
Introduction
Atherosclerosis is a devastating and life-threatening disease that is characterized by an accumulation of fibrous elements and lipids in large arteries and is the leading cause of mortality and morbidity worldwide [1,2]. Vascular smooth muscle cells (VSMCs) comprise the majority of arterial walls and contribute to the development of atherosclerosis through aberrant cell functions, such as proliferation, migration, and apoptosis [3,4]. However, the detailed molecular mechanisms underlying VSMCs contributions to atherosclerosis remain unclear.
MicroRNAs (miRNAs) are non-coding RNA molecules that are 18~24 nucleotides in length and suppress translation or induce mRNA cleavage by binding to the 3’ untranslated regions (UTRs) of target genes [5]. Numerous studies have demonstrated that miRNAs are aberrantly present in many human cancers and are involved in tumor cell initiation, development, and metastasis [6-8]. Emerging evidence suggests that miRNAs are involved in a variety of normal cellular processes, such as cell development, growth, survival, differentiation, proliferation and apoptosis [9-11]. Recently, many studies have shown that miRNAs play important roles in VSMCs functions. For example, Torella et al. demonstrated that miR-133 could control VSMCs phenotypic switching in vitro and vascular remodeling in vivo [12]. Chen et al. showed that miRNA-34a reduces neointima formation by inhibiting VSMCs proliferation and migration [13]. Xu et al. reported that miR-135b-5p and miR-499a-3p promote VSMCs proliferation and migration in atherosclerosis by directly targeting MEF2C [14]. Liu et al. found that miRNA-1 regulates the proliferation of VSMCs by targeting insulin-like growth factor 1 [15]. However, despite these findings, the functions of many other miRNAs present in VSMCs remain unknown.
In this study, we demonstrate that hsa-miR-148b wass ignificantly decreased in plaques from atherosclerotic patients and that the overexpression of hsa-miR-148b inhibited VSMCs proliferation and migration. More importantly, we show that heat shock protein 90 (HSP90) is a direct target of hsa-miR-148b in VSMCs. The restoration of HSP90 partially reversed the hsa-miR-148b-induced inhibition of VSMCs proliferation and migration.
Materials and methods
Ethics statement
The protocol for our clinical research was approved by the Ethical Committee of General Hospital of Jinan Military Region and Guizhou Provincial People’s Hospital. All patients gave written informed consent to participate in this study. Both the study and consent complied with the Declaration of Helsinki.
Patient samples
Forty-six atherosclerotic patients undergoing carotid endarterectomy were enrolled in this study (62±2.4 years old; 31% women). Forty-six healthy individuals enrolled from the Jinan district served as controls (65±3.6 years old; 27% women). All specimens were frozen in liquid nitrogen immediately after surgery.
Cell culture and transfection
Human VSMCs were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% 100 U/ml penicillin and 1% 100 mg/ml streptomycin sulfate and incubated in a humidified incubator with 5% CO2 at 37°C.
Hsa-miR-148b mimics and controls were chemically synthesized by Suzhou GenePharma Co., Ltd. (Suzhou, China). The sequences were as follows: 5’-GGUGAAGUUCUGUUAUACACU-3’ (mimics); 5’-CUAAGGCAACGAUCAGCAUUACGA-3’ (mimics control). The human HSP90 gene (NM_001017963.2) was inserted into the pcDNA3.1+HA vector to construct the pcDNA3.1+HA-HSP90 over-expression plasmid from Life Technologies (Invitrogen, CA, USA), and the empty vector served as the negative control. For transfection, hsa-miR-148b mimics or controls and pcDNA3.1+HA-HSP90 or pcDNA3.1+HA empty vector were transfected with Opti-MEM and Lipofectamine 2000 reagents (Invitrogen, CA, USA), according to the manufacturer’s instructions.
RNA isolation and real-time quantitative PCR (qRT-PCR)
Total RNA was isolated from plaques with TRIzol® reagent (Invitrogen, CA, USA) according to the manufacturer’s protocols, and aMiScript SYBR Green PCR Kit (Qiagen, Valencia, CA, USA) was used for qRT-PCR analysis. The sequences of hsa-miR-148b amplification primers were 5’-AGUCAGUGCAUCACAGAACUU-3’ (sense), and the antisense primers were provided by the miScript SYBR Green PCR Kit. The qRT-PCR was performed on an Applied Biosystems 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Small nuclear U6 snRNA was used as an internal control. The relative expression levels of hsa-miR-148b in plaques was quantified using the 2-ΔΔCT method [16].
Luciferase reporter assays
The potential hsa-miR-148b binding site of HSP90 was predicted by three computer-aided algorithms: TargetScan, miRanda and PicTar. The mRNA 3’-UTR sequence of the HSP90 (WT) was PCR amplified and inserted into the psiCHECK-2 luciferase vector (Promega, Madison, WI, USA). The forward primer sequence for the mRNA 3’-UTR of HSP90 was 5’-TCTCTGGCTGAGGGATGACTTACCT-3’, and the reverse primer sequence was 5’-TTAAGGCCAAGGAATTAAGTGACTG-3’. HSP90 mRNA 3’-UTR containing a sequence with a mutation (MUT) in the putative binding site of hsa-miR-148b were chemically synthesized by Suzhou GenePharma Co., Ltd. (Suzhou, China). For luciferase reporter assays, VSMCs were seeded in 24-well plates and co-transfected with either the WT or MUT reporter plasmids and the hsa-miR-148b mimic or mimic control using Lipofectamine 2000. After 24 h, the Firefly and Renilla luciferase activities were detected using aGloMax-Multi Jr Single Tube Multimode Reader (Promega, Madison, WI, USA), where firefly luciferase activity served as an internal control to normalize for transfection efficiency.
Western blotting
Proteins were extracted from VSMCs using RIPA lysis buffer (Invitrogen, CA, USA) and was separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) before being transferred to polyvinylidene fluoride membranes (PVDF) (Millipore, MA, USA). After blocking with 5% nonfat milk for 2 h at 37°C, the membranes were incubated overnight with the primary antibodies: PCNA (1:1000, Sigma, USA), ki-67 (1:1000, Sigma, USA), COL1A1 (1:500, Abcam, USA), COL5A1 (1:1000, Abcam, USA), MMP-2 (1:1000, Abcam, USA), MMP-9 (1:2000, Abcam, USA) and HSP90 (1:1000, Millipore, USA). Membranes were then incubated with corresponding HRP-linked secondary antibodies (Santa Cruz Biotechnology, USA). The protein level of GAPDH was used as a loading control. Western blotting signals were detected using the ECL plus Kit (Pierce, USA).
Cell proliferation assays
VSMCs proliferation was assayed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma, USA). Approximately 8000 cells were seeded into each well of 96-well plates 24 h prior to transfection. At 0, 24, 48, or 72 h post-transfection, 25 μl of MTT (5 mg/ml) was added to each well and plates were incubated for 3~4 h at 37°C. Precipitates in each well were solubilized with 150 μl of dimethyl sulfoxide (DMSO) (Sigma, USA) and measured at 480 nm using a VersaMax ELISA microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Cell migration assays
VSMCs were co-transfected with either the hsa-miR-148b mimic or mimic control, and pcDNA3.1+HA-HSP90 or pcDNA3.1+HAempty vector according to the manufacture’s information. When a cell confluency of ~90-95% was reached ~6 h post-transfection, a cell spatula was used to scratch the cell layer. Cells were then washed 3x with warm PBS and incubated at 37°C for 24 h. At 0 and 18 h post-washing, the scratches were imaged with a digital camera system (Olympus Corp., Tokyo, Japan).
Statistical analysis
Experimental results are presented as the mean ± standard deviation (SD) and analyzed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) with Student’s t-test and ANOVA analyses. All experiments were performed in triplicate in three independent assays. P-values of less than 0.05 were considered to be statistically significant.
Results
Expression of atherosclerosis-related miRNAs in human carotid atherosclerotic plaques
Because miRNAs play important roles in VSMCs functioning, we selected four miRNAs (hsa-miR-21, hsa-miR-98, hsa-miR-132 and hsa-miR-148b) that were reported to be aberrantly expressed in the serum of atherosclerotic patients based on the results of microarray-based miRNA expression profiling analysis [14,17]. The expression levels of these miRNAs in 12 cases of human carotid atherosclerotic plaques and healthy controls were investigated by qRT-PCR analysis. Although no significant difference in the expression of hsa-miR-21, hsa-miR-98 and hsa-miR-132 was observed, the expression of hsa-miR-148b was significantly down-regulated compared to the healthy control (Figure 1A, P<0.01). The decreased hsa-miR-148b observed in human carotid atherosclerotic plaques was further confirmed in a larger study group (Figure 1B, P<0.01), which prompted us to focus on hsa-miR-148b.
Figure 1.
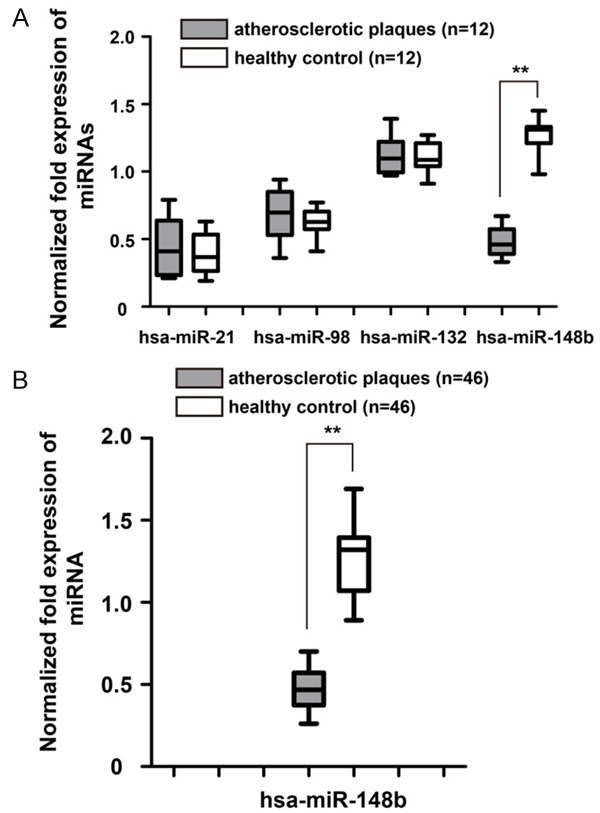
Expression of atherosclerosis-related miRNAs in human carotid atherosclerotic plaques. A. Analysis of the expression levels of four miRNAs (hsa-miR-21, hsa-miR-98, hsa-miR-132 and hsa-miR-148b) in human carotid atherosclerotic plaques and the healthy control by qRT-PCR. miRNA expression was normalized by a calibrator sample from the healthy control. B. The expression of hsa-miR-148b was significantly down-regulated in human carotid atherosclerotic plaques. U6 was used as an internal reference. **P<0.01.
Restoration of hsa-miR-148b inhibited VSMCs proliferation in vitro
MTT assay was performed to explore the function of hsa-miR-148b in VSMCs proliferation. As shown in Figure 2A, the expression of hsa-miR-148b was significantly increased in VSMCs after treatment with hsa-miR-148b mimics (P<0.01). The ectopic over-expression of hsa-miR-148b could dramatically inhibit the proliferative ability of VSMCs (Figure 2B, P<0.05). In addition, we found that the restoration of hsa-miR-148b decreased the protein expression levels of PCNA and ki-67 in VSMCs (Figure 2C).
Figure 2.
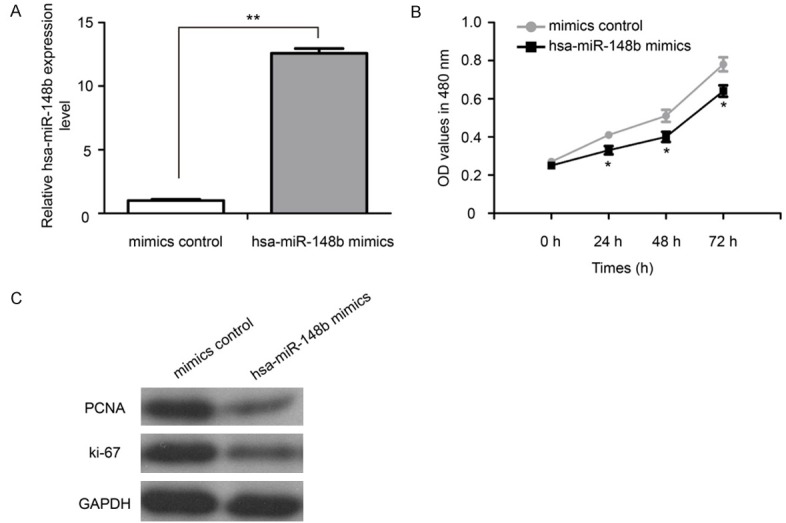
Restoration of hsa-miR-148b inhibited VSMCs proliferation in vitro. A. The expression of hsa-miR-148b was assayed by qRT-PCR analysis. B. Hsa-miR-148b mimic or mimic control treated VSMCs were subjected to an MTT assay. C. The protein expression levels of PCNA and ki-67 in VSMCs were measured by western blot.MTT: 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide. *P<0.05 and **P<0.01.
Restoration of hsa-miR-148b suppressed VSMCs migration in vitro
Images of the scratches were captured at 0 and 18 h after transfection. We found that over-expression of hsa-miR-148b markedly inhibited VSMCs migration (Figure 3A). Restoration of hsa-miR-148b suppressed the protein expression of COL1A1, COL5A1, MMP-2 and MMP-9 (Figure 3B).
Figure 3.
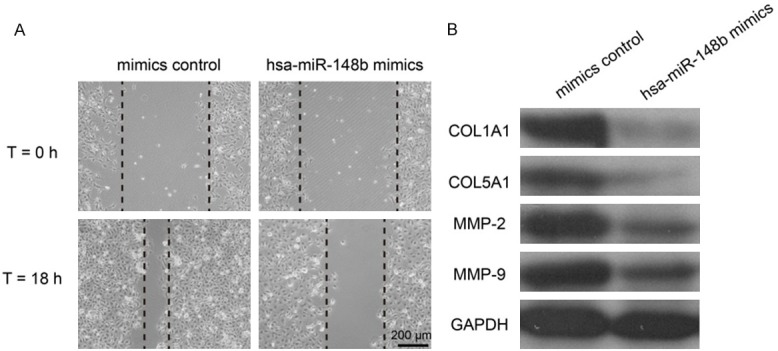
Restoration of hsa-miR-148b suppressed VSMCs migration in vitro. A. Wound-healing assay showed that over-expression of hsa-miR-148b inhibited VSMCs migration. B. The protein expression levels of COL1A1, COL5A1, MMP-2 and MMP-9 were in VSMCs were measured by western blot.
HSP90 was a direct target of hsa-miR-148b in VSMCs
Accumulating evidence has demonstrated that miRNAs control genes expression by binding target mRNAs to regulate transcriptional decay and translational repression [5,18]. A bioinformatic analysis was performed to identify the potential targeted gene of hsa-miR-148b. MiRanda algorithms revealed a potential seed sequence of hsa-miR-148b in the 3’-UTR of HSP90 (Figure 4A). Previous studies reported that HSP90 was over-expressed in plaques of atherosclerotic patients [19,20]. Interestingly, we observed that hsa-miR-148b expression was negatively correlated with HSP90 mRNA in plaques of atherosclerotic patients (Figure 4B, P<0.001). As shown in Figure 4C, the mRNA and protein expression levels of HSP90 were significantly down-regulated in hsa-miR-148b mimic transfectants compared with the mimic control (P<0.01).
Figure 4.
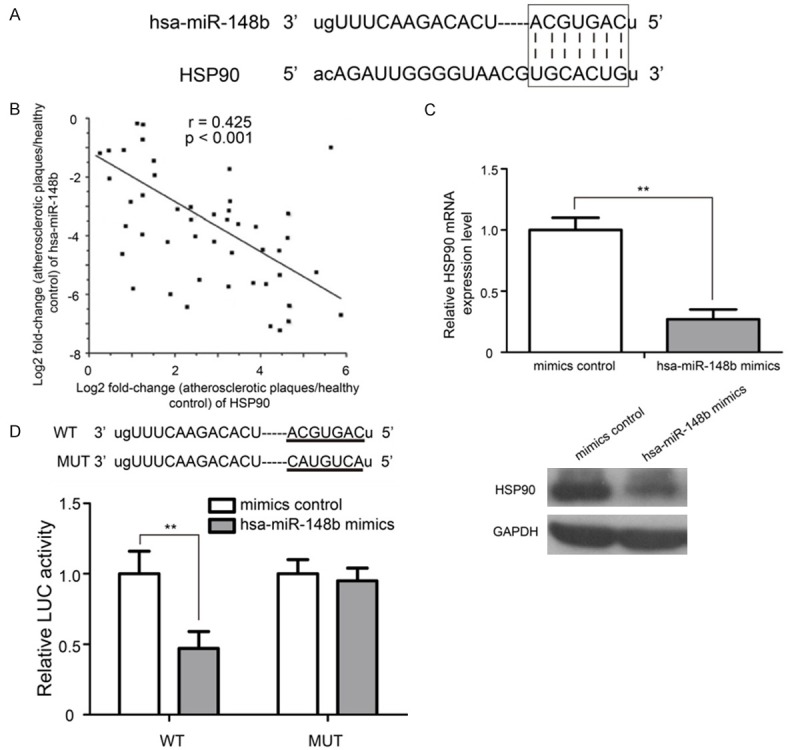
HSP90 mRNA is a direct target of hsa-miR-148b in VSMCs. A. The predicted hsa-miR-148b binding site in the HSP90 3’-UTR is shown. B. The expression of hsa-miR-148b was significantly negatively correlated with HSP90 mRNA levels in plaques of atherosclerotic patients. C. qRT-PCR and western blot analysis of HSP90 expression in VSMCs transfected with the hsa-miR-148b mimic or mimic control. D. Hsa-miR-148b inhibited luciferase activity in the HSP90 with WT binding site 3’-UTR, but luciferase activity was not decreased in the MUT binding site 3’-UTR of HSP90 mRNA. WT: wild type; MUT: mutant. LUC: luciferase. **P<0.01.
To investigate whether HSP90 is a target gene of hsa-miR-148b, we generated a wide type (WT) recombinant reporter plasmid containing the 975 nucleotide 3’-UTR of HSP90 mRNA. Then, we manually altered the potential binding sites by exchanging the G and U and the A and C to construct the mutant (MUT) recombinant reporter plasmid. The WT and MUT reporter plasmids were co-transfected into VSMCs with 100 Nm of the hsa-miR-148b mimic or mimic control. Whereas luciferase activity was inhibited by the hsa-miR-148b mimic with the WT vector, the inhibitory effect of the hsa-miR-148b mimic was not observed in the MUT vector (Figure 4D, P<0.01).
Over-expression of HSP90 partially abrogated hsa-miR-148b induced inhibitory effects on VSMCs
Although the luciferase reporter assays demonstrated that HSP90 is a direct target of hsa-miR-148b in VSMCs, the role of the hsa-miR-148b-induced inhibition of HSP90 in VSMCs remains unknown. As shown in Figure 5, the overexpression of HSP90 could partly reverse the inhibitory effects of hsa-miR-148b on VSMCs proliferation and migration (P<0.05). Taken together, these results strongly suggest that hsa-miR-148b plays a crucial role in VSMCs proliferation and migration by target HSP90 in atherosclerosis.
Figure 5.
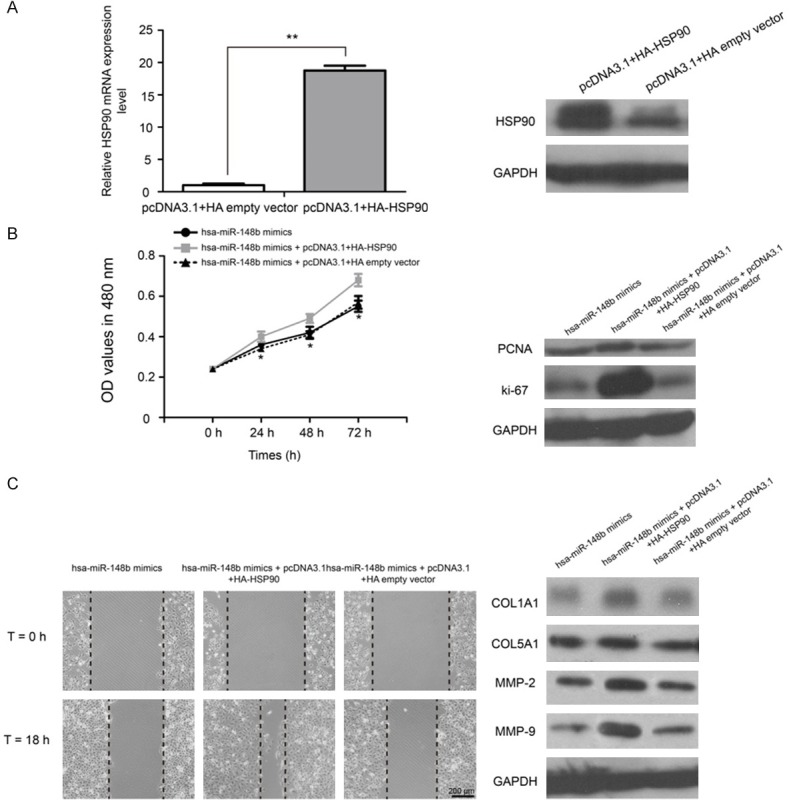
Overexpression of HSP90 partially abrogates hsa-miR-148b induced inhibitory effects on VSMCs. A. The expression of HSP90 mRNA and protein was detected by qRT-PCR and western blot analyses. B. Cell proliferation was assayed by an MTT assay in VSMCs transfected with hsa-miR-148b mimic, hsa-miR-148b mimicand pcDNA3.1+HA-HSP90 or hsa-miR-148b mimicand pcDNA3.1+HA empty vector. C. Wound scratch assay was performed in VSMCs treated with hsa-miR-148b mimics or hsa-miR-148b mimics+pcDNA3.1+HA-HSP90 or hsa-miR-148b mimics+pcDNA3.1+HA empty vector. *P<0.05 and **P<0.01.
Discussion
VSMCs proliferation and migration are involved in all stages of the progression of atherosclerosis [21,22]. The regulatory roles of miRNAs in the control of VSMCs proliferation and migration have been described in recent years [18,23]. Abnormal miRNA expression profiles have been reported to be correlated with atherosclerosis development [13,24,25]. Moreover, there is accumulating evidence of an intimate association between miRNAs and the functions of VSMCs [26,27]. The identification of the roles of novel miRNAs in the regulation of VSMCs may lead to an improved understanding of molecular mechanisms governing atherosclerosis and provide novel diagnostic and therapeutic strategies.
Since their discovery in 1993, miRNAs have been implicated in regulating various pathways, including cell proliferation, migration, apoptosis, and differentiation and therefore participated in the process of many human diseases [28]. It has been estimated that miRNAs could regulate the expression of up to 30% of all genes in the human genome [29]. In the present study, we analyzed the expressions of four atherosclerosis-related miRNAs and found that the expression of hsa-miR-148b was significantly down-regulated in human carotid atherosclerotic plaques compared with healthy control. Furthermore, the exogenous over-expression of hsa-miR-148b markedly inhibited the proliferation and migration of VSMCs.
The hsa-miR-148b is located in the host gene COPZ1 at human chromosome 12q13.13 [30]. Recently, several studies have demonstrated that hsa-miR-148b is involved in regulating cancer development. The down-regulation of miR-148b plays a role as an independent prognostic factor for non-small-cell lung cancer [31] and may serve as a prognostic biomarker for the early detection of hepatocellular carcinoma [32]. Additionally, circulating miR-148b and miR-133a serve as biomarkers for breast cancer detection [33]. Interestingly, MiR-148b functions as a tumor suppressor inpancreatic carcinoma by targeting AMPKα1 [34]. Therefore, our data are consistent with previous research, which demonstrated a role of has-miR-148b in the cell growth and migration of hepatocellular carcinoma, pancreatic carcinoma and lung cancer.
Aberrant VSMCs growth and migration are associated with the development of cardiovascular diseases, such as atherosclerosis and hypertension [26]. Accumulating studies have indicated that miRNAs may play important roles in VSMCs migration and invasion processes. PCNA and ki-67 were reported as two biomarkers involved in cell proliferation [35]. Additionally, COL1A1, COL5A1, MMP-2 and MMP-9 were shown to contribute to VSMCs migration [36,37]. In a further study, we found that the restoration of has-miR-148b decreased PCNA and ki-67 protein expression and down-regulated the protein expression of COL1A1, COL5A1, MMP-2 and MMP-9. These findings all suggested that the restoration of hsa-miR-148b inhibited the proliferation and migration of the VSMCs.
HSP90 is a ubiquitous molecular chaperone that aids in the folding, assembly, and activation of many proteins, including those involved in signal transduction and transcriptional regulation [38]. Previous research showed that HSP90 is highly expressed in plaques of atherosclerotic patients and is associated with features of plaque instability [19,20]. Here, we demonstrated that hsa-miR-148b functions as an important regulator of HSP90 expression by modifying VSMCs proliferation and migration. Luciferase reporter assays confirmed that hsa-miR-148b directly binds the 3’-UTR of HSP90 mRNA. Moreover, the over-expression of HSP90 partly reversed the hsa-miR-148b-induced inhibition of VSMCs proliferation and migration.
In conclusion, we report that hsa-miR-148b was down-regulated in plaques of atherosclerotic patients and that hsa-miR-148b overexpression inhibited VSMCs proliferation and migration by targeting HSP90. Our study provides a better understanding of hsa-miR-148b function in atherosclerosis development, which may benefit the development of miRNA directed diagnostic and therapeutic approaches against atherosclerosis.
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province (ZR2016HP18), Foundation of Guizhou provincial Science and Technology Department [(2016)1090], United Foundation of Guizhou provincial Science and Technology Department and Guizhou Provincial People’s Hospital [LH(2016)7149], Foundation of Guizhou Provincial Health Department (gzwkj2015-1-024), and Science and Technology Program of Guiyang City (2012103)056 and (2016205)007.
Disclosure of conflict of interest
None.
References
- 1.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: global burden of disease Study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973;180:1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- 5.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 6.Thomas J, Ohtsuka M, Pichler M, Ling H. MicroRNAs: clinical relevance in colorectal cancer. Int J Mol Sci. 2015;16:28063–28076. doi: 10.3390/ijms161226080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho WC. OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer. 2007;6:60. doi: 10.1186/1476-4598-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghisi M, Corradin A, Basso K, Frasson C, Serafin V, Mukherjee S, Mussolin L, Ruggero K, Bonanno L, Guffanti A, De Bellis G, Gerosa G, Stellin G, D’Agostino DM, Basso G, Bronte V, Indraccolo S, Amadori A, Zanovello P. Modulation of microRNA expression in human T-cell development: targeting of NOTCH3 by miR-150. Blood. 2011;117:7053–7062. doi: 10.1182/blood-2010-12-326629. [DOI] [PubMed] [Google Scholar]
- 9.Tian L, Li M, Ge J, Guo Y, Sun Y, Liu M, Xiao H. MiR-203 is downregulated in laryngeal squamous cell carcinoma and can suppress proliferation and induce apoptosis of tumours. Tumour Biol. 2014;35:5953–5963. doi: 10.1007/s13277-014-1790-7. [DOI] [PubMed] [Google Scholar]
- 10.Yao CX, Wei QX, Zhang YY, Wang WP, Xue LX, Yang F, Zhang SF, Xiong CJ, Li WY, Wei ZR, Zou Y, Zang MX. miR-200b targets GATA-4 during cell growth and differentiation. RNA Biol. 2013;10:465–480. doi: 10.4161/rna.24370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin DN, Qian L, Hu DL, Yu ZB, Han SP, Zhu C, Wang X, Hu X. Effects of miR-19b overexpression on proliferation, differentiation, apoptosis and Wnt/beta-catenin signaling pathway in P19 cell model of cardiac differentiation in vitro. Cell Biochem Biophys. 2013;66:709–722. doi: 10.1007/s12013-013-9516-9. [DOI] [PubMed] [Google Scholar]
- 12.Torella D, Iaconetti C, Catalucci D, Ellison GM, Leone A, Waring CD, Bochicchio A, Vicinanza C, Aquila I, Curcio A, Condorelli G, Indolfi C. MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ Res. 2011;109:880–893. doi: 10.1161/CIRCRESAHA.111.240150. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Yang F, Guo M, Wen G, Zhang C, Luong LA, Zhu J, Xiao Q, Zhang L. miRNA-34a reduces neointima formation through inhibiting smooth muscle cell proliferation and migration. J Mol Cell Cardiol. 2015;89:75–86. doi: 10.1016/j.yjmcc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Xu Z, Han Y, Liu J, Jiang F, Hu H, Wang Y, Liu Q, Gong Y, Li X. MiR-135b-5p and MiR-499a-3p promote cell proliferation and migration in atherosclerosis by directly targeting MEF2C. Sci Rep. 2015;5:12276. doi: 10.1038/srep12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu K, Ying Z, Qi X, Shi Y, Tang Q. MicroRNA-1 regulates the proliferation of vascular smooth muscle cells by targeting insulin-like growth factor 1. Int J Mol Med. 2015;36:817–824. doi: 10.3892/ijmm.2015.2277. [DOI] [PubMed] [Google Scholar]
- 16.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 17.Song Z, Li G. Role of specific microRNAs in regulation of vascular smooth muscle cell differentiation and the response to injury. J Cardiovasc Transl Res. 2010;3:246–250. doi: 10.1007/s12265-010-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469:336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madrigal-Matute J, Lopez-Franco O, Blanco-Colio LM, Munoz-Garcia B, Ramos-Mozo P, Ortega L, Egido J, Martin-Ventura JL. Heat shock protein 90 inhibitors attenuate inflammatory responses in atherosclerosis. Cardiovasc Res. 2010;86:330–337. doi: 10.1093/cvr/cvq046. [DOI] [PubMed] [Google Scholar]
- 20.Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz SM. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest. 1997;100:S87–89. [PubMed] [Google Scholar]
- 22.Gittenberger-de Groot AC, DeRuiter MC, Bergwerff M, Poelmann RE. Smooth muscle cell origin and its relation to heterogeneity in development and disease. Arterioscler Thromb Vasc Biol. 1999;19:1589–1594. doi: 10.1161/01.atv.19.7.1589. [DOI] [PubMed] [Google Scholar]
- 23.Han M, Toli J, Abdellatif M. MicroRNAs in the cardiovascular system. Curr Opin Cardiol. 2011;26:181–189. doi: 10.1097/HCO.0b013e328345983d. [DOI] [PubMed] [Google Scholar]
- 24.Xie B, Zhang C, Kang K, Jiang S. miR-599 inhibits vascular smooth muscle cells proliferation and migration by targeting TGFB2. PLoS One. 2015;10:e0141512. doi: 10.1371/journal.pone.0141512. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Zhang P, Zheng C, Ye H, Teng Y, Zheng B, Yang X, Zhang J. MicroRNA-365 inhibits vascular smooth muscle cell proliferation through targeting cyclin D1. Int J Med Sci. 2014;11:765–770. doi: 10.7150/ijms.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu X, Li Z. MicroRNAs regulate vascular smooth muscle cell functions in atherosclerosis (review) Int J Mol Med. 2014;34:923–933. doi: 10.3892/ijmm.2014.1853. [DOI] [PubMed] [Google Scholar]
- 27.Hosin AA, Prasad A, Viiri LE, Davies AH, Shalhoub J. MicroRNAs in atherosclerosis. J Vasc Res. 2014;51:338–349. doi: 10.1159/000368193. [DOI] [PubMed] [Google Scholar]
- 28.Pizzini S, Bisognin A, Mandruzzato S, Biasiolo M, Facciolli A, Perilli L, Rossi E, Esposito G, Rugge M, Pilati P, Mocellin S, Nitti D, Bortoluzzi S, Zanovello P. Impact of microRNAs on regulatory networks and pathways in human colorectal carcinogenesis and development of metastasis. BMC Genomics. 2013;14:589. doi: 10.1186/1471-2164-14-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 30.Serino G, Sallustio F, Cox SN, Pesce F, Schena FP. Abnormal miR-148b expression promotes aberrant glycosylation of IgA1 in IgA nephropathy. J Am Soc Nephrol. 2012;23:814–824. doi: 10.1681/ASN.2011060567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghasemkhani N, Shadvar S, Masoudi Y, Talaei AJ, Yahaghi E, Goudarzi PK, Shakiba E. Down-regulated MicroRNA 148b expression as predictive biomarker and its prognostic significance associated with clinicopathological features in non-small-cell lung cancer patients. Diagn Pathol. 2015;10:164. doi: 10.1186/s13000-015-0393-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Ziari K, Zarea M, Gity M, Fayyaz AF, Yahaghi E, Darian EK, Hashemian AM. Downregulation of miR-148b as biomarker for early detection of hepatocellular carcinoma and may serve as a prognostic marker. Tumour Biol. 2015;37:5765–5768. doi: 10.1007/s13277-015-3777-4. [DOI] [PubMed] [Google Scholar]
- 33.Shen J, Hu Q, Schrauder M, Yan L, Wang D, Medico L, Guo Y, Yao S, Zhu Q, Liu B, Qin M, Beckmann MW, Fasching PA, Strick R, Johnson CS, Ambrosone CB, Zhao H, Liu S. Circulating miR-148b and miR-133a as biomarkers for breast cancer detection. Oncotarget. 2014;5:5284–5294. doi: 10.18632/oncotarget.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao G, Zhang JG, Liu Y, Qin Q, Wang B, Tian K, Liu L, Li X, Niu Y, Deng SC, Wang CY. miR-148b functions as a tumor suppressor in pancreatic cancer by targeting AMPKalpha1. Mol Cancer Ther. 2013;12:83–93. doi: 10.1158/1535-7163.MCT-12-0534-T. [DOI] [PubMed] [Google Scholar]
- 35.Barbareschi M, Girlando S, Mauri FA, Arrigoni G, Laurino L, Dalla Palma P, Doglioni C. Tumour suppressor gene products, proliferation, and differentiation markers in lung neuroendocrine neoplasms. J Pathol. 1992;166:343–350. doi: 10.1002/path.1711660405. [DOI] [PubMed] [Google Scholar]
- 36.Newby AC. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc Res. 2006;69:614–624. doi: 10.1016/j.cardiores.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Johnson C, Galis ZS. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler Thromb Vasc Biol. 2004;24:54–60. doi: 10.1161/01.ATV.0000100402.69997.C3. [DOI] [PubMed] [Google Scholar]
- 38.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]


