Abstract
Objective: To investigate whether calcium is involved in downstream signal transduction in neurite outgrowth regulated by Rho kinase. Methods: In vitro primary hippocampal neurons were cultured and treated with Rho kinase agonist (LPA) or antagonist (Y-27632). Then, the cytoskeleton and neurite outgrowth were observed. After addition of calcium antagonist BAPTA/AM to reduce intracellular calcium, the cytoskeleton distribution and neurite outgrowth were observed. Results: The activation or inhibition of Rho kinase could significantly alter the number and length of neurites of hippocampal neurons. Rho kinase regulated the cytoskeleton to regulate the neurite outgrowth, and LPA could significantly increase intracellular calcium. After BAPTA/AM treatment, the length and branch number of neurites of neurons reduced markedly. BAPTA/AM was able to reduce intracellular calcium and decrease neuronal cytoskeleton. Treatment with both BAPTA/AM and LPA could stop the retraction of neurites, but the length and branch number of neurites remained unchanged after treatment with Y-27632 and LPA. Conclusion: Calcium may affect the cytoskeleton arrangement to regulate neurite outgrowth, and calcium is involved in the downstream signal transduction of Rho kinase regulated neurite outgrowth of hippocampal neurons.
Keywords: Rho kinase, calcium, rat, hippocampal neuron, neurite outgrowth
Introduction
Rho kinase belongs to Rho GTPases family and may serve as a molecular switch in the cellular response depending on the different GDP-GTP binding statuses. Studies have shown that Rho kinase is involved in multiple cell processes such as differentiation, migration and polarization [1,2]. As a negative regulator of neurite outgrowth of neurons, Rho kinase activation may inhibit the neurite outgrowth, and its inhibition will facilitate the neurite outgrowth [3,4].
In the neuronal development, the cytoskeleton movement leads to the formation of prototype of neurites, the polarization of which will lead to formation of neurites. The growth cone at the neurite terminal is able to explore the correct target structure to form synaptic connections. These neurites form precise neural networks via synaptic connections to maintain the normal function of neural tissues [5]. Neurite outgrowth is dependent on the cytoskeleton movement including the polymerization, depolymerization and arrangement alteration of cytoskeletons [6,7]. The relationship between Rho kinase and cytoskeleton concentrates on the microfilament movement, but the neurite outgrowth and formation have involvement of microtubules [8,9]. The microtubules are indispensable for the maintenance of cell morphology, and their structure is relatively stable, but a few instable dynamic microtubules are involved in the formation and growth maintenance of neurites [10]. After the initiation of Rho signal pathway, extracellular neurite growth and inhibition signals usually cause the polymerization and depolymerization of microtubules, which may affect the cell polarization and neurite outgrowth. However, the mechanism and signal pathways underlying the Rho kinase regulated neurite outgrowth are still poorly understood.
Calcium is an important intracellular second messenger and involved in multiple cellular events including neuronal development [11-13]. Whether calcium also participates in the Rho kinase regulated neurite outgrowth is still unclear. In this study, primary hippocampal neurons were cultured in vitro, Rho kinase agonist or antagonist was used to alter the Rho activity, and calcium chelator was used to reduce intracellular calcium. Then, real-time imaging microscopy, fluorescence double staining and calcium fluorescence imaging were employed for the observation of neurite outgrowth of hippocampal neurons, cytoskeletal rearrangement and intracellular calcium, respectively, aiming to investigate the relationship of Rho kinase and calcium regulated neurite outgrowth with cytoskeletal rearrangement and whether calcium is involved in the downstream signal transduction in Rho kinase regulated neurite outgrowth.
Materials and methods
Reagents
SD rats (specific pathogen free) were purchased from the Experimental Animal Center of Sun Yat-sen University. DMEM/F12 (Invitrogen), Neurobasal medium, B27 supplement (Gibco), polylysine (Sigma), cytosine arabinoside (Ara-C) (Invitrogen), fetal bovine serum (Gibco), anti-rat α-tubulin antibody (Sigma), Rhodamine-Phalloidin (Invitrogen), FITC conjugated fluorescent secondary antibody (Amersham pharmacia), fluorescent secondary antibody (Jackson), LPA, Y27632 (Sigma), BAPTA/AM (Merck), and Fluo-3/AM (Molecular probes) were used in the present study. Other analytical reagents were domestic.
Separation and culture of hippocampal neurons
The hippocampus was collected from 1-day SD rat pups and cut into blocks which were then transferred into 15-ml centrifuge tube. The tissues were digested with 0.125% trypsin at 37°C in an environment with 5% CO2 for 10 min. The reaction was stopped, and cell suspension was pipetted, followed by centrifugation at 800 rpm for 5 min. The supernatant was removed, and the sediment was re-suspended in Neurobasal medium containing 10% serum and 2% B27. After filtering, the cell density was adjusted, and cells were then seeded into PDL pre-coated coverslips. The medium was refreshed with serum free medium 24 h later. Thereafter, the medium was refreshed once every 3 days.
Grouping
The hippocampal neurons were seeded at a density of 2×105 cells/cm2, and the medium was refreshed 24 h later. Then, cells were divided into 4 groups: (1) control group: cells were maintained in DMEM/F12 containing 10% FBS at 37°C in an environment with 5% CO2 for 5 days; (2) Y-27632 group: cells were treated with Y-27632 at 10 μmol/L for 5 days, and remaining conditions were identical to those in control group; (3) LPA group: after 5-day culture, cells were treated with LPA at 1 μmol/L for 2 h; (4) Y-27632+LPA group: after 5-day culture, cells were treated with Y-27632 at 10 μmol/L for 30 min and then with LPA at 1 μmol/L for 2 h.
Delayed microscopic imaging
Electronically controlled inverted microscope with micro-culture system was employed for delayed imaging. After 5-day culture, cells were treated as above mentioned. Then, cells were observed under this microscope. Image was captured at 1 frame/3 min with Time-lapse program of LEICA-Qwin software. The photographs were obtained via the LEICA DFC300 camera.
Fluorescence staining of rat hippocampal neurons
Cells after calcium phosphate transfection were fixed in 4% paraformaldehyde. After treatment with perforating agent twice (5 min for each), cells were blocked in 5% donkey serum at room temperature for 1 h. After incubation with primary antibody at 4°C over night, cells were washed with TTBS to remove non-specific binding. Following incubation with secondary antibody, cells were washed with TTBS again. Then, cells were stained with DAPI, mounted, and dried in dark, followed by observation under a laser scanning confocal microscope and photographing.
Calcium imaging of cells
Neurons were seeded into Petri dish at a density of 2×105 cells/cm2. After 5-day culture, cells were washed in Hank’s solution twice and the incubated in 10 μmol/L Fluo-3/AM KRH’s at 37°C in an environment with 5% CO2 in dark for 30 min. Following washing with KRH’s twice to remove Fluo-3/AM, the cells were observed under the Zeiss LSM 510 confocal microscopy. Scanning was done at 1 frame/s with Time series program at Fluo-3 excitation wavelength of 488 nm and emission wavelength of 524 nm. The intracellular calcium fluorescence was dynamically observed, and images with the resolution of 512×512 were consecutively captured.
Measurement of branch number and length of neurites
Image-Proplus software was used to determine the length and branch number of neurites: total length of neurites and number of axons (neurites with the diameter two times larger than that of cell body), dendrites (neurites with the diameter no more than two times larger than that of cell body) and second neuronal branches. The neurites with the length shorter than 2 times of the diameter of the neurite were not included for analysis.
Statistical analysis
Statistical analysis was performed with SPSS version 19.0. All the data are expressed as mean ± standard deviation. Comparisons between groups were done with t test. A value of P < 0.05 was considered statistically significant.
Results
Rho kinase regulated neurite outgrowth of neurons
First, we investigated whether Rho kinase affected the neurite outgrowth of neurons. Under an electronically controlled inverted microscope, the neuronal body protruded 4 primary protrusions, one of which was similar to axons and grew outward the cell body, but other protrusions were short, and there were no evident second-level protrusions. 30 min later, thin and small second and third-level protrusions grew from the base of first-level protrusions. The first-level protrusions extended, and a small protrusion was present at the opposite side. 60 min later, second and third-level protrusions grew rapidly, their length increased significantly, and at the same time fourth-level protrusions formed. 90 min later, no neuronal branching was observed again, but the number and length of protrusions increased dramatically (Figure 1; control group).
Figure 1.
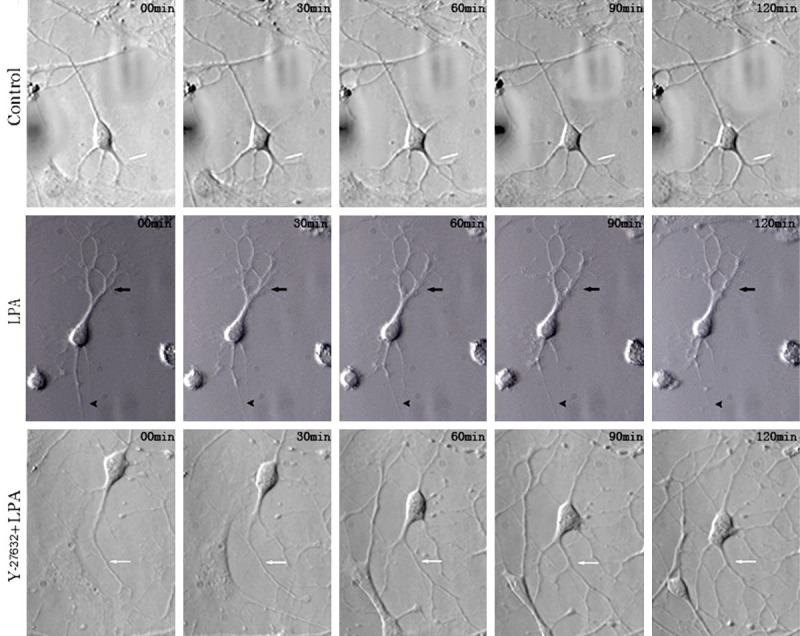
Images from delayed microscopy of neurites of rat hippocampal neurons after treatment with LPA and Y27632 (200×). In the control group, the number and length of protrusions increased dramatically with the time. In LPA group, LPA was added to activate Rho kinase. Protrusions rapidly shortened with the time. In Y27632+LPA group, the first, second, third and fourth-level protrusions continued to extend and grow, and neurons showed favorable polarity.
In LPA group, LPA was added to activate Rho kinase. 30 min later, the first-level protrusions rapidly shortened, and their terminals became thin. 60 min later, the second-level protrusions shortened, a few third-level protrusions disappeared. 120 min later, the retracted first-level protrusions became thin (arrow), second-level protrusions shortened, and a fraction of third-level protrusions disappeared (Figure 1). However, in the presence of Y-27632 pre-treatment and treatment with LPA, neuronal body became oval, the small second-level protrusions at proximal end of the cell body disappeared 30 min later, but the second-level protrusions from the large first-level protrusions rapidly grew. 60 min later, the cell body moved downward, a new thin first-level protrusion grew from the cell body, the original first and second-level protrusions continued to grow, and third-level protrusions appeared. 90 min later, neurons became polygonal, and fourth-level protrusions appeared. 120 min later, the neuronal body merged with a fraction of first-level protrusions, the number of first-level protrusions increased, the first, second, third and fourth-level protrusions continued to extend and grow, and neurons showed favorable polarity (Figure 1).
Rho kinase regulates neurite outgrow via regulating cytoskeleton
Then, we further investigated whether Rho regulated neurite outgrowth via regulating cytoskeleton in which immunocytochemistry was employed for cytoskeleton staining. As shown in Figure 2, green fluorescence was indicative of microtubules and red fluorescence was indicative of microfilaments. In control group, the green fluorescence was distributed in the whole cell body, protrusions and their terminals, but it reduced gradually with the outward extension of protrusions and the increase in the number of protrusions (Figure 2A1). The red fluorescence was also distributed in the whole neurons, especially the protrusion terminals were rich in microfilaments with red fluorescence, and the protrusions at different levels (especially the small protrusions) were clear (Figure 2A2). This was also observed in the merged images. However, at the protrusion terminals, the microfilaments were more than the microtubules. In a fraction of protrusions, there were only microfilaments and microtubules were not observable at their terminals (Figure 2A3). At the magnified images, the microtubules were found to be distributed along the protrusions (Figure 3A1), but the microfilaments were mainly distributed along the boundary of protrusions, the small spinous protrusions composed of microfilaments grew outward longitudinal to the protrusions with regular arrangement (Figure 3A2). This was also confirmed in the merged images (Figure 3A3).
Figure 2.
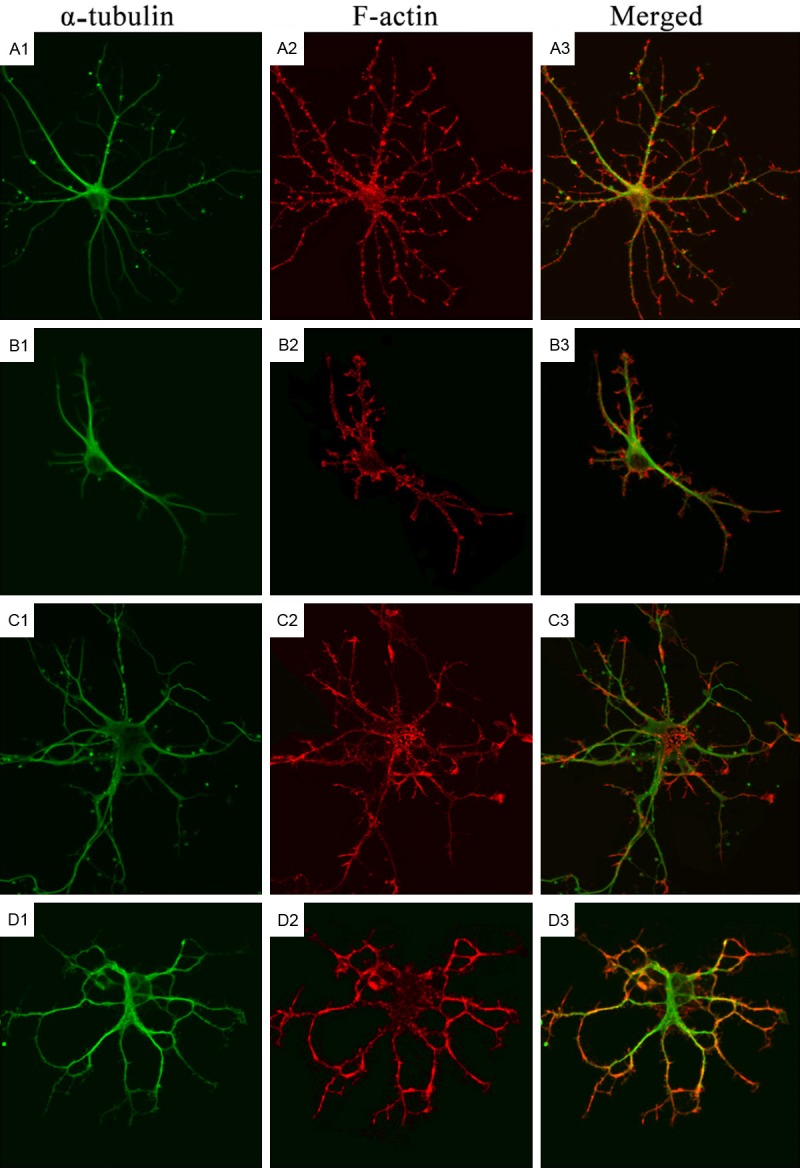
Cytoskeleton of rat hippocampal neurons after treatment with LPA and Y27632 (Confocal Microscopy). A1-A3: Immunofluorescence staining of α-tubulin and F-actin in hippocampal neurons in control group; B1-B3: Immunofluorescence staining of α-tubulin and F-actin in hippocampal neurons in Y-27632 group; C1-C3: Immunofluorescence staining of α-tubulin in hippocampal neurons in LPA group; D1-D3: Immunofluorescence staining of α-tubulin and F-actin in hippocampal neurons in Y-27632+LPA group.
Figure 3.
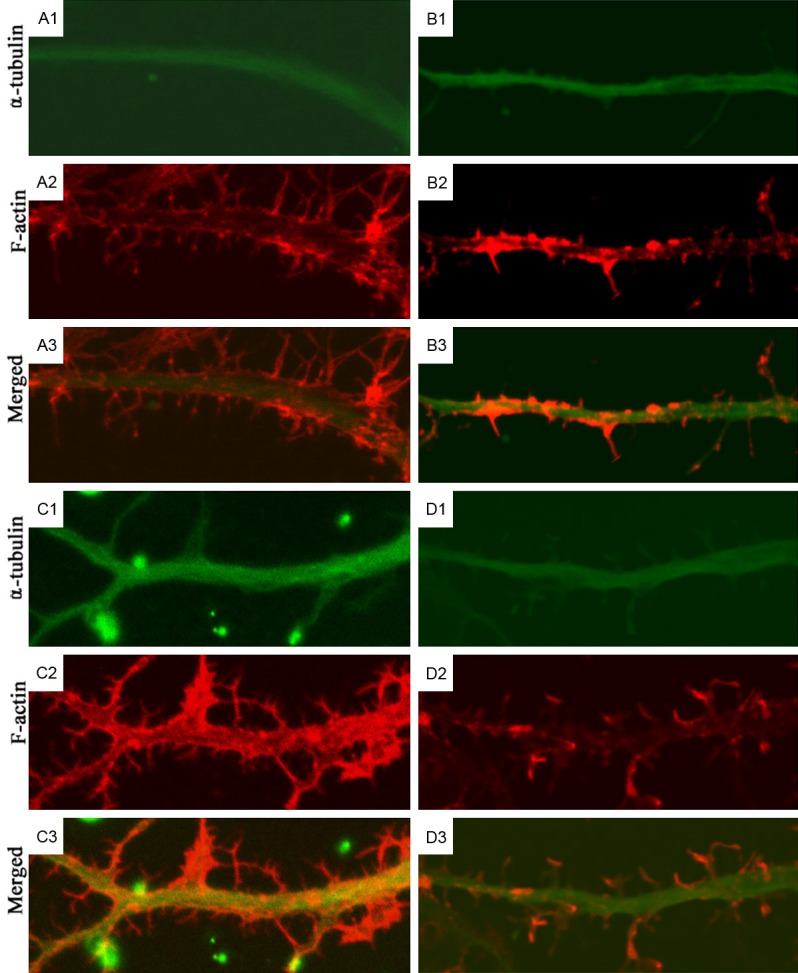
Cytoskeleton of neurites of rat hippocampal neurons after treatment with LPA and Y27632 (Confocal Microscopy). Results showed Rho kinase may regulate the cytoskeleton (microfilaments and microtubules) to regulate the neurite outgrowth. A1-A3: Immunofluorescence staining of α-tubulin and F-actin in hippocampal neurons in control group; B1-B3: Immunofluorescence staining of α-tubulin and F-actin in hippocampal neurons in Y-27632 group; C1-C3: Immunofluorescence staining of α-tubulin in hippocampal neurons in LPA group; D1-D3: Immunofluorescence staining of α-tubulin and F-actin in hippocampal neurons in Y-27632+LPA group.
In Y-27632 group, the neuronal body became larger and irregular after treatment, the number of branches increased and some small branches grew outward from the neuronal body (Figure 2B1). In a lot of protrusion terminals, spinous protrusions composed of microfilaments were observed (Figure 2B2). In the magnified images, the main protrusions composed of microtubules displayed a short branch (Figure 3B1). As compared with control group, the red fluorescence in the protrusions increased. Some small spinous protrusions composed of microfilaments grew outward, and some of spinous protrusions were large and long (Figure 3B2). The increased red fluorescence was also observed in the merged images, but there were no evident microtubules in the spinous protrusions (Figure 3B3). Although the microtubules in the neuronal body and main protrusions were similar between LPA group and control group, the number of protrusions at different levels reduced significantly after treatment in LPA group, the second-level protrusions became short, third-level protrusions disappeared, and the number of microtubules also reduced (Figure 2C1), microfilaments reduced significantly in the neuronal protrusions, and especially there was a little red fluorescence in the small protrusions, but the microfilaments at protrusion terminals were more than microtubules (Figure 2C2). At the magnified protrusions, the microtubules distribution was even in the main protrusions (Figure 3C1), the microfilaments were mainly distributed at the boundary of main protrusions, but the microfilaments reduced significantly, or even disappeared at some regions, and a few spinous protrusions grew outward from the main protrusions (Figure 3C2).
In Y-27632+LPA group, LPA was added at 30 min after Y-27632 pre-treatment, neurons were still rich in protrusions, both microfilaments and microtubules were observed at the neuronal body and protrusions of different levels, which were similar to findings in control group. The microtubules were mainly distributed in the neuronal body and main protrusions (Figure 2D1), but microfilaments were more evident in the second and third-level protrusions, and there were spinous protrusions composed of microfilaments at the small protrusions and protrusion terminals (Figure 2D2). In the merged images, the red fluorescence became more evident at the protrusion terminals (Figure 3D1). In the magnified images, the microfilaments were more than in LPA group, and the number of spinous protrusions also increased significantly (Figure 3D2). These findings indicate that Rho kinase may regulate the cytoskeleton (microfilaments and microtubules) to regulate the neurite outgrowth.
Rho kinase regulates the intracellular distribution of free calcium
We further investigated which pathway was involved in the Rho kinase regulated neurite outgrowth via Cytoskeleton. Under a confocal microscopy, the intracellular calcium displayed green fluorescence after the neurons were incubated with Fluo-3/AM. In the neuronal body, the intensity of calcium fluorescence was higher than in the protrusions. In control group, neurons were scanned for 1 min. When the intracellular calcium fluorescence remained stable, 10 μl of PBS was added. Under a microscope, the calcium fluorescence was evenly distributed, and there was no significant increase or decrease in the intensity of calcium fluorescence. At 410 s, the intensity of intracellular calcium fluorescence reduced, and that in the protrusions disappeared, but green fluorescence was still observable in cells. In a few cells, the fluorescence intensity reduced significantly. In the fluorescence intensity-time curve, the intracellular calcium fluorescence intensity curve was relatively straight, the addition of PBS did not increase the intensity, and the intensity reduced gradually over time (Figure 4, Control group).
Figure 4.
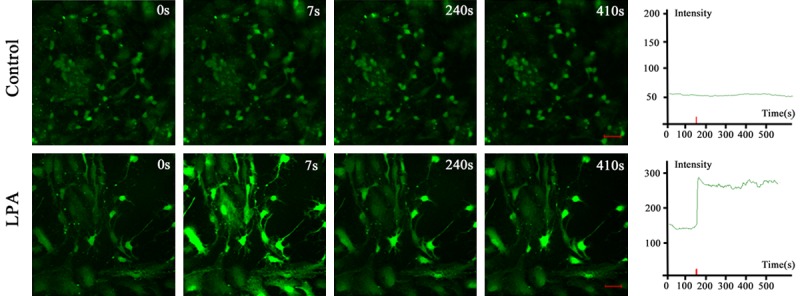
Calcium real-time imaging of intracellular calcium of neurons and calcium fluorescence intensity curve-time curve in control group and LPA group (scale bar: 50 μm). In control group, the calcium fluorescence was evenly distributed, and there was no significant increase or decrease in the intensity of calcium fluorescence. In LPA group, calcium fluorescence intensity of hippocampal neurons was stable at baseline. Several seconds after LPA treatment, the intracellular calcium increased abruptly and reached a peak. The peak level of calcium fluorescence intensity was about 2 times that at baseline. Thereafter, the calcium fluorescence intensity slightly reduced and then remained stable and at a high level.
In LPA group, the intracellular calcium fluorescence became stable within 1 min. After incubation of 1 μmol/L LPA, the calcium increased significantly at 7 s. Under the microscope, the intracellular fluorescence intensity increased dramatically in neurons and glial cells. The nuclear fluorescence intensity was the highest in glial cells. The fluorescence intensity increased markedly in only the cell body and main protrusions, but in distal protrusions and small branches. At 240 s, the fluorescence intensity became to reduce in a majority of glial cells, and the fluorescence intensity also decreased in neurons, but it in the cell body and protrusions was still higher than that at the beginning. In some small protrusions and protrusion terminals, the fluorescence intensity reduced or even the fluorescence disappeared. At 410 s, the intracellular calcium fluorescence intensity in neurons was still at a high level and did not reduce. In LPA group, the calcium fluorescence intensity-time curve showed the calcium fluorescence intensity of hippocampal neurons was stable at baseline. Several seconds after LPA treatment, the intracellular calcium increased abruptly and reached a peak. The peak level of calcium fluorescence intensity was about 2 times that at baseline. Thereafter, the calcium fluorescence intensity slightly reduced and then remained stable and at a high level. The curve was nearly horizontal, which remained for 3-4 min. Thereafter, the calcium fluorescence intensity began to fluctuate with small amplitude, but it still remained at a high level (Figure 4, LPA group). These findings indicate that Rho kinase may affect the calcium concentration to regulate cytoskeleton and subsequent neurite outgrowth of neurons.
Calcium is involved in the Rho kinase regulated neurite outgrowth of hippocampal neurons
To elucidate whether Rho kinase regulates neurite outgrowth in a calcium dependent manner, pharmacological experiment was performed. When compared with control group, the neuronal volume was smaller in BAPTA/AM group, only several thin first-level protrusions grew outward through the cell body, and these protrusions were short and often disappeared near the cell body. Few branches were observed in the protrusions, and occasionally, several small and thin second-level protrusions grew from the terminal of first-level protrusion, but no third-level protrusions were observed (Figure 5).
Figure 5.
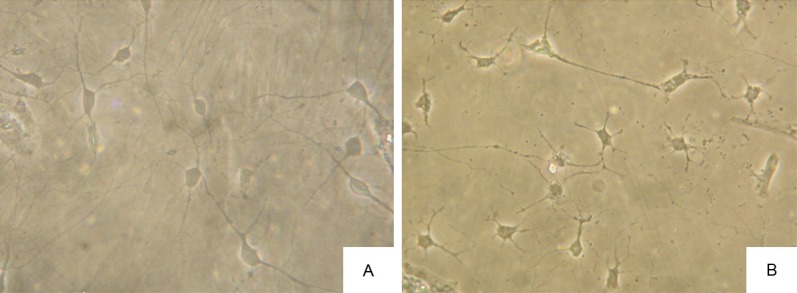
Effects of BAPTA/AM on the rat hippocampal neurons and their neurites (Phase contrast microscope; 200×). When compared with control group, the neuronal volume was smaller in BAPTA/AM group, only several thin first-level protrusions grew outward through the cell body, and these protrusions were short and often disappeared near the cell body. Few branches were observed in the protrusions, and occasionally, several small and thin second-level protrusions grew from the terminal of first-level protrusion, but no third-level protrusions were observed. A: Rat hippocampal neurons and their protrusions in control group; B: Rat hippocampal neurons and their protrusions after BAPTA/AM treatment.
After treatment, the fluorescence intensity of microtubules and microfilaments in the cell body and main protrusions in BAPTA/AM group did not reduce when compared with control group. In BAPTA/AM group, the microfilaments at protrusion terminals were evident, and a few spinous protrusions grew from the main protrusions. At magnified protrusions, the microtubules were evenly distributed, but microfilaments displayed discontinuous distribution, more microfilaments were found at the boundary of protrusions, microfilaments at some regions even disappeared, and a few spinous protrusions growing from the main protrusions disappeared near the main protrusions. In BAPTA/AM+LPA group, neurons were pre-treated with BAPTA/AM for 30 min and then with LPA. Results showed the neurite branches of neurons increased, the microfilaments and microtubules were observed in the neuronal body and protrusions of different levels, and microfilaments were obvious in the protrusions of different levels (Figure 6). In the magnified images, the red microfilaments in protrusion terminals showed increased fluorescence intensity, and the spinous protrusions composed of microfilaments increased significantly when compared with LPA group and BAPTA/AM group (Figure 7). These findings confirm that Rho kinase may regulate neurite outgrowth via regulating intracellular calcium.
Figure 6.
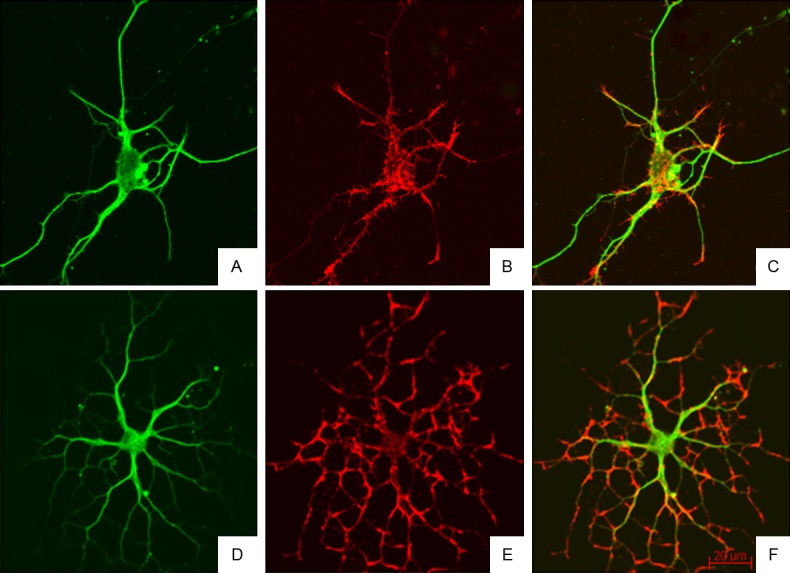
Cytoskeleton of rat hippocampal neurons after treatment with BAPTA/AM and LPA (Confocal Microscopy). Neurite branches of neurons increased, the microfilaments and microtubules were observed in the neuronal body and protrusions of different levels, and microfilaments were obvious in the protrusions of different levels. A-C: Immunofluorescence staining of α-tubulin and F-actin in hippocampal neurons in BAPTA/AM group; D-F: Immunofluorescence staining of α-tubulin and F-actin in hippocampal neurons in BAPTA/AM+LPA group.
Figure 7.
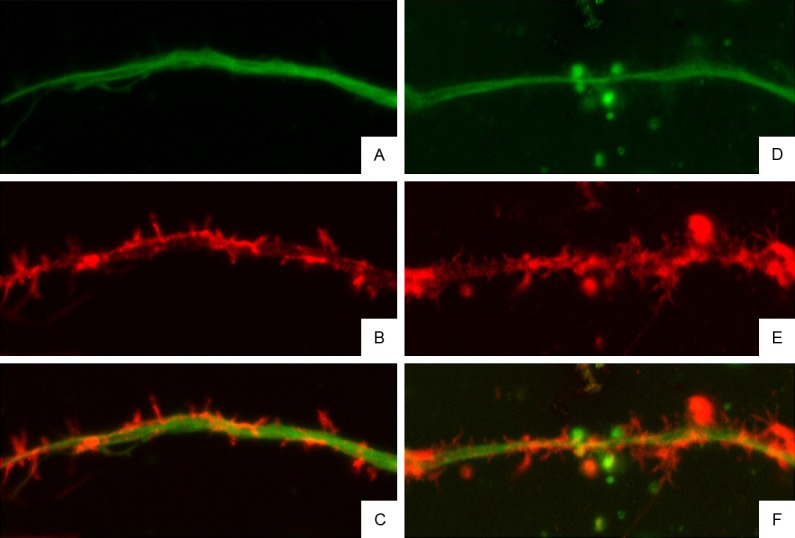
Cytoskeleton of neurites of rat hippocampal neurons after treatment with BAPTA/AM and LPA (Confocal Microscopy). In the magnified images, the red microfilaments in protrusion terminals showed increased fluorescence intensity, and the spinous protrusions composed of microfilaments increased significantly when compared with LPA group and BAPTA/AM group. A-C: Immunofluorescence staining of α-tubulin and F-actin in hippocampal neurons in BAPTA/AM group; D-F: Immunofluorescence staining of α-tubulin and F-actin in hippocampal neurons in BAPTA/AM+LPA group.
Discussion
Rho kinase plays an important regulatory role in the neurite outgrowth. Inhibition of Rho kinase may promote neurite outgrowth and increase the number of neurites, and activation of Rho kinase pathway may cause neurite retraction and reduce the number of neurites [8,9]. In addition, calcium has complex regulatory effects on the neurite outgrowth of hippocampal neurons, and excess reduction or increase in intracellular calcium may cause the neurite outgrowth arrest [14,15]. Our results showed Rho kinase could regulate the cytoskeleton----microtubules and microfilaments, to regulate the neurite outgrowth. In addition, our findings also indicated that Rho kinase agonist PLA was able to significantly increase the intracellular calcium, and inhibition of calcium increase was able to antagonize the protrusion inhibition secondary to the treatment of Rho kinase agonist. This indicates that calcium is involved in the Rho kinase regulated neurite outgrowth.
The effects of calcium on the neurite outgrowth are related to the regulation of cytoskeletal rearrangement, and calcium signal is an up-stream signal in the regulation of cytoskeletal rearrangement [12]. Under a confocal microscope, although the fluorescence of microtubules and microfilaments did not reduce significantly in the cell body and main protrusions of neurons after BAPTA/AM treatment, the number of protrusions and their branches reduced markedly, the fluorescence of microfilaments was weak in a majority of regions of protrusions, and a few spinous protrusions grew outward from the main protrusions. In the magnified images, the microfilaments in the protrusions reduced, only a few short spinous protrusions grew outward from the main protrusions. These indicate that reduction in intracellular calcium is able to decrease the microfilaments in the protrusion terminals and small branches.
There is evidence showing that LPA is able to specifically induce the increase in intracellular calcium, and the higher the extracellular LPA concentration, the higher the intracellular calcium concentration was [16-18]. Our results showed the calcium fluorescence intensity was stable at baseline; several seconds after addition of LPA, intracellular calcium concentration increased dramatically and reached a peak which was about 2 times of that at baseline, thereafter it reduced gradually. In control group, the calcium fluorescence intensity remained stable without significant fluctuation, although a slow reduction was observed at late stage; the calcium fluorescence also remained unchanged. These findings suggest LPA is able to increase the intracellular calcium of hippocampal neurons. The effects of LPA on the intracellular calcium of hippocampal neurons are ascribed to the influx of extracellular calcium. If cells are maintained in calcium free buffer, the effects of LPA on the intracellular calcium are significantly compromised or even disappeared. The calcium imaging in living cells is dependent on the type and status of neurons and the experimental preparation. Zhou et al found LPA induced the slow increase in intracellular calcium of chick embryonic neural retinal cells which lasted for a short time. Moreover, they also found that the release of intracellular calcium seemed to be a major cause of increase in intracellular calcium, but more studies are required to confirm these findings [16].
The LPA induced neurite retraction is closely related to the activation of RhoA/Rho. In the present study, the LPA induced neurite retraction disappeared after inhibition of intracellular calcium with BAPTA/AM. Immunofluorescence staining of cytoskeleton showed the LPA induced neurite retraction was also related to the cytoskeletal rearrangement in neurons after inhibition of intracellular calcium with BAPTA/AM. Under a confocal microscope, neurons in BAPTA/AM+LPA group were rich in neurites, and microtubules and microfilaments were distributed along the neuronal body and protrusions of different levels. The microfilaments were obvious in the protrusions of different levels, and the red fluorescence of microfilaments was strong at the protrusion terminals. In the magnified images, the fluorescence of microfilaments was strong in the main protrusions, the spinous protrusions were short, but their number increased significantly when compared with LPA group and BAPTA/AM group. In studies on neuroblastoma, LPA may activate calcium-actinin signal pathway to induce the depolymerization of actin, leading to the neurite collapse. Other studies also indicate that the increase in intracellular calcium is able to activate calmodulin kinase (CaM) or calpain and calmodulin phosphatase, leading to the cytoskeletal rearrangement [19,20] and subsequent neurite retraction. Although our study did not confirm which downstream signal was involved in the LPA induced increase in intracellular calcium and the subsequent cytoskeletal rearrangement and neurite retraction in rat hippocampal neurons, the LPA induced cytoskeletal rearrangement (especially the microfilament rearrangement) was significantly compromised after reduction in intracellular calcium, the actin in protrusions increased, but the spinous protrusions composed of microfilaments increased significantly. Rho kinase plays an important role in the regulation of T-type calcium channel on cell membrane [21,22]. After LPA induced activation, Rho kinase may cause the close of T-type calcium channel Cav3.1 in Y79 neuroblastoma cells and lateral lacing neurons, but it may up-regulate the Cav3.2 channel dorsal root ganglion neurons, leading to the increase in calcium influx and membrane depolarization, which will affect the bioeffects of LPA [22].
Our results indicate that Rho kinase may regulate cytoskeletons to affect neurite growth. Calcium is an important signal molecule in the regulation of neurite outgrowth of hippocampal neurons, and has complex regulatory role in the neurite outgrowth. The calcium regulated neurite outgrowth is closely related to cytoskeletons, and calcium is involved in the Rho kinase regulated neurite outgrowth of hippocampal neurons.
Acknowledgements
This study was supported by the National Nature Science Foundation of China (No. 81571191), the Natural Science Foundation of Guangdong Province (No. 2014A030313357), Project from Development Center for Medical Science and Technology of the National Health and Family Planning Commission of People’s Republic of China (No. W2013ZT083) and Scientific Fund of the No. 1 School of Clinical Medicine at Jinan University (No. 2015202), and China Post-doctoral Scientific Fund (No. 2016T90821).
Disclosure of conflict of interest
None.
References
- 1.Zhang C, Wu JM, Liao M, Wang JL, Xu CJ. The ROCK/GGTase pathway are essential to the proliferation and differentiation of neural stem cells mediated by simvastatin. J Mol Neurosci. 2016;60:474–485. doi: 10.1007/s12031-016-0811-y. [DOI] [PubMed] [Google Scholar]
- 2.Mack NA, Georgiou M. The interdependence of the Rho GTPases and apicobasal cell polarity. Small GTPases. 2014;5:10. doi: 10.4161/21541248.2014.973768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auer M, Hausott B, Klimaschewski L. Rho GTPases as regulators of morphological neuroplasticity. Ann Anat. 2011;193:259–266. doi: 10.1016/j.aanat.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickson HM, Wilbur A, Reinke AA, Young MA, Vojtek AB. Targeted inhibition of the Shroom3-Rho kinase protein-protein interaction circumvents Nogo66 to promote axon outgrowth. BMC Neurosci. 2015;16:34. doi: 10.1186/s12868-015-0171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stiess M, Bradke F. Neuronal polarization: the cytoskeleton leads the way. Dev Neurobiol. 2011;71:430–444. doi: 10.1002/dneu.20849. [DOI] [PubMed] [Google Scholar]
- 6.Nelson WJ. Remodeling epithelial cell organization: transitions between front-rear and apical-basal polarity. Cold Spring Harb Perspect Biol. 2009;1:a000513. doi: 10.1101/cshperspect.a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lestanova Z, Bacova Z, Bakos J. Mechanisms involved in the regulation of neuropeptide-mediated neurite outgrowth: a minireview. Endocr Regul. 2016;50:72–82. doi: 10.1515/enr-2016-0011. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed Z, Berry M, Logan A. ROCK inhibition promotes adult retinal ganglion cell neurite outgrowth only in the presence of growth promoting factors. Mol Cell Neurosci. 2009;42:128–133. doi: 10.1016/j.mcn.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Zuo YC, Xiong NX, Shen JY, Yu H, Huang YZ, Zhao HY. MARK2 rescues Nogo-66-induced inhibition of neurite outgrowth via regulating microtubule-associated proteins in neurons in vitro. Neurochem Res. 2016;41:2958–2968. doi: 10.1007/s11064-016-2016-8. [DOI] [PubMed] [Google Scholar]
- 10.Schmandke A, Strittmatter SM. ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist. 2007;13:454–469. doi: 10.1177/1073858407303611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolsover SR. Calcium signalling in growth cone migration. Cell Calcium. 2005;37:395–402. doi: 10.1016/j.ceca.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Spira ME, Oren R, Dormann A, Ilouz N, Lev S. Calcium, protease activation, and cytoskeleton remodeling underlie growth cone formation and neuronal regeneration. Cell Mol Neurobiol. 2001;21:591–604. doi: 10.1023/A:1015135617557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sargin D, Oliver DK, Lambe EK. Chronic social isolation reduces 5-HT neuronal activity via upregulated SK3 calcium-activated potassium channels. Elife. 2016;5:e21416. doi: 10.7554/eLife.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty P, Williams G, Williams EJ. CAMs and axonal growth: a critical evaluation of the role of calcium and the MAPK cascade. Mol Cell Neurosci. 2000;16:283–295. doi: 10.1006/mcne.2000.0907. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg DJ, Grabham PW. Braking news: calcium in the growth cone. Neuron. 1999;22:423–425. doi: 10.1016/s0896-6273(00)80697-2. [DOI] [PubMed] [Google Scholar]
- 16.Zhou WL, Sugioka M, Yamashita M. Lysophosphatidic acid-induced Ca(2+) mobilization in the neural retina of chick embryo. J Neurobiol. 1999;41:495–504. doi: 10.1002/(sici)1097-4695(199912)41:4<495::aid-neu5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 17.Gomez TM, Spitzer NC. Regulation of growth cone behavior by calcium: new dynamics to earlier perspectives. J Neurobiol. 2000;44:174–183. [PubMed] [Google Scholar]
- 18.Lee JM, Park SJ, Im DS. Lysophosphatidylethanolamine increases intracellular Ca(2+) through LPA(1) in PC-12 neuronal cells. Biochem Biophys Res Commun. 2015;461:378–82. doi: 10.1016/j.bbrc.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 19.Kater SB, Mills LR. Regulation of growth cone behavior by calcium. J Neurosci. 1991;11:891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Rodriguez P, Falcon D, Castro MJ, Urena J, Lopez-Barneo J, Castellano A. Hypoxic induction of T-type Ca(2+) channels in rat cardiac myocytes: role of HIF-1α and RhoA/ROCK signalling. J Physiol. 2015;593:4729–45. doi: 10.1113/JP271053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iftinca M, Hamid J, Chen L, Varela D, Tadayonnejad R, Altier C, Turner RW, Zamponi GW. Regulation of T-type calcium channels by Rho-associated kinase. Nat Neurosci. 2007;10:854–860. doi: 10.1038/nn1921. [DOI] [PubMed] [Google Scholar]


