Abstract
Objectives
To evaluate adaptive behavior outcomes of children prenatally exposed to lamotrigine, valproate, or carbamazepine, and to determine if these outcomes were dose-dependent.
Methods
Data were collected from women enrolled in the North American Anti-epileptic Drug (AED) Pregnancy Registry who had taken lamotrigine, valproate, or carbamazepine monotherapies throughout pregnancy to suppress seizures. The adaptive behavior of 252 exposed children (including 104 lamotrigine-exposed, 97 carbamazepine-exposed, and 51 valproate-exposed), ages 3- to 6-years-old, was measured using the Vineland-II Adaptive Behavior Scales, administered to each mother by telephone. Mean Adaptive Behavior Composite (ABC), domain standard scores for communication, daily living, socialization and motor skills, and adaptive levels were analyzed and correlated with first trimester drug dose.
Results
After adjusting for maternal age, education, folate use, cigarette and alcohol exposure, gestational age, and birth weight by propensity score analysis, the mean ABC score for valproate-exposed children was 95.6 (95% CI [91, 101]), versus 100.8 (95% CI [98, 103]) and 103.5 (95% CI [101, 106]) for carbamazepine- and lamotrigine-exposed children, respectively (ANOVA; p=0.017). Significant differences were observed among the three drug groups in the ABC (p=0.017), socialization (p=0.026), and motor (p=0.018) domains, with a trend toward significance in the communication domain (p=0.053). Valproate-exposed children scored lowest and lamotrigine-exposed children scored highest in every category. Valproate-exposed children were most likely to perform at a low or moderately low adaptive level in each category. Higher valproate dose was associated with significantly lower ABC (p=0.020), socialization (p=0.009), and motor (p=0.041) scores before adjusting for confounders. After adjusting for the above variables, increasing VPA dose was associated with decreasing Vineland scores in all domains, but the relationships were not statistically significant. No dose effect was observed for carbamazepine or lamotrigine.
Conclusions
Unlike carbamazepine and lamotrigine, prenatal valproate exposure was associated with adaptive behavior impairments with specific deficits in socialization and motor function, along with a relative weakness in communication. Increasing valproate dose was associated with a decline in adaptive functioning. This finding of a linear dose-dependent teratogenic effect suggests that valproate should be avoided at any dose during pregnancy. However, some women with epilepsy controlled only by valproate will decide, in consultation with their provider, that the benefits of continuing valproate during pregnancy outweigh the fetal risks. Faced with difficult choices, clinicians should be supportive as these patients consider their options.
Keywords: valproate, lamotrigine, carbamazepine, antiepileptic, pregnancy, behavior
1. Introduction
Fetal exposure to anti-epileptic drugs (AEDs) carries elevated risk for birth defects [1], [2], [3], [4], [5], [6], [7], [8], and may be associated with cognitive dysfunction [9], [10], [11], [12]. Valproate, especially, has been associated with lower IQ [10], [13], [14], [15], [16],[17], increased special education needs [18], behavioral problems [3], [18], [19], [20], and increased risk of autism spectrum disorder [21], [22], [23], [24], when compared to several other AEDs, namely phenytoin, carbamazepine, and lamotrigine.
Unfortunately, most studies investigating neurodevelopmental outcomes of exposed children have relied on language testing and IQ to assess cognitive function, while adaptive behavior outcomes have been significantly less well-studied. Although IQ tests measure general intelligence, they neither assess functional abilities nor adaptive behaviors required for independent daily living, such as socialization, communication, self-care, and motor skills. Deficits in these areas have significant implications for long-term behavioral outcomes. Impairments in socialization and communication, along with repetitive, stereotyped behaviors, form the basis for diagnosis of autism spectrum disorder [25].
While studies have suggested that IQ is a strong predictor of adaptive impairments for individuals with cognitive disabilities, research has also shown that the gap between IQ and adaptive skills is greater among higher functioning individuals [26], [27]. In individuals with autism spectrum disorder, severe social deficits have been observed even with relatively high IQ [26], [27]. Thus, using IQ scores alone to evaluate neurodevelopmental outcomes for children exposed to AEDs in utero is not sufficient to identify those individuals with adaptive behavior impairments despite high cognitive potential. As a result, information on the long-term behavioral effects of prenatal AED exposure is lacking, and significant limitations exist for physicians counseling women of childbearing age with epilepsy (WWE). We present a cohort study to evaluate adaptive behavior outcomes among children born to WWE who received one of three AED treatments as monotherapy during pregnancy. The a priori hypotheses were that prenatal exposure to carbamazepine (CBZ), lamotrigine (LTG), or valproate (VPA) monotherapies would be associated with adaptive behavior impairments, and that exposure to higher doses during the first trimester would be associated with lower adaptive behavior levels.
2. Methods
2.1. Recruitment, Inclusion, and Exclusion Criteria
Recruitment letters were sent by mail to WWE who had prospectively enrolled in the North American AED Pregnancy Registry (“the Registry”) while taking LTG, VPA, or CBZ as monotherapy to suppress seizures throughout pregnancy, and whose exposed children were 3- to 6-years-old. The Registry’s methodology has been described previously [28]. Children were excluded if they were exposed to other known teratogens, such as isotretinoin or warfarin, or if the AED was not taken throughout pregnancy. Mothers were excluded if they had mental illness or memory disorders, or if they refused to release medical records.
2.2. Study Procedures
Mothers were screened by telephone to determine eligibility and collect data on participant characteristics and confounding variables. Data on each enrolled child’s development were then collected from mothers by telephone, using the Vineland Adaptive Behavior Scales, Second Edition (Vineland-II) Survey Interview Form, a semi-structured interview designed to assess a child’s self-sufficiency and adaptive functioning in the domains of communication, daily living, socialization, and motor skills [29]. The four domains are further divided into 11 subdomains, as shown in Table 1.
Table 1.
The 4 Domains and 11 Subdomains that make up the overall Adaptive Behavior Composite scores on the Vineland Adaptive Behavior Scales, Survey Interview Form, Second Edition.
| Domain | Subdomain |
|---|---|
| Communication | Receptive |
| Expressive | |
| Written | |
| Daily Living Skills | Personal |
| Domestic | |
| Community | |
| Socialization | Interpersonal Relationships |
| Play and Leisure Time | |
| Coping Skills | |
| Motor Skills | Gross |
| Fine |
The subdomains yield v-scale scores that sum to yield the four domain composite scores, which are then standardized (mean=100, SD=15), and summed to produce the global Adaptive Behavior Composite (ABC) for individuals from birth through 6-years-old. The ABC score provides the overall assessment of an individual’s adaptive functioning. Lower Vineland scores indicate increased impairment in adaptive behavior. Standard scores can be grouped into ranges representing high, moderately high, adequate, moderately low, and low levels of adaptive functioning, based on the scores’ standard deviations from the expected mean, as shown in Table 2.
Table 2.
Adaptive level descriptions, modified from the Vineland-II Instruction Manual [29]. Standard scores are classified into adaptive levels based on their standard deviations from the expected mean of 100.
| Adaptive Level | Standard Deviations from the Mean | Standard Score Range | Percentile Rank Range |
|---|---|---|---|
| High | 2.0 or above | 130 and above | 98 and above |
| Moderately High | 1.0 – 2.0 | 115 – 129 | 85 – 97 |
| Adequate | −1.0 – 1.0 | 86 – 114 | 16 – 84 |
| Moderately Low | −2.0 – −1.0 | 71 – 85 | 3 – 15 |
| Low | −2.0 or below | 70 and below | 2 and below |
The Vineland-II was standardized based on a national sample of over 3,000 individuals selected to match U.S. Census data. Standard scores and adaptive behavior levels are measured relative to a non-clinical, age-matched reference group. The ABC and domain standard scores were the primary and secondary outcome variables. Adaptive levels and subdomains were then examined for a more nuanced analysis of differences in adaptive functioning among the three exposure groups.
Interviews took 60–90 minutes to complete, on average, and were conducted by non-blinded research coordinators (UD and RD) at Massachusetts General Hospital who were trained using the official Vineland-II training video and survey manual. Interviews were scored by hand or using the Vineland-II Survey Forms ASSIST software.
2.3. Statistical Analyses
Statistical analyses were completed by a research coordinator (UD) and a biostatistician (EM) at Massachusetts General Hospital’s Biostatistics Center using Statistical Analysis Software, version 9.4. The primary analysis examined baseline characteristics of the study participants, including drug exposure, epilepsy type, seizure frequency during pregnancy, child’s age, mother’s marital status, insurance coverage, maternal age at delivery, maternal education, prenatal vitamin and folate use, cigarette and alcohol exposure, presence of major malformations in the exposed child, gestational age at birth, birth weight, and birth length. Differences in covariates among the three exposure groups were examined using Pearson’s chi-squared tests and one-way ANOVA.
Mean adaptive behavior scores were calculated for the children in each exposure group. One-sample t-tests were used to test whether group means differed from the test normative value of 100. Mean adaptive behavior composite (ABC) scores and domain standard scores were analyzed using one-way ANOVA to identify differences among the three drug groups. Scores were compared in linear models without adjustment and after adjustment using propensity scores calculated from a model that included the following potential confounders: maternal age, education, folate use, cigarette and alcohol exposure, gestational age, and birth weight.
Confounding variables with incompletely available data were examined independently in lieu of inclusion in the propensity score analysis. These variables included: marital status, insurance coverage, seizure occurrence during pregnancy, presence of major malformations in the exposed child, and epilepsy type.
Measures that differed significantly among the three groups were tested for pairwise differences using Tukey post-hoc comparisons. Adaptive levels for each domain and subdomain were examined for frequency of moderately low or low levels (standard scores ≤ 85, or ≥ 1 SD from the expected mean of 100) of adaptive functioning in each of the three drug groups.
To examine dose-response relationships, the first trimester drug dose was derived by averaging the dose at the last menstrual period and any changes in dose through the first trimester, as reported by the mother. Standard adaptive behavior scores were correlated with the derived first trimester drug dose using linear models with exposure-dependent dose effects before and after adjustment for the same confounders included in the propensity score analysis. To isolate and examine the effect of exposure specifically to high doses of valproate, the propensity score analysis described above was repeated to compare only those VPA-exposed children exposed to less than 1000 mg/day with all CBZ- or LTG-exposed children. Significance was declared for two-tailed p<0.05 for all analyses.
2.4. Standard Protocol Approvals, Registrations, and Patient Consents
This study was reviewed and approved annually by the Partners Human Research Committee, the institutional review board for the Massachusetts General Hospital. All participating women provided informed written consent.
3. Results
3.1. Recruitment Process
Recruitment letters were mailed to 972 women (with 1,032 children) who had enrolled in the Registry while taking LTG, VPA, or CBZ as monotherapy to treat epilepsy throughout pregnancy, and whose exposed children were 3- to 6-years-old (mean 4.9 years, SD 1.1 years). Of those contacted, mothers of 495 children responded, 455 expressed interest, and 346 completed the initial screening to assess eligibility.
Of the 495 respondents, 149 were never screened, including 40 who explicitly refused to participate and 109 who never completed the screening interview. Of the 40 who refused to participate, 15 (37.5%) had children exposed to CBZ, 18 (45%) had LTG-exposed children, and 7 (17.5%) had VPA-exposed children (χ2=1.42; p = 0.482).
Among the remaining 109 mothers who were never screened, 46 (42.2%) were CBZ-exposed, 32 (29.4%) were LTG-exposed, and 31 (28.4%) were VPA-exposed (χ2=4.09; p = 0.130). Four (all mothers of VPA-exposed children) changed their minds and chose not to participate after initially expressing interest, including two mothers who said they did not have time, and two who refused to participate for “privacy concerns”. Of the remaining 105 women, 69 (65.7%) were found to be ineligible and 36 (33.0%) never responded to attempts to contact them to complete the screening. Among the 69 ineligible mothers, the most common reasons for ineligibility were: the child was too old (over 6-years-old) by the time the screening interview could be scheduled (62.3%); the AED was not taken throughout pregnancy (18.8%); the mother took more than one AED during pregnancy (10.1%); or the mother suffered from mental illness or memory disorder (4.3%). One mother (1.4%) was ineligible due to a hearing disorder. Finally eligibility could not be confirmed for two mothers due to incomplete screening interviews with missing data.
Of the 346 mothers that responded to recruitment letters and completed the initial screening, 94 were excluded from the study. The most common reasons for exclusion were: the child was too old (over 6-years-old) by the time of the interview (11.0%); failure to return consent forms (8.1%); inability to schedule an interview (7.8%); loss of interest or inability to meet the time commitment (2.9%); and failure to respond to follow-up calls (1.7%). Three subjects (0.9%) were excluded after discovering through medical records that the children were, in fact, exposed to polypharmacy during gestation. One participant was excluded after review of the mother’s neurology records by our epileptologists (BD and AK) raised doubt that she truly had epilepsy. Outcome data were missing for 25 subjects who had initially been eligible to participate, but who had subsequently aged out before interviews could be completed. After exclusions, 252 children met our inclusion criteria, including 97 CBZ-exposed, 104 LTG-exposed, and 51 VPA-exposed children.
3.2. Baseline Characteristics
The overall response rate was 48.0% (n=495). Response rates differed significantly among the LTG (57.1%), VPA (50.7%) and CBZ (41.1%) groups (χ2 = 20.87; p < 0.001). Women were more likely to respond to recruitment letters if they were married (χ2 = 26.64; p < 0.001), had private insurance (χ2 = 18.37; p = 0.001), were 30–34 years-old (χ2 = 36.50; p < 0.001), or had college degrees (χ2 = 48.22; p < 0.001). Data on major malformations (defined as a structural abnormality with surgical, medical, or cosmetic importance, and confirmed by LH through medical record reviews) were available for 253 children, including 215 who were enrolled in the study and 38 who were not included. Mothers of children with major malformations were significantly less likely to respond to recruitment letters (χ2 = 56.96; p < 0.001).
In the recruitment cohort, the three drug groups differed significantly with respect to insurance coverage (χ2 = 21.22; p = 0.002), marital status (χ2 = 29.53; p < 0.001), prenatal folate use (χ2 = 6.49; p < 0.039), cigarette (χ2 = 18.47; p = 0.001) and alcohol exposure (χ2 = 7.86; p = 0.020), epilepsy type (χ2 = 51.36; p < 0.001), seizures during pregnancy (χ2 = 12.57; p = 0.002), and presence of major malformations in the exposed child (χ2 = 12.84; p = 0.002).
In the final Vineland cohort of 252 children, however, significant differences in maternal education, marital status, presence of major malformations, prenatal folate exposure, epilepsy type, seizures during pregnancy, and child’s age at the time of the interview were observed, while all other group differences disappeared. Baseline characteristics of these children and their mothers, along with associated p-values, are summarized in Table 3.
Table 3.
Baseline Characteristics of 252 Children and their Mothers By Prenatal AED Exposure.
| CBZ | LTG | VPA | p-value | |
|---|---|---|---|---|
| N=97 | N=104 | N=51 | ||
| % (no.) | ||||
| Maternal Education | 0.032 | |||
| High School or Less | 2.1% (2) | 6.7% (7) | 9.8% (5) | |
| Some College | 21.1% (20) | 17.3% (18) | 23.5% (12) | |
| College Graduate | 38.9% (37) | 38.5% (40) | 52.9% (27) | |
| Post-Graduate | 37.9% (36) | 37.5% (39) | 13.7% (7) | |
| Insurance | p=0.101 | |||
| Canadian | 7.5% (3) | 1.2% (1) | 0.0% (0) | |
| Medicaid | 2.5% (1) | 1.2% (1) | 6.7% (2) | |
| Private | 90.0% (36) | 97.6% (81) | 93.3% (28) | |
| Marital Status | p=0.040 | |||
| Married | 96.0% (48) | 95.7% (88) | 84.2% (32) | |
| Unmarried | 4.0% (2) | 4.3% (4) | 15.8% (6) | |
| Multivitamin Use | p=0.114 | |||
| Yes | 63.8% (60) | 76.0% (79) | 76.5% (39) | |
| No | 36.2% (34) | 24.0% (25) | 23.5% (12) | |
| Folic Acid Use | p=0.017 | |||
| None | 31.2% (24) | 13.2% (12) | 25.6% (11) | |
| Some | 68.8% (53) | 86.8% (79) | 74.4% (32) | |
| Cigarette Exposure | p=0.675 | |||
| Yes | 11.6% (11) | 7.7% (8) | 7.8% (4) | |
| No | 35.8% (34) | 44.2% (46) | 45.1% (23) | |
| Don't remember | 52.6% (50) | 48.1% (50) | 47.1% (24) | |
| Alcohol Exposure | p=0.360 | |||
| Yes | 24.2% (23) | 23.1% (24) | 33.3% (17) | |
| No | 75.8% (72) | 76.9% (80) | 66.7% (34) | |
| Major Malformation | p=0.025 | |||
| Yes | 6.3% (5) | 3.3% (3) | 15.9% (7) | |
| No | 93.7% (74) | 96.7% (89) | 84.1% (37) | |
| Epilepsy Typea | p<0.001 | |||
| IGE | 5.2% (3) | 18.1% (15) | 60.0% (21) | |
| NCE | 41.4% (24) | 32.5% (27) | 25.7% (9) | |
| PE | 53.4% (31) | 49.4% (41) | 14.3% (5) | |
| Prenatal Seizures | p<0.001 | |||
| Yes | 16.0% (15) | 39.8% (41) | 16.3% (8) | |
| No | 84.0% (79) | 60.2% (62) | 83.7% (41) | |
| Mean Maternal Age at Delivery (yr) | 32.3±5.4 | 32.1±4.4 | 31.3±5.0 | p=0.474 |
| Gestational Age (wks) | 38.7±2.4 | 38.5±1.5 | 38.8±1.6 | p=0.434 |
| Birth Weight (kg) | 3.41±0.58 | 3.34±0.60 | 3.35±0.61 | p=0.701 |
| Birth Length (cm) | 50.8±3.1 | 50.6±3.4 | 51.3±2.8 | p=0.433 |
| Interview Age (yrs) | 5.3±1.1 | 4.6±1.1 | 4.9±1.1 | p<0.001 |
| First trimester drug dose (mg/day), avg (range) | 705 (100–2000) |
379 (75–1500) |
771 (100–1500) |
N/A |
IGE = Idiopathic Generalized Epilepsy, PE = Partial Epilepsy, NCE = Nonclassifiable Epilepsy.
3.3. Global ABC and Domain Comparisons
Adaptive behavior data were available for 252eligible children, including 97 CBZ-exposed, 104 LTG-exposed and 51 VPA-exposed. The different sample sizes reflect the steady decline in enrollment of VPA cases in the Registry over the last decade.
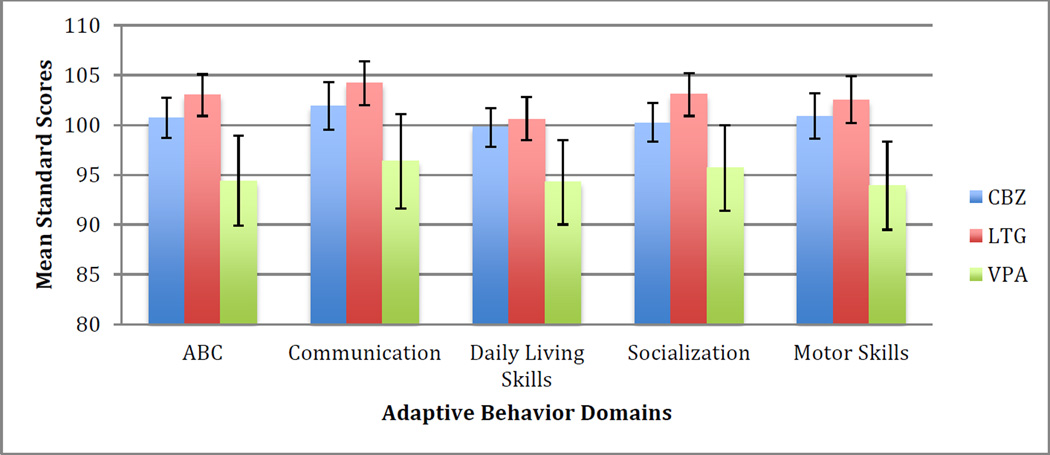
The mean ABC and domain standard scores for each drug group are presented in Figure 1. ABC standard scores of CBZ-exposed children (100.7± 9.9; t (96)=0.70, p=0.486, 95% CI [99, 103]) were not significantly different from the expected mean of 100. However, the mean scores for LTG-exposed children were significantly higher than expected (103.0 ± 11.0; t(103)=2.76, p=0.006, 95% CI [101, 105]), while those for VPA-exposed children were significantly lower than expected (94.4 ± 16.2; t(50)= −2.47, p=0.014, 95% CI [90, 99]). Group comparisons showed significant differences in the ABC and domain standard scores among the three groups (F2,249=3.53–5.94; p<0.031), with pairwise comparisons showing VPA-exposed children scoring significantly below LTG-exposed children in all domains, and significantly below CBZ-exposed children in the ABC and motor skills domains along with a trend toward significance in the domain of daily living skills.
Figure 1.
Mean ABC and Domain Standard Scores for each drug group, before adjusting for covariates.
The association of VPA with weaker performance persisted in nearly all domains after adjustment for propensity scores from maternal age, education, folate use, cigarette and alcohol exposure, gestational age, and birth weight. The mean adjusted ABC score for VPA-exposed children was 95.6 (95% CI [91, 100]), versus 100.8 (95% CI [98, 103]) for CBZ-exposed and 103.5 (95% CI [101, 106]) for LTG-exposed children (F2,187=4.14; p=0.017).
After adjusting for the same propensity scores, significant differences were observed among the three groups in the socialization and motor domains, and approached significance in the communication domain. In the socialization domain, the mean adjusted standard score for the VPA group was 97.6 (95% CI [93, 102]), compared to 100.3 (95% CI [98, 103]) and 103.7 (95% CI [101, 106]) for the CBZ and LTG groups, respectively (F2,187=3.73; p=0.026). In the communication domain, VPA-exposed children had a mean adjusted standard score of 97.0 (95% CI [91, 103]), compared to 102.5 (95% CI [99, 106]) and 104.4 (95% CI [102, 107]) for the CBZ and LTG groups, respectively (F2,187=2.99; p=0.053). In the motor domain, the mean adjusted standard score for the VPA groupwas94.4 (95% CI [89, 99]), compared to 101.1 (95% CI [98, 104]) and 102.8 (95% CI [100, 106]) for the CBZ and LTG groups, respectively (F2,187=4.12; p=0.018). Although a similar trend was observed in the domain of daily living, with VPA-exposed children performing lowest and LTG-exposed children performing highest, the cross-group differences were not significant in this category.
Tukey’s HSD post-hoc pairwise comparisons showed that, though the mean adjusted ABC, communication, socialization, and motor standard scores for the VPA group were persistently lower than those for both the LTG and CBZ groups, the differences were significant only when comparing results for the VPA and LTG groups. VPA-exposed children also performed substantially worse than CBZ-exposed children in the motor domain, but this difference was not statistically significant (p=0.060).
Independent analyses were completed for those confounding variables with large amounts of missing data. These included marital status, insurance coverage, seizure occurrence during pregnancy, presence of major malformations, and epilepsy type.
After adjusting for marital status, significant associations were observed between exposure and Vineland scores in the ABC, daily living, socialization, and motor skills domains (p-values=0.004–0.049). Relative to the CBZ and LTG groups, the VPA group scored lowest in the communication domain, but the difference was not statistically significant (p=0.077).
Vineland scores in the ABC, socialization, and motor domains also differed significantly among exposure groups (p=0.024–0.041) after adjusting for insurance coverage. However, group mean scores in the communication and daily living skills domains did not differ significantly after adjusting for this covariate (p=0.109).
The three exposure groups had significantly different scores in every tested domain after adjusting separately for seizure occurrence during pregnancy (p-values=0.003–0.036) and presence of major malformations in the exposed child (p-values=0.008–0.038), with VPA-exposed children persistently performing worse in all domains. For both categories, post-hoc pairwise comparisons demonstrated that VPA-exposed children scored significantly below LTG-exposed children in all domains, and significantly below CBZ-exposed children in the ABC, daily living skills, and motor skills domains.
Data on epilepsy type were available for only 177 participants. After adjusting for this covariate, significant differences were observed in the ABC, communication, socialization, and motor skills domains (p=0.002–0.033). Once again, although VPA-exposed children also scored lower than the other groups in the daily living skills domain, this difference was not statistically significant (p=0.067).
3.4. Adaptive Levels and Subdomain Comparisons
VPA-exposed children were more likely than the other two groups to perform at an adaptive level that was low or moderately low (standard scores ≤ 85) in each category, with significant differences observed in all except the communication domain and the subdomains of written and personal skills (Table 4).
Table 4.
Frequency of low and moderately low adaptive levels in the overall ABC, domain, and subdomain categories for each drug group. Significant p-values (p<0.05) are indicated in bold print.
| CBZ % (n) |
LTG % (n) |
VPA % (n) |
P-value | |
|---|---|---|---|---|
| ABC | 5.1% (5) | 2.9% (3) | 19.6% (10) | <0.001 |
| Communication | 10.2% (10) | 7.7% (8) | 17.6% (9) | 0.166 |
| Receptive | 11.2% (11) | 3.8% (4) | 17.6% (9) | 0.017 |
| Expressive | 6.1% (6) | 5.8% (6) | 33.3% (17) | <0.001 |
| Written | 9.2% (9) | 13.5% (14) | 19.6% (10) | 0.198 |
| Daily Living Skills | 5.1% (5) | 10.6% (11) | 17.6% (9) | 0.049 |
| Personal | 14.3% (14) | 15.4% (16) | 27.5% (14) | 0.103 |
| Domestic | 4.1% (4) | 4.8% (5) | 15.7% (8) | 0.016 |
| Community | 6.1% (6) | 10.6% (11) | 25.5% (13) | 0.002 |
| Socialization | 5.1% (5) | 4.8% (5) | 21.6% (11) | <0.001 |
| Interpersonal | 9.2% (9) | 5.8% (6) | 21.6% (11) | 0.002 |
| Play | 5.1% (5) | 3.8% (4) | 23.5% (12) | <0.001 |
| Coping | 12.2% (12) | 12.5% (13) | 27.5% (14) | 0.029 |
| Motor Skills | 8.2% (8) | 7.7% (8) | 31.4% (16) | <0.001 |
| Gross | 13.3% (13) | 6.7% (7) | 33.3% (17) | <0.001 |
| Fine | 10.2% (10) | 8.7% (9) | 23.5% (12) | 0.022 |
The VPA-exposed group had a disproportionate number scoring in the low or moderately low range compared to the CBZ and LTG groups. These findings suggest that, despite the adequacy of the overall group mean scores, the VPA-exposed group is more likely to possess clinically significant adaptive behavior impairments potentially requiring intervention.
Analysis of the subdomains also revealed significant differences in the communication subdomains of receptive and expressive communication, while there was no difference in the subdomain of written communication. More specifically, pairwise comparisons revealed that the VPA-exposed group was significantly more likely than the LTG-exposed group to score low or moderately low in the receptive (p=0.021) and expressive (p<0.001) communication subdomains. The VPA-exposed group was significantly more likely than the CBZ-exposed group to score low or moderately low in only the expressive subdomain (p<0.001). This suggests that the absence of a significant difference in the communication domain is largely explained by the relative strength of the writing skills of the VPA-exposed group despite the weaknesses that this group possesses in the areas of receptive and expressive communication.
The frequency distributions outlined above are also impressive in the sheer proportions of VPA-exposed children falling within the low or moderately low categories for each domain and subdomain. These data reveal that fully 15–33% of VPA-exposed children received standard scores that were greater than one standard deviation below the expected means in every single category.
Repeating the above analysis after adjusting for covariates within our propensity score model revealed that VPA-exposed children were significantly more likely than LTG-exposed children to score low or moderately low in the ABC, socialization, and motor domains, and in the expressive, interpersonal, play, and gross motor subdomains (p=0.002–0.037). In the adjusted analysis, the VPA group was also more likely than the LTG group to score low or moderately in the receptive, coping, and fine motor skills subdomains, but these differences were not statistically significant.
When compared to CBZ-exposed children, the VPA group was significantly more likely to score low or moderately low in the ABC, socialization, and motor domains, and in the expressive, play, and gross motor subdomains (p=0.001–0.050). VPA-exposed children were also more likely than CBZ-exposed children to score low or moderately low in the community and fine motor subdomains, but these differences did not meet statistical significance. No differences were observed between the LTG and CBZ groups. The adjusted odds ratios and p-values are presented in Table 5 below.
Table 5.
Odds ratios for low and moderately low adaptive levels in the ABC, domain, and subdomain categories for each exposure group, adjusted for propensity scores from maternal age, maternal education, folate use, cigarette and alcohol exposure, gestational age, and gestational weight. Significant p-values (p<0.05) are indicated in bold print.
| CBZ vs. LTG | CBZ vs. VPA | LTG vs. VPA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| ABC | 1.46 | 0.24–9.03 | 0.876 | 0.16 | 0.03–0.92 | 0.038 | 0.11 | 0.02–0.74 | 0.019 |
| Communication | 2.91 | 0.64–13.21 | 0.222 | 0.68 | 0.15–3.05 | 0.815 | 0.23 | 0.04–1.27 | 0.109 |
| Receptive | 6.31 | 0.92–43.22 | 0.064 | 0.97 | 0.19–4.94 | 0.999 | 0.15 | 0.02–1.30 | 0.099 |
| Expressive | 1.20 | 0.22–6.36 | 0.966 | 0.18 | 0.04–0.83 | 0.023 | 0.15 | 0.03–0.71 | 0.012 |
| Written | 0.67 | 0.19–2.39 | 0.744 | 0.49 | 0.10–2.45 | 0.551 | 0.73 | 0.17–3.20 | 0.869 |
| Daily Living Skills | 0.64 | 0.15–2.75 | 0.757 | 0.48 | 0.09–2.66 | 0.570 | 0.74 | 0.16–3.44 | 0.889 |
| Personal | 1.12 | 0.37–3.36 | 0.969 | 0.42 | 0.11–1.52 | 0.254 | 0.37 | 0.11–1.30 | 0.154 |
| Domestic | 0.98 | 0.16–5.97 | 0.999 | 0.37 | 0.06–2.45 | 0.433 | 0.38 | 0.06–2.39 | 0.433 |
| Community | 0.55 | 0.13–2.25 | 0.576 | 0.23 | 0.05–1.15 | 0.082 | 0.42 | 0.11–1.71 | 0.319 |
| Socialization | 1.54 | 0.18–13.15 | 0.885 | 0.15 | 0.02–1.00 | 0.050 | 0.10 | 0.01–0.89 | 0.037 |
| Interpersonal | 2.43 | 0.44–13.38 | 0.441 | 0.32 | 0.06–1.65 | 0.232 | 0.13 | 0.02–0.91 | 0.037 |
| Play | 1.17 | 0.17–7.88 | 0.979 | 0.15 | 0.03–0.90 | 0.035 | 0.13 | 0.02–0.83 | 0.027 |
| Coping | 1.47 | 0.43–5.06 | 0.743 | 0.39 | 0.10–1.56 | 0.251 | 0.27 | 0.07–1.07 | 0.067 |
| Motor Skills | 0.81 | 0.19–3.41 | 0.938 | 0.07 | 0.01–0.42 | 0.001 | 0.09 | 0.02–0.49 | 0.003 |
| Gross | 1.86 | 0.50–6.96 | 0.513 | 0.20 | 0.05–0.82 | 0.020 | 0.11 | 0.02–0.50 | 0.002 |
| Fine | 0.98 | 0.26–3.64 | 0.999 | 0.21 | 0.04–1.06 | 0.063 | 0.22 | 0.04–1.11 | 0.072 |
3.5. Dose-Response Relationship
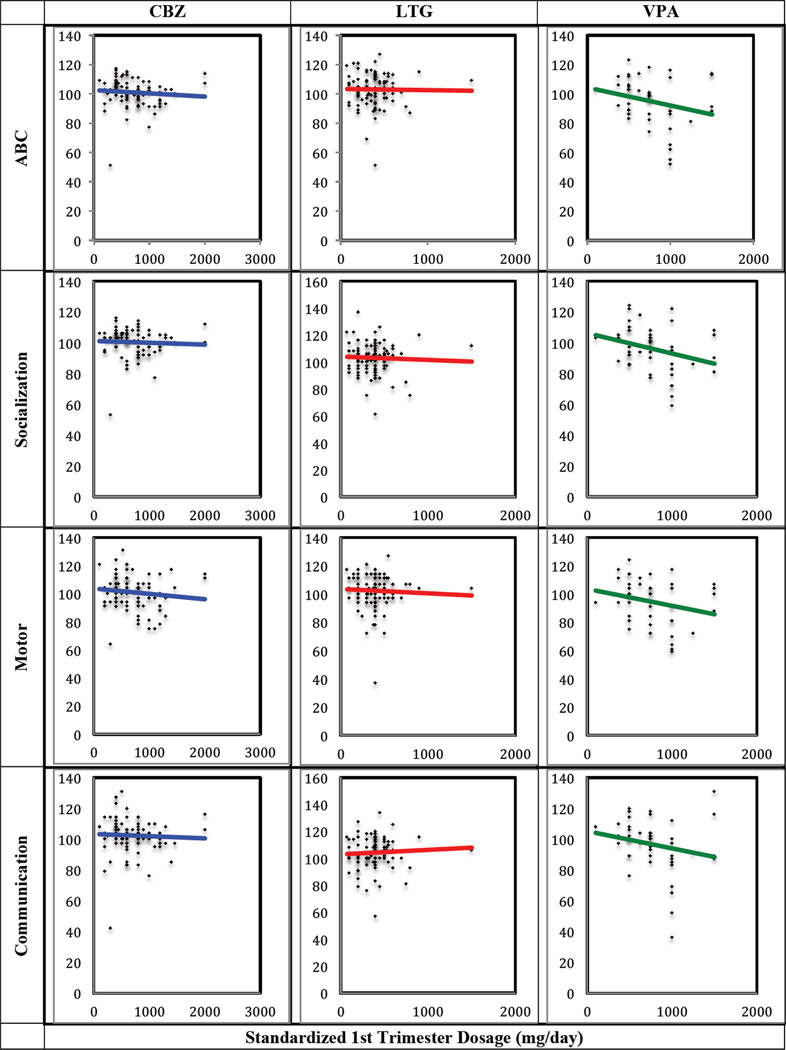
Regression analysis using general linear models with exposure-dependent dose effects identified a significant negative relationship between first trimester VPA drug dose and unadjusted ABC (t=−2.33; p=0.020), socialization (t=−2.64; p=0.009), and motor standard scores (t=−2.06; p=0.041), such that a higher VPA dose was associated with lower scores. There was also a trend toward a significant inverse relationship between VPA drug dose and communication standard scores (t=−1.92; p=0.056) (Table 6; Figure 2).
Table 6.
Estimates of exposure-dependent dose effect on Vineland performance in each tested domain (Adaptive Behavior Composite, Communication, Daily Living Skills, Socialization, and Motor Skills) for the three exposure groups. Significant p-values are shown in bold print.
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| Variable | Measure | Estimatea | Lower | Upper | t-value | P-value |
| Adaptive Behavior Composite (ABC) Score | CBZ | −0.209 | −0.863 | 0.445 | −0.63 | 0.529 |
| LTG | −0.081 | −1.246 | 1.084 | −0.14 | 0.891 | |
| VPA | −1.229 | −2.267 | −0.192 | −2.33 | 0.020 | |
| Communication Standard Score | CBZ | −0.136 | −0.858 | 0.587 | −0.37 | 0.712 |
| LTG | 0.331 | −0.957 | 1.618 | 0.51 | 0.613 | |
| VPA | −1.120 | −2.266 | 0.027 | −1.92 | 0.056 | |
| Daily Living Standard Score | CBZ | 0.071 | −0.573 | 0.715 | 0.22 | 0.829 |
| LTG | −0.063 | −1.210 | 1.084 | −0.11 | 0.914 | |
| VPA | −0.865 | −1.887 | 0.156 | −1.67 | 0.096 | |
| Socialization Standard Score | CBZ | −0.111 | −0.733 | 0.511 | −0.35 | 0.726 |
| LTG | −0.255 | −1.363 | 0.853 | −0.45 | 0.651 | |
| VPA | −1.320 | −2.307 | −0.334 | −2.64 | 0.009 | |
| Motor Standard Score | CBZ | −0.370 | −1.091 | 0.352 | −1.01 | 0.314 |
| LTG | −0.311 | −1.596 | 0.975 | −0.48 | 0.634 | |
| VPA | −1.195 | −2.339 | −0.050 | −2.06 | 0.041 | |
The estimates are the drug-specific slopes per 100 mg from a model that included exposure-by-dose interaction terms.
Figure 2.
Scatterplots and regression lines for Adaptive Behavior Composite (ABC), socialization, motor, and communication domain standard scores versus standardized 1st trimester dose (mg/day) for each exposure group.
VPA doses of 1000 mg/day or greater were significantly associated with lower ABC scores and standard scores across all domains. No dose-response effects were observed for the CBZ-exposed and LTG-exposed groups. Of the 51 children in the VPA-exposed group, 16 were exposed to doses greater than 1000 mg/day, and 31 were exposed to doses below 1000 mg/day. Reliable data on dose exposure were not available for 4 participants, so they were excluded from the dose response analysis. After excluding children exposed to VPA doses greater than 1000 mg/day, we found that the three groups performed similarly in all domains.
We also analyzed the association between standardized dose and the odds of achieving Vineland scores in the low or moderately low range after adjusting for maternal age, education, folate use, cigarette and alcohol exposure, and gestational age and weight. As VPA dose increased, the odds of achieving low or moderately low Vineland scores also increased in nearly every domain and subdomain, but only met a level of significance in the subdomains of interpersonal (adjusted OR 1.51 [95% CI:1.10–2.07]; p=0.010), play (adjusted OR 1.39 [95% CI: 1.03–1.87]; p=0.031), and gross motor skills (adjusted OR 1.36 [95% CI: 1.05–1.76]; p=0.018). The only category for which increasing VPA dose was not associated with increased risk for poor performance was the subdomain of written communication (adjusted OR 0.95 [95% CI: 0.70–1.31]; p=0.789). There was no dose effect on the prevalence of low or below average scores for the LTG and CBZ exposure groups.
4. Discussion
Prenatal exposure to VPA was associated with poorer adaptive behavior outcomes in all of the measured domains, when compared to the test mean and to children exposed to CBZ or LTG. Significant differences from the LTG-exposed group were noted in the ABC, socialization, communication, and motor domains after adjusting for propensity scores from a variety of potential confounders. While the VPA-exposed group scored the lowest in the daily living domain, these scores did not differ significantly when compared to the other drug groups. The VPA-exposed group was most likely to perform low or moderately low in every category, with significant differences in all except the communication domain. Closer examination of the communication subdomain scores revealed that the VPA group was significantly more likely than the LTG group to perform at a low or moderately low adaptive level in the subdomains of receptive and expressive communication, making up for these weaknesses with a relative strength in their writing skills. The VPA group was significantly more likely than the CBZ group to score low or moderately low in the expressive communication subdomain. Furthermore, the negative effect of VPA on adaptive behavior outcomes was dose-dependent.
Our results are consistent with previous reports that VPA exposure negatively impacts cognitive outcomes [13], [15], [16], [30], [31], [32], [33]. Like our findings, prior studies have identified communication deficits among VPA-exposed children, in the form of reduced verbal IQ and language impairments [13], [14], [34], [35], suggesting that valproate exposure may especially impact verbal skills.
Previous investigations also support our findings of poorer socialization [19] and motor skills [36] in VPA-exposed children. Relative deficits in these domains persisted throughout most of our analyses, before and after adjusting for many different covariates. While overall mean scores for the VPA-exposed group were within the average range, this group was significantly more likely to perform low or moderately low in these categories. Although, according to the Vineland’s percentile ranking, 20% of individuals in the general population would be expected to score in this range, the significant differences in prevalence of low or moderately low scores among the three exposure groups is noteworthy. These findings raise concerns for the ability of these children to perform in school, interact with their peers, and function independently in society. Vinten and colleagues also reported deficits in daily living skills among children exposed to VPA [19]. While group differences in this domain were not significant in the majority of our adjusted analyses, the mean daily living score for VPA-exposed children was below that of the other two drug groups, and the VPA-exposed group was significantly more likely to score low or moderately low in this category. These findings suggest that additional research is needed to understand the drug’s impact, if any, in this domain.
Of particular interest is the combination of socialization and communication (specifically receptive and expressive communication) deficits in the VPA-exposed children, which characterize symptoms commonly associated with autism [29]. Previous reports suggest that VPA exposure is associated with increased risk for autism spectrum disorder, when compared to children exposed to other AEDs or unexposed controls [21], [22], [23],[24]. Without thorough neurodevelopmental evaluation of these children, we cannot address an association between VPA exposure and autism in this study. However, the pattern of weaknesses observed in socialization and receptive and expressive communication is concerning for autistic traits.
These concerns are further compounded in light of the dose effects we observed for VPA, which are also supported by several previous investigations. Cohen and colleagues [37] found a dose-dependent decline in social and motor skills, as well as in parental ratings of adaptive functioning for children exposed to VPA. Other studies have reported dose-dependent language [34], [38] and IQ effects [16] in VPA-exposed children. Unlike our findings, however, several studies also found dose effects for motor function [37] and lower verbal abilities [38] following prenatal exposure to CBZ. While our findings suggest that CBZ poses a relatively low risk for adaptive behavior impairment with no dose effect, further research is necessary to examine the impact of the drug at higher doses.
Several studies have also suggested a possible threshold dose of 800 mg [13] – 1000 mg [1], [2], [5], [8], [16], [39],[40], [41],[42],[43] of VPA daily, beyond which major congenital malformations are more likely to occur. Furthermore, prior studies have described a dose-response relationship between IQ and VPA exposure, suggesting a similar threshold dose [16],[17],[44]. Our findings agree that first trimester VPA doses above 1000 mg/day are associated with a decline in adaptive function across all domains. However, rather than simply identifying a specific cut-off below which VPA might be considered safe, our findings importantly suggest a striking linear relationship between dose and behavioral outcomes. All women of reproductive age who are taking valproate for seizure prophylaxis should be informed of these risks, and should consequently be counseled about the critical need to evaluate the indication of valproate and to consider switching to another antiepileptic drug before becoming pregnant. Experience has shown that some women, whose seizures are only controlled by valproate, will choose to take the fetal risks [45]. This difficult choice underscores the need for more information on three critical issues: 1) the fetal risks from exposure to lower doses of valproate, i.e. < 1000 mg/d; 2) the experience with switching from valproate to other medications during pregnancy, considering the risks to both the mother and the fetus; 3) the option of using a gestational carrier to avoid these risks [46]. While it will take time to develop the information needed on the issues, it is crucial that clinicians provide continuing support to the pregnant woman, as she weighs her options.
Our study provides a unique evaluation of adaptive behavior outcomes of AED-exposed children, rather than relying on IQ or language tests to measure adaptive function or daily living skills. The use of the Vineland-II, a widely recognized, validated instrument for measurement of adaptive behavior, allows consideration of the true functional abilities of these children to live independently within the community, regardless of their intellectual capacity.
This study is also unique in its evaluation of 3- to 6-year-old children, distinguishing it from prior comparable studies that have evaluated younger children up to 3-years-old [16], or older children over 6 years of age [19]. Given that deficits in adaptive functioning are likely to become more evident with age, the evaluation of children over 3 years offers greater insight into long-term outcomes of AED exposure. At the same time, the age at diagnosis of autism spectrum disorders typically ranges from 3- to 6-years-old [47], thus making it more likely that older children with impairments will have already been identified, and will have already received early intervention services. This would potentially falsely elevate test scores, and could mask a drug’s true impact on adaptive function. Capturing children in this critical age range between 3- and 6-years-old thus allows us to evaluate children at a time when their impairments are evident but have likely not yet been amply addressed, potentially providing a more accurate measure of an exposed child’s baseline adaptive behavior before having received extensive interventions. However, our analysis did not control for receipt of previous early intervention services as a potential confounder. This would be recommended for a future study.
Our findings are further strengthened by the recruitment of all participants from the hospital-based North American AED Pregnancy Registry. This resource facilitated prospective data collection directly from a large number of U.S. and Canadian mothers with a wide-range of potential confounders, along with retrospective observational data on the adaptive behaviors of their exposed children.
Despite these strengths, further research is required including thorough, prospective, blinded neurodevelopmental evaluations of each child, along with inclusion of a control group and a larger study sample to determine the true prevalence of adaptive behavior impairments and autistic symptoms among AED-exposed children. A study that combines both cognitive and behavioral measures would be preferred. The dose effect observed in our study should be cautiously interpreted, in the absence of directly observed daily drug compliance during pregnancy, dosage information beyond the first trimester, data on maternal serum drug concentrations, or data on drug metabolism and clearance rates for participating mothers. Our study was also limited by the reliance on observational data collected through subjective, non-blinded interviews with the mother, and the lack of data collected on family history of neurodevelopmental disorders or delays. A future study should include collection of a detailed family history, given that a family history of neurodevelopmental delays would be an important potential confounding variable that could alter the interpretation of results of this study.
Furthermore, although our study controlled for a number of potential confounders, the variations in baseline characteristics and participation rates observed across drug groups suggest a sampling bias commonly encountered in pregnancy registries and prospective studies. However, while one cannot exclude the possibility that mothers who participated in the study were motivated by some underlying concern for their child’s development, we can be reassured by the fact that, when compared to the other groups, the LTG-exposed group had the highest response rate and the highest performance in all domains. Additionally, given the high level of education of participating mothers, one might argue that the adaptive behavior scores of the study subjects are in fact higher than we might expect from children of mothers with less education. Similarly, the fact that mothers of children with major malformations were significantly less likely to respond to recruitment letters, suggests that the most severely affected children tended not to participate. Thus, our study sample may actually be biased towards children with more favorable adaptive behavior outcomes. It is therefore unlikely that our findings have overestimated the relative impairments observed in the VPA-exposed group, and may even suggest that VPA-exposed children could fare worse than what we observed in our study.
Additionally, despite the large number of confounders included in our model, there are a number of other excluded variables that could be considered as possible sources of bias. For example, in our propensity score model, we did not adjust for marital status, insurance coverage, race, socioeconomic status, or other characteristics associated with social and emotional stressors or unstable home life, which could negatively impact a child’s adaptive functioning. Furthermore, our propensity score model did not include seizure occurrence, epilepsy type, and presence of major malformations in the exposed child. These variables were excluded from our model due to the large amount of missing data. Instead, associations between these variables and Vineland scores were examined independently. However, a future study with a larger sample size may benefit from inclusion of these variables as possible confounders, to allow for broader adjustments and remove the possible effects of such unfavorable factors that may influence the outcomes of interest.
Additionally, the age at which each child was assessed was not specifically adjusted for in our propensity score analysis, even though this variable differed significantly among exposure groups. This variable was not included in our model because the Vineland scores are in fact already age-adjusted, and so did not require additional adjustment in our analysis. Furthermore, post-hoc pairwise comparisons of the exposure groups in our study cohort revealed that only the CBZ and LTG groups differed significantly in this category. Since the interview age of the VPA-exposed group did not differ significantly from either the CBZ- or LTG-exposed group, this variable was deemed unlikely to be a true confounder given that all the significant differences noted among the group Vineland scores involved comparisons with VPA. In other words, the poorer performance of the VPA-exposed group relative to the other two exposure groups could not be explained by differences in age at the time of the interview.
Despite these limitations, our results contribute to the growing body of literature linking VPA exposure to poor neurodevelopmental outcomes in exposed children, with evidence of adaptive function declining in a linear dose-dependent fashion. Our findings compel us to advocate for complete avoidance of this drug among women of childbearing age with epilepsy whenever possible [45]. Finally, our findings suggest that valproate-exposed children are at higher risk for adaptive behavior problems, especially in the areas of socialization, communication, and motor skills. Since deficits in these areas can be tempered if identified and treated early, we recommend that pediatricians routinely conduct age-appropriate developmental monitoring and screening for this group of children, and maintain a low threshold for early intervention referrals in any child with even subtle signs of adaptive behavior impairments.
Highlights.
-
➢
Children prenatally exposed to valproate have poorer adaptive functioning compared to children prenatally exposed to lamotrigine or carbamazepine.
-
➢
Children prenatally exposed to valproate have poorer socialization, communication, and motor function, compared to lamotrigine or carbamazepine exposure.
-
➢
Prenatal exposure to higher valproate doses is associated with lower adaptive functioning, especially affecting socialization and motor skills.
Acknowledgments
The investigators thank the women and families who generously volunteered their time to participate in this study.
We would also like to acknowledge the tragic passing in 2013 of our co-author, Autumn Klein, MD, PhD. Her contribution to the field of Neurology in general, and Women’s Neurology in particular, was invaluable, and she continues to be sorely missed. It was an honor to collaborate with her on this project, and we hope that the knowledge gained from this work adds to her legacy.
We would also like to thank Abha Gupta, MD, PhD, Associate Research Scientist in the Department of Pediatrics and the Child Study Center at Yale University School of Medicine, for her assistance reviewing, proofreading, and editing this article.
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
This work was supported by GlaxoSmithKline.
References
- 1.Samren EB, van Duijn CM, Koch S, Hiilesmaa VK, Klepel H, Bardy AH, Mannagetta GB, Deichl AW, Gaily E, Granstrom ML, Meinardi H, Grobbee DE, Hofman A, Janz D, Lindhout D. Maternal use of antiepileptic drugs and the risk of major congenital malformations: a joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia. 1997;38:981–990. doi: 10.1111/j.1528-1157.1997.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 2.Meador KJ, Baker GA, Finnell RH, Kalayjian LA, Liporace JD, Loring DW, Mawer G, Pennell PB, Smith JC, Wolff MC, Group NS. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407–412. doi: 10.1212/01.wnl.0000227919.81208.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean JC, Hailey H, Moore SJ, Lloyd DJ, Turnpenny PD, Little J. Long term health and neurodevelopment in children exposed to antiepileptic drugs before birth. J Med Genet. 2002;39:251–259. doi: 10.1136/jmg.39.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell E, Kennedy F, Russell A, Smithson WH, Parsons L, Morrison PJ, Liggan B, Irwin B, Delanty N, Hunt SJ, Craig J, Morrow J. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland Epilepsy and Pregnancy Registers. J Neurol Neurosurg Psychiatry. 2014;85:1029–1034. doi: 10.1136/jnnp-2013-306318. [DOI] [PubMed] [Google Scholar]
- 5.Morrow J, Russell A, Guthrie E, Parsons L, Robertson I, Waddell R, Irwin B, McGivern RC, Morrison PJ, Craig J. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77:193–198. doi: 10.1136/jnnp.2005.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artama M, Auvinen A, Raudaskoski T, Isojarvi I, Isojarvi J. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology. 2005;64:1874–1878. doi: 10.1212/01.WNL.0000163771.96962.1F. [DOI] [PubMed] [Google Scholar]
- 7.Wyszynski DF, Nambisan M, Surve T, Alsdorf RM, Smith CR, Holmes LB. Antiepileptic Drug Pregnancy R. Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology. 2005;64:961–965. doi: 10.1212/01.WNL.0000154516.43630.C5. [DOI] [PubMed] [Google Scholar]
- 8.Wide K, Winbladh B, Kallen B. Major malformations in infants exposed to antiepileptic drugs in utero, with emphasis on carbamazepine and valproic acid: a nation-wide, population-based register study. Acta Paediatr. 2004;93:174–176. doi: 10.1080/08035250310021118. [DOI] [PubMed] [Google Scholar]
- 9.Scolnik D, Nulman I, Rovet J, Gladstone D, Czuchta D, Gardner HA, Gladstone R, Ashby P, Weksberg R, Einarson T, et al. Neurodevelopment of children exposed in utero to phenytoin and carbamazepine monotherapy. JAMA. 1994;271:767–770. [PubMed] [Google Scholar]
- 10.Titze K, Koch S, Helge H, Lehmkuhl U, Rauh H, Steinhausen HC. Prenatal and family risks of children born to mothers with epilepsy: effects on cognitive development. Dev Med Child Neurol. 2008;50:117–122. doi: 10.1111/j.1469-8749.2007.02020.x. [DOI] [PubMed] [Google Scholar]
- 11.Veiby G, Daltveit AK, Schjolberg S, Stoltenberg C, Oyen AS, Vollset SE, Engelsen BA, Gilhus NE. Exposure to antiepileptic drugs in utero and child development: a prospective population-based study. Epilepsia. 2013;54:1462–1472. doi: 10.1111/epi.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore SJ, Turnpenny P, Quinn A, Glover S, Lloyd DJ, Montgomery T, Dean JC. A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genet. 2000;37:489–497. doi: 10.1136/jmg.37.7.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adab N, Kini U, Vinten J, Ayres J, Baker G, Clayton-Smith J, Coyle H, Fryer A, Gorry J, Gregg J, Mawer G, Nicolaides P, Pickering L, Tunnicliffe L, Chadwick DW. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2004;75:1575–1583. doi: 10.1136/jnnp.2003.029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinten J, Adab N, Kini U, Gorry J, Gregg J, Baker GA. Liverpool, Manchester Neurodevelopment Study G. Neuropsychological effects of exposure to anticonvulsant medication in utero. Neurology. 2005;64:949–954. doi: 10.1212/01.WNL.0000154514.82948.69. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson K, Viinikainen K, Monkkonen A, Aikia M, Nieminen P, Heinonen S, Kalviainen R. Children exposed to valproate in utero--population based evaluation of risks and confounding factors for long-term neurocognitive development. Epilepsy Res. 2005;65:189–200. doi: 10.1016/j.eplepsyres.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW. Group NS. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360:1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meador KJ, Baker GA, Browning N, Cohen MJ, Bromley RL, Clayton-Smith J, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW. Group NS. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244–252. doi: 10.1016/S1474-4422(12)70323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viinikainen K, Eriksson K, Monkkonen A, Aikia M, Nieminen P, Heinonen S, Kalviainen R. The effects of valproate exposure in utero on behavior and the need for educational support in school-aged children. Epilepsy Behav. 2006;9:636–640. doi: 10.1016/j.yebeh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Vinten J, Bromley RL, Taylor J, Adab N, Kini U, Baker GA. Liverpool, Manchester Neurodevelopment G. The behavioral consequences of exposure to antiepileptic drugs in utero. Epilepsy Behav. 2009;14:197–201. doi: 10.1016/j.yebeh.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Cohen MJ, Meador KJ, Browning N, May R, Baker GA, Clayton-Smith J, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW, group Ns. Fetal antiepileptic drug exposure: Adaptive and emotional/behavioral functioning at age 6years. Epilepsy Behav. 2013;29:308–315. doi: 10.1016/j.yebeh.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christensen J, Gronborg TK, Sorensen MJ, Schendel D, Parner ET, Pedersen LH, Vestergaard M. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309:1696–1703. doi: 10.1001/jama.2013.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bromley RL, Mawer G, Clayton-Smith J, Baker GA. Liverpool, Manchester Neurodevelopment G. Autism spectrum disorders following in utero exposure to antiepileptic drugs. Neurology. 2008;71:1923–1924. doi: 10.1212/01.wnl.0000339399.64213.1a. [DOI] [PubMed] [Google Scholar]
- 23.Rasalam AD, Hailey H, Williams JH, Moore SJ, Turnpenny PD, Lloyd DJ, Dean JC. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol. 2005;47:551–555. doi: 10.1017/s0012162205001076. [DOI] [PubMed] [Google Scholar]
- 24.Bromley RL, Mawer GE, Briggs M, Cheyne C, Clayton-Smith J, Garcia-Finana M, Kneen R, Lucas SB, Shallcross R, Baker GA. Liverpool, Manchester Neurodevelopment G. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84:637–643. doi: 10.1136/jnnp-2012-304270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Psychiatric A, American Psychiatric A, Force DSMT. Diagnostic and statistical manual of mental disorders : DSM-5. 2013 [Google Scholar]
- 26.Kanne SM, Gerber AJ, Quirmbach LM, Sparrow SS, Cicchetti DV, Saulnier CA. The role of adaptive behavior in autism spectrum disorders: implications for functional outcome. J Autism Dev Disord. 2011;41:1007–1018. doi: 10.1007/s10803-010-1126-4. [DOI] [PubMed] [Google Scholar]
- 27.Klin A, Saulnier CA, Sparrow SS, Cicchetti DV, Volkmar FR, Lord C. Social and communication abilities and disabilities in higher functioning individuals with autism spectrum disorders: the Vineland and the ADOS. J Autism Dev Disord. 2007;37:748–759. doi: 10.1007/s10803-006-0229-4. [DOI] [PubMed] [Google Scholar]
- 28.Holmes LB, Mittendorf R, Shen A, Smith CR, Hernandez-Diaz S. Fetal effects of anticonvulsant polytherapies: different risks from different drug combinations. Arch Neurol. 2011;68:1275–1281. doi: 10.1001/archneurol.2011.133. [DOI] [PubMed] [Google Scholar]
- 29.Sparrow SS, Balla DA, Cicchetti DV, Doll EA. Circle Pines. Minn: AGS Publishing; 2005. Vineland-II : Vineland adaptive behavior scales : survey forms manual. [Google Scholar]
- 30.Adab N, Jacoby A, Smith D, Chadwick D. Additional educational needs in children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2001;70:15–21. doi: 10.1136/jnnp.70.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cummings C, Stewart M, Stevenson M, Morrow J, Nelson J. Neurodevelopment of children exposed in utero to lamotrigine, sodium valproate and carbamazepine. Arch Dis Child. 2011;96:643–647. doi: 10.1136/adc.2009.176990. [DOI] [PubMed] [Google Scholar]
- 32.Shallcross R, Bromley RL, Irwin B, Bonnett LJ, Morrow J, Baker GA. Liverpool Manchester Neurodevelopment G, Epilepsy UK, Pregnancy R. Child development following in utero exposure: levetiracetam vs sodium valproate. Neurology. 2011;76:383–389. doi: 10.1212/WNL.0b013e3182088297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ardinger HH, Atkin JF, Blackston RD, Elsas LJ, Clarren SK, Livingstone S, Flannery DB, Pellock JM, Harrod MJ, Lammer EJ, et al. Verification of the fetal valproate syndrome phenotype. Am J Med Genet. 1988;29:171–185. doi: 10.1002/ajmg.1320290123. [DOI] [PubMed] [Google Scholar]
- 34.Nadebaum C, Anderson VA, Vajda F, Reutens DC, Barton S, Wood AG. Language skills of school-aged children prenatally exposed to antiepileptic drugs. Neurology. 2011;76:719–726. doi: 10.1212/WNL.0b013e31820d62c7. [DOI] [PubMed] [Google Scholar]
- 35.Gaily E, Kantola-Sorsa E, Hiilesmaa V, Isoaho M, Matila R, Kotila M, Nylund T, Bardy A, Kaaja E, Granstrom ML. Normal intelligence in children with prenatal exposure to carbamazepine. Neurology. 2004;62:28–32. doi: 10.1212/wnl.62.1.28. [DOI] [PubMed] [Google Scholar]
- 36.Thomas SV, Ajaykumar B, Sindhu K, Nair MK, George B, Sarma PS. Motor and mental development of infants exposed to antiepileptic drugs in utero. Epilepsy Behav. 2008;13:229–236. doi: 10.1016/j.yebeh.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Cohen MJ, Meador KJ, Browning N, Baker GA, Clayton-Smith J, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW. Fetal antiepileptic drug exposure: motor, adaptive, and emotional/behavioral functioning at age 3 years. Epilepsy Behav. 2011;22:240–246. doi: 10.1016/j.yebeh.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meador KJ, Baker GA, Browning N, Cohen MJ, Clayton-Smith J, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW, Group NS. Foetal antiepileptic drug exposure and verbal versus non-verbal abilities at three years of age. Brain. 2011;134:396–404. doi: 10.1093/brain/awq352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omtzigt JG, Los FJ, Grobbee DE, Pijpers L, Jahoda MG, Brandenburg H, Stewart PA, Gaillard HL, Sachs ES, Wladimiroff JW, et al. The risk of spina bifida aperta after first-trimester exposure to valproate in a prenatal cohort. Neurology. 1992;42:119–125. [PubMed] [Google Scholar]
- 40.Samren EB, van Duijn CM, Christiaens GC, Hofman A, Lindhout D. Antiepileptic drug regimens and major congenital abnormalities in the offspring. Ann Neurol. 1999;46:739–746. [PubMed] [Google Scholar]
- 41.Mawer G, Clayton-Smith J, Coyle H, Kini U. Outcome of pregnancy in women attending an outpatient epilepsy clinic: adverse features associated with higher doses of sodium valproate. Seizure. 2002;11:512–518. doi: 10.1016/s1059-1311(02)00135-8. [DOI] [PubMed] [Google Scholar]
- 42.Alsdorf R, Wyszynski DF. Teratogenicity of sodium valproate. Expert Opin Drug Saf. 2005;4:345–353. doi: 10.1517/14740338.4.2.345. [DOI] [PubMed] [Google Scholar]
- 43.Vajda FJ, Hitchcock A, Graham J, Solinas C, O'Brien TJ, Lander CM, Eadie MJ. Foetal malformations and seizure control: 52 months data of the Australian Pregnancy Registry. Eur J Neurol. 2006;13:645–654. doi: 10.1111/j.1468-1331.2006.01359.x. [DOI] [PubMed] [Google Scholar]
- 44.Baker GA, Bromley RL, Briggs M, Cheyne CP, Cohen MJ, Garcia-Finana M, Gummery A, Kneen R, Loring DW, Mawer G, Meador KJ, Shallcross R, Clayton-Smith J. Liverpool, Manchester Neurodevelopment G. IQ at 6 years after in utero exposure to antiepileptic drugs: a controlled cohort study. Neurology. 2015;84:382–390. doi: 10.1212/WNL.0000000000001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomson T, et al. Valproate in the treatment of epilepsy in girls and women of childbearing potential. Epilepsia. 2015 Jul;56(7):1006–1019. doi: 10.1111/epi.13021. Epub 2015 May 16. [DOI] [PubMed] [Google Scholar]
- 46.Dar S, Lazer T, Swanson S, Silverman J, Wasser C, Moskovtsev SI, Sojecki A, Librach CL. Assisted reproduction involving gestational surrogacy: an analysis of the medical, psychosocial and legal issues: experience from a large surrogacy program. Hum Reprod. 2015;30:345–352. doi: 10.1093/humrep/deu333. [DOI] [PubMed] [Google Scholar]
- 47.Mandell DS, Novak MM, Zubritsky CD. Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics. 2005;116:1480–1486. doi: 10.1542/peds.2005-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]