Abstract
Nonalcoholic fatty liver disease (NAFLD) is a chronic disorder characterized by hepatic fat accumulation and abnormal lipid metabolism. Although miR-21 has been implicated in nonalcoholic fatty liver disease, it is unknown whether miR-21 could function as a therapeutic target. Here, we perform transfection analysis of miR-21 mimic or control mimic to evaluate the effects of miR-21 expression levels on human HepG2 nonalcoholic fatty liver cells. We used siRNA techniques to knock down miR-21 in HepG2 and control 293T cell lines, and then monitored lipid production and the expression levels of genes involved in lipid metabolism. The effects of miR-21 expression levels on LDL receptor-related protein 6 (LRP6) expression were evaluated using qRT-PCR and western blot analyses. Luciferase reporter assays were conducted to confirm the effects of miR-21 expression levels on LRP6. The results indicated that transfection of miR-21 mimic induced changes in the expression levels of lipogenic enzymes, including acetyl-CoA carboxylase 1 (ACC1), stearoyl CoA desaturase (1SCD1), sterol regulatory element-binding protein 1 (SREBP1), and liver X receptor alpha (LXRα). Transfection of miR-21 mimic suppressed the transcription and translation of LRP6 at the mRNA and protein levels, whereas miR-21 knockdown increased the expression levels of LRP6. Transfection of miR-21 mimic in HepG2 cells also induced lipid production and triggered the expression of critical lipid metabolic enzymes. These data suggest that mutation of miR-21 may be a new therapeutic strategy to treat nonalcoholic fatty liver diseases by targeting endogenous LRP6.
Keywords: Nonalcoholic fatty liver disease, miR-21, low-density lipoprotein (LDL), LDL receptor-related protein 6 (LRP6)
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a major chronic liver dysfunction disease globally, and its prevalence is increasing along with epidemics of obesity, diabetes, and metabolic syndrome. The NAFLD spectrum spans from steatosis to nonalcoholic steatohepatitis (NASH), which can lead to cirrhosis and hepatocellular cancer. Currently, there is a lack of therapeutic strategies to alleviate NAFLD, especially those that remove fatty liver cells without causing severe tissue damage.
MicroRNAs (miRs) are short, non-coding RNAs that act as post-translational regulators of gene expression. MiRs are conserved, small (20 to 25 nucleotide), non-coding RNAs that negatively regulate the expression of messenger RNAs (mRNAs) at the post-transcriptional level [1,2]. MiR-21 has been implicated in the development of NAFLD, and the association between nonalcoholic fatty liver and circulating miR-21, miR-34a, miR-122, and miR-451 has been documented [3]. MiR-21 and miR-155 are significantly upregulated in mice fed with a choline- and amino acid-deficient diet (CDAA), which promoted the development of NASH and hepatocellular carcinoma [4]. MiR21 targets and represses the grainy head like 3 (GRHL3) transcription factor, which leads to dephosphorylation of endothelial nitric oxide synthase (NOS3). NOS3 is a crucial mediator of endothelial function [5,6]. The low-density lipoprotein (LDL) receptor gene family encodes for at least 13 structurally related cell surface receptors that perform diverse biological functions in different organs, tissues, and cell types [7]. LDL receptors are transmembrane cell surface proteins involved in receptor-mediated endocytosis of lipoprotein and protein ligands. The Wnt signaling co-receptor LRP6 (low-density lipoprotein receptor-related protein 6) functions as a receptor or co-receptor (with Frizzled) for Wnt, and therefore functions in the canonical Wnt/β-catenin signaling cascade for endocytosis [8,9]. Several studies have highlighted the key roles of Wnt, LRP6, and its transcriptional cofactor, T-cell transcription factor 4 (TCF4), in the regulation of plasma LDL, triglyceride (TG)/very low-density lipoprotein (VLDL) synthesis, glucose homeostasis, and vascular cell proliferation [8-10]. Ex vivo examination of primary cells from mutant LRP6 carriers showed that an arginine to cysteine mutation at residue 611 (R611C) impaired LRP6 phosphorylation by Wnt and inhibited the nuclear localization of both β-catenin and TCF4 [10].
In this study, we investigated how miR-21 affects lipogenesis. We report that miR-21 inhibits LRP6 expression in HepG2 cells, thereby inducing lipid production.
Materials and methods
Cell lines and reagents
The human kidney epithelial cell line 293T (ATCC CRL-3216) was maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% penicillin, 5% streptomycin, and 10% heat-inactivated fetal bovine serum (Gibco, USA). The human fatty liver cancer cell line HepG2 was maintained in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 5% penicillin, 5% streptomycin, and 10% heat-inactivated bovine calf serum (Gibco, USA).
DNA constructs
The 3’-UTR and mutated 3’-UTR of LRP6 (mRNA residue positions 5,042-5,048) were synthesized and sub-cloned into the 3’ end of the pGL3-Luc vector (Promega, USA).
Quantitative real-time PCR
MicroRNAs were isolated using mirVana™ miRNA Isolation Kit (Life Technologies). Mature miR-21 was quantified with the TaqMan miRNA assay kit for mmu-miR-21 (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. Other qRT-PCR analyses were performed using the SYBR® Green kit (Bio-Rad). Primers used in this study are listed in Table 1 [8].
Table 1.
Human primer sequences for qRT-PCR analysis
| Target Gene | Reverse | Forward | Accession |
|---|---|---|---|
| LRP6 | Forward | 5’-CAGTTGGAGTGGTGCTGAAAGG-3’ | NM_002336 |
| Reverse | 5’-CCATCCAAAGCAGCCCGTTCAA-3’ | ||
| ACC1 | Forward | 5’-TTCACTCCACCTTGTCAGCGGA-3’ | NM_000664 |
| Reverse | 5’-GTCAGAGAAGCAGCCCATCACT-3’ | ||
| FASN | Forward | 5’-TTCTACGGCTCCACGCTCTTCC-3’ | NM_004104 |
| Reverse | 5’-GAAGAGTCTTCGTCAGCCAGGA-3’ | ||
| SCD1 | Forward | 5’-CCTGGTTTCACTTGGAGCTGTG-3’ | NM_005063 |
| Reverse | 5’-TGTGGTGAAGTTGATGTGCCAGC-3’ | ||
| LXRα | Forward | 5’-TGGACACCTACATGCGTCGCAA-3’ | NM_001130101 |
| Reverse | 5’-CAAGGATGTGGCATGAGCCTGT-3’ | ||
| SREBP1 | Forward | 5’-ACTTCTGGAGGCATCGCAAGCA-3’ | NM_001005291 |
| Reverse | 5’-AGGTTCCAGAGGAGGCTACAAG-3’ |
ACC1, acetyl-CoA carboxylase 1; FASN, fatty acid synthase; SCD1, stearoyl CoA desaturase 1; LXRa, liver X receptor; SREBP1, sterol regulatory element-binding protein 1; LRP6, low-density lipoprotein related receptor 6.
Western blotting
HepG2 cells were lysed on ice using lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% NP-40, and 1 mM DTT) supplemented with a protease inhibitor mixture (Sigma). Then, 20 µg lysates were separated on 9% SDS-polyacrylamide gels (SDS-PAGE). Proteins were transferred for western blotting. The blots were incubated with anti-LRP6 antibody (1:500; ab24386, Abcam, UK) or anti-b-actin antibody (ab8227, Abcam) overnight at 4°C. After washing, blots were incubated with the secondary antibody, which was a 1:2,000 dilution of HRP-linked anti-IgG, followed by detection using ECL reagents (Pierce, USA).
MiR-21 mimic and control mimic
The miR-21 mimic (dsRNA oligonucleotides) and control mimic were purchased from GenePharma (Shanghai, China). HepG2 cells were transfected with miR-21 mimic or control mimic (5, 10, or 25 nM) using Lipofectamine RNAiMAX (Invitrogen, USA) according to the manufacturer’s instructions.
Lipid synthesis and secretion assays
We adapted a detailed protocol from Skoog et al. [11] to determine both the cellular and secreted lipids, and evaluated the levels of cholesterol (C), triglyceride (TG), phospholipid (PL), and cholesteryl ester (CE).
Statistical analysis
Data were analyzed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). All data were analyzed with Student’s t-test; a P value less than 0.05 was considered statistically significant.
Results
Transfection with miR-21 mimic increases lipogenesis
We transfected miR-21 mimic and control mimic into HepG2 cells, and investigated the effects on lipogenesis. The miR-21 mimic, but not the control mimic, increased the levels of both cellular and secreted cholesterol, triglyceride, and phospholipid, whereas the cholesteryl ester levels did not change significantly (Figure 1A and 1B). Quantitative RT-PCR analysis indicated that transfection of miR-21 mimic induced the expression of genes encoding lipogenic enzymes, including ACC1, SCD1, SREBP1, and LXRα, but not fatty acid synthase (FASN) (Figure 1C).
Figure 1.
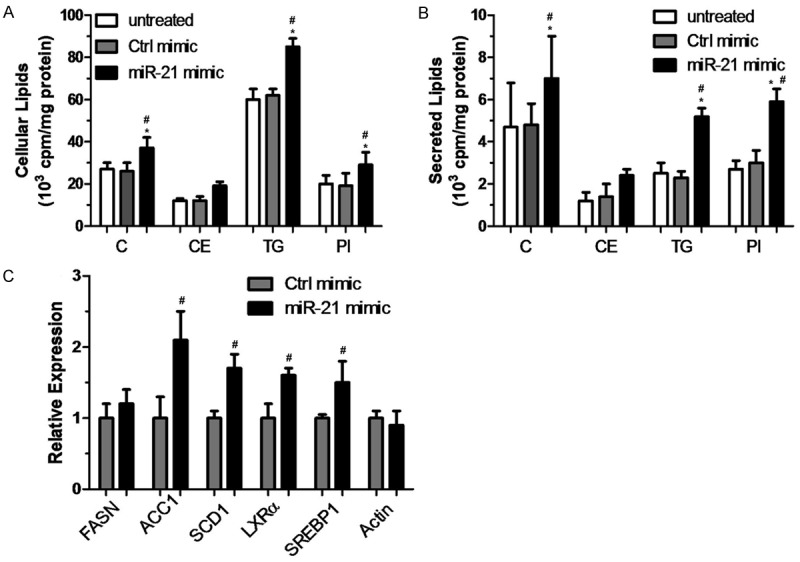
Transfection of miR-21 mimic stimulates lipogenesis. Contents of labeled lipids in cells and culture media. HepG2 cells were transfected with miR-21 mimic or control mimic (25 nM) for 48 h. Cholesterol (C), cholesteryl ester (CE), triglyceride (TG), and phospholipid (PL) levels were measured after 24 h in cells (A) and media (B). The induction of genes encoding lipogenic enzymes was quantified using qRT-PCR analyses (C). Data represent mean ± SD of two independent experiments (N = 4). *P < 0.05 represents the cellular lipids in the miR21 mimic group compared with the untreated group. #P < 0.05 represents the cellular lipids in the miR21 mimic group compared with the control mimic group.
LRP6 is downregulated in transfected HepG2 cells expressing miR-21 mimic
To identify the target of miR-21 that is responsible for the observed increase in lipid production, we employed the Target Scan program (MIT). The results indicated that LRP6 was a potential target of miR-21. The 3’-UTR region of human LRP6 mRNA contains the seed sequence that may be recognized by miR-21 (Figure 2A). We performed qRT-PCR and western blot analyses to experimentally verify this result, and found that miR-21 transfection into HepG2 cells suppressed LRP6 expression at both the transcriptional (mRNA) and translational (protein) levels (Figure 2B and 2C). These combined results indicate that LRP6 is a cellular target of miR-21.
Figure 2.
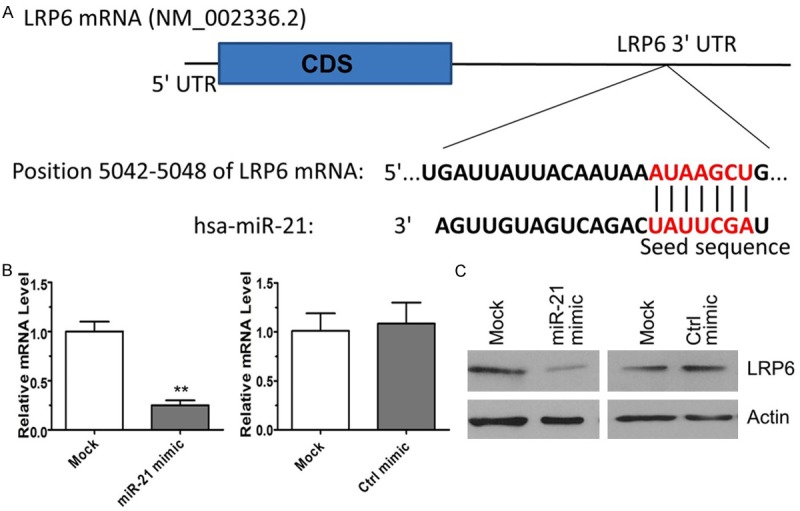
MiR-21 directly targets the 3’-UTR of LRP6. (A) Putative binding site of miR-21 in the 3’-UTR of LRP6 mRNA. HepG2 cells were transfected with 25 nM miR-21 mimic or control mimic. Cellular RNA or total protein was isolated at 48 h post-transfection for qRT-PCR (B) or western blot (C) analysis. **P < 0.01 represents the relative mRNA levels in the miR-21 mimic group compared with that in the mock transfected group.
Blocking miR-21 expression increases LRP6 expression
To corroborate the results of the miR-21 mimic experiments, we transfected miR-21 antagomir or control antagomir into HepG2 cells and measured LRP6 expression using qRT-PCR and western blot analyses. The results clearly show that blocking miR-21 expression induced LRP6 expression (Figure 3). These combined results confirm that miR-21 levels are inversely correlated with LRP6 levels (Figures 2 and 3). Therefore, miR-21 appears to be a novel regulator of endogenous LRP6 expression.
Figure 3.
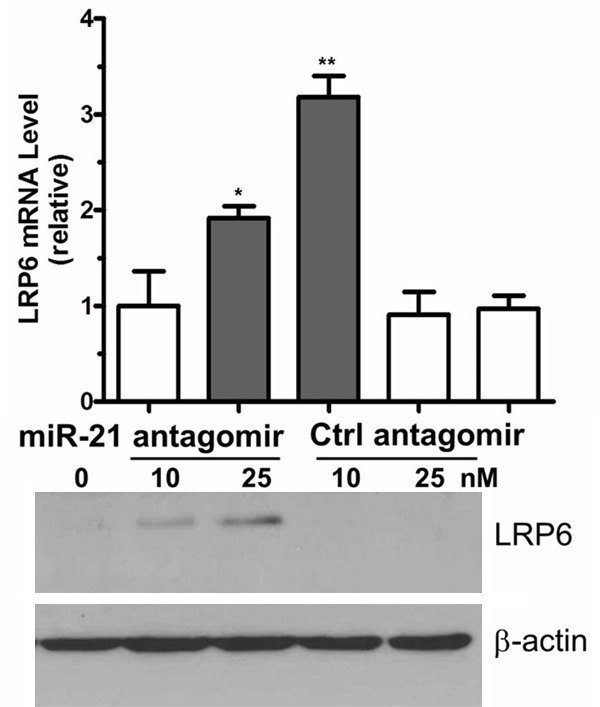
MiR-21 antagomir induces LRP6 expression. HepG2 cells were transfected with the indicated amount of miR-21 antagomir or control for 48 h. LRP6 mRNA and protein were quantified by qRT-PCR and western blotting, respectively. *P < 0.05 and **P < 0.01 represent the LRP6 mRNA levels in the miR-21 antagomir group compared with the miR-21 mimic group.
LRP6 is a direct target of miR-21
To mechanistically verify the regulation of LRP6 expression by miR-21, we sub-cloned the LRP6 3’-UTR into a firefly luciferase reporter construct. Similarly, we cloned a mutant LRP6 3’-UTR with a mutation in the miR-21 seed sequence. The LXRa 3’-UTR was generated as a negative control (Figure 4A). These constructs were transfected into HepG2 cells along with miR-21 mimic or control mimic. The results indicate that miR-21 mimic specifically inhibited luciferase expression, but the mutated miR-21 mimic did not (Figure 4B and 4C). The miR-21 mimic had no effect when luciferase was fused to the LXRa 3’-UTR.
Figure 4.
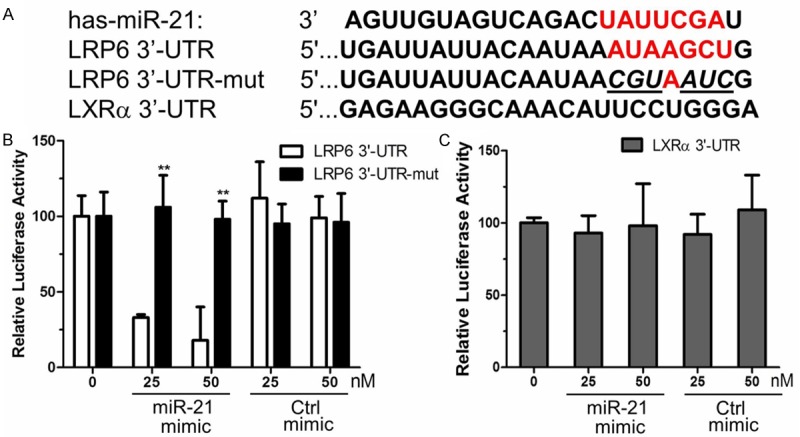
MiR-21 specifically inhibits LRP6 expression. A. Putative binding site of miR-21 in the 3’-UTR of LRP6 mRNA and the mutated nucleotide residues are shown in red and italics, respectively. B. HepG2 cells were co-transfected with 0.2 µg LRP6 3’-UTR-Luc or LRP6 3’-UTR-mut-Luc and 25 nM miR-21 mimic or control. C. HepG2 cells were transiently co-transfected with LXRa 3’-UTR-Luc and 25 nM of the indicated RNA oligonucleotides. Luciferase assays were conducted 48 h post-transfection. Data represent the firefly luciferase activities normalized with respect to the β-galactosidase activities. **P < 0.01 represents the relative luciferase activity of the LRP6 3’-UTR-mut group compared with the LRP6 3’-UTR group.
Discussion
Previous studies report that microRNAs play important roles in adipogenesis and osteogenesis [12]. In this study, we investigated the function of miR-21 in lipogenesis. Wu et al. [13] reported that miR-21 may function as a link between nonalcoholic fatty liver disease and the development of hepatocellular carcinoma. MicroRNAs also have been associated with lipoprotein metabolism, including miR-30, miR-21, and miR-221 [14]. These miRNAs were identified using gene chip assays [15].
Our study demonstrated for the first time that miR-21 may be a new therapeutic target for the treatment of nonalcoholic fatty liver disease. We also explored the specific mechanism of the potential therapeutic effects. Transfection of miR-21 mimic into HepG2 cells increased the expression of lipogenic enzymes and suppressed LRP6 expression at the transcriptional (mRNA) and translational (protein) levels. We investigated the mechanism of miR-21-mediated inhibition of LRP6 expression. Wang et al. [16] reported that miR-183 could downregulate LRP6 expression via a specific target site, the first binding site in the 3’-UTR of LRP6 mRNA. It is possible that miR-21 could have suppressed LRP6 expression via the same mechanism. Serum miR-21 levels were significantly higher in hepatocellular carcinoma patients who carried the hepatitis B or C virus compared with those with chronic hepatitis and healthy individuals [17-19]. Based on previous reports and the present study, we speculate that miR-21 may be involved in lipogenesis by activation of the LRP6 signaling pathway.
LRP6 plays important roles in metabolic regulation, specifically in the nutrient-sensing pathway, which has recently garnered considerable interest [10]. Patients carrying an LRP6 mutation exhibit elevated levels of LDL cholesterol, triglycerides, and fasting glucose, which cooperatively constitute the risk factors of metabolic syndrome and atherosclerosis [20]. LRP6 is one of the co-receptors of the Wnt/b-catenin pathway, and is essential for its activation. LRP6 is linked with several disease processes via its potential role in suppressing lipogenesis. Kawakita et al. [21] reported that miR-21 promoted oral cancer invasion via the Wnt/β-catenin signaling pathway. Lan et al. [22] found that miR-21 linked the Wnt/β-catenin signaling pathway with glioblastomas. Chen et al. [23] reported that miR-183 regulated adipogenesis by inhibiting the canonical Wnt/β-catenin signaling pathway via its targeting of LRP6. Our future studies will investigate the function of miR-21 in lipogenesis and the Wnt/β-catenin signaling pathway.
In conclusion, this study determined that LRP6 is a novel target for miR-21, suggesting that miR-21-mediated lipogenesis may proceed via its inhibition of LRP6 expression. Future studies will determine if this regulatory pathway can be therapeutically targeted to reduce lipogenesis.
Acknowledgements
This study was sponsored by Developmental fund by Sichuan Science and Technology Agency Luzhou City-Luzhou Medical College Combined fund (No.14JC0087).
Disclosure of conflict of interest
None.
References
- 1.Musso G, Gambino R, Cassader M. Emerging molecular targets for the treatment of nonalcoholic fatty liver disease. Ann Rev Med. 2010;61:375–392. doi: 10.1146/annurev.med.60.101107.134820. [DOI] [PubMed] [Google Scholar]
- 2.Kerr TA, Korenblat KM, Davidson NO. MicroRNAs and liver disease. Transl Res. 2011;157:241–252. doi: 10.1016/j.trsl.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada H, Suzuki K, Ichino N, Ando Y, Sawada A, Osakabe K, Sugimoto K, Ohashi K, Teradaira R, Inoue T, Hamajima N, Hashimoto S. Associations between circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and non-alcoholic fatty liver. Clin Chim Acta. 2013;424:99–103. doi: 10.1016/j.cca.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Wang B, Majumder S, Nuovo G, Kutay H, Volinia S, Patel T, Schmittgen TD, Croce C, Ghoshal K, Jacob ST. Role of microRNA-155 at early stages of hepatocarcinogenesis induced by choline-deficient and amino acid-defined diet in C57BL/6 mice. Hepatology. 2009;50:1152–1161. doi: 10.1002/hep.23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lukosz M, Mlynek A, Czypiorski P, Altschmied J, Haendeler J. The transcription factor Grainyhead like 3 (GRHL3) affects endothelial cell apoptosis and migration in a NO-dependent manner. Biochem Biophys Res Commun. 2011;412:648–653. doi: 10.1016/j.bbrc.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Darido C, Georgy SR, Wilanowski T, Dworkin S, Auden A, Zhao Q, Rank G, Srivastava S, Finlay MJ, Papenfuss AT, Pandolfi PP, Pearson RB, Jane SM. Targeting of the tumor suppressor GRHL3 by a miR-21-dependent proto-oncogenic network results in PTEN loss and tumorigenesis. Cancer Cell. 2011;20:635–648. doi: 10.1016/j.ccr.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 7.May P, Woldt E, Matz RL, Boucher P. The LDL receptor-related protein (LRP) family: an old family of proteins with new physiological functions. Ann Med. 2007;39:219–228. doi: 10.1080/07853890701214881. [DOI] [PubMed] [Google Scholar]
- 8.Go GW, Srivastava R, Hernandez-Ono A, Gang G, Smith SB, Booth CJ, Ginsberg HN, Mani A. The combined hyperlipidemia caused by impaired Wnt-LRP6 signaling is reversed by Wnt3a rescue. Cell Metab. 2014;19:209–220. doi: 10.1016/j.cmet.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mani A, Radhakrishnan J, Wang H, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Go GW. Low-Density Lipoprotein Receptor-Related Protein 6 (LRP6) Is a Novel Nutritional Therapeutic Target for Hyperlipidemia, Non-Alcoholic Fatty Liver Disease, and Atherosclerosis. Nutrients. 2015;7:4453–4464. doi: 10.3390/nu7064453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skoog M, Berggren-Soderlund M, Nilsson-Ehle P, Xu N. Lipid synthesis and secretion in HepG2 cells is not affected by ACTH. Lipids Health Dis. 2010;9:48. doi: 10.1186/1476-511X-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Guan X, Guo F, Zhou J, Chang A, Sun B, Cai Y, Dai C, Li X, Wang B. miR-30e reciprocally regulates the differentiation of adipocytes and osteoblasts by directly targeting low-density lipoprotein receptor-related protein 6. Cell Death Dis. 2013;4:e845. doi: 10.1038/cddis.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu H, Ng R, Chen X, Steer CJ, Song G. MicroRAN-21 is a potential link between non-alcoholic fatty liver disease and hepatocellular carcinoma via modulation of the HBP1-p53-Srebp1c pathway. Gut. 2015 doi: 10.1136/gutjnl-2014-308430. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horie T, Baba O, Kuwabara Y, Yokode M, Kita T, Kimura T, Ono K. MicroRNA and lipoprotein metabolism. J Atheroscler Thromb. 2014;21:17–22. doi: 10.5551/jat.20859. [DOI] [PubMed] [Google Scholar]
- 15.Keller P, Gburcik V, Petrovic N, Gaqllagher IJ, Nedergaard J, Cannon B, Timmons JA. Gene-ship studies of adipogenesis-regulated microRNAs in mojuse primary adipocytes and human obesity. BMC Endocr Disord. 2011;11:7. doi: 10.1186/1472-6823-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Wang X, Li Z, Liu H, Teng Y. MciroRNA-183 suppresses retinoblastoma cell growth, invasion and migration by targeting LRP6. FEBS J. 2014;281:1355–1365. doi: 10.1111/febs.12659. [DOI] [PubMed] [Google Scholar]
- 17.Qu KZ, Zhang K, Li H, Afdhal NH, Albitar M. Circulating microRNAs as biomarkers for hepatocellualr carcinoma. J Clin Gastroenterol. 2011;45:355–360. doi: 10.1097/MCG.0b013e3181f18ac2. [DOI] [PubMed] [Google Scholar]
- 18.Tomimaru Y, Eguchi H, Nagano H, Wada H, Kobayashi S, Marubashi S, Tanemura M, Tomokuni A, Takemasa I, Umeshita K, Kanto T, Doki Y, Mori M. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. J Hepatol. 2012;56:167–175. doi: 10.1016/j.jhep.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Wang Y, Yu W, Chen J, Luo J. Expression of serum miR-221 in human hepatocellular carcinoma and its prognostic significance. Biochem Biophys Res Commun. 2011;406:70–73. doi: 10.1016/j.bbrc.2011.01.111. [DOI] [PubMed] [Google Scholar]
- 20.Tomaszewski M, Charchar FJ, Barnes T, Gawron-Kiszka M, Sedkowska A, Podolecka E, Kowalczyk J, Rathbone W, Kalarus Z, Grzeszczak W, Goodall AH, Samani NJ, Zukowska-Szczechowska E. A common variant in low-density lipoprotein receptor-related protein 6 gene (LRP6) is associated with LDL-cholesterol. Arterioscler Thromb Vasc Biol. 2009;29:1316–1321. doi: 10.1161/ATVBAHA.109.185355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawakita A, Yanamoto S, Yamada S, Naruse T, Takahashi H, Kawasaki G, Umeda M. MciroRNA-21 promotes oral cancer invasion via the Wnt/β-catenin signal pathway by targeting DKK2. Pathol Oncol Res. 2014;20:253–361. doi: 10.1007/s12253-013-9689-y. [DOI] [PubMed] [Google Scholar]
- 22.Lan F, Pan Q, Yu H, Yue X. Sulforaphane enhances temozolomide-induced apoptosis because of down-regulation of miR-21 via Wnt/β-catenin signal pathway in glioblastoma. J Neurochem. 2015;134:811–818. doi: 10.1111/jnc.13174. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Xiang H, Peng YL, Peng J, Jiang SW. Mature miR-183, negatively regulated by transcription factor GATA3, promotes 3T3-L1 adipogenesis through inhibition of the canonical Wnt/β-catenin signaling pathway by targeting LRP6. Cell Cell Signal. 2014;26:1155–1165. doi: 10.1016/j.cellsig.2014.02.003. [DOI] [PubMed] [Google Scholar]


