Abstract
Ischemic stroke therapy and prognosis outcomes largely depend on the time periods after symptom onset. This study aims to explore the difference of global gene expression profiles and impairment of biological functions between short-term and long-term after stroke onset. We compared three short-term (3 h, 5 h and 24 h) and a long-term (6-month) gene expression levels by a multi-platform microarray data integration method. RankProd was used to calculate the differentially expressed genes between stroke patients and controls. DAVID Bioinformatics Resources was utilized to determine affected biological functions. Consensus cluster and hierarchical cluster methods were employed to compare the gene expression patterns of the commonly biological functions among these four time course groups. The results showed that severe impairment of inflammation and immune related functions in 5 h and 24 h after symptom onset. However, these functions were less affected in the 3 h and the 6-month groups. In addition, several key genes (CCL20, THBS1, EREG, and IL6 et al.) were dramatically down-regulated in 5 h and 24 h groups, whereas these genes showed no change or even a slight contrary expression in 3 h or 6-month groups. This study has identified the large differences of altered immune and inflammation functions based on gene levels between short and long-term after stroke onset. The findings provide valuable insight into the clinical practice and prognosis evaluation of ischemic stroke.
Keywords: Ischemic stroke, time periods, immune and inflammation, biological functions, gene expression
Introduction
Stroke is a major risk factor that threatens the global health of the elderly population. Actually, stroke influences people of all ages, but risk markedly increases with aging. The risk of stroke is doubled for every decade after the age of 55 [1]. Stroke is also an important cause of serious, long-term disability in patients who survive and experience the physical and mental consequences of the insult for many years thereafter [2]. Vascular cognitive impairment, with or without dementia, is a serious long-term consequence of stroke. Unlike physical dysfunction, cognitive dysfunction caused by the stroke often cannot be recovered [3].
Ischemic strokes account for the vast majority (85%) of stroke events and it often occurs in the middle cerebral artery [4,5]. When the cerebral artery in brain ruptures is clogged by thrombus, atherosclerotic plaque or other particles, nerve cells in this brain region will turn to die in few minutes due to the deficiency of oxygen supplement [6]. Treatment of ischemic stroke must be initiated within three hours of symptom onset in order to prevent irreversible neuronal damage and function loss [7]. Without therapeutic intervention in time, the ischemic region will undergo irreversible damage and dysfunction, and brain injury area continues to spread out and to be widened. Recombinant tissue-type plasminogen activator (rt-PA) has been used to dissolve the clot to re-establish quickly the blood flow to the brain and reduce neuronal injury. It has become the gold standard therapy for patients presenting within 3 hours of symptom onset [8].
Numerous studies using human and animal stroke models have found the large changes in different time periods after symptom onset. A previous study showed several gene expression changes and impairment of CD28 signaling, toll-like receptor signaling and other pathways within 24 hours after ischemic stroke onset [9]. Stamova et al. found an incremental gene expression changes in peripheral immune cells after cardioembolic stroke onset within 3, 5 and 24 h, and these changes vary largely in male and females [10]. However, Krug et al. found different biological function impairment (such as immune and inflammatory responses, platelet α granule membrane, response to virus) after 6-months of symptom onset [11]. In addition, a rat stroke model showed different protein expression of brain derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) on 3, 7, 14 and 28 days post-injury [12].
Although the deregulated genes or biological pathways vary in these studies, all these impairments were closely related to immune functions. However, there were no studies that focus on comparing the altered genes expression and affected biological functions between short and long-term after symptom onset in human. Our recent study showed that impairment of immune related pathways and anti-stroke target genes expression varies greatly between sex and ages in ischemic stroke [13]. Given the time course after stroke onset significantly alters the biological functions and numerous genes expression, in current study we aimed to compare the influenced pathways and key genes in short-term and long-term after symptom onset by a multi-platform gene expression data integration method.
Materials and methods
Microarray data collection
Human ischemic stroke microarray datasets were searched and downloaded from NCBI-GEO database (http://www.ncbi.nlm.nih.gov/geo) in January 2016. The data selection criteria were: (1) all datasets were genome-wide; (2) the samples of each dataset must include ischemic stroke patients and controls; (3) each dataset should provide the time period information after stroke; (4) raw data was available. Based on the above criteria, we finally chose two datasets for the integrate analysis (GSE22255 and GSE58294). Dataset of GSE22255 (contributed by Krug T) analyzed 20 patients and 20 controls after symptom onset 6-months. GSE58294 dataset (contributed by Stamova B) contained 69 ischemic stroke samples (time courses within 3, 5, and 24 h of each 23 samples) and 23 controls. All these two datasets were tested using the Affymetrix Human Genome U133 Plus 2.0 Array. Details of the two datasets were seen in the Supplementary Table 1.
Data preprocessing
R v3.2.2 was performed for data preprocessing. We used Robust Multichip Average (RMA) algorithm in oligo package [14] to normalize the raw expression data and generate the normalized gene expression intensity of the two datasets. Gene annotation, integration and renormalization of the two datasets were carried out using custom written Python code. We removed probes that with no gene annotation or that matched multiple gene symbols. Next, we calculated the average expression values of multiple probe IDs that matched to an official gene symbol, and took this value to represent the expression intensity of the corresponded gene symbol. The renormalization method was reported in our previous publication [15]. The distribution of RMA processed and global renormalized gene expression values in two studies were shown in the Supplementary Figure 1.
Bioinformatics analysis
RankProd [16] was used to detect differentially expressed genes between ischemic stroke patients and controls. Differentially expressed genes were defined as the absolute values of the logarithmic transformed fold-change (log2(FC)) ≥ log2(1.5) and a FDR adjusted P value ≤ 0.05. Up- and down-regulated genes were considered as log2(FC) ≥ log2(1.5) and log2(FC) ≤ log2(1.5), respectively. Differentially expressed genes in 3, 5, 24 h and 6-month groups were calculated as case samples in each group compared to all control samples.
Functional Annotation Tool in DAVID Bioinformatics Resources 6.7 [17] was used to perform GO Biological Processes enrichment analysis in each group. The input parameters were differentially expressed genes listed in each group. Biological processes with a P value ≤ 0.01 were considered to be significantly enriched. Venn diagram was used to show the overlapped enriched biological processes in each group. We filtered 3 overlapped biological processes (immune response, defense response and response to wounding) and extracted the genes in these processes in QuickGO (http://www.ebi.ac.uk/QuickGO-Beta/). We then obtained the union genes of these processes and used Consensus Cluster Plus [18] to assess the gene expression clustering profiles across all ischemic stroke samples. We utilized heatmap in “pheatmap” package to show the log2(FC) of genes in 4 time course groups in the filtered 3 processes, and the hierarchical clustering method was chosen as “ward.D2”.
Results
Differentially expressed genes overview
The numbers of differentially expressed genes in four time periods after stroke were shown in Table 1. We detected total of 20307 genes in each group. There were 83 up-regulated genes and 33 down-regulated genes in the 3 h group, 26 up-regulated genes and 35 down-regulated genes in the 5 h group, 127 up-regulated genes and 93 down-regulated genes in the 24 h group, and 18 up-regulated genes and 15 down-regulated genes in the 6-month group. The Venn diagram of these dysregulated genes was shown in the Supplementary Figure 2. There were two overlapped up-regulated genes in these four groups (XIST and TSIX).
Table 1.
Differentially expressed genes in four different time periods after symptom onset
| Time Periods | Total Genes | Up-regulated | Down-regulated |
|---|---|---|---|
| 3 h | 20307 | 83 | 33 |
| 5 h | 20307 | 26 | 35 |
| 24 h | 20307 | 127 | 93 |
| 6-month | 20307 | 18 | 15 |
Enriched GO biological processes in four groups
We got 7, 51, 57 and 3 enriched GO Biological Processes in 3, 5, 24 h and 6-month group, respectively. Venn diagram of the enriched biological processes in each group was shown in the Supplementary Figure 3. There were 5 commonly enriched biological processes in 3, 5 and 24 h groups: immune response, defense response, response to wounding, inflammatory response and acute inflammatory response. The processes of inflammatory response and acute inflammatory response were child processes of defense response.
Gene expression profiles in common biological processes
We extracted the genes in immune response, defense response and response to wounding and mapped to our datasets. Totally, we mapped 1365, 1490 and 725 genes of these 3 biological processes, respectively. The numbers of union genes in these processes were 2252. Figure 1 showed the consensus matrix of these 2252 genes expression patterns in all datasets. The cluster results demonstrated that these 2252 genes expression profiles in all case samples were clearly divided into two categories. To further elucidate the gene expression heterogeneity across these 4 groups, we observed the log2(FC) of genes in these biological processes in all subgroups (Figure 2). The cluster results suggested that the genes expression patterns in the 3 biological processes were similar in the 3 h group and the 6-month group. The 5 h and 24 h group also showed the similar expression alterations in all these biological processes. The numbers of dysregulated genes in these biological processes were shown in Table 2. There were more up-regulated genes than down-regulated genes in the 3 h group. Interestingly, these genes in the 6-month group also showed a similar trend of over-expression, although most of these genes have never reached to significant levels of our criteria. However, in the 5 and 24 h groups, there were more down-regulated genes than up-regulated genes in all 3 biological processes. These results implicate that impairments of immune response, defense response and response to wounding functions vary largely among different time periods after stroke onset.
Figure 1.
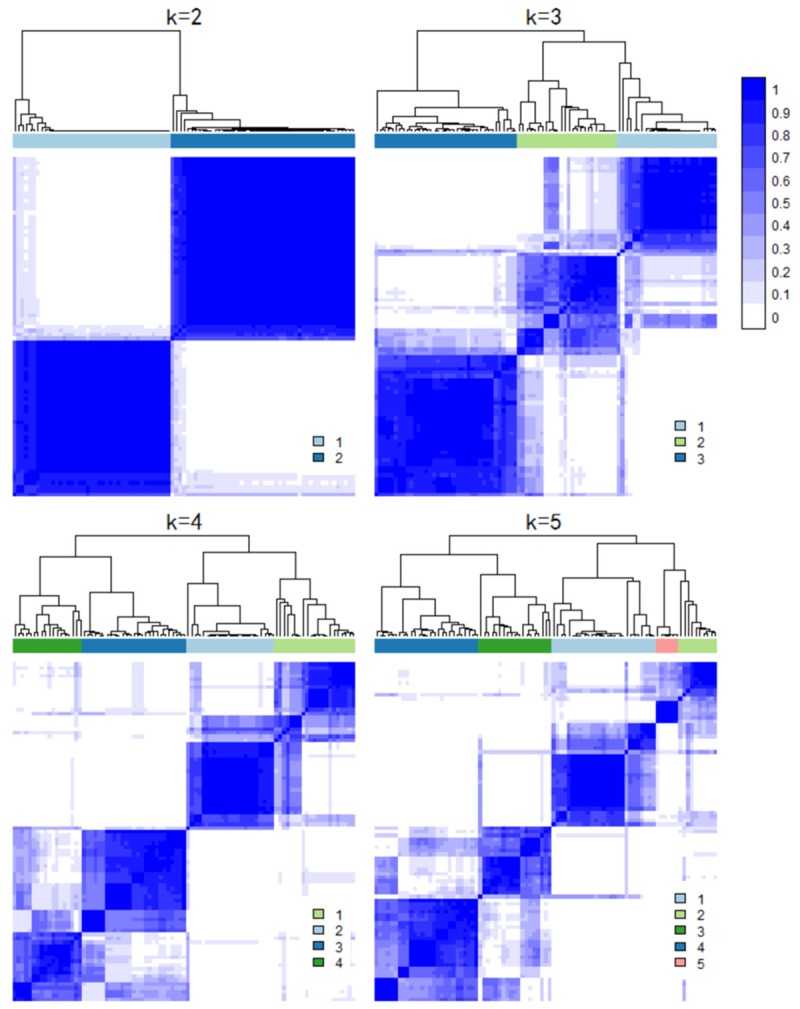
Consensus matrix of the union genes in 3 commonly biological processes. A white to blue color scale represented the consensus values.
Figure 2.
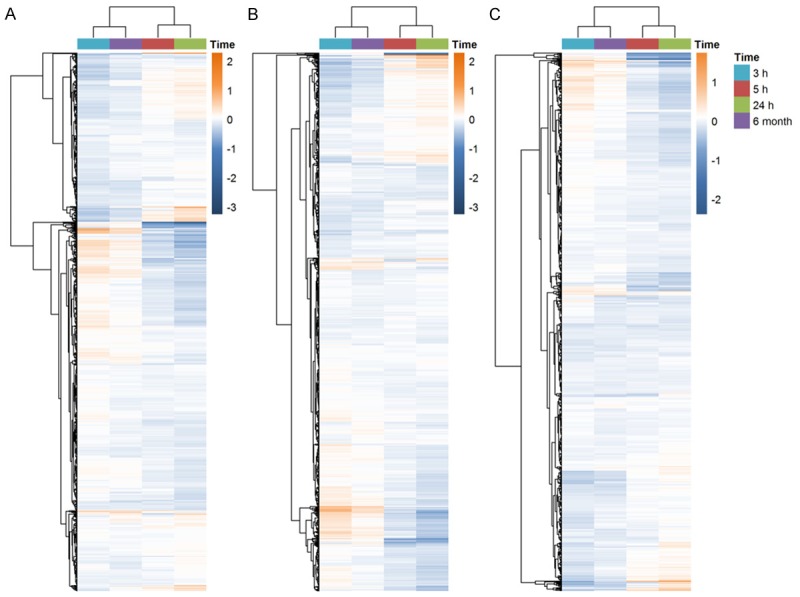
Heatmap of commonly 3 GO Biological Processes. Four time periods are represented by different colors. The color bar showed the range of log2 (fold-change). A. The immune response; B. The defense response; C. The response to wounding.
Table 2.
The number of differentially expressed genes in the three commonly GO Biological Processes in ischemic stroke
| GO Biological Processes | 3 h | 5 h | 24 h | 6-month | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Up | Down | Up | Down | Up | Down | Up | Down | |
| Immune response | 14 | 3 | 4 | 16 | 12 | 26 | 1 | 1 |
| Defense response | 15 | 5 | 4 | 14 | 14 | 25 | 1 | 0 |
| Response to wounding | 3 | 2 | 1 | 9 | 7 | 13 | 1 | 0 |
Fold changes of key genes in four time periods after stroke
The numbers of common genes in the immune response, defense response and response to wounding were 175 (Supplementary Figure 4). Among them, 5 genes were down-regulated in 5 h group, and 9 genes were down-regulated in 24 h group. However, no genes were dysregulated in 3 h or 6-month group. Figure 3 showed the logFC of these 9 genes. The results indicated that CCL20, EREG, IL1β, IL-6 and THBS1 were down-regulated in both 5 and 24 h groups, especially IL-6. In addition, CCR2, IL1α, PF4V1 and SERPING1 were only dysregulated in 24 h group. Totally, there were 8 genes displayed low-expression patterns in 5 and 24 h group. Conversely, these genes did not vary or only had a slight up-regulation in 3 h and 6-month groups.
Figure 3.
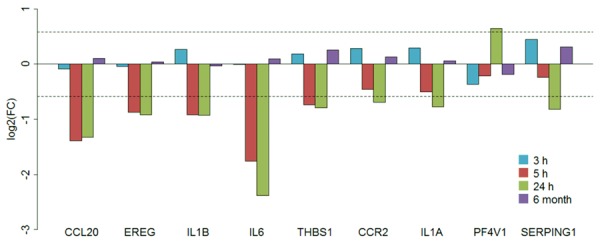
LogFC barplot of the key genes in 3 commonly GO Biological Processes. Four time periods groups are represented by different colors.
Discussion
Although some reports have demonstrated gene expression alteration in brain tissues after stroke onset, it has been unknown whether these are any links between gene profile patterns and immune functions between early and late stages of stroke. In this study, we provided evidence that a high similarity in gene expression patterns in the immune response, defense response and response to wounding biological processes of ischemic stroke patients between 5 and 24 h groups after symptom onset. These two groups all showed serious injury in the above processes. However, gene profiles were considerably different between 3 and 5 h although there was a relatively small change between 3 h and 6-month groups after stroke onset. We identified 9 key genes in the above biological processes, most of these genes showed a huge down-regulated in 5 and 24 h groups. Conversely, no key gene was dysregulated in 3 h or 6-month groups, suggesting the 5 h is a key time point for stroke revision although these gene changes no longer lasted in the late stage of stroke.
It is notable that several dysregulated genes were identified among these four time course groups. Two genes were commonly up-regulated in four time periods after symptom onset (XIST and TSIX). X inactive specific transcript (XIST) belongs to long non-coding RNA, being important for X-chromosome inactivation in female cells. Up-regulated XIST results in coating of the entire X chromosome by XISTRNA in cis, whereas TSIX (XIST antisense RNA) transcription acts as a negative regulator of XIST [19]. Dysregulated XIST and TSIX were closely related to many complex diseases. A recent study observed that XIST expression is significantly reduced in breast tumor samples and cancer cell lines, suggesting the tumor suppressor role of XIST in breast cancer [20]. In females, highly overexpressed XIST and other transcripts reflected the huge gender-specific transcriptomic differences in new-onset heart failure [21]. Therefore, it is speculated that the dysregulated XIST and TSIX could also play an important role in stroke onset.
The present study also revealed the severe damage of immune and inflammatory processes in ischemic stroke. Previous studies have found the associations between systemic inflammatory conditions and cardiovascular diseases including stroke [22,23]. More recently, it has been recognized that inflammation not only predisposes to ischemic stroke, but also directly drives many pathogenic aspects of ischemic stroke [24]. Several tissues are considered immune-privileged sites because of their unique immune surveillance activities. Traditional view is that the central nervous system (CNS) is an immune-privileged site. However, subsequent studies proved it is a false assumption that the CNS lacks immune surveillance and that neuronal homeostasis was not compatible with typical immune cell patrolling [25]. In addition, accumulated mechanistic studies found that there were several distinct immune responses in CNS compared with other peripheral tissues, including: (1) lack of lymphatic vessels in the brain parenchyma that would allow for egress of immune cells from the CNS; (2) CNS glial cells inability to propagate an effective immune response; (3) and low levels of dendritic cells (DC) which can dampen brain inflammatory responses [26,27]. Furthermore, classical lymphatic vessels were found in the CNS meninges, providing a mechanism by which immune cells can drain from the brain interstitial fluid directly with the peripheral immune system [28].
Under inflammatory conditions, including those associated with stroke, the mechanisms that govern normal CNS immune surveillance are perturbed [29]. CNS leukocyte trafficking can increase under these inflammatory conditions, leading to leukocyte penetration into the brain parenchyma through multiple barriers, including: (1) blood to the subarachnoid space via leptomeningeal vessels; (2) blood to the parenchymal perivascular space through the neurovascular unit (NVU), with tight junctions forming the so-called BBB; (3) blood to the CSF, which bathes the brain and spinal cord, via the choroid plexus; and (4) blood to the CSF via meningeal spaces and ependymal cell layers, which line the cerebral ventricles [30]. Therefore, the immune and inflammatory changes in blood under ischemic stroke can accurately reflect the conditions in the brain. In this study, we analyzed the blood transcripts in four time course after stroke onset and revealed severe damage of the immune response, defense response and response to wounding biological processes in 5 and 24 h group after stroke onset. In these two groups, several key genes (CCL20, EREG, IL1β, IL6, THBS1, CCR2, IL1α, and SERPING1) were all down-regulated, regardless of CCR2, IL1α, and SERPING1 did not reach significance in 5 h group.
CCL20 belongs to the subfamily of small cytokine CC genes. Cytokines are a family of secreted proteins involved in immune regulatory and inflammatory processes. CC cytokines are proteins characterized by two adjacent cysteines. Increased CCL20 protein expression was directly toxic to primary neurons and oligodendrocytes subjected to oxygen glucose deprivation in traumatic brain injury rats [31]. In addition, CCL20, CXCL16 and other cytokines were highly expressed in the CNS during both preclinical and acute experimental auto immune encephalomyelitis (EAE) [32]. Furthermore, a recent study showed that in stroke rats model treated with human umbilical cord blood exhibited significantly altered inflammatory response genes, including CCL20 [33]. Based on the above results and our findings, we speculate that CCL20 may be considered as a candidate therapeutic target and/or biomarker for neurological disorders in future investigations.
Epiregulin (EREG) is a member of the epidermal growth factor (EGF) family. EREG involves in a large range of biological processes including inflammation, wound healing and cell proliferation. In a transplantation of mesenchymal stem cells (MSC) swine model, the investigators have observed intracoronary injection of MSC secreted biologically active factors (vascular endothelial growth factor, endothelin, epiregulin, et al.) that could achieve early protection of ischemic myocardium and improve cardiac repair and contractility [34]. Furthermore, intermittent hypoxia (IH) treated cell conditioned medium exhibited significant increases in mRNAs for EREG, AREG and NRG1, suggesting that these EGFs could be involved in the proliferation of IH-induced vascular smooth muscle cells [35]. These studies demonstrated that high expression of EREG is beneficial for ischemic heart disease. Our new observation implicates that the low-expressed EREG could cause ischemic stroke injury.
Both of IL1α and IL1β are members of the interleukin 1 cytokine family. IL1α and IL1β are main regulators of the inflammation process and involve in various immune responses, inflammatory processes, and hematopoiesis [36]. These two genes encode IL1α and IL1β, respectively. Experimental results showed abnormal levels of IL1α or IL1β lead to inflammatory diseases [36]. IL1 is also known to involve in the neurodegeneration of acute and chronic brain disorders, including ischemic stroke and some neurodegenerative diseases, although the cytokine is also thought to be involved in the recovery of neurological functions [36,37]. Interleukin-6 (IL6) is a key early mediator of the inflammatory and overall immune responses [38]. Previous studies showed many different cells including astrocytes, mast cells, monocytes, macrophages, fibroblasts and endothelial cells can secrete IL6 after cerebral ischemia [24,39]. Others have observed that IL6 plays a central role in the pathogenesis of several ischemic cardiovascular disorders, including ischemic stroke [40]. Furthermore, a recent report suggested that IL6 is essential for promoting social interaction on the neurogenesis as well as long-term functional recovery after ischemic stroke [41]. In addition, a meta-analysis including 20-subject studies showed the correction between higher circulating IL6 levels and poor stroke prognosis [42]. Our results showed IL1α, IL1β and IL6 were all low-expressed in 5 and 24 h groups, indicating that severe immune function injury has appeared in these two time courses.
Angiogenesis is essential for recovery from ischemic stroke and other neurovascular diseases. Thrombospondin 1 (THBS1) is the first identified endogenous angiogenesis inhibitor. Because of important roles of THBS1 and 2 in synapse formation and axonal outgrowth, deficiency of these proteins leads to impaired recovery after stroke [43]. Nonetheless, recent studies also found increased blood thrombospondin concentrations in both acute ischemic stroke and acute intracerebral hemorrhage patients are highly associated with short-term and long-term clinical outcomes [44,45]. It is noteworthy that the blood THBS1 levels vary largely in different time points after ischemic stroke onset. Blood THBS1 level was strikingly increased in baseline and 2 h after onset, whereas its levels were drastically reduced in 12 and 24 h [46]. We observed the key time point is 5 h after stroke onset since THBS1 was down-regulated in 5 h but not in 3 h. Thus, THBS1 could be served as a prognostic biomarker of ischemic stroke.
Taken together, present study has showed huge differences in global gene expression and impaired biological functions in short-term and long-term ischemic stroke onset. We revealed severe impairment of inflammation and immune functions and identified several key genes that are drastically low-expressed in 5 and 24 h after symptom onset. CCL20, THBS1 and IL families have been identified to be related to prognosis in patients with stroke. Moreover, targeting these factors and downstream signal pathways could be therapeutically relevant in patients with stroke.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81570376, No. 31401142 and No. 31401137).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Sanossian N, Ovbiagele B. Prevention and management of stroke in very elderly patients. Lancet Neurol. 2009;8:1031–1041. doi: 10.1016/S1474-4422(09)70259-5. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. 2010;9:105–118. doi: 10.1016/S1474-4422(09)70266-2. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman RF, Hillis AE. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol. 2010;9:895–905. doi: 10.1016/S1474-4422(10)70164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musuka TD, Wilton SB, Traboulsi M, Hill MD. Diagnosis and management of acute ischemic stroke: speed is critical. Can Med Assoc J. 2015;187:887–893. doi: 10.1503/cmaj.140355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green AR. Pharmacological approaches to acute ischaemic stroke: reperfusion certainly, neuroprotection possibly. Br J Pharmacol. 2008;153(Suppl 1):S325–338. doi: 10.1038/sj.bjp.0707594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moustafa RR, Baron JC. Pathophysiology of ischaemic stroke: insights from imaging, and implications for therapy and drug discovery. Br J Pharmacol. 2008;153(Suppl 1):S44–54. doi: 10.1038/sj.bjp.0707530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamieson DG. Diagnosis of ischemic stroke. Am J Med. 2009;122:S14–20. doi: 10.1016/j.amjmed.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Bonaventura A, Montecucco F, Dallegri F. Update on the effects of treatment with recombinant tissue-type plasminogen activator (rt-PA) in acute ischemic stroke. Expert Opin Biol Ther. 2016;16:1323–1340. doi: 10.1080/14712598.2016.1227779. [DOI] [PubMed] [Google Scholar]
- 9.Barr TL, Conley Y, Ding J, Dillman A, Warach S, Singleton A, Matarin M. Genomic biomarkers and cellular pathways of ischemic stroke by RNA gene expression profiling. Neurology. 2010;75:1009–1014. doi: 10.1212/WNL.0b013e3181f2b37f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamova B, Jickling GC, Ander BP, Zhan X, Liu D, Turner R, Ho C, Khoury JC, Bushnell C, Pancioli A, Jauch EC, Broderick JP, Sharp FR. Gene expression in peripheral immune cells following cardioembolic stroke is sexually dimorphic. PLoS One. 2014;9:e102550. doi: 10.1371/journal.pone.0102550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krug T, Gabriel JP, Taipa R, Fonseca BV, Domingues-Montanari S, Fernandez-Cadenas I, Manso H, Gouveia LO, Sobral J, Albergaria I, Gaspar G, Jimenez-Conde J, Rabionet R, Ferro JM, Montaner J, Vicente AM, Silva MR, Matos I, Lopes G, Oliveira SA. TTC7B emerges as a novel risk factor for ischemic stroke through the convergence of several genome-wide approaches. J Cereb Blood Flow Metab. 2012;32:1061–1072. doi: 10.1038/jcbfm.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sist B, Fouad K, Winship IR. Plasticity beyond peri-infarct cortex: spinal up regulation of structural plasticity, neurotrophins, and inflammatory cytokines during recovery from cortical stroke. Exp Neurol. 2014;252:47–56. doi: 10.1016/j.expneurol.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Li WX, Dai SX, Wang Q, Guo YC, Hong Y, Zheng JJ, Liu JQ, Liu D, Li GH, Huang JF. Integrated analysis of ischemic stroke datasets revealed sex and age difference in anti-stroke targets. PeerJ. 2016;4:e2470. doi: 10.7717/peerj.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26:2363–2367. doi: 10.1093/bioinformatics/btq431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li WX, Dai SX, Liu JQ, Wang Q, Li GH, Huang JF. Integrated analysis of Alzheimer’s disease and schizophrenia dataset revealed different expression pattern in learning and memory. J Alzheimers Dis. 2016;51:417–425. doi: 10.3233/JAD-150807. [DOI] [PubMed] [Google Scholar]
- 16.Hong F, Breitling R, McEntee CW, Wittner BS, Nemhauser JL, Chory J. RankProd: a bioconductor package for detecting differentially expressed genes in meta-analysis. Bioinformatics. 2006;22:2825–2827. doi: 10.1093/bioinformatics/btl476. [DOI] [PubMed] [Google Scholar]
- 17.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 18.Wilkerson MD, Hayes DN. Consensus cluster plus: a class discovery tool with confidence assessments and item tracking. Bioinformatics. 2010;26:1572–1573. doi: 10.1093/bioinformatics/btq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loos F, Maduro C, Loda A, Lehmann J, Kremers GJ, Ten Berge D, Grootegoed JA, Gribnau J. Xist and Tsix transcription dynamics is regulated by the X-to-autosome ratio and semistable transcriptional states. Mol Cell Biol. 2016;36:2656–2667. doi: 10.1128/MCB.00183-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang YS, Chang CC, Lee SS, Jou YS, Shih HM. Xist reduction in breast cancer upregulates AKT phosphorylation via HDAC3-mediated repression of PHLPP1 expression. Oncotarget. 2016;7:43256–43266. doi: 10.18632/oncotarget.9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heidecker B, Lamirault G, Kasper EK, Wittstein IS, Champion HC, Breton E, Russell SD, Hall J, Kittleson MM, Baughman KL, Hare JM. The gene expression profile of patients with new-onset heart failure reveals important gender-specific differences. Eur Heart J. 2010;31:1188–1196. doi: 10.1093/eurheartj/ehp549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen PC, Tseng TC, Hsieh JY, Lin HW. Association between stroke and patients with pelvic inflammatory disease: a nationwide population-based study in Taiwan. Stroke. 2011;42:2074–2076. doi: 10.1161/STROKEAHA.110.612655. [DOI] [PubMed] [Google Scholar]
- 23.Rahman MM, Kopec JA, Cibere J, Goldsmith CH, Anis AH. The relationship between osteoarthritis and cardiovascular disease in a population health survey: a cross-sectional study. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ. Inflammatory Disequilibrium in Stroke. Circ Res. 2016;119:142–158. doi: 10.1161/CIRCRESAHA.116.308022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shechter R, Schwartz M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer ‘if’ but ‘how’. J Pathol. 2013;229:332–346. doi: 10.1002/path.4106. [DOI] [PubMed] [Google Scholar]
- 26.Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 27.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 31.Leonardo CC, Musso J, Das M, Rowe DD, Collier LA, Mohapatra S, Pennypacker KR. CCL20 is associated with neurodegeneration following experimental traumatic brain injury and promotes cellular toxicity in vitro. Transl Stroke Res. 2012;3:357–363. doi: 10.1007/s12975-012-0203-8. [DOI] [PubMed] [Google Scholar]
- 32.Wojkowska DW, Szpakowski P, Ksiazek-Winiarek D, Leszczynski M, Glabinski A. Interactions between neutrophils, Th17 cells, and chemokines during the initiation of experimental model of multiple sclerosis. Mediators Inflamm. 2014;2014:590409. doi: 10.1155/2014/590409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahaduzzaman MD, Mehta V, Golden JE, Rowe DD, Green S, Tadinada R, Foran EA, Sanberg PR, Pennypacker KR, Willing AE. Human umbilical cord blood cells induce neuroprotective change in gene expression profile in neurons after ischemia through activation of Akt pathway. Cell Transplant. 2015;24:721–735. doi: 10.3727/096368914X685311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen BK, Maltais S, Perrault LP, Tanguay JF, Tardif JC, Stevens LM, Borie M, Harel F, Mansour S, Noiseux N. Improved function and myocardial repair of infarcted heart by intracoronary injection of mesenchymal stem cell-derived growth factors. J Cardiovasc Transl Res. 2010;3:547–558. doi: 10.1007/s12265-010-9171-0. [DOI] [PubMed] [Google Scholar]
- 35.Kyotani Y, Ota H, Itaya-Hironaka A, Yamauchi A, Sakuramoto-Tsuchida S, Zhao J, Ozawa K, Nagayama K, Ito S, Takasawa S, Kimura H, Uno M, Yoshizumi M. Intermittent hypoxia induces the proliferation of rat vascular smooth muscle cell with the increases in epidermal growth factor family and erbB2 receptor. Exp Cell Res. 2013;319:3042–3050. doi: 10.1016/j.yexcr.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kostulas N, Pelidou SH, Kivisakk P, Kostulas V, Link H. Increased IL-1beta, IL-8, and IL-17 mRNA expression in blood mononuclear cells observed in a prospective ischemic stroke study. Stroke. 1999;30:2174–2179. doi: 10.1161/01.str.30.10.2174. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 39.Ferrarese C, Mascarucci P, Zoia C, Cavarretta R, Frigo M, Begni B, Sarinella F, Frattola L, De Simoni MG. Increased cytokine release from peripheral blood cells after acute stroke. J Cereb Blood Flow Metab. 1999;19:1004–1009. doi: 10.1097/00004647-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Quan Z, Quan Y, Wei B, Fang D, Yu W, Jia H, Quan W, Liu Y, Wang Q. Protein-protein interaction network and mechanism analysis in ischemic stroke. Mol Med Rep. 2015;11:29–36. doi: 10.3892/mmr.2014.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng C, Zhang JC, Shi RL, Zhang SH, Yuan SY. Inhibition of interleukin-6 abolishes the promoting effects of pair housing on post-stroke neurogenesis. Neuroscience. 2015;307:160–170. doi: 10.1016/j.neuroscience.2015.08.055. [DOI] [PubMed] [Google Scholar]
- 42.Bustamante A, Sobrino T, Giralt D, Garcia-Berrocoso T, Llombart V, Ugarriza I, Espadaler M, Rodriguez N, Sudlow C, Castellanos M, Smith CJ, Rodriguez-Yanez M, Waje-Andreassen U, Tanne D, Oto J, Barber M, Worthmann H, Wartenberg KE, Becker KJ, Chakraborty B, Oh SH, Whiteley WN, Castillo J, Montaner J. Prognostic value of blood interleukin-6 in the prediction of functional outcome after stroke: a systematic review and meta-analysis. J Neuroimmunol. 2014;274:215–224. doi: 10.1016/j.jneuroim.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Liauw J, Hoang S, Choi M, Eroglu C, Choi M, Sun GH, Percy M, Wildman-Tobriner B, Bliss T, Guzman RG, Barres BA, Steinberg GK. Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. J Cereb Blood Flow Metab. 2008;28:1722–1732. doi: 10.1038/jcbfm.2008.65. [DOI] [PubMed] [Google Scholar]
- 44.Dong XQ, Yu WH, Zhu Q, Cheng ZY, Chen YH, Lin XF, Ten XL, Tang XB, Chen J. Changes in plasma thrombospondin-1 concentrations following acute intracerebral hemorrhage. Clin Chim Acta. 2015;450:349–355. doi: 10.1016/j.cca.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 45.Gao JB, Tang WD, Wang HX, Xu Y. Predictive value of thrombospondin-1 for outcomes in patients with acute ischemic stroke. Clin Chim Acta. 2015;450:176–180. doi: 10.1016/j.cca.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 46.Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, Penalba A, Boada C, Domingues-Montanari S, Ribo M, Alvarez-Sabin J, Montaner J. A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis. 2011;216:205–211. doi: 10.1016/j.atherosclerosis.2011.01.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


