Abstract
Poly r(C) binding protein (PCBP) 1 or heterogeneous ribonucleoprotein (hnRNP) E1 is a RNA binding protein that plays a vital role in a wide variety of biological processes. PCBP1 has been shown to function as a tumor suppressor by negatively regulating translation of pro-metastatic proteins in different cancers. Loss of PCBP1 expression or its Akt2-mediated phosphorylation at serine 43 residue has both been indicated to de-repress its regulation of EMT inducer proteins. Our previous work has established that PCBP1 functions as a tumor suppressor in thyroid cancer, where its translation is inhibited by microRNA-490-3p. Here we show that thyroid cancer patients can be divided into 2 cohorts based on miR-490-3p expression and PCBP1 mRNA expression-one cohort with high PCBP1 mRNA expression and basal miR-490-3p expression and a second cohort with low PCBP1 mRNA expression and high miR-490-3p expression. However, PCBP1 protein expression is also downregulated in the cohort with high PCBP1 mRNA expression, with expression levels similar to what is observed in patients with the low PCBP1 mRNA expression. Our analysis shows that PCBP1 mRNA is actively translated in patients with high PCBP1 mRNA expression, but that the protein is post translationally degraded by the proteasome machinery. Our results thus elucidate a novel mechanism responsible for down regulation of PCBP1 expression in thyroid cancer. It will be important in future to identify the mechanism that causes degradation of PCBP1 protein and to identify if similar mechanisms are active in other tumors characterized by low PCBP1 protein expression.
Keywords: Poly r(C) binding protein, thyroid carcinoma, miR-490-3p
Introduction
Thyroid cancer accounts for 90-95% of all endocrine tumors [1,2] and has exhibited a steady increase in incidence, especially in men [1]. Approximately 30% of palpable nodules associated with thyroid disorders turn out to be malignant [3]. Papillary, follicular, medullary, or anaplastic are the different forms of thyroid cancer [4], with papillary thyroid cancer (PTC) accounting for 70% of all thyroid tumors [5,6]. Death due to PTC mostly result from secondary progression to lymph nodes in necks [7,8]. However, the underlying mechanisms for metastatic dissemination of PTC is not well understood [9].
We have recently shown that the RNA binding protein, poly r(c) binding protein (PCBP) 1 or heterogeneous nuclear ribonucleoprotein (hnRNP) E1 functions as a putative tumor suppressor in thyroid cancer [10]. PCBP1 has also been previously shown to function as a tumor suppressor by inhibiting translation of genes required for metastatic progression in pancreatic cancer, ovarian cancer, breast cancer, lung cancer, gastric cancer, and Burkitt lymphoma [11-17]. Loss of PCBP1 expression in prostate cancer is also associated with enrichment of CD44+CD133+CD24- stem cells [18].
Our previous experiments showed that in thyroid cancer, translation of PCBP1 mRNA is suppressed by the microRNA miR-490-3p [10]. MiR-490-3p expression similarly functions as an oncomirin hepatocellular carcinoma [19]. However, in Ewing’s sarcoma and colon cancer, miR-490-3p is downregulated [20,21], which would suggest that there might be additional mechanisms to downregulate or inactivate PCBP1 expression and function during tumor progression.
The objective of the current study was to determine if additional mechanism(s) regulate PCBP1 protein expression in thyroid cancer. Our results cumulatively reveal that PCBP1 is post-translationally degraded in a cohort of thyroid cancer patients who have low miR-490-3p expression.
Materials
Patient samples
The study protocol was approved by the Institutional Review Board of Tianjin Medical University General Hospital, China. Papillary thyroid carcinoma tissue specimens and corresponding adjacent non-tumorous thyroid tissue samples were obtained from 47 Chinese patients at Tianjin Medical University General Hospital between 2012 and 2015. Patients that did not undergo pre-operative local or systemic treatment were included in this study. Of the 47 patients, PCBP1 mRNA expression was determined as described below and 10 patients each with low and high PCBP1 expression were included for the final analysis.
Cell culture and treatment
BCAP, WRO, FTC133, TPC1, NPA, FRO, and ARO cells were obtained from the cell and tissue bank at our center and cultured in RPMI1640 medium, containing glutamine, 5% FBS (Lonza, Germany), and penicillin/streptomycin. Cells were maintained at 37°C in a humidified atmosphere of 5% carbon dioxide. Where indicated cells were treated with 10 µM of MG-132 (Sigma-Aldrich, Shanghai, China) for 6 hours before being harvested.
Polyribosomal profiling
BCAP, WRO, FTC133, TPC1, NPA, FRO, and ARO cells were treated with 100 µg/mL cycloheximide (Sigma-Aldrich, Shanghai, China) for one hour at 37°C before being washed with cold PBS containing 100 µg/mL Cycloheximide. Cell lysis and polyribosomal profiling was done by lysing cells in polysome lysis buffer (10 mM Tris-Cl, pH 7.4, 5 mM MgCl2, 100 mM KCl, 1% (v/v) Triton X-100, 0.5% (w/v) deoxycholate, 1000 U/ml RNasin, 2 mM DTT and 100 µg/ml Cycloheximide in DEPC treated water), incubated on ice for 10 minutes and centrifuged at 16,000× g at 4°C to obtain the clarified post-nuclear lysate. One-tenth (v/v) of the lysate was set aside for total RNA isolation. Post-nuclear clarified lysates (50 OD units) were layered on top of 10-50% sucrose gradients and centrifuged at 28,000 rpm (100,000× g) for 4 hours at 4°C using an SW41 rotor (Beckman, Fullerton, CA, USA).
RNA extraction and quantitative real time polymerase chain reaction (qRT-PCR)
Trizol was used to isolate total RNA and miRNA from tissue specimens and cultured cells. The expression level of PCBP1 mRNA, GAPDH mRNA, miR-490-3p, and RNU6B were detected by TaqMan miRNA assays (Life technology, Shanghai, China). The -ΔΔCt method was used to analyze the data in each case and normalization was done to GAPDH and RNU6B expression for mRNA and miRNA, respectively, unless otherwise mentioned.
The cancer genome atlas analysis
The TCGA data portal (tcga-data.nci.nih.gov/tcga/) was used to download thyroid carcinoma RNASeqV2 normalized gene expression data on 153 tumor-normal matched pairs. Statistical analysis was performed using the R statistical software. Tumor-normal log2-fold changes were calculated as FCij = log2 [(Tij + 1)/(Nij + 1)], where FCij represents the fold change for patient i in gene j. Tij and Nij represent the normalized read counts for gene j in tumor and matched normal samples of patient i, respectively.
Cell and tissue lysis and western blot
Cell lysis and western blot was done as described previously [10]. Blots were probed with anti-PCBP1 antibody (#ab-133421, Abcam) and anti-GAPDH antibody (#ab-9485, Abcam) (to confirm equal loading per sample).
Immunoprecipitation to detect poly-ubiquitinated PCBP1
To detect ubiquitinated-PCBP1, whole cell lysates from TPC1 cells ± MG-132 were prepared as above. Cell lysates (2 mg) were incubated for overnight at 4°C with 50 μg of either mouse anti-PCBP1 antibody or mouse IgG (Sigma-Aldrich, Shanghai, China) crosslinked to protein A/G beads (Pierce Crosslink IP Kit, Life Technologies, Shanghai, China). The immune complexes were collected by centrifugation and eluted with glycine. The eluant were resolved by SDS-PAGE and probed by anti-Ubiquitin antibody (#ab-7780, Abcam) to detect poly-ubiquitinated PCBP1.
Statistical analyses
All statistical analyses were performed using the SPSS version 20.0 (IBM Corporation, NY). A P-value <0.05 was considered statistically significant.
Results
We have previously shown that miR-490-3p inhibits PCBP1 translation in thyroid cancer [10]. To determine, if PCBP1 mRNA was differentially expressed among thyroid cancer patients, we performed metagenomic analysis of The Cancer Genome Atlas (TCGA) data. Along expected lines, CDH1, encoding the epithelial cell marker E-cadherin, and CDH2, encoding the mesenchymal cell marker N-cadherin, were respectively, down- and up-regulated uniformly among the entire cohort of thyroid cancer patients (Figure 1). However, PCBP1 mRNA was either up or down regulated among the cancer patients. Based on PCBP1 mRNA expression the entire cohort could be sub-divided into 2-one with low PCBP1 expression and one with high PCBP1 expression (Figure 1), suggesting there are other mechanisms beyond miR-490 that might regulates PCBP1 expression in thyroid cancer patients.
Figure 1.
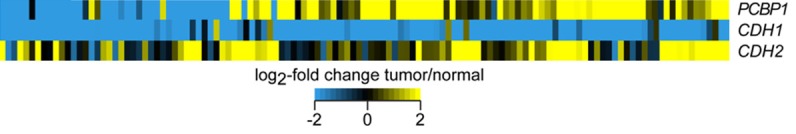
Thyroid cancer patients can be divided into two groups based on PCBP1 mRNA expression levels. Metagenomic analysis of expression of CDH1, CDH2, and PCBP1 in thyroid cancer patients. Heat map depicting log2-fold change in mRNA expression of indicated genes between tumor-normal matched pairs for 153 TCGA patients.
From our cohort of 47 patients with thyroid cancer, we choose 10 each with low and high PCBP1 mRNA expression and determined miR-490-3p expression level in these 20 patients. In each of the patients with low PCBP1 mRNA expression, miR-490-3p expression was significantly high (P<0.05) (Figure 2A). In comparison, in patients with high PCBP1 mRNA expression, miR-490-3p expression was detected only at the basal level. Our results indicated that PCBP1 mRNA expression showed an inverse relation to miR-490-3p expression, and the observed cases of low PCBP1 mRNA expression can be due to the high miR-490-3p expression. Whether transcriptional regulation is also a contributing factor in the later cohort remains to be determined.
Figure 2.
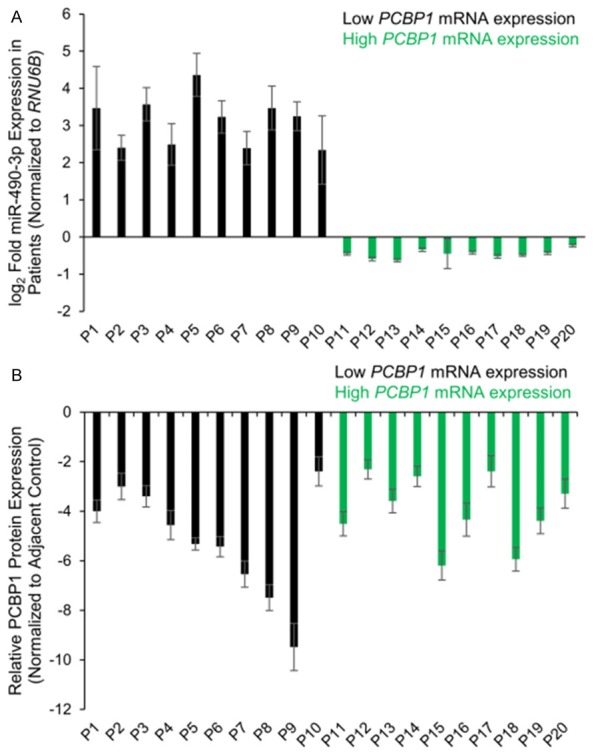
PCBP1 protein expression is universally suppressed in thyroid cancer patients, irrespective of miR-490-3p expression. A: Steady state expression of miR-490-3p in thyroid cancer and adjacent normal tissues from twenty different patients, ten with high and ten with low PCBP1 mRNA expression. Data is represented as mean ± standard deviation. B: Relative PCBP1 protein expression, normalized to adjacent control tissue, in 20 thyroid cancer tissue specimens. Data is represented as mean ± standard deviation.
We next decide to focus on the cohort of patients with basal miR-490-3p expression and high PCBP1 mRNA expression. Interestingly, PCBP1 protein expression was significantly suppressed (P<0.001) in all thyroid cancer patients, irrespective of the PCBP1 mRNA expression level (Figure 2B). This indicated that in the cohort of patients with high PCBP1 mRNA and low miR-490-3p expression, there are additional post-transcriptional mechanism (miRNA or RNA-binding protein) or post-translational mechanism that is causing the observed repression of PCBP1 protein.
To find the appropriate in vitro model system to further test this observation, we determined miR-490-3p and PCBP1 mRNA and protein expression in 7 different thyroid cancer cell lines (Figure 3A, 3B). Out of the 7 cell lines tested, miR-490-3p expression was significantly high (P<0.05) in BCPAP, WRO, and FTC133 cells; these were also the cell lines where PCBP1 mRNA expression was significantly downregulated (P<0.01) (Figure 3A). In the remaining 4 cell lines, NPA, FRO, ARO, and TPC1, PCBP1 mRNA expression was high and miR-490-3p was expressed at basal level (Figure 3A). However, PCBP1 protein expression was hardly different in these later 4 cell lines or detectable in any of the 7 cell lines tested (Figure 3B).
Figure 3.
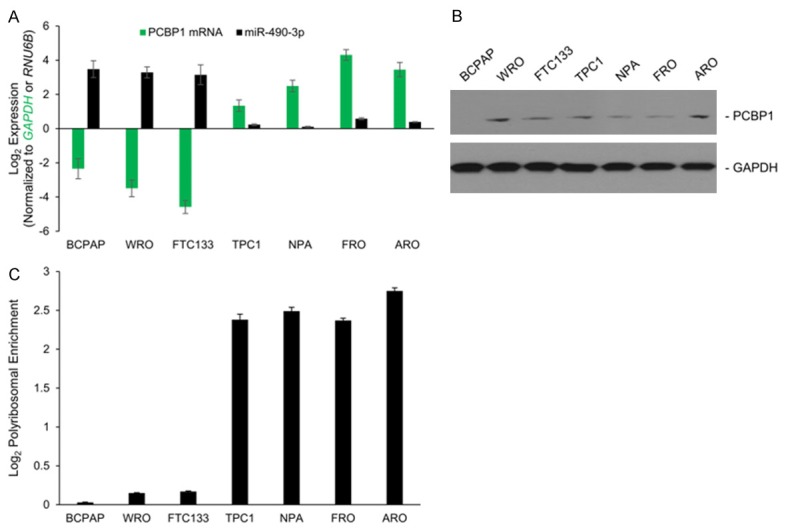
PCBP1 is perhaps post-translationally regulated in thyroid cancer cells with basal miR-490-3p expression. A: Steady state expression of miR-490-3p and PCBP1 mRNA in indicated thyroid cancer cell lines. Data was normalized to GAPDH for PCBP1 and RNU6B for miR-490-3p and is represented as mean ± standard deviation. B: Steady state expression of PCBP1 in indicated thyroid cancer cell lines. Blots were probed with anti-GAPDH antibody to verify equivalent loading. Data is representative of four independent experiments. C: Log2-fold change in PCBP1 mRNA enrichment in actively translating polysome fractions in the indicated cell lines. Data was derived in respect to translationally inactive non-polysomal fraction and total mRNA and represented as mean ± standard deviation.
To determine if additional post-transcriptional mechanism(s) are operant, we determined polysomal enrichment of PCBP1 mRNA in each of the aforementioned 7 cell lines (Figure 3C). As has been shown previously [10], and expected based on miR-490-3p expression level, PCBP1 was excluded from the actively translating polysome fractions in the BCPAP, WRO, and FTC133 cells. However, PCBP1 was detected at significant levels in the actively translating polysome fractions in each of the 4 cell lines with high PCBP1 mRNA expression, indicating that protein synthesis was not being inhibited and that post-translational mechanism(s) might be involved.
Hence, we next determined if the difference in the PCBP1 protein expression level was due to altered degradation kinetics in the NPA, FRO, ARO, and TPC1 cells. All the 7 aforementioned cell lines were treated with MG-132, which inhibits proteasomal degradation. Inhibition of proteasomal degradation induced PCBP1 protein expression only in the NPA, FRO, ARO, and TPC1 cells, but not in the BCPAP, WRO, and FTC133 cells (Figure 4A), suggesting that differential targeting by the protein degradation machinery is responsible for lack of PCBP1 protein expression in the NPA, FRO, ARO, and TPC1 cell lines.
Figure 4.
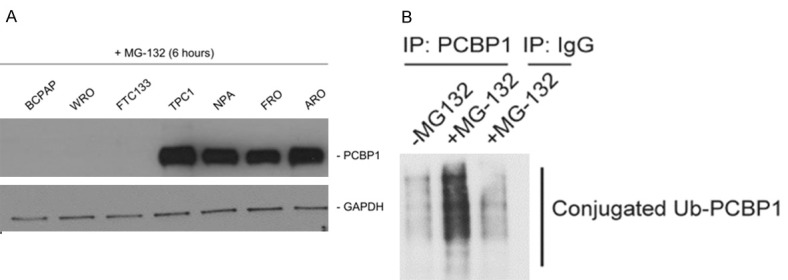
PCBP1 is post translationally modified in thyroid cancer cells. A: Western blot analysis of PCBP1 protein expression in indicated cell lines following treatment with the proteasome inhibitor, MG-132 for six hours. B: PCBP1 immunoprecipitates from untreated and MG-132 treated TPC1 cells were probed with anti-Ubiquitin antibody to determine if PCBP1 protein was being ubiquitinated in these cells. Smear indicates ubiquitinated PCBP1 in the MG-132 treated TPC1 cells.
We next determined if PCBP1 was actually getting ubiquitinated in the TPC1 cells. PCBP1 was immunoprecipitated from MG-132 treated or untreated TPC1 cells and resolved by SDS-PAGE and probed with anti-Ubiquitin antibody. MG-132 treatment lead to detection of a robust poly-ubiquitinated PCBP1 smear in the TPC1 cells (Figure 4B).
Discussion
Our results cumulatively show that PCBP1 is indeed a tumor suppressor in the context of thyroid cancer and that these cells have evolved redundant mechanisms to suppress its expression during tumorigenesis and progression. In patients with high miR-490-3p, PCBP1 mRNA’s translation is inhibited; however, in patients with basal miR-490-3p expression, the PCBP1 mRNA is translated but the protein is targeted for proteasome-mediated ubiquitination and degradation. The degradation machinery remains to be determined in future endeavors.
PCBP1 can regulate the stability of the pro-oncogenic p63 transcript in pancreatic, ovarian and breast cancer cell line [11]. Furthermore, downregulation or loss of PCBP1 expression has been shown to upregulate translation of genes and long non-coding RNA required for epithelial to mesenchymal transition and metastatic progression in different cancers, and Burkitt lymphoma [12-17]. However, that PCBP1 itself can be regulated at the level of protein stability has not been previously indicated in any of the aforementioned cases.
It will be important to understand the degradation machinery and particularly what regulates the machinery. It can be hypothesized that the same regulatory mechanism will have a way to sense miR-490-3p expression levels to regulate its own activation. Similarly, it will be important to determine if similar redundant mechanisms regulate PCBP1 expression in other cancers.
Of note, in certain cases like colon cancer and Ewing’s sarcoma, miR-490-3p expression has been shown to be suppressed [20,21]. To better understand the regulation of PCBP1 expression, it will be vital to use colon cancer and Ewing’s sarcoma as a model to determine if PCBP1 expression is downregulated and if so whether it is by proteasomal degradation, as can be extrapolated from our own findings in thyroid cancer.
Acknowledgements
This study was supported by the project Mechanism and Effect of Hypothyroidism Induced by Chronic Intermittent Hypoxia (No. 2013KZ138) from the Science and Technology Foundation of Tianjin Municipal Health Bureau.
Disclosure of conflict of interest
None.
References
- 1.Hodgson NC, Button J, Solorzano CC. Thyroid cancer: is the incidence still increasing? Ann Surg Oncol. 2004;11:1093–1097. doi: 10.1245/ASO.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Mackenzie EJ, Mortimer RH. Thyroid nodules and thyroid cancer. Med J Aust. 2004;180:242–247. doi: 10.5694/j.1326-5377.2004.tb05894.x. [DOI] [PubMed] [Google Scholar]
- 4.Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgibbons SC, Brams DM, Wei JP. The treatment of thyroid cancer. Am Surg. 2008;74:389–399. [PubMed] [Google Scholar]
- 6.Kitamura Y, Shimizu K, Ito K, Tanaka S. Emi M. Allelotyping of follicular thyroid carcinoma: frequent allelic losses in chromosome arms 7q, 11p, and 22q. J Clin Endocrinol Metab. 2001;86:4268–4272. doi: 10.1210/jcem.86.9.7853. [DOI] [PubMed] [Google Scholar]
- 7.Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008;21(Suppl 2):S37–S43. doi: 10.1038/modpathol.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suster S. Thyroid tumors with a follicular growth pattern: problems in differential diagnosis. Arch Pathol Lab Med. 2006;130:984–988. doi: 10.5858/2006-130-984-TTWAFG. [DOI] [PubMed] [Google Scholar]
- 9.Vasko VV, Saji M. Molecular mechanisms involved in differentiated thyroid cancer invasion and metastasis. Curr Opin Oncol. 2007;19:11–17. doi: 10.1097/CCO.0b013e328011ab86. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, Wang X, Tan J, Zhao M, Lian L, Zhang W. Poly r(C) binding protein (PCBP) 1 is a negative regulator of thyroid carcinoma. Am J Transl Res. 2016;8:3567–3573. [PMC free article] [PubMed] [Google Scholar]
- 11.Cho SJ, Jung YS, Chen X. Poly (C)-Binding Protein 1 Regulates p63 Expression through mRNA Stability. PLoS One. 2013;8:e71724. doi: 10.1371/journal.pone.0071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhury A, Hussey GS, Ray PS, Jin G, Fox PL, Howe PH. TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat Cell Biol. 2010;12:286–293. doi: 10.1038/ncb2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussey GS, Chaudhury A, Dawson AE, Lindner DJ, Knudsen CR, Wilce MC, Merrick WC, Howe PH. Identification of an mRNP complex regulating tumorigenesis at the translational elongation step. Mol Cell. 2011;41:419–431. doi: 10.1016/j.molcel.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Vardy LA, Tan CP, Loo JM, Guo K, Li J, Lim SG, Zhou J, Chng WJ, Ng SB, Li HX, Zeng Q. PCBP1 suppresses the translation of metastasis-associated PRL-3 phosphatase. Cancer Cell. 2010;18:52–62. doi: 10.1016/j.ccr.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Gai L, Liu J, Cui Y, Zhang Y, Feng J. Expression of poly(C)-binding protein 1 (PCBP1) in NSCLC as a negative regulator of EMT and its clinical value. Int J Clin Exp Pathol. 2015;8:7165–7172. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang ZZ, Shen ZY, Shen YY, Zhao EH, Wang M, Wang CJ, Cao H, Xu J. HOTAIR Long noncoding RNA promotes gastric cancer metastasis through suppression of poly r(C)-binding protein (PCBP) 1. Mol Cancer Ther. 2015;14:1162–70. doi: 10.1158/1535-7163.MCT-14-0695. [DOI] [PubMed] [Google Scholar]
- 17.Wagener R, Aukema SM, Schlesner M, Haake A, Burkhardt B, Claviez A, Drexler HG, Hummel M, Kreuz M, Loeffler M, Rosolowski M, López C, Möller P, Richter J, Rohde M, Betts MJ, Russell RB, Bernhart SH, Hoffmann S, Rosenstiel P, Schilhabel M, Szczepanowski M, Trümper L, Klapper W, Siebert R ICGC MMML-Seq-Project; “Molecular Mechanisms in Malignant Lymphomas” Network Project of the Deutsche Krebshilfe. The PCBP1 gene encoding poly(rC) binding protein I is recurrently mutated in Burkitt lymphoma. Genes Chromosomes Cancer. 2015;54:555–564. doi: 10.1002/gcc.22268. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Cai ZK, Chen YB, Gu M, Zheng DC, Zhou J, Wang Z. Poly r(C) binding protein-1 is central to maintenance of cancer stem cells in prostate cancer cells. Cell Physiol Biochem. 2015;35:1052–1061. doi: 10.1159/000373931. [DOI] [PubMed] [Google Scholar]
- 19.Zhang LY, Liu M, Li X, Tang H. miR-490-3p modulates cell growth and epithelial to mesenchymal transition of hepatocellular carcinoma cells by targeting endoplasmic reticulum-Golgi intermediate compartment protein 3 (ERGIC3) J Biol Chem. 2013;288:4035–47. doi: 10.1074/jbc.M112.410506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamfjord J, Stangeland AM, Hughes T, Skrede ML, Tveit KM, Ikdahl T, Kure EH. Differential expression of miRNAs in colorectal cancer: comparison of paired tumor tissue and adjacent normal mucosa using high-throughput sequencing. PLoS One. 2012;7:e34150. doi: 10.1371/journal.pone.0034150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakatani F, Ferracin M, Manara MC, Ventura S, Del Monaco V, Ferrari S, Alberghini M, Grilli A, Knuutila S, Schaefer KL, Mattia G, Negrini M, Picci P, Serra M, Scotlandi K. miR-34a predicts survival of Ewing’s sarcoma patients and directly influences cell chemo-sensitivity and malignancy. J Pathol. 2012;226:796–805. doi: 10.1002/path.3007. [DOI] [PubMed] [Google Scholar]


