Abstract
The updated edition of The World Health Organization Classification of Tumours of the Head and Neck includes discussions on mucosal melanoma of both the sinonasal and oral cavity. Since the prior edition, sinonasal origin is now recognized as the most common site of occurrence of mucosal melanoma in the head and neck (66%) with oral cavity representing 25% of cases. Histologic features of mucosal melanomas vary widely from spindled, epithelioid, and pleomorphic to rhabdoid, plasmacytoid and undifferentiated. Additionally, mucosal melanomas are commonly amelanotic (or minimal pigmentation) (~50%) leading to overlapping features and diagnostic challenges in differentiating mucosal melanomas from other small cell/undifferentiated sinonasal tumors. Since the last edition, formal staging of head and neck mucosal melanomas was added to the American Joint Committee on Cancer entities, though the traditional histologic features that have prognostic significance in cutaneous melanomas fail to stratify mucosal melanomas (i.e. tumor thickness, ulceration). Interestingly, while melanomas of all sites are a malignancy derived from melanocytes, mucosal melanomas are now recognized to have distinct molecular alterations compared to cutaneous or uveal melanomas. BRAF V600E mutations are rare (<6%) in mucosally derived melanomas compared to a rate of 50% in cutaneous melanomas. CD117 (C-Kit) mutations are the most common alteration encountered (~25%) in mucosal sites with potential therapeutic targetability. The recognition of the distinct genetic changes in this subgroup of melanomas means that therapy advances in cutaneous melanomas may not translate to head and neck mucosal melanomas and clinical trials specific to this subgroup of patients are needed.
Keywords: Sinonasal melanoma, Mucosal melanoma, CD117 mutations, Oral melanoma
Introduction
The updated edition of The World Health Organization Classification of Tumours of the Head and Neck delineates mucosal melanoma of both the sinonasal and oral cavity as distinct sections. While there are more similarities than differences between these two subsites of mucosal melanoma, details are provided for both subsites when available. Additionally, the differential diagnoses to consider especially for the sinonasal region are highlighted to aid in recognition of morphologic overlap and variable sensitivity and specificity in immunohistochemical markers. Aberrant marker expression in mucosal melanomas is also noted.
Mucosal melanoma (MM) is a malignancy of melanocytes derived from mucosal sites. It is now recognized that MM is the rarest melanoma subtype with an estimated 800 cases per year in the US. This is in striking contrast to cutaneous melanomas, which are 100x more common (Table 1). Furthermore, while there is a rising incidence of cutaneous melanomas, head and neck (HN) MM appears stable over the past decade [1]. These differences begin to highlight why mucosal melanoma needs to be viewed as a unique disease from cutaneous melanoma, as well as uveal melanoma, with other divergences including etiology, staging, and molecular alterations are highlighted below.
Table 1.
Clinical and molecular comparison of melanoma based on site of origin
| Mucosala
[2, 3] |
Cutaneous [4, 5] | Ocular (uveal) [6] | |
|---|---|---|---|
| Clinical | |||
| Age of onset | 60–70 years | 55 years | 62 years |
| Incidence all (USA) | 1% (800/yr) | 90%+ (80,000/yr) | 3% (2500/yr) |
| Incidence rate | Stable | Rising | Stable |
| 5 year survival | 25–30% regardless of stage | By stage 80% | Class 1: 90% Class 2: <20% |
| Molecular | |||
| BRAF V600E mutationsb | <6% | 50% | 0% |
| NRAS | 15–20% | 30% | <5% |
| KIT mutation/ampc | 25% (10–37) | 6–8% | <1% |
| BAP1 mutation | ? | 3% | 50% (metastases) |
| GNAQ and GNA11 | 0, rare | 2%, 4% | 50%, 36% respectively |
| Other | Monosomy Chr 3 | ||
| TERT promoter mutations | 8–20% | 48% | 1% |
Bolded entries are notable differences between melanoma subtypes
Amp amplification; Chr chromosome; yr year
aMucosal melanomas include anogenital; 55% arise in the head and neck region Treatment/clinical trials require validation in each subsite secondary to differences in molecular profiles
bBRAF inhibitors (cutaneous origin)
cKIT inhibitors (i.e. imatinib) (mucosal origin—most patients develop resistance)
Within MM, head and neck sites represent 55% of cases with anogenital being then the second most common mucosal site of origin. HN MM represents <1% of all melanomas and arise predominantly in two primary sites, the sinonasal region and oral cavity [7]. While melanomas may arise in other mucosal sites including larynx and nasopharynx, they share overlapping histologic features and biologic characteristics and are exceptionally rare.
Clinical and Etiologic Factors
In the head and neck, MM are most commonly encountered in the sinonasal region followed by the oral cavity. Previously, the incidence of oral MM was thought to be more prevalent than other HN sites. This may have reflected differences in ethnic distribution seen in oral MM, a focus of earlier publications [8–10]. Current SEER data supports up to 2/3rds of HN MM arise in the sinonasal region followed by 1/4th in the oral cavity [1].
Factors contributing to the etiology of MM remain unclear; Melanocytosis has been noted in some cases but has been difficult to confirm this association [11]. While risk factors are known for cutaneous melanoma hallmarked by increased UV exposure, unknown factors contribute to tumorigenesis of MM, marking a continued challenge to identify those patients at greatest risk for the development of MM.
Sinonasal
Sinonasal MM is rare, representing about 4% of sinonasal tumors [12, 13]. Although the peak incidence occurs in the 7th decade, sinonasal melanoma should be included in the differential diagnosis of most sinonasal masses as there is a wide age distribution (13–93 years) [14]. Moreover, a patient’s presenting symptoms are indistinguishable from those of other sinonasal tumors including sinonasal congestion and/or epistaxis. Studies show no gender predisposition to slight male predominance in this site [13, 14].
Sinonasal MM most commonly arises from the lateral nasal wall including turbinates (30–40%), followed by the nasal septum (10–20%), with fewer cases in the paranasal sinuses, and nasopharynx [13–16]. Multiple subsites may also be involved as patients often present with advanced disease. When pigmentation is noted on endoscopy, clinically the diagnosis is suspected; however the clinical and pathologic differential diagnosis is broad when tumors present as a flesh colored mass lesion or as “polyps”, for which melanoma must be included in the differential diagnosis.
Oral Cavity
Patients with mucosal melanomas of the oral cavity often are asymptomatic, presenting slightly younger in the 6th decade. A flat or nodular black gray mucosal lesion is most often identified on the palate or gingiva [8, 9, 17, 18]. Lesions are often detected by the patient or on oral examination during a dental procedure and require differentiation from amalgam tattoos: metal “pigment” from prior dental work accumulating in macrophages. Up to 1/3 of lesions may show ulceration. However, the significance of this finding in MM remains unclear or at least different from ulceration in cutaneous melanomas. Lymph node metastases are also common at presentation (33% of patients) and thus clinical evaluation should include neck imaging (ultrasound or CT scan) [11]. Thus, despite easier visualization of the oral cavity, presentation at a late stage remains common. Men may be slightly more affected by oral MM than women [19–21].
Histologic Review/Diagnosis
Gross Features
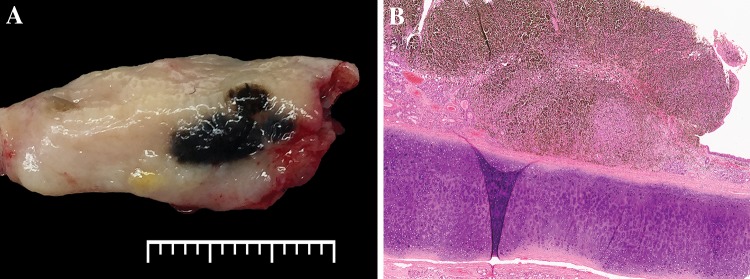
Surgical specimens may be limited to an anatomic region (septum or turbinate), or larger composite resections. MM may be polypoid, predominantly in the SN region, but pigmented plaque-like lesions are also encountered (Fig. 1). Gross assessment should include tumor size and tumor relationship to underlying structures such as bone. Broad sampling may also be required for assessment of pagetoid or in situ spread beyond the defined mass, especially when ulcerated.
Fig. 1.
Gross features in mucosal melanoma. a Grossly a darkly pigmented lesion is seen on the mucosa of the turbinate (lateral nasal wall). The presence of pigmentation makes mucosal melanoma the suspected diagnosis clinically. (The scale bar is marking millimeter increments). b This hematoxylin and eosin photomicrograph of a polypoid septal mass shows overlying surface ulceration and marked pigmentation aiding in tumor recognition as a mucosal melanoma
Histologic Features
Histologic evaluation of HN MM shows a wide range of growth patterns and cellular morphology. This broad spectrum of morphology leads to challenges recognizing mucosal melanomas especially when up to 50% of tumors are amelanotic, lacking intracytoplasmic pigmentation, which aids in the diagnosis of this entity. Ulceration is common in most specimens, but does not allow for stratification as noted in cutaneous melanomas. Similarly, necrosis is present in over half of tumors. Mitoses are common with some studies suggesting that high mitoses (>10 per 10 high power fields) are associated with a worse clinical outcome [14]. Other histologic features including lymphovascular invasion and true tumor thickness can be difficult to document secondary to the friability of the tumors on histologic sections. Perineural invasion should be carefully sought, found most frequently in the desmoplastic variant.
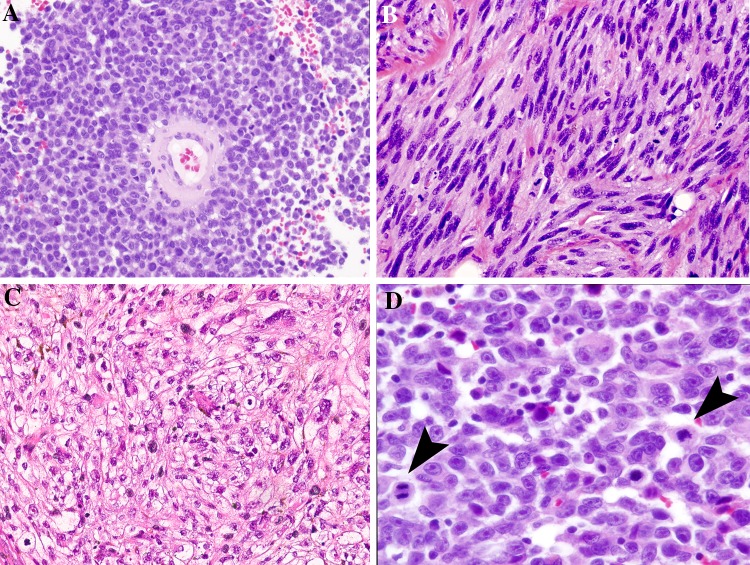
Growth patterns commonly found in HN MM include spindled, perivascular (peritheliomatous), and solid, with other patterns encountered less often (i.e. pseudopapillary, storiform, and alveolar). Peritheliomatous pattern is defined by several layers of tumor cells adherent around a blood vessel wall. As the tumor is discohesive, these structures separate from each other and may predominant over the solid growth pattern (Fig. 2).
Fig. 2.
Morphologic features in mucosal melanoma. Note that pigmentation is not observed in these examples. a The perivascular/peritheliomatous pattern shows tumor cells loosely clinging around a blood vessel. b Spindled cells create a fasciculated pattern of growth of mimicking other neuronal and soft tissue tumors. c A solid sheet of predominantly clear tumor cells show occasional scattered rhabdoid forms. d Epithelioid cells show characteristic prominent nucleoli and mitoses (arrows)
Cellular morphology also shows a wide range of features. The cellular features are characterized as spindled (elongated, fusiform cells), epithelioid cells (ranging from small with high nuclear to cytoplasmic ratio without prominent nucleoli to large pleomorphic cells with large cherry-red nucleoli), plasmacytoid and rhabdoid morphologies, leading to broad differential considerations.
Lastly, MM may be undifferentiated with a high mitotic rate. Multiple cell types may be encountered in the same tumor. Thus, based on the wide spectrum of potential cytologic features, MM should frequently enter the differential diagnosis with other sinonasal tumors. Morphologically, MM may overlap with small round blue cell tumors (rhabdomyosarcoma, Ewings/PNET, olfactory neuroblastoma), high-grade tumors (neuroendocrine carcinomas, sinonasal undifferentiated carcinomas, poorly differentiated squamous cell carcinoma, NUT midline carcinoma, SMARCB1 (INI-1) deficient tumors, and diffuse large B cell lymphoma), and spindle cell entities including sarcomas. Immunohistochemical evaluation therefore is a core component to correctly classifying sinonasal tumors (Fig. 3).
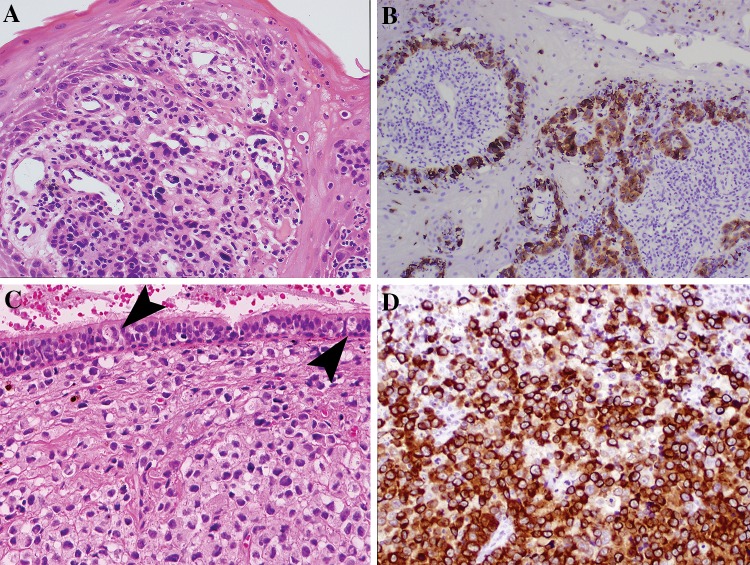
Fig. 3.
Intraepithelial involvement in mucosal melanoma and immunohistochemical confirmation of lineage. a Atypical cells are mixed with acute inflammation in this oral biopsy. b An S100 protein immunohistochemical stain on part A highlights the in situ component and invasive tumor nests. c Pagetoid spread may be identified when the surface mucosa is intact. Large atypical melanocytes (arrows) percolate into the overlying respiratory epithelium. Notice that the underlying tumor is without pigmentation or prominent nucleoli. d An immunohistochemical cocktail of melanoma markers (tyrosinase and HMB45) in case C, strongly highlight the tumor cells confirming tumor lineage
Intraepithelial Involvement
On examination of sinonasal MM, an intraepithelial/in situ component, composed of basally situated atypical melanocytes may be identified (20–60%) (Fig. 3A, B) [14, 15]. However, often the tumor size and tissue integrity (ulceration, friable) may limit the evaluation for this potential histologic finding. Pagetoid spread into the overlying epithelium may also occur (<20%). In oral MM, the rate of finding an in situ component is up to 90% [21]. This observation may result from better oriented tissue sections and smaller tumor size at presentation than sinonasal tract primaries.
Immunohistochemical Evaluation
The immunohistochemical evaluation of sinonasal tumors requires consideration of the differential diagnosis (below) and knowledge of potential overlapping and/or aberrant staining patterns in these lesions.
Melanocytic Markers
As with melanomas in other sites, S100 protein, melan-A, tyrosinase, and/or HMB45 react in the majority of tumors. These traditional markers range in sensitivity with S100 protein being the highest at 90%. Thus, as a screening marker, this is a useful but not always sufficient marker to identify MM, as up to 10% may be negative. S100 protein, while sensitive, is not specific, with reactivity identified in a range of neural and non-neural tumors. Thus confirmatory, more specific markers of tumor lineage are required. HMB45 and tyrosinase have a similar sensitivity of 75% in sinonasal MM; however, the sensitivity of these markers does decline to 65–70% specifically in spindled-cell melanomas. Melan A and MITF vary at 65% versus 57% positivity, respectively [14]. MITF is also not specific for melanoma and has been expressed in some sarcomas, neurogenic tumors and other entities. SOX10 is a newer marker, showing a high sensitivity for MM (88–100%) [22, 23]. However, SOX10 specificity in the wide range of neoplasms in the sinonasal region has only partially been studied, showing expression in non-melanomas, such as 8% of alveolar rhabdomyosarcomas [23]. Thus, a panel of markers is employed to maximize both sensitivity and specificity in making a diagnosis of MM. Another marker seen in MM is high CD117 (C-Kit) expression, which is identified in at least 25% of tumors. While not a diagnostic marker, CD117 expression (>50% of tumor cells) often correlates with molecular alterations (CD117 mutations), which identify a tumor that may benefit from targeted therapy (see below) [24].
Other Common Immunohistochemical Markers for the Differential Diagnosis
When overt differentiation (i.e. such as keratinization) is not identified in a sinonasal tumor, immunohistochemical markers to determine the tumor lineage are warranted. A particularly useful panel of immunohistochemical markers includes: cytokeratin, synaptophysin, S100 protein or pan-melanoma cocktail, and desmin. This initial evaluation on small biopsies and non-pigmented tumors facilitates correct classification [25]. The rationale for concurrent marker testing is secondary to aberrant staining that occurs in several tumor types including melanoma and rhabdomyosarcoma. In melanoma, the primary pitfall is aberrant cytokeratin staining seen in ~10% and synaptophysin reported in 13% of MM [14, 26]. Interestingly, these are the same aberrant markers that occur in one-third to one-half of alveolar rhabdomyosarcomas [27]. Thus, screening for melanoma during the initial immunohistochemical evaluation of undifferentiated sinonasal tumors should include markers with good sensitivity and specificity for melanoma. Similarly, CD99 is now recognized to stain a variety of tumors and is viewed as non-specific for Ewing/PNET and may stain up to 25% of melanomas. As newer markers become available evaluating their expression across the spectrum of SN tumors will be important to recognize their specificity, and thus utility in diagnosing MM.
Prognosis and Staging of Mucosal Melanomas
Factors used for prognosis in cutaneous melanomas (e.g. Breslow’s thickness, and ulceration) do not apply to mucosal melanomas and further studies are needed to identify prognostic factors in mucosal origin tumors. Histologic, anatomic and clinical factors have been investigated in MM to predict outcome (age, mitoses, cell type, ulceration), however, studies remain limited in this rare disease precluding validation for clinical utility [14, 19, 28].
Metastatic disease remains the most important factor in predicting outcome [28]. Patterns of recurrence may be local/regional or distant spread. Neck involvement/recurrence (lymph nodes) is higher in oral primary MM (40%) than in sinonasal (20%) [29]. Combined local/regional and distant metastasis occurred in 50% of HN MM [29, 30]. Overall, prognosis remains poor with a median survival of 24 months [18, 21, 31].
Prior to 2010 there was no formal staging for HN MM. The 7th edition of the American Joint Committee on Cancer (AJCC) added classification which recognized the aggressiveness of MM with all primary tumors having only a T3 or T4 primary designation [32] (Table 2). Several studies have supported prognostic value of the AJCC system, but the overall prognostic grouping utilizing combined T, N and M results has been removed from the 8th edition [4, 16].
Table 2.
AJCC Staging for head and neck mucosal melanomas
| T category | (Primary tumor assessment) |
| T3 | Tumor limited to the mucosa and submucosal* |
| T4a | (Moderately advanced) involves deep soft tissue, cartilage, bone or overlying skin |
| T4b | (Very advanced) involves skull base, brain or dura, cranial nerves (IX–XII), prevertebral, carotid artery, mediastinal and masticator space |
| N category | (Regional lymph nodes) |
| NX | Cannot be assessed |
| N0 | Negative for metastasis |
| N1 | Metastasis present |
| M category | (Distant metastasis) |
| M0 | Absent |
| M1 | Present |
Prognostic stage groups were removed from the 8th edition of AJCC
*Size and tumor thickness do not determine T stage; i.e.T3 tumors may be polypoid or flat
Genetics and Therapeutic Considerations
Melanomas as a family of tumors harbor a high mortality regardless of site of origin. Thus, investigations into genetic alterations contributing to tumorigenesis to identify targets for therapeutic intervention are on-going. Extensive molecular evaluation has occurred in cutaneous melanomas including The Cancer Genome Atlas (TCGA) initiatives [33]. BRAF V600E mutations and RAS mutations were among the most frequently identified in melanomas of skin origin. This is in stark contrast to only rare BRAF V600E mutations in MM (<6%) (Table 1) [2, 34]. It remains unclear if the BRAF V600E sinonasal cases may partially represent cutaneous origin with recurrence within the nasal cavity, thereby phenotypically having the risk factors that align with cutaneous origin and not truly of mucosal origin.
Distinct mutational patterns have been identified in melanomas based on site of origin and are highlighted in Table 1. These distinctions are critical to recognize for several reasons. First, currently targetable BRAF V600E with BRAF inhibitors will only address a small subset of advanced MM harboring this mutation [35, 36]. Second, evaluation beyond BRAF V600E in HN MM should include CD117 (C-Kit) mutations, which will identify a different subset (~25%) of patients who may benefit from imatinib therapy. Importantly, while imatinib therapy has shown initial responses in patients in clinical trials, the responses are not durable [37]. Additionally, tumors with mutations in certain locations of CD117 exon 11 and 13 show higher correlation with response than amplifications of this gene alone or mutations in other exons [7]. Therefore, novel therapies specific to MM need to be considered. Furthermore, therapies showing benefit in cutaneous melanomas may not be applicable (i.e. BRAF inhibitors without a BRAF V600E mutation). In addition, these notable genetic differences between cutaneous, mucosal and uveal melanomas highlight the need for validating therapies specifically in each group of melanoma patients to determine efficacy [38].
Therefore, in tumors for which cutaneous versus mucosal origin cannot be definitively determined by pathology, further evaluation is warranted to correctly provide staging and prognosis, which are distinctly different for cutaneous melanomas versus MM (i.e., melanoma involving the lip or nasal ala without clear in situ component of skin versus mucosal sites). As the molecular profile varies based on site of origin, the presence of a BRAF V600E mutation (either by immunohistochemical evaluation or molecular assessment) would more likely represent a cutaneous primary and thus have therapeutic implications.
In Summary
The updated WHO Classification of Head and Neck Tumours on MM expands upon the growing biologic distinction from their cutaneous counterparts in incidence, etiology, risk-factors, staging parameters and molecular alterations. As MM are molecularly distinct from both cutaneous and uveal melanomas, molecular investigations on a broader scale are needed to characterize MM and pursue novel biomarkers in this disease. Secondary to these striking molecular differences, the implementation and translation of potential therapies from cutaneous melanoma to MM will require validation specifically in this subtype of melanoma.
Histopathologically, the WHO highlights the frequent observation of mucosal melanomas as amelanotic with a wide cytologic spectrum leading to a broad differential diagnosis in the sinonasal region. Therefore, immunohistochemical evaluation often with a panel of markers is necessary to confirm tumor lineage and avoid diagnostic pitfalls particularly in this area.
Compliance with Ethical Standards
Conflict of interest
The author has no conflict of interest to disclose.
Research Involving Animal and Human Rights
This article does not contain any studies with human participants or animals performed by the author.
Footnotes
Special Issue: World Health Organization Classification Update
References
- 1.Bishop KD, Olszewski AJ. Epidemiology and survival outcomes of ocular and mucosal melanomas: a population-based analysis. Int J Cancer. 2014;134(12):2961–2971. doi: 10.1002/ijc.28625. [DOI] [PubMed] [Google Scholar]
- 2.Rivera RS, Nagatsuka H, Gunduz M, Cengiz B, Gunduz E, Siar CH, Tsujigiwa H, Tamamura R, Han KN, Nagai N. C-kit protein expression correlated with activating mutations in KIT gene in oral mucosal melanoma. Virchows Arch. 2008;452(1):27–32. doi: 10.1007/s00428-007-0524-2. [DOI] [PubMed] [Google Scholar]
- 3.Jangard M, Zebary A, Ragnarsson-Olding B, Hansson J. TERT promoter mutations in sinonasal malignant melanoma: a study of 49 cases. Melanoma Res. 2015;25(3):185–188. doi: 10.1097/CMR.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 4.Shuman AG, Light E, Olsen SH, Pynnonen MA, Taylor JM, Johnson TM, Bradford CR. Mucosal melanoma of the head and neck: predictors of prognosis. Arch Otolaryngol Head Neck Surg. 2011;137(4):331–337. doi: 10.1001/archoto.2011.46. [DOI] [PubMed] [Google Scholar]
- 5.Griewank KG, Murali R, Puig-Butille JA, Schilling B, Livingstone E, Potrony M, Carrera C, Schimming T, Möller I, Schwamborn M, Sucker A, Hillen U, Badenas C, Malvehy J, Zimmer L, Scherag A, Puig S, Schadendorf D. TERT promoter mutation status as an independent prognostic factor in cutaneous melanoma. J Natl Cancer Inst. 2014;106(9). [DOI] [PMC free article] [PubMed]
- 6.van Essen TH, van Pelt SI, Versluis M, Bronkhorst IH, van Duinen SG, Marinkovic M, Kroes WG, Ruivenkamp CA, Shukla S, de Klein A, Kiliç E, Harbour JW, Luyten GP, van der Velden PA, Verdijk RM, Jager MJ. Prognostic parameters in uveal melanoma and their association with BAP1 expression. Br J Ophthalmol. 2014;98(12):1738–1743. doi: 10.1136/bjophthalmol-2014-305047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvajal RD, Spencer SA, Lydiatt W. Mucosal melanoma: a clinically and biologically unique disease entity. J Natl Compr Cancer Netw. 2012;10(3):345–356. doi: 10.6004/jnccn.2012.0034. [DOI] [PubMed] [Google Scholar]
- 8.Takagi M, Ishikawa G, Mori W. Primary malignant melanoma of the oral cavity in Japan. With special reference to mucosal melanosis. Cancer. 1974;34(2):358–370. doi: 10.1002/1097-0142(197408)34:2<358::AID-CNCR2820340221>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Sortino-Rachou AM, Cancela Mde C, Voti L, Curado MP. Primary oral melanoma: population-based incidence. Oral Oncol. 2009;45(3):254–258. doi: 10.1016/j.oraloncology.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 10.López F, Rodrigo JP, Cardesa A, Triantafyllou A, Devaney KO, Mendenhall WM, Haigentz M, Strojan P, Pellitteri PK, Bradford CR, Shaha AR, Hunt JL, de Bree R, Takes RP, Rinaldo A, Ferlito A. Update on primary head and neck mucosal melanoma. Head Neck. 2016;38(1):147–155. doi: 10.1002/hed.23872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hicks MJ, Flaitz CM. Oral mucosal melanoma: epidemiology and pathobiology. Oral Oncol. 2000;36(2):52–69. doi: 10.1016/S1368-8375(99)00085-8. [DOI] [PubMed] [Google Scholar]
- 12.Gal TJ, Silver N, Huang B. Demographics and treatment trends in sinonasal mucosal melanoma. Laryngoscope. 2011;121(9):2026–2033. doi: 10.1002/lary.21925. [DOI] [PubMed] [Google Scholar]
- 13.Moreno MA, Roberts DB, Kupferman ME, DeMonte F, El-Naggar AK, Williams M, Rosenthal DS, Hanna EY. Mucosal melanoma of the nose and paranasal sinuses, a contemporary experience from the M. D. Anderson Cancer Center. Cancer. 2010;116(9):2215–2223. doi: 10.1002/cncr.24976. [DOI] [PubMed] [Google Scholar]
- 14.Thompson LD, Wieneke JA, Miettinen M. Sinonasal tract and nasopharyngeal melanomas: a clinicopathologic study of 115 cases with a proposed staging system. Am J Surg Pathol. 2003;27(5):594–611. doi: 10.1097/00000478-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Mochel MC, Duncan LM, Piris A, Kraft S. Primary mucosal melanoma of the sinonasal tract: a clinicopathologic and immunohistochemical study of thirty-two cases. Head Neck Pathol. 2015;9(2):236–243. doi: 10.1007/s12105-014-0570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koivunen P, Bäck L, Pukkila M, Laranne J, Kinnunen I, Grénman R, Mäkitie AA. Accuracy of the current TNM classification in predicting survival in patients with sinonasal mucosal melanoma. Laryngoscope. 2012;122(8):1734–1738. doi: 10.1002/lary.23343. [DOI] [PubMed] [Google Scholar]
- 17.Rapini RP, Golitz LE, Greer RO, Krekorian EA, Poulson T. Primary malignant melanoma of the oral cavity. A review of 177 cases. Cancer. 1985;55(7):1543–1551. doi: 10.1002/1097-0142(19850401)55:7<1543::AID-CNCR2820550722>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 18.Lourenço SV, A MS, Sotto MN, Bologna SB, Giacomo TB, Buim ME, Coutinho-Camillo CM, Silva SD, Landman G, Soares FA, Simonsen Nico MM. Primary oral mucosal melanoma: a series of 35 new cases from South America. Am J of Dermatopathol. 2009;31(4):323–330. doi: 10.1097/DAD.0b013e3181a0d37c. [DOI] [PubMed] [Google Scholar]
- 19.Song H, Wu Y, Ren G, Guo W, Wang L. Prognostic factors of oral mucosal melanoma: histopathological analysis in a retrospective cohort of 82 cases. Histopathology. 2015;67(4):548–556. doi: 10.1111/his.12692. [DOI] [PubMed] [Google Scholar]
- 20.de-Andrade BA, Toral-Rizo VH, León JE, Contreras E, Carlos R, Delgado-Azañero W, Mosqueda-Taylor A, de-Almeida OP. Primary oral melanoma: a histopathological and immunohistochemical study of 22 cases of Latin America. Medicina oral patología oral y cirugía bucal. 2012;17(3):e383–e388. doi: 10.4317/medoral.17588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad ML, Patel S, Hoshaw-Woodard S, Escrig M, Shah JP, Huvos AG, Busam KJ. Prognostic factors for malignant melanoma of the squamous mucosa of the head and neck. Am J Surg Pathol. 2002;26(7):883–892. doi: 10.1097/00000478-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Liu HG, Kong MX, Yao Q, Wang SY, Shibata R, Yee H, Martiniuk F, Wang BY. Expression of Sox10 and c-kit in sinonasal mucosal melanomas arising in the Chinese population. Head Neck Pathol. 2012;6(4):401–408. doi: 10.1007/s12105-012-0375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miettinen M, McCue PA, Sarlomo-Rikala M, Biernat W, Czapiewski P, Kopczynski J, Thompson LD, Lasota J, Wang Z, Fetsch JF. Sox10–a marker for not only schwannian and melanocytic neoplasms but also myoepithelial cell tumors of soft tissue: a systematic analysis of 5134 tumors. Am J Surg Pathol. 2015;39(6):826–835. doi: 10.1097/PAS.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres-Cabala CA, Wang WL, Trent J, et al. Correlation between KIT expression and KIT mutation in melanoma: a study of 173 cases with emphasis on the acral lentiginous/ mucosal type. Mod Pathol. 2009;22:1446–1456. doi: 10.1038/modpathol.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cordes B, Williams MD, Tirado Y, Bell D, Rosenthal DI, Al-Dhahri SF, Hanna EY, El-Naggar AK. Molecular and phenotypic analysis of poorly differentiated sinonasal neoplasms: an integrated approach for early diagnosis and classification. Hum Pathol. 2009;40(3):283–292. doi: 10.1016/j.humpath.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safadi RA, Bader DH, Abdullah NI, Sughayer MA. Immunohistochemical expression of keratins 6, 7, 8, 14, 16, 18, 19, and MNF-116 pancytokeratin in primary and metastatic melanoma of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(5):510–519. doi: 10.1016/j.oooo.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Bahrami A, Gown AM, Baird GS, Hicks MJ, Folpe AL. Aberrant expression of epithelial and neuroendocrine markers in alveolar rhabdomyosarcoma: a potentially serious diagnostic pitfall. Mod Pathol. 2008;21(7):795–806. doi: 10.1038/modpathol.2008.86. [DOI] [PubMed] [Google Scholar]
- 28.Chan RC, Chan JY, Wei WI. Mucosal melanoma of the head and neck: 32-year experience in a tertiary referral hospital. Laryngoscope. 2012;122(12):2749–2753. doi: 10.1002/lary.23625. [DOI] [PubMed] [Google Scholar]
- 29.Patel SG, Prasad ML, Escrig M, Singh B, Shaha AR, Kraus DH, Boyle JO, Huvos AG, Busam K, Shah JP. Primary mucosal malignant melanoma of the head and neck. Head Neck. 2002;24(3):247–257. doi: 10.1002/hed.10019. [DOI] [PubMed] [Google Scholar]
- 30.Meleti M, Leemans CR, de Bree R, Vescovi P, Sesenna E, van der Waal I. Head and neck mucosal melanoma: experience with 42 patients, with emphasis on the role of postoperative radiotherapy. Head Neck. 2008;30(12):1543–1551. doi: 10.1002/hed.20901. [DOI] [PubMed] [Google Scholar]
- 31.Jethanamest D, Vila PM, Sikora AG, Morris LG. Predictors of survival in mucosal melanoma of the head and neck. Ann Surg Oncol. 2011;18(10):2748–2756. doi: 10.1245/s10434-011-1685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7. New York, NY: Springer; 2010. [Google Scholar]
- 33.Cancer Genome Atlas Network Genomic Classification of Cutaneous Melanoma. Cell. 2015;161(7):1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zebary A, Jangard M, Omholt K, Ragnarsson-Olding B, Hansson J. KIT, NRAS and BRAF mutations in sinonasal mucosal melanoma: a study of 56 cases. Br J Cancer. 2013;109(3):559–564. doi: 10.1038/bjc.2013.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodi FS, Corless CL, Giobbie-Hurder A, Fletcher JA, Zhu M, Marino-Enriquez A, Friedlander P, Gonzalez R, Weber JS, Gajewski TF, O’Day SJ, Kim KB, Lawrence D, Flaherty KT, Luke JJ, Collichio FA, Ernstoff MS, Heinrich MC, Beadling C, Zukotynski KA, Yap JT, Van den Abbeele AD, Demetri GD, Fisher DE. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Onco. 2013;31(26):3182–3190. doi: 10.1200/JCO.2012.47.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turri-Zanoni M, Medicina D, Lombardi D, Ungari M, Balzarini P, Rossini C, Pellegrini W, Battaglia P, Capella C, Castelnuovo P, Palmedo G, Facchetti F, Kutzner H, Nicolai P, Vermi W. Sinonasal mucosal melanoma: molecular profile and therapeutic implications from a series of 32 cases. Head Neck. 2013;35(8):1066–1077. doi: 10.1002/hed.23079. [DOI] [PubMed] [Google Scholar]
- 37.Carvajal RD, Antonescu CR, Wolchok JD, Chapman PB, Roman RA, Teitcher J, Panageas KS, Busam KJ, Chmielowski B, Lutzky J, Pavlick AC, Fusco A, Cane L, Takebe N, Vemula S, Bouvier N, Bastian BC, Schwartz GK. KIT as a therapeutic target in metastatic melanoma. JAMA. 2011;305(22):2327–2334. doi: 10.1001/jama.2011.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lazarev S, Gupta V, Hu K, Harrison LB, Bakst R. Mucosal melanoma of the head and neck: a systematic review of the literature. Int J Radiat Oncol Biol Phys. 2014;90(5):108–118. doi: 10.1016/j.ijrobp.2014.03.042. [DOI] [PubMed] [Google Scholar]