Abstract
Prevention of colon cancer among high-risk group has been long lasting research goal. Emerging data have evidenced the anticancer activities of Vitamin D3 (Vit.D) and Thymoquinone (TQ). The aim of the current study was to evaluate the synergistic potential of Thymoquinone and Vitamin D3 in the control of colon cancer progression using azoxymethane-induced rat model. Vit.D and TQ were given individually or in combination 4 week prior to induction and continued for a total of 20 week. At the end of the study, all animals were euthanized and their resected colons were examined macroscopically and microscopically for tumor growth. Colonic tissue preparations were used for measuring gene expression and/or protein levels of selected pro and anti-tumor biomarkers using quantitative RT-PCR, ELISA and immunohistochemistry. Compared with their individual supplementation, combined Vit.D/TQ showed prominent anti-tumor effect manifested by significant reduction (P < 0.05) of the numbers of grown tumors and large aberrant crypts foci. Mechanistically, gene expression and/or protein quantification studies revealed that combined Vit.D/TQ supplementation induced significant reduction (P < 0.01 and P < 0.05) of pro-cancerous molecules (Wnt, β-catenin, NF-κB, COX-2, iNOS, VEGF and HSP-90) as well as significant increase (P < 0.01 and P < 0.05, respectively) of anti-tumorigenesis biomarkers (DKK-1, CDNK-1A, TGF-β1, TGF-β/RII and smad4) as compared to un-supplemented or individually supplemented groups, respectively. In conclusion, TQ augmented the chemopreventive effect of Vit.D during the initiation phase of colon cancer in rat model, with the potential to suppress progression of pre-neoplastic lesions in colon carcinogenesis.
Keywords: Colon cancer, thymoquinone, Vitamin D3, tumor biomarkers, azoxymethane-induced rat model
Introduction
Colon cancer is a common malignancy in both developed and developing countries. It is one of the leading causes of deaths among cancer patients worldwide [1]. Surgical removal of colon cancer during the early stages is the most effective therapeutic approach but it requires early diagnosis, otherwise chemotherapy and radiotherapy are the approach of choice during the advanced stages [2,3].
Understating the cancer biology and its related pathophysiological mechanisms is necessary not only for describing new biomarkers that allow for early diagnosis, but also for the development of alternative preventative and/or therapeutic strategies. Colon cancer, as most solid tumors, is characterized by uncontrolled cell proliferation, ablation of apoptosis, enhanced inflammation and tumor angiogenesis with subsequent poor prognosis [4]. Multiple molecular pathways underlay the pathological alterations associated with colon cancer tumorigenesis. Several molecules including, but not limited to, transforming growth factor beta (TGF-β), TGF-β type II receptor (TGF-β/RII), Wnt, β-catenin, Dickkopf WNT signaling pathway inhibitor 1 (DKK-1), inducible nitric oxide synthase (iNOS), heat shock protein (HSP)-90, cyclooxygenase (COX)-2, vascular endothelial growth factor (VEGF), nuclear factor kappa-B (NF-kβ) and cyclin-dependent kinase inhibitor (CDKN1-A) are among the important elements of the multi-mechanistic pathways of colon cancer and serve as valuable biomarkers for the evaluation of the disease stages and outcomes [5,6].
According to the WHO, prevention is the most effective long-term strategy for the control of cancer especially among those at high risk of developing progressive cancer. Patients with long-standing inflammatory bowel disease including ulcerative colitis and Crohn’s disease have an increased risk of developing colorectal cancer. In other words, prophylactic prevention is an intensely beneficial method to combat cancer in high-risk population [7-9].
Chemoprevention is one of the effective prophylactic strategies to manage and control cancer in high-risk population. It is refer to the use of natural or synthetic chemical agents that can interfere with the process of carcinogenesis by inducing a variety of biological mechanisms [10]. Micronutrients as well as phytochemicals have received great attention lately for their propitious anticancer properties. In term of cancer management, these products could have serve dual purposes. In addition to providing chemoprotection against development of progressive cancer, they can also enhance the therapeutic effect of currently used chemotherapeutics with the subsequent diminution of their inescapable toxicity via reducing the dose and time required for treatment. From the prophylactic point of view, several micronutrients and phytochemicals were shown to counteract the onset of carcinogenesis especially among high-risk population, by inhibiting initiation of carcinogenesis or by arresting or reversing later stages of cancer progression [11-13]. Recently, significant numbers of observational and epidemiological studies have illustrated an inverse association between intake of vitamin D3 (Vit.D) and the risk of CRC and have suggested a pro-active role of Vit.D in colorectal cancers. The protective role of Vit.D against cancer has been attributed to its influence on underlying mechanisms of carcinogenesis including cell proliferation, differentiation, apoptosis, DNA repair mechanisms, inflammation, and immune function [14-16]. Evidenced anti- proliferative effect of Vit.D and its analogs via inducing G1 cell-cycle arrest. This effect is mediated by up-regulation of cell-cycle inhibitors, such as p21WAF1/CIP and p27KIP [17,18]. Initiation of apoptosis is another anti- cancer activity of Vit.D, which is mediated by up-regulation of pro-apoptotic proteins and down-regulation of the anti-apoptotic proteins [19]. In addition to the direct inhibition of endothelial cells proliferation, Vit.D and its analogs also inhibit the vascular endothelial growth factor (VEGF), leading to the inhibition of angiogenesis [20].
Phytochemicals are non-nutritive plant chemicals that have protective or disease preventive properties. Multiple epidemiological and experimental studies have demonstrated the medical importance of certain active compounds derived from medicinal plants in inhibiting the development and reducing the risk of cancer [21,22]. Thymoquinone (TQ), a component derived from the medical plant Nigella sativa, is one of the promising compounds of plant origin that exhibited multiple pharmacotherapeutic properties [23]. Several studies have documented the inhibitory effect of TQ on cell proliferation of many types of cancer cell lines, including breast and ovarian adenocarcinoma, colorectal cancer, human pancreatic adenocarcinoma, uterine sarcoma, neoplastic keratinocytes, human osteosarcoma, fibrosarcoma and lung carcinoma [24-26]. Interestingly, acute and chronic toxicity studies on laboratory animals have showed high therapeutic index and safety margin of TQ, particularly when given orally [23,27]. Moreover, incorporation of TQ with conventional chemotherapeutic regimens has augmented the therapeutic effect and reduced the toxicity of the latter [28,29]. In addition, In vivo anti-tumor effects of TQ have also been investigated in tumor xenograft mice models for colon, prostate, pancreatic and lung cancer. Different modes of actions were proposed for the anticancer effects of TQ, including anti-proliferation, apoptosis induction, cell cycle arrest, inhibition of reactive oxygen species (ROS) generation and anti-metastasis/anti-angiogenesis [30-32].
Based on these collective data, calcitriol (the hormonal form of Vitamin D3) and TQ show promising role in the modern medicine of cancer control. However, to the best of our knowledge, there is no published report on the possible beneficial anti-cancer interactions between TQ and vitamin D3 or its new analogue(s). Therefore, the aim of the current study was to investigate the synergistic potential of TQ when used in combination with vitamin D in providing chemopreventive or chemoprotective effect against colon carcinogenesis using azoxymethane-induced colon cancer rat models.
Materials and methods
Azoxymethane induction of colon cancer model in rat
The current experimental animal study was carried out in strict accordance with the recommendations of the EU Directive 2010/63/EU for animal experiments. The Ethical Committee for the Care and Use of Laboratory Animals, Collage of Applied Medical Sciences, Umm Al-Qura University has approved used experimental animal protocols (Approval # AMSEC 8/07-10-2014]. All animals received human handling during the experiment and all effort were made to minimize suffering. Euthanasia and collection of samples were made under complete anesthesia. Morbidity-based humane endpoint protocol was adopted and approved during the initial animal study proposal. Azoxymethane (AOM) was used in the current study for induction of colon cancer model in rats. AOM is a known carcinogenic chemical commonly used to induce an experimental model of colon cancer in rodents that mimics human cancer sporadic phenotype with prohibitive resemblances at clinical, histopathological and molecular levels. AOM has been extensively used in the prevention and treatment studies of colon cancer with 14 weeks required duration for cancer development in rodents [33,34]. Briefly, AOM (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in normal saline and injected subcutaneously to the animals at a dose of 15 mg/kg body weight, once weekly for a total of 2 weeks to induce tumorigenesis in the colon as previously described [35].
Treatment preparation and regimens
Oral drops of vitamin D3 (cholecalciferol 4500 IU/ mL) were purchased and used for the study (Novartis International AG, Basel, Switzerland). Cholecalciferol and its dose were chosen over calcitriol, the hormonal form of vitamin D, to achieve the therapeutic properties of calcitriol but preclude the risk of soft tissue calcification [36,37]. Cholecalciferol (Vit.D) was prepared by adding 4 ml to 16 ml saline every morning to form a final concentration of 1000 IU/mL. Treated rats received 0.5 ml/day (500 IU/day; 3 days/week) by oral gavage. Thymoquinone (2-isopropyl-5-methyl-1,4-benzoquinone) was obtained from Sigma USA. TQ solution was weekly freshly prepared by dissolving it in 0.5% dimethyl sulphoxide (DMSO) followed by dilution using olive oil, and then given orally by gastric gavage at the following dosage regimen: 35 mg/kg/day, three days/week. The dissolving process and dose of TQ were chosen on the basis of our tested pilot experiments and previously published reports [38,39].
Experimental design
A total of 75 male Wistar rats weighing 200-250 g. were purchased from the Animal house facility, King Abdul-Aziz University (Jeddah, KSA). The animals were housed in clean and sterile polyvinyl cages (5 rats/cage), maintained on standard laboratory pellet diet and water ad libitum and kept in a temperature-controlled air-conditioned (22°C) and 12 h dark/light cycle. Animals were daily monitored during the weekdays by the investigators during administration of different treatments. In addition, a properly trained and qualified personnel was hired to monitor the animals’ health signs, survivability, eating and drinking behavior especially during the weekend days. All animals received humane care during the study protocol and during euthanasia. After acclimation, the rats were randomly categorized into 5 groups (15 rats/group). The first group served as ‘Control group’, which received saline only throughout the whole experiment without AOM injection. All the rest of the groups received AOM injection for induction of colon cancer model. The 2nd group received only saline and designated as ‘AOM group’, the 3rd group received Vit.D and designated as ‘Vit.D group’, the 4th group received TQ and designated as ‘TQ group’ and the 5th group received Vit.D +TQ and designated as ‘Vit.D/TQ group’. Treatments started 4 weeks before AOM induction and continued during the 2 weeks induction period and extended for additional 14 weeks after induction.
Blood and tissue sampling
At the end of the experiment (14 weeks after AOM induction and 20 weeks from the start of treatment), rats of all groups were fasted overnight and subsequently euthanized under complete anesthesia. Three ml of whole blood were collected from each rat in a plain tube after cutting the vena cava. The samples were centrifuged and the serum was stored in -20°C till used. In addition, immediately after collection of blood, whole colon from rectum to caecum was gently resected, flushed with cold potassium phosphate buffer (0.1 M, pH 7.2) to remove residual bowel contents and slit opened longitudinally. The length and width of the isolated colons were measured to calculate the colon surface area. The opened colons were then submerged overnight in 10% (v/v) neutralized formalin with the mucosa on the upper side between layers of filter papers. All colon specimens were then processed for gross and histopathological examination and later for immunohistochemistry, ELISA and quantitative gene expression.
Biochemical analysis
Serum samples were used to measure the serum levels of liver enzymes (ALP, ALT and AST), renal function parameters (creatinine, BUN and urea) and serum concentrations of 25-OH vitamin D using Cobas e411 (Roche Diagnostics International Ltd, Switzerland) according to the manufacturer’s protocol.
Counting and sampling of tumors
Numbers of expected tumors were evaluated at 2 levels by 2 different observers blind to the source group. First, grown tumors on colon mucosa were counted by naked eye. Then, in order to locate small tumors and large Aberrant Crypts Foci (ACF) that were undetected by naked eye counting, formalin-fixed tissues were cut into proximal, middle and distal segments of the same length. Each segment was stained with 0.2% methylene blue solution for 1.5-2 min, placed on a microscope slide with the mucosal side upward, and then observed under a dissecting microscope (× 20). ACF were reported as foci containing 4 or more aberrant crypts as previously described [40]. Using a micro-feather scalpel blade, tumors of interest were excised from the surrounding normal tissues under the dissecting microscopy to be used for histopathological, immunohistochemical and molecular examinations. Two specimens were processed for histopathology, 2 specimens for immunohistochemistry and the remaining were distributed equally either in RIPA buffer (Santa-Cruz Biotechnology Inc, Burlingame, CA) for protein extraction or RNALater (Ambion, Thermo Fisher Scientific, USA) for preservation in -80°C till processed for quantitative real time-polymerase chain reaction (Q-RT-PCR).
Histopathological examination
Following de-staining from methylene blue with 80% ethanol, the tissue specimens were embedded in paraffin and sectioned at 4-5 μm, and stained by haematoxylin and eosin (H&E) as previously described [41]. An expert histopathologistexamined aberrant crypt foci (ACF) and glandular epithelial morphologyblind to specimen groups. ACF were microscopically classified into hyperplastic ACF (no dysplasia) or dysplastic ACF (elongated, crowded and pseudo-stratified nuclei; increased nucleus-to-cytoplasm ratio; reduced number of goblet cells; back-to-back glands and markedly decreased inter-glandular stroma) as established and described previously [42]. Colonic adenoma was characterized as being consisted of proliferative/hyperplastic colonic glands, and colonic adenocarcinoma was characterized by observingdysplastic glands that invaded the sub-mucosal muscle layer [34].
Evaluation of selected biomarkers associating colon carcinogenesis
In the current study, gene expression and/or protein levels of selected biomarkers were investigated using Q-RT-PCR, immunohistochemistry and/or ELISA. Selected molecules included canonical Wnt, B-catenin, DKK-1, TGF-B, TGF receptor, smad 4 and HSP-90 as selected biomarkers of cancer proliferation and progression. The study also included VEGF, iNOS and COX-2 as selected biomarkers of cancer angiogenesis. In addition, NF-KB and CDKN1-A molecules were also studies as apoptotic biomarkers.
Immunohistochemistry
Polyclonal goat IgG antibodies to detect TGF-β1 (C-16), smad4 (C-20), β-catenin (C-18), and polyclonal rabbit IgG antibodies against TGF-β/RII receptor (L-21), iNOS (N-20) and HSP-90-α/β (N-17) were obtained from Santa-Cruz Biotechnology Inc. (Burlingame, CA) and used for evaluation of their corresponding molecules in tissues of investigated groups. An avidin-biotin horseradish peroxidase technique was used to localize the proteins of interest as previously described. Evaluation and scoring of immunohistochemical staining were carried out as previously described [36], using Labor Lux microscope (Leitz, Wetzlar, Germany), at a magnification of × 100, × 200 and × 400. In case of wide disagreement between the two observers, the slides were re-analyzed by a third independent reviewer. The final result was obtained by averaging the individual observer results. Representative sections were photographed using an Olympus digital camera at × 200 magnification.
Enzyme linked immunosorbant assay (ELISA)
Two specimens, 1 cm each, including tumors (except for the control group) were excised under dissecting microscope and were used immediately for protein extraction. The concentrations of total proteins extracted from the colon tissue homogenates were measured using the BioSpec-nano (Shimadzu Corporation, Japan) at 280 OD. All protein samples were diluted using normal sterile saline to make a final concentration of 500 μg/ml of total protein. Concentrations of TGF-β1, HSP-90α proteins and COX-2 enzyme in the tissue homogenates were measured using commercial ELISA kits: HSP-90 and COX-2 kit (Cusabio, Hubei, China) and TGF-β1 kit (R&D systems, Minneapolis, USA. All samples were processed in duplicate on a fully automated ELISA system (Human Diagnostics, Germany) according to the manufacturers’ instructions and as previously described [36].
Quantitative RT-PCR
Total RNA was isolated from the stored colonic specimens in RNALater following homogenization of the specimens and by using the Purelink RNA mini kit from Life Technologies (Thermo Fisher Scientific, CA, USA) according to the manufacturer’s instructions. RNA was treated with RNAse-free DNAse during the extraction protocol to avoid the collection of genomic DNA and the concentrations and quality of the extracted total RNA were measured using the BioSpec-nano (Shimadzu Corporation, Japan), and its quality and integrity were concluded through the A260/A280 ratio. cDNA synthesis was conducted using a high capacity RNA-to-cDNA Reverse Transcription Kit from Applied Biosystems, (Thermo Fisher Scientific, Warrington, UK) following the manufacturer’s protocol. Following cDNA synthesis, quantitative RT-PCR was used to quantify Wnt (NM_001105714.1), β-Catenin (AF397179.1), DKK-1 (NM_001106350.1), CDKN1-A (NM_080782.3), COX-2 (AF233596.1) and NF-κB (NM_001008349.1) genes with primer sets listed in Table 1 using a step one Real Time PCR system (Applied Biosystems, USA) in triplicate wells. Power SYBR green master mix from Applied Biosystems, (Thermo Fisher Scientific, Warrington, UK) was used for the reactions and the results were normalized against the Ct values of β-actin (NM_031144.3) and expressed as fold-change compared with the normal control group as previously described [36].
Table 1.
The sequences of PCR primers used for the detection of β-actin, Wnt, β-catenin, DKK-1, CDNK-1A, COX-2 and NF-κB including the corresponding genes accession numbers
| Forwards | Reverse | |
|---|---|---|
| β-actin (NM_031144.3) | 5’ CGG TCA GGT CAT CAC TAT CG 3’ | 5’ TTC CAT ACC CAG GAA GGA AG 3’ |
| Wnt (NM_001105714.1) | 5’ AGC TGG GTT TCT GCT ACG TT 3’ | 5’ AAT CTG TCA GCA GGT TCG TG 3’ |
| β-Catenin (AF397179.1) | 5’ TTC CTG AGC TGA CCA AAC TG 3’ | 5’ GCA CTA TGG CAG ACA CCA TC 3’ |
| DKK-1 (NM_001106350.1) | 5’ ATT CCA GCG CTG TTA CTG TG 3’ | 5’ GAA TTG CTG GTT TGA TGG TG 3’ |
| CDNK-1A (NM_080782.3) | 5’ AGA AGG GAA CGG GTA CAC AG 3’ | 5’ ACC CAT AAG AAG GGC AGT TG 3’ |
| COX-2 (AF233596.1) | 5’ AAT CGC TGT ACA AGC AGT GG 3’ | 5’ GCA GCC ATT TCT TTC TCT CC 3’ |
| NF-κB (NM_001008349.1) | 5’ CAG AGC TGG CAG AGA GAC TG 3’ | 5’ TAC GAA GGA GAC TGC CAC TG 3’ |
Statistical analysis
Results were expressed as mean ± standard deviation (SD). Normality and homogeneity of data were assessed with the Kolmogorov-Smirnov test and Levene test, respectively. Comparisons of data between groups were made using one-way ANOVA, with post-hoc comparisons using Dunnett’s multiple comparison test. The difference between data were considered statistically significant when P < 0.05, and highly significant when P < 0.01. Statistical analysis was performed with SPSS 15.0 for Windows (SPSS Inc., Chicago, USA).
Results
Effects of mono- and combined therapy of Vit.D and TQ on colon tumor growth and large aberrant crypts foci (ACF) formation
Although morbidity-based humane endpoint protocol was adopted during the experiment, no cases of severe illness/moribundity were recorded throughout the study. Gross examination of tumorous growth on mucosal surface by naked eye and dissecting microscope revealed significant decrease in gross tumor count in Vit.D, TQ and Vit.D/TQ treated groups as compared to AOM group. Interestingly, Vit.D/TQ combined treatment group showed the lowest count of tumors compared to other individually treated group (Table 2). Moreover, the number of tumors/colon surface area ratio (NT/CS) was also significantly lower in Vit.D/TQ combined treatment group as compared to both AOM group and other individually treated groups (Table 2).
Table 2.
Mean ± SD of colon surface area (length X width in cm), count of colonic tumours by gross and dissecting microscope, number of tumour/colon surface area ratio (NT/CS) and number of large apparent crypts (ACF) in the different study groups
| Animal groups | Colon surface area (cm2) | Tumour count | NT/SC Ratio | Large ACF (≥ 4 crypts/focus) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Gross | Dissecting Microscope | Total | ||||
| Control | 19.1 ± 2.2 | N/A | N/A | N/A | N/A | 0 |
| AOM | 18.89 ± 3.43 | 12.5 ± 3.21 | 17.36 ± 4.6 | 29.16 ± 2.92 | 1.5 ± 0.14 | 41.3 ± 8.0 |
| Vit.D | 21.5 ± 2.29 | 8.3 ± 2.7a | 10.2 ± 3.8a | 17.5 ± 3.7a | 0.83 ± 0.14 | 22.1 ± 6.2a |
| TQ | 20.7 ± 2.72 | 9.1 ± 2.5a | 8.5 ± 2.3a | 17.6 ± 2.2a | 0.9 ± 0.1 | 21.7 ± 7.1a |
| Vit.D/TQ | 21.1 ± 2.8 | 6.9 ± 1.5a,c | 5.9 ± 2.1b,c,d | 12.2 ± 3.9b,c,d | 0.5 ± 0.2a,b,c | 10.3 ± 5.4b,c,d |
P < 0.05 compared with AOM group;
P < 0.05 compared with Vit.D group;
P < 0.05 compared with TQ group;
P < 0.01 compared with AOM group.
Examination by dissecting microscope following methylene blue staining showed several micro-tumors and significant alteration of the mucosal surface of the AOM group compared to normal mucosal and crypt appearance in the control group (Table 2 and Figure 1A and 1C). While Vit.D and TQ monotherapy significantly decreased the numbers of tumors as compared to AOM group, distortion of colonic mucosal architecture was still evident (Figure 1E and 1G). However, combined Vit.D/TQ treatment significantly decreased the number of tumors and preserved/restored the mucosal architecture compared with AOM and monotherapy groups (Table 2 and Figure 1I). On the other hand, histopathological examination of colon mucosa from AOM-induced colon cancer group revealed multiple tubular adenomas and many large ACF (> 4 crypt/focus) with hyperplastic and dysplastic glandular epithelium (Figure 1D). Monotherapy with either Vit.D or TQ showed a significant reduction in the numbers of large ACF and the thickness of the colon mucosa compared with AOM group. However, hyperplasia and low-grade dysplasia of the glandular epithelial cells were still evident despite the observed decrease in the quantities of large ACF (Figure 1F and 1H). Combined Vit.D/TQ treatment showed the lowest number of large ACF compared with AOM and monotherapy groups (Table 2). Furthermore, glandular dysplasia was less frequently observed and it was characterized of being of low grade in the groups treated with Vit.D/TQ compared with the other groups (Figure 1J). Nevertheless, Vit.D/TQ combined treatment did not prevent/restore glandular epithelial hyperplasia.
Figure 1.
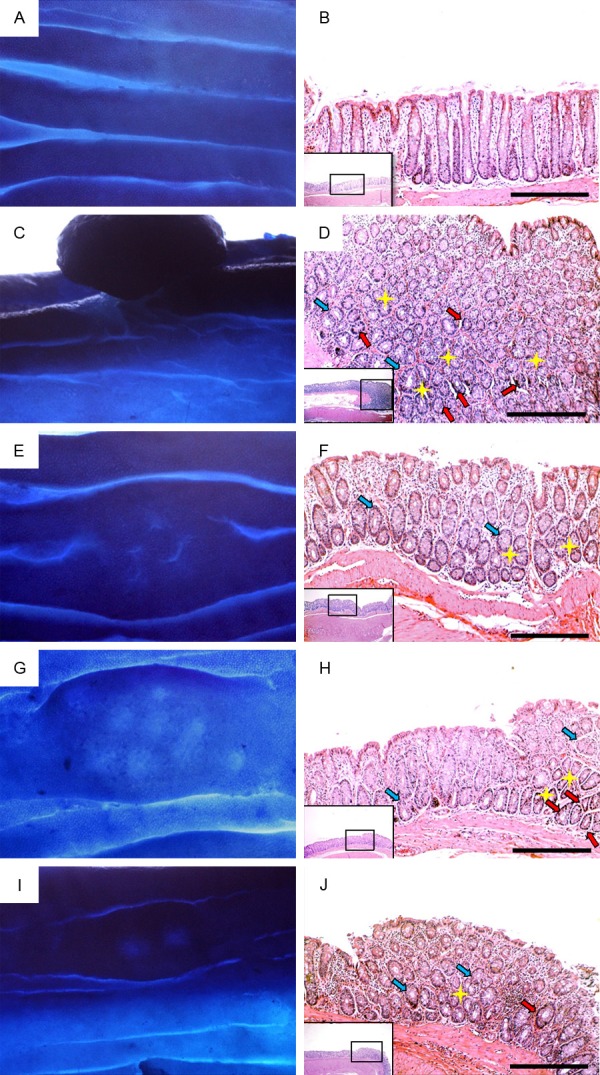
Appearance of colon mucosa by dissecting microscopyfollowing staining with 0.2% methylene blue (left column) and by light microscopy at magnifications X100 and X200 following staining with H&E (right column)in control group (A and B), AOM group (C and D), VitD group (E and F), TQ group (G and H) and VitD/TQ group (I and J). (Yellow star = large ACF [> 4 crypts/focus]; light blue arrow = hyperplasia and red arrow = dysplasia).
Effects of mono- and combined therapy of Vit.D and TQ on target genes and molecules associated with colon carcinogenesis.
Expression of selected genes by quantitative RT-PCR
Investigation of gene expression of Wnt, β-catenin, NF-κB, COX-2, DKK-1 and CDNK-1A were conducted based on their relative mRNA expression patterns by Q-RT-PCR. Current findings revealed a significant up-regulation of Wnt (Figure 2A), β-catenin (Figure 2B), COX-2 (Figure 2E) and NF-κB genes (Figure 2F), and a significant down regulation of the expression of DKK-1 (Figure 2C) and CDKN1-A (Figure 2D) in AOM group as compared to normal control group. On the other hand, monotherapy with either Vit.D or TQ significantly down regulated the expression of canonical Wnt, β-catenin, NF-κB and COX-2 genes, while up regulated the expression of the DKK-1 and CDKN1-A genes as compared to the untreated AOM group. Interestingly, these corrective alterations of selected genes expression were significantly enhanced by the Vit.D/TQ combined therapy (Figure 2).
Figure 2.

Relative messenger RNA expression of (A) Wnt; (B) β-catenin; (C) DKK-1; (D) CDKN1A; (E) COX-2 and (F) NF-kB. a = P < 0.05 compared with control group; b = P < 0.05 compared with AOM group; c = P < 0.05 compared with Vit.D group; d = P < 0.05 compared with TQ group; e = P < 0.01 compared with control group and f = P < 0.01 compared with AOM group.
ELISA findings of selected proteins in affected colon tissues
Quantitative measurement of TGF-β1, COX-2, HSP-90 and VEGF protein concentrations by ELISA in the colon tissue homogenates of the different groups revealed significant reduction in the concentrations of TGF-β1 (Figure 3A) and significant elevation of HSP-90α (Figure 3B), COX-2 (Figure 3C), and VEGF (Figure 3D) in the colon tissue homogenates of AOM group as compared to control group. On the other hand, the concentrations of these selected molecules were significantly reduced in affected groups treated with either Vit.D or TQ monotherapy. However, it is worth to be mentioned that combined Vit.D/TQ therapy induced even more reduction in the level of these proteins in the colon tissues of affected groups as compared to both AOM untreated group and monotherapy treated group (Vit.D group and TQ group) as well (Figure 3).
Figure 3.
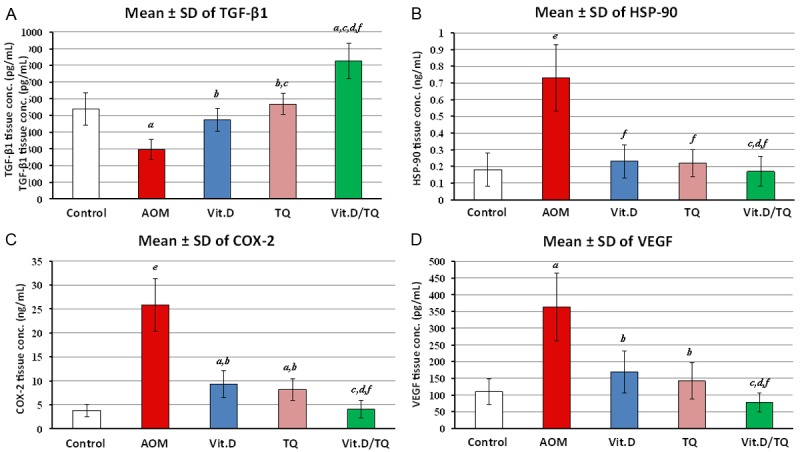
Mean ± SD of ELISA findings of tissue homogenate concentrations of (A) TGF-β1, (B) HSP-90, (C) COX-2 and (D) VEGF molecules in the different study groups. a = P < 0.05 compared with control group; b = P < 0.05 compared with AOM group; c = P < 0.05 compared with VitD group; d = P < 0.05 compared with TQ group; e = P < 0.01 compared with control group and f = P < 0.01 compared with AOM group.
Immunohistochemistry findings of selected proteins in affected colon tissues
In correlation with ELISA findings, immunohistochemical study revealed similar pattern of TGF-β1 expression along with its TGF-β/RII (Figure 4A-J, respectively) as well as the HSP-90 molecule (Figure 5K-O) in the colon tissues of investigated animal groups. Immunohistochemical study also revealed compatible expression patterns of β-catenin protein in correlation with gene expression findings (Figure 5A-E). In addition, immunohistochemical investigations demonstrated significant low-expression of smad4 and over expression of iNOS molecules in the colon tissues, particularly in the glandular epithelium, of AOM groupas compared to the control group (Figure 4K, 4L) and (Figure 5F, 5G), respectively. Interestingly, both mono and combined therapy with Vit.D and or TQ significantly countered the expression pattern of these 2 molecules in the affected tissues. However, combined Vit.D/TQ therapy demonstrated more significant counter effect (Figures 4L-O and 5G-J), respectively. Immunohistochemical scores of all investigation molecules are shown in Table 3.
Figure 4.
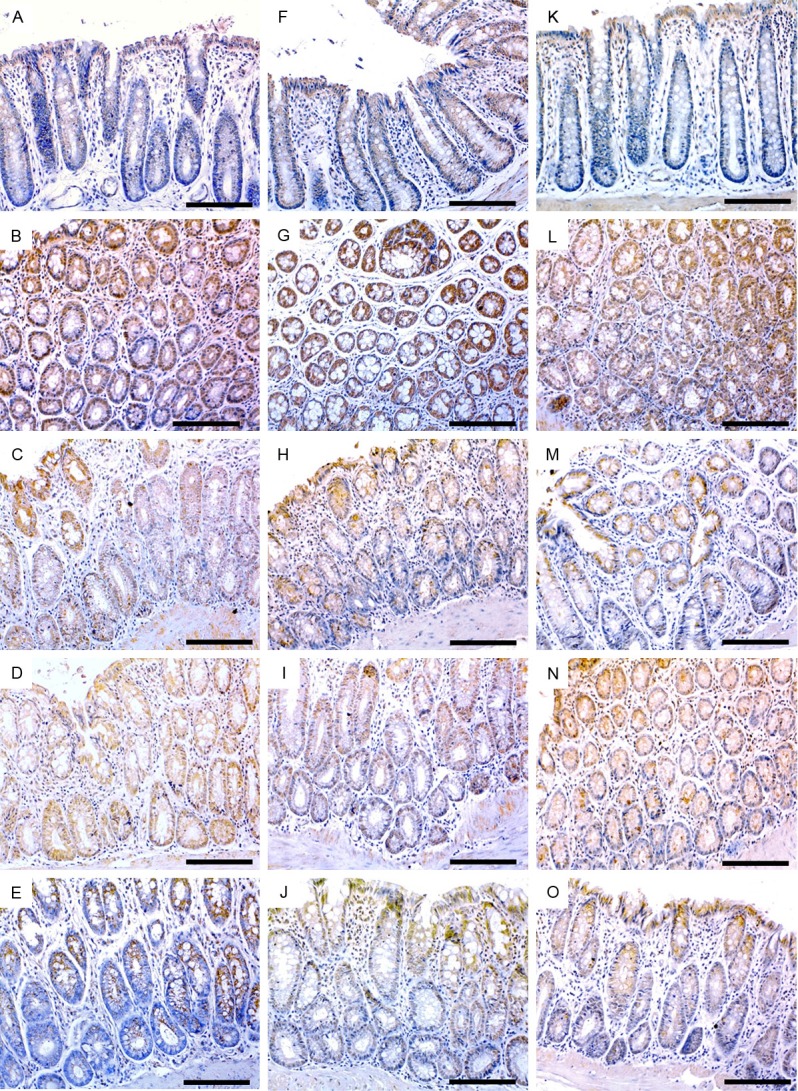
Immunohistochemistry localization of TGF-β1 (left column), TGF-β/RII (middle column) and smad4 (right column) in control (A, F, K), AOM (B, G, L), vitamin D (C, H, M), TQ (D, I, N) and D/TQ (E, J, O) groups. (×200 magnification, scale bar = 8 µm).
Figure 5.
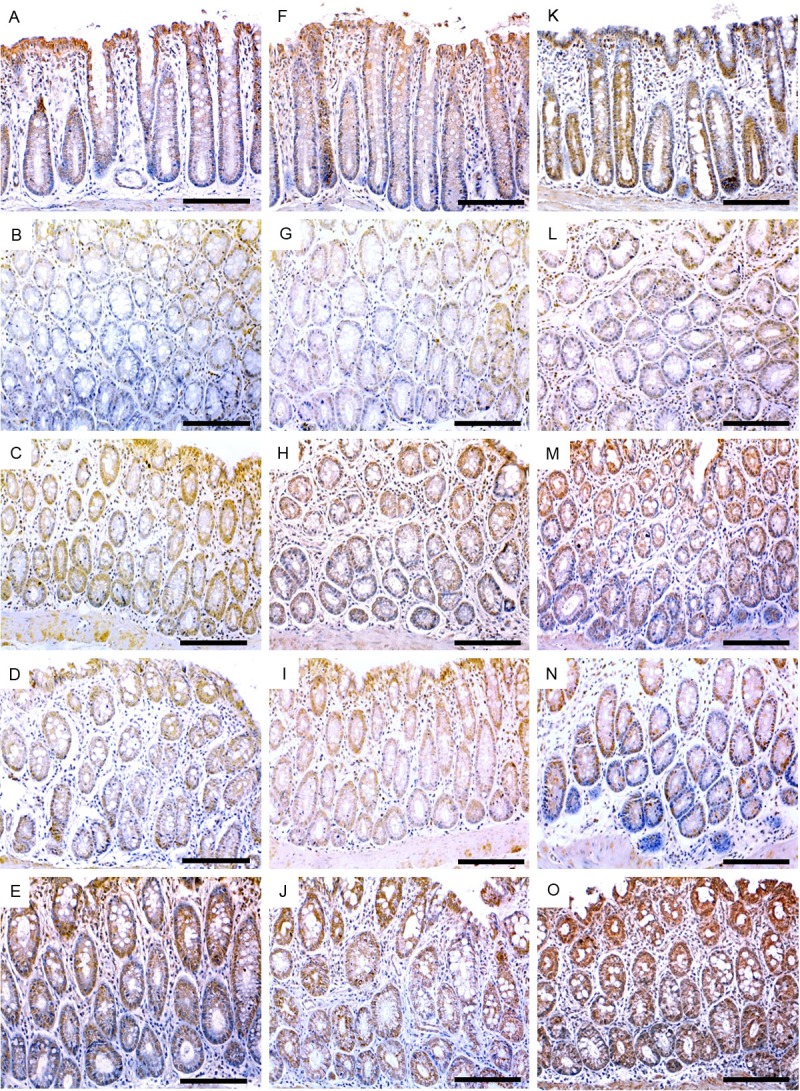
Immunohistochemistry localization of β-catenin (left column), iNOS (middle column) and HSP-90 (right column) in control (A, F, K), AOM (B, G, L), vitamin D (C, H, M), TQ (D, I, N) and D/TQ (E, J, O) groups. (×200 magnification, scale bar = 8 µm).
Table 3.
Mean ± SD of immunohistochemistry scores for TGF-β1, TGF-β/RII, smad 4, β-catenin, iNOS and HSP-90 proteins in colon specimens
| Normal group | AOM group | D group | TQ group | D/TQ group | |
|---|---|---|---|---|---|
| TGF-β1 | 315.5 ± 31.2 | 136.4 ± 23.4a | 323.9 ± 38.5b | 345.3 ± 37.2b | 381.7 ± 24.1a,c,f |
| TGF-β/RII | 333.7 ± 28.7 | 121.4 ± 19.2a | 289.3 ± 31.9a,b | 211.6 ± 33.2a,b,c | 378.4 ± 36.8c,d,f |
| Smad 4 | 301.1 ± 26 | 147.2 ± 20.3e | 258.7 ± 31.6a,b | 248.4 ± 27.7a,b | 362.7 ± 37.1a,c,d,f |
| β-catenin | 61 ± 16.7 | 370.6 ± 28.7e | 259.6 ± 30.1b,e | 251.6 ± 35.1b,e | 92.7 ± 31.4c,d,f |
| iNOS | 39.4 ± 9.5 | 373.1 ± 26.8e | 188.1 ± 33.6a,b | 117.5 ± 41.1a,b,c | 41.3 ± 13.1c,d,f |
| HSP-90 | 112.7 ± 27.6 | 376.5 ± 22.6e | 174.7 ± 28.6a,b | 238.7 ± 34.5a,b,c | 89.4 ± 27.6a,c,d,f |
P < 0.05 compared with control group;
P < 0.05 compared with AOM group;
P < 0.05 compared with VitD group;
P < 0.05 compared with TQ group;
P < 0.01 compared with control group;
P < 0.01 compared with AOM group.
Discussion
Emerging data of cancer prevention have provided growing evidences of the anticancer activities of both Vit.D and TQ not only in vitro but also in vivo [26,30,43,44]. In the present study, the synergistic effect of TQ on the preventive anticancer activity of Vit.D in comparison to their monotherapy were investigated during early stages of colon carcinogenesis in rat model. To the best of our knowledge, this report is the first to elaborate on the potential synergistic preventive/therapeutic anti-cancer effect of Vit.D/TQ combination therapy in AOM-induced rat model of colon cancer.
In agreement with previous studies [36-39], biochemical analyses of serum levels of calcium as well as liver function enzymes and renal function parameters revealed non-significant alteration in all treated groups with cholecalciferol (Vit.D) and/or TQ as compared to non-treated groups (data not shown). These findings confirm the non-calcemic property of the applied cholecalciferol dosage regimen and also preclude any hepatic/renal toxicity of mono and combined therapy with vit.D and/or TQ.
Gross and histopathological study of colon tissues from all investigated groups revealed apparent anti-cancer effect of both Vit.D and TQ manifested by significant reduction of gross tumor counts, NT/CS and quantities of large ACF formation in monotherapy groups as compared to the AOM untreated group. Interestingly, the recorded anticancer effects were significantly enhanced at using Vit.D/TQ combination therapy with less frequent, low-grade cellular dysplasia in the glandular epithelium of treated group as compared to both AOM and monotherapy groups. Our results correlate with previous studies, which have reported significant reduction in the number of colon tumors following treatment with Vit.D. or its analogues in rodent models of cancer [43-45]. Therapeutic potential of TQ against colon cancer, manifested by reduction in the numbers and sizes of ACF in DMH-induced colon cancer model, was also reported [46].
To provide mechanistic insights into the observed anti-cancer effects of both mono and combined therapy with Vit.D and/or TQ in the early stages of colon cancer, gene expression pattern of selected pro-oncogenes (canonical β-catenin pathway of Wnt, NF-κB, and COX-2) as well as some of the well-established colon cancer suppressive genes (DKK-1 and CDNK-1A) were investigated in colon tissues using Q-RT-PCR. Moreover, protein levels of selected carcinogenesis biomarkers (β-catenin, TGF-β, TGF-β/RII, Smad4, HSP-90, VEGF, iNOS and COX2) were also evaluated in the colon tissues of investigated rats using ELISA and/or immunohistochemistry.
Multiple molecular pathways underlay the pathological alterations associated with colon cancer tumorigenesis. Abnormal proliferation and un-differentiation of epithelial cells is one of the main features of any carcinogenesis including colon cancer. Activation of the Wnt/β-catenin pathway is one of the major known pathways associated with cell differentiation and proliferation in colon cancer and other cancers. It has been shown that abnormal expression of Wnt molecule during early stages of tumorigenesis results in the stabilization of β-catenin with eventual translocation into the nucleus to act as a transcriptional co-activator of transcription factors belonging to the TCF/LEF family with subsequent development and progression of cancer [6]. In consistency, significant up-regulation of Wnt and β-catenin expression were observed in the current study among AOM-induced colon cancer group as compared to normal control group. Remarkably, corrective alterations manifested by significant down regulation of these genes were recorded in both Vit.D and TQ monotherapy groups. Vitamin D has been shown to inhibit the expression of Wnt, β-catenin and their target genes in normal colon cells, an action that was attributed to the modulation of the expression of Vit.D receptor in target tissues [47,48]. With regard to TQ, its interference with tumor growth and progression was recently recorded in ApcMin mice colon cancer model. It has been shown that TQ exert its inhibition effect on tumor growth by modulating Wnt signaling pathway through activation of GSK-3β [49]. Noteworthy, down regulation of the expression of these genes were even more significant in Vit.D/TQ combined therapy as compared to Vit.D or TQ monotherapy.
On the other hand, DKK-1 is a known inhibitor of the Wnt/β-catenin pathway and its low expression is usually associated with poor clinical outcome and is considered a therapeutic resistance sign [5]. In agreement, current study revealed significant down regulation of DKK-1 in AOM group as compared to control group. It was reported that Dkk-1 inhibits Wnt/β-catenin pathway not only by blocking Wnt signaling receptor complexes but it also has additional β-catenin-independent tumor-suppressor effect [50,51]. Moreover, previous studies have reported the association between the differentiation colon cancer cells and the Vit.D-induced DKK-1 gene expression [52,53]. In the light of this, the significant increase in DKK-1 expression, as revealed in the current study among Vit.D and TQ treated groups with higher significant elevation in combined therapy as compared to monotherapy, affirm the anti-proliferative effect of Vit.D and TQ especially when used in combination.
Another major pathway that promotes cell proliferation is the resistance of affected cells to TGF-β, a known growth inhibitor of normal epithelial cells. In early stages of carcinogenesis, reduction of TGF-β expression and its type-2 receptor and mutation of its intracellular mediators (smad2 and smad4) were reported as possible mechanisms of resistance in both human patients and rodent model of colon cancer [52,53]. In the current study, contrary to its significantly low level in AOM untreated group, significant elevations of TGF-β were recorded in colon tissue homogenate, particularly in the glandular epithelium in monotherapy groups. In addition, immunohistochemical investigations demonstrated significant high-expression of TGF-β, TGF-β/RII and the intracellular mediator smad4 in the colon tissues of the same groups. In agreement with our findings, several previous studies have shown that Vit.D promote the expression of TGF-β and its signaling molecules via increasing the expression of vitamin D receptor (VDR) [54,55]. In addition, a study in human colonic epithelial cells has reported the up-regulation of TGF-β/RII expression after treatment with vitamin D, which was attributed to the increasing the levels of calcium sensing receptor (CaSR) [56]. Recently, we have recorded positive influence of Vit.D3 treatment either alone or in combination with 5-Fluorouracil on the expression of TGF-β and its related type II receptor and intracellular mediator smad4 [36]. With regard to TQ, scarce reports on its in vivo influence on TGF-β in colon cancer are available. However, TGF-β restoring ability of TQ was recently demonstrated in radiation-driven migration and invasion associating chemotadiotherapy of breast cancer [57]. Remarkably, the up regulation of these growth inhibition biomarkers were significantly higher in Vit.D/TQ combined therapy group as compared to monotherapy groups which suggest a synergistic negative influence of the two drugs on tumor growth and progression.
Heat Shock protein-90, a known chaperon protein that regulates protein folding and trafficking in normal cells, is another molecule involved in the pathogenic mechanisms of colon cancer. Blocking of HSP-90 activities has been shown to delay the progression of colon cancer both in vitro and in vivo by several mechanisms [58-60]. In accordance, current study revealed significant increase of HSP-90 in colon tissues of AOM untreated group as compared to control group. However, this effect was reversed in Vit.D and TQ treated groups. Likewise, more significant reduction of HSP-90 concentration was evident with combined therapy rather than monotherapy.
Angiogenesis is a crucial step for development and progression of solid tumors. VEGF is a very well known angiogenic factor that plays an essential role in tumor neovascularization [61]. Moreover, iNos and COX-2 are among the pro-angiogenic molecules that promote tumor angiogenesis. These molecules have been shown to play an important role in the progression of colon cancer in human and rat model [62,63]. Current study, and in harmony with this, revealed significant reduction of VEGF concentration in colon tissue homogenates and significant low expression of COX2 molecule at both gene expression and protein concentration levels as well as significant reduction of iNOS expression in the colon tissues, particularly in the glandular epithelium, of monotherapy groups as compared to AOM group. Little information is available on the direct influence of vitamin D on the expression of the pro-angiogenic molecules, VEGF, iNOS and COX-2. However, our previous finding [36], in addition to the current ones revealed significant down regulation of these molecules, which support the hypothesis that Vit.D inhibit the processes of angiogenesis and hence the progression of the colon cancer by inhibiting the AOM-induced up-regulation of these molecules in affected tissues. In other study, VSL#3 (a probiotic that increases VDR expression in colon cancer cells) were found to increase the expression of angiostatin, a known anti-angiogenic factor, in the colon tissue of colitis-associated cancer [64]. With regard to TQ, a recent study recorded its suppression of neoplastic progression in DMH-induced colon cancer, which was attributed to the down-regulation of VEGF expression in affected tissues [27]. Interestingly, Vit.D/TQ combined therapy induced even more significant reduction of the level of these pro-angiogenic proteins in the colon tissues of affected groups as compared to both AOM untreated group and monotherapy treated groups as well.
Inhibition of apoptosis is another mechanism of tumorigenesis. In this regard, NF-kB is an important molecule that have anti-apoptotic role during cell cycle. NF-kB mediates the transcription of downstream targets that belong to anti-apoptotic genes [59]. In consistency, our results revealed over expression of the gene coding for NF-kB molecule in the colon tissue homogenate of AOM untreated group as compared to control group. However, the pattern of expression was reversed in Vit.D and TQ treated groups with the maximum increase being recorded in Vit.D/TQ combined therapy group. On the other hand, CDKN1-A, also known as p21, is another molecule that has a known pro-apoptotic role during cell cycle. It is a potent cyclin-dependent kinase (CDK) inhibitor that act as regulator of cell cycle progression at G1 via binding and inhibiting the activity of cyclin-cyclin-dependent kinase2 or -cyclin-dependent kinase4 complexes [65,66]. The expression of the gene coding for this protein is tightly controlled by the tumor suppressor protein p53, through which this protein mediates the p53-dependent cell cycle G1 phase arrest in response to a variety of stress stimuli [67,68]. In agreement with previous studies that demonstrated down regulation of p21 coding gene in colon cancer and other inflammatory diseases [69,70], current study revealed significant low expression of CDKN1-A gene in colon tissue homogenate of AOM-induced CRD group as compared to control group. However, Vit.D and TQ treatment were associated with variable degrees of significant over expression of CDKN1-A gene, an effect that is greatly enhanced using combined Vit.D/TQ therapy. These findings are in agreement with the recently demonstrated pro-apoptotic effect of both Vit.D and TQ, which reported to be attained by up-regulation of cell cycle inhibitors including P21, P27, CDKs and P53 with subsequent induction of G1 cell-cycle arrest [71-73]. These reports, in addition to the currently revealed augmented apoptotic effect of Vit.D/TQ combined therapy as compared to monotherapy clearly emphasizing the potential synergistic effect of Vit.D/TQ combination in combating the development of colon cancer.
In conclusion, tumor growth-associated cellular proliferation and un-differentiation, as assessed in the current study by expression of Wnt, β-catenin and DKK-1 molecules as well as other tumor growth factors namely TGF-β along with its type 2 receptor and intracellular mediator (Smad4), were shown to be significantly diminished in Vit.D/TQ treated groups of AOM-induced colon cancer rats. Moreover, evaluation of the expression of VEGF, iNOS and COX2 molecules as known biomarkers of angiogenesis as well as NF-kB and CDKN1-A as known anti- and pro-apoptotic molecules, respectively, revealed an obvious anti-angiogenic and pro-apoptotic influence of Vit.D and TQ. Conceivably, the current study revealed synergistic and/or potentiating anticancer effects of Vit.D and TQ when used in combination. It is plausible to propose that Vit.D/TQ combination has a pronounced chemopreventive effect in the initiation phase of colon cancer, with the potential to diminish tumor burden, suppress progression of pre-neoplastic lesions in colon carcinogenesis. Hence, dietary co-supplementation with Vit.D plus TQ could provide a promising approach in the primary prevention of colon cancer in high-risk individuals such as those with suppurative colitis, uncontrolled irritable bowel diseases or those with family history or germ line APC mutation. Moreover, in addition to the preventive/protective potentials of the proposed combination, enhancement of the therapeutic effect of currently used chemotherapeutic agents is another potential approach that need to be investigated in future studies not only for the possible improving of therapeutic outcome, but also for reducing the toxicity of conventional chemotherapy via reducing the dose and time required for treatment. In deed this could have represented a promising horizon to combat and manage colon cancer in human. Nevertheless, further investigations are required to reveal the underlying mechanism of the conspicuously demonstrated synergistic effect of TQ, when used in combination with Vit.D, at the molecular level using colon cancer cell lines and to evaluate the pharmacokinetics of using this combination with other conventional chemotherapy to reach more effective, yet saver treatment of colon cancer in human.
Acknowledgements
Supported by the National Science, Technology and Innovation (NSTI) Plan-King Abdul Aziz City for Science and Technology (KACST/MARRIFAH) grant: 12-MED2965-10, Kingdom of Saudi Arabia.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Fleshman JW, Smallwood N. Current concepts in rectal cancer. Clin Colon Rectal Surg. 2015;28:5–11. doi: 10.1055/s-0035-1545064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JH. Chemotherapy for colorectal cancer in the elderly. World J Gastroenterol. 2015;21:5158–5166. doi: 10.3748/wjg.v21.i17.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HJ, Yu MH, Kim H, Byun J, Lee C. Noninvasive molecular biomarkers for the detection of colorectal cancer. BMB Rep. 2008;41:685–692. doi: 10.5483/bmbrep.2008.41.10.685. [DOI] [PubMed] [Google Scholar]
- 5.Pendas-Franco N, Aguilera O, Pereira F, Gonzalez-Sancho JM, Munoz A. Vitamin D and Wnt/beta-catenin pathway in colon cancer: role and regulation of DICKKOPF genes. Anticancer Res. 2008;28:2613–2623. [PubMed] [Google Scholar]
- 6.Bialkowska AB, Yang VW. High-throughput screening strategies for targeted identification of therapeutic compounds in colorectal cancer. Future Oncol. 2012;8:259–272. doi: 10.2217/fon.12.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29:2727–2737. [PubMed] [Google Scholar]
- 8.Vainio H, Miller AB. Primary and secondary prevention in colorectal cancer. Acta Oncol. 2003;42:809–815. doi: 10.1080/02841860310010673. [DOI] [PubMed] [Google Scholar]
- 9.Lippman SM, Benner SE, Hong WK. Chemoprevention. Strategies for the control of cancer. Cancer. 1993;72(Suppl):984–990. doi: 10.1002/1097-0142(19930801)72:3+<984::aid-cncr2820721306>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res. 2008;52:507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- 11.Nichenametla SN, Taruscio TG, Barney DL, Exon JH. A review of the effects and mechanisms of polyphenolics in cancer. Crit Rev Food Sci Nutr. 2006;46:161–183. doi: 10.1080/10408390591000541. [DOI] [PubMed] [Google Scholar]
- 12.Lambert JD, Hong J, Yang GY, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005;81(Suppl):284S–291S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- 13.Sun SY, Hail N Jr, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Natl Cancer Inst. 2004;96:662–672. doi: 10.1093/jnci/djh123. [DOI] [PubMed] [Google Scholar]
- 14.Guraya SY. Chemopreventive role of vitamin D in colorectal carcinoma. Journal of Microscopy and Ultrastructure. 2014;2:1–6. [Google Scholar]
- 15.Giovannucci EL, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of Vitamin D statusand cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 16.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, Newmark HL, Giovannucci E, Wei M, Holick MF. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32:210–216. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3:601–614. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 18.Chen A, Davis BH, Sitrin MD, Brasitus TA, Bissonnette M. Transforming growth factor-beta 1 signaling contributes to Caco-2 cell growth inhibition induced by 1,25(OH)(2)D(3) Am J Physiol Gastrointest Liver Physiol. 2002;283:G864–874. doi: 10.1152/ajpgi.00524.2001. [DOI] [PubMed] [Google Scholar]
- 19.Diaz GD, Paraskeva C, Thomas MG, Binderup L, Hague A. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer Res. 2000;60:2304–2312. [PubMed] [Google Scholar]
- 20.Chung I, Han G, Seshadri M, Gillard BM, Yu WD, Foster BA, Trump DL, Johnson CS. Role of vitamin D receptor in the antiproliferative effects of calcitriol in tumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res. 2009;69:967–975. doi: 10.1158/0008-5472.CAN-08-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan MH, Lai CS, Wu JC, Ho CT. Molecular mechanisms for chemoprevention of colorectal cancer by natural dietary compounds. Mol Nutr Food Res. 2011;55:32–45. doi: 10.1002/mnfr.201000412. [DOI] [PubMed] [Google Scholar]
- 22.Ko JK, Auyeung KK. Target-oriented mechanisms of novel herbal therapeutics in the chemotherapy of gastrointestinal cancer and inflammation. Curr Pharm Des. 2013;19:48–66. doi: 10.2174/13816128130109. [DOI] [PubMed] [Google Scholar]
- 23.Randhawa MA, Alghamdi MS. Anticancer activity of Nigella sativa (black seed)-a review. Am J Chin Med. 2011;39:1075–1091. doi: 10.1142/S0192415X1100941X. [DOI] [PubMed] [Google Scholar]
- 24.Gali-Muhtasib H, Diab-Assaf M, Boltze C, Al-Hmaira J, Hartig R, Roessner A, Schneider-Stock R. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int J Oncol. 2004;25:857–866. [PubMed] [Google Scholar]
- 25.Roepke M, Diestel A, Bajbouj K, Walluscheck D, Schonfeld P, Roessner A, Schneider-Stock R, Gali-Muhtasib H. Lack of p53 augments thymoquinone-induced apoptosis and caspase activation in human osteosarcoma cells. Cancer Biol Ther. 2007;6:160–169. doi: 10.4161/cbt.6.2.3575. [DOI] [PubMed] [Google Scholar]
- 26.Kaseb AO, Chinnakannu K, Chen D, Sivanandam A, Tejwani S, Menon M, Dou QP, Reddy GP. Androgen receptor and E2F-1 targeted thymoquinone therapy for hormone-refractory prostate cancer. Cancer Res. 2007;67:7782–7788. doi: 10.1158/0008-5472.CAN-07-1483. [DOI] [PubMed] [Google Scholar]
- 27.Asfour W. ASaHL. Thymoquinone suppresses cellular proliferation, inhibits VEGF production and obstructs tumor progression and invasion in the rat model of DMH-induced colon carcinogenesis. Pharmacology & Pharmacy. 2013;4:7–17. [Google Scholar]
- 28.Banerjee S, Padhye S, Azmi A, Wang Z, Philip PA, Kucuk O, Sarkar FH, Mohammad RM. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr Cancer. 2010;62:938–946. doi: 10.1080/01635581.2010.509832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kensara OA, El-Shemi AG, Mohamed AM, Refaat B, Idris S, Ahmad J. Thymoquinone subdues tumor growth and potentiates the chemopreventive effect of 5-fluorouracil on the early stages of colorectal carcinogenesis in rats. Drug Des Devel Ther. 2016;10:2239–2253. doi: 10.2147/DDDT.S109721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi T, Cho SG, Yi Z, Pang X, Rodriguez M, Wang Y, Sethi G, Aggarwal BB, Liu M. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol Cancer Ther. 2008;7:1789–1796. doi: 10.1158/1535-7163.MCT-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woo CC, Kumar AP, Sethi G, Tan KH. Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem Pharmacol. 2012;83:443–451. doi: 10.1016/j.bcp.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 32.El-Najjar N, Chatila M, Moukadem H, Vuorela H, Ocker M, Gandesiri M, Schneider-Stock R, Gali-Muhtasib H. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis. 2010;15:183–195. doi: 10.1007/s10495-009-0421-z. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Huang XF. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biol Ther. 2009;8:1313–1317. doi: 10.4161/cbt.8.14.8983. [DOI] [PubMed] [Google Scholar]
- 34.Washington MK, Powell AE, Sullivan R, Sundberg JP, Wright N, Coffey RJ, Dove WF. Pathology of rodent models of intestinal cancer: progress report and recommendations. Gastroenterology. 2013;144:705–717. doi: 10.1053/j.gastro.2013.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hajrezaie M, Hassandarvish P, Moghadamtousi SZ, Gwaram NS, Golbabapour S, Najihussien A, Almagrami AA, Zahedifard M, Rouhollahi E, Karimian H, Fani S, Kamalidehghan B, Majid NA, Ali HM, Abdulla MA. Chemopreventive evaluation of a Schiff base derived copper (II) complex against azoxymethane-induced colorectal cancer in rats. PLoS One. 2014;9:e91246. doi: 10.1371/journal.pone.0091246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Refaat B, El-Shemi AG, Kensara OA, Mohamed AM, Idris S, Ahmad J, Khojah A. Vitamin D3 enhances the tumouricidal effects of 5-Fluorouracil through multipathway mechanisms in azoxymethane rat model of colon cancer. J Exp Clin Cancer Res. 2015;34:71. doi: 10.1186/s13046-015-0187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salum E, Kampus P, Zilmer M, Eha J, Butlin M, Avolio AP, Podramagi T, Arend A, Aunapuu M, Kals J. Effect of vitamin D on aortic remodeling in streptozotocin-induced diabetes. Cardiovasc Diabetol. 2012;11:58. doi: 10.1186/1475-2840-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suddek GM. Protective role of thymoquinone against liver damage induced by tamoxifen in female rats. Can J Physiol Pharmacol. 2014;92:640–644. doi: 10.1139/cjpp-2014-0148. [DOI] [PubMed] [Google Scholar]
- 39.Abukhader MM. The effect of route of administration in thymoquinone toxicity in male and female rats. Indian J Pharm Sci. 2012;74:195–200. doi: 10.4103/0250-474X.106060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan F, Li G, Liu AB, Lee MJ, Yang Z, Chen YK, Lin Y, Shih W, Yang CS. delta- and gamma-tocopherols, but not alpha-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 rats. Cancer Prev Res (Phila) 2012;5:644–654. doi: 10.1158/1940-6207.CAPR-11-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008 doi: 10.1101/pdb.prot4986. pdb prot4986. [DOI] [PubMed] [Google Scholar]
- 42.Xiao H, Hao X, Simi B, Ju J, Jiang H, Reddy BS, Yang CS. Green tea polyphenols inhibit colorectal aberrant crypt foci (ACF) formation and prevent oncogenic changes in dysplastic ACF in azoxymethane-treated F344 rats. Carcinogenesis. 2008;29:113–119. doi: 10.1093/carcin/bgm204. [DOI] [PubMed] [Google Scholar]
- 43.Ordonez-Moran P, Larriba MJ, Pendas-Franco N, Aguilera O, Gonzalez-Sancho JM, Munoz A. Vitamin D and cancer: an update of in vitro and in vivo data. Front Biosci. 2005;10:2723–2749. doi: 10.2741/1731. [DOI] [PubMed] [Google Scholar]
- 44.Hummel DM, Thiem U, Hobaus J, Mesteri I, Gober L, Stremnitzer C, Graca J, Obermayer-Pietsch B, Kallay E. Prevention of preneoplastic lesions by dietary vitamin D in a mouse model of colorectal carcinogenesis. J Steroid Biochem Mol Biol. 2013;136:284–288. doi: 10.1016/j.jsbmb.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mokady E, Schwartz B, Shany S, Lamprecht SA. A protective role of dietary vitamin D3 in rat colon carcinogenesis. Nutr Cancer. 2000;38:65–73. doi: 10.1207/S15327914NC381_10. [DOI] [PubMed] [Google Scholar]
- 46.Gali-Muhtasib H, Ocker M, Kuester D, Krueger S, El-Hajj Z, Diestel A, Evert M, El-Najjar N, Peters B, Jurjus A, Roessner A, Schneider-Stock R. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J Cell Mol Med. 2008;12:330–342. doi: 10.1111/j.1582-4934.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larriba MJ, Ordonez-Moran P, Chicote I, Martin-Fernandez G, Puig I, Munoz A, Palmer HG. Vitamin D receptor deficiency enhances Wnt/beta-catenin signaling and tumor burden in colon cancer. PLoS One. 2011;6:e23524. doi: 10.1371/journal.pone.0023524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng W, Wong KE, Zhang Z, Dougherty U, Mustafi R, Kong J, Deb DK, Zheng H, Bissonnette M, Li YC. Inactivation of the vitamin D receptor in APC(min/+) mice reveals a critical role for the vitamin D receptor in intestinal tumor growth. Int J Cancer. 2012;130:10–19. doi: 10.1002/ijc.25992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lang M, Borgmann M, Oberhuber G, Evstatiev R, Jimenez K, Dammann KW, Jambrich M, Khare V, Campregher C, Ristl R, Gasche C. Thymoquinone attenuates tumor growth in ApcMin mice by interference with Wnt-signaling. Mol Cancer. 2013;12:41. doi: 10.1186/1476-4598-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Z, Sun B, Qi L, Li Y, Zhao X, Zhang D, Zhang Y. Dickkopf-1 expression is down-regulated during the colorectal adenoma-carcinoma sequence and correlates with reduced microvessel density and VEGF expression. Histopathology. 2015;67:158–166. doi: 10.1111/his.12474. [DOI] [PubMed] [Google Scholar]
- 51.Aguilera O, Gonzalez-Sancho JM, Zazo S, Rincon R, Fernandez AF, Tapia O, Canals F, Morte B, Calvanese V, Orgaz JL, Niell N, Aguilar S, Freije JM, Grana O, Pisano DG, Borrero A, Martinez-Useros J, Jimenez B, Fraga MF, Garcia-Foncillas J, Lopez-Otin C, Lafarga M, Rojo F, Munoz A. Nuclear DICKKOPF-1 as a biomarker of chemoresistance and poor clinical outcome in colorectal cancer. Oncotarget. 2015;6:5903–5917. doi: 10.18632/oncotarget.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skeen VR, Paterson I, Paraskeva C, Williams AC. TGF-beta1 signalling, connecting aberrant inflammation and colorectal tumorigenesis. Curr Pharm Des. 2012;18:3874–3888. doi: 10.2174/138161212802083734. [DOI] [PubMed] [Google Scholar]
- 53.Slattery ML, Herrick JS, Lundgreen A, Wolff RK. Genetic variation in the TGF-beta signaling pathway and colon and rectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20:57–69. doi: 10.1158/1055-9965.EPI-10-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fischer KD, Agrawal DK. Vitamin D regulating TGF-beta induced epithelial-mesenchymal transition. Respir Res. 2014;15:146. doi: 10.1186/s12931-014-0146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aivo J, Hanninen A, Ilonen J, Soilu-Hanninen M. Vitamin D3 administration to MS patients leads to increased serum levels of latency activated peptide (LAP) of TGF-beta. J Neuroimmunol. 2015;280:12–15. doi: 10.1016/j.jneuroim.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 56.Singh N, Liu G, Chakrabarty S. Cellular responses to TGFbeta and TGFbeta receptor expression in human colonic epithelial cells require CaSR expression and function. Cell Calcium. 2013;53:366–371. doi: 10.1016/j.ceca.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Rajput S, Kumar BN, Banik P, Parida S, Mandal M. Thymoquinone restores radiation-induced TGF-beta expression and abrogates EMT in chemoradiotherapy of breast cancer cells. J Cell Physiol. 2015;230:620–629. doi: 10.1002/jcp.24780. [DOI] [PubMed] [Google Scholar]
- 58.Moser C, Lang SA, Stoeltzing O. Heat-shock protein 90 (Hsp90) as a molecular target for therapy of gastrointestinal cancer. Anticancer Res. 2009;29:2031–2042. [PubMed] [Google Scholar]
- 59.Chen JS, Hsu YM, Chen CC, Chen LL, Lee CC, Huang TS. Secreted heat shock protein 90alpha induces colorectal cancer cell invasion through CD91/LRP-1 and NF-kappaB-mediated integrin alphaV expression. J Biol Chem. 2010;285:25458–25466. doi: 10.1074/jbc.M110.139345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen WS, Chen CC, Chen LL, Lee CC, Huang TS. Secreted heat shock protein 90alpha (HSP90alpha) induces nuclear factor-kappaB-mediated TCF12 protein expression to down-regulate E-cadherin and to enhance colorectal cancer cell migration and invasion. J Biol Chem. 2013;288:9001–9010. doi: 10.1074/jbc.M112.437897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 62.Campanholo VM, Silva RM, Silva TD, Neto RA, Paiotti AP, Ribeiro DA, Forones NM. Oral concentrated grape juice suppresses expression of NF-kappa B, TNF-alpha and iNOS in experimentally induced colorectal carcinogenesis in Wistar rats. Asian Pac J Cancer Prev. 2015;16:947–952. doi: 10.7314/apjcp.2015.16.3.947. [DOI] [PubMed] [Google Scholar]
- 63.Cianchi F, Cortesini C, Fantappie O, Messerini L, Sardi I, Lasagna N, Perna F, Fabbroni V, Di Felice A, Perigli G, Mazzanti R, Masini E. Cyclooxygenase-2 activation mediates the proangiogenic effect of nitric oxide in colorectal cancer. Clin Cancer Res. 2004;10:2694–2704. doi: 10.1158/1078-0432.ccr-03-0192. [DOI] [PubMed] [Google Scholar]
- 64.Appleyard CB, Cruz ML, Isidro AA, Arthur JC, Jobin C, De Simone C. Pretreatment with the probiotic VSL#3 delays transition from inflammation to dysplasia in a rat model of colitis-associated cancer. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1004–1013. doi: 10.1152/ajpgi.00167.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 66.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 67.Pasz-Walczak G, Kordek R, Faflik M. P21 (WAF1) expression in colorectal cancer: correlation with P53 and cyclin D1 expression, clinicopathological parameters and prognosis. Pathol Res Pract. 2001;197:683–689. doi: 10.1078/0344-0338-00146. [DOI] [PubMed] [Google Scholar]
- 68.Darzynkiewicz Z, Zhao H, Zhang S, Lee MY, Lee EY, Zhang Z. Initiation and termination of DNA replication during S phase in relation to cyclins D1, E and A, p21WAF1, Cdt1 and the p12 subunit of DNA polymerase delta revealed in individual cells by cytometry. Oncotarget. 2015;6:11735–11750. doi: 10.18632/oncotarget.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han Z, Wei W, Dunaway S, Darnowski JW, Calabresi P, Sedivy J, Hendrickson EA, Balan KV, Pantazis P, Wyche JH. Role of p21 in apoptosis and senescence of human colon cancer cells treated with camptothecin. J Biol Chem. 2002;277:17154–17160. doi: 10.1074/jbc.M112401200. [DOI] [PubMed] [Google Scholar]
- 70.Ioachim EE, Katsanos KH, Michael MC, Tsianos EV, Agnantis NJ. Immunohistochemical expression of cyclin D1, cyclin E, p21/waf1 and p27/kip1 in inflammatory bowel disease: correlation with other cell-cycle-related proteins (Rb, p53, ki-67 and PCNA) and clinicopathological features. Int J Colorectal Dis. 2004;19:325–333. doi: 10.1007/s00384-003-0571-3. [DOI] [PubMed] [Google Scholar]
- 71.Kang W, Lee S, Jeon E, Yun YR, Kim KH, Jang JH. Emerging role of vitamin D in colorectal cancer. World J Gastrointest Oncol. 2011;3:123–127. doi: 10.4251/wjgo.v3.i8.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kundu J, Choi BY, Jeong CH, Kundu JK, Chun KS. Thymoquinone induces apoptosis in human colon cancer HCT116 cells through inactivation of STAT3 by blocking JAK2- and Srcmediated phosphorylation of EGF receptor tyrosine kinase. Oncol Rep. 2014;32:821–828. doi: 10.3892/or.2014.3223. [DOI] [PubMed] [Google Scholar]
- 73.Kundu J, Kim DH, Kundu JK, Chun KS. Thymoquinone induces heme oxygenase-1 expression in HaCaT cells via Nrf2/ARE activation: Akt and AMPKalpha as upstream targets. Food Chem Toxicol. 2014;65:18–26. doi: 10.1016/j.fct.2013.12.015. [DOI] [PubMed] [Google Scholar]


