Abstract
Cell sheet technology is a promising therapeutic strategy for the treatment of ischemic diseases such as myocardial infarction. We recently developed a novel protocol, termed “hypoxic preconditioning,” capable of augmenting the therapeutic efficacy of cell sheets. Following this protocol, the pro-angiogenic and anti-fibrotic activity of cell sheets were enhanced by brief incubation of cell sheets under hypoxic culture conditions. However, the precise molecular mechanism underlying the hypoxic preconditioning of cell sheets is unclear. In the present study, we examined signal transducers in cell sheets to identify those responsive to hypoxic preconditioning, using cardiosphere-derived cell (CDC) sheets. We initially tested whether sheet-like structures were suitable for hypoxic preconditioning by comparing them with individual cells. Hypoxic preconditioning was more effective in sheeted cells than in individual cells. Expression of hypoxia inducible factor-1α (HIF-1α) and mammalian target of rapamycin (mTOR) were induced upon hypoxic preconditioning of cell sheets, as was the phosphoinositide 3-kinase (PI3K)/Akt pathway. In addition, hypoxic preconditioning increased phosphorylation of epidermal growth factor receptor (EGFR) and heat shock protein 60 (HSP60) in CDC sheets. Our findings provide novel insights into the utility of hypoxic preconditioning in cell sheet-based technologies for the treatment of ischemic diseases.
Keywords: Cell sheet, hypoxic preconditioning, cardiosphere, HIF-1α
Introduction
Cell transplantation is a promising therapeutic strategy for the treatment of myocardial infarctions, as an alternative to heart transplantation, which is limited by critical shortage of donor hearts [1]. Various cell types are potentially useful for cell-based treatment of myocardial infarction owing to the high regenerative capacity of the cardiac tissue. For example, cardiac stem/progenitor cells generate new cardiomyocytes, and endothelial progenitor cells induce therapeutic angiogenesis in infarcted heart tissue after transplantation, resulting in heart regeneration [2]. Among the available cell types for cell-based therapy, mesenchymal stem/stromal cells (MSCs) and cardiosphere-derived cells (CDCs) have already been studied for safety and therapeutic availability in early-phase clinical trials [3,4]. It is thought that CDCs are one of the most suitable cell types for cell-based heart therapy, as they include cardiac stem/progenitor cells among the heterogeneous cell population and possess a high regeneration capacity [5]. However, therapeutic outcomes of cell-based therapies using either MSCs or CDCs in clinical settings have been limited, and the efficacy of cell-based therapy is still controversial. A possible reason for the limited therapeutic efficacy of cell-based therapy is the poor retention of graft cells in ischemic tissues [1]. Conversely, cell-based therapy may provide great value for patients with myocardial infarction if graft cells could be retained for a longer period in the infarcted heart.
Cell sheet technology has been developed as an advanced cell delivery method [6]. This technology enables the delivery of graft cells held by cell-cell contact and matrix components, resulting in longer retention of graft cells, even in ischemic heart tissue [7]. Cell sheets composed of pluripotent stem cell-derived cardiomyocytes or MSCs showed longer survival in ischemic heart tissue of animal models and improved cardiac function after implantation [8,9]. Although other biological scaffolds such as hydrogels are useful for prolonging the survival of graft cells in ischemic tissue [10], there is a risk of bacterial contamination during the manufacturing process. Cell sheets can be developed without any biological materials, indicating that this technology is a safe delivery method. However, the therapeutic efficacy of cell sheet-based therapy for myocardial infarction is still insufficient for clinical application, and there is still potential for improvement of this technology.
Hypoxic preconditioning, which is the brief incubation of cells under hypoxic conditions before transplantation, is a simple method to enhance the functions of graft cells without specialized equipment [11]. We recently demonstrated that hypoxic pre-treatment can be applied to cell sheets to enhance therapeutic functions. In addition, transplantation of preconditioned cell sheets improved left ventricular function in infarcted hearts of small and middle-sized animal models [12,13]. These results led us to apply hypoxic preconditioning to cell sheet-based therapy for the treatment of myocardial infarction.
In previous studies, the molecular mechanisms underlying hypoxic preconditioning of cell sheets were not fully elucidated, but the involvement of the phosphoinositide 3-kinase (PI3K)/Akt or Erk-mediated pathways has been reported [12,13]. In the present study, we aimed to clarify how hypoxic preconditioning enhances cell sheet functions, using human CDCs. We first determined whether sheet-like structures were suitable for hypoxic preconditioning by comparing them with sparse cells. Notably, hypoxic preconditioning was more effective on sheeted cells than on sparse cells. Next, we investigated whether hypoxia inducible factor-1α (HIF-1α) and mammalian target of rapamycin (mTOR) contribute to hypoxic preconditioning, in addition to the PI3K/Akt pathway. Our findings provide novel insights into the hypoxic preconditioning of cell sheet-based materials for the treatment of myocardial infarction.
Materials and methods
Ethical approval
The protocol for isolation of human cells was approved by the Ethics Review Board for Clinical Research at Yamaguchi University (no. 2010025). The study was conducted in accordance with the Declaration of Helsinki, and informed written consent for participation in the study was obtained from all patients.
Preparation of cardiosphere-derived cells
Cardiosphere-derived cells were isolated from biopsied heart specimens according to our established protocol [12,14]. Briefly, right atrial biopsy samples (~100 mg) were dissected from patients scheduled for open-heart surgery and digested with 0.5% trypsin for 5 min. Enzymatically-treated samples were placed onto fibronectin-coated cell culture dishes and incubated in Iscove’s Modified Dulbecco’s medium (IMDM; Life Technologies, Grand Island, NY, USA) with 10% fetal bovine serum (FBS; Life Technologies), 2 mM l-glutamine (Sigma-Aldrich, St. Louis, MO, USA), 2 nM β-mercaptoethanol (Wako, Osaka, Japan), and 1% penicillin/streptomycin (Life Technologies) at 37°C. Migrated cells from heart tissues were collected and cultured in the cardiosphere-formation medium to induce spherical aggregation [35% IMDM; 65% DMEM/F12 (Life Technologies); 3.5% FBS; 1 mM l-glutamine; 0.1 mM β-mercaptoethanol; 1 unit/ml thrombin (Sigma-Aldrich); 1% B-27 (Life Technologies); 80 ng/ml basic fibroblast growth factor (Sigma-Aldrich); 25 ng/ml epidermal growth factor (Sigma-Aldrich); 4 ng/ml cardiotrophin-1 (Sigma-Aldrich); 5 ng/ml heparin (Sigma-Aldrich); 1% penicilin/streptomycin] [5]. After 4 days in free-floating culture, cardiospheres were collected and plated onto fibronectin-coated cell culture dishes to isolate CDCs that propagated from spheres.
Preparation and hypoxic treatment of cell sheets
Temperature-responsive cell culture dishes (UpCell®; CellSeed, Tokyo, Japan) were used to prepare human CDC sheets. CDCs (3 × 105 cells/well) were plated onto 24-well UpCell® dishes and cultured with 10% FBS/IMDM. To prepare hypoxically-preconditioned cell sheets, CDC sheets were placed in an incubator adjusted to a 2% oxygen level and 33°C and cultivated for 24 hours. Dimethyloxaloylglycine (DMOG; 10 μM, Sigma-Aldrich), a PHD inhibitor, was used to stabilize HIF-1α in normoxic culture conditions.
To compare hypoxia sensitivity between individual cells and cell sheets, equal numbers of CDCs were plated into 6-well and 12-well UpCell® dishes and cultured under hypoxic conditions for 24 hours. Cell lysates and supernatants from each culture were collected for western blotting and ELISA.
Western blotting
Human CDC sheets were dissolved in RIPA buffer containing a protease/phosphatase inhibitor cocktail, and 30 μg of proteins were applied to polyacrylamide gels. To detect target proteins, the following antibodies were used: anti-phospho-histone H3 (rabbit polyclonal; Cell Signaling Technology, Danvers, MA, USA), anti-HIF-1α (mouse monoclonal; Santa Cruz Biotechnology, Dallas, TX, USA), HRP-conjugated anti-β-actin (rabbit polyclonal; Novus Biologicals, Littleton, CO, USA), anti-phospho-Akt (rabbit polyclonal; Cell Signaling Technology), anti-Akt (rabbit polyclonal; Cell Signaling Technology), anti-phospho-ERK (rabbit polyclonal; Cell Signaling Technology), anti-ERK (rabbit polyclonal; Cell Signaling Technology), anti-phospho-mTOR (rabbit polyclonal; Cell Signaling Technology), anti-mTOR (rabbit polyclonal; Cell Signaling Technology), anti-endoglin (rabbit polyclonal; Santa Cruz Biotechnology), HRP-conjugated anti-rat IgG (DAKO-Japan, Tokyo, Japan), HRP-conjugated anti-rabbit IgG (DAKO-Japan, Tokyo, Japan), and HRP-conjugated anti-mouse IgG (DAKO-Japan). Proteins were visualized using an HRP substrate (ECL Prime Western Blotting Detection System; GE Healthcare, Buckinghamshire, UK), and band intensities were quantified using ImageJ software.
Enzyme-linked immunosorbent assay (ELISA)
To assess the production of VEGF, hepatocyte growth factor (HGF), and insulin-like growth factor-I (IGF-I), conditioned media were collected from hypoxically-treated CDCs and CDC sheet cultures, and enzyme-linked immunosorbent assay (ELISA) was performed using a Quantikine ELISA kit (R&D systems, Minneapolis, MN, USA) according to the manufacturer’s protocol.
Immunocytochemistry
Cell sheets were fixed in 4% paraformaldehyde (Wako). After rinsing with phosphate-buffered saline (PBS), cell sheets were permeabilized with 0.1% Triton-X/Protein blocking solution (Dako-Japan), and then incubated with an anti-HIF-1α antibody (Santa Cruz). After incubation with the primary antibody, cell sheets were incubated with a secondary antibody conjugated with Alexa Fluor 488 (Life Technologies). Cell nuclei were labeled with DAPI after incubation with the secondary antibody. Immunofluorescence images were acquired using a BIOREVO microscopy system (BZ-X700; Keyence, Osaka).
Kinase array analysis
To identify kinase proteins responsive to hypoxic stimulation, a kinase array analysis was performed using a Human Phospho-Kinase Antibody Array kit (R&D systems). Cell lysates from normoxically- or hypoxically-cultured human CDC sheets were applied to the array.
Statistical analysis
All values were expressed as mean ± SD. A two-tailed Student’s t-test was performed to compare VEGF production, HIF-1α expression, phospho-mTOR expression, and phospho-Akt expression in CDC sheets. Statistical analysis was performed using IBM SPSS Statics 20 (IBM-Japan, Tokyo, Japan). P-values less than 0.05 were considered statistically significant.
Results
Sheet-like structures increase the sensitivity of cardiosphere-derived cells to hypoxic preconditioning
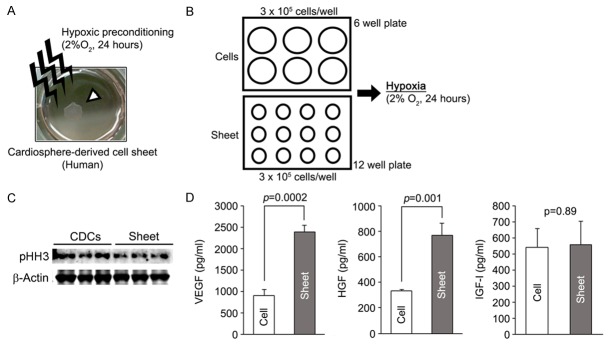
Cardiosphere-derived cells, which are a heterogeneous cell population that includes cardiac stem/progenitor cells, can be easily isolated from the postnatal heart and display a high regenerative capacity in infarcted hearts [5]. In this study, we used CDC sheets to investigate the molecular mechanism underlying the hypoxic preconditioning of cell sheets. Monolayer cell sheets were developed in a temperature responsive cell culture dish and pre-incubated in a condition adjusted to 2% oxygen for 24 hours following sheet delivery to infarcted heart tissue (Figure 1A) [12]. It was previously reported that the functions of CDCs cultured as individual cells could be enhanced by hypoxic treatment [15]. These findings raised the question of whether culture format influenced cell sensitivity to hypoxic preconditioning. Thus, we compared vascular endothelial growth factor (VEGF) production in individual CDCs with that in CDC monolayer sheets to compare hypoxia sensitivity. Equal numbers of CDCs were separately plated onto cell culture dishes with different surface diameters, and then each culture dish was exposed to hypoxic conditions for 24 hours (Figure 1B). Cell sheets showed a low amount of phosphorylated-histone H3 compared with non-layered CDCs, indicating that cells in the sheet could have exited the cell cycle (Figure 1C). Cell sheets produced significantly higher amounts of VEGF and hepatocyte growth factor (HGF) than non-layered CDCs in response to hypoxic treatment (Figure 1D), suggesting that a sheet-like structure increased CDC sensitivity to hypoxia and demonstrating a greater effect of hypoxic preconditioning in cell sheets.
Figure 1.
Sheet-like structures display enhanced sensitivity to hypoxic preconditioning. A: Representative image of the hypoxic preconditioning of a human cardiosphere-derived cell (CDC) sheet. Monolayer CDC sheets were incubated in 2% oxygen for 24 hours to reinforce therapeutic efficacy. The white arrowhead indicates a CDC monolayer sheet. B: Schema of the experiment performed to compare hypoxia sensitivity between sheet-like structure and individual cells. Equal numbers of CDCs (3 × 105 cells/well) were plated onto 12-well and 6-well plates and then incubated in 2% oxygen for 24 hours. Cell sheets were well developed in 12-well plates, whereas cells were sparsely distributed in 6-wells plates. C: Cell proliferation status in sparse CDCs and CDC monolayer sheets. Phosphorylated histone H3 (pHH3) expression was reduced in CDC sheets. D: A sheet-like structure enhanced the angiogenic activity of CDCs in response to hypoxic preconditioning. Pro-angiogenic factor levels, except for that of IGF-I, were significantly higher in preconditioned CDC sheets than preconditioned sparse CDCs.
HIF-1α was upregulated in preconditioned CDC sheets
HIF-1α, a major oxygen sensor, regulates the expression of hypoxia response factors, and a wide variety of cell types recognizes extracellular oxygen status through the HIF pathway [16]. In the case of CDCs, hypoxia also stabilizes HIF-1α, resulting in upregulation of hypoxia response factors such as VEGF and erythropoietin (EPO) [15]. However, it is unclear whether the HIF pathway similarly enhances cellular functions in CDC monolayer sheets via hypoxic preconditioning. To evaluate HIF-1α expression in cell sheets, CDC monolayers were cultured in normoxic or hypoxic conditions for 24 hours and then subjected to a HIF-1α analysis using immunofluorescence. Similar to dimethyloxaloylglycine (DMOG)-treated cell sheets (HIF-1α+ cells: 82%), hypoxic preconditioning stabilized HIF-1α proteins in CDC monolayers (HIF-1α+ cells: 69%), while HIF-1α proteins were degraded in normoxically-cultured CDC monolayers (HIF-1α+ cells: 27%) (Figure 2A). Similar result was obtained by western blot analysis (Figure 2B).
Figure 2.
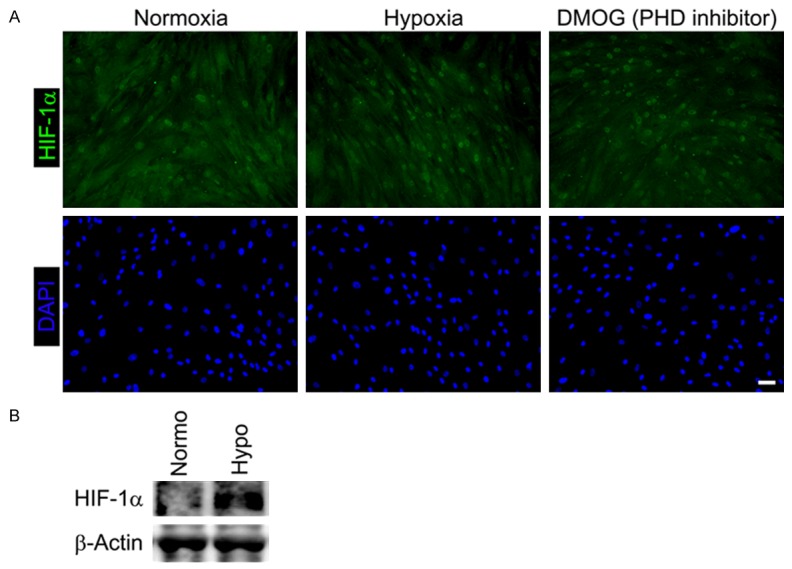
Hypoxic preconditioning stabilizes HIF-1α in cell sheets. CDC monolayer sheets were incubated under normoxia or hypoxia for 24 hours. HIF-1α expression was evaluated using immunocytochemistry (A) and western blotting (B). DMOG, a PHD inhibitor, was used as a positive control for HIF-1α expression in immunocytochemistry. Normo: Normoxia; Hypo: Hypoxia. Scale bar = 50 μm.
Hypoxic preconditioning activates Akt and Erk in cell sheets
To identify activated signal transducers in preconditioned CDC monolayer sheets, we performed a phosphorylated kinase array analysis. The phosphorylation status of 40 signaling proteins in normally-cultured (normoxia) and preconditioned (hypoxia) CDC monolayer sheets was analyzed. Three different proteins, including EGFR, Akt, and HSP60, showed a more than 5-fold increase in phosphorylation under hypoxic conditions (Figure 3A). A previous study demonstrated that hypoxic preconditioning of cardiac progenitor cells activated the Erk signaling pathway [17]. However, Erk phosphorylation was not dramatically altered in preconditioned CDC monolayer sheets in our kinase array. However, components of the ERK pathway were slightly increased based on western blot results (Figure 3A and 3B). Since we recently demonstrated that PI3K inhibition inhibited the hypoxia-associated stimulation of CDC monolayers [12], these results indicate that Akt is the major signal transducer responsive to hypoxic preconditioning in CDC monolayer sheets rather than the Erk pathway.
Figure 3.

Hypoxic preconditioning induces phosphorylation of some kinase proteins in CDC monolayer sheets. A: To identify pathways responding to hypoxic preconditioning, a kinase array analysis was performed using cell lysates from preconditioned and non-preconditioned CDC monolayer sheets. Within 40 kinase proteins, three different proteins, EGFR, Akt, and HSP60, displayed a more than 5-fold increase in phosphorylation. Data represent the average spot intensity of duplicate examinations. B: Upregulation of Akt phosphorylation was confirmed by western blot analysis, whereas phosphorylated-Erk was slightly increased.
Hypoxic preconditioning activates the PI3K/Akt/mTOR/HIF-1α pathway in CDC monolayer sheets
We previously demonstrated that enhancement of cellular functions in CDC sheets by hypoxic preconditioning was mediated by the PI3K/Akt pathway [12]. However, the specific downstream effectors of the hypoxia-PI3K/Akt pathway in CDC sheets have not been identified. Cell sheets were incubated in low oxygen with LY294002, a small molecule inhibitor of PI3K, for 24 hours, and collected proteins were evaluated using western blotting. Phosphorylation of mTOR and Akt was reduced by LY294002 treatment, as was the hypoxia-associated stabilization of HIF-1α (Figure 4A). Because phosphorylation of Akt and mTOR and stabilization of HIF-1α were inhibited by PI3K inhibition, both mTOR and HIF-1α are candidate downstream effector molecules of the PI3K/Akt pathway implicated in the preconditioning of CDC sheets. In addition, PI3K inhibition also decreased endoglin expression, an antagonist of the TGF-β signaling pathway, in preconditioned CDC sheets (Figure 4B). Taken together with our previous report showing upregulation of endoglin in preconditioned CDC sheets, these results suggest that endoglin is a potential downstream target of the hypoxia-activated PI3K/Akt/mTOR/HIF-1α pathway in CDC sheets along with VEGF (Figure 4C).
Figure 4.
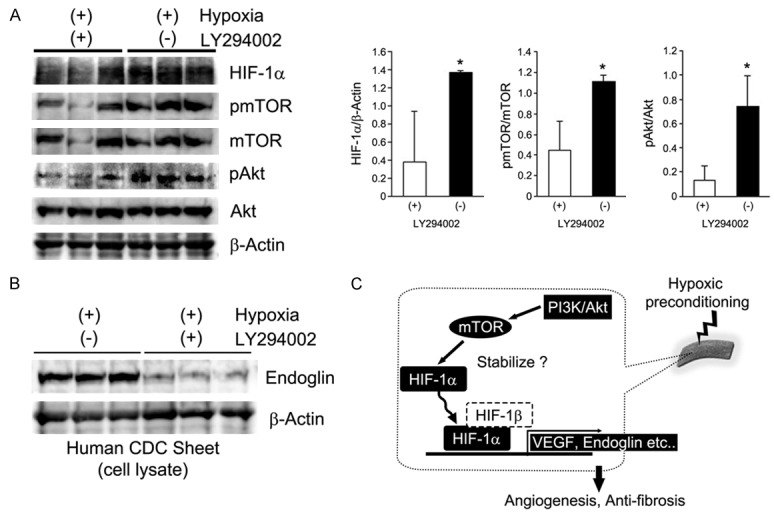
Hypoxic preconditioning activates the PI3K/Akt/mTOR/HIF1α pathway in CDC monolayer sheets. CDC monolayer sheets were incubated in low oxygen for 24 hours with or without a PI3K inhibitor (LY294002). A: Expression of HIF-1α, and phosphorylation of mTOR and Akt were significantly decreased in LY294002-treated preconditioned CDC monolayer sheets. *P < 0.05. B: Endoglin expression in preconditioned CDC monolayer sheets was suppressed by PI3K inhibition. C: Proposed molecular pathway of hypoxic preconditioning in cell sheets.
Discussion
Cell sheet technology is a promising therapeutic strategy for the treatment of heart failure. Indeed, the clinical efficacy of cell sheet-based therapy has been demonstrated in patients with dilated cardiomyopathy who were implanted with autologous myoblast sheets [18]. There is a recent trend in cell sheet-based therapy for heart failure, involving the implantation of layered cell sheets into the failing heart [19,20]. However, it will take more time until layered cell sheets can be generally used in clinical settings, as protocols to efficiently laminate cell sheets have not been established. In addition, the technical complexity of developing layered cell sheets for clinical use cannot be overlooked. In contrast, hypoxic preconditioning is an effective method to directly enhance cellular functions and is beneficial for clinical application due to its simplicity. We recently demonstrated that hypoxic preconditioning could also be applied to cell sheets, resulting in the enhancement of therapeutic efficacy for the treatment of infarcted hearts [12,13]. In the present study, we determined that sheeted structures were more sensitive to hypoxic preconditioning than individual cells. Although further experiments are needed to elucidate the basis for these observed differences in hypoxia sensitivity, gap junctions formed in cell sheets could increase the effect of hypoxic preconditioning, as cell sheet structure enables the formation of morphological and electrical connections between cells [21]. Thus, a combination of hypoxic preconditioning and cell sheet-based therapy could be a better therapeutic strategy for heart failure than ordinary cell transplantation.
Extracellular oxygen sensing is mainly mediated by the HIF pathway. In this pathway, hypoxia stabilizes HIFs by inhibiting the activity of prolyl hydroxylase domain proteins (PHDs), leading to transcriptional regulation of hypoxia adaptation target genes such as VEGF and erythropoietin [22]. We observed that HIF-1α expression in preconditioned cell sheets, indicating that hypoxic preconditioning exerts its potentiation effects on cell sheet-based systems through the HIF pathway. In addition, we also found that phosphorylated mTOR and HIF-1α were decreased in preconditioned cell sheets when sheets were treated with a PI3K inhibitor. The PI3K/Akt/mTOR pathway stabilizes and upregulates HIF-1α [23,24]. Thus, hypoxic preconditioning could similarly maintain HIF-1α expression in cell sheets through this molecular pathway in addition to exerting an inhibitory effect on HIF-1α degradation. The outcome is that angiogenesis and anti-fibrotic activity are increased in ischemic tissues.
In addition to Akt, kinase array analysis revealed that two candidate molecules were phosphorylated in preconditioned cell sheets. One of these molecules, EGFR, has been reported to be ligand-independently upregulated by hypoxia in cancer cells [25]. Although we have no supporting data, there is a possibility that hypoxic preconditioning is initially sensed by EGFR on the cell membrane of CDC sheets upstream of PI3K. However, the molecular chaperone HSP60 also displayed increased phosphorylation in preconditioned-cell sheets. Phosphorylation of HSP60 is mediated by protein kinase A (PKA), and hypoxia increases PKA activity [26,27]. Thus, hypoxic preconditioning could activate PKA and subsequently induce HSP60 phosphorylation in cell sheets. Both HSP60 and PKA are involved in the protection of cardiomyocytes from ischemia-reoxygenation [28,29]. Hypoxic preconditioning could potentially enhance the cardioprotective function of cell sheets through HSP60 phosphorylation. Further studies are needed to determine the significance of HSP60 phosphorylation in cell sheets.
The present study demonstrated that cell sheets are more sensitive to hypoxic stimulation than individual cells. This finding indicates that hypoxic preconditioning of cell sheets is a valuable strategy to enhance the therapeutic efficacy of cell-based systems for the treatment of ischemic diseases. This study also elucidated part of the molecular mechanisms of hypoxic preconditioning of cell sheets, as the PI3K/Akt/mTOR/HIF-1α pathway was activated in response to hypoxia. These findings provide important information on the hypoxic preconditioning of cell sheets and could lead to clinical application of this therapeutic strategy.
Acknowledgements
We thank cardiac surgeons at the Yamaguchi University Hospital for collecting the human heart specimens. We also thank Yukari Hironaka and Kazuko Tanaka for technical assistance. This work was supported in part by the JSPS-KAKENHI (15K10218 to A.M., 15K10217 to B.S), the Yamaguchi University New Frontier Project Research Grant (to T.H.), and the Yamaguchi University School of Medicine Translational Research Propulsion Grant (to K.H.).
Disclosure of conflict of interest
None.
Authors’ contribution
Y.T., and T.H. conceived the study and designed the experiments. Y.T., T.H., and K.H. wrote the manuscript. Y.T., A.M., H.K., A.N., K.U., and B.S. performed the experiments and analyzed the data. All authors discussed the results and approved the manuscript.
References
- 1.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 2.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Yamato M, Kohno C, Nishimoto A, Sekine H, Fukai F, Okano T. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials. 2005;26:6415–6422. doi: 10.1016/j.biomaterials.2005.04.061. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, Kikuchi A, Umezu M, Okano T. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res. 2002;90:e40. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- 8.Masumoto H, Matsuo T, Yamamizu K, Uosaki H, Narazaki G, Katayama S, Marui A, Shimizu T, Ikeda T, Okano T, Sakata R, Yamashita JK. Pluripotent stem cell-engineered cell sheets reassembled with defined cardiovascular populations ameliorate reduction in infarct heart function through cardiomyocyte-mediated neovascularization. Stem Cells. 2012;30:1196–1205. doi: 10.1002/stem.1089. [DOI] [PubMed] [Google Scholar]
- 9.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 10.Cheng K, Blusztajn A, Shen D, Li TS, Sun B, Galang G, Zarembinski TI, Prestwich GD, Marban E, Smith RR, Marban L. Functional performance of human cardiosphere-derived cells delivered in an in situ polymerizable hyaluronan-gelatin hydrogel. Biomaterials. 2012;33:5317–5324. doi: 10.1016/j.biomaterials.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudo T, Hosoyama T, Samura M, Katsura S, Nishimoto A, Kugimiya N, Fujii Y, Li TS, Hamano K. Hypoxic preconditioning reinforces cellular functions of autologous peripheral blood-derived cells in rabbit hindlimb ischemia model. Biochem Biophys Res Commun. 2014;444:370–375. doi: 10.1016/j.bbrc.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 12.Hosoyama T, Samura M, Kudo T, Nishimoto A, Ueno K, Murata T, Ohama T, Sato K, Mikamo A, Yoshimura K, Li TS, Hamano K. Cardiosphere-derived cell sheet primed with hypoxia improves left ventricular function of chronically infarcted heart. Am J Transl Res. 2015;7:2738–2751. [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka Y, Shirasawa B, Takeuchi Y, Kawamura D, Nakamura T, Samura M, Nishimoto A, Ueno K, Morikage N, Hosoyama T, Hamano K. Autologous preconditioned mesenchymal stem cell sheets improve left ventricular function in a rabbit old myocardial infarction model. Am J Transl Res. 2016;8:2222–2233. [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura T, Hosoyama T, Kawamura D, Takeuchi Y, Tanaka Y, Samura M, Ueno K, Nishimoto A, Kurazumi H, Suzuki R, Ito H, Sakata K, Mikamo A, Li TS, Hamano K. Influence of aging on the quantity and quality of human cardiac stem cells. Sci Rep. 2016;6:22781. doi: 10.1038/srep22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan SC, Gomes RS, Yeoh KK, Perbellini F, Malandraki-Miller S, Ambrose L, Heather LC, Faggian G, Schofield CJ, Davies KE, Clarke K, Carr CA. Preconditioning of cardiosphere-derived cells with hypoxia or prolyl-4-hydroxylase inhibitors increases stemness and decreases reliance on oxidative metabolism. Cell Transplant. 2016;25:35–53. doi: 10.3727/096368915X687697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung SY, Choi SH, Yoo SY, Baek SH, Kwon SM. Modulation of human cardiac progenitors via hypoxia-ERK circuit improves their functional bioactivities. Biomol Ther (Seoul) 2013;21:196–203. doi: 10.4062/biomolther.2013.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawa Y, Miyagawa S, Sakaguchi T, Fujita T, Matsuyama A, Saito A, Shimizu T, Okano T. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg Today. 2012;42:181–184. doi: 10.1007/s00595-011-0106-4. [DOI] [PubMed] [Google Scholar]
- 19.Haraguchi Y, Shimizu T, Sasagawa T, Sekine H, Sakaguchi K, Kikuchi T, Sekine W, Sekiya S, Yamato M, Umezu M, Okano T. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat Protoc. 2012;7:850–858. doi: 10.1038/nprot.2012.027. [DOI] [PubMed] [Google Scholar]
- 20.Sekine H, Shimizu T, Sakaguchi K, Dobashi I, Wada M, Yamato M, Kobayashi E, Umezu M, Okano T. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013;4:1399. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuda N, Shimizu T, Yamato M, Okano T. Tissue engineering based on cell sheet technology. Adv Mater. 2007;19:3089–3099. [Google Scholar]
- 22.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Roche O, Xu C, Moriyama EH, Heir P, Chung J, Roos FC, Chen Y, Finak G, Milosevic M, Wilson BC, Teh BT, Park M, Irwin MS, Ohh M. Hypoxia promotes ligand-independent EGF receptor signaling via hypoxia-inducible factor-mediated upregulation of caveolin-1. Proc Natl Acad Sci U S A. 2012;109:4892–4897. doi: 10.1073/pnas.1112129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan IU, Wallin R, Gupta RS, Kammer GM. Protein kinase A-catalyzed phosphorylation of heat shock protein 60 chaperone regulates its attachment to histone 2B in the T lymphocyte plasma membrane. Proc Natl Acad Sci U S A. 1998;95:10425–10430. doi: 10.1073/pnas.95.18.10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaikh D, Zhou Q, Chen T, Ibe JC, Raj JU, Zhou G. cAMP-dependent protein kinase is essential for hypoxia-mediated epithelial-mesenchymal transition, migration, and invasion in lung cancer cells. Cell Signal. 2012;24:2396–2406. doi: 10.1016/j.cellsig.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Lin KM, Lin B, Lian IY, Mestril R, Scheffler IE, Dillmann WH. Combined and individual mitochondrial HSP60 and HSP10 expression in cardiac myocytes protects mitochondrial function and prevents apoptotic cell deaths induced by simulated ischemia-reoxygenation. Circulation. 2001;103:1787–1792. doi: 10.1161/01.cir.103.13.1787. [DOI] [PubMed] [Google Scholar]
- 29.Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25:9554–9575. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]