Abstract
Objectives: To investigate the effect of gastric motility protein 2 (GKN2) on the proliferation, apoptosis and invasion of gastric cancer cell and on the JAK/signal transducer and activator of transcription 3 (STAT3) signaling pathway. Methods: Expression of GKN2 was qualified using Western blot analysis in four gastric cancer cell lines and immortalized human gastric mucosal epithelial cell line GES-1. The cells were then transfected with pcDNA3.1-GKN2 and control vector using Lipofectaminetm2000 and assayed for viability, apoptosis, cell cycle changes, invasion ability as well as expression of cell cycle protein D1 (Cylin D1), Bcl-2, Bax, matrix metalloproteinase 2 (MMP2), MMP9, JAK2 and p-STAT3. Results: Western blot analyses showed that the expression of GKN2 was significantly lower in 4 gastric cancer cell lines (BGC-823, SGC-7901, AGS and MKN-45) than in GES-1. Of them, SGC-7901 had the lowest expression. The line was chosen for subsequent transfection experiments. Compared with control (transfection with empty vector), pcDNA3.1-GKN2-transfected cells had significantly more GKN2 protein and mRNA, decreased cell viability, increased apoptosis, more cells arrested at G1 phase and reduced invasiveness. Expression analyses showed that expression of Cyclin D1, Bcl-2, MMP2, MMP9, JAK2 and STAT3 was significantly down-regulated, while Bax was significantly up-regulated. Conclusion: Over-expression of GKN2 can increase apoptosis, reduce proliferation and invasion ability of gastric cancer cells as a result of down-regulated JAK2/STAT3 signaling pathway.
Keywords: Gastric motility protein 2, gastric cancer, proliferation, apoptosis, invasion, JAK/STAT3 signaling pathway
Introduction
Gastric cancer is one of the most frequent cancers in the world, in both men and women. In China, especially in rural areas, the incidence of gastric cancer ranks the first among malignant cancer [1]. For early gastric cancer patients, the survival period may be over 5 years or even much longer after surgical treatment and chemotherapy. However, due to insidious onset, most diagnosed patients are already at late stage and no radical treatment options are available to them, resulting in a 5 year- survival rate as low as 5% [2]. Therefore, biomarkers for early gastric cancer is of great significance to improve the diagnosis and prognosis. Gastrokines (GKNs) are gastric tissue-specific tumor suppressors and express abundantly in normal gastric tissues, and lowly or even not, in gastric cancer. Their expression is also affected by gastric cancer-related factors such as Helicobacter pylori (HP), inflammatory cytokines and trefoil factor family [3,4]. GKNs include three members, GKN1, GKN2 and GKN3. Among them, GKN2, also known as GDDR, is the mostly studied. It was discovered as a GKN member in 2002. GKN2 was shown not expressed or lowly expressed in gastric cancer tissue and closely related with the prognosis of gastric cancer patients [5-9]. Overexpression of GKN2 was shown to inhibit the proliferation, migration and invasion of gastric cancer cells through the interaction with GKN1, suggesting that GKN2 might be a tumor suppressor [5-9]. Studies have shown that the biological characteristics of cancer cells, such as proliferation, growth, differentiation, migration and invasion are regulated by various intracellular signaling pathways, such as the JAK/STAT signaling pathway [10-12]. JAK is a protein phosphorylase with STAT as substrate. It phosphorylates STAT to bind DNA and regulate the transcription of relevant genes to exert the biological effects. The JAK/STAT signaling pathway is activated constantly in a variety of tumor tissues, including gastric cancer. The downstream signaling pathways, particularly those mediated by STAT3, have thus become therapeutic targets for the treatment of a variety of tumors [10,13-15]. In addition, bioinformatics and in vitro studies have revealed that the GKN2 gene has sequence that can specifically bind STAT3 and that STAT3 is one of key components of GKN2-mediated regulation mechanisms. It has been proposed that the tumor suppression of GKN2 is achieved though the STAT3 signaling pathway [4,16]. The aim of this study was to investigate the expression of GKN2 in gastric cancer cells and normal gastric epithelial cells, and to explore the effect of GKN2 overexpression on proliferation, apoptosis, invasion and the JAK/STAT3 signaling pathway of gastric cancer cells.
Materials and methods
Cell lines
Human gastric cancer cell lines (BGC-823, SGC-7901, AGS and MKN-45) and immortalized gastric epithelial cell line (GES-1) were purchased from the Cell Banks, the Chinese Academy of Sciences (Shanghai, China).
Reagents and instruments
Rabbit anti-Bcl-2, Bax, Cyclin D1, MMP2, MMP9 and GAPDH monoclonal antibodies were purchased from (Epitmics, USA); rabbit anti-JAK2, STAT3, p-STAT3 monoclonal antibodies were obtained from Cell Signaling Technology, USA; horseradish peroxidase-labeled goat anti rabbit IgG (H + L chains) and BCA Kit were purchased from Beyotime Biotechnology (Beijing, China); methyl thiazolyl tetrazolium was obtained from GIBCO USA; fetal bovine serum and DMEM medium were purchased from Hyclone, USA; Transwell was purchased from Corning, USA; pcDNA3.1-GKN and control vector were provided by Zimmer Biological Technology, Shanghai, China; ChemiDocTM XRS gel imaging system was obtained from Bio-Rad, USA; inverted fluorescence microscope TE2000 was from Nikon, Japan and flow cytometer FACScalibur was from BD, USA.
RT-PCR for GNK2 expression
Total RNA isolated using Trizol kit (Invitrogen, USA) according to supplier’s instructions. Reverse transcription was preformed using the microRNA reverse transcription kit (Applied Biosystems by Life Technologies, Carlsbad, California, USA). Subsequent dilution of pre-amplified cDNA was 1:10. RT-qPCR was performed using TaqMan microRNA Assays (Applied Biosystems, USA). The PCR was carried out in a total volume of 25 μL. The cycling conditions were 50°C for 2 min, 95°C for 10 min followed by 45 cycles, each one consisting of 45 s at 94°C, 45 s at 59°C and 60 s at 72°C. The primers used for GKN2 were 5’-TGAGAAACAGGCTCTGGACA (forward), and 5’-CAGGAACCAATCCACGTCTT (reverse) and for GADPH (internal reference) were 5’-AGCCACATCGCTCAGACA (forward) and 5’-TGGACTCCACGACGTACT (reverse). Samples were run in triplicate and the mean value was calculated for each case. The data were managed according to previously described protocol [17].
Transfection
When confluence reached about 50-70%, the gastric cancer cells (SGC-7901) and immortalized human immortalized gastric epithelial cells (GES-1) cultured in DMEM supplemented with 10% FCS at 37°C in 5% CO2 cell culture incubator were transfected with pcDNA3.1-GKN and control vector using Lipofectamine 2000 according to manufacturer’s instructions. The transfected cells were cultured in DMEM supplemented with 10% FCS at 37°C in 5% CO2 for 24 h and then harvested for analysis.
Cell viability assay
The cell viability was measured by MTT assay. In brief, cells (1-1.5 × 104 per well) were plated in 96-well culture plates in complete medium. After pre-determined times, 20 μL MTT (5 mg/ml) was added and the cells were incubated for 4 h. The optical density at 560 nm was measured as a measure for cell viability.
Detection of apoptosis by flow cytometry
Transfected cells were harvested, labeled with Annexin V and propidiumiodide (PI) following the manufacturer’s instructions (Biosea Biotechnology, Beijing, China) and assessed on flow cytometer FACScalibur for apoptotic cells.
Cell cycle analysis
Transfected cells were digested with EDTA-free trypsin, washed with PBS and fixed with ice-cold 70% ethanol overnight at 4°C. Cells were washed with PBS and incubated with RNase A (100 μg/ml) at 37°C for 30 min. DNA was labeled with PI (50 μg/ml) and the fluorescence was measured with a FACScalibur flow cytometer. Data collection and analysis of the cell cycle distribution were performed using CellQuest and the Modfit software (Becton Dickinson).
Invasion assays
Transfected cells (5 × 105) in serum-free medium were placed into the upper chamber of an insert coated with Matrigel. DMEM medium was added to the lower chamber. After 48 h of incubation, the cells remaining on the upper membrane were removed with cotton wool, whereas the cells that had migrated through the membrane were stained with 2% crystal violet in 25% methanol/PBS, imaged and counted under an inverted microscope. The experiments were independently repeated three times.
Western blot analysis
Cells were lysed in the RIPA buffer that contains protease and phosphatase inhibitors cocktail and the supernatants were collected after centrifugation at 12000 rpm for 20 min. The protein was applied to polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a PVDF membrane, and then detected by the proper primary and secondary antibodies after visualization with a chemiluminescence kit. The intensity of blot signals was quantitated using Quantity one analysis software.
Statistical analysis
All data were expressed as means ± standard error of the mean (SEM) obtained from at least three independent experiments. Statistical comparisons between experimental and control groups were assessed by using the Student’s t-test. P < 0.05 was considered statistically significant.
Results
Expression of GKN2 in gastric cancer cell lines and normal gastric mucosa epithelial cell line
Western blot analysis showed that the expression of GKN2 was significantly lower in all gastric cancer cell lines assayed (BGC-823, SGC-7901, AGS and MKN-45) than in immortalized gastric epithelial cell line GES-1 (Figure 1). Among these cell lines tested, SGC-7901 had the lowest expression level and was therefore chosen for subsequent experiments.
Figure 1.
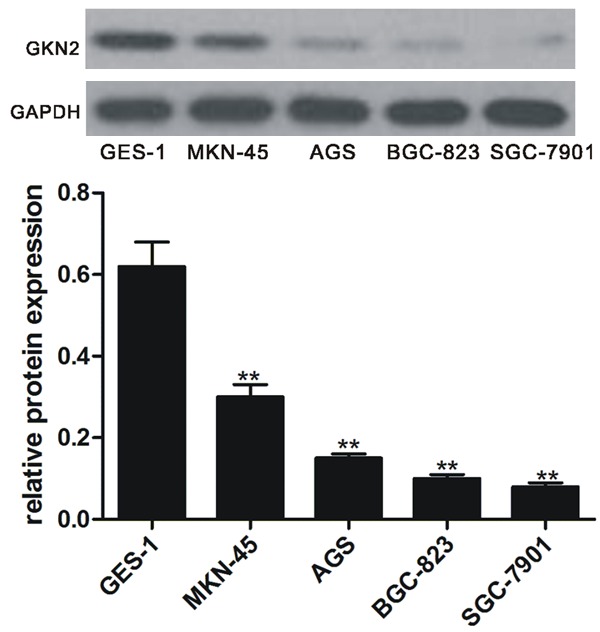
Expression of GKN2 in gastric cancer cell lines and normal gastric epithelial cell line. Upper pane: Western blot of GKN2; lower pane: relative GKN2 level. **denotes P < 0.01 vs normal gastric epithelial cell line GES-1.
Expression of GKN2 in transfected cells
We then transfected SGC-7901 with pcDNA3.1-GKN2 and control (vector without GKN2). Western blot and RT-PCR analyses showed that the levels of GKN2 at protein and mRNA level were significantly higher in the cells transfected with pcDNA3.1-GKN2 than with control (P < 0.01, Figure 2).
Figure 2.
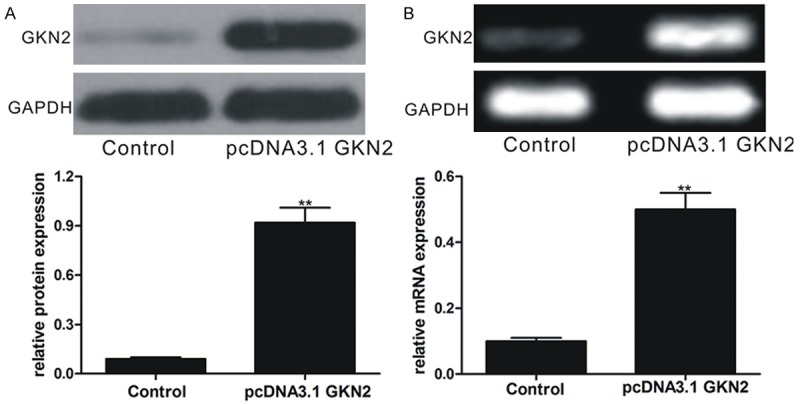
Expression of GKN2 in gastric cancer cell line SGC-7901 after transfection with pcDNA3.1-GKN2 and control at protein and mRNA levels. A. Upper pane: Western blot of GKN2; lower pane: relative GKN2 level. B. Upper pane: electrophoresis of GKN2 PCR product; lower pane: relative GKN2 mRNA level. **denotes P < 0.01 vs normal gastric epithelial cell line GES-1.
Overexpression of GKN2 reduced cell viability
MTT assays showed that the viability of SGC-7901 cells transfected with pcDNA3.1-GKN2 was reduced and was significantly lower than those transfected with control (P < 0.01, Figure 3). Between 24 and 96 h after the transfection, pcDNA3.1-GKN2-transfected cells did not grow at all, while the control cells were proliferative (Figure 3).
Figure 3.
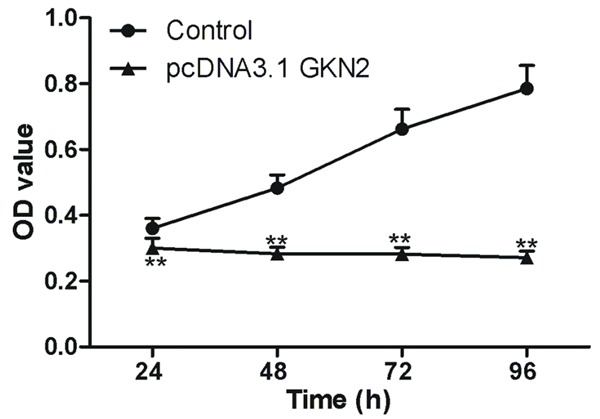
Viability of SGC-7901 cells after overexpressing GKN2 in gastric cancer cell line SGC-7901. **denotes P < 0.01 vs normal gastric epithelial cell line GES-1.
Overexpression of GKN2 augmented apoptosis
Annexin V/PI flow cytometry showed that there were significantly more apoptotic cells after transfection with pcDNA3.1-GKN2 than with control vector (37.8% vs 5.96%, P < 0.01, Figure 4; Table 1).
Figure 4.
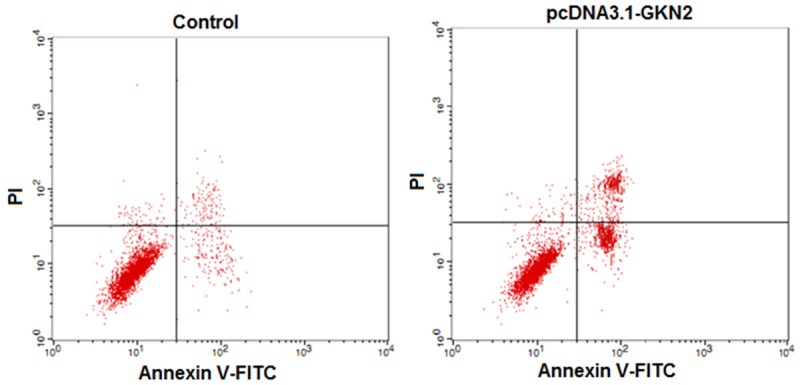
Flow cytometry assays of apoptosis in gastric cancer cell line SGC-7901 after transfection with pcDNA3.1-GKN2 and control.
Table 1.
Percentages of cells arrested in different cell cycle and apoptosis rate (%)
| Transfection with | % cells arrested at | Apoptosis rate (%) | |||
|---|---|---|---|---|---|
|
| |||||
| G1 | S | G2 | Early | Late | |
| Control | 57.81 ± 5.75 | 22.45 ± 2.21 | 11.49 ± 1.15 | 3.49 ± 0.35 | 2.47 ± 0.25 |
| PcDNA3.1-GKN2 | 81.55 ± 8.16** | 13.03 ± 0.56** | 6.50 ± 0.58** | 19.35 ± 1.94** | 18.45 ± 1.85** |
denotes P < 0.01 vs normal gastric epithelial cell line GES-1.
GKN2 overexpression arrested cells at S1
Analysis with FACS calibur flow cytometer showed that in compared with control, significantly more SGC-7901 cells were arrested at S1 phase when they were transfected with pcDNA3.1-GKN2 (Table 1; Figure 5).
Figure 5.
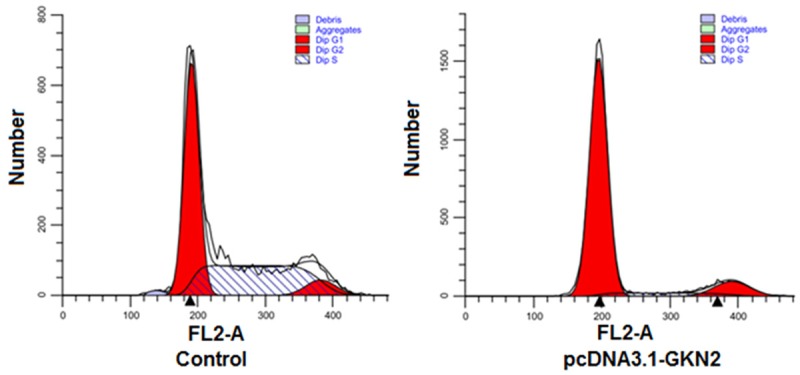
Distribution of SGC-7901 cells at different cell cycles after transfection with pcDNA3.1-GKN2 and control.
Overexpression of GKN2 attenuated invasion ability
Transwell assays showed that SGC-7901 cells had significantly lower invasion ability after transfection with pcDNA3.1-GKN2 as compared to control (P < 0.01, Figure 6). The numbers of migrated cells were 135.54 ± 11.26 (pcDNA3.1-GKN2) vs 39.53 ± 2.95 (control), respectively.
Figure 6.
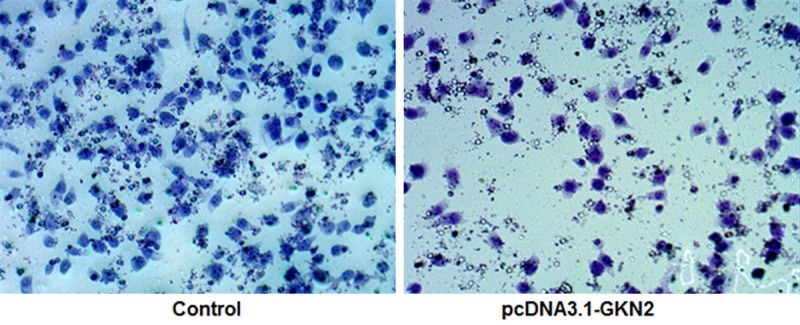
Crystal violet straining of migrated cells in transwell assays of SGC-7901 cells after transfection with pcDNA3.1-GKN2 and control (× 200).
Regulation of GKN2 on cancer-related gene expression
Western blot analyses of SGC-7901 cells transfected with pcDNA3.1-GKN2 showed that Bcl-2 and Cyclin D1 were significantly up-regulated, while Bax, MMP2 and MMP9 were significantly down-regulated (P < 0.01, Figure 7A and 7B).
Figure 7.
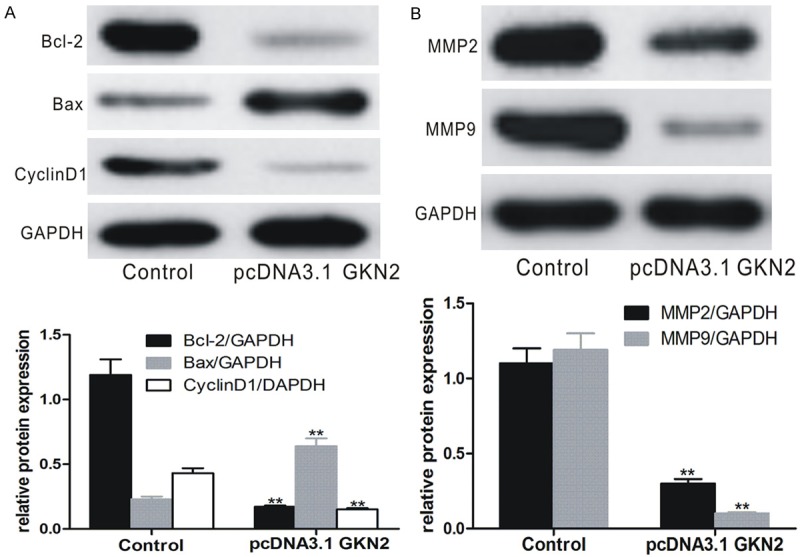
Expression of cancer-related genes in SGC-7901 cells after transfection with pcDNA3.1-GKN2 and control. A. Expression of Bcl-2, Cyclin D1 and Bax; B. Expression of MMP2 and MMP9. Upper panes: Western blots; lower panes: relative protein level. **denotes P < 0.01 vs control-transfected SGC-7901 cells.
GKN2 overexpression down-regulated JAK2/STAT3 pathway
Western blot analyses of SGC-7901 cells transfected with pcDNA3.1-GKN2 showed that the JAK2 and p-STAT3 expression was significantly down-regulated (P < 0.01, Figure 8).
Figure 8.
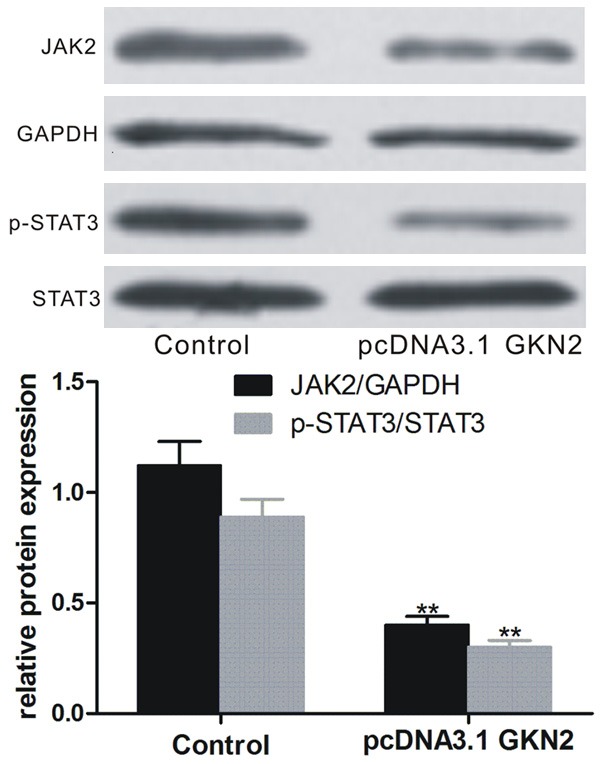
Expression of JAK2 and pSTST3 in SGC-7901 cells after transfection with pcDNA3.1-GKN2 and control. Upper pane: Western blot; lower pane: relative protein level. **denotes P < 0.01 vs control-transfected SGC-7901 cells.
Discussion
The occurrence and progression of gastric cancer, like most tumors, are multi-factor and multi-stage complex biological processes. The activation of oncogenes and inactivation of onco-suppressor genes lead to uncontrollable growth of the cells, resulting in malignant proliferation, invasion and metastasis of cancer cells [18,19]. GKNs are gastric tissue-specific proteins, including GKN1 (AMP-18), GKN2 (GDDR) and GKN3. They are highly conserved in mammals and all have a special BRICHOS domain and a common carboxyl terminal fragment. Studies have shown that GKN2 is a secretory protein of gastric epithelial cells and may be a gastric cancer suppressor. For example, Moss et al found that in 78% proliferative gastric cancer samples and 42% small intestinal gastric cancer samples, GKN2 was not detectable or lowly expressed [9]. Dai et al shown that GKN did not express or lowly expressed in gastric cancer cell lines BGC-823, SGC-7901 and AGS, but highly expressed in GES-1 [6]. They further showed that over-expression of GKN2 inhibited the growth of SGC-7901 cells grafted on nude mice. Over-expression of GKN2 is also shown to reduce the expression of MMP9, Cyclin E and Cyclin D1 in gastric cancer cells, and arrest the cells at G1 phase. All these findings suggest that GKN2 may be a gastric cancer suppressor gene. Our data show that GKN2 expression was significantly lower in the gastric cancer cell lines than in non-cancer cell line GES-1. This is consistent with early work [6]. We selected SGC-7901 for further experiments because of its lowest GKN2 expression to better understand the role of GKN2 in gastric cancer pathogenesis. We observed significantly increased GKN2 expression at protein and mRNA levels when the cells were transformed with pcDNA3.1-GKN2 as compared to control (empty vector), suggesting the transfection effectively elevates the GKN2 level in the cell line. Furthermore, cell viability assays showed that the pcDNA3.1-GKN2-transfected SGC-7901 cells had significantly reduced viability and increased apoptosis, and more cells arrested in G1 phase as compared with control-transfected cells, as observed early [6]. In addition, Transwell assays showed that GKN2 overexpression significantly reduced the invasiveness of SGC-7901 cells as reported previously [5]. These findings demonstrate that GKN2 overexpression can reduce the viability and invasiveness, induce apoptosis and arrest the cells in G1 phase to suppress the progression of cancer.
Apoptosis is regulated by apoptosis-related genes, most commonly by the genes in the Bcl-2 family, such as Bcl-2, which is a well-studied apoptosis inhibitor. Bcl-2 complexes with pro-apoptotic gene Bax to form heterodimer to promote apoptosis. Cyclin D1 is the most critical protein in G1 phase when the increase in its expression is an indication of rapid conversion from G1 to S phase as it promotes DNA replication and mitosis [20,21]. Cell invasion ability is closely related to the degradation of extracellular matrix, and MMPs are the major proteolytic enzymes involved in the degradation of extracellular matrix [22]. MMP2 can not only promote the invasion and metastasis of tumor cells by degrading extracellular matrix collagen, but also induce angiogenesis to promote the invasion and metastasis of tumor cells. MMP9 degrades extracellular matrix and basement membrane, thereby promoting tumor migration, proliferation and metastasis. Therefore, by properly regulating the expression of these genes, it may be possible to inhibit the proliferation and invasion of tumor cells or to induce apoptosis and arrest cell proliferation to a certain extent. To better understanding the molecular mechanisms underlying GKN2-mediated tumor suppression, we analyzed the expression of these genes. The results showed that compared with the control, GKN overexpression up regulated the expression of Bax, and down-regulated the expression of Bcl-2, Cyclin D1, MMP2 and MMP9, thereby inhibiting the proliferation of SGC-7901 cells and arresting the cells in G1 phase.
JAK/STAT signaling pathway is closely related to the proliferation, apoptosis, differentiation, migration and invasion and metastasis of cancer cells [10-12]. Studies have shown that this pathway is consistently activated in a variety of tumors. This pathway, particularly the STAT3-mediated signal pathway, has become a hot treatment target for cancers including gastric cancer [10,13-15]. JAK family consists of JAK1, JAK2, JAK3 and TYK2. Among them, JAK2 is the mostly studied. When stimulated, activated JAK2 as tyrosine kinase phosphatase recruits and phosphorylates cytoplasmic STAT3 monomers to activate the SH2 domain to form dimers with p-STAT3 through the tyrosine residues. The dimers are then translocated into the nuclei where they bind the promoter region of target genes to regulate the transcription. As a result, these activities may promote cell proliferation and angiogenesis, block apoptosis, induce immune escape, invasion and metastasis. Earlier studies show that activated STAT3 increases the expression of Bcl-xl, Mcl-1, Survivin, Cyclin D1, VEGF and MMPs and promotes tumor proliferation, angiogenesis, and invasion and metastasis [1,22-24]. In addition, studies have also shown that in mouse gastric cancer model developed from gastritis GKN2 expression was lower and was related to the activation of STAT3. When HP was removed completely, GKN2 was the mostly up-regulated gene [4]. These and other evidences [16] suggest that GKN2 may function as a tumor suppressor gene by regulating the STAT3 signaling pathway [4]. Our results showed that over-expression of GKN2 significantly downregulated the expression of JAK2 and STAT3 expression, suggesting that GKN2 inhibits the JAK2/STAT3 signal pathway to further up-regulate Bax, and down-regulating Bcl-2, Cyclin D1, MMP2 and MMP9, thereby resulting in reduced proliferation and invasiveness, increased apoptosis and cell arrest in the G1 phase in SGC-7901 cells.
Acknowledgements
This study was supported by the Science Research Project of Hunan Provincial Department of Education (grant No: 16c1398).
Disclosure of conflict of interest
None.
References
- 1.Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M, Gershenwald JE, Xie K, Sawaya R, Huang S. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–3196. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 2.Jiang C, Chen X, Alattar M, Wei J, Liu H. MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis of gastric cancer. Cancer Gene Ther. 2015;22:291–301. doi: 10.1038/cgt.2015.19. [DOI] [PubMed] [Google Scholar]
- 3.Luo Y, Wang C. Role of gastrokine 1 in the gastric mucosal relative diseases. Chin J Bases Clin General Surg. 2015;22:1143–1147. [Google Scholar]
- 4.Liu J, Lin H. Research progress of GKNs in gastric cancer. J Med Res. 2015;44:21–23. [Google Scholar]
- 5.Fahlbusch FB, Ruebner M, Huebner H, Volkert G, Bartunik H, Winterfeld I, Hartner A, Menendez-Castro C, Noegel SC, Marek I, Wachter D, Schneider-Stock R, Beckmann MW, Kehl S, Rascher W. Trophoblast expression dynamics of the tumor suppressor gene gastrokine 2. Histochem Cell Biol. 2015;144:281–291. doi: 10.1007/s00418-015-1336-0. [DOI] [PubMed] [Google Scholar]
- 6.Dai J, Zhang N, Wang J, Chen M, Chen J. Gastrokine-2 is downregulated in gastric cancer and its restoration suppresses gastric tumorigenesis and cancer metastasis. Tumour Biol. 2014;35:4199–4207. doi: 10.1007/s13277-013-1550-0. [DOI] [PubMed] [Google Scholar]
- 7.Kim O, Yoon JH, Choi WS, Ashktorab H, Smoot DT, Nam SW, Lee JY, Park WS. GKN2 contributes to the homeostasis of gastric mucosa by inhibiting GKN1 activity. J Cell Physiol. 2014;229:762–771. doi: 10.1002/jcp.24496. [DOI] [PubMed] [Google Scholar]
- 8.Zhang F, Tang JM, Wang L, Shen JY, Zheng L, Wu PP, Zhang M, Yan ZW. Detection of beta-catenin, gastrokine-2 and embryonic stem cell expressed ras in gastric cancers. Int J Clin Exp Pathol. 2010;3:782–791. [PMC free article] [PubMed] [Google Scholar]
- 9.Moss SF, Lee JW, Sabo E, Rubin AK, Rommel J, Westley BR, May FE, Gao J, Meitner PA, Tavares R, Resnick MB. Decreased expression of gastrokine 1 and the trefoil factor interacting protein TFIZ1/GKN2 in gastric cancer: influence of tumor histology and relationship to prognosis. Clin Cancer Res. 2008;14:4161–4167. doi: 10.1158/1078-0432.CCR-07-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Meng X, Shi H, Li W, Ming Z, Zhong Y, Deng W, Zhang Q, Fan N, Niu Z, Chen G, Yang S. The role of JAK/STAT3 signaling pathway on apoptosis of lung adenocarcinoma cell line PC-9 induced by icotinib. Am J Transl Res. 2016;8:1730–1737. [PMC free article] [PubMed] [Google Scholar]
- 11.Khanna P, Chua PJ, Bay BH, Baeg GH. The JAK/STAT signaling cascade in gastric carcinoma (Review) Int J Oncol. 2015;47:1617–1626. doi: 10.3892/ijo.2015.3160. [DOI] [PubMed] [Google Scholar]
- 12.Arumuggam N, Bhowmick NA, Rupasinghe HP. A review: phytochemicals targeting JAK/STAT signaling and IDO expression in cancer. Phytother Res. 2015;29:805–817. doi: 10.1002/ptr.5327. [DOI] [PubMed] [Google Scholar]
- 13.Li MX, Bi XY, Huang Z, Zhao JJ, Han Y, Li ZY, Zhang YF, Li Y, Chen X, Hu XH, Zhao H, Cai JQ. Prognostic role of phospho-STAT3 in patients with cancers of the digestive system: a systematic review and meta-analysis. PLoS One. 2015;10:e0127356. doi: 10.1371/journal.pone.0127356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Liu X, Jiao H, Peng L, Huo Z, Yang W, Shen Q, Li T, Liu Q. Prognostic and clinical significance of STAT3 and MMP9 in patients with gastric cancer: a meta-analysis of a Chinese cohort. Int J Clin Exp Med. 2015;8:546–557. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao G, Zhu G, Huang Y, Zheng W, Hua J, Yang S, Zhuang J, Ye J. IL-6 mediates the signal pathway of JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol Rep. 2016;35:1787–1795. doi: 10.3892/or.2016.4544. [DOI] [PubMed] [Google Scholar]
- 16.Menheniott TR, O’Connor L, Chionh YT, Dabritz J, Scurr M, Rollo BN, Ng GZ, Jacobs S, Catubig A, Kurklu B, Mercer S, Minamoto T, Ong DE, Ferrero RL, Fox JG, Wang TC, Sutton P, Judd LM, Giraud AS. Loss of gastrokine-2 drives premalignant gastric inflammation and tumor progression. J Clin Invest. 2016;126:1383–1400. doi: 10.1172/JCI82655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Cheng H, Wang X, Li T, Chen L. Bcl-2 expression and patient survival in gastric cancer: a systematic review of the literature with meta-analysis. Med Oncol. 2015;32:389. doi: 10.1007/s12032-014-0389-6. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki T, Kuniyasu H. Significance of AKT in gastric cancer (Review) Int J Oncol. 2014;45:2187–2192. doi: 10.3892/ijo.2014.2678. [DOI] [PubMed] [Google Scholar]
- 20.Cadoni G, Boccia S, Petrelli L, Di Giannantonio P, Arzani D, Giorgio A, De Feo E, Pandolfini M, Galli P, Paludetti G, Ricciardi G. A review of genetic epidemiology of head and neck cancer related to polymorphisms in metabolic genes, cell cycle control and alcohol metabolism. Acta Otorhinolaryngol Ital. 2012;32:1–11. [PMC free article] [PubMed] [Google Scholar]
- 21.Scheller K, Becker S, Scheller C. Symmetric palatal swelling as the first clinical manifestation of a mantle cell non-Hodgkin’s lymphoma: a case report and review of literature. J Oral Maxillofac Pathol. 2011;15:311–315. doi: 10.4103/0973-029X.86703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Priceman SJ, Xin H, Zhang W, Deng J, Liu Y, Huang J, Zhu W, Chen M, Hu W, Deng X, Zhang J, Yu H, He G. Icaritin inhibits JAK/STAT3 signaling and growth of renal cell carcinoma. PLoS One. 2013;8:e81657. doi: 10.1371/journal.pone.0081657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL, Xie K. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- 24.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]


