Abstract
The 2017 fourth edition of the World Health Organization Classification of Tumours, specifically as it relates to the ear (Chap. 9), has several changes. Importantly, the number of entities has been significantly reduced by omitting tumors or lesions if they do not occur exclusively or predominantly at this site or if they are discussed in detail elsewhere in the book. These entities include: embryonal rhabdomyosarcoma, osteoma, exostosis, angiolymphoid hyperplasia with eosinophilia, Schneiderian papilloma, inverted papilloma, lipoma of the internal auditory canal, hemangioma, hematolymphoid tumors, and secondary tumors. Paraganglioma was included in the neck chapter. New entries include otosclerosis and cholesteatoma, while refinements to nomenclature, classification and criteria were incorporated into the ceruminous gland tumors and epithelial tumors of the middle and inner ear. Specifically, the middle and inner ear were combined, as practical limitations of origin and imaging make a definitive separation artificial. The classification reflects the state of current understanding for these uncommon entities, with this update only highlighting selected entities that were the most significantly changed.
Keywords: Ear; Temporal bone; Tumor; WHO; Hearing loss; Carcinoma, squamous cell; Cholesteatoma; Ear, middle; Temporal bone; Otosclerosis
Introduction
The 2017 fourth edition of the World Health Organization Classification of Tumours of the Head and Neck, specifically as it relates to the ear (Chapter 9), has several changes [1]. Significantly, the number of entities has been reduced by omitting tumors or lesions when they do not occur exclusively or predominantly at this site or if they are discussed in detail elsewhere in the book. New entries include otosclerosis and cholesteatoma, while refinements to nomenclature, classification and criteria were incorporated into the ceruminous gland tumors and epithelial tumors of the middle and inner ear. Specifically, lesions of the middle and inner ear were combined, as practical limitations of origin and imaging make a definitive separation artificial. The classification reflects the state of current understanding for these uncommon entities, with this update only highlighting selected entities that were the most significantly changed. The discussion will be separated into external auditory canal and middle and inner ear lesions.
Tumours of the External Auditory Canal
Squamous Cell Carcinoma
Squamous cell carcinoma (SCC) of the external auditory canal (EAC) was contrasted to the external ear (pinna), the latter removed from this edition [2]. EAC SCCs are rare, affecting only about 1 in a million population per year, showing a female predominance, distinctly different from pinna tumors which are more common in men [3–5]. Chronic inflammation (otitis media or externa) and radiation treatment specifically for nasopharyngeal tumors, are considered in the etiology for EAC SCCs, quite different from sun exposure or frostbite in the development of pinna carcinomas [6–8]. Rarely, transformation from papilloma into carcinoma may be seen [9]. Pain, otitis (externa or media), and hearing changes bring the patients to seek clinical attention, with nerve symptoms late in the disease course [10–12]. The tumors will frequently completely occlude the EAC lumen (Fig. 1), extending through the tympanic membrane and surrounding soft tissues. The histologic features of SCC are identical to other sites, showing cohesive pleomorphic neoplastic cells bound by intercellular bridges, containing keratin pearls and dyskeratotic cells, and easily identified mitoses (Fig. 2). Spindle cell SCC (Fig. 2), verrucous SCC and acantholytic patterns may be seen [3, 11, 13, 14]. EAC SCCs are more biologically aggressive than pinna tumors, showing a high frequency of local recurrence and lymph node metastases [3, 5, 8, 13].
Fig. 1.

A clinical photograph showing an ulcerated mass completely occluding the external auditory canal (courtesy Dr. Carsten Palme)
Fig. 2.
a A well differentiated squamous cell carcinoma, showing a pavemented growth of squamous cells. Mitoses are noted. b A spindle cell SCC is a more biologically aggressive variant in the EAC
Ceruminous Neoplasms
The ceruminous glands of the outer one-third to one-half of the EAC are modified apocrine sweat glands, with yellow–brown lipofuscin-like ceroid pigment granules, that combine with secretions to create ear wax. Tumors of these glands show a dual population of a basal, myoepithelial layer surrounding inner luminal secretory cells. The tumors are separated into adenomas [15] and adenocarcinomas [16].
Ceruminous Adenocarcinoma
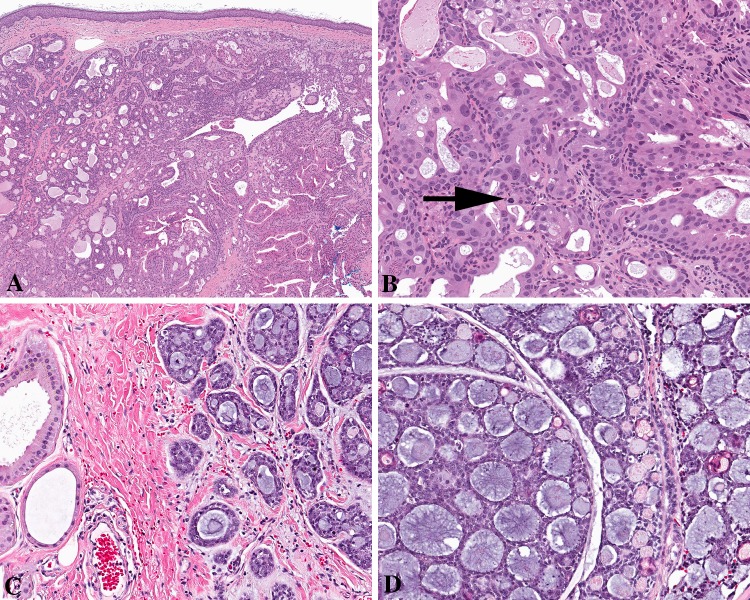
The malignant tumors of the EAC ceruminous glands are separated into three types, based on specific histologic features: ceruminous adenocarcinoma, ceruminous adenoid cystic carcinoma, and ceruminous mucoepidermoid carcinoma [16, 17]. Carcinomas are rare, affecting middle aged women more often than men, and presenting with pain, hearing loss and tinnitus [17–19]. Imaging studies should be performed to define the extent of the tumor (Fig. 3), while also excluding direct extension from a parotid gland or nasopharynx salivary gland primary. Due to anatomic restraints, the tumors are often small (1.4 cm) at presentation. The unencapsulated tumors invade into adjacent tissues, including bone. Ceruminous adenocarcinoma show an apocrine glandular neoplasm with easily identified pleomorphism, single cell infiltration, perineural invasion and comedonecrosis (Fig. 4). Increased mitoses and absent ceroid pigment granules are hallmarks of adenocarcinoma. Ceruminous adenoid cystic carcinoma (Fig. 4) and mucoepidermoid carcinoma are histologically indistinguishable from salivary gland primaries, and thus imaging or intraoperative exclusion of a salivary gland primary are necessary [17, 20, 21]. The biphasic composition of the tumors can be highlighted with luminal CK7 and CD117 immunoreactivity, while the basal cells are highlighted by p63, S100 protein and CK5/6 [17, 22, 23].
Fig. 3.

A coronal MRI T1 weighted fat suppressed image shows a large tumor (arrow) expanding into the bony EAC, but separate and distinct from the parotid gland
Fig. 4.
Ceruminous adenocarcinoma. a A widely infiltrative tumor, showing a mixed glandular architecture. b The biphasic appearance is noted, with basal cells still present in this ceruminous adenocarcinoma. Mitotic figures (arrow) are increased. c An adenoid cystic ceruminous adenocarcinoma is noted adjacent to uninvolved ceruminous glands (left). d The classical histologic features of adenoid cystic carcinoma, including clefting, cribriform architecture, and glycosaminoglycan material, are seen
Recurrences are common, especially if there are positive surgical margins or high grade histologic features, with death often a consequence of local destruction of vital structures.
Ceruminous Adenoma
The benign tumors of the EAC ceruminous glands are separated into three types, based on specific histologic features: ceruminous adenoma, ceruminous pleomorphic adenoma, and ceruminous syringocystadenoma papilliferum [15, 24]. Adenomas are also rare, presenting in middle aged patients without a sex difference [25–27]. A mass of the posterior wall of the EAC is common, while hearing changes or tinnitus are less frequent symptoms. Tumors are small, nonulcerating, superficial masses, frequently fragmented during removal. Bone and middle ear extension is not seen. While circumscribed, they are not encapsulated neoplasms, often involving but not originating from the surface epithelium (Fig. 5). A dense, sclerotic background fibrosis separates the bilayered apocrine glandular proliferation into moderately cellular cystic-glandular structures [24]. The inner luminal cells show decapitation apocrine blebs of granular, eosinophilic cytoplasm. The yellow–brown pigment granules are seen in the cytoplasm of adenomas (Fig. 5). A myoepithelial layer surrounds the luminal cells. Ceruminous pleomorphic adenoma (Fig. 6) is identical to salivary gland or cutaneous primaries (chondroid syringoma), requiring careful exclusion of extension from a parotid gland primary. Ceruminous syringocystadenoma papilliferum has papillary projections of cuboidal-columnar cells associated with a rich, dense plasmacytic infiltrate (Fig. 6). Immunohistochemistry studies highlight the biphasic epithelial and myoepithelial populations [22, 24]. Complete excision is curative, although recurrences may be seen when incompletely excised.
Fig. 5.
Ceruminous adenoma. a The glandular to solid architecture of a ceruminous adenoma is noted below the intact squamous epithelium. b Yellow-bodies are noted with the cytoplasm of this ceruminous adenoma. c The biphasic basal and luminal components are easily seen in this adenoma. Note the yellow–brown ceroid granules
Fig. 6.
a Ceruminous pleomorphic adenoma, shows ceruminous differentiation in the epithelial component, blended with a myxochondroid matrix. b A ceruminous syringocystadenoma papilliferum with numerous papillary projects and plasma cells
Entities Excluded
A number of entities were removed from this section, including embryonal rhabdomyosarcoma, osteoma, exostosis, angiolymphoid hyperplasia with eosinophilia, Kimura disease, fibrous dysplasia, idiopathic pseudocystic chondromalacia, and chondrodermatitis nodularis chronica helicis. Several of these lesions are not neoplastic conditions, but had been included as part of the differential diagnostic considerations for the external ear tumors. The neoplastic conditions are covered in greater detail in other sections of the book.
Tumours of the Middle and Inner Ear
Squamous cell carcinoma may develop from the middle ear epithelium, although considered a very rare event. Meningioma, vestibular or acoustic neuroma (schwannoma), and middle ear adenoma, all included updated demographic and clinical information, but were otherwise not significantly changed. Although included in the previous edition, cholesteatoma was not actually cited in the index, with updates included in the current edition. The various papillary tumors of the middle and inner ear were better defined, as discussed below.
Aggressive Papillary Tumour
This tumor type was included in the previous edition, but the separation and distinction from endolymphatic sac tumor was further elaborated on in this edition [28]. This tumor is an intermediate grade neoplasm, showing locally aggressive (invasive) behavior, composed of non-stratified epithelium. The tumor is very rare, presenting most often in young female patients (mean, 34 years), who often have symptoms for years. The tumor arises within the middle ear, but expands beyond this space into adjacent structures, which is why separation from endolymphatic sac tumor of the inner ear is difficult [29–35]. Patients present with hearing changes, showing a middle ear cavity filled with a papillary tumor that shows bone invasion. The tumor is arranged in a papillary architecture, showing complex interdigitating papillae lined by cuboidal-columnar epithelial cells (Fig. 7). Pleomorphism is limited, with luminal secretions sometimes noted [36]. In spite of the uncertainty about the exact point of origin, this tumor is more aggressive than either middle ear adenoma or endolymphatic sac tumor, and may respond to targeted molecular therapy [29, 37].
Fig. 7.
Aggressive papillary tumor. The tumor shows complex papillary structures (a), lined by neoplastic cells that are cuboidal to columnar, with limited pleomorphism (b)
Endolymphatic Sac Tumor
The endolymphatic sac tumor (ELST) is a low grade papillary epithelial neoplasm [38] that arises from within the endolymphatic sac or duct [39], showing a very high association with von Hippel-Lindau syndrome (VHL) [40–42]. The tumor is intermediate between a benign and malignant neoplasm, but lacking metastatic potential. About 10–15% of VHL patients develop ELST, with about 30% of these patients showing bilateral tumors [43, 44]. Without a sex difference, patients are usually in their early 30s to 40s at presentation. Symptoms consist of ipsilateral hearing loss, tinnitus and vestibular dysfunction. It is important to identify signs of VHL at other anatomic sites during evaluation for ELST. Imaging studies are imperative to document the extent of disease and plan surgery. Tumors are often large, expanding into the posterior cranial fossa. Bone “invasion” is common, with the tumor arranged in a cystically-dilated cavity as coarse, interdigitating, papillary projections (Fig. 8). Fibrovascular cores support a single epithelial layer of cuboidal-columnar cells that often have cleared to eosinophilic cytoplasm. The nuclei are round to oval, lacking pleomorphism [39, 40, 44]. Secretions may be seen, resembling thyroid gland follicles (Fig. 8). Tumor cells lack CD10, TTF1 and thyroglobulin, while showing pancytokeratin, CK7, EMA and weak S100 protein immunoreactivity. Germline mutations of the VHL tumor suppressor gene are usually detected, although not in sporadic cases. Surgery, while it may be curative, has significant morbidity. Radiation may be used in combination with surgery or as stand alone therapy [42, 45].
Fig. 8.
Endolymphatic sac tumor. a Broad papillary structures expanding into bone. b The neoplastic cells are columnar, with lightly eosinophilic cytoplasm surrounding oval nuclei. Note the luminal secretions
Otosclerosis
Otosclerosis is a poorly understood disorder of bone remodelling and collagen synthesis developing in the otic capsule [46], recently shown to behave as a low grade neoplasm, with invasion of pre-existing structures of the cochlear and vestibular otic capsule, and continuing to expand throughout life [47–50]. Otosclerosis is rare (3/1000 population), but differentially expressed in Caucasians much more so than Asians and Africans [50, 51]. When patients present with hearing loss, especially conductive hearing loss, up to 22% show otosclerosis [48, 51, 52]. As such, hearing loss usually develops in the 3rd to 5th decades, with females affected more often than males (3:1), usually presenting with bilateral and symmetrical disease. The bony plaque develops within the otic capsule posterior to the cochlea (Fig. 9), expanding into the stapes footplate, and then broadly in all directions [50, 53]. Seldom biopsied, the characteristic findings are often seen at autopsy. Histologically, there is a well defined tumor-like mass of immature trabecular bone within a richly vascularized stroma within the otic capsule, variably cellular depending on the stage of remodelling (Fig. 10). If untreated, profound hearing loss will develop, with surgical management geared to improvement of conductive hearing loss [47, 50, 54].
Fig. 9.

Intraoperative image of the immobilized stapes bone seen in otosclerosis (courtesy Dr. Gabriel Calzada)
Fig. 10.
The left part of the image shows the cochlea, with a tumor-like mass of immature trabecular bone expanding the otic capsule, immediately at the point of the stapes joint (courtesy Dr. Ann Sandison)
Cholesteatoma
Cholesteatoma is a misnomer as it contains no “cholesterol” and it is not a true “neoplasm” [55]. However, it simulates a neoplasm clinically with a propensity to destroy surrounding tissues (including bone) and to recur after excision. Due to negative pressure and Eustachian tube dysfunction, there is an accumulation of desquamated keratin, which results in obstruction. Trapped bacteria cause infection, resulting in increased inflammatory cells, with release of cytokines, causing epithelial proliferation. Collagenase production by the squamous epithelium causes bone destruction [56–59]. The cystic cavity is filled with keratinous debris, lined by keratinizing squamous epithelium, which is abnormal in this site. Acquired and congenital forms of cholesteatoma are recognized. There are about 15 cases/100,000 in the population, with a slight male predominance. Congenital forms affect male infants, with acquired forms affecting mainly young adults [58, 60]. Acquired cholesteatoma is usually due to recurrent otitis media, which may result in a perforated ear drum (tympanic membrane) [61]. Patients present with hearing loss associated with a foul-smelling discharge. Destruction of middle and inner ear structures brings about vestibular dysfunction, facial nerve paralysis and potential intracranial complications. Imaging studies are essential to the diagnosis (Fig. 11), with computed tomography and magnetic resonance imaging complimentary studies [62, 63]. The samples contain flakey, white keratinous debris with soft tissue and bony fragments (Fig. 11). Three components are necessary for the definitive diagnosis: (1) keratinous debris; (2) stratified squamous epithelium with a prominent granular layer; and (3) inflamed granulation or fibrous tissue (Figs. 11, 12). The squamous epithelium is cytologically bland, atrophic and generally lacking rete pegs [56, 58, 64]. Concurrent findings may be seen, including cholesterol granuloma, aural polyps, tympanosclerosis, and middle ear adenoma (Fig. 12) [65]. Surgery is the treatment of choice, although recurrence is high, requiring long term follow-up.
Fig. 11.
Cholesteatoma. a A high resolution computed tomography scan shows opacification of the middle ear, contained by an intact tympanic membrane (arrow). b Otoscopic view of a left ear acquired cholesteatoma with ear drum rupture and keratinous debris (courtesy Dr. Ann Sandison). c Histology shows a squamous epithelium associated with inflammation and keratinaceous debris
Fig. 12.
Cholesteatoma. a Thin squamous epithelium with no rete, prominent granular layer and luminal keratin debris. Inflammation and foreign body giant-cell reaction is noted. b This cholesteatoma is associated with a middle ear adenoma, a concurrent finding
Entities Excluded
A number of entities were removed from the middle ear and inner ear section, including cholesterol granuloma, Schneiderian-type papilloma, inverted papilloma, choristoma (salivary gland or glial), Paget disease of bone, microneuroma, neurofibromatosis 2, lipoma of the internal auditory canal, hemangioma, hematolymphoid tumors, Langerhans cell histiocytosis, and secondary tumors. While some of the lesions are covered in more detail elsewhere in the book, some of the entities are not neoplasms and were thus excluded as specific sections, including only as part of the differential diagnosis discussion.
Footnotes
Special Issue: World Health Organization Classification Update
References
- 1.Tumours of the Ear. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of tumours of the head and neck, 4th ed. Lyon: IARC Press; 2017;261–73.
- 2.Sandison A, Thompson LDR. Tumours of the external auditory canal: squamous cell carcinoma. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumours, 4th ed. Lyon: IARC Press, 2017;263–64.
- 3.Ouaz K, Robier A, Lescanne E, et al. Cancer of the external auditory canal. Eur Ann Otorhinolaryngol Head Neck Dis. 2013;130:175–182. doi: 10.1016/j.anorl.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Johns ME, Headington JT. Squamous cell carcinoma of the external auditory canal. A clinicopathologic study of 20 cases. Arch Otolaryngol. 1974;100:45–49. doi: 10.1001/archotol.1974.00780040049010. [DOI] [PubMed] [Google Scholar]
- 5.Wermker K, Kluwig J, Schipmann S, et al. Prediction score for lymph node metastasis from cutaneous squamous cell carcinoma of the external ear. Eur J Surg Oncol. 2015;41:128–135. doi: 10.1016/j.ejso.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 6.Shu MT, Lin HC, Lee JC, et al. Radiation-induced squamous cell carcinoma of the external auditory canal. Otol Neurotol. 2011;32:e24–e25. doi: 10.1097/MAO.0b013e3181e711a0. [DOI] [PubMed] [Google Scholar]
- 7.Lo WC, Ting LL, Ko JY, et al. Malignancies of the ear in irradiated patients of nasopharyngeal carcinoma. Laryngoscope. 2008;118:2151–2155. doi: 10.1097/MLG.0b013e3181839b8c. [DOI] [PubMed] [Google Scholar]
- 8.Fleiner F, Jumah M, Goktas O. Cancer of the external auditory canal-diagnostic and treatment. Indian J Otolaryngol Head Neck Surg. 2009;61:270–274. doi: 10.1007/s12070-009-0081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miah MS, Crawford M, White SJ, et al. Malignant transformation from benign papillomatosis of the external auditory canal. Otol Neurotol. 2012;33:643–647. doi: 10.1097/MAO.0b013e31824b76d3. [DOI] [PubMed] [Google Scholar]
- 10.Clark RR, Soutar DS, Hunter KD. A retrospective analysis of histological prognostic factors for the development of lymph node metastases from auricular squamous cell carcinoma. Histopathology. 2010;57:138–146. doi: 10.1111/j.1365-2559.2010.03593.x. [DOI] [PubMed] [Google Scholar]
- 11.Hosokawa S, Mizuta K, Takahashi G, et al. Carcinoma of the external auditory canal: histological and treatment groups. B-ENT. 2014;10:259–264. [PubMed] [Google Scholar]
- 12.Repanos C, Mitchell D, Gandhi M, et al. Great auricular nerve perineural spread of squamous cell carcinoma. ANZ J Surg. 2012;82:179–180. doi: 10.1111/j.1445-2197.2011.05988.x. [DOI] [PubMed] [Google Scholar]
- 13.Mazzoni A, Danesi G, Zanoletti E. Primary squamous cell carcinoma of the external auditory canal: surgical treatment and long-term outcomes. Acta Otorhinolaryngol. 2014;34:129–137. [PMC free article] [PubMed] [Google Scholar]
- 14.Stafford ND, Frootko NJ. Verrucous carcinoma in the external auditory canal. Am J Otol. 1986;7:443–445. [PubMed] [Google Scholar]
- 15.Sandison A, Thompson LDR. Tumours of the external auditory canal: Ceruminous adenoma. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumours, 4th ed. Lyon: IARC Press, 2017;265.
- 16.Sandison A, Stenman G, Thompson LDR. Tumours of the external auditory canal: Ceruminous adenocarcinoma. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO Classification of Head and Neck Tumours, 4th ed. Lyon: IARC Press, 2017;264.
- 17.Crain N, Nelson BL, Barnes EL, et al. Ceruminous gland carcinomas: a clinicopathologic and immunophenotypic study of 17 cases. Head Neck Pathol. 2009;3:1–17. doi: 10.1007/s12105-008-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dehner LP, Chen KT. Primary tumors of the external and middle ear. Benign and malignant glandular neoplasms. Arch Otolaryngol. 1980;106:13–19. doi: 10.1001/archotol.1980.00790250015004. [DOI] [PubMed] [Google Scholar]
- 19.Mansour P, George MK, Pahor AL. Ceruminous gland tumours: a reappraisal. J Laryngol Otol. 1992;106:727–732. doi: 10.1017/S0022215100120717. [DOI] [PubMed] [Google Scholar]
- 20.Jan JC, Wang CP, Kwan PC, et al. Ceruminous adenocarcinoma with extensive parotid, cervical, and distant metastases: case report and review of literature. Arch Otolaryngol Head Neck Surg. 2008;134:663–666. doi: 10.1001/archotol.134.6.663. [DOI] [PubMed] [Google Scholar]
- 21.Perzin KH, Gullane P, Conley J. Adenoid cystic carcinoma involving the external auditory canal. A clinicopathologic study of 16 cases. Cancer. 1982;50:2873–2883. doi: 10.1002/1097-0142(19821215)50:12<2873::AID-CNCR2820501230>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 22.Lott Limbach AA, Hoschar AP, Thompson LD, et al. Middle ear adenomas stain for two cell populations and lack myoepithelial cell differentiation. Head Neck Pathol. 2012;6:345–353. doi: 10.1007/s12105-012-0365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito K, Ito T, Tsukuda M, et al. An immunohistochemical study of adenoid cystic carcinoma of the external auditory canal. Eur Arch Otorhinolaryngol. 1993;250:240–244. doi: 10.1007/BF00171533. [DOI] [PubMed] [Google Scholar]
- 24.Thompson LD, Nelson BL, Barnes EL. Ceruminous adenomas: a clinicopathologic study of 41 cases with a review of the literature. Am J Surg Pathol. 2004;28:308–318. doi: 10.1097/00000478-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Cankar V, Crowley H. Tumors Of ceruminous glands: a clinicopathological study Of 7 cases. Cancer. 1964;17:67–75. doi: 10.1002/1097-0142(196401)17:1<67::AID-CNCR2820170109>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 26.Wetli CV, Pardo V, Millard M, et al. Tumors of ceruminous glands. Cancer. 1972;29:1169–1178. doi: 10.1002/1097-0142(197205)29:5<1169::AID-CNCR2820290507>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Iqbal A, Newman P. Ceruminous gland neoplasia. Br J Plast Surg. 1998;51:317–320. doi: 10.1054/bjps.1997.0075. [DOI] [PubMed] [Google Scholar]
- 28.Sandison A. Tumours of the middle and inner ear: aggressive papillary tumour. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumours, 4th ed. Lyon: IARC Press, 2017;266–67.
- 29.Duderstadt M, Forster C, Welkoborsky HJ, et al. Adenomatous tumors of the middle ear and temporal bone: clinical, morphological and tumor biological characteristics of challenging neoplastic lesions. Eur Arch Otorhinolaryngol. 2012;269:823–831. doi: 10.1007/s00405-011-1729-1. [DOI] [PubMed] [Google Scholar]
- 30.Tysome JR, Harcourt J, Patel MC, et al. Aggressive papillary tumor of the middle ear: a true entity or an endolymphatic sac neoplasm? Ear Nose Throat J. 2008;87:378–393. [PubMed] [Google Scholar]
- 31.Siedentop KH, Jeantet C. Primary adenocarcinoma of the middle ear. Report of three cases. Ann Otol Rhinol Laryngol. 1961;70:719–733. doi: 10.1177/000348946107000307. [DOI] [PubMed] [Google Scholar]
- 32.Dadas B, Alkan S, Turgut S, et al. Primary papillary adenocarcinoma confined to the middle ear and mastoid. Eur Arch Otorhinolaryngol. 2001;258:93–95. doi: 10.1007/s004050000288. [DOI] [PubMed] [Google Scholar]
- 33.Polinsky MN, Brunberg JA, McKeever PE, et al. Aggressive papillary middle ear tumors: a report of two cases with review of the literature. Neurosurgery. 1994;35:493–497. doi: 10.1227/00006123-199409000-00018. [DOI] [PubMed] [Google Scholar]
- 34.Gaffey MJ, Mills SE, Fechner RE, et al. Aggressive papillary middle-ear tumor. A clinicopathologic entity distinct from middle-ear adenoma. Am J Surg Pathol. 1988;12:790–797. doi: 10.1097/00000478-198810000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Goebel JA, Smith PG, Kemink JL, et al. Primary adenocarcinoma of the temporal bone mimicking paragangliomas: radiographic and clinical recognition. Otolaryngol Head Neck Surg. 1987;96:231–238. doi: 10.1177/019459988709600302. [DOI] [PubMed] [Google Scholar]
- 36.Muller M, Zammit-Maempel I, Hill J, et al. An unusual middle-ear mass. J Laryngol Otol. 2010;124:108–110. doi: 10.1017/S0022215109990442. [DOI] [PubMed] [Google Scholar]
- 37.Kawabata S, Hollander MC, Munasinghe JP, et al. Epidermal growth factor receptor as a novel molecular target for aggressive papillary tumors in the middle ear and temporal bone. Oncotarget. 2015;6:11357–11368. doi: 10.18632/oncotarget.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandison A. Tumours of the middle and inner ear: endolymphatic sac tumour. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumours, 4th ed. Lyon: IARC Press, 2017; 267–68.
- 39.Michaels L. Origin of endolymphatic sac tumor. Head Neck Pathol. 2007;1:104–111. doi: 10.1007/s12105-007-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heffner DK. Low-grade adenocarcinoma of probable endolymphatic sac origin A clinicopathologic study of 20 cases. Cancer. 1989;64:2292–2302. doi: 10.1002/1097-0142(19891201)64:11<2292::AID-CNCR2820641119>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Manski TJ, Heffner DK, Glenn GM, et al. Endolymphatic sac tumors. A source of morbid hearing loss in von Hippel-Lindau disease. JAMA. 1997;277:1461–1466. doi: 10.1001/jama.1997.03540420057030. [DOI] [PubMed] [Google Scholar]
- 42.Megerian CA, Haynes DS, Poe DS, et al. Hearing preservation surgery for small endolymphatic sac tumors in patients with von Hippel-Lindau syndrome. Otol Neurotol. 2002;23:378–387. doi: 10.1097/00129492-200205000-00026. [DOI] [PubMed] [Google Scholar]
- 43.Kupferman ME, Bigelow DC, Carpentieri DF, et al. Endolymphatic sac tumor in a 4-year-old boy. Otol Neurotol. 2004;25:782–786. doi: 10.1097/00129492-200409000-00022. [DOI] [PubMed] [Google Scholar]
- 44.Skalova A, Sima R, Bohus P, et al. Endolymphatic sac tumor (aggressive papillary tumor of middle ear and temporal bone): report of two cases with analysis of the VHL gene. Pathol Res Pract. 2008;204:599–606. doi: 10.1016/j.prp.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Lonser RR, Kim HJ, Butman JA, et al. Tumors of the endolymphatic sac in von Hippel-Lindau disease. NEngl J Med. 2004;350:2481–2486. doi: 10.1056/NEJMoa040666. [DOI] [PubMed] [Google Scholar]
- 46.Sandison A. Tumours of the middle and inner ear: otosclerosis. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumours, 4th ed. Lyon: IARC Press, 2017;268–69.
- 47.Bittermann AJ, Wegner I, Noordman BJ, et al. An introduction of genetics in otosclerosis: a systematic review. Otolaryngol Head Neck Surg. 2014;150:34–39. doi: 10.1177/0194599813509951. [DOI] [PubMed] [Google Scholar]
- 48.Karosi T, Sziklai I. Etiopathogenesis of otosclerosis. Eur Arch Otorhinolaryngol. 2010;267:1337–1349. doi: 10.1007/s00405-010-1292-1. [DOI] [PubMed] [Google Scholar]
- 49.Michaels L, Soucek S. Atypical mature bone in the otosclerotic otic capsule as the differentiated zone of an invasive osseous neoplasm. Acta Otolaryngol. 2014;134:118–123. doi: 10.3109/00016489.2013.849386. [DOI] [PubMed] [Google Scholar]
- 50.Rudic M, Keogh I, Wagner R, et al. The pathophysiology of otosclerosis: review of current research. Hear Res. 2015;330:51–56. doi: 10.1016/j.heares.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 51.Declau F, van SM, Timmermans JP, et al. Prevalence of otosclerosis in an unselected series of temporal bones. Otol Neurotol. 2001;22:596–602. doi: 10.1097/00129492-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Declau F, van SM, Timmermans JP, et al. Prevalence of histologic otosclerosis: an unbiased temporal bone study in Caucasians. Adv Otorhinolaryngol. 2007;65:6–16. doi: 10.1159/000098663. [DOI] [PubMed] [Google Scholar]
- 53.Michaels L, Soucek S. Origin and growth of otosclerosis. Acta Otolaryngol. 2011;131:460–468. doi: 10.3109/00016489.2010.532156. [DOI] [PubMed] [Google Scholar]
- 54.Carvalho B, Hamerschmidt R, Telles JE, et al. Anatomopathology of the superstructure of the stapes in patients with otosclerosis. Int Arch Otorhinolaryngol. 2015;19:1–4. doi: 10.1055/s-0034-1382096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandison A. Tumours of the middle and inner ear: cholesteatoma. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumours, 4th ed. Lyon: IARC Press, 2017;269–70.
- 56.Michaels L. Biology of cholesteatoma. Otolaryngol Clin N.Am. 1989;22:869–881. [PubMed] [Google Scholar]
- 57.Michaels L. Origin of congenital cholesteatoma from a normally occurring epidermoid rest in the developing middle ear. Int J Pediatr Otorhinolaryngol. 1988;15:51–65. doi: 10.1016/0165-5876(88)90050-X. [DOI] [PubMed] [Google Scholar]
- 58.Kuo CL, Shiao AS, Yung M, et al. Updates and knowledge gaps in cholesteatoma research. Biomed Res Int. 2015;2015:854024. doi: 10.1155/2015/854024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuo CL. Etiopathogenesis of acquired cholesteatoma: prominent theories and recent advances in biomolecular research. Laryngoscope. 2015;125:234–240. doi: 10.1002/lary.24890. [DOI] [PubMed] [Google Scholar]
- 60.Nevoux J, Lenoir M, Roger G, et al. Childhood cholesteatoma. Eur Ann Otorhinolaryngol Head Neck Dis. 2010;127:143–150. doi: 10.1016/j.anorl.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Louw L. Acquired cholesteatoma pathogenesis: stepwise explanations. J Laryngol Otol. 2010;124:587–593. doi: 10.1017/S0022215109992763. [DOI] [PubMed] [Google Scholar]
- 62.Jindal M, Riskalla A, Jiang D, et al. A systematic review of diffusion-weighted magnetic resonance imaging in the assessment of postoperative cholesteatoma. Otol Neurotol. 2011;32:1243–1249. doi: 10.1097/MAO.0b013e31822e938d. [DOI] [PubMed] [Google Scholar]
- 63.Heilbrun ME, Salzman KL, Glastonbury CM, et al. External auditory canal cholesteatoma: clinical and imaging spectrum. AJNR Am J Neuroradiol. 2003;24:751–756. [PMC free article] [PubMed] [Google Scholar]
- 64.Wells MD, Michaels L. Mode of growth of acquired cholesteatoma. J Laryngol Otol. 1991;105:261–267. doi: 10.1017/S0022215100115567. [DOI] [PubMed] [Google Scholar]
- 65.Torske KR, Thompson LD. Adenoma versus carcinoid tumor of the middle ear: a study of 48 cases and review of the literature. Mod Pathol. 2002;15:543–555. doi: 10.1038/modpathol.3880561. [DOI] [PubMed] [Google Scholar]