Abstract
Rapid growth of tumor cells needs to consume large amounts of oxygen and glucose, due to lack of blood supply within the tumor, cells live in an environment that lack of oxygen and nutrients. This environment results in endoplasmic reticulum (ER) stress and activates the UPR (unfolded protein response). More and more evidence suggests UPR provides a growth signal pathway required for tumor growth. In the present study, we investigated the relationship between XBP1, one transcription factor in UPR, and the expression of LOX. We found that ER stress induces high expression of XBP1, one transcription factor in UPR, in both 2D culture and 3D culture; but only promotes growth of lung adenocarcinoma cells in in vitro 3D culture other than 2D culture. In 3D culture, we further showed that knockdown XBP1 expression can block Tm/Tg-induced cell growth. LOX genes may be key downstream effector of XBP1. Knockdown LOX expression can partially block XBP1-induced cell growth. Then we showed XBP1 suppressed by RNA interference (RNAi) can reduce the expression of LOX. For the first time, it is being shown that XBP1 can regulate the expression of LOX to promote cell growth.
Keywords: XBP1, LOX, A549, ER stress, 3D culture
Introduction
Lung cancer has become the most common cause of death from cancer, accounting for approximately 23% of all cancer-related deaths in the world. In particular, non-small cell lung cancer (NSCLC) accounts for about nine out of ten cases of all lung cancers [1]. Rapid growth of tumor cells need to consume large amounts of oxygen and glucose, but blood vessel growth lags far behind the rate of tumor growth, leading to cell hypoxia and under-nutrition. These external pressures will cause endoplasmic reticulum stress in cells and activate the UPR (unfolded protein response). More and more evidence showed that, UPR provides a growth signal pathway required for tumor growth and helps tumor cells to escape apoptosis induced by endoplasmic reticulum stress [2].
In the UPR process, IRE1α/XBP1 pathway controls the expression of many UPR-related genes, these genes participated in the process of protein folding, transportation and degradation to alleviate the endoplasmic reticulum stress. A series of recent studies showed that, IRE1α/XBP1 pathway is essential on tumor cell response to external pressure. Lack of XBP1 in human HT1080 fibrosarcoma cells cannot form tumor in SCID mice [3]. XBP1-missing cells showed increased apoptosis and decreased ability to form colonies in endoplasmic reticulum stress or hypoxic conditions. In addition, the expression of a dominant negative mutant (dominant-negative) of XBP1 can reduce or inhibit angiogenesis [4,5].
Lysyl oxidase (LOX) is an enzyme that mediates the cross-linking of collagens and elastin [6,7]. Recent studies showed that patients with high expression of LOX in malignant tumors have a high relapse rate and very poor prognosis. Head and neck cancer patients with high LOX have a higher probability of metastatic invasion and lower survival rate [8-10]. Further studies showed hypoxia inducible factor HIF1α directly bind to the promoter region of LOX and significantly induced the expression of LOX [11].
Hypoxia not only activates HIF1α, but also activates the UPR [12]. Hypoxia induced signaling pathway is essential for cell survival under hypoxic conditions and that XBP1 is required in this process for tumor growth [4]. These studies suggest that the full UPR is activated under hypoxia. These findings prompted us to investigate the downstream target genes that regulates cell survival under UPR. In this study, we firstly studied the relationship between UPR and the expression of LOX, and we showed XBP1, the critical transcriptional factor in UPR process, can promote cell growth in vitro by regulating the expression of LOX.
Materials and methods
Plasmid construction
The Spliced XBP1 (XBP1s) coding sequence was amplified by PCR from cDNA of A549 cells, using primers 5’-gatcGCTAGCATGGTGGTGGTGGCAGCC-3’ (forward) and 5’-gatcGCGGCCGCTTAGACACTAATCAGCTGGGGAAAG-3’ (reverse).
PCR reaction was performed using PrimeStar kit (Toyobo) according to manufacturer’s protocol. The PCR product was purified and cloned into pCDH lentivirus vector using NheI/NotI. The selected clone was fully sequenced in order to verify that no mutations were introduced by PCR.
For RNAi, we transfected siRNA of LOX or XBP1 into A549 or CRL5908 cells with lipofectamine 2000 (life). LOX RNAi sequence is: 5’-CTGGAAACTATATCCTAA-3’, XBP1 RNAi sequence is: 5’-GCCAAGCTAATGTGGTAGT-3’, Scramble sequence is: 5’-CATGAAGAAGGGCACCTAA-3’.
The luciferase construct of pGL3-LOX promoter contained nts -1000 to 249 of the LOX gene (+1 denoted to transcription start site). Genomic DNA fragment of this region was obtained by PCR amplification using AmpliTaq Gold DNA Polymerase (Applied Biosystems) with primer sequences: 5’-gatcGGTACCCAAAGTTTCTAGCACTGCCC-3’ (forward), 5’-gatcGCTAGCCTTCTGCACGTACGTGGACGCC-3’ (reverse).
Real-time RT-PCR
Quantitative RT-PCR analysis was used to determine the relative expression level of XBP1, LOX. Total RNA was extracted from cells using Trizol (Invitrogen) according to the manufacturer’s instructions. Single-stranded cDNA was synthesized by using Reverse Transcription Kit (Fermentas). The expression levels of LOX was detected by RT-Real Time PCR (BioRad). Splicing of XBP1 was detected by RT-PCR. Each sample in each group was measured in triplicate and the experiment was repeated at least three times. Primer pairs: LOX: 5’-GAAGGCCACAAAGCAAGTTT-3’ (Forward), 5’-CAGGACTCAATCCCTGTGTG-3’ (Reverse); GAPDH: 5’-ATGCCTCCTGCACCACCAAC-3’ (Forward), 5’-GGCAGTGATGGCATGGACTG-3’ (Reverse); XB P1: 5’-TGAGCTGGAACAGCAAGTGGT-3’ (Forward), 5’-CCCAAGCGCTGTCTTAACTCC-3’ (Reverse).
Cell culture
A549 cells were cultured in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum (Hyclone), 100 IU/ml penicillin and 10 mg/mL streptomycin. CRL-5908 cells were cultured in ATCC-formulated RPMI-1640 Medium containing 10% fetal bovine serum, 100 IU/ml penicillin and 10 mg/mL streptomycin. All cells were maintained at 37°C under an atmosphere of 5% CO2.
MTT cell viability assay
Cell viability after indicated treatment was measured by the MTT assay. Briefly, the cells were collected and seeded in 96-well plate at a density of 5 × 103 cells/well. Different seeding densities were optimized at the beginning of the experiments. After indicated treatment, 20 µl of MTT tetrazolium salt (Sigma) dissolved in phosphate-buffered saline (PBS) at a concentration of 5 mg/ml was added to each well and incubated in a CO2 incubator for additional 4 h. Finally, the medium was aspirated from each well and 150 µl of DMSO (Sigma) was added to dissolve formazan crystals and the absorbance of each well was obtained at 490 nm.
Western blotting
Cells in 2D and 3D culture were lysed in RIPA buffer (Pierce). Protein extracts were boiled in SDS/β-mercaptoethanol sample buffer, and 30 μg samples were loaded into each lane of 10% polyacrylamide gels. The proteins were separated by electrophoresis, and the proteins in the gels were blotted onto nitrocellulose membranes (PALL) by electrophoretic transfer. The membrane was incubated with rabbit polyclonal antibodies against-LOX (sigma), mouse anti-β-actin monoclonal antibody (santa cruz), rabbit polyclonal antibodies against-XBP1 (Biolegend) for 16 h at 4°C. The specific protein-antibody complex was detected by using horseradish peroxidase conjugated rabbit anti-mouse or rabbit anti-mouse IgG. Detection by the chemiluminescence reaction was carried using the ECL kit (Pierce). The β-actin signal was used as a loading control.
Spheroids-forming assay in 3D culture
The wells in 48-well plates were coated with 50 μl of growth factor reduced BD Matrigel (BD Biosciences #356231) and allowed to polymerize at 37°C for 15 minutes. Then, 5 × 105 cells were resuspended in 200 μl of growth factor reduced BD Matrigel and plated onto the matrigel-coated wells. Plates were incubated for 30 minutes after which 1 ml of media was added to the top of the matrigel. Cells were treated with Tm/Tg (sigma) or DMSO for 6 hours and then change into normal media. Media was replenished every 48 hours. Images were taken on day 6, spheroids in the size range of 100-300 µm were counted.
Luciferase reporter assay
The promoter of human LOX mRNA was constructed using synthetic oligonucleotides and cloned in pGL3-Basic vector (Promega, USA). PGL3-LOX promoter-luciferase was transfected into the HEK293 cell line, with pGL3-SV40-Renilla as the internal loading control. The cells were harvested and lysed 24 h after transfection. Reporter activity was then measured using the Dual-Luciferase Assay System (Promega).
Statistical analysis
Data were analyzed by using SPSS Statistical Package version 16. Independent two group’s analyses are used t-test. P<0.05 was considered statistically significant.
Results
Endoplasmic reticulum stress promotes cell growth in 3D culture but not in 2D culture
Tunicamycin (Tm) is a mixture of homologous nucleoside antibiotics that inhibits GlcNAc phosphotransferase (GPT), which catalyzes the transfer of N-acetylglucosamine-1-phosphate from UDP-N-acetyl glucosamine to dolichol phosphate in the first step of glycoprotein synthesis. So it is used as an experimental tool to induce unfolded protein response (UPR) [13]. Thapsigargin (Tg) is non-competitive inhibitor of the endoplasmic reticulum Ca2 ATPase. Thapsigargin (Tg) can block the ability of the cell to pump calcium into endoplasmic reticula which causes unfolded protein response (UPR) [14]. To study how ER stress can regulate cancer cell growth, we cultured two lung adenocarcinoma cell lines A549 and CRL-5908 in 2D culture, used Tm/Tg to induce ER stress in both cells, and then performed MTT assay. The result demonstrated that Tm/Tg significantly inhibited cell growth in both A549 and CRL-5908 cells (Figure 1A).
Figure 1.
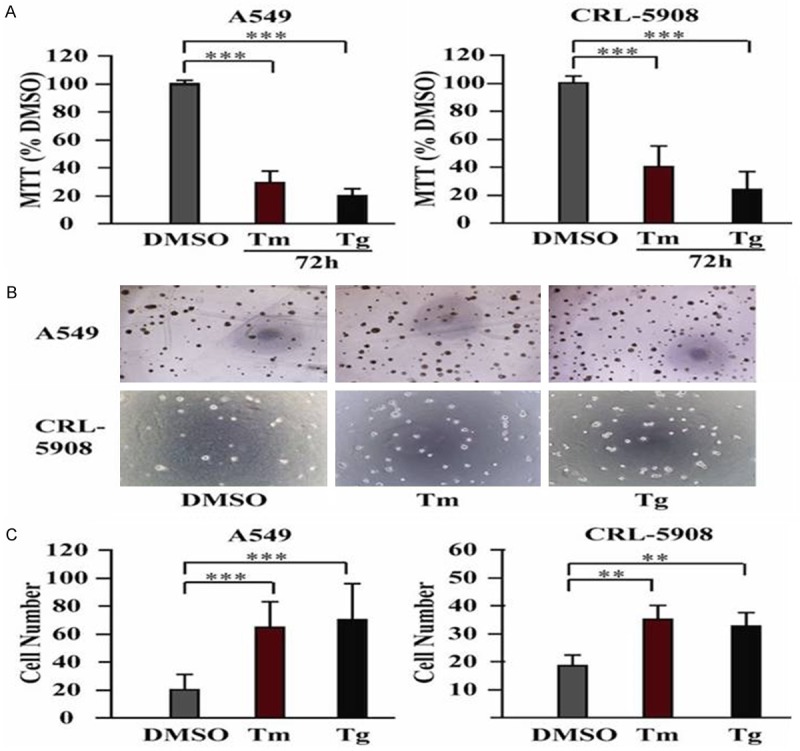
ER stress promotes growth of lung cancer cells in 3D culture other than in 2D culture. (A) Tm/Tg treatment inhibits growth of A549 and CRL-5908 cells in 2D culture. (B) After Tm/Tg treatment, there are more spheroids compared with DMSO control group in both A549 and CRL-5908 cells in 3D culture. B Showed the representative photomicrographs of cells at × 4 original magnification. (C) Quantitative analysis of (B). **P<0.05, ***P<0.001. Data are expressed as the mean numbers of independent triplicate experiments.
It is now well accepted that traditional two-dimensional (2D) cell culture have contributed tremendously to our understanding of cancer biology but have significant limitations in mimicking in vivo conditions such as the tumor microenvironment [15]. In vitro, three-dimensional (3D) cell culture models represent a more accurate, intermediate platform between simplified 2D culture models and complex and expensive in vivo models. 3D in vitro models can overcome 2D in vitro limitations caused by the oversupply of nutrients, and not physiological cell-cell and cell-material interactions, and allow for dynamic interactions between cells, stroma, and extracellular matrix. Spheroids have been shown to mimic the tissue-like properties of tumors [16-18]. According to these facts, we decided to investigate whether ER stress can regulate cell growth differently in 3D culture, we cultured A549 and CRL-5908 cells in 3D matrigel and treated with Tm/Tg. Interestingly, the result showed that both A549 and CRL-5908 treated with Tm/Tg grew more spheroids than cells treated with DMSO ctrl (Figure 1B and 1C). These data showed ER stress promotes the spheroids-forming ability of lung cancer cells only in 3D culture other than 2D culture.
XBP1 is essential in ER stress-induced cell growth in 3D culture
We then studied why cells showed differences with UPR in 2D culture and 3D culture. Firstly, we compared the splicing of XBP1, the ER stress marker, in these two culture systems. We isolated the cells and extracted the RNA, then we did RT-PCR, the result showed mRNA of XBP1 is spliced into XBP1s after Tm/Tg treatment in both 2D and 3D culture (Figure 2A). We also collected cell lysate to perform westernblot assay, expression of XBP1s is also upregulated at protein level after Tm/Tg treatment in both 2D and 3D culture (Figure 2B).
Figure 2.
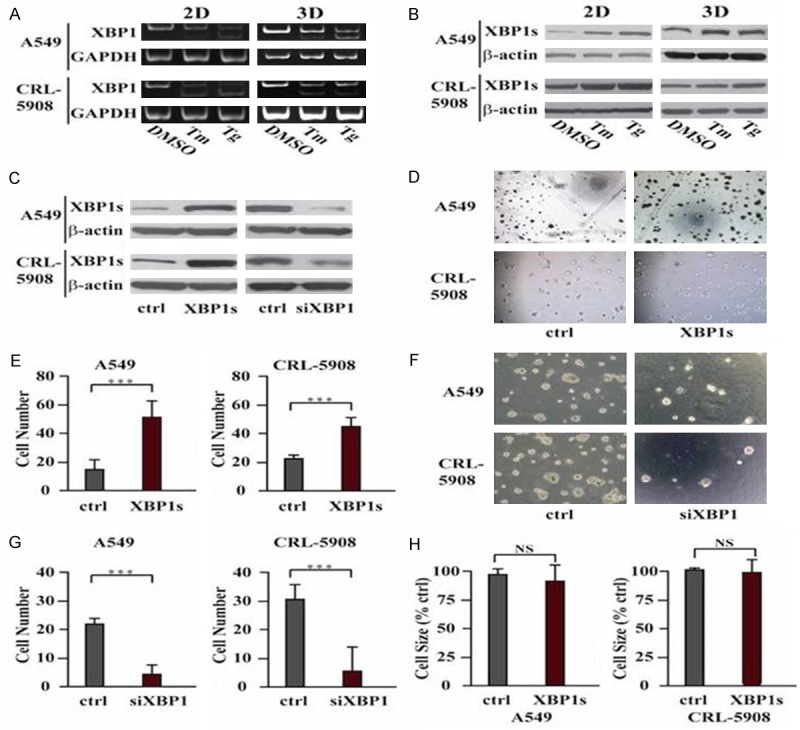
Induced Splicing and expression of XBP1 by ER stress is critical for growth of cancer cells in 3D culture. (A) RT-PCR showed Tm/Tg promotes splicing of XBP1. (B) higher expression of XBP1s after Tm/Tg treatment. (C) Western blot showed the expression level of spliced XBP1 (XBP1s). (D) Overexpression of XBP1s promotes number of spheroids in 3D culture. (E) Quantitative analysis of (D). ***P<0.001. (F) Knockdown of XBP1s by siRNA decreases number of spheroids in 3D culture. (G) Quantitative analysis of (F). ***P<0.001. (H) The size of spheroids showed no significance between control cells and XBP1s overexpressing cells. Data are expressed as the mean numbers of independent triplicate experiments.
To investigate the role of XBP1 in ER stress-induced cell growth, we transiently overexpressed spliced XBP1 (XBP1s) (Figure 2C), then seeded these cells in 3D culture, the results suggested that cells overexpressing spliced XBP1 form more spheroids than control cells (Figure 2D and 2E). We also used siRNA to knockdown XBP1 (Figure 2C) and then treated cells with Tm/Tg, the result showed without XBP1, the cells formed much less spheroids than control cells (Figure 2F and 2G). The size of spheroids showed no differences between control and ER stress cells (Figure 2H). These data suggested XBP1 is the main player in ER stress-induced cell growth in 3D culture.
LOX is the downstream effector in XBP1-induced cell growth in 3D culture
3D structure and the interaction of cells with extracellular matrix play very important role in cell survival and cell growth. To investigate the mechanism by which XBP1 induced cell growth in 3D culture, we found that the expression of LOX was highly upregulated with Tm/Tg treatment in both 2D and 3D culture (Figure 3A and 3B).
Figure 3.
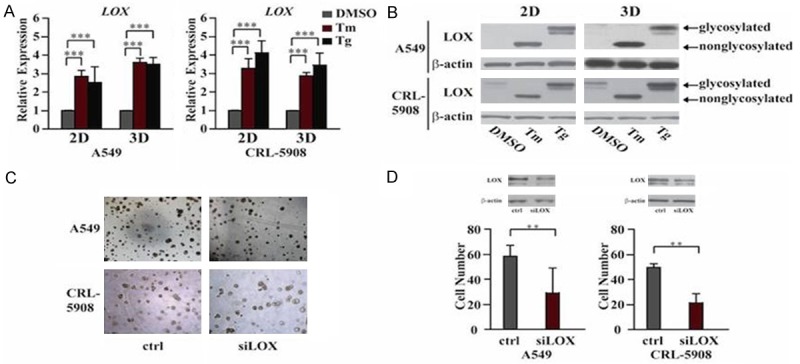
Upregulation of LOX by ER stress is necessary for cell growth in 3D culture. (A) QPCR showed Tm/Tg upregulated the expression of LOX in both 2D and 3D culture at mRNA level. (B) Western blot showed higher expression of LOX after Tm/Tg treatment. A lower size of LOX in lane 2 indicates inhibited glycosylation by Tm. (C) Knockdown of LOX by siRNA decreases number of spheroids in 3D culture. (D) Quantitative analysis of (C). **P<0.05. WB results showed the knockdown efficiency of LOX. Data are expressed as the mean numbers of independent triplicate experiments.
To further investigate whether LOX is the downstream effector in XBP1-induced cell growth in 3D culture, we designed shRNA to transiently knockdown LOX in spliced XBP1-overexpressing A549 and CRL-5908 cells, then we performed spheroids-forming assay in 3D culture, the results suggested that cells lacking LOX expression form fewer spheroids than cells with control shRNA (Figure 3C and 3D). So these data suggest that XBP1-LOX axis plays critical role in ER stress-induced cell growth.
LOX expression is regulated by XBP1 in both 2D and 3D culture
To further study the mechanism that regulates the expression of LOX in ER stress, we used the DECODE transcription factor database to predict transcription factors that may bind to the promoter region of LOX. There are many HIF1 binding sites in the promoter region of LOX, which is consistent with previous reports. Interestingly, we found a predict XBP1 binding sites in the promoter region of LOX (Figure 4A). Then we investigated whether XBP1 indeed regulates the expression of LOX. Real-time quantitative PCR and western blot assay showed transient overexpression of XBP1s in both A549 and CRL-5908 cells upregulated the expression of LOX at both mRNA and protein levels (Figure 4B and 4C). We also designed shRNA to knockdown XBP1, the results indicated that upregulation of expression of LOX induced by Tm/Tg was inhibited after knockdown of XBP1 (Figure 4D and 4E). Luciferease activity assay also showed much higher luciferase activity in cells transfected with pGL3-LOX promoter than pGL3-basic (Figure 4F), so these data suggest LOX is the direct downstream target gene of XBP1.
Figure 4.
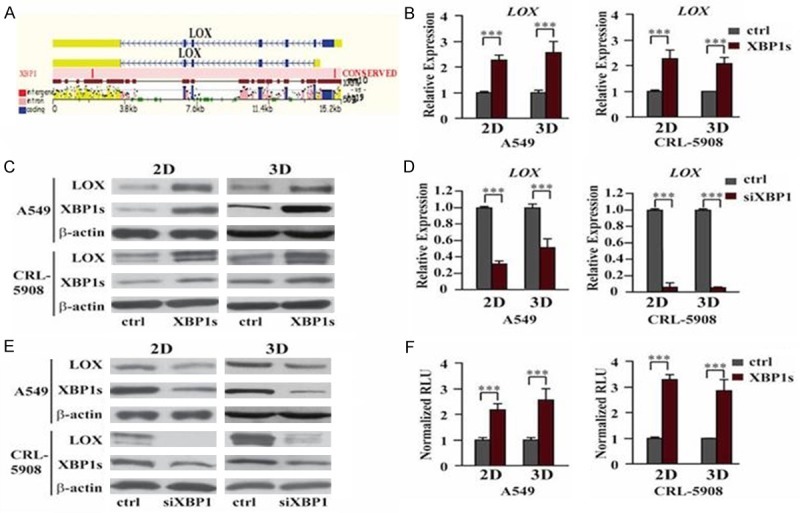
XBP1 promotes A549 growth in 3D culture by regulating expression of LOX. A: Prediction of binding site of XBP1 in the promoter of LOX. We used the DECODE transcription factor database to predict transcription factors that may bind to the promoter region of LOX. The result showed XBP1 may bind at the 5’UTR of mRNA of LOX. B: QPCR showed overexpression of XBP1s upregulated the expression of LOX in both 2D and 3D culture at mRNA level. ***P<0.001. C: Westernblot showed higher expression of LOX in XBP1s overexpressing cells. D: QPCR showed knockdown of XBP1s downregulated the expression of LOX in both 2D and 3D culture at mRNA level. ***P<0.001. E: Westernblot showed decreased expression of LOX after knockdown of XBP1s. F: Luciferase reporter assay showed direct binding of XBP1 on the promoter of LOX mRNA. ***P<0.001. Data are expressed as the mean numbers of independent triplicate experiments.
Discussion
Tumor cells need huge amounts of oxygen and glucose for rapid growth, but the growth of tumor blood vessels lags far behind, resulting in a hypoxic and under-nutrition environment, these external pressures will result in cellular endoplasmic reticulum stress and activate the UPR. More and more evidence showed that, UPR provides the necessary growth signal to promote tumor growth. In fact, in many cancers high expression of XBP1, ATF6, CHOP and GRP78 are discovered, such as breast, liver, stomach and esophagus, etc. But the detailed mechanism by which UPR can promote tumor growth and growth is still unclear.
In this paper, we showed UPR can activate high expression of XBP1 and splicing of XBP1 in both 2D and 3D culture system, but interestingly, spliced XBP1 only functions to promote cell growth in 3D culture other than 2D culture.
It’s well known that the microenvironment surrounding the cancer cells play very important role in cell survival and growth in vivo. So we proposed that XBP1 functions through interaction with the microenvironment surrounding the cancer cells. Here is ECM components and structure in 3D culture system. LOX is the key enzyme crosslinking collagen and elastin to maintain complete extracellular matrix (ECM) structure integrity and plays an important role in variety of abnormal connective tissue diseases [8]. In recent years more and more evidence suggest the role of LOX in different types of tumor progression including in breast cancer, central nervous system, head and neck squamous cell carcinoma, prostate carcinoma, renal cell carcinoma, lung cancer, melanoma and osteosarcoma. Patients with high expression and differentiation of LOX in malignant tumors have high recurrence rate and poor growth, and therefore LOX can be used as a diagnostic marker of poor prognosis and maybe a target to treat tumor.
Given the key role of LOX in the development of connective tissue disease and tumor metastasis, further research on regulation of expression of LOX takes more attention. Numerous studies indicate that, expression of LOX is mainly due to the regulation of hypoxia-inducible factor HIF1α [11]. But low oxygen not only activates HIF1α, but also activates the UPR.
Here we show that LOX is a downstream gene of UPR and plays critical role in cell growth in 3D culture. Through bioinformatics approach we predict LOX may be a downstream gene of XBP1, we then demonstrated that XBP1 can increase LOX expression by direct binding of XBP1 on the promoter of LOX mRNA.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Boelens J, Lust S, Offner F, Bracke ME, Vanhoecke BW. Review. The endoplasmic reticulum: a target for new anticancer drugs. In Vivo. 2007;21:215–226. [PubMed] [Google Scholar]
- 3.Chen Y, Feldman DE, Deng C, Brown JA, De Giacomo AF, Gaw AF, Shi G, Le QT, Brown JM, Koong AC. Identification of mitogen-activated protein kinase signaling pathways that confer resistance to endoplasmic reticulum stress in Saccharomyces cerevisiae. Mol Cancer Res. 2005;3:669–677. doi: 10.1158/1541-7786.MCR-05-0181. [DOI] [PubMed] [Google Scholar]
- 4.Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H, Mori K, Glimcher LH, Denko NC, Giaccia AJ, Le QT, Koong AC. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004;64:5943–5947. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- 5.Romero-Ramirez L, Cao H, Regalado MP, Kambham N, Siemann D, Kim JJ, Le QT, Koong AC. X box-binding protein 1 regulates angiogenesis in human pancreatic adenocarcinomas. Transl Oncol. 2009;2:31–38. doi: 10.1593/tlo.08211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez C, Martinez-Gonzalez J, Raposo B, Alcudia JF, Guadall A, Badimon L. Regulation of lysyl oxidase in vascular cells: lysyl oxidase as a new player in cardiovascular diseases. Cardiovasc Res. 2008;79:7–13. doi: 10.1093/cvr/cvn102. [DOI] [PubMed] [Google Scholar]
- 7.Payne SL, Hendrix MJ, Kirschmann DA. Paradoxical roles for lysyl oxidases in cancer--a prospect. J Cell Biochem. 2007;101:1338–1354. doi: 10.1002/jcb.21371. [DOI] [PubMed] [Google Scholar]
- 8.Stassar MJ, Devitt G, Brosius M, Rinnab L, Prang J, Schradin T, Simon J, Petersen S, Kopp-Schneider A, Zoller M. Identification of human renal cell carcinoma associated genes by suppression subtractive hybridization. Br J Cancer. 2001;85:1372–1382. doi: 10.1054/bjoc.2001.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirschmann DA, Seftor EA, Fong SF, Nieva DR, Sullivan CM, Edwards EM, Sommer P, Csiszar K, Hendrix MJ. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002;62:4478–4483. [PubMed] [Google Scholar]
- 10.Kirschmann DA, Seftor EA, Nieva DR, Mariano EA, Hendrix MJ. Differentially expressed genes associated with the metastatic phenotype in breast cancer. Breast Cancer Res Treat. 1999;55:127–136. doi: 10.1023/a:1006188129423. [DOI] [PubMed] [Google Scholar]
- 11.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 12.Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, Harding H, Novoa I, Varia M, Raleigh J, Scheuner D, Kaufman RJ, Bell J, Ron D, Wouters BG, Koumenis C. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24:3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan SW, Egan PA. Hepatitis C virus envelope proteins regulate CHOP via induction of the unfolded protein response. FASEB J. 2005;19:1510–1512. doi: 10.1096/fj.04-3455fje. [DOI] [PubMed] [Google Scholar]
- 14.Ganley IG, Wong PM, Gammoh N, Jiang X. Distinct autophagosomal-lysosomal fusion mechanism revealed by thapsigargin-induced autophagy arrest. Mol Cell. 2011;42:731–743. doi: 10.1016/j.molcel.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasser AK, Mundy BL, Smith KM, Studebaker AW, Axel AE, Haidet AM, Fernandez SA, Hall BM. Human bone marrow stromal cells enhance breast cancer cell growth rates in a cell line-dependent manner when evaluated in 3D tumor environments. Cancer Lett. 2007;254:255–264. doi: 10.1016/j.canlet.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- 17.Weigelt B, Bissell MJ. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin Cancer Biol. 2008;18:311–321. doi: 10.1016/j.semcancer.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc. 2009;4:309–324. doi: 10.1038/nprot.2008.226. [DOI] [PubMed] [Google Scholar]


