Abstract
Increasing evidences have suggested that microRNAs (miRNAs) act a critical role in tumor initiation, progression and metastasis. Deregulated expression of miR-217 has been identified in various tumors. However, the expression and role of miR-217 in the development of cutaneous squamous cell carcinoma (cSCC) remain unclear. In our study, we showed that miR-217 expression was upregulated in the cSCC tissues compared to adjacent non-tumor samples. We also demonstrated that miR-217 expression was upregualted in the cSCCcSCC cell lines. Overexpression of miR-217 promoted cSCCcSCC cell growth, cell cycle and invasion. We identified Polymerase I and Transcript Release Factor (PTRF) as a direct target gene of miR-217 in the SCC13 cell. In addition, PTRF expression was downregulated in the cSCCcSCC tissues. Moreover, we demonstrated that there was a significant inverse correlation between miR-217 and PTRF expression in the cSCCcSCC. Furthermore, overexpression of PTRF could rescue miR-217’s oncogenic effect on cSCC. Therefore, these results suggested that upregulation of miR-217 could contribute to development of cSCCcSCC through targeting PTRF.
Keywords: MicroRNAs, miR-217, PTRF, cutaneous squamous cell carcinoma
Introduction
Cutaneous squamous cell carcinoma (cSCC) is the 2nd most common skin cancer and is responsible for about 20% of the skin tumor-associated mortalities yearly [1-4]. The incidence of cSCC has been increased during the past years [5,6]. Despite the advances in therapeutic approaches such as surgery, chemotherapy and radiotherapy, the prognosis of the cSCC remains unsatisfactory [7-10]. This cancer is often aggressive and multiple, with increased metastasis and recurrences [11-14]. Thus, it is crucial to identify more molecules in cSCC development and find more new targets for treatment strategies of cSCC.
MicroRNA (miRNA) is a category of highly conserved, endogenously expressed small non-coding RNA molecules that induces mRNA cleavage or represses gene translation through binding to the 3’UTR of target mRNAs [15-19]. Increasing studies have demonstrated that miRNAs are deregulated in various tumors such as gastric cancer, hepatocellular carcinoma, breast cancer, bladder cancer and cSCC [10,20-22]. miRNAs act pivotal roles in tumor initiation and progression due to their function as tumor suppressors or oncogenes [15,23,24]. miRNAs participate in various cell biological processes such as cell development, proliferation, apoptosis, invasion, migration and differentiation through regulating their target genes [16,25-27].
In our study, we examined the expression of miR-217 and polymerase I and transcript release factor (PTRF) in cSCC tissues and cell lines using qRT-PCR. We found that miR-217 was upregualted in cSCC tissues and cell lines and overexpression of miR-217 promoted cSCC cell growth, cell cycle and invasion.
Materials and methods
Tissue sample and cell line cutltured and tranfection
CSCC tissues and adjacent non-tumor samples were obtained from cSCC patients undergoing surgery at our department and Peking University Cancer Hospital. Our study was approved by the Peking Union Medical Hospital Institutional Review Board and in accordance with the Declaration of Helsinki Principles. Written informed consent was collected from each patient. Four cSCC cell lines (Tca8113, A431, SCC13 and HSC-5) and human benign epidermal keratinocyte cell line (HaCaT) were purchased from the Cell Bank of the Chinese Academy of Sciences and cultured in the DMEM (Dulbecco’s modified Eagle medium) supplemented with fetal bovine serum (FBS), streptomycin and penicillin. MiR-217 mimics and scramble mimics were purchased from the Ambion (TX, USA) and tranfected to the cells by using the Lipofectamine 2000 (Invitrogen L, CA, USA) following to manufacturer’s protocol.
Western blot
Total protein was isolated from cell or tissue by using the RIPA Lysis Buffer (Beyotime, China). Total protein was electrophoresed through 12% SDS gels and then transferred to the PVDF membrane (Millipore). Membrane was then blotted with individual primary antibodies (PTRF and GAPDH, Abcam, 1:2000). The band was visualized using the ECL (enhanced chemiluminescence) system following to the instructions of manufacturer.
Luciferase activity analysis
The 3’-UTR of PTRF containing the miR-217-binding sites and its mutant was cloned into pLUC vectors. Cells were cultured in the 96-well plate after transfection. Cell was co-transfected with the pLUC-PTRF or mutant pLUC-PTRF and miR-217 mimic or scramble, along with Opti-MEM and Fugene HD. The luciferase activities were evaluated by using the dual luciferase reporter analysis system (Promega, USA).
Cell proliferation, cell cycle, and cell invasion assay
For cell proliferation assay, the cells were plated on the 96-well plate and treated with the CCK8 (Cell Counting Kit-8, Dojindo, Japan). The absorbance at 450 nm was evaluated by using the microplate reader. The cell proliferation was measured for 0, 24, 48 hours respectively. To evaluate the cell cycle, flow cytometry was used. The cell was fixed in the ethanol before staining with the propidium iodide and RNase A and then measured by usig the FACScan. For cell invasion assay, cells seeded in the 24-transwell of upper Matrigel-coated membrane with no-serum medium. Ten percent of FBS was added to the lower medium. The invasive cell was fixed with formaldehyde and then dyed with crystal violet and counted.
RNA extraction and qRT-PCR
Total RNAs, including miRNA, were extracted from the cells or tissues using the TRIzol (Invitrogen, USA). To determin the expression of the miR-217 and PTRF, qRT-PCR was performed using the SYBR PCR Kit on the ABI 7300 real-time PCR system. The primer sequences used in our study were described as following: PTRF, forward 5’-CGGGAACAGGGCAACATCTA-3’ and reverse 5’-TGTGTCCCTTCTTTCTGC-3’ and GAPDH forward 5’-TTGTGATGGGTGTGAACCACGAGA-3’ reverse 5’-CATGAGCCCTTCCACAATGCCAAA-3’.
Statistical analysis
Data are present as the mean ± SD (standard deviation). Statistical significance for different groups was determined by the Student’s t-test or one-way ANOVA. P-value less than 0.05 were indicated to be statistically significant.
Result
MiR-217 is upregulated in cSCC tissues
To study the expression of miR-217 in cSCC, we analyzed 15 paired cSCC tissues and adjacent non-tumor tissues by qRT-PCR. As shown in the Figure 1A, miR-217 expression was upregulated in the cSCC tissues. Moreover, the expression of miR-217 was significant higher in the cSCC tissues than in adjacent non-tumor tissues (Figure 2B).
Figure 1.
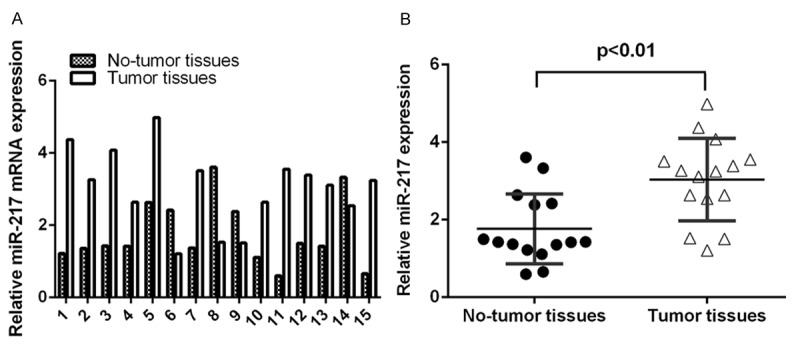
MiR-217 is upregulated in cSCC tissues. A. The expression of miR-217 in the 15 paired cSCC tissues and adjacent non-tumor tissues was measured by using qRT-PCR. B. The expression of miR-217 was significant higher in the cSCC tissues than in adjacent non-tumor tissues.
Figure 2.
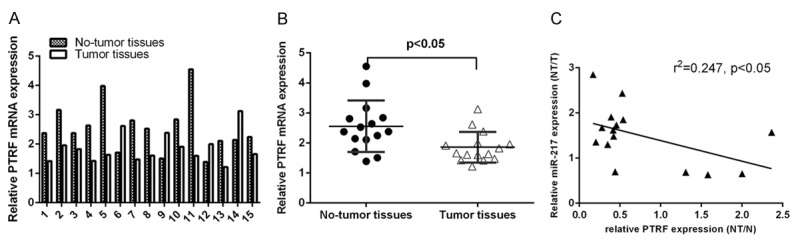
PTRF is downregulated in cSCC tissues. A. The expression of PTRF in the 15 paired cSCC tissues and adjacent non-tumor tissues was measured by using qRT-PCR. B. The expression of PTRF was significant higher in the cSCC tissues than in adjacent non-tumor tissues. C. The expression of PTRF was inversely correlated with the expression of miR-217 in the cSCC tissues.
PTRF is downregulated in cSCC tissues
As shown in the Figure 2A, PTRF expression was downregulated in the cSCC tissues. Moreover, the expression of PTRF was significantly lower in the cSCC tissues than in adjacent non-tumor tissues (Figure 2B). In addition, we demonstrated that there was a significant inverse correlation between miR-217 and PTRF expression in the cSCC (Figure 2C).
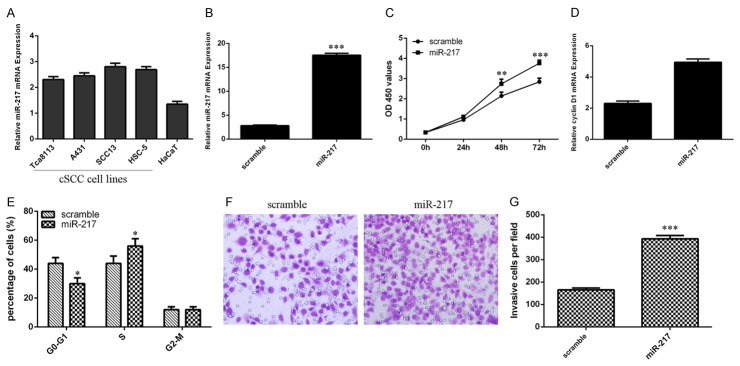
Overexpression of miR-217 promotes cSCC cell growth, cell cycle and invasion miR-217 expression was upregualted in the cSCC cell lines (Tca8113, A431, SCC13 and HSC-5) than in the human benign epidermal keratinocyte cell line (HaCaT) (Figure 3A). Moreover, the expression of miR-217 in the SCC13 cells was increased following transfection with miR-217 mimic compared with the scramble parental cells (Figure 3B). Ectopic expression of miR-217 increased the cSCC cell line SCC13 cell proliferation (Figure 3C). We also found that the expression of cyclin D1 was upregulated in the SCC13 cell following transfection with miR-217 mimics (Figure 3D). In addition, overexpression of miR-217 promoted the SCC13 cell cycle (Figure 3E). Furthermore, ectopic expression of miR-217 increased the SCC13 cell invasion (Figure 3F and 3G).
Figure 3.
Overexpression of miR-217 promotes cSCC cell growth, cell cycle and invasion. A. miR-217 expression in the cSCC cell lines (Tca8113, A431, SCC13 and HSC-5) and human benign epidermal keratinocyte cell line (HaCaT) was determined by using qRT-PCR. B. The expression of miR-217 in the SCC13 cell after treated with miR-217 mimic was measured by using qRT-PCR. C. Overexpression of miR-217 increased the SCC13 cell proliferation. D. Elevated expression of miR-217 promoted the cyclin D1 expression in the SCC13 cell. E. Overexpression of miR-217 promoted the SCC13 cell cycle. F. miR-217 overexpression increased the SCC13 cell invasion. G. The relative invasive cells were shown. *P<0.05, **P<0.01 and ***P<0.001.
MiR-217 directly targets PTRF expression
We searched the potential targets of miR-217 using the TargetScan tool and identified PTRF as a potential target of miR-217 (Figure 4A). As shown in Figure 4B, miR-217 overexpression inhibited the luciferase activity of the wild-type vector (WT PTRF 3’UTR) but not the mutant vector (Mut PTRF 3’UTR) (Figure 4B). Moreover, overexpression of miR-217 suppressed the PTRF expression in the SCC13 cell (Figure 4C and 4D).
Figure 4.
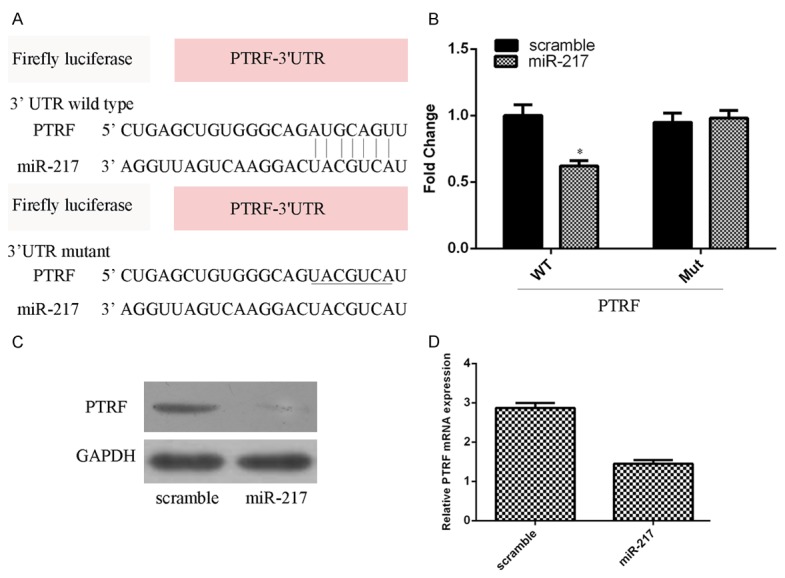
MiR-217 directly targets PTRF expression. A. There are 7 sequential bases between PTRF gene 3’UTR and 5’ of human miR-217. B. miR-217 overexpression inhibited the luciferase activity of the wild-type vector (WT PTRF 3’UTR) but not the mutant vector (Mut PTRF 3’UTR). C. miR-217 overexpression suppressed the PTRF protein expression in the SCC13 cell. D. miR-217 overexpression suppressed the PTRF mRNA expression in the SCC13 cell. *P<0.05.
Overexpression of PTRF could rescue miR-217’s oncogene effect on cSCC
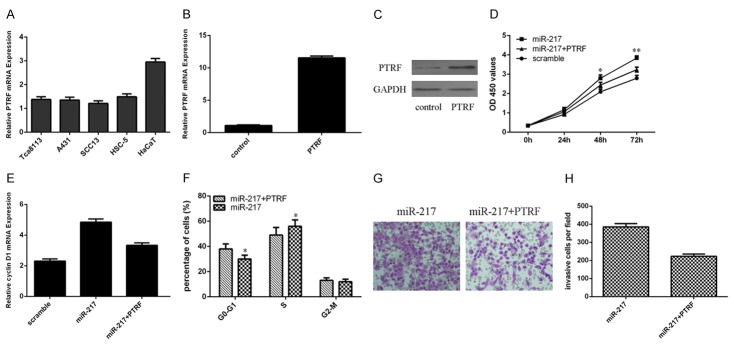
PTRF expression was lower in the cSCC cell lines (Tca8113, A431, SCC13 and HSC-5) than in the human benign epidermal keratinocyte cell line (HaCaT) (Figure 5A). PTRF expression in the SCC13 cells was increased following transfection with PTRF vector compared with the control vector parental cells (Figure 5B and 5C). We rescued PTRF expression in the miR-217 overexpressing SCC13 cell by transfecting PTRF vector. The proliferation of miR-217 overexpressing SCC13 cells was partially decreased after treated with PTRF vector (Figure 5D). Moreover, overexpression of PTRF suppressed cell cycle in the SCC13 cells overexpressing miR-217 (Figure 5E). In addition, ectopic expression of PTRF suppressed cell invasion in the SCC13 cells overexpressing miR-217 (Figure 5F and 5H).
Figure 5.
Overexpression of PTRF could rescue miR-217’s oncogene effect on cSCC. A. PTRF expression in the cSCC cell lines (Tca8113, A431, SCC13 and HSC-5) and human benign epidermal keratinocyte cell line (HaCaT) was determined by using qRT-PCR. B. The mRNA expression of PTRF was measued by qRT-PCR. C. The PTRF vetor promoted the protein expression of PTRF in the SCC13 cell. D. The proliferation of miR-217 overexpressing SCC13 cells was partially decreased after treated with PTRF vector. E. The cyclin D1 expression in the SCC13 cell was determined by qRT-PCR. F. Overexpression of PTRF suppressed cell cycle in the SCC13 cells overexpressing miR-217. G. Ectopic expression of PTRF suppressed cell invasion in the SCC13 cells overexpressing miR-217. H. The relative invasive cells were shown. *P<0.05 and **P<0.01.
Discussion
In our study, we showed that miR-217 expression was upregulated in the cSCC tissues. We also demonstrated that miR-217 expression was upregualted in the cSCC cell lines (Tca8113, A431, SCC13 and HSC-5) than in the human benign epidermal keratinocyte cell line (HaCaT). Overexpression of miR-217 promoted cSCC cell growth, cell cycle and invasion. We identified PTRF as a direct target gene of miR-217 in the SCC13 cell. In addition, we demonstrated that the PTRF expression was downregulated in the cSCC tissues. Moreover, we demonstrated that there was a significant inverse correlation between miR-217 and PTRF expression in the cSCC. Furthermore, Overexpression of PTRF could rescue miR-217’s oncogenic effect on cSCC. Therefore, these results suggested that upregulation of miR-217 could contribute to development of cSCC.
Previous studies suggested that miR-217 played an important role in the initiation and progression of tumors such as gastric cancer, breast cancer, glioma, epithelial ovarian cancer and osteosarcoma [28-32]. For example, Zhang et al [33] demonstrated that the miR-217 expression was upregulated in the breast tumor tissues and high expression of miR-217 was correlated with the triple negative subtype, highly histological grade and advanced tumor stage. Overexpression of miR-217 increased the breast cancer cell proliferation and cell cycle. Li et al [34] demonstrated that miR-217 was downregulated in the clear cell renal cell carcinoma and ectopic expression of miR-217 promoted clear cell renal cell carcinoma cell proliferation and migration. In addition, Shen et al [35] showed that miR-217 expression was downregulated in the osteosarcoma tissues and cell lines and overexpression of miR-217 suppressed the osteosarcoma cell invasion and proliferation through targeting WASF3 expression. However, there are no published literature about the role and function of miR-217 in the cSCC. In this study, we studied the role and function of miR-217 in the cSCC. We showed that miR-217 expression was upregulated in the cSCC tissues. We also demonstrated that miR-217 expression was upregualted in the cSCC cell lines (Tca8113, A431, SCC13 and HSC-5) than in the human benign epidermal keratinocyte cell line (HaCaT). Overexpression of miR-217 promoted cSCC cell growth, cell cycle and invasion. These results suggested that miR-217 might act as a potential oncogene in the development of cSCC.
Previous studies showed that PTRF acted a crucial role in the development of cancer including prostate cancer, glioblastoma, lung cancer, rhabdomyosarcoma and breast cancer [36-41]. Previous report reported a lack of PTRF expression in the prostate tumor, and ectopic expression of PTRF in the prostate cancer cells inhibited prostate tumor metastasis and growth [37]. In addition, Nassar et al [42] demonstrated that overexpression of PTRF decreased lymphatic vessel and blood densities in the orthotopic tumors. Their results suggested that absence of PTRF contributed to tumour metastasis and progression through enhancing the lymphangiogenesis and angiogenesis potential of the prostate cancer cells. Wang et al [43] showed that PTRF expression was downregulated in the colorectal cancers and downregulatd expression of PTRF was correlated to the advanced stage of colorectal tumor. Ectopic expression of PTRF suppressed colorectal tumor cell colony growth and proliferation. In this study, we identified PTRF as a direct target gene of miR-217 in the SCC13 cell. In addition, we demonstrated that the PTRF expression was downregulated in the cSCC tissues. Moreover, there was a significant inverse correlation between miR-217 and PTRF expression in the cSCC. Furthermore, Overexpression of PTRF could rescue miR-217’s oncogenic effect on cSCC.
In conclusion, we observed that miR-217 expression was upregulated in the cSCC tissues. Overexpression of miR-217 promoted cSCC cell growth, cell cycle and invasion through inhibiting PTRF expression. Therefore, these results suggested that upregulation of miR-217 might contribute to the development of cSCC through inhibiting PTRF expression.
Disclosure of conflict of interest
None.
References
- 1.Languino LR, Singh A, Prisco M, Inman GJ, Luginbuhl A, Curry JM, South AP. Exosome-mediated transfer from the tumor microenvironment increases TGFbeta signaling in squamous cell carcinoma. Am J Transl Res. 2016;8:2432–2437. [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L, Xiang P, Han X, Wu L, Li X, Xiong Z. Decreased expression of microRNA-20a promotes tumor progression and predicts poor prognosis of cutaneous squamous cell carcinoma. Int J Clin Exp Pathol. 2015;8:11446–11451. [PMC free article] [PubMed] [Google Scholar]
- 3.Toll A, Salgado R, Espinet B, Diaz-Lagares A, Hernandez-Ruiz E, Andrades E, Sandoval J, Esteller M, Pujol RM, Hernandez-Munoz I. MiR-204 silencing in intraepithelial to invasive cutaneous squamous cell carcinoma progression. Mol Cancer. 2016;15:53. doi: 10.1186/s12943-016-0537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh A, Willems E, Hafeez BB, Ong IM, Mehta SL, Verma AK. Ultraviolet radiation-induced tumor necrosis factor alpha, which is linked to the development of cutaneous SCC, modulates differential epidermal microRNAs expression. Oncotarget. 2016;7:17945–17956. doi: 10.18632/oncotarget.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trimmer C, Sotgia F, Lisanti MP, Capozza F. Cav1 inhibits benign skin tumor development in a two-stage carcinogenesis model by suppressing epidermal proliferation. Am J Transl Res. 2013;5:80–91. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou L, Wang Y, Ou C, Lin Z, Wang J, Liu H, Zhou M, Ding Z. microRNA-365-targeted nuclear factor I/B transcriptionally represses cyclin-dependent kinase 6 and 4 to inhibit the progression of cutaneous squamous cell carcinoma. Int J Biochem Cell Biol. 2015;65:182–191. doi: 10.1016/j.biocel.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Yu X, Li Z. The role of miRNAs in cutaneous squamous cell carcinoma. J Cell Mol Med. 2016;20:3–9. doi: 10.1111/jcmm.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen B, Pan W, Lin X, Hu Z, Jin Y, Chen H, Ma G, Qiu Y, Chang L, Hua C, Zou Y, Gao Y, Ying H, Lv D. MicroRNA-346 functions as an oncogene in cutaneous squamous cell carcinoma. Tumour Biol. 2016;37:2765–2771. doi: 10.1007/s13277-015-4046-2. [DOI] [PubMed] [Google Scholar]
- 9.Luo Q, Li W, Zhao T, Tian X, Liu Y, Zhang X. Role of miR-148a in cutaneous squamous cell carcinoma by repression of MAPK pathway. Arch Biochem Biophys. 2015;583:47–54. doi: 10.1016/j.abb.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Liu R, Luo C, Zhou X, Xia K, Chen X, Zhou M, Zou Q, Cao P, Cao K. MiR-20a inhibits cutaneous squamous cell carcinoma metastasis and proliferation by directly targeting LIMK1. Cancer Biol Ther. 2014;15:1340–1349. doi: 10.4161/cbt.29821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang SH, Zhou JD, He QY, Yin ZQ, Cao K, Luo CQ. MiR-199a inhibits the ability of proliferation and migration by regulating CD44-Ezrin signaling in cutaneous squamous cell carcinoma cells. Int J Clin Exp Pathol. 2014;7:7131–7141. [PMC free article] [PubMed] [Google Scholar]
- 12.Wang A, Landen NX, Meisgen F, Lohcharoenkal W, Stahle M, Sonkoly E, Pivarcsi A. MicroRNA-31 is overexpressed in cutaneous squamous cell carcinoma and regulates cell motility and colony formation ability of tumor cells. PLoS One. 2014;9:e103206. doi: 10.1371/journal.pone.0103206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gastaldi C, Bertero T, Xu N, Bourget-Ponzio I, Lebrigand K, Fourre S, Popa A, Cardot-Leccia N, Meneguzzi G, Sonkoly E, Pivarcsi A, Mari B, Barbry P, Ponzio G, Rezzonico R. miR-193b/365a cluster controls progression of epidermal squamous cell carcinoma. Carcinogenesis. 2014;35:1110–1120. doi: 10.1093/carcin/bgt490. [DOI] [PubMed] [Google Scholar]
- 14.Xu N, Zhang L, Meisgen F, Harada M, Heilborn J, Homey B, Grander D, Stahle M, Sonkoly E, Pivarcsi A. MicroRNA-125b down-regulates matrix metallopeptidase 13 and inhibits cutaneous squamous cell carcinoma cell proliferation, migration, and invasion. J Biol Chem. 2012;287:29899–29908. doi: 10.1074/jbc.M112.391243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang K, Dong X, Sui C, Hu D, Xiong T, Liao S, Zhang H. MiR-223 suppresses endometrial carcinoma cells proliferation by targeting IGF-1R. Am J Transl Res. 2014;6:841–849. [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad A, Sethi S, Chen W, Ali-Fehmi R, Mittal S, Sarkar FH. Up-regulation of microRNA-10b is associated with the development of breast cancer brain metastasis. Am J Transl Res. 2014;6:384–390. [PMC free article] [PubMed] [Google Scholar]
- 17.Tao K, Yang J, Guo Z, Hu Y, Sheng H, Gao H, Yu H. Prognostic value of miR-221-3p, miR-342-3p and miR-491-5p expression in colon cancer. Am J Transl Res. 2014;6:391–401. [PMC free article] [PubMed] [Google Scholar]
- 18.Olasz EB, Seline LN, Schock AM, Duncan NE, Lopez A, Lazar J, Flister MJ, Lu Y, Liu P, Sokumbi O, Harwood CA, Proby CM, Neuburg M, Lazarova Z. MicroRNA-135b regulates leucine zipper tumor suppressor 1 in cutaneous squamous cell carcinoma. PLoS One. 2015;10:e0125412. doi: 10.1371/journal.pone.0125412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanitz A, Imig J, Dziunycz PJ, Primorac A, Galgano A, Hofbauer GF, Gerber AP, Detmar M. The expression levels of microRNA-361-5p and its target VEGFA are inversely correlated in human cutaneous squamous cell carcinoma. PLoS One. 2012;7:e49568. doi: 10.1371/journal.pone.0049568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang M, Ren MP, Zhao L, Li CP, Deng MM. miR-485-5p acts as a negative regulator in gastric cancer progression by targeting flotillin-1. Am J Transl Res. 2015;7:2212–2222. [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X, He W, Li J, Huang S, Wan X, Luo H, Wu D. MiRNA-125b inhibits proliferation and migration by targeting SphK1 in bladder cancer. Am J Transl Res. 2015;7:2346–2354. [PMC free article] [PubMed] [Google Scholar]
- 22.Song X, Wang Z, Jin Y, Wang Y, Duan W. Loss of miR-532-5p in vitro promotes cell proliferation and metastasis by influencing CXCL2 expression in HCC. Am J Transl Res. 2015;7:2254–2261. [PMC free article] [PubMed] [Google Scholar]
- 23.Hu A, Huang JJ, Xu WH, Jin XJ, Li JP, Tang YJ, Huang XF, Cui HJ, Sun GB. miR-21 and miR-375 microRNAs as candidate diagnostic biomarkers in squamous cell carcinoma of the larynx: association with patient survival. Am J Transl Res. 2014;6:604–613. [PMC free article] [PubMed] [Google Scholar]
- 24.Song YF, Hong JF, Liu DL, Lin QA, Lan XP, Lai GX. miR-630 targets LMO3 to regulate cell growth and metastasis in lung cancer. Am J Transl Res. 2015;7:1271–1279. [PMC free article] [PubMed] [Google Scholar]
- 25.Ren Q, Liang J, Wei J, Basturk O, Wang J, Daniels G, Gellert LL, Li Y, Shen Y, Osman I, Zhao J, Melamed J, Lee P. Epithelial and stromal expression of miRNAs during prostate cancer progression. Am J Transl Res. 2014;6:329–339. [PMC free article] [PubMed] [Google Scholar]
- 26.Li S, Qin X, Li Y, Zhang X, Niu R, Zhang H, Cui A, An W, Wang X. MiR-133a suppresses the migration and invasion of esophageal cancer cells by targeting the EMT regulator SOX4. Am J Transl Res. 2015;7:1390–1403. [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao X, Li J, Huang S, Wan X, Luo H, Wu D. MiRNA-29c regulates cell growth and invasion by targeting CDK6 in bladder cancer. Am J Transl Res. 2015;7:1382–1389. [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang AX, Lu FQ, Yang YP, Ren XY, Li ZF, Zhang W. MicroRNA-217 overexpression induces drug resistance and invasion of breast cancer cells by targeting PTEN signaling. Cell Biol Int. 2015 doi: 10.1002/cbin.10506. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Wei R, Deng Z, Su J. miR-217 targeting Wnt5a in osteosarcoma functions as a potential tumor suppressor. Biomed Pharmacother. 2015;72:158–164. doi: 10.1016/j.biopha.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, Zhao H, Feng L, Xu S. MicroRNA-217 inhibits cell proliferation and invasion by targeting Runx2 in human glioma. Am J Transl Res. 2016;8:1482–1491. [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Li D, Zhang W. Tumor suppressor role of miR-217 in human epithelial ovarian cancer by targeting IGF1R. Oncol Rep. 2016;35:1671–1679. doi: 10.3892/or.2015.4498. [DOI] [PubMed] [Google Scholar]
- 32.Chen DL, Zhang DS, Lu YX, Chen LZ, Zeng ZL, He MM, Wang FH, Li YH, Zhang HZ, Pelicano H, Zhang W, Xu RH. microRNA-217 inhibits tumor progression and metastasis by downregulating EZH2 and predicts favorable prognosis in gastric cancer. Oncotarget. 2015;6:10868–10879. doi: 10.18632/oncotarget.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q, Yuan Y, Cui J, Xiao T, Jiang D. MiR-217 promotes tumor proliferation in breast cancer via targeting DACH1. J Cancer. 2015;6:184–191. doi: 10.7150/jca.10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Zhao J, Zhang JW, Huang QY, Huang JZ, Chi LS, Tang HJ, Liu GQ, Zhu DJ, Ma WM. MicroRNA-217, down-regulated in clear cell renal cell carcinoma and associated with lower survival, suppresses cell proliferation and migration. Neoplasma. 2013;60:511–515. doi: 10.4149/neo_2013_066. [DOI] [PubMed] [Google Scholar]
- 35.Shen L, Wang P, Yang J, Li X. MicroRNA-217 regulates WASF3 expression and suppresses tumor growth and metastasis in osteosarcoma. PLoS One. 2014;9:e109138. doi: 10.1371/journal.pone.0109138. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Moon H, Lee CS, Inder KL, Sharma S, Choi E, Black DM, Le Cao KA, Winterford C, Coward JI, Ling MT, Craik DJ, Parton RG, Russell PJ, Hill MM. PTRF/cavin-1 neutralizes non-caveolar caveolin-1 microdomains in prostate cancer. Oncogene. 2014;33:3561–3570. doi: 10.1038/onc.2013.315. [DOI] [PubMed] [Google Scholar]
- 37.Nassar ZD, Hill MM, Parton RG, Parat MO. Caveola-forming proteins caveolin-1 and PTRF in prostate cancer. Nat Rev Urol. 2013;10:529–536. doi: 10.1038/nrurol.2013.168. [DOI] [PubMed] [Google Scholar]
- 38.Yi JS, Mun DG, Lee H, Park JS, Lee JW, Lee JS, Kim SJ, Cho BR, Lee SW, Ko YG. PTRF/cavin-1 is essential for multidrug resistance in cancer cells. J Proteome Res. 2013;12:605–614. doi: 10.1021/pr300651m. [DOI] [PubMed] [Google Scholar]
- 39.Peng J, Liu HZ, Zhong J, Deng ZF, Tie CR, Rao Q, Xu W, You T, Li J, Cai CB, Lu Q, Liu W, Zhang Y, Lei ZY. MicroRNA187 is an independent prognostic factor in lung cancer and promotes lung cancer cell invasion via targeting of PTRF. Oncol Rep. 2016;36:2609–2618. doi: 10.3892/or.2016.5083. [DOI] [PubMed] [Google Scholar]
- 40.Faggi F, Chiarelli N, Colombi M, Mitola S, Ronca R, Madaro L, Bouche M, Poliani PL, Vezzoli M, Longhena F, Monti E, Salani B, Maggi D, Keller C, Fanzani A. Cavin-1 and Caveolin1 are both required to support cell proliferation, migration and anchorage-independent cell growth in rhabdomyosarcoma. Lab Invest. 2015;95:585–602. doi: 10.1038/labinvest.2015.45. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Liu T, Bai Y, Liao H, Qiu S, Chang Z, Liu Y, Yan X, Guo H. Polymerase I and transcript release factor acts as an essential modulator of glioblastoma chemoresistance. PLoS One. 2014;9:e93439. doi: 10.1371/journal.pone.0093439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nassar ZD, Moon H, Duong T, Neo L, Hill MM, Francois M, Parton RG, Parat MO. PTRF/Cavin-1 decreases prostate cancer angiogenesis and lymphangiogenesis. Oncotarget. 2013;4:1844–1855. doi: 10.18632/oncotarget.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang F, Zheng Y, Orange M, Yang C, Yang B, Liu J, Tan T, Ma X, Chen T, Yin X, Tang X, Zhu H. PTRF suppresses the progression of colorectal cancers. Oncotarget. 2016 doi: 10.18632/oncotarget.9424. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]