Abstract
The sinonasal tract remains an epicenter of a diverse array of neoplasia. This paper discusses changes to the WHO classification system of tumors involving this area. In particular, seromucinous hamartoma, NUT carcinoma, biphenotypic sinonasal sarcoma, HPV-related carcinoma with adenoid cystic features, SMARCB1-deficient carcinoma, and renal cell-like adenocarcinoma are discussed.
Keywords: Nasal, Sinonasal, Sinus, Human papillomavirus, WHO, NUT carcinoma, Seromucinous hamartoma, Biphenotypical sinonasal sarcoma, Adenoid cystic, INI1
Introduction
The sinonasal tract can be involved by a vast array of neoplasia, with phenotypic diversity surpassing what can be seen at most sites throughout the upper aerodigestive tract and even throughout most parts of the body. The 4th edition of the World Health Organization Classification of Head and Neck Tumours includes three new, relatively well-defined entities: seromucinous hamartoma, NUT carcinoma, and biphenotypic sinonasal sarcoma. In addition, a handful of new, less-defined tumor types were included either as provisional entities or mentioned in the differential diagnosis of other tumors. Here, we will describe what has remained unchanged and what has changed in the new WHO classification system.
Squamous Cell Carcinoma
Squamous cell carcinoma (SCC) remains the most common form of neoplasia within the sinonasal tract, although it makes up a smaller proportion of neoplasia here than at other sites within the upper aerodigestive tract, approximately 65–70% of carcinomas [1]. As at most other sites, smoking of tobacco products is the greatest risk factor for disease, although other exposures such as to solvents, wood dust, and leather dusts put patients at risk. Benign sinonasal papillomas as well as high-risk human papillomavirus (HR-HPV) infection also put patients at risk.
Histologic classification of SCC in the sinonasal tract remains similar to that at other sites. Most tumors are classified as either keratinizing or non-keratinizing, a distinction of possible relevance due to the fact that non-keratinizing SCC at this site is much more likely than keratinizing SCC to be secondary to HR-HPV infection (41 vs. <5%) (Fig. 1) [1–4]. Although a number of descriptive names have been applied to non-keratinizing SCC (e.g., Schneiderian carcinoma, transitional cell carcinoma, cylindrical cell carcinoma, etc.), the WHO chose to use the most accepted and straightforward terminology here. Other variants of SCC should be diagnosed as such (e.g., basaloid SCC, papillary SCC, spindle cell carcinoma, etc.). It is of note that at this site approximately 45% of basaloid SCCs and 80% of papillary SCCs are associated with HR-HPV [2, 5]. Lymphoepithelial carcinoma, a variant associated with Epstein-Barr virus (EBV) infection also retains its descriptive name, actually at odds with the names used for the same entity in the nasopharynx [6].
Fig. 1.
Squamous cell carcinoma. a Typical keratinizing squamous cell carcinoma of the sinonasal tract with abundant keratin formation. b Non-keratinizing squamous cell carcinoma with ribbons of immature squamous cells
Other Carcinomas
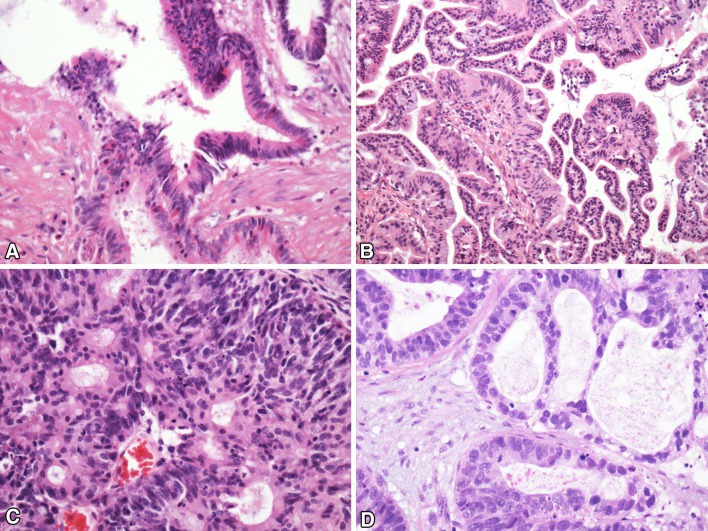
Adenocarcinomas are classified as previously with the most important distinction between intestinal-type adenocarcinomas (ITACs) and non-intestinal sinonasal adenocarcinomas (SNACs) (Fig. 2). In general, this can be justified given the distinct histological and immunohistochemical features of ITAC as well as its very strong association with wood and other dust exposures [7–12].
Fig. 2.
Adenocarcinoma. a Intestinal-type adenocarcinoma with mucus production. b Low-grade sinonasal adenocarcinoma with typical papillary architectures. c, d Two examples of high-grade sinonasal adenocarcinoma showing some of the heterogeneity that can be seen
Non-intestinal adenocarcinomas remain a group of phenotypically diverse tumors, although there is much more homogeneity with the tumors classified as low-grade. Low-grade non-intestinal SNACs are typically papillary and / or tubular and composed of monomorphic cuboidal or columnar cells with rare mitotic figures [13–16]. Tumors are free of necrosis. They uncommonly recur and do not lead to patient death. High-grade non-intestinal SNACs can show a variety of histologic features [13, 17] Many have abundant solid growth with only focal glandular differentiation. More nested growth and cells with abundant eosinophilic cytoplasm can be seen. By definition, such tumors are high-grade and mitotic figures and necrosis are frequently present. One potentially reproducible group of tumors within the category non-intestinal SNACs has been noted that resembles conventional clear cell carcinoma of the kidney (sinonasal renal cell-like adenocarcinoma). These are discussed below.
Neuroendocrine carcinomas at this site are uncommon. As tumors similar to pulmonary carcinoid tumors or “atypical” carcinoid tumors are almost unheard of here, they are not discussed. Instead the authors use the term “neuroendocrine carcinoma” at this site to encompass high-grade lesions, both small cell carcinoma and large cell neuroendocrine carcinoma [18, 19]. Uncommonly, either tumor may be associated with squamous cell carcinoma or adenocarcinoma [20]. There are now reports of tumors secondary to HR-HPV and previous irradiation is considered a risk factor [2, 21, 22].
Sinonasal undifferentiated carcinoma (SNUC) remains a diagnosis of exclusion. Neither overt squamous or glandular differentiation can be present [23]. Tumors associated with EBV-infection are better classified as lymphoepithelial carcinomas as mentioned above. Those tumors associated with translocations of the gene encoding nuclear protein of the testis (NUTM1) are called NUT carcinomas (see below). The absence of INI1 expression (or SMARCB1 loss) as seen with tumors of the kidney and elsewhere may come to define a unique entity as well (see also below). Finally, the definitive distinction of SNUC from large cell neuroendocrine carcinoma remains problematic, as expression of neuroendocrine markers is acceptable with tumors diagnosed as SNUCs.
Other Tumors More or Less Distinct to the Sinonasal Tract
Teratocarcinoma is discussed within the section devoted to epithelial malignancies. By definition, the tumors must lack germ cell elements aside from those resembling teratoma. The tumors also, by definition, have carcinomatous components and sarcomatous elements [24]. They remain poorly understood other than that they do not resemble germ cell tumors at other sites of the body. Indeed, they continue to appear to be clinically, histologically, and molecularly unique lesions that seem to most resemble carcinomas [25].
Olfactory neuroblastomas (ONBs) are still considered neuroectodermal tumors and are discussed in a section with Ewing sarcoma/peripheral neuroectodermal tumor (PNET) and sinonasal melanoma. It remains essential to distinguish these tumors from lesions showing definite epithelial differentiation and from Ewing sarcoma/PNET. Fortunately, ONBs show little or no expression of keratins by immunohistochemistry and do not have EWSR1 rearrangements as seen with Ewing sarcomas/PNETs [26, 27]. Of note, recent papers have shown that keratin expressing Ewing sarcomas/PNETs (adamantinoma-like Ewing family tumors) do occur in the sinonasal area [28]. It is imperative to recognize such lesions and not to classify them as carcinomas.
Extranodal NK/T cell lymphoma, nasal type is so designated because of its proclivity to involve the sinonasal tract [29, 30]. It is the most common lymphoma to involve the area in eastern Asia and parts of Central and South America. The lymphomas must be distinguished from other high-grade malignancies that can affect the area and from other lymphoproliferative tumors. Fortunately, with modern immunohistochemical techniques, this is seldom a problem. Other lymphoid tumors are to be classified as per the WHO system for classification for those tumors. These lesions are all discussed in more depth elsewhere.
Other Tumors
Mesenchymal neoplasms involving the sinonasal tract that are classified as soft tissue tumors are elsewhere. The exception here is the now well-recognized biphenotypic sinonasal sarcoma (discussed below). Of note, while glomangiopericytomas can be seen at soft tissue locations elsewhere, they are by far most often seen in the sinonasal tract [31]. Recent research has shown recurrent CTNNB1 mutations in these lesions with beta-catenin nuclear accumulation seen by immunohistochemistry [32]. Only a few other malignant lesions are discussed within the text including fibrosarcoma, a diagnosis becoming very uncommon.
Salivary neoplasms of the sinonasal tract are classified as such and the changes made within the salivary gland portion of the text should be instituted with sinonasal tumors. There are no unique salivary gland tumors of the sinonasal tract unless one regards seromucinous hamartoma (discussed below) as a salivary gland-type tumor.
New Lesions
Seromucinous Hamartoma
In the previous WHO classification system of tumors of the head and neck, seromucinous hamartoma (SMH) was considered synonymous with respiratory epithelial adenomatoid hamartoma (REAH). Weinreb et al. have since described 7 cases of SMH involving the sinonasal tract [33]. Patients included four men and three women and ages ranged 14–85 years. The majority of lesions occurred on the posterior nasal septum and one occurred on the lateral nasal wall. Nasal obstruction and epistaxis were the most common presenting symptoms.
The underlying glandular component of SMHs consists predominately of seromucous glands (Fig. 3). Larger glands lined by a respiratory-type epithelium, however, are invariably present similar to what are seen with REAHs [33]. Smaller tubules, ducts and glands are present in lobular and more haphazard arrangements, lined by bland cuboidal cells that rarely contain dark, eosinophilic granular material. The smaller glands may have little intervening stroma and can appear to be back to back. The glands may be surrounded by stromal hyalinization. A chronic lymphoplasmacellular infiltrate is typically present.
Fig. 3.
Seromucinous hamartoma. a Low-power image showing abundant small seromucinous glands with occasional larger, cystically dilated glands. b High-power showing bland seromucinous glands with back-to-back architecture. c S100 immunostain highlights the bland seromucinous glands. d p63 immunostain shows an absence of basal and myoepithelial cells
The immunohistochemical findings are similar to those seen with REAHs but reflect the disproportionate number of seromucinous glands present. Both respiratory and seromucinous glands are immunoreactive with antibodies to CK7 and CK19 and not with antibodies to CK20 [33, 34]. p63 and high molecular weight CK immunostaining can be used to highlight myoepithelial cells surrounding the large glands and some of the seromucinous glands, whereas muscle specific actin will not stain the basal cells. Of note, the seromucinous glandular proliferation typical of SMH shows little or no basal cell staining [33]. Weinreb et al. reported that the glandular cells of seromucinous hamartomas are typically immunoreactive with antibodies to S100 protein, whereas Ozolek at al., reported the larger glands of REAHs to be non-reactive [33, 34].
Good criteria do not exist for the definitive distinction between REAHs with florid seromucinous glandular proliferation, seromucinous hamartomas, and low-grade non-intestinal SNACs. In a series of 29 low-grade non-intestinal adenocarcinomas, 6 were associated with polypoid lesions that had some features similar to those of REAH [35]. That said, the seromucinous hamartomas described by Weinreb at al. have similar histologic and immunohistochemical features including a lack of myoepithelial cells surrounding the seromucinous glandular proliferations [33]. Recurrence for either lesion is uncommon (only 20% of the lesions reported by Weinreb et al. recurred) and metastases are only very rarely seen with sinonasal low-grade non-intestinal adenocarcinomas. Some authors have likened the glandular proliferation of SMH to microglandular adenosis of the breast [36]. Indeed, it may be that “adenosis” or “adenoma” is truly the best appellation for these benign glandular proliferations.
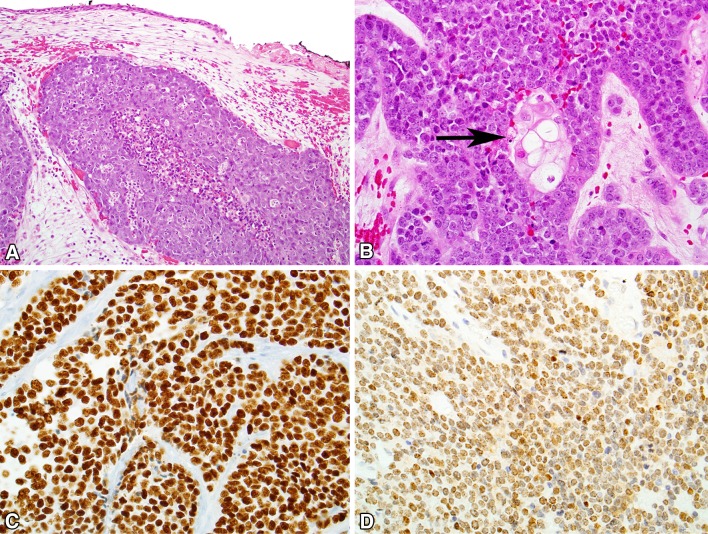
NUT Carcinoma
NUT carcinoma, also known as NUT midline carcinoma or t(15;19) carcinoma, was initially described as a mediastinal tumor in 1991 [37, 38]. NUT carcinoma is rare, with fewer than 100 cases reported [39–43]. It can affect patients of any age though is most common in children and young adults (median, 21.9 years), and has a slight female predominance. NUT carcinoma most often arises in the sinonasal tract and mediastinum, though it can affect virtually any site [39, 42, 44]. Patients with NUT carcinoma present with non-specific symptoms of a rapidly growing mass, including nasal obstruction, epistaxis, orbital symptoms, and pain [45, 46]. Imaging typically reveals an extensively infiltrative tumor with frequent involvement of the orbit and cranial cavity [45, 46]. About half of cases have regional and/or distant metastases on presentation [42].
Cytologic smears of NUT carcinoma are typically highly cellular, with monotonous, small to midsize, primitive-appearing cells in clusters or as single cells. The tumor cells have round to oval nuclei that appeared mostly naked and devoid of cytoplasm [47, 48]. Histologically, NUT carcinoma grows as undifferentiated cells in nests and sheets within the sinonasal submucosa (Fig. 4). There is no carcinoma-in situ component. The carcinoma is highly infiltrative, and demonstrates necrosis and high mitotic rates. An intratumoral acute inflammatory infiltrate is common. Two histologic hallmarks of NUT carcinoma are (1) monotonous tumor cells and (2) a peculiar pattern of keratinization often described as “abrupt.” Rarely, foci of glandular or even mesenchymal differentiation may be encountered. By immunohistochemistry, NUT carcinoma is positive for cytokeratins, often positive for squamous markers like p40, p63 and CK5/6, and occasionally positive for neuroendocrine markers, p16, or even TTF-1. The differential diagnosis of NUT carcinoma is very broad, and includes the numerous other “small round cell tumors” that occur in the sinonasal tract including olfactory neuroblastoma, melanoma, primitive neuroectodermal tumor, squamous cell carcinoma, and sinonasal undifferentiated carcinoma.
Fig. 4.
NUT carcinoma. a NUT carcinoma grows as nests of tumor cells in the sinonasal submucosa, without a surface epithelial component. a neutrophilic infiltrate is seen. b Most cases of NUT carcinoma demonstrate focal squamous differentiation (arrow) in an abrupt pattern. c NUT carcinoma is usually positive for p40. (D) The diagnosis of NUT carcinoma can be confirmed with diffuse immunoreactivity for NUT protein, typically with a distinctly speckled pattern
NUT carcinoma is defined by rearrangements of the NUTM1 gene on chromosome 15q14 [49]. The most common fusion partner is BRD4 (in about 70% of cases). The diagnosis of NUT carcinoma depends on demonstrating evidence of this rearrangement. While this can be done by fluorescence in situ hybridization (FISH), polymerase chain reaction (PCR), conventional cytogenetics, or targeted next-generation sequencing approaches, it can also be confirmed immunohistochemically with >50% nuclear expression of the NUT protein by the monoclonal antibody C52 (Cell Signaling Technologies, Danvers, MA) [39, 40, 50].
The prognosis of NUT carcinoma is poor, with a median overall survival of 9.8 months [42]. There is some evidence that NUT carcinomas with variant rearrangements not involving BRD4 may have longer survival [42, 51].
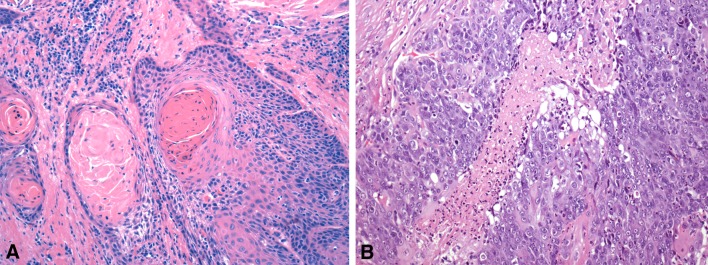
Biphenotypic Sinonasal Sarcoma
Biphenotypic sinonasal sarcoma (BSNS) was initially described in 2012 as “low-grade sinonasal sarcoma with neural and myogenic differentiation,” which remains a synonym for BSNS [52]. BSNS is rare, with fewer than 50 cases reported. BSNS tends to arise in middle-aged women (2:1 female to male ratio, mean 52 years), and although it can arise anywhere in the sinonasal tract, it has a predilection for the superior aspects of the nasal cavity and ethmoid sinuses [52–55]. Affected patients present with non-specific symptoms like nasal obstruction and facial pressure.
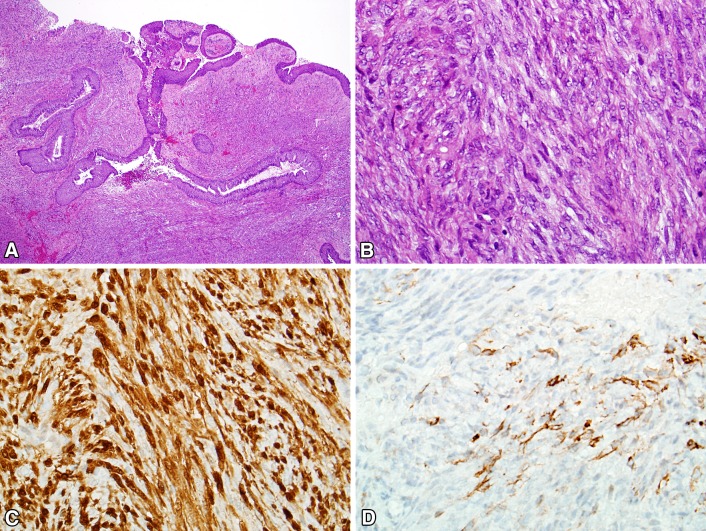
The histopathology of BSNS is that of an infiltrative proliferation of spindled cells arranged as fascicles or in a “herringbone” pattern (Fig. 5). Hemangiopericytoma-like (“staghorn”) vessels are common. A characteristic feature of BSNS is its propensity to entrap benign downward invaginations of sinonasal epithelium. These glands can become proliferative and undergo squamous or oncocytic metaplasia, mimicking sinonasal papillomas. The nuclei of BSNS are pale, slender, and uniform. Mitotic figures are uncommon, and necrosis is absent. By immunohistochemistry, BSNS shows varying degrees of staining for S100, actin, and calponin. Beta-catenin is usually positive in a nuclear distribution focally [56]. BSNS may also exhibit focal staining for desmin, myogenin, EMA, and cytokeratins [52–55]. Despite the consistent S100 positivity, SOX10 is always negative. BSNS characteristically harbors rearrangements of PAX3, with the most common partner being MAML3 [54]. Studies have also identified both PAX3-NCOA1 and PAX3-FOXO1 fusions of alveolar rhabdomyosarcoma, which appear to be associated with focal rhabdomyoblastic differentiation in BSNS [53, 57]. Before it was recognized, BSNS was likely to be misdiagnosed as cellular schwannoma, malignant peripheral nerve sheath tumor, solitary fibrous tumor, glomangiopericytoma, and synovial sarcoma.
Fig. 5.
Biphenotypic sinonasal sarcoma. a Biphenotypic sinonasal sarcoma often demonstrates entrapment of downward extensions of surface epithelium, a pattern that can mimic inverted papilloma. b The tumor typically consists of fascicles of uniform spindled cells with elongated, hypo chromatic nuclei growing in a herringbone pattern. c Biphenotypical sinonasal sarcoma demonstrates varying degrees of immunostaining for S100. d Most cases are positive for smooth muscle markers like actin
BSNS tends to demonstrate slow, progressive growth. While almost half of patients with BSNS experienced local recurrences, none of the tumors have metastasized, and only one patient to date has died of their disease [52, 54, 56].
Emerging Entities
HPV-Related Sinonasal Carcinomas Including HPV-Related Carcinoma with Adenoid Cystic Features
HPV-related carcinomas of the head and neck have a striking predilection for the oropharynx, where up to 80% of carcinomas are HPV-positive compared to 5% or fewer in sites like the oral cavity and larynx [58–62]. In the oropharynx, HPV-related carcinomas are histologically and clinically distinct from their HPV-negative counterparts, and as a result, in the new edition of the WHO, squamous cell carcinomas of the oropharynx are classified by HPV status. As mentioned above, several recent papers have shown that the sinonasal tract is the second anatomic “hot spot” from which HPV-related carcinomas can arise, with 20–30% of sinonasal carcinomas harboring transcriptionally-active, high-risk forms of HPV [25–27]. In the new edition of the WHO classification, however, “HPV-related squamous cell carcinoma” is not regarded as a separate tumor entity as it is in the oropharynx, largely because it lacks clinical and pathologic distinctness. For example, while most HPV-related sinonasal carcinomas have a histologic appearance that conforms to the WHO entity of non-keratinizing squamous cell carcinoma, only about 41% of sinonasal non-keratinizing squamous cell carcinomas are HPV-positive [2–4]. Perhaps more importantly, HPV positivity has not been proven to confer the same excellent prognosis for carcinomas of the sinonasal tract as it does for carcinomas of the oropharynx, although studies have been suggestive [2, 4, 63].
The histologic variants of HPV-positive carcinoma that are encountered in the oropharynx (e.g., small cell carcinoma, adenosquamous carcinoma, papillary squamous cell carcinoma) have also been described in the sinonasal tract [2]. There is one histologic variant, however, that has only been described in the sinonasal tract. This variant has features of both a surface-derived and salivary gland carcinoma, and has been referred to as “sinonasal HPV-related carcinomas with adenoid cystic-like features.” [64] Nine cases of HPV-related carcinomas with adenoid cystic-like features have been published, consisting of seven women and two men ranging in age from 40 to 75 years (mean, 57) [64, 65]. Patients have presented with nasal obstruction and/or epistaxis, and often at high-stage.
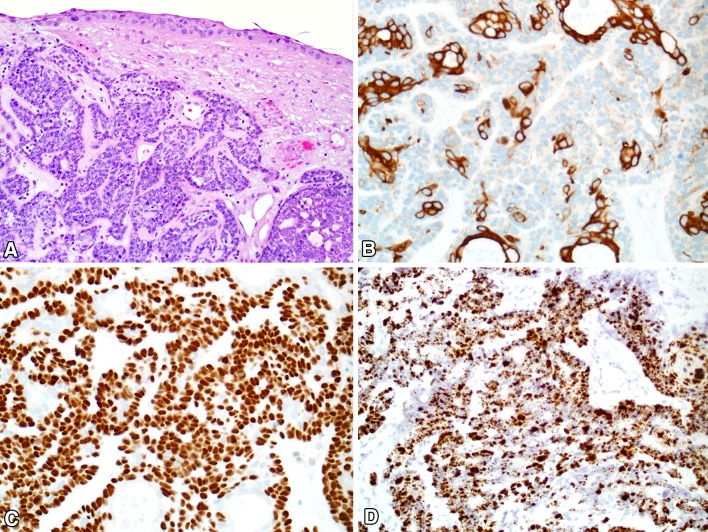
At the histologic level, sinonasal HPV-related carcinoma with adenoid cystic like features consists of highly cellular proliferations of basaloid cells growing mostly as solid nests and trabeculae, with most cases also exhibiting focal cribriform structures with microcystic pseudoductal spaces, reminiscent of adenoid cystic carcinoma (Fig. 6). The predominant basaloid cells have hyperchromatic, angulated nuclei, a high nuclear-to-cytoplasmic ratio, and often demonstrate cell spindling or clearing. In addition to the basaloid cells, these tumors also demonstrate inconspicuous ducts comprised of eosinophilic cuboidal cells. Squamous differentiation has not been seen within the invasive tumor, but most cases demonstrate squamous dysplasia of the surface epithelium. Mitotic rates are usually high, and necrosis is often present. Unlike true adenoid cystic carcinoma, perineural invasion is uncommon.
Fig. 6.
HPV-related adenoid cystic-like carcinoma. a Sinonasal HPV-related carcinoma with adenoid cystic-like features grows as nests and cribriform structures. Squamous dysplasia is seen in the overlying surface epithelium. b An immunostain for CK7 highlights the ductal structures. c An immunostain for p40 highlights only the basaloid myoepithelial cells, sparing the ducts. d The carcinoma is strongly positive for high-risk HPV by RNA in situ hybridization
At the immunohistochemical level, sinonasal HPV-related carcinoma with adenoid cystic like features is essentially identical to true adenoid cystic carcinoma, with two cell populations. The basaloid tumor cells are myoepithelial in nature, with immunostaining for one or more of these markers: S100, calponin, p63, p40, and actin. In contrast, the tumor ducts are negative for myoepithelial immunostains but positive for c-kit and CK7. By definition, the tumor cells are positive for high-risk types of HPV. Interestingly, most reported cases have harbored the uncommon HPV type 33, and neither type 16 or 18 have been reported in this tumor [64, 65]. Unlike true adenoid cystic carcinomas, HPV-related carcinoma with adenoid cystic like features does not harbor MYB gene fusions. The differential diagnosis of sinonasal HPV-related carcinoma with adenoid cystic-like features includes salivary gland neoplasms like adenoid cystic carcinoma, as well as other HPV-related variants like basaloid squamous cell carcinoma and adenosquamous carcinoma.
HPV-related carcinoma with adenoid cystic like features was considered as a possible new entity in the 4th edition of the WHO classification, but it was ultimately included as a provisional entity, listed in the differential diagnosis of non-keratinizing squamous cell carcinoma. The reservation for its inclusion focused on the low number of reported cases, its variable histology that often shows at least focal overlap with conventional non-keratinizing SCC, and its as-of-now uncertain prognostic significance.
SMARCB1 (INI-1) Deficient Sinonasal Sarcoma
SMARCB1 (INI-1) is a tumor suppressor gene located on chromosome 22q11.2; its inactivation has been implicated in the pathogenesis of a family of malignant neoplasms that includes pediatric atypical teratoid/rhabdoid tumor, rhabdoid tumors of the kidney and soft tissue [66–72], epithelioid sarcoma [73–75], renal medullary carcinoma [76], myoepithelial carcinoma of soft tissue [73, 77], epithelioid malignant peripheral nerve sheath tumor [73], and extraskeletal myxoid chondrosarcoma [78]. Recently two groups described a form of SMARCB1 (INI-1) deficient sinonasal carcinoma [79, 80].
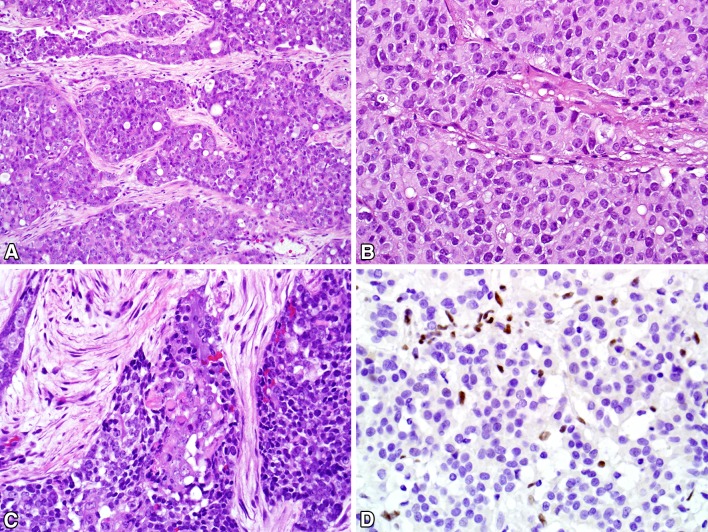
The sixteen SMARCB1 (INI-1) deficient sinonasal carcinomas have arose in 6 men and 10 women ranging in age from 28 to 78 (mean, 54) who presented with facial pain, eye symptoms, and nasal obstruction. Histologically, the reported SMARCB1 (INI-1) deficient sinonasal carcinomas grew as highly infiltrative epithelioid nests with tumor necrosis and high mitotic rates (Fig. 7). While most reported SMARCB1 (INI-1) deficient sinonasal carcinomas have been basaloid, resembling sinonasal undifferentiated carcinoma or non-keratinizing squamous cell carcinoma, a minority were oncocytic and plasmacytoid, resembling myoepithelial carcinoma. Even in the basaloid forms, however, rare plasmacytoid or rhabdoid cells were often identified. The reported SMARCB1 (INI-1) deficient sinonasal carcinomas have been consistently cytokeratin-positive and SMARCB1-negative, with variable positivity for squamous and neuroendocrine markers. All cases have been negative for HPV and NUT. In the eight cases in which SMARCB1 (INI-1) FISH was successfully performed, 5 showed homozygous deletion and 1 showed a heterozygous deletion pattern. The reported SMARCB1 (INI-1) deficient sinonasal carcinomas have been aggressive, with frequent local invasion into the brain and/or skull base. Seven of 15 reported patients experienced local recurrence, 7 of 15 have had regional or distant metastasis, and 6 of 15 patients have died of their disease [80–82].
Fig. 7.
SMARCB1 Deficient carcinoma. a SMARCB1-deficient sinonasal carcinomas typically grow as nests and cords of undifferentiated cells. b Some cases exhibit prominent plasmacytoid or rhabdoid morphology. c Other examples are more basaloid in appearance, with only focal plasmacytoid/rhabdoid features (center). d By definition, these tumors demonstrate a complete absence of SMARCB1 immunoreactivity, with the background stromal and inflammatory cells showing intact staining
SMARCB1 (INI-1) deficient sinonasal carcinoma was given consideration for inclusion in the 4th edition of the WHO classification. It remains unclear, however, whether SMARCB1 (INI-1) deficient sinonasal carcinoma is a distinct entity, or rather a pattern that can be seen in a variety of tumor types. Additional publications will be needed to address this question. As a result, while in the WHO classification SMARCB1 (INI-1) deficient sinonasal carcinoma is mentioned in the differential diagnosis of non-keratinizing squamous cell carcinoma, sinonasal undifferentiated carcinoma, NUT carcinoma, and melanoma, it was not regarded as a distinct tumor type.
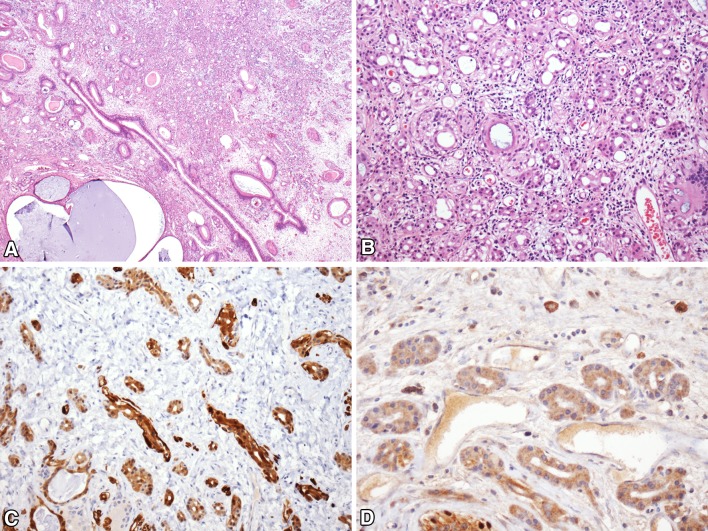
Renal Cell-like Adenocarcinoma
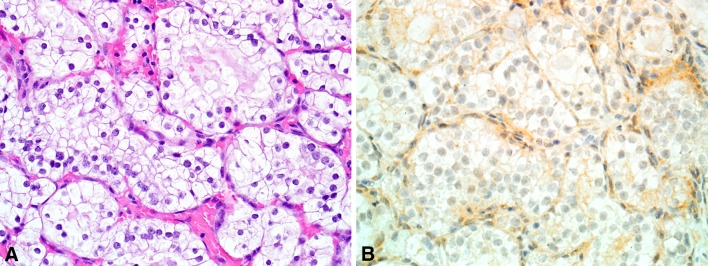
A variant of low-grade, non-intestinal sinonasal adenocarcinoma that mimicked renal cell carcinoma was initially described as renal cell-like adenocarcinoma in 2002, and a total of 16 cases have now been reported [83–87]. Renal cell-like adenocarcinoma has occurred in 11 women and five men ranging from 22 to 89 years old (mean, 58). Patients most commonly presented with epistaxis. As the name suggests, renal cell-like adenocarcinoma histologically resembles clear cell renal cell carcinoma, with nests and follicles of polyhedral cells with abundant optically clear cytoplasm (Fig. 8). Some cases have been described as containing prominent intrafollicular hemorrhage, also a classic feature of clear cell renal cell carcinoma. Nuclear pleomorphism and mitotic activity have been minimal. Sinonasal renal cell-like adenocarcinoma has been consistently positive for CK7, sometimes positive for S100, and negative for high-molecular weight cytokeratins, actin and calponin [83, 84]. More recently, it has been noted that CAIX is also positive in renal cell-like adenocarcinoma [87].
Fig. 8.
Renal cell-like adenocarcinoma. a Renal cell carcinoma-like sinonasal adenocarcinoma consists of nests and follicles of polygonal cells with small round nuclei and optically clear cytoplasm. b Unlike metastatic renal cell carcinoma, renal cell carcinoma-like sinonasal adenocarcinoma is negative for PAX8
Clearly, a diagnostic consideration for renal cell-like adenocarcinoma is metastatic renal cell carcinoma, which is not uncommon in the head and neck. Sinonasal renal cell-like adenocarcinoma is consistently negative for the immunohistochemical markers PAX8, RCC, and vimentin, unlike true renal cell carcinoma. Other diagnostic considerations include the minor salivary gland tumors hyalinizing clear cell carcinoma, mucoepidermoid carcinoma, and myoepithelial carcinoma. Sinonasal renal cell-like adenocarcinoma appears to be very indolent [83, 84].
Conclusion
There are a few new well-defined and emerging sinonasal neoplasms. Both NUT carcinomas and SMARCB1-deficient carcinomas were for the most part classified as poorly differentiated squamous cell carcinomas or SNUCs. Although the tumors have somewhat similar (very poor) prognoses, the specific molecular abnormalities seen with them may make the tumors responsive to targeted therapies in the future. The recognition of biphenotypic sinonasal sarcoma as a distinct neoplasm helps to clean up the somewhat heterogeneous group of tumors previously classified as malignant peripheral nerve sheath tumors or fibrosarcomas at the site. Finally, the recognition that some carcinomas associated with high-risk HPV can show differentiation reminiscent of salivary-gland neoplasia expands our understanding of the potential plasticity of sinonasal mucosa as it undergoes onconeogenesis.
Compliance with Ethical Standards
Disclosure
Drs. Bishop and Stelow have no conflict of interest.
Research Involving Animal and Human Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Special Issue: World Health Organization Classification Update
References
- 1.Lewis JS., Jr Sinonasal squamous cell carcinoma: a review with emphasis on emerging histologic subtypes and the role of human papillomavirus. Head Neck Pathol. 2016;10:60–67. doi: 10.1007/s12105-016-0692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop JA, Guo TW, Smith DF, Wang H, Ogawa T, Pai SI, Westra WH. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37:185–192. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29:1367–1372. doi: 10.1097/01.pas.0000173240.63073.fe. [DOI] [PubMed] [Google Scholar]
- 4.Larque AB, Hakim S, Ordi J, Nadal A, Diaz A, del Pino M, Marimon L, Alobid I, Cardesa A, Alos L. High-risk human papillomavirus is transcriptionally active in a subset of sinonasal squamous cell carcinomas. Mod Pathol. 2014;27:343–351. doi: 10.1038/modpathol.2013.155. [DOI] [PubMed] [Google Scholar]
- 5.Jo VY, Mills SE, Stoler MH, Stelow EB. Papillary squamous cell carcinoma of the head and neck: frequent association with human papillomavirus infection and invasive carcinoma. Am J Surg Pathol. 2009;33:1720–1724. doi: 10.1097/PAS.0b013e3181b6d8e6. [DOI] [PubMed] [Google Scholar]
- 6.Rytkonen AE, Hirvikoski PP, Salo TA. Lymphoepithelial carcinoma: two case reports and a systematic review of oral and sinonasal cases. Head Neck Pathol. 2011;5:327–334. doi: 10.1007/s12105-011-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes L. Intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Am J Surg Pathol. 1986;10:192–202. doi: 10.1097/00000478-198603000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Batsakis JG, Mackay B, Ordonez NG. Enteric-type adenocarcinoma of the nasal cavity. An electron microscopic and immunocytochemical study. Cancer. 1984;54:855–860. doi: 10.1002/1097-0142(19840901)54:5<855::AID-CNCR2820540516>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Cathro HP, Mills SE. Immunophenotypic differences between intestinal-type and low-grade papillary sinonasal adenocarcinomas: an immunohistochemical study of 22 cases utilizing CDX2 and MUC2. Am J Surg Pathol. 2004;28:1026–1032. doi: 10.1097/01.pas.0000126856.09058.71. [DOI] [PubMed] [Google Scholar]
- 10.Acheson ED, Cowdell RH, Hadfield E, Macbeth RG. Nasal cancer in woodworkers in the furniture industry. Br Med J. 1968;2:587–596. doi: 10.1136/bmj.2.5605.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadfield EH. A study of adenocarcinoma of the paranasal sinuses in woodworkers in the furniture industry. Ann R Coll Surg Engl. 1970;46:301–319. [PMC free article] [PubMed] [Google Scholar]
- 12.Hadfield EH, Macbeth RG. Adenocarcinoma of ethmoids in furniture workers. Ann Otol Rhinol Laryngol. 1971;80:699–703. doi: 10.1177/000348947108000512. [DOI] [PubMed] [Google Scholar]
- 13.Heffner DK, Hyams VJ, Hauck KW, Lingeman C. Low-grade adenocarcinoma of the nasal cavity and paranasal sinuses. Cancer. 1982;50:312–322. doi: 10.1002/1097-0142(19820715)50:2<312::AID-CNCR2820500225>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Jo VY, Mills SE, Cathro HP, Carlson DL, Stelow EB. Low-grade sinonasal adenocarcinomas: the association with and distinction from respiratory epithelial adenomatoid hamartomas and other glandular lesions. Am J Surg Pathol. 2009;33:401–408. doi: 10.1097/PAS.0b013e3181874ee8. [DOI] [PubMed] [Google Scholar]
- 15.Kleinsasser O. Terminal tubulus adenocarcinoma of the nasal seromucous glands. A specific entity. Arch Otorhinolaryngol. 1985;241:183–193. doi: 10.1007/BF00454353. [DOI] [PubMed] [Google Scholar]
- 16.Stelow EB, Mills SE, Jo VY, Carlson DL. Adenocarcinoma of the upper aerodigestive tract. Adv Anat Pathol. 2010;17:262–269. doi: 10.1097/PAP.0b013e3181e3bf80. [DOI] [PubMed] [Google Scholar]
- 17.Stelow EB, Jo VY, Mills SE, Carlson DL. A histologic and immunohistochemical stduy of high-grade non-intestinal sinonasal adenocarcinomas. Am J Surg Pathol. 2011;35:97180. doi: 10.1097/PAS.0b013e31821cbd72. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Ordonez B, Caruana SM, Huvos AG, Shah JP. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. Hum Pathol. 1998;29:826–832. doi: 10.1016/S0046-8177(98)90452-X. [DOI] [PubMed] [Google Scholar]
- 19.Weinreb I, Perez-Ordonez B. Non-small cell neuroendocrine carcinoma of the sinonasal tract and nasopharynx. Report of 2 cases and review of the literature. Head Neck Pathol. 2007;1:21–26. doi: 10.1007/s12105-007-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franchi A, Rocchetta D, Palomba A, Degli Innocenti DR, Castiglione F, Spinelli G. Primary combined neuroendocrine and squamous cell carcinoma of the maxillary sinus: report of a case with immunohistochemical and molecular characterization. Head Neck Pathol. 2015;9:107–113. doi: 10.1007/s12105-013-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson ED, Stelow EB, Mills SE, Westra WH, Bishop JA. Large cell neuroendocrine carcinoma of the head and neck: a clinicopathologic series of 10 cases with an emphasis on HPV status. Am J Surg Pathol. 2016;40:471–478. doi: 10.1097/PAS.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang CP, Hsieh CY, Chang YL, Lou PJ, Yang TL, Ting LL, Ko JY. Postirradiated neuroendocrine carcinoma of the sinonasal tract. Laryngoscope. 2008;118:804–809. doi: 10.1097/MLG.0b013e3181671491. [DOI] [PubMed] [Google Scholar]
- 23.Frierson HF, Jr, Mills SE, Fechner RE, Taxy JB, Levine PA. Sinonasal undifferentiated carcinoma. An aggressive neoplasm derived from schneiderian epithelium and distinct from olfactory neuroblastoma. Am J Surg Pathol. 1986;10:771–779. doi: 10.1097/00000478-198611000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Heffner DK, Hyams VJ. Teratocarcinosarcoma (malignant teratoma?) of the nasal cavity and paranasal sinuses: a clinicopathologic study of 20 cases. Cancer. 1984;53:2140–2154. doi: 10.1002/1097-0142(19840515)53:10<2140::AID-CNCR2820531025>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 25.Salem F, Rosenblum MK, Jhanwar SC, Kancherla P, Ghossein RA, Carlson DL. Teratocarcinosarcoma of the nasal cavity and paranasal sinuses: report of 3 cases with assessment for chromosome 12p status. Hum Pathol. 2008;39:605–609. doi: 10.1016/j.humpath.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Perlman E, Pack S, Davis M, Zhang H, Meltzer P, Tsokos M. Absence of EWS/FLI1 fusion in olfactory neuroblastomas indicates these tumors do not belong to the Ewing’s sarcoma family. Hum Pathol. 1999;30:1356–1360. doi: 10.1016/S0046-8177(99)90068-0. [DOI] [PubMed] [Google Scholar]
- 27.Taxy JB, Bharani NK, Mills SE, Frierson HF, Jr, Gould VE. The spectrum of olfactory neural tumors. A light-microscopic immunohistochemical and ultrastructural analysis. Am J Surg Pathol. 1986;10:687–695. doi: 10.1097/00000478-198610000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Bishop JA, Alaggio R, Zhang L, Seethala RR, Antonescu CR. Adamantinoma-like Ewing family tumors of the head and neck: a pitfall in the differential diagnosis of basaloid and myoepithelial carcinomas. Am J Surg Pathol. 2015;39:1267–1274. doi: 10.1097/PAS.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crane GM, Duffield AS. Hematolymphoid lesions of the sinonasal tract. Semin Diagn Pathol. 2016;33:71–80. doi: 10.1053/j.semdp.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreisel FH. Hematolymphoid lesions of the sinonasal tract. Head and Neck Pathol. 2016;10:109–117. doi: 10.1007/s12105-016-0698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson LD, Miettinen M, Wenig BM. Sinonasal-type hemangiopericytoma: a clinicopathologic and immunophenotypic analysis of 104 cases showing perivascular myoid differentiation. Am J Surg Pathol. 2003;27:737–749. doi: 10.1097/00000478-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Lasota J, Felisiak-Golabek A, Aly FZ, Wang ZF, Thompson LD, Miettinen M. Nuclear expression and gain-of-function beta-catenin mutation in glomangiopericytoma (sinonasal-type hemangiopericytoma): insight into pathogenesis and a diagnostic marker. Mod Pathol. 2015;28:715–720. doi: 10.1038/modpathol.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinreb I, Gnepp DR, Laver NM, Hoschar AP, Hunt JL, Seethala RR, Barnes EL, Chetty R, Perez-Ordonez B. Seromucinous hamartomas: a clinicopathological study of a sinonasal glandular lesion lacking myoepithelial cells. Histopathology. 2009;54:205–213. doi: 10.1111/j.1365-2559.2008.03198.x. [DOI] [PubMed] [Google Scholar]
- 34.Ozolek JA, Barnes EL, Hunt JL. Basal/myoepithelial cells in chronic sinusitis, respiratory epithelial adenomatoid hamartoma, inverted papilloma, and intestinal-type and nonintestinal-type sinonasal adenocarcinoma: an immunohistochemical study. Arch Pathol Lab Med. 2007;131:530–537. doi: 10.5858/2007-131-530-MCICSR. [DOI] [PubMed] [Google Scholar]
- 35.Jo VY, Mills SE, Cathro HP, Carlson DL, Stelow EB. Low-grade sinonasal adenocarcinomas: the association with and distinction from respiratory epithelial adenomatoid hamartomas and other glandular lesions. Am J Surg Pathol. 2008;33:401408. doi: 10.1097/PAS.0b013e3181874ee8. [DOI] [PubMed] [Google Scholar]
- 36.Ambrosini-Spaltro A, Morandi L, Spagnolo DV, Cavazza A, Brisigotti M, Damiani S, Jain S, Eusebi V. Nasal seromucinous hamartoma (microglandular adenosis of the nose): a morphological and molecular study of five cases. Virchows Arch. 2010;457:727–734. doi: 10.1007/s00428-010-0984-7. [DOI] [PubMed] [Google Scholar]
- 37.Kees UR, Mulcahy MT, Willoughby ML. Intrathoracic carcinoma in an 11-year-old girl showing a translocation t(15;19) Am J Pediatric Hematol. 1991;13:459–464. doi: 10.1097/00043426-199124000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Kubonishi I, Takehara N, Iwata J, Sonobe H, Ohtsuki Y, Abe T, Miyoshi I. Novel t(15;19)(q15;p13) chromosome abnormality in a thymic carcinoma. Cancer Res. 1991;51:3327–3328. [PubMed] [Google Scholar]
- 39.French C. NUT midline carcinoma. Nature reviews Cancer. 2014;14:149–150. doi: 10.1038/nrc3659. [DOI] [PubMed] [Google Scholar]
- 40.French CA. The importance of diagnosing NUT midline carcinoma. Head Neck Pathol. 2013;7:11–16. doi: 10.1007/s12105-013-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stelow EB. A review of NUT midline carcinoma. Head Neck Pathol. 2011;5:31–35. doi: 10.1007/s12105-010-0235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauer DE, Mitchell CM, Strait KM, Lathan CS, Stelow EB, Luer SC, Muhammed S, Evans AG, Sholl LM, Rosai J, Giraldi E, Oakley RP, Rodriguez-Galindo C, London WB, Sallan SE, Bradner JE, French CA. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res. 2012;18:5773–5779. doi: 10.1158/1078-0432.CCR-12-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stelow EB, Bellizzi AM, Taneja K, Mills SE, Legallo RD, Kutok JL, Aster JC, French CA. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. 2008;32:828–834. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 44.Solomon LW, Magliocca KR, Cohen C, Muller S. Retrospective analysis of nuclear protein in testis (NUT) midline carcinoma in the upper aerodigestive tract and mediastinum. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;119:213220. doi: 10.1016/j.oooo.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 45.Bishop JA, Westra WH. NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol. 2012;36:1216–1221. doi: 10.1097/PAS.0b013e318254ce54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang W, French CA, Cameron MJ, Han Y, Liu H. Clinicopathological significance of NUT rearrangements in poorly differentiated malignant tumors of the upper respiratory tract. Int J Surg Pathol. 2013;21:102–110. doi: 10.1177/1066896912451651. [DOI] [PubMed] [Google Scholar]
- 47.Bishop JA, French CA, Ali SZ. Cytopathologic features of NUT midline carcinoma: A series of 26 specimens from 13 patients. Cancer Cytopathol. 2016;124:901908. doi: 10.1002/cncy.21761. [DOI] [PubMed] [Google Scholar]
- 48.Bellizzi AM, Bruzzi C, French CA, Stelow EB. The cytologic features of NUT midline carcinoma. Cancer. 2009;117:508–515. doi: 10.1002/cncy.20044. [DOI] [PubMed] [Google Scholar]
- 49.French CA, Ramirez CL, Kolmakova J, Hickman TT, Cameron MJ, Thyne ME, Kutok JL, Toretsky JA, Tadavarthy AK, Kees UR, Fletcher JA, Aster JC. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27:2237–2242. doi: 10.1038/sj.onc.1210852. [DOI] [PubMed] [Google Scholar]
- 50.Haack H, Johnson LA, Fry CJ, Crosby K, Polakiewicz RD, Stelow EB, Hong SM, Schwartz BE, Cameron MJ, Rubin MA, Chang MC, Aster JC, French CA. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol. 2009;33:984–991. doi: 10.1097/PAS.0b013e318198d666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.French CA. Pathogenesis of NUT midline carcinoma. Annu Rev Pathol. 2012;7:247–265. doi: 10.1146/annurev-pathol-011811-132438. [DOI] [PubMed] [Google Scholar]
- 52.Lewis JT, Oliveira AM, Nascimento AG, Schembri-Wismayer D, Moore EA, Olsen KD, Garcia JG, Lonzo ML, Lewis JE. Low-grade sinonasal sarcoma with neural and myogenic features: a clinicopathologic analysis of 28 cases. Am J Surg Pathol. 2012;36:517–525. doi: 10.1097/PAS.0b013e3182426886. [DOI] [PubMed] [Google Scholar]
- 53.Huang SC, Ghossein RA, Bishop JA, Zhang L, Chen TC, Huang HY, Antonescu CR. Novel PAX3-NCOA1 fusions in biphenotypic sinonasal sarcoma with focal rhabdomyoblastic differentiation. Am J Surg Pathol. 2016;40:51–59. doi: 10.1097/PAS.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Bledsoe KL, Graham RP, Asmann YW, Viswanatha DS, Lewis JE, Lewis JT, Chou MM, Yaszemski MJ, Jen J, Westendorf JJ, Oliveira AM. Recurrent PAX3-MAML3 fusion in biphenotypic sinonasal sarcoma. Nat Genet. 2014;46:666–668. doi: 10.1038/ng.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Powers KA, Han LM, Chiu AG, Aly FZ. Low-grade sinonasal sarcoma with neural and myogenic features-diagnostic challenge and pathogenic insight. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:e265–e269. doi: 10.1016/j.oooo.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 56.Rooper LM, Huang SC, Antonescu CR, Westra WH, Bishop JA. Biphenotypic sinonasal sarcoma: an expanded immunoprofile including consistent nuclear beta-catenin positivity and absence of SOX10 expression. Hum Pathol. 2016;55:44–50. doi: 10.1016/j.humpath.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong WJ, Lauria A, Hornick JL, Xiao S, Fletcher JA, Marino-Enriquez A. Alternate PAX3-FOXO1 oncogenic fusion in biphenotypic sinonasal sarcoma. Genes Chromosomes Cancer. 2016;55:25–29. doi: 10.1002/gcc.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ukpo OC, Flanagan JJ, Ma XJ, Luo Y, Thorstad WL, Lewis JS., Jr High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–1350. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 59.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 60.Bishop JA, Ma XJ, Wang H, Luo Y, Illei PB, Begum S, Taube JM, Koch WM, Westra WH. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36:1874–1882. doi: 10.1097/PAS.0b013e318265fb2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poling JS, Ma XJ, Bui S, Luo Y, Li R, Koch WM, Westra WH. Human papillomavirus (HPV) status of non-tobacco related squamous cell carcinomas of the lateral tongue. Oral Oncol. 2014;50:306–310. doi: 10.1016/j.oraloncology.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lewis JS, Jr, Ukpo OC, Ma XJ, Flanagan JJ, Luo Y, Thorstad WL, Chernock RD. Transcriptionally-active high-risk human papillomavirus is rare in oral cavity and laryngeal/hypopharyngeal squamous cell carcinomas–a tissue microarray study utilizing E6/E7 mRNA in situ hybridization. Histopathology. 2012;60:982–991. doi: 10.1111/j.1365-2559.2011.04169.x. [DOI] [PubMed] [Google Scholar]
- 63.Alos L, Moyano S, Nadal A, Alobid I, Blanch JL, Ayala E, Lloveras B, Quint W, Cardesa A, Ordi J. Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favorable outcome. Cancer. 2009;115:2701–2709. doi: 10.1002/cncr.24309. [DOI] [PubMed] [Google Scholar]
- 64.Bishop JA, Ogawa T, Stelow EB, Moskaluk CA, Koch WM, Pai SI, Westra WH. Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal tract. Am J Surg Pathol. 2013;37:836–844. doi: 10.1097/PAS.0b013e31827b1cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang SJ, Ok S, Lee HM, Lee E, Park IH. Human papillomavirus-related carcinoma with adenoid cystic-like features of the inferior turbinate: a case report. Auris Nasus Larynx. 2015;42:53–55. doi: 10.1016/j.anl.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 66.Biegel JA, Kalpana G, Knudsen ES, Packer RJ, Roberts CW, Thiele CJ, Weissman B, Smith M. The role of INI1 and the SWI/SNF complex in the development of rhabdoid tumors: meeting summary from the workshop on childhood atypical teratoid/rhabdoid tumors. Cancer Res. 2002;62:323–328. [PubMed] [Google Scholar]
- 67.Biegel JA, Zhou JY, Rorke LB, Stenstrom C, Wainwright LM, Fogelgren B. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res. 1999;59:74–79. [PubMed] [Google Scholar]
- 68.Rorke LB, Packer RJ, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg. 1996;85:56–65. doi: 10.3171/jns.1996.85.1.0056. [DOI] [PubMed] [Google Scholar]
- 69.Biegel JA, Burk CD, Parmiter AH, Emanuel BS. Molecular analysis of a partial deletion of 22q in a central nervous system rhabdoid tumor. Genes Chromosomes Cancer. 1992;5:104–108. doi: 10.1002/gcc.2870050203. [DOI] [PubMed] [Google Scholar]
- 70.Biegel JA, Rorke LB, Emanuel BS. Monosomy 22 in rhabdoid or atypical teratoid tumors of the brain. N Engl J Med. 1989;321:906. doi: 10.1056/nejm198909283211317. [DOI] [PubMed] [Google Scholar]
- 71.Shashi V, Lovell MA, von Kap-herr C, Waldron P, Golden WL. Malignant rhabdoid tumor of the kidney: involvement of chromosome 22. Genes Chromosomes Cancer. 1994;10:49–54. doi: 10.1002/gcc.2870100108. [DOI] [PubMed] [Google Scholar]
- 72.Schofield DE, Beckwith JB, Sklar J. Loss of heterozygosity at chromosome regions 22q11-12 and 11p15.5 in renal rhabdoid tumors. Genes Chromosomes Cancer. 1996;15:10–17. doi: 10.1002/(SICI)1098-2264(199601)15:1<10::AID-GCC2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 73.Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol. 2009;33:542–550. doi: 10.1097/PAS.0b013e3181882c54. [DOI] [PubMed] [Google Scholar]
- 74.Modena P, Lualdi E, Facchinetti F, Galli L, Teixeira MR, Pilotti S, Sozzi G. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res. 2005;65:4012–4019. doi: 10.1158/0008-5472.CAN-04-3050. [DOI] [PubMed] [Google Scholar]
- 75.Le Loarer F, Zhang L, Fletcher CD, Ribeiro A, Singer S, Italiano A, Neuville A, Houlier A, Chibon F, Coindre JM, Antonescu CR. Consistent SMARCB1 homozygous deletions in epithelioid sarcoma and in a subset of myoepithelial carcinomas can be reliably detected by FISH in archival material. Genes Chromosomes Cancer 2014. [DOI] [PMC free article] [PubMed]
- 76.Cheng JX, Tretiakova M, Gong C, Mandal S, Krausz T, Taxy JB. Renal medullary carcinoma: rhabdoid features and the absence of INI1 expression as markers of aggressive behavior. Mod Pathol. 2008;21:647–652. doi: 10.1038/modpathol.2008.44. [DOI] [PubMed] [Google Scholar]
- 77.Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813–1824. doi: 10.1097/PAS.0b013e31805f6775. [DOI] [PubMed] [Google Scholar]
- 78.Kohashi K, Oda Y, Yamamoto H, Tamiya S, Oshiro Y, Izumi T, Taguchi T, Tsuneyoshi M. SMARCB1/INI1 protein expression in round cell soft tissue sarcomas associated with chromosomal translocations involving EWS: a special reference to SMARCB1/INI1 negative variant extraskeletal myxoid chondrosarcoma. Am J Surg Pathol. 2008;32:1168–1174. doi: 10.1097/PAS.0b013e318161781a. [DOI] [PubMed] [Google Scholar]
- 79.Agaimy A, Rau TT, Hartmann A, Stoehr R. SMARCB1 (INI1)-negative rhabdoid carcinomas of the gastrointestinal tract: clinicopathologic and molecular study of a highly aggressive variant with literature review. Am J Surg Pathol. 2014;38:910920. doi: 10.1097/PAS.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 80.Bishop JA, Antonescu CR, Westra WH. SMARCB1 (INI-1)-deficient carcinomas of the sinonasal tract. Am J Surg Pathol. 2014;38:1282–1289. doi: 10.1097/PAS.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Agaimy A, Koch M, Lell M, Semrau S, Dudek W, Wachter DL, Knoll A, Iro H, Haller F, Hartmann A. SMARCB1(INI1)-deficient sinonasal basaloid carcinoma: a novel member of the expanding family of SMARCB1-deficient neoplasms. Am J Surg Pathol. 2014;38:1274–1281. doi: 10.1097/PAS.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bell D, Hanna EY, Agaimy A, Weissferdt A. Reappraisal of sinonasal undifferentiated carcinoma: SMARCB1 (INI1)-deficient sinonasal carcinoma: a single-institution experience. Virchows Archiv. 2015;467:649656. doi: 10.1007/s00428-015-1853-1. [DOI] [PubMed] [Google Scholar]
- 83.Zur KB, Brandwein M, Wang B, Som P, Gordon R, Urken ML. Primary description of a new entity, renal cell-like carcinoma of the nasal cavity: van Meegeren in the house of Vermeer. Arch Otolaryngol Head Neck Surg. 2002;128:441–447. doi: 10.1001/archotol.128.4.441. [DOI] [PubMed] [Google Scholar]
- 84.Storck K, Hadi UM, Simpson R, Ramer M, Brandwein-Gensler M. Sinonasal renal cell-like adenocarcinoma: a report on four patients. Head Neck Pathol. 2008;2:75–80. doi: 10.1007/s12105-008-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moh’d Hadi U, Kahwaji GJ, Mufarrij AA, Tawil A, Noureddine B. Low grade primary clear cell carcinoma of the sinonasal tract. Rhinology. 2002;40:44–47. [PubMed] [Google Scholar]
- 86.Brandwein-Gensler M, Wei S. Envisioning the next WHO head and neck classification. Head Neck Pathol. 2014;8:1–15. doi: 10.1007/s12105-014-0529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shen T, Shi Q, Velosa C, Bai S, Thompson L, Simpson R, Wei S, Brandwein-Gensler M. Sinonasal renal cell-like adenocarcinomas: robust carbonic anhydrase expression. Hum Pathol. 2015;46:1598–1606. doi: 10.1016/j.humpath.2015.06.017. [DOI] [PubMed] [Google Scholar]