Abstract
Osteoarthritis (OA) is a joint disease caused by the breakdown of joint cartilage and underlying bone, and places great burdens to daily life of patients. Nuclear orphan receptor nuclear receptor subfamily 4, group A, member 1 (NR4A1) is vital for cell apoptosis, but little is known about its role in OA. This study aims to reveal the expression and function of NR4A1 during OA chondrocyte apoptosis. NR4A1 expression by qRT-PCR and western blot, and chondrocyte apoptosis by TUNEL assay were detected in normal and OA joint cartilage. NR4A1 was located in cartilage sections by immunohistofluorescence. Chondrocytes from normal joint cartilage were cultured in vitro for interleukin 6 (IL6) or tumor necrosis factor (TNF) treatment and si-NR4A1 transfection, after which the possible mechanism involving NR4A1 was analyzed. Results showed that NR4A1 expression and chondrocyte apoptosis were significantly elevated in OA cartilage (P < 0.05 and P < 0.01). NR4A1 was located in nuclei of normal cartilage chondrocytes, but was translocated to mitochondria and co-located with B-cell lymphoma 2 in OA chondrocytes. NR4A1 expression in cultured chondrocytes could be promoted by both IL6 and TNF treatment. si-NR4A1 partly reduced TNF-induced cell apoptosis. Inhibiting p38 by SB203580 could decrease TNF-induced NR4A1 to some extent, while inhibiting JNK could not. So NR4A1 is likely to facilitate OA chondrocyte apoptosis, which is associated with p38 MAPK and mitochondrial apoptosis pathway. This study provides a potential therapeutic target for OA treatment and offers information for regulatory mechanisms in OA.
Keywords: Osteoarthritis, NR4A1, tumor necrosis factor, mitochondrial apoptosis pathway, p38
Introduction
Osteoarthritis (OA), also known as degenerative arthritis or degenerative joint disease, is caused by breakdown of joint cartilage and underlying bone due to aging, joint injury or inherited factors. The incidence of OA, especially knee and hip OA, increases in the elderly [1], but recent studies have revealed growing OA incidence in younger adults and the annual newly diagnosed cases are nearly 9 in 1000 adults in England, Canada, Netherlands and Spain [2]. Significant correlation between chondrocyte apoptosis and OA grade has been reported [3], thus elevated chondrocyte apoptosis is a primary feature of OA. Impaired chondrocytes induce release of reactive oxygen species (ROS) [4] and activate production of inflammatory factors such as tumor necrosis factor (TNF) and interleukins (ILs) which in turn help to aggravate chondrocyte apoptosis [5,6]. Alleviation of OA mainly relies on exercise, joint protectors and anti-inflammation drugs. Besides, artificial joint replacement is applicable to certain patients in advanced stages.
Research on the molecular mechanism of OA is a rapidly developing area in recent years, whereby several pivotal regulators and potential therapeutic target in OA development have been uncovered, such as nuclear factor κB [7], metalloproteinases [8], some microRNAs [9] and mitogen-activated protein kinase (MAPK) pathways [10]. Nuclear receptor subfamily 4, group A, member 1 (NR4A1), also known as nuclear orphan receptor TR3 or Nur77, is an important transcription factor whose transcription activity stimulates pathologic angiogenesis and thus facilitates wound healing [11,12]. It also plays pivotal roles in regulating cell apoptosis via interacting with proteins on mitochondria and activating mitochondrial apoptosis pathway [13]. However, little is known about its roles in OA.
This study aims at revealing the expression and function of NR4A1 in OA, especially its regulatory role in chondrocyte apoptosis. We assessed chondrocyte apoptosis and NR4A1 expression in normal and OA joint cartilage. Location of NR4A1 in these tissue sections were observed after immunohistofluorescence. Articular chondrocytes from normal cartilage were isolated and cultured for TNF or IL6 treatment and si-NR4A1 transfection to investigate the underlying mechanism of NR4A1 in modulating chondrocyte apoptosis. These results will help to understand the specific role of NR4A1 in chondrocyte apoptosis and offer a promising therapeutic target for OA treatment.
Material and methods
Tissue sampling
Human joint cartilage tissues were collected from 5 male (23 to 51 years old, 39.6 ± 5.07) and 5 female (27 to 50 years old, 39.8 ± 4.35) OA patients undergoing total knee replacement surgery from March 2014 to March 2015. Normal joint cartilage tissues were collected from 5 male (25 to 50 years old, 37.0 ± 5.32) and 5 female (23 to 49 years old, 35.6 ± 4.77) patients with amputation from accidents. No significant difference in age existed between genders or groups. The tissue samples were immediately stored at -80°C for RNA and protein extraction. A part of fresh tissues were quickly frozen in liquid nitrogen for production of frozen sections. The patients were informed of the sampling procedures before surgery, and the whole procedures were performed under the instructions of our institute.
Cell isolation, culture and treatment
Articular chondrocytes in joint cartilage from normal patients were isolated according to the description in previous studies [14,15] with minor changes. Briefly, the cartilage tissues were cut into pieces, washed in phosphate buffered saline (PBS) for five times, and digested in 0.25% trypsin (Gibco, Carlsbad, CA) for 30 min at 37°C. The cells were washed in PBS again and digested in collagenase II (Sigma-Aldrich, Shanghai, China) for 4 h at 37°C. Then the suspension was filtered and cells were collected, washed in PBS and resuspended in Dulbecco’s modified Eagle medium-F12 supplemented with 10% fetal bovine serum (Gibco). The cells were adjusted to 1 × 104/mL and cultured in humidified atmosphere with 5% CO2 at 37°C. The medium was changed every 48 h and the cells were passaged at a confluence of 90%.
IL6 and TNF treatment in chondrocytes was performed based on a previous study [16]. Human recombinant IL6 or TNF (PeproTech, Rocky Hill, NJ) was added to the medium to a final concentration of 100 ng/mL and the cells were treated for 12 or 24 h until further analysis. Cells were then treated with SB203580 (a selective inhibitor of p38 MAPK) or SP600125 (a selective inhibitor of JNK, Calbiochem, Boston, MA) at a concentration of 10 μM for 24 h according to previous description [17,18]. The same amount of dimethylsulfoxide was added as control.
Cell transfection
NR4A1-specific siRNA and scrambled control siRNA were synthesized by RiboBio (Guangzhou, China) and transfected in cultured chondrocytes with the mediation of Lipofactamine RNAiMAX (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. 1 × 104 cells were transfected with 1 pmol siRNA in each well of 96-well plates, and incubated at 37°C for 48 h until further analysis.
TUNEL assay
Apoptotic cells in tissue sections or cultured cells were detected by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) method using One-step TUNEL Cell Apoptosis Assay Kit-FITC (Solarbio, Beijing, China) according to the manufacturer’s instructions. The frozen sections or cell smears were fixed in 4% paraformaldehyde for 1 h, washed in PBS twice and incubated on ice with 1% Triton-X 100 (Solarbio) for 2 min. Then new-made TUNEL detection buffer was added to the section for 1-h-incubation in dark at 37°C. Cell nuclei were stained by 4’,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich). After washed in PBS, the sections were mounted and observed under a fluorescence microscope (Olympus, Tokyo, Japan). The apoptotic cell number was counted in randomly-selected view fields.
Immunohistofluorescence
The frozen sections (8 μm) of tissues were used for detect the localization of NR4A1 according to the commonly used procedure. Briefly, the sections were blocked in 10% bovine serum albumin (Sigma-Aldrich) for 1 h at room temperature and incubated in primary antibodies for NR4A1 (ab109180) or B-cell lymphoma 2 (BCL2, ab32124, Abcam, Cambridge, UK) at 1:200 dilution for 1 h at room temperature. After washed in PBS, the sections were incubated in secondary antibodies Alexa Fluor 647 (ab62050) or Alexa Fluor 488 (ab150073) at 1:200 for 1 h in dark at room temperature. The primary antibodies anti-prohibitin (PHB, ab75771) were used to mark mitochondria. The nuclei were stained by DAPI. Then the sections were washed three times with PBS, mounted and observed by a fluorescence microscope (Olympus).
Real-time quantitative PCR
Tissue samples were extracted in Trizol (Invitrogen) for total RNA extraction. DNA or protein contamination was removed by RNA Purification Kit (TIANGEN, Beijing, China) and then 1 μg RNAs of each sample were used for complementary DNAs (cDNAs) synthesis by the catalysis of PrimeScript Reverse Transcriptase (Takara, Dalian, China). qRT-PCR was conducted on QuantStudio 6 Flex Realtime PCR System (Applied Biosystems, Carlsbad, CA) with 20 ng template cDNAs and the specific primer for human NR4A1 (Fw: 5’-CAG CTT GCT TGT CGA TGT C-3’ and Rv: 5’-GTG TCC ATG AAG ATC TTG TCA ATG-3’). Gapdh (Fw: 5’-GAA GGT GAA GGT CGG AGT C-3’ and Rv: 5’-GAA GAT GGT GAT GGG ATT TG-3’) was used as an internal reference. Data were analyzed by the 2-ΔΔCt method.
Western blot
Protein of tissues and cultured cells was extracted by M-Per Mammalian Protein Extraction Reagent (Thermo Scientific, Carlsbad, CA) according to the manufacturer’s instructions. Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted on a polyvinylidene fluoride membrane. The membrane was blocked in 5% skim milk for 2 h at room temperature and incubated in the primary antibodies (1:800) for NR4A1 (ab109180) or cleaved poly (ADP-ribose) polymerase 1 (PARP1, ab4830) at 4°C overnight. GAPDH (ab181602) was used as an internal reference. After washed in PBS, the membrane was incubated in the horse reddish peroxidase-conjugated secondary antibodies (1:2000) for 1 h at room temperature. Then positive signals were developed by EasyBlot ECL Kit (Sangon Biotech, Shanghai, China) and the grey scale was analyzed by software ImageJ 1.49 (National Institute of Health, Bethesda, MD).
Statistical analysis
All the experiments were repeated for 5 times and results were represented as the mean ± standard deviation. Differences between groups were examined by one-way analysis of variance and t test in SPSS 20 (IBM, New York, USA). P < 0.05 was considered statistically significant.
Results
Chondrocyte apoptosis and NR4A1 expression are promoted during OA
To start with, we examined the cartilage sections of and performed in situ TUNEL assay to assess chondrocyte apoptosis in OA and normal cartilage. More FITC-positive cells were observed in OA cartilage sections than normal sections and significant difference was found when all the patients were examined (P < 0.01, Figure 1), which indicated elevated chondrocyte apoptosis during OA.
Figure 1.
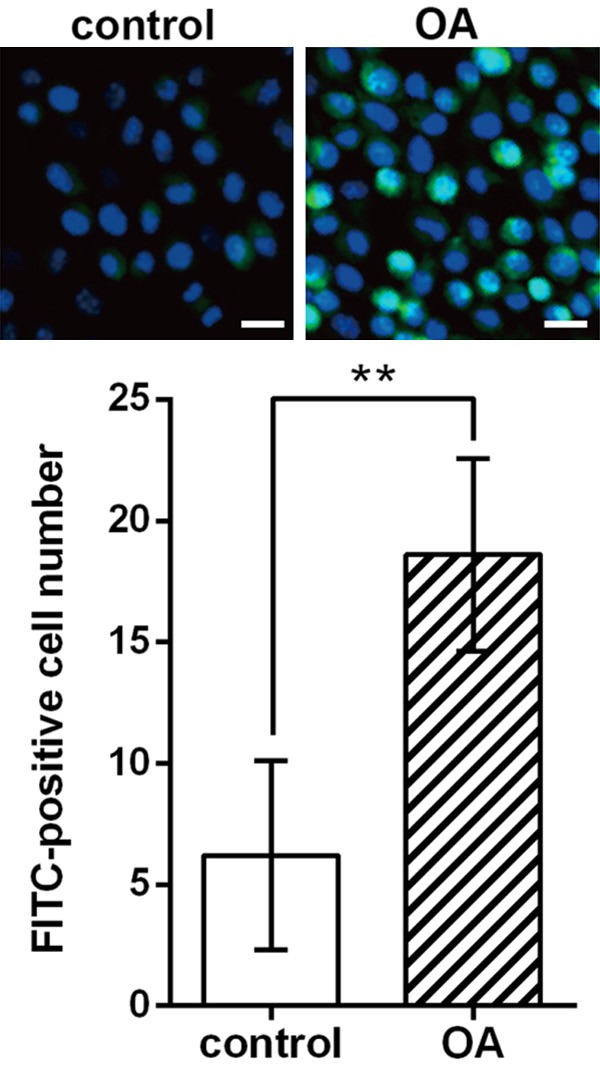
Apoptotic chondrocytes (blue-green) in cartilage sections of OA patients and non-OA patients (control) using TUNEL assay and the significant difference in apoptotic cell (FITC-positive) number between the two groups. The experiments are repeated five times. Bar indicates 20 μm. The nuclei are stained by DAPI (blue). **P < 0.01. OA, osteoarthritis. FITC, fluorescein isothiocyanate.
Then mRNA and protein levels of NR4A1 were detected in normal and OA cartilage samples. qRT-PCR results showed that NR4A1 mRNA expression was significantly higher in OA tissues than control (P < 0.05, Figure 2A), and western blot showed similar NR4A1 protein expression pattern which was obviously elevated in OA cartilage (P < 0.05, Figure 2B). We assumed that there might be possible associations between the elevated chondrocyte apoptosis and the promoted NR4A1 expression, which were to be analyzed in the following experiments.
Figure 2.
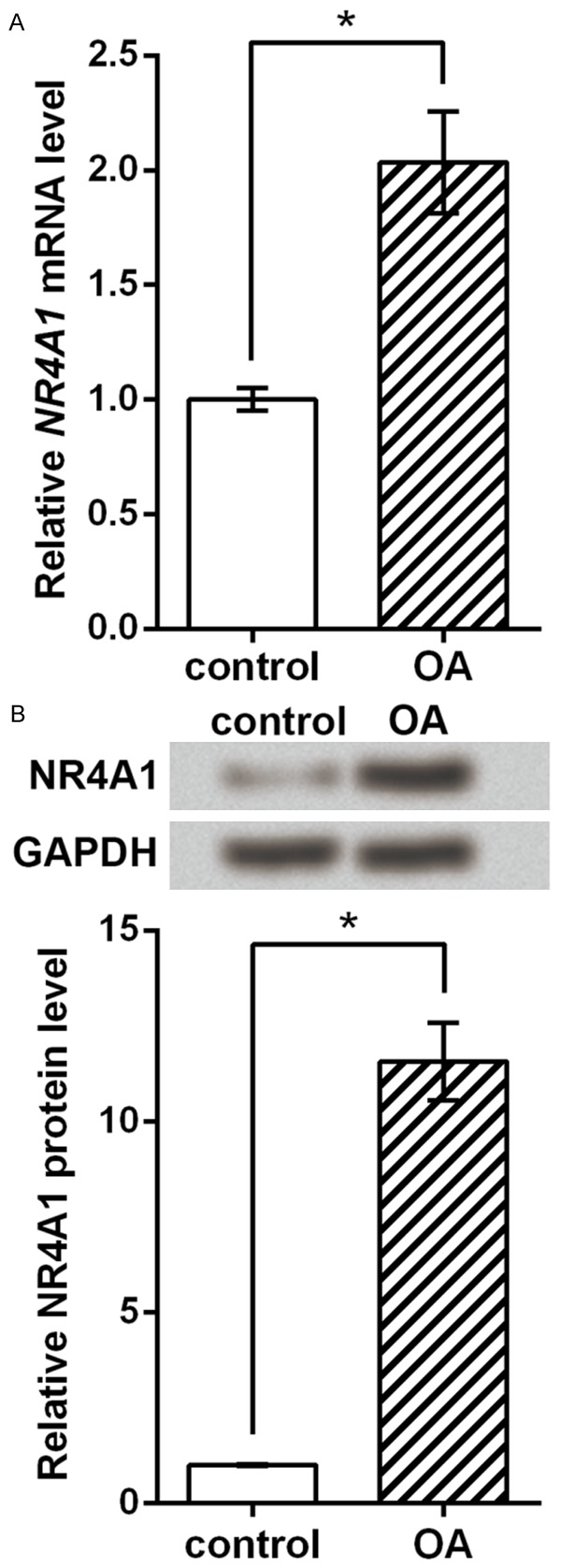
NR4A1 expression is promoted during OA. A. NR4A1 mRNA level detected by qRT-PCR. B. NR4A1 protein level detected by western blot and the histogram drawn based on repeated results. The experiments are repeated five times. *P < 0.05. OA, osteoarthritis. NR4A1, nuclear subfamily 4, group A, member 1.
NR4A1 co-locates with mitochondria and BCL2 during OA
Immunohistofluorescence was performed to detect the localization of NR4A1 in chondrocytes. Results showed that in chondrocytes of normal cartilage, NR4A1 signals were located in nuclei, overlapping DAPI signals. But in the chondrocytes of OA patients, most NR4A1 signals were located to cytoplasm and seemed to overlap PHB signals which indicated mitochondria (Figure 3A). BCL2, an important factor in mitochondrial apoptosis pathway, was also labelled by fluorescence, and consistent results were observed that NR4A1 signals in normal chondrocytes were translocated to cytoplasm, co-localizing with BCL2 (Figure 3B). These results indicated that NR4A1 was translocated to cytoplasm and localized to mitochondria during OA, which implied the involvement of NR4A1 in mitochondria apoptosis pathway.
Figure 3.
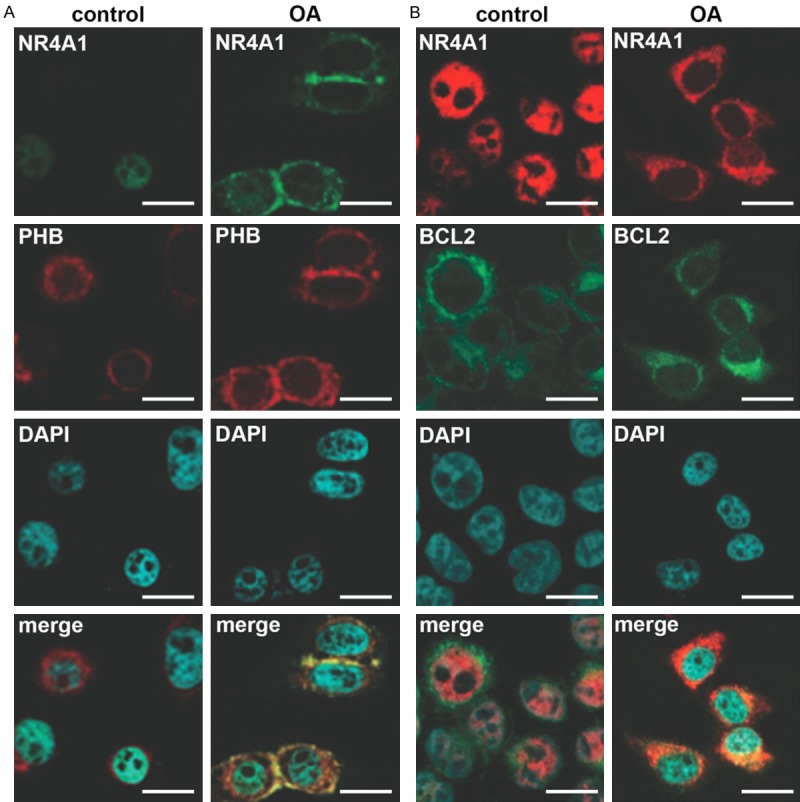
Localization of NR4A1 in normal and OA patients. A. NR4A1 (green) is translocated to cytoplasm and co-localized with PHB-marked mitochondria (red) in OA patients. B. NR4A1 (red) is translocated to cytoplasm and co-localized with BCL2 (green) in OA patients. Immunohistofluorescence is performed in normal and OA cartilage sections and in each section NR4A1 and PHB or BCL2 are marked with the specific primary antibodies and then the secondary antibodies labelled by Alexa Fluor 647 (red) or 488 (green). DAPI stains nuclei. The experiments are repeated five times. Bar indicates 10 μm. OA, osteoarthritis. NR4A1, nuclear receptor subfamily 4, group A, member 1. PHB, prohibitin. BCL2, B-cell lymphoma 2. merge, pictures showing green, red and blue signals at the same time.
NR4A1 and p38 is involved in TNF-induced apoptosis
Based on the above results, we suspected that the promoted expression of NR4A1 chondrocytes might be related to elevated inflammatory factors during OA. Here TNF and IL6 were used to treat cultured chondrocytes for their possible involvement in chondrocyte apoptosis during OA [19,20]. Results showed that after 12 or 24 h of treatment, both IL6 and TNF promoted NR4A1 protein level in cultured chondrocytes from normal cartilage, with a time-dependent manner (Figure 4), implying that NR4A1 might be induced by inflammatory cytokines like IL6 and TNF during OA.
Figure 4.
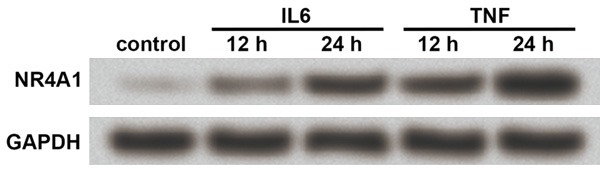
IL6 and TNF induce NR4A1 in chondrocytes. The cultured chondrocytes from normal joint cartilage are treated with IL6 or TNF for 12 or 24 h, after which western blot is performed to examine NR4A1 expression. GAPDH is an internal reference. The experiments are repeated five times. Both IL6 and TNF induce NR4A1 protein expression in a time-dependent manner based on results from the two time points. NR4A1, nuclear receptor subfamily 4, group A, member 1. IL6, interleukin 6. TNF, tumor necrosis factor.
We used TNF to represent typical inflammatory cytokines and treated the cultured chondrocytes for 12 h which were then transfected with si-NR4A1 or scramble siRNA. Cleaved PARP1 protein was used as an indicator of cell apoptosis and western blot showed that si-NR4A1 transfection successfully inhibited NR4A1 protein level, and that TNF treatment could increase cleaved PARP1 level. But the change in cleaved PARP1 change was not that obvious when NR4A1 was knocked down (Figure 5A). We further tested cell apoptosis via TUNEL assay and calculated the apoptotic cell percent in each sample, and results showed similar changes to the cleaved PARP1 (Figure 5B): TNF significantly induced cell apoptosis with or without the knockdown of NR4A1 (P < 0.001 or P < 0.01), and the increased cell apoptosis was obviously suppressed, but not totally abrogated, when NR4A1 was knocked down (P < 0.05). It could be deduced that the TNF-induced chondrocyte apoptosis might partly rely on NR4A1 or NR4A1-mediated pathways.
Figure 5.
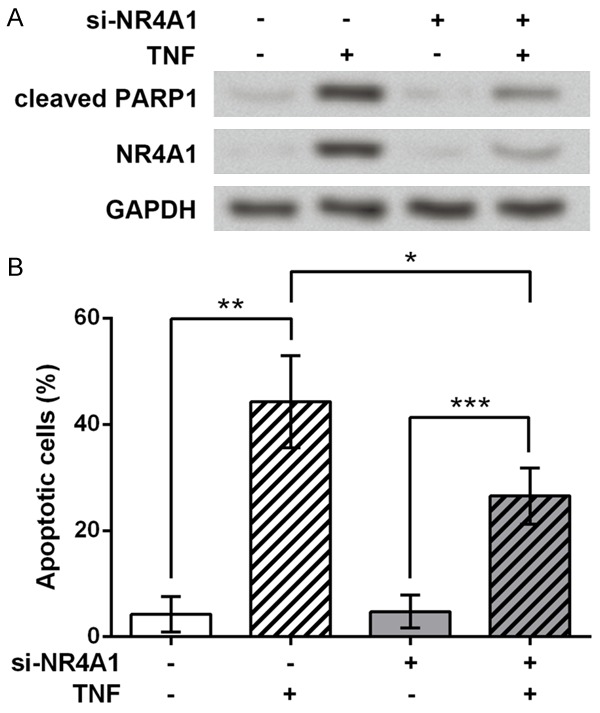
NR4A1 is involved in TNF-induced chondrocyte apoptosis. A. si-NR4A1 partly reduces the TNF-induced up-regulation of cleaved PARP1, as indicated by western blot. GAPDH is an internal reference. B. TUNEL assay shows that the apoptotic cell percent of each group exhibits similar apoptosis changes to western blot results. The experiments are repeated five times. Cells are first treated by TNF for 12 h and then transfected with si-NR4A1. Scramble si-NR4A1 is transfected as control. *P < 0.05. **P < 0.01. ***P < 0.001. NR4A1, nuclear receptor subfamily 4, group A, member 1. TNF, tumor necrosis factor. PARP1, poly (ADP-ribose) polymerase 1.
It has been reported that p38 MAPK pathway plays vital roles in chondrocyte apoptosis during OA [21,22], so we detect the association of p38 with TNF and NR4A1 in the cultured chondrocytes. SB203580 and SP600125 were used as the specific inhibitor of p38 and c-Jun N-terminal kinase (JNK), respectively, according to previous studies [23,24]. Western blot results showed that the up-regulation of NR4A1 by TNF was less obvious when p38 was inhibited but was still evident when JNK was inhibited compared to control cells (Figure 6), indicating that the regulation of NR4A1 by TNF in chondrocytes was partly dependent on p38 MAPK, rather than JNK MAPK.
Figure 6.
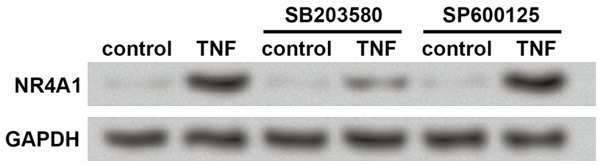
p38 MAPK is involved in the up-regulation of NR4A1 by TNF. Western blot is used to detect NR4A1 protein level in treated cells. GAPDH is an internal reference. The experiments are repeated five times. The cultured chondrocytes are treated with TNF for 12 h and then SB203580 (the specific inhibitor of p38) or SP600125 (the specific inhibitor of JNK) for 24 h. SP600125 does not affect the up-regulation of NR4A1 by TNF, while SB203580 inhibits the promotive effects of TNF on NR4A1. NR4A1, nuclear receptor subfamily 4, group A, member 1. TNF, tumor necrosis factor.
Discussion
Nuclear orphan receptor NR4A1 has not been reported in the regulation of chondrocytes or OA. This study reveals the up-regulation of NR4A1 and its translocation to mitochondria in joint cartilage chondrocytes of OA patients. NR4A1 expression is promoted by TNF treatment in cultured chondrocytes, which is involved in TNF-induced cell apoptosis. Further, we find the up-regulated NR4A1 by TNF is partly dependent on p38 MAPK.
OA is accompanied by increased chondrocyte apoptosis in joint cartilage, which was supported by TUNEL assay in this study on ten OA patients and ten non-OA patients. We then observed that the NR4A1 expression level in these OA cartilages was significantly higher than in normal samples. Actually, the up-regulation of NR4A1 has been reported in diseases like B-cell lymphomas, where its proapoptotic functions inhibit lymphomagenesis [25]. So the results of this study led us to hypothesize the involvement and possible mechanism of NR4A1 in modulating chondrocyte apoptosis.
The location of NR4A1 was labelled in normal and OA cartilage sections and we observed the translocation of NR4A1 to mitochondria, co-locating with BCL2 during OA. Mitochondrial proteins hold vital positions in chondrocyte apoptosis, with over 20 proteins aberrantly expressed during OA [26]. NR4A1 is capable of responding to apoptotic stimuli, translocating from the nucleus to mitochondria and inducing the release of cytochrome c and cell apoptosis [27]. In the process, NR4A1 recruits and interacts with key apoptotic factors in BCL2 family, such as BCL2, BAX and NIX, to participate in mitochondrial apoptosis pathway [13,28-30]. Based on the existed information, it can be deduced that the up-regulated NR4A1 in OA chondrocytes is translocated to mitochondria, which allows NR4A1 to interact with BCL2 or other apoptotic factors to activate mitochondrial apoptosis pathway and further induce chondrocyte apoptosis during OA.
Mitochondrial dysfunction can be generated from mitochondrial DNA (mtDNA) mutation or proinflammatory mediators such as cytokines, prostaglandins, ROS and nitric oxide (NO) [31]. Proinflammatory factors like TNF may damage mtDNA, interrupt mitochondrial functions including energy production and mitochondrial transcription, and lead to chondrocyte apoptosis in OA [32]. Consistent to these reports, this study detected elevated chondrocyte apoptosis after TNF treatment and found this process was partly dependent on the elevated NR4A1. So NR4A1 is a likely subsidiary in TNF-induced chondrocyte apoptosis during OA.
Existed studies indicate the pivotal position of MAPK pathways in heat or mechanical stress and NO-induced articular chondrocyte apoptosis [33,34]. Moreover, MAPK pathways including p38, extracellular signal-regulated kinase and JNK MAPK are important mediators for TNF-induced cell apoptosis [35-37]. This study also tried to investigate the niche of MAPK pathways during the up-regulation of NR4A1 by TNF, and the relative expression results showed that p38, rather than JNK, was partly involved in the promoted NR4A1 by TNF. All information considered, it is deduced that p38 MAPK is one of the possible pathways mediating TNF-induced NR4A1, and that TNF/p38/NR4A1 signaling and NR4A1-involved mitochondrial apoptosis pathway are possible mechanisms in OA chondrocyte apoptosis.
In summary, NR4A1 is up-regulated in joint cartilage chondrocytes of OA patients. NR4A1 can be induced by cytokines like TNF and promote chondrocyte apoptosis in association with p38 MAPK and mitochondrial apoptosis pathway. These results offer a potential target for OA treatment and further mechanism investigation is necessary for controlling OA.
Acknowledgements
Construction and clinical application of digital precision spinal surgery system, major scientific and technological research of Henan provincial science and technology department in 2015 (No. 162102310018).
Disclosure of conflict of interest
None.
References
- 1.Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. 2014;73:1659–1664. doi: 10.1136/annrheumdis-2013-203355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu D, Peat G, Bedson J, Jordan KP. Annual consultation incidence of osteoarthritis estimated from population-based health care data in England. Rheumatology (Oxford) 2015;54:2051–2060. doi: 10.1093/rheumatology/kev231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998;41:1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin W, McCabe D, Sauter E, Reese E, Walter M, Buckwalter JA, Martin JA. Rotenone prevents impact-induced chondrocyte death. J Orthop Res. 2010;28:1057–1063. doi: 10.1002/jor.21091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polzer K, Schett G, Zwerina J. The lonely death: chondrocyte apoptosis in TNF-induced arthritis. Autoimmunity. 2007;40:333–336. doi: 10.1080/08916930701356721. [DOI] [PubMed] [Google Scholar]
- 7.Roman-Blas JA, Jimenez SA. NF-κB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839–848. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Bigg HF, Rowan AD. The inhibition of metalloproteinases as a therapeutic target in rheumatoid arthritis and osteoarthritis. Curr Opin Pharmacol. 2001;1:314–320. doi: 10.1016/s1471-4892(01)00055-8. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Jia J, Yang S, Liu X, Ye S, Tian H. MicroRNA-21 controls the development of osteoarthritis by targeting GDF-5 in chondrocytes. Exp Mol Med. 2014;46:e79. doi: 10.1038/emm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi S, Nishiyama T, Miura Y, Fujishiro T, Kanzaki N, Hashimoto S, Matsumoto T, Kurosaka M, Kuroda R. DcR3 induces cell proliferation through MAPK signaling in chondrocytes of osteoarthritis. Osteoarthritis Cartilage. 2011;19:903–910. doi: 10.1016/j.joca.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Zeng H, Qin L, Zhao D, Tan X, Manseau EJ, Van Hoang M, Senger DR, Brown LF, Nagy JA, Dvorak HF. Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J Exp Med. 2006;203:719–729. doi: 10.1084/jem.20051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niu G, Ye T, Qin L, Bourbon PM, Chang C, Zhao S, Li Y, Zhou L, Cui P, Rabinovitz I, Mercurio AM, Zhao D, Zeng H. Orphan nuclear receptor TR3/Nur77 improves wound healing by upregulating the expression of integrin β4. FASEB J. 2014;29:131–140. doi: 10.1096/fj.14-257550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang WJ, Wang Y, Chen HZ, Xing YZ, Li FW, Zhang Q, Zhou B, Zhang HK, Zhang J, Bian XL, Li L, Liu Y, Zhao BX, Chen Y, Wu R, Li AZ, Yao LM, Chen P, Zhang Y, Tian XY, Beermann F, Wu M, Han J, Huang PQ, Lin T, Wu Q. Orphan nuclear receptor TR3 acts in autophagic cell death via mitochondrial signaling pathway. Nat Chem Biol. 2014;10:133–140. doi: 10.1038/nchembio.1406. [DOI] [PubMed] [Google Scholar]
- 14.Attur MG, Dave M, Cipolletta C, Kang P, Goldring MB, Patel IR, Abramson SB, Amin AR. Reversal of autocrine and paracrine effects of IL-1 in human arthritis by type II IL-1 decoy receptor. Potential for pharmacological intervention. J Biol Chem. 2000;275:40307–40315. doi: 10.1074/jbc.M002721200. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Cai H, Zheng X, Zhang B, Xia C. The involvement of mutual inhibition of ERK and mTOR in PLCgamma1-Mediated MMP-13 expression in human osteoarthritis chondrocytes. Int J Mol Sci. 2015;16:17857–17869. doi: 10.3390/ijms160817857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuerwegh AJ, Dombrecht EJ, Stevens WJ, Van Offel JF, Bridts CH, De Clerck LS. Influence of pro-inflammatory (IL-1α, IL-6, TNF-α, IFN-γ) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthritis Cartilage. 2003;11:681–687. doi: 10.1016/s1063-4584(03)00156-0. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury TT, Arghandawi S, Brand J, Akanji OO, Bader DL, Salter DM, Lee DA. Dynamic compression counteracts IL-1beta induced iNOS and COX-2 expression in chondrocyte/agarose constructs. Arthritis Res Ther. 2008;10:R35. doi: 10.1186/ar2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieminen R, Leinonen S, Lahti A, Vuolteenaho K, Jalonen U, Kankaanranta H, Goldring MB, Moilanen E. Inhibitors of mitogen-activated protein kinases downregulate COX-2 expression in human chondrocytes. Mediators Inflamm. 2005;2005:249–255. doi: 10.1155/MI.2005.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-Armada MJ, Caramés B, Lires-Deán M, Cillero-Pastor B, Ruiz-Romero C, Galdo F, Blanco FJ. Cytokines, tumor necrosis factor-α and interleukin-1β, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthritis Cartilage. 2006;14:660–669. doi: 10.1016/j.joca.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Venn G, Nietfeld JJ, Duits AJ, Brennan FM, Arner E, Covington M, Billingham ME, Hardingham TE. Elevated synovial fluid levels of interleukin-6 and tumor necrosis factor associated with early experimental canine osteoarthritis. Arthritis Rheum. 1993;36:819–826. doi: 10.1002/art.1780360613. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Wang Z, Wu J, Kao X, Gao Y. The p38 MAPK signal transduction pathway is involved in rabbit articular chondrocyte apoptosis induced by NO. Chinese Journal of Control of Endemic Diseases. 2006;21:332–336. [Google Scholar]
- 22.Qin ST, Jiang Q, Huang JH, Liu HF, Chen WD. Effects of p38 mitogen activated protein kinase inhibitor on chondrocyte apoptosis in rats with osteoarthritis. Shandong Medical Journal. 2008;48:6–8. [Google Scholar]
- 23.Pan YX, Chen KF, Lin YX, Wu W, Zhou XM, Zhang XS, Zhang X, Shi JX. Intracisternal administration of SB203580, a p38 mitogen-activated protein kinase inhibitor, attenuates cerebral vasospasm via inhibition of tumor-necrosis factor-α. J Clin Neurosci. 2013;20:726–730. doi: 10.1016/j.jocn.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Assi K, Pillai R, Gomez-Munoz A, Owen D, Salh B. The specific JNK inhibitor SP600125 targets tumour necrosis factor-alpha production and epithelial cell apoptosis in acute murine colitis. Immunology. 2006;118:112–121. doi: 10.1111/j.1365-2567.2006.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deutsch AJ, Rinner B, Wenzl K, Pichler M, Troppan K, Steinbauer E, Schwarzenbacher D, Reitter S, Feichtinger J, Tierling S, Prokesch A, Scheideler M, Krogsdam A, Thallinger GG, Schaider H, Beham-Schmid C, Neumeister P. NR4A1-mediated apoptosis suppresses lymphomagenesis and is associated with a favorable cancer-specific survival in patients with aggressive B-cell lymphomas. Blood. 2014;123:2367–2377. doi: 10.1182/blood-2013-08-518878. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz-Romero C, Calamia V, Mateos J, Carreira V, Martínez-Gomariz M, Fernández M, Blanco FJ. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics. Mol Cell Proteomics. 2009;8:172–189. doi: 10.1074/mcp.M800292-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F, Xie Z, Fontana JA, Reed JC, Zhang X. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289:1159–1164. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- 28.Lin B, Kolluri SK, Lin F, Liu W, Han Y, Cao X, Dawson MI, Reed JC, Zhang X. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 29.Wilson AJ, Arango D, Mariadason JM, Heerdt BG, Augenlicht LH. TR3/NR4A1 in colon cancer cell apoptosis. Cancer Res. 2003;63:5401–5407. [PubMed] [Google Scholar]
- 30.Oshima Y, Akiyama T, Hikita A, Iwasawa M, Nagase Y, Nakamura M, Wakeyama H, Kawamura N, Ikeda T, Chung Ui, Hennighausen L, Kawaguchi H, Nakamura K, Tanaka S. Pivotal role of Bcl-2 family proteins in the regulation of chondrocyte apoptosis. J Biol Chem. 2008;283:26499–26508. doi: 10.1074/jbc.M800933200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7:161–169. doi: 10.1038/nrrheum.2010.213. [DOI] [PubMed] [Google Scholar]
- 32.Kim J, Xu M, Xo R, Mates A, Wilson GL, Pearsall AW 4th, Grishko V. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthritis Cartilage. 2010;18:424–432. doi: 10.1016/j.joca.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Takebe K, Nishiyama T, Hayashi S, Hashimoto S, Fujishiro T, Kanzaki N, Kawakita K, Iwasa K, Kuroda R, Kurosaka M. Regulation of p38 MAPK phosphorylation inhibits chondrocyte apoptosis in response to heat stress or mechanical stress. Int J Mol Med. 2011;27:329–335. doi: 10.3892/ijmm.2010.588. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Wang Z, Chen J, Wu J. Apoptosis induced by NO via phosphorylation of p38 MAPK that stimulates NF-kappaB, p53 and caspase-3 activation in rabbit articular chondrocytes. Cell Biol Int. 2007;31:1027–1035. doi: 10.1016/j.cellbi.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Ding L, Heying E, Nicholson N, Stroud NJ, Homandberg GA, Buckwalter JA, Guo D, Martin JA. Mechanical impact induces cartilage degradation via mitogen activated protein kinases. Osteoarthritis Cartilage. 2010;18:1509–1517. doi: 10.1016/j.joca.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeh CJ, Lin PY, Liao MH, Liu HJ, Lee JW, Chiu SJ, Hsu HY, Shih WL. TNF-alpha mediates pseudorabies virus-induced apoptosis via the activation of p38 MAPK and JNK/SAPK signaling. Virology. 2008;381:55–66. doi: 10.1016/j.virol.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 37.Chin BY, Choi ME, Burdick MD, Strieter RM, Risby TH, Choi AM. Induction of apoptosis by particulate matter: role of TNF-alpha and MAPK. Am J Physiol. 1998;275:L942–949. doi: 10.1152/ajplung.1998.275.5.L942. [DOI] [PubMed] [Google Scholar]


