Abstract
Cytomegalovirus (CMV) infection of the gastrointestinal tract has a variety of presentations. We present a case of gastric perforation, which is a relatively infrequent presentation of CMV infection. If the cause of gastric perforation is not readily apparent, testing for CMV should be considered. If CMV workup is positive, an evaluation for immunocompromise is prudent as CMV infections are more common in immunocompromised individuals.
Introduction
Gastric perforation can have a variety of causes, the most common of which is peptic ulcer disease. Infectious causes, such as cytomegalovirus (CMV), are less common. CMV can have a wide array of clinical presentations in the gastrointestinal tract from mouth to anus, which are usually seen in the setting of immunocompromise. Common symptoms of CMV are diarrhea, abdominal pain, fever, and weight loss, typically resulting from gastritis and enteritis. CMV may also cause ischemia, leading to ulceration and perforation. It may also superinfect patients with inflammatory bowel disease on immunosuppressive therapy. While CMV is commonly seen in patients with known immunocompromise, it is much less commonly seen in immunocompetent hosts.
Case Report
A 56-year-old previously healthy woman presented with 6 episodes of frank hematemesis followed by coffee ground emesis. She denied alcohol, tobacco, or nonsteroidal anti-inflammatory drugs use, and her physical exam was pertinent for right-sided mild flank pain but was otherwise benign. Esophagogastroduodeno-scopy (EGD) showed an ulcer with associated gastritis in the cardia of the stomach; biopsies were negative for Helicobacter pylori (Figure 1). Abdominal/pelvic computed tomography (CT) showed a perforation at the ulcer site and a fistulous connection to a splenic abscess (Figure 2).
Figure 1.
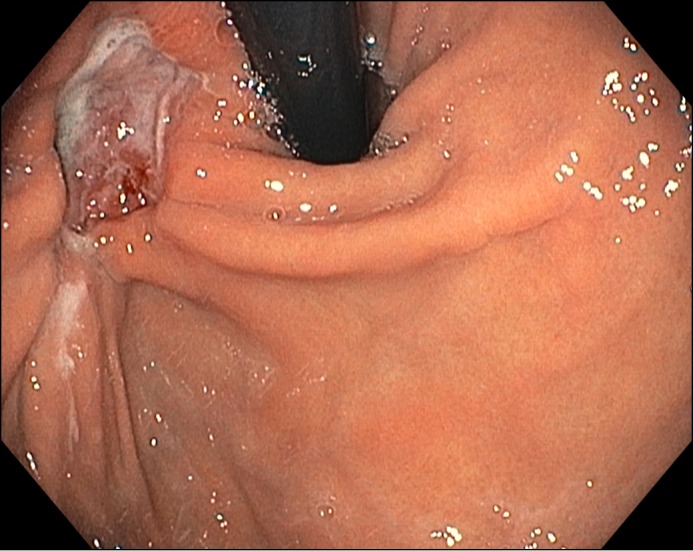
EGD showing an ulcer with associated gastritis in the cardia of the stomach.
Figure 2.
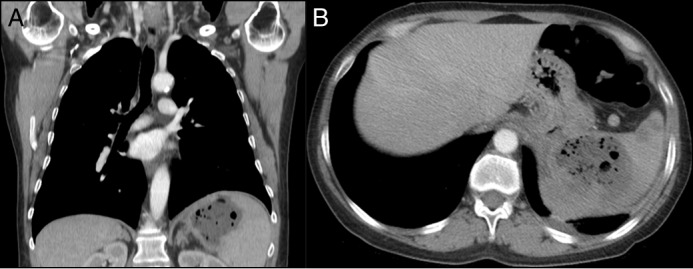
(A) Coronal and (B) axial CT showing perforation at the ulcer site and a fistulous connection to a splenic abscess.
The splenic abscess was drained percutaneously and grew multiple species consistent with oral flora. Given the unusual location of the ulcer and lack of risk factors, the ulcer was thought to represent either a malignant process or a direct extension of a primary splenic abscess into the stomach. A repeat EGD was undertaken to perform additional biopsies, which were negative for malignancy but revealed CMV gastritis with extensive ulceration (Figure 3). Human immunodeficiency virus (HIV) screening was negative. Surgical management of the ulcer was deferred due to its location near the gastroesophageal junction; the splenic abscess was managed conservatively. The ulcer was endoscopically closed with Ovesco clipping (Ovesco Endoscopy AG, Tübingen, Germany) and overstitching. She was discharged on nasojejunal tube feeds, intravenous antibiotics, and ganciclovir.
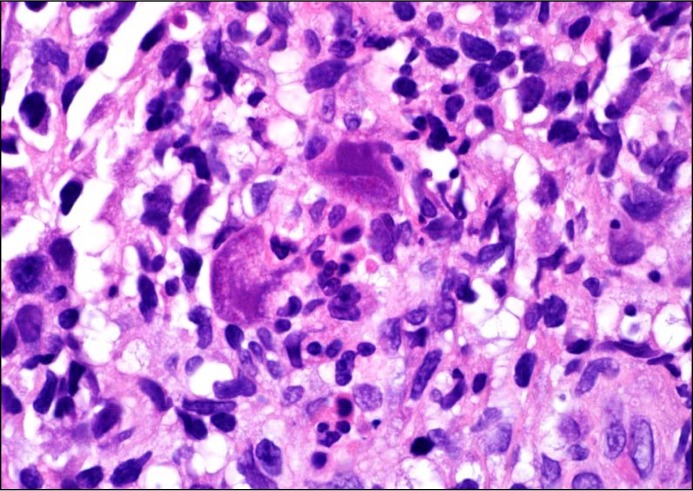
Figure 3.
Hematoxylin and eosin stain of gastric biopsy demonstrating CMV “owl’s eye” appearance.
Early during the course of her outpatient follow-up, an abdominal CT showed a non-specific para-aortic mass potentially consistent with adenopathy in the setting of ongoing splenic abscess. Despite the multimodal endoscopic closure, the gastric ulcer did not close and led to persistent fluid collection outside of the stomach, which required placement of a percutaneous drain by interventional radiology. Follow-up abdominal CT obtained 6 weeks after the previous showed progression of the para-aortic mass in the setting of resolved abscess. Given the concern for malignancy, a CT-guided biopsy was positive for diffuse large B-cell lymphoma and positron emission tomography-CT showed diffuse abdominal disease (Figure 4). The patient was initiated on chemotherapy with the R-CHOP treatment (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone). Her gastric perforation has fully healed and she is currently in clinical and radiographic remission.
Figure 4.
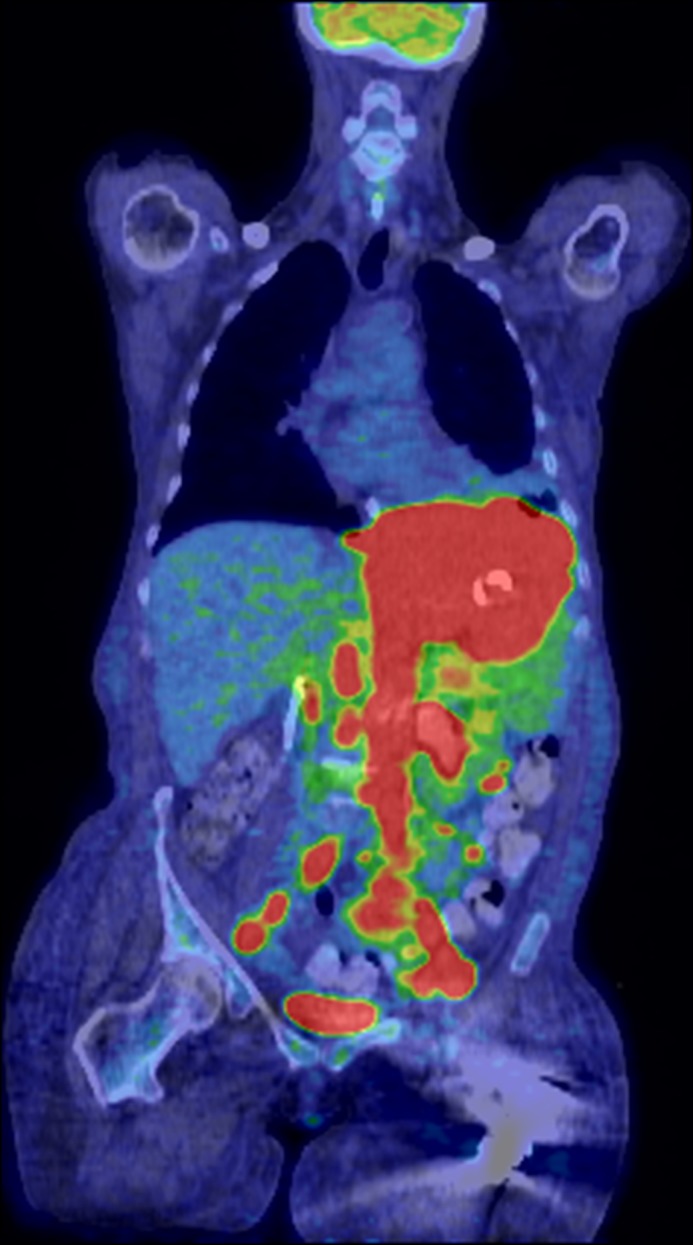
Positron emission tomography scan demonstrating diffuse large B-cell lymphoma.
Discussion
This case represents both a rare location and a rare underlying etiology of gastric perforation. While gastric erosions and ulceration are common, perforations are less common and can occur secondary to peptic ulcer disease, trauma, neoplasms, surgical or endoscopic complications, or infections including CMV. Known complications of CMV infections in the gastric tract include perforation, ulceration, hemorrhagic proctocolitis, and lesions mimicking neoplasia.1 Specifically, CMV enteritis and gastritis in immunocompromised patients has been well described in the literature, and patients with lymphoma are known to be at risk for CMV infection given altered cell-mediated immunity.2,3 CMV-associated perforations are much less frequent but have been seen in patients with malignancy.4-9 However, these cases were reported in East Asian patients who had a known malignant diagnosis and underwent immunosuppressive therapy. Furthermore, not only is this presentation rare, potentially leading to low clinical suspicion of CMV, but the diagnosis of CMV-induced gastric ulceration can be difficult because its presentation can be quite varied on gross endoscopic examination, often requiring pathologic diagnosis.10,11 If evaluation for underlying causes of gastritis or gastric ulcer is unrevealing, it is reasonable to consider further testing for CMV with mucosal biopsies. If CMV infection is found, then clinicians should consider evaluation for potential causes of an immunocompromised state including HIV, history of immunomodulating therapy, and malignancy.
Disclosures
Author contributions: E. Krajicek reviewed the literature, drafted the manuscript, and is the article guarantor. R. Shivashankar and S. Hansel edited the manuscript.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Chetty R, Roskell DE. Cytomegalovirus infection in the gastrointestinal tract. J Clin Pathol. 1994; 47(11):968–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med. 1993; 119(9):924–35. [DOI] [PubMed] [Google Scholar]
- 3.Hwang ET, Lee JY, Chung JS, et al.. [Cytomegalovirus induced gastric ulcer as a principal manifestation in the initial stage of Hodgkin’s disease]. Korean J Gastroenterol. 2009; 54(2):117–22. [DOI] [PubMed] [Google Scholar]
- 4.Jun YJ, Sim J, Ahn HI, et al.. Cytomegalovirus enteritis with jejunal perforation in a patient with endometrial adenocarcinoma. World J Clin Cases. 2013; 1(7):220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawate S, Ohwada S, Sano T, et al. . Ileal perforation caused by cytomegalovirus infection in a patient with recurrent gastric cancer: Report of a case. Surg Today. 2002; 32(12):1088–90. [DOI] [PubMed] [Google Scholar]
- 6.Nabeshima K, Sakaguchi E, Inoue S, Eizuru Y, Minamishima Y, Koono M. Jejunal perforation associated with cytomegalovirus infection in a patient with adult T-cell leukemia-lymphoma. Acta Pathol Jpn. 1992; 42(4):267–71. [DOI] [PubMed] [Google Scholar]
- 7.Nishii T, Rino Y, Ando K, et al. . Successful treatment of multiple small-bowel perforations caused by cytomegalovirus in a patient with malignant lymphoma: Report of a case. Surg Today. 2006; 36(10):930–3. [DOI] [PubMed] [Google Scholar]
- 8.Hirayama K, Nakamura T, Fukazawa A, et al.. [Ileal perforation due to cytomegalovirus enteritis under chemotherapy for malignant lymphoma. Report of a case]. Nihon Shokakibyo Gakkai Zasshi. 2001; 98(10):1185–9. [PubMed] [Google Scholar]
- 9.Yasunaga M, Hodohara K, Uda K, et al. . Small intestinal perforation due to cytomegalovirus infection in patients with non-Hodgkin's lymphoma. Acta Haematol. 1995; 93(2-4):98–100. [DOI] [PubMed] [Google Scholar]
- 10.Hokama A, Taira K, Yamamoto Y, et al. . Cytomegalovirus gastritis. World J Gastrointest Endosc. 2010; 2(11):379–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebisutani C, Kawamura A, Shibata N, et al. . Gastric ulcer associated with cytomegalovirus in an immunocompetent patient: Method for diagnosis. Case Rep Gastroenterol. 2012; 6(2):365–8. [DOI] [PMC free article] [PubMed] [Google Scholar]