Abstract
Chapter 3 “Tumours of the hypopharynx, larynx, trachea, and parapharyngeal space” of the World Health Organization (WHO) Blue Book 2017 “Classification of Head and Neck Tumours” shows a shortened list of entities, especially due to reducing the number of benign and malignant soft tissue tumours, malignant melanoma and some others, which are transferred to more frequently affected regions of the head and neck. The basic concept of the new edition is to assimilate all advances concerning the discussed tumours in a shorter framework, appropriate for daily work. The main emphasis is on the most frequent lesions and tumors originating from the covering squamous epithelium. Laryngeal and hypopharyngeal conventional squamous cell carcinoma (CSCC), its variants and precursor lesions, occupy a major part of the chapter. New data on etiopathogenesis, with the focus on human papillomavirus (HPV) infection, are discussed in relation to the entities of the squamous epithelium. Although only a small fraction of these lesions are HPV-related, further studies are required for evaluation of the potential prognostic and therapeutic benefit of mRNA HPV determination. In contrast to earlier data, laryngeal and hypopharyngeal verrucous SCC, spindle cell SCC and basaloid SCC are not anymore considered as HPV-related tumours. New data on the pathogenesis of spindle cell SCC exhibiting divergent differentiation by epithelial—mesenchymal transition, are also briefly discussed. The most important innovation is brought by the section on precursor lesions, in which a unified two-tier classification, consisting of low- and high-grade dysplasia, is introduced. The proposed two-tier system can also be transformed into a three-tier classification for treatment purposes, with a distinction between carcinoma in situ and high-grade dysplasia. The reviewed morphological criteria of the proposed system are based on the amended Ljubljana classification. The section on laryngeal neuroendocrine carcinomas (NEC) represents a considerable improvement in terminology and classification. NEC are divided into well-, moderate- and poorly-differentiated neuroendocrine carcinoma. The latter is additionally divided into small cell NEC and large cell NEC (LCNEC). It is of extreme importance that LCNEC, which was associated in the WHO 2005 edition with atypical carcinoid/moderately differentiated neuroendocrine carcinoma, grade II, has now been transferred into the group of poorly differentiated NEC, grade III, displaying a specific morphology and poorer prognosis.
Keywords: Larynx; Precursor lesions, classification; Conventional squamous cell carcinoma; Variants of conventional squamous cell carcinoma; HPV infection; Neuroendocrine carcinomas
Introduction
The 2017 edition of the WHO Blue Book “Tumours of the Head and Neck” is a keenly awaited publication, updated with new information provided throughout by recent the epidemiology, etiology, pathogenesis, histologic classification, immunohistochemistry, molecular genetics, differential diagnosis with potential pitfalls, and prognosis of head and neck tumours. The list of entities is significantly reduced, especially in relation to benign and malignant soft tissue tumours, malignant melanoma and some others, which are discussed in different, more frequently affected regions of the head and neck. The basic concept of the new edition is to assimilate all advances in a reasonable framework appropriate for daily routine work.
Parapharyngeal space is the new anatomical subsite, grouped with the larynx, hypopharynx and trachea, while anatomically oropharynx would be perhaps more appropriate. It is not actually addressed with the two most common tumors of this area, pleomorphic adenoma and schwannoma, although it is mentioned in the section of Inflammatory myofibroblastic tumor.
Chapter 3 of laryngeal and hypopharyngeal tumours is mainly dedicated to the most frequent lesions and tumours originating from the covering squamous epithelium. Entities arising from the underlying soft tissues, elements of the salivary glands and cartilage have a minor role and are not the subject of significant differences in comparison with the previous edition.
Laryngeal and hypopharyngeal conventional squamous cell carcinoma (CSCC) and its variants occupy a major part of the chapter. New data on etiopathogenesis, with a specific focus on the role of human papillomavirus (HPV) infection in precursor lesions, CSCC and subtypes, are discussed in this section [1–6]. The recently clarified probable pathogenetic mechanism of spindle cell SCC (SpSCC) is also presented [7, 8].
The main advance provided in this chapter relates to precursor lesions. The era of gallimaufry, with several classifications of laryngeal precursor lesions with non-comparable histologic criteria and terminology, is over and hopefully all now history. WHO 2017 introduces a two-tier classification with overall consensus, consisting of low- and high-grade dysplasia; low- and high-grade squamous intraepithelial lesion is offered as a synonym. The proposed two-tier system can also be transformed into a three-tier classification for treatment purposes, with a distinction between carcinoma in situ (CIS) and high-grade dysplasia. The group of CIS is reserved for rare cases with pronounced architectural disorder, severe cellular and nuclear atypias and an increased number of mitoses, including atypical forms. Such a distinction can facilitate clinical decisions about treatment modalities for patients with laryngeal high-grade lesions and CIS [6, 9, 10].
A special section is devoted to laryngeal neuroendocrine carcinomas. Due to previous disagreement about this topic, especially in terms of the classification, the new edition brings considerable clarification. The terminology and classification of laryngeal neuroendocrine carcinomas has thus been changed to well-, moderate-, and poorly-differentiated neuroendocrine carcinoma (NEC). Particular attention is devoted to two histological subtypes of poorly differentiated NEC: small cell NEC (SmCNEC) and large cell NEC (LCNEC). LCNEC, which was grouped in the previous edition, WHO 2005, with atypical carcinoid/moderately-differentiated neuroendocrine carcinoma, grade II, is now transferred to a subtype of poorly differentiated NEC, grade III, since these tumours show a specific morphology and are associated with a poorer outcome [11–13].
Etiopathogenesis of Conventional Squamous Cell Carcinoma, Subtypes and Precursor Lesions
Smoking and alcohol abuse are still the main risk factors in laryngeal and hypopharyngeal carcinogenesis. Over the last three decades, the high-risk HPV genotypes (hrHPV) have been confirmed as the major etiologic factor of the subset of oropharyngeal SCC, dominating in the western part of the world [14, 15]. The significance of hrHPV infection in the development of tumours in oral cavity, hypopharynx and larynx needs to be additionally elucidated [1, 2, 16–18]. Controversial data on hrHPV DNA prevalence in hypopharyngeal and laryngeal SCC has been published; prevalence varied considerably, from 5 to 60% [4, 19, 20]. Such a difference is most likely due to variability of sampling and methods used for detection of hrHPVs in different studies. Moreover, hrHPV DNA was also detected in healthy laryngeal tissue and is considered in this form only as a bystander [21, 22]. The mere presence of HPV DNA in clinical samples does not therefore reliably imply a viral involvement in carcinogenesis. HPV-induced carcinogenesis is related to disruption of intracellular control of hrHPV E6 and E7 oncogene functions, caused by integration of viruses into the host genome. Thus only integrated and transcriptionally active forms of hrHPV contribute in HPV-related carcinogenesis. HPV E6 and E7 oncoproteins are now established as standard biomarkers for oncogenic activity of hrHPVs, together with more than 70% positivity of p16 protein in tumour cells [4, 18, 19, 23, 24]. HPV-related laryngeal and hypopharyngeal SCC is histologically mostly non-keratinizing SCC, but focally may be also keratinizing. Unlike in the oropharynx, the morphology of laryngeal SCC does not predict a viral etiology [19]. The most common HPV genotype detected in laryngeal CSCC is HPV-16. Recent data from seven studies of laryngeal SCC revealed that only 66 of 1359 laryngeal SCC (4.9%) were positive for hrHPV mRNA [1, 18, 19, 25–28]. These results confirm that only a small fraction of laryngeal SCC are HPV-related (Fig. 1a–c). However, further studies are required for reliable evaluation of the potential prognostic and therapeutic benefit of HPV mRNA determination [19]. A combination of multifactorial influences on laryngeal and hypopharyngeal carcinogenesis, such as smoking and alcohol abuse, together with hrHPV detection, can additionally cloud the role of viral infection in the biological behaviour of laryngeal and hypopharyngeal SCCs.
Fig. 1.

Invasive non-keratinizing squamous cell carcinoma of the laryngeal ventricular fold. a Large islands of tumor cells with well-defined borders on the right and central side, on the left bottom side, more infiltrative growth pattern is present. b The same part of the tumor showing strong and diffuse p16 immunostaining, both nuclear and cytoplasmic. c The same part of the tumor; cancer cells show positive in situ hybridization for E6/E7 mRNA for high-risk HPV with numerous dot-like signals highlighting the viral infection
Among subtypes of laryngeal CSCC, the papillary variant is most frequently related to HPV infection [29–31]. The presence of HPV mRNA was detected in 5 of 22 (26.3%) papillary SCC, while p16 immunohistochemistry was positive in 13.6% of cases. These tumours showed a keratinizing and nonkeratinizing morphology. Interestingly, papillary SCC of the larynx shows some similarities to HPV-related CSCC in terms of prevalence and morphological data [31, 32]. The larynx is also the most frequent site of adenosquamous SCC in the head and neck region [27, 33]; however, none of them has been shown to be HPV mRNA positive [27]. The same holds for verrucous SCC (VSCC). Three studies have recently investigated the possible etiological role of HPV in VSCC. Using highly sensitive and specific molecular methods, it was shown that HPV of α, γ and μ genera are not etiopathogenetically associated with the VSCC of the head and neck region. Furthermore, no evidence of transcriptionally active high-risk-α-HPV was found in VSCC by real-time polymerase chain reaction for HPV E6/E7 mRNA. Thus, it appears that VSCC of the head and neck (Fig. 2a) is not associated with infection with HPV [34–36].
Fig. 2.
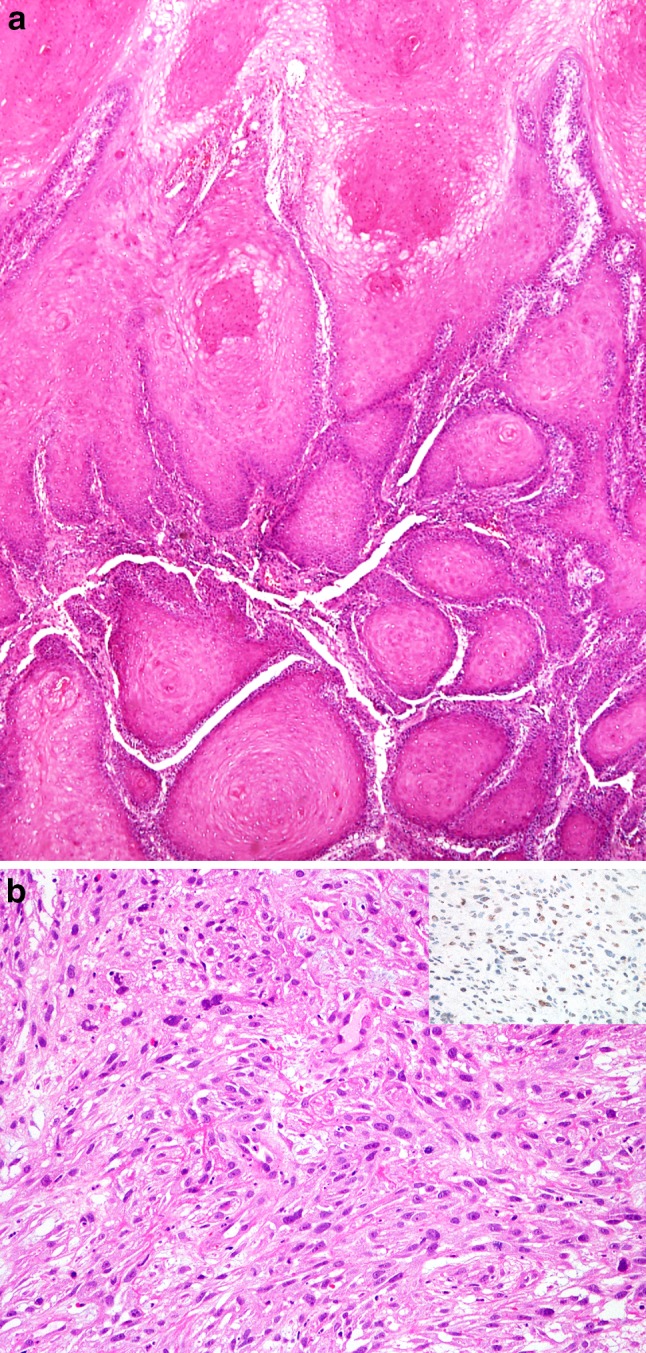
a Verrucous carcinoma. Prominent surface keratinization, projection and invagination of well-differentiated squamous epithelium invading the stroma with pushing margins. b Spindle cell carcinoma of the larynx. Proliferation of malignant spindle cells with marked mitotic activity. b Inset Positive immunohistochemical reaction for Snail in some tumor cells
In addition, most spindle cell carcinoma (SpCC), an uncommon variant of CSCC, including those arising in the oropharynx, is not related to transcriptionally active HPV [37]. However, in relation to SpCC histogenesis, which is only briefly mentioned in WHO 2017, it is important to highlight that SpCC is a monoclonal neoplasm originating from a non-committed stem cell, giving rise to both epithelial and mesenchymal components [38, 39]. Recent studies suggest that the characteristic spindle cell phenotype of the neoplastic cells in SpCC is the result of epithelial–mesenchymal transition. It has been postulated as a versatile mechanism that facilitates cellular reposition during embryonal development and can be reactivated in later life, contributing to various pathologic processes, including the progression of malignant tumours and the development of SpCC [7, 8]. Several features of SpCC support this hypothesis, e.g., an altered composition of cell-to-cell contacts (adherens junctions, desmosomes) and upregulation of transcription repressors, e.g., Snail, Slug, SIP, and Twist [7, 8, 40] (Fig. 2b, b insert). These transcription repressors have been demonstrated in experimental models to be potent inducers of epithelial–mesenchymal transition.
As with laryngeal SCC and HPV infection, only a few studies of laryngeal precursor lesions have to date been devoted to this topic. The overall prevalence of HPV infection in studies published since 2005 was 8.5%, ranging from 0 to 38.5% [22, 26, 41–44]. As recently described by Chernock et al., there is currently no indication for routine clinical HPV testing of laryngeal dysplasia or carcinoma in situ of the upper aerodigestive tract, although recognition of HPV as important carcinogen in several related premalignant lesions may have an important role in disease prevention, particularly as argument for HPV vaccination advocacy [5].
In recurrent respiratory papillomatosis, the rate of dysplasia varied significantly between the adult- and juvenile-onset of disease. About one-tenth (10/96) of patients with the adult onset showed dysplasia or carcinoma in situ and half of them were diagnosed with invasive SCC-ex papillomatosis. In contrast, none of the 63 patients with the juvenile onset of the disease exhibited dysplasia or carcinoma in situ, but 5% (3/63) were diagnosed with invasive pulmonary carcinoma-ex papillomatosis. In this large series, the age of disease onset was the strongest predictor of dysplastic transformation. However, history of smoking in 6/10 patients with adult-onset and dysplasia has to be considered as an additional etiopathogenetic factor [45].
Histological Classification and Biological Behaviour of Precursor Lesions
After many decades, head and neck pathologists have been able to consolidate different viewpoints on laryngeal precursor lesions and a unified classification with well-defined morphological criteria is presented in WHO 2017. The three classifications of laryngeal dysplasia/squamous intraepithelial neoplasia/SIL presented in WHO 2005, as a result of compromise, have various morphological criteria with different numbers of grades and heterogeneous terminologies. The treatment approaches of cases classified according to these grading systems are not harmonized and their results have proved not to be comparable [6, 9, 46]. In recent inter-observer studies, in which pathologists used all three classifications, no significant advantage was found for any of the applied grading systems and inter-observer agreements among pathologists were not encouraging [47–49]. Despite a certain degree of subjectivity, grading precursor lesions in the upper aerodigestive tract remains the most important prognostic factor for the biological behaviour of disease and the leading guidance for clinicians in selecting appropriate treatment. The new WHO 2017 classification is a two-tier system, based on the morphological criteria of the amended Ljubljana classification, which were confirmed to have better inter-observer agreement than had been found in previous studies and with the largest retrospective follow-up study, which presented a highly significant difference in the risk of malignant progression between low-grade SILs and high-grade SILs [6, 9]. The morphological criteria of the WHO 2017 classification are reviewed in Table 1 and Fig. 3a–e. Although the new grading system is presented as a two-tier system, a transition to three-tier classification is also offered when, for treatment purposes, the high-grade category is divided into high-grade dysplasia and CIS. The group of CIS is therefore reserved for rare cases with pronounced architectural disorder, severe cellular and nuclear atypias and an increased number of mitoses, also atypical forms. Such a distinction can facilitate clinical decisions about treatment modalities for patients with laryngeal high-grade lesions and CIS. The risk and progression to invasive growth of patients with laryngeal dysplasia was presented in a systemic review of nine cases series with 940 cases and a meta-analysis. The overall malignant transformation rate was 14% and the mean time to malignant transformation 5.8 years. The progression to overt malignancy was higher with increased severity of dysplasia: patients with severe dysplasia/carcinoma in situ in 30.4% versus mild/moderate dysplasia in 10.6% (p < 0.0002). The type of treatment modality did not have a significant effect on results [50]. Recent study of laryngeal dysplasia confirmed that progression to overt cancer was stable during 20 years at an approximate rate of 8% [51]. The largest published retrospective study, of 1444 patients with laryngeal SILs, classified according to the amended Ljubljana classification, showed a significant difference (p = 0.0001) in progression to cancer between patients with low-grade and those with high-grade SILs: 19/1204 (1.6%) over a period of 2–15 years and 30/240 (12.5%) over a period of 2–26 years with high-grade SILs. Nine of 49 patients who progressed to cancer were diagnosed as carcinoma in situ. They were treated more aggressively than patients with high-grade SILs; eight patients were additionally treated with radiotherapy, and one patient with chordectomy. After treatment, all of them were free of disease [9]. Hopefully, further studies of laryngeal dysplasia, classified by the proposed two/three-tier system will bring promising and comparable results of treatment.
Table 1.
Morphological criteria of the unified two/three tier classification of laryngeal precursor lesions, modified from the amended Ljubljana classification [9]
| Low-grade dysplasia/SIL (previous categories squamous hyperplasia, mild dysplasia)—low malignant potential—spectrum of morphological changes ranging from squamous hyperplasia to an augmentation of basal/parabasal cells, occupying up to the middle of the epithelial thickness, upper part unchanged | |
| Architectural criteria | Stratification preserved Transition of basal/augmented parabasal cell layer with perpendicular orientation to the basement membrane to horizontally oriented prickle cell layer in the upper part Spinous layer: spectrum of changes ranging from the whole epithelial thickness up to a variant with prickle cell present only in the upper part Basal/parabasal layer: spectrum of changes ranging from unchanged layer (2–3 rows) to augmentation up to the middle of epithelium |
| Cytological criteria | No atypia/at most minimal atypia (reactive changes) Parabasal cells: slightly increased cytoplasm and enlarged nuclei compared to basal cells, uniformly distributed chromatin, no intercellular bridges Mitoses: regular, rare, in/near basal layer Dyskeratotic cells: few |
| High-grade dyplasia/SIL (previous categories moderate and severe dysplasia, carcinoma in situ)—high malignant potential—spectrum of atypical epithelial cells, occupying at least lower epithelial half up to the whole epithelial thickness | |
| Architectural criteria | Abnormal maturation Variable degrees of disordered stratification and polarity Altered epithelial cells occupy from half to the whole epithelial thickness Variable degree of irregularly shaped rete ridges Intact basement membrane No stromal changes |
| Cytological criteria | Cellular and nuclear atypias Marked variations in size and shape Hyperchromasia Nucleoli increased in number and size Mitoses increased throughout the epithelium, with or without atypical forms Dyskeratotic and apoptotic cells frequent throughout the epithelium |
| Carcinoma in situ, distinguished from high-grade dysplasia if three-tier system is used—showing features of conventional carcinoma, e.g., structural and cellular abnormalities but without invasion | |
| Architectural criteria | Complete loss of stratification and polarity of the whole epithelium The epithelial surface may be covered with a few compressed, horizontally oriented cells Intact basement membrane No stromal changes |
| Cytological criteria | Severe cellular and nuclear atypias Severe variations in cellular and nuclear size and size and shape Frequent nuclear hyperchromasia Nucleoli increased in number and size Mitoses increased throughout the epithelium, abnormal mitoses frequently seen Dyskeratotic and apoptotic cells frequent throughout the epithelium |
Fig. 3.
Spectre of dysplasia and carcinoma in situ. a Low-grade dysplasia. Hyperplastic squamous epithelium showing increased number of basal-parabasal cells, occupying the lower epithelial third; epithelial cells show minimal atypias with only slightly enlarged nuclei compared to basal cells; the upper two thirds without epithelial changes. b High-grade dysplasia. Hyperplastic squamous epithelium, two thirds, up to the entire epithelial thickness occupy atypical cells with still preserved polarity; cellular and nuclear atypias are present, increased mitotic activity is evident in the lower epithelial half; surface is covered by the thin parakeratotic layer. c High-grade dysplasia. The entire epithelial thickness is occupied by the atypical epithelial cells with still preserved polarity. d Carcinoma in situ. Pronounced architectural and cellular abnormalities of the whole epithelium with prominent nuclear and cellular atypias. The epithelial surface is covered by the parakeratotic layer. e The same epithelial changes are seen on the right side, occupying up to the two thirds of the epithelial thickness
Neuroendocrine Tumours of the Larynx
The larynx is the most common site for neuroendocrine tumours in the head and neck; they are divided into epithelial (carcinomas) and neural-type tumours (paraganglioma) [12]. The terminology and classification of laryngeal neuroendocrine carcinomas have been changed and adjusted with recognition of the different biological behaviour and histological features of a particular entity in comparison with the classification used in previous edition [11–13]. Large cell neuroendocrine carcinoma, the main target of disagreement, which was previously grouped with atypical carcinoid/moderately differentiated NEC, grade II, is now transferred into a subtype of poorly differentiated NEC, grade III. Thus, in the WHO Blue Book 2017, the classification of neuroendocrine tumours is now categorized as follows: well-differentiated, moderately-differentiated (Fig. 4a) and poorly-differentiated neuroendocrine carcinoma, which is additionally divided into two subtypes: small cell NEC (SmCNEC) and large cell NEC (LCNEC) [12, 13, 52, 53].
Fig. 4.
Laryngeal neuroendocrine carcinoma. a Moderately-differentiated neuroendocrine carcinoma. Submucosal tumor composed of neural-type rosettes, glandular and trabecular growth with mild to moderate nuclear polymorphism, and rare mitoses. b Poorly-differentiated neuroendocrine carcinoma, small cell. Irregular ribbons and nests of hyperchromatic cells with oval to spindle-shaped nuclei, increased nuclear-cytoplasmic ratio, and indistinct cellular borders. Small foci of crush artefacts are present. c Poorly-differentiated neuroendocrine carcinoma, large cell. Cords and trabecules of tumor cells with abundant cytoplasm, round pleomorphic nuclei and prominent nucleoli; mitoses and apoptotic cells are evident. d Poorly-differentiated neuroendocrine carcinoma, large cell. Immunohistochemistry shows diffuse positive staining for synaptophysin
The most recent meta-analysis reported 436 cases of the laryngeal NECs: 23 well-differentiated NECs (carcinoid), 163 moderately differentiated NECs (atypical carcinoid), 183 SmNECs, 29 LCNECs, and 38 unspecified carcinoid tumors. Males were more frequently affected than females (3:1) for all subtypes except well-differentiated NEC, for which no gender predilection was found. The tumor was most often located in the supraglottis, ranging from 60 to 96%. The majority of patients had a history of smoking, ranging from 73 to 94% [53]. Recent data also showed that the poorly differentiated laryngeal NECs are not associated with hrHPV infection, unlike the tumors at the other sites [54, 55].
Histologically, laryngeal SmCNEC grows in nests and trabeculae of closely packed small to medium sized cells, with hyperchromatic round or oval nuclei with prominent moulding to each other, nucleoli are inconspicuous and cytoplasm is very scant. Rosette-like structures may be present (Fig. 4b). Necroses, mitoses, crash artefacts, as well as vascular and perineural invasion, are characteristically frequently seen [12, 13, 52]. LNEC usually grows in nests, with occasional rosette formation and nuclear palisading. It is composed of large, polygonal cells with a low nuclear-cytoplasmic ratio, coarse nuclear chromatin, sometimes with a speckled salt-and-pepper quality, and prominent nucleoli. There is a high mitotic rate (>10 mitoses/2 mm2 or 10 per high power field). Extensive necroses are usually present [52]. Both types of poorly differentiated NEC are positive for at least one neuroendocrine marker (chromogranin, synaptophysin, or CD56) and low molecular weight cytokeratins, while TTF-1 is variably positive (Fig. 4c, d). In differential diagnosis, the possibility of a metastasis from the lung must be excluded. Poorly differentiated NEC should be distinguished from basaloid squamous carcinoma, malignant lymphoma and malignant melanoma.
Well-differentiated NEC (carcinoid) can be treated with local excision, moderately differentiated NEC (atypical carcinoid) is best treated with surgery, and both types of poorly differentiated laryngeal NEC seem to benefit most from chemotherapy. The 5-year disease-specific survival was 100% for well-differentiated NEC (carcinoid), 53% for moderately differentiated NEC (atypical carcinoid), 19% for SmCNEC, and 15% for LCNEC [53]. LCNEC are very aggressive; most patients develop distant metastases and die within 2 years [12].
Conclusions
In comparison with the WHO Blue Book 2005, the 3rd chapter contains a reduced number of entities, especially in relation to non-epithelial tumours. Of tumours originating from the covering squamous epithelium, laryngeal and hypopharyngeal CSCC and its variants, together with precursor lesions, occupy a major part of the chapter. Their etiological relation with hrHPV infection is highlighted, although viral etiology as detected by presence of HPV E6/E7 mRNA using in situ hybridization and positive p16 immunohistochemistry does not exceed 10% of involvement in single entities, except for papillary SCC. However, evaluation of the possible prognostic and therapeutic benefits of HPV mRNA determination in laryngeal tumours may be of some clinical benefit in the near future.
The unified two-tier classification of precursor lesions consists of low- and high-grade dysplasia. The proposed system can also be transformed into a three-tier classification for treatment purposes, with a distinction between carcinoma in situ (CIS) and high-grade dysplasia. The value of the morphological criteria was verified by an inter-observer study with good agreement and with the results of the largest follow-up study, demonstrating a highly significant difference in the risk of malignant progression between low- and high-risk precursor lesions.
The classification and terminology of laryngeal neuroendocrine NEC are renewed according to earlier proposals. NECs are divided into well-, moderate- and poorly-differentiated NEC and the latter additionally into SmCNEC and LCNEC. LCNEC, which was attached to atypical carcinoid/moderately differentiated NEC, grade II, in the WHO 2005 edition, is now in the group of poorly differentiated NEC, grade III, showing a specific morphology and poorer prognosis.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest.
Footnotes
Special Issue: World Health Organization Classification Update
References
- 1.Halec G, Holzinger D, Schmitt M, et al. Biological evidence for a causal role of HPV16 in a small fraction of laryngeal squamous cell carcinoma. Br J Cancer. 2013;109(1):172–183. doi: 10.1038/bjc.2013.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westra WH. The pathology of HPV-related head and neck cancer: implications for the diagnostic pathologist. Semin Diagn Pathol. 2015;32(1):42–53. doi: 10.1053/j.semdp.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 3.El-Mofty SK. Human papillomavirus related head and neck squamous cell variants. Semin Diagn Pathol. 2015;32(1):23–31. doi: 10.1053/j.semdp.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Fusconi M, Campo F, Gallo A, et al. Laryngeal Cancer, HPV DNA vs E6/E7 mRNA test: a systematic review. J Voice. 2016 doi: 10.1016/j.jvoice.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Chernock RD, Nussenbaum B, Thorstad WL, et al. Extensive HPV-related carcinoma in situ of the upper aerodigestive tract with ‘nonkeratinizing’ histologic features. Head Neck Pathol. 2014;8(3):322–328. doi: 10.1007/s12105-013-0499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gale N, Gnepp DR, Poljak M, et al. Laryngeal squamous intraepithelial lesions: an updated review on etiology, classification, molecular changes, and treatment. Adv Anat Pathol. 2016;23(2):84–91. doi: 10.1097/PAP.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 7.Zidar N, Gale N, Kojc N, et al. Cadherin-catenin complex and transcription factor snail-1 in spindle cell carcinoma of the head and neck. Virchows Arch. 2008;453(3):267–274. doi: 10.1007/s00428-008-0649-y. [DOI] [PubMed] [Google Scholar]
- 8.Zidar N, Boštjančič E, Gale N, et al. Down-regulation of microRNAs of the miR-200 family and miR-205, and an altered expression of classic and desmosomal cadherins in spindle cell carcinoma of the head and neck-hallmark of epithelial-mesenchymal transition. Hum Pathol. 2011;42(4):482–488. doi: 10.1016/j.humpath.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Gale N, Blagus R, El-Mofty SK, et al. Evaluation of a new grading system for laryngeal squamous intraepithelial lesions-a proposed unified classification. Histopathology. 2014;65(4):456–464. doi: 10.1111/his.12427. [DOI] [PubMed] [Google Scholar]
- 10.Gale N, Zidar N, Poljak M, et al. Current views and perspectives on classification of squamous intraepithelial lesions of the head and neck. Head Neck Pathol. 2014;8(1):16–23. doi: 10.1007/s12105-014-0530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis JS, Jr, Spence DC, Chiosea S, et al. Large cell neuroendocrine carcinoma of the larynx: definition of an entity. Head Neck Pathol. 2010;4(3):198–207. doi: 10.1007/s12105-010-0188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis JS, Jr, Ferlito A, Gnepp DR, et al. Terminology and classification of neuroendocrine neoplasms of the larynx. Laryngoscope. 2011;121(6):1187–1193. doi: 10.1002/lary.21790. [DOI] [PubMed] [Google Scholar]
- 13.Kao HL, Chang WC, et al. Head and neck large cell neuroendocrine carcinoma should be separated from atypical carcinoid on the basis of different clinical features, overall survival, and pathogenesis. Am J Surg Pathol. 2012;36(2):185–192. doi: 10.1097/PAS.0b013e318236d822. [DOI] [PubMed] [Google Scholar]
- 14.Gooi Z, Chan JY, Fakhry C. The epidemiology of the human papillomavirus related to oropharyngeal head and neck cancer. Laryngoscope. 2016;126(4):894–900. doi: 10.1002/lary.25767. [DOI] [PubMed] [Google Scholar]
- 15.Chai RC, Lambie D, Verma M, et al. Current trends in the etiology and diagnosis of HPV-related head and neck cancers. Cancer Med. 2015;4(4):596–607. doi: 10.1002/cam4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M. Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol. 2012;6(Suppl 1):S104–S120. doi: 10.1007/s12105-012-0368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lingen MW, Xiao W, Schmitt A, et al. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2013;49(1):1–8. doi: 10.1016/j.oraloncology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Chernock RD, Wang X, Gao G, et al. Detection and significance of human papillomavirus, CDKN2A(p16) and CDKN1A(p21) expression in squamous cell carcinoma of the larynx. Mod Pathol. 2013;26(2):223–231. doi: 10.1038/modpathol.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis JS, Jr, Ukpo OC, Ma XJ, et al. Transcriptionally-active high-risk human papillomavirus is rare in oral cavity and laryngeal/hypopharyngeal squamous cell carcinomas-a tissue microarray study utilizing E6/E7 mRNA in situ hybridization. Histopathology. 2012;60(6):982–991. doi: 10.1111/j.1365-2559.2011.04169.x. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Gao L, Li H, et al. Human papillomavirus infection and laryngeal cancer risk: a systematic review and meta-analysis. J Infect Dis. 2013;207(3):479–488. doi: 10.1093/infdis/jis698. [DOI] [PubMed] [Google Scholar]
- 21.Morshed K, Polz-Dacewicz M, Szymański M, et al. Short-fragment PCR assay for highly sensitive broad-spectrum detection of human papillomaviruses in laryngeal squamous cell carcinoma and normal mucosa: clinico-pathological evaluation. Eur Arch Otorhinolaryngol. 2008;265(Suppl 1):S89–S96. doi: 10.1007/s00405-007-0569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duray A, Descamps G, Arafa M, et al. High incidence of high-risk HPV in benign and malignant lesions of the larynx. Int J Oncol. 2011;39(1):51–59. doi: 10.3892/ijo.2011.1031. [DOI] [PubMed] [Google Scholar]
- 23.zur Hausen H. Infections causing human cancer. Weinheim: Wiley; 2011. pp. 145–243. [Google Scholar]
- 24.Bishop JA, Ma XJ, Wang H, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36(12):1874–1882. doi: 10.1097/PAS.0b013e318265fb2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gheit T, Abedi-Ardekani B, Carreira C, et al. Comprehensive analysis of HPV expression in laryngeal squamous cell carcinoma. J Med Virol. 2014;86(4):642–646. doi: 10.1002/jmv.23866. [DOI] [PubMed] [Google Scholar]
- 26.Gale N. Head and neck cancer and HPV infection. 27th Eroppean congress of pathology, Belgarde, 2015; Sy-24, Abstract.
- 27.Masand RP, El-Mofty SK, Ma XJ, et al. Adenosquamous carcinoma of the head and neck: relationship to human papillomavirus and review of the literature. Head Neck Pathol. 2011;5(2):108–116. doi: 10.1007/s12105-011-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castellsagué X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016 doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 29.Ereño C, López JI, Sánchez JM, et al. Papillary squamous cell carcinoma of the larynx. J Laryngol Otol. 2001;115(2):164–166. doi: 10.1258/0022215011907631. [DOI] [PubMed] [Google Scholar]
- 30.Jo VY, Mills SE, Stoler MH, et al. Papillary squamous cell carcinoma of the head and neck: frequent association with human papillomavirus infection and invasive carcinoma. Am J Surg Pathol. 2009;33(11):1720–1724. doi: 10.1097/PAS.0b013e3181b6d8e6. [DOI] [PubMed] [Google Scholar]
- 31.Mehrad M, Carpenter DH, Chernock RD, et al. Papillary squamous cell carcinoma of the head and neck: clinicopathologic and molecular features with special reference to human papillomavirus. Am J Surg Pathol. 2013;37(9):1349–1356. doi: 10.1097/PAS.0b013e318290427d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson LD, Wenig BM, Heffner DK, et al. Exophytic and papillary squamous cell carcinomas of the larynx: a clinicopathologic series of 104 cases. Otolaryngol Head Neck Surg. 1999;120(5):718–724. doi: 10.1053/hn.1999.v120.a92773. [DOI] [PubMed] [Google Scholar]
- 33.Schick U, Pusztaszeri M, Betz M, et al. Adenosquamous carcinoma of the head and neck: report of 20 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(3):313–320. doi: 10.1016/j.oooo.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 34.del Pino M, Bleeker MC, Quint WG, et al. Comprehensive analysis of human papillomavirus prevalence and the potential role of low-risk types in verrucous carcinoma. Mod Pathol. 2012;25(10):1354–1363. doi: 10.1038/modpathol.2012.91. [DOI] [PubMed] [Google Scholar]
- 35.Patel KR, Chernock RD, Zhang TR, et al. Verrucous carcinomas of the head and neck, including those with associated squamous cell carcinoma, lack transcriptionally active high-risk human papillomavirus. Hum Pathol. 2013;44(11):2385–2392. doi: 10.1016/j.humpath.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Odar K, Kocjan BJ, Hošnjak L, et al. Verrucous carcinoma of the head and neck—not a human papillomavirus-related tumour? J Cell Mol Med. 2014;18(4):635–645. doi: 10.1111/jcmm.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson RF, Chernock RD, Wang X, et al. Spindle cell carcinomas of the head and neck rarely harbor transcriptionally-active human papillomavirus. Head Neck Pathol. 2013;7(3):250–257. doi: 10.1007/s12105-013-0438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson L, Chang B, Barsky SH. Monoclonal origins of malignant mixed tumors (carcinosarcomas). Evidence for a divergent histogenesis. Am J Surg Pathol. 1996;20(3):277–285. doi: 10.1097/00000478-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Choi HR, Sturgis EM, Rosenthal DI, et al. Sarcomatoid carcinoma of the head and neck: molecular evidence for evolution and progression from conventional squamous cell carcinomas. Am J Surg Pathol. 2003;27(9):1216–1220. doi: 10.1097/00000478-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Kojc N, Zidar N, Gale N, et al. Transcription factors Snail, Slug, Twist, and SIP1 in spindle cell carcinoma of the head and neck. Virchows Arch. 2009;454(5):549–555. doi: 10.1007/s00428-009-0771-5. [DOI] [PubMed] [Google Scholar]
- 41.Gallo A, Degener AM, Pagliuca G, et al. Detection of human papillomavirus and adenovirus in benign and malignant lesions of the larynx. Otolaryngol Head Neck Surg. 2009;141(2):276–281. doi: 10.1016/j.otohns.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 42.Waters HH, Seth R, Hoschar AP, et al. Does HPV have a presence in diffuse high grade pre-malignant lesions of the larynx? Laryngoscope. 2010;120(Suppl 4):S201. doi: 10.1002/lary.21668. [DOI] [PubMed] [Google Scholar]
- 43.Mooren JJ, Gültekin SE, Straetmans JM, et al. P16(INK4A) immunostaining is a strong indicator for high-risk-HPV-associated oropharyngeal carcinomas and dysplasias, but is unreliable to predict low-risk-HPV-infection in head and neck papillomas and laryngeal dysplasias. Int J Cancer. 2014;134(9):2108–2117. doi: 10.1002/ijc.28534. [DOI] [PubMed] [Google Scholar]
- 44.Pagliuca G, Martellucci S, Degener AM, et al. Role of human papillomavirus in the pathogenesis of laryngeal dysplasia. Otolaryngol Head Neck Surg. 2014;150(6):1018–1023. doi: 10.1177/0194599814525749. [DOI] [PubMed] [Google Scholar]
- 45.Karatayli-Ozgursoy S, Bishop JA, Hillel A, et al. Risk factors for dysplasia in recurrent respiratory papillomatosis in an adult and pediatric population. Ann Otol Rhinol Laryngol. 2016;125(3):235–241. doi: 10.1177/0003489415608196. [DOI] [PubMed] [Google Scholar]
- 46.Gale N, Pilch BZ, Sidransky D, et al. Epithelial precursor lesions. In: Barnes L, Eveson JW, Reichart P, Sidransky D, et al., editors. World health organization classification of tumours. head and neck tumours. Lyon: IARC Press; 2005. pp. 140–143. [Google Scholar]
- 47.McLaren KM, Burnett RA, Goodlad JR, et al. Consistency of histopathological reporting of laryngeal dysplasia. The Scottish pathology consistency group. Histopathology. 2000;37(5):460–463. doi: 10.1046/j.1365-2559.2000.00998.x. [DOI] [PubMed] [Google Scholar]
- 48.Fleskens SA, Bergshoeff VE, Voogd AC, et al. Interobserver variability of laryngeal mucosal premalignant lesions: a histopathological evaluation. Mod Pathol. 2011;24(7):892–898. doi: 10.1038/modpathol.2011.50. [DOI] [PubMed] [Google Scholar]
- 49.Sarioglu S, Cakalagaoglu F, Elagoz S, et al. Inter-observer agreement in laryngeal pre-neoplastic lesions. Head Neck Pathol. 2010;4(4):276–280. doi: 10.1007/s12105-010-0208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weller MD, Nankivell PC, McConkey C, et al. The risk and interval to malignancy of patients with laryngeal dysplasia; a systematic review of case series and meta-analysis. Clin Otolaryngol. 2010;35(5):364–372. doi: 10.1111/j.1749-4486.2010.02181.x. [DOI] [PubMed] [Google Scholar]
- 51.Karatayli-Ozgursoy S, Pacheco-Lopez P, Hillel AT, et al. Laryngeal dysplasia, demographics, and treatment: a single-institution, 20-year review. JAMA Otolaryngol Head Neck Surg. 201;141(4):313–8. [DOI] [PubMed]
- 52.Kusafuka K, Ferlito A, Lewis JS, Jr, et al. Large cell neuroendocrine carcinoma of the head and neck. Oral Oncol. 2012;48(3):211–215. doi: 10.1016/j.oraloncology.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 53.van der Laan TP, Plaat BE, van der Laan BF, et al. Clinical recommendations on the treatment of neuroendocrine carcinoma of the larynx: A meta-analysis of 436 reported cases. Head Neck. 2015;37(5):707–715. doi: 10.1002/hed.23666. [DOI] [PubMed] [Google Scholar]
- 54.Alos L, Hakim S, Larque AB, a et. p16 overexpression in high-grade neuroendocrine carcinomas of the head and neck: potential diagnostic pitfall with HPV-related carcinomas. Virchows Arch. 2016;469(3):277–284. doi: 10.1007/s00428-016-1982-1. [DOI] [PubMed] [Google Scholar]
- 55.Thompson ED, Stelow EB, Mills SE, et al. Large cell neuroendocrine carcinoma of the head and neck: a clinicopathologic series of 10 cases with an emphasis on HPV status. Am J Surg Pathol. 2016;40(4):471–478. doi: 10.1097/PAS.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]




