Abstract
There have been several additions and deletions in Chapter 4 on Tumours of the oral cavity and mobile tongue in the 2017 fourth edition of the World Health Organization Classification of Tumours of the Head and Neck. This chapter excludes the oropharynx, which now is a stand-alone chapter acknowledging the uniqueness of the oropharynx from the oral cavity. New entries in Chapter 4 include rhabdomyoma, haemangioma, schwannoma, neurofibroma and myofibroblastic sarcoma in the section titled Soft tissue and neural tumours. Discussion of salivary gland entities have been reduced and includes mucoepidermoid carcinoma and pleomorphic adenoma as the other salivary gland types are discussed elsewhere. In the Haematolymphoid tumours section, like the salivary gland section, only tumors that commonly present in the oral cavity are discussed in Chapter 4. Excluded entities in the updated classification include papillary hyperplasia, median rhomboid glossitis, keratoacanthoma, focal oral mucinosis, and secondary tumors. This article will summarize the changes in the new classification since the 2005 edition focusing on selected entities that have had significant changes along with new entries.
Keywords: Oral cavity, Mobile tongue, WHO, Squamous cell, Dysplasia, Proliferative verrucous leukoplakia
Introduction
There have been several additions and deletions in Chapter 4 on Tumours of the oral cavity and mobile tongue in the 2017 fourth edition of the World Health Organization Classification of Tumours of the Head and Neck compared to the 2005 3rd edition [1]. Significantly, this chapter excludes the oropharynx, which now is a stand-alone chapter acknowledging the uniqueness of the oropharynx from the oral cavity. This change reflects the momentous impact oropharyngeal carcinoma and the role high-risk human papilloma virus has had in the field of head and neck oncology since the last WHO publication. New entries in Chapter 4 include rhabdomyoma, haemangioma, schwannoma, neurofibroma, and myofibroblastic sarcoma in the section titled Soft tissue and neural tumours. Dramatic reductions in the number of entities discussed under the haematolymphoid section and the salivary gland section as these entities are discussed elsewhere in the book. Excluded entities in the updated classification include papillary hyperplasia, median rhomboid glossitis, keratoacanthoma, focal oral mucinosis, and secondary tumors. This update will highlight the areas in Chapter 4 that have significantly changed since the 3rd edition was published in 2005 along with describing new entries.
Tumours of the Oral Cavity and Mobile Tongue
Malignant Surface Epithelial Tumours
The major change in this section as already mentioned is the exclusion of the oropharynx [2]. Although human papillomavirus (HPV), particularly type 16, is mentioned as an etiologic factor in the development of oral cavity squamous cell carcinoma (OSCC), acknowledgement is made that HPV-driven OSCC accounts for a very small percentage (1–10%) of cancers [3–8]. The major risk factor remains smoking with a synergistic association with alcohol consumption [9–11]. The incidence of OSCC has decreased in some countries, however a high incidence of OSCC is still found in South Asian countries including India, Pakistan, Sri Lanka, China, and Taiwan [12, 13]. In addition to tobacco smoking, betel-quid chewing, with or without tobacco, is prevalent in these countries. Other countries with a high incidence in cancer rates per the GLOBOCAN project are Hungary, Slovakia, Slovenia, France, Brazil, Uruguay, and Puerto Rico [14].
The subtypes of OSCC are unchanged from the 3rd edition and include basaloid SCC, spindle cell carcinoma, adenosquamous carcinoma, carcinoma cuniculatum, verrucous carcinoma, papillary SCC, acantholytic SCC, and lymphoepithelial SCC [15–22]. Lymphoepithelial carcinoma was a stand-alone section in the last edition but now is included in the discussion on OSCC subtypes. In the current classification under oral cavity, lymphoepithelial SCC is considered rare for this anatomic location. Not all cases are EBV-positive and most patients present with nodal disease [22].
The need for immunohistochemistry in OSCC is limited in most cases except when squamous differentiation is minimal or absent as is the case with spindle cell carcinoma. Grading of conventional squamous cell carcinoma is unchanged although grading is not an independent predictor of prognosis [23]. Risk factors associated with poor prognosis include perineural invasion, lymphovascular invasion, non-cohesive pattern of invasion, depth of invasion, and high grade dysplasia at the surgical margins [24–27]. The importance of depth of invasion in prognosis has led to reclassification of the T stage in the new 8th edition of the American Joint Cancer Committee (AJCC) (Table 1; Fig. 1) [28].
Table 1.
AJCC 8th edition definition of primary tumor (T) for oral cavity [28]
| T1 = tumor ≤2 cm, ≤5 mm depth of invasion |
| T2 = tumor ≤2 cm, depth of invasion >5 mm and ≤10 mm OR tumor >2 cm, but ≤4 cm, depth of invasion ≤10 mm |
| T3 = tumor >4 cm OR any tumor with depth of invasion >10 mm |
Fig. 1.
Measuring depth of invasion (DOI). DOI is not synonymous with tumor thickness and is determined by creating an imaginary horizontal line along the basement membrane of adjacent squamous mucosa. A vertical or plumb line (arrow) from the horizontal line is extended to measure DOI. a Measuring DOI in an exophytic tumor. b Measuring DOI in an ulcerated tumor
Oral Potentially Malignant Disorders and Oral Epithelial Dysplasia
This section has been renamed from combining sections entitled Epithelial precursor lesions and Proliferative verrucous leukoplakia and precancerous lesions from the 3rd edition [29]. The name oral potentially malignant disorders (OPMD) recognizes that in some conditions the risk of malignant transformation is extremely low and even reversible [30]. The global malignant transformation rate of oral leukoplakia is estimated to be 1–2% [31].
The diagnostic criteria for epithelial dysplasia has been modified from the 3rd edition with two classification schemas proposed for grading of oral epithelial dysplasia. The WHO dysplasia grade is three tiered: mild, moderate and severe with severe dysplasia and carcinoma in situ considered to be synonymous. A proposed binary system (high-grade and low-grade) similar to grading laryngeal lesions is advocated by some but requires validation before being adopted [32].
A recently described type of dysplasia which is positive for high-risk HPV with distinct histology has been added to the 4th edition, but the significance of this finding is unknown as the risk of malignant transformation is undetermined [33]. The epithelium exhibits full-thickness dysplasia with marked apoptosis and karyorrhexis (Fig. 2a). There is strong nuclear and cytoplasmic staining for p16 (Fig. 2b) and by in situ hybridization high-risk HPV is detected.
Fig. 2.

HPV-associated oral dysplasia. a The squamous epithelium shows brightly eosinophilic parakeratosis with epithelial hyperplasia exhibiting full-thickness atypia with marked karyorrhexis and apoptosis (×100). b The oral dysplasia demonstrated strong and diffuse nuclear and cytoplasmic staining for p16 by immunohistochemistry (×100)
Using p16 as a surrogate marker of HPV status in oral cavity dysplasia and OSCC is discouraged. HPV-driven OSCC has a very low incidence. Studies have shown that up to a third of OSCC are p16 positive and viral DNA is detectable by polymerase chain reaction in up to 28% of cases [7, 8]. However when studies use in situ hybridization, a more sensitive method of detecting high-risk HPV, very few cases (1–10%) are positive [3–8]. Therefore, relying on p16 as a surrogate marker for HPV overestimates the number of HPV-related cancers in the oral cavity. In addition, studies have shown no survival benefit in p16 positive OSCC compared to p16 negative OSCC [7, 8].
Papillomas
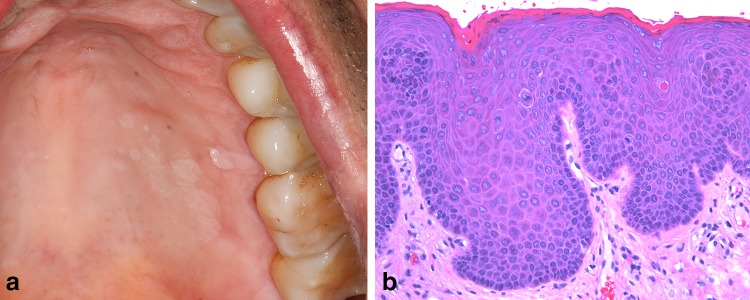
As expected there have not been any significant alterations in this section apart from multifocal epithelial hyperplasia (MFEH) [34]. In the 3rd edition there was a brief section entitled Papillomas and papillomatosis in immunodeficiency. Since 2005, it has been recognized that these florid presentations of HPV-induced lesions, particularly in HIV-positive patients on combination antiretroviral medication are a form of MFEH [35–37]. The traditional form of MFEH is prevalent in children and adolescents, and is endemic in parts of the world [37, 38]. Associated with low socioeconomic status, poor nutrition and crowded living conditions, this form of MFEH is usually associated with HPV types 13 and 32. Other genotypes have been implicated including 1, 6, 11, 16, 18, and 55 and are seen in HIV-positive patients [37, 39]. The most common location for MFEH are lips, buccal mucosa and tongue borders, however the hard palate and gingiva are well documented sites of occurrence in the HIV-positive patient (Fig. 3a, b) [37].
Fig. 3.
Multifocal epithelial hyperplasia. a Multiple white coalescing papules of the hard palate in a 47-year-old HIV-positive white male on combination anti-retroviral medicine. b On medium-power the epithelium is acanthotic with a smooth surface and elongated rete. Mitosoid bodies, representing cells with karyorrhectic nuclei are readily observed (×100)
Epithelial entities that have been omitted in the 4th edition include keratoacanthoma, a lesion of questionable histogenesis in the oral cavity. Papillary hyperplasia and median rhomboid glossitis which are reactive processes rather than neoplastic have also been omitted.
Non-epithelial Tumours
Since the 2005 publication of the 3rd edition additional cases of Ectomesenchymal chondromyxoid tumour (ECT), a rare tumor, have been published bringing to date about 60 cases [40]. With additional cases the epidemiology is unchanged with no gender predilection and reported age range of 7–78 years [41, 42]. Although a few cases have been reported in the posterior tongue and hard palate, ECT arises almost exclusively in the anterior dorsal tongue. The cell or origin is still unknown. The anterior dorsal tongue lacks minor salivary glands and ECT exhibits variable cytokeratin staining, making a salivary gland origin less likely [43]. It is proposed that ECT arises from undifferentiated ectomesenchymal cells from embryonic neural crest mesenchyme [44].
Focal oral mucinosis, considered to be the oral counterpart of focal cutaneous mucinosis has been omitted in the 4th edition.
Soft Tissue and Neural Tumours
There have been new entities added under this section in the 4th edition. Rhabdomyoma, a benign tumor of skeletal differentiation with a predilection for head and neck sites was in the pharynx chapter in the 3rd edition but now is in Chapter 4 [45]. Rhabdomyomas are divided into fetal, juvenile, and adult subtypes. These subtypes are based on histology rather than age of occurrence, though fetal rhabdomyoma usually presents in newborns and early childhood. PTCH1 aberrations in fetal rhabdomyomas and multifocal fetal rhabdomyomas have been reported in the context of nevoid basal cell carcinoma syndrome, a genetic disorder linked to PTCH1 mutations [46].
The Lymphangioma section has been updated to reflect the ever-increasing array of immunohistochemical stains available [47]. The endothelial cells of lymphangioma are positive for CD31 or CD34, podoplanin (D2-40), PROX1, VEGFR3 and LYVE1 while the vessel wall is immune-positive for SMA [48–52]. Lymphangiomas are associated with genetic disorders including Turner syndrome (45,X syndrome), trisomy 21 and Proteus syndrome caused by mutation in AKT1 [49, 53].
Haemangioma, a benign vascular neoplasm has been added to this section which is new from previous editions [54]. Oral hemangiomas grow by endothelial cell hyperplasia and are considered a true neoplasm distinct from vascular ectasias, vascular malformations and pyogenic granulomas. Hemangiomas are found in greater frequency in females, whites, and premature infants. Although hemangiomas are common in the head and neck region (60%), they are less frequently encountered in the oral cavity apart from infantile hemangioma, which is the most common benign tumor of the oral cavity including mobile tongue in childhood [55–58].
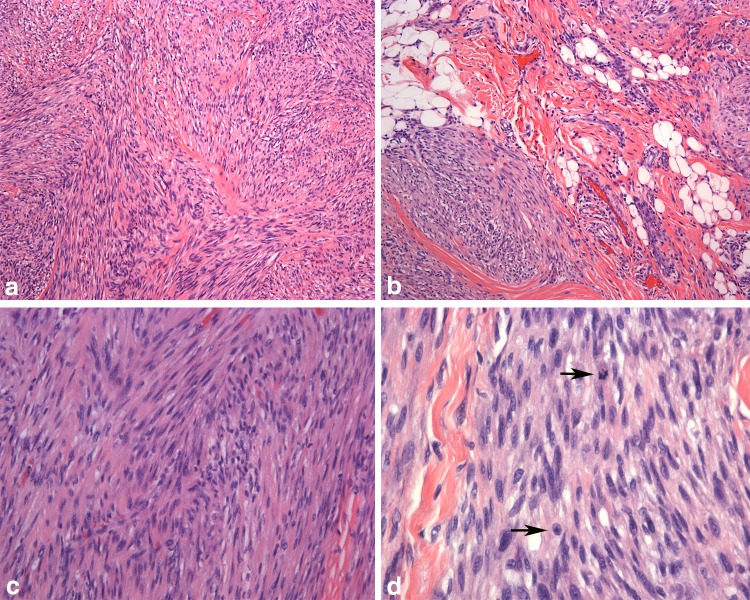
A new entity for Chapter 4 is Myofibroblastic sarcoma, a low-grade malignant tumor with a predilection for the oral cavity and mobile tongue [59]. The tumor occurs in a wide age group with a mean of 40 years [60, 61]. The tumor growth can be circumscribed to diffusely infiltrative. The histology is varied with cellular, fibromatosis-like or fibrosarcoma-like fascicles or storiform growth (Fig. 4). The cells have fusiform nuclei that may be elongated or wavy with evenly distributed chromatin or the nuclei may be round and vesicular. Nuclear atypia with hyperchromasia and variable mitotic activity is seen. The stroma is collagenous and no histiocytic giant cells or prominent inflammation is present. Immunohistochemistry is helpful for diagnosis demonstrating a myofibroblastic immunophenotype. Variable expression is seen with SMA, desmin, calponin and CD34 and rarely, focal expression with h-caldesmon [60, 61]. The tumor rarely metastasizes (lung, soft tissue, bone) but local recurrences are common [61].
Fig. 4.
Myofibroblastic sarcoma. a Tumor cells are arranged in interlacing fascicles with a herringbone arrangement (×40). b Infiltration into the surrounding fat and muscle by tumor exhibiting a storiform configuration (×100). c Tumor nuclei are tapered and hyperchromatic (×200). d The nuclei have fine, evenly distributed chromatin and small nucleoli. The number of mitotic figures (arrows) in myofibroblastic sarcoma are variable (×400)
Salivary-Type Tumours
This section has been greatly reduced from the 3rd edition and now has a truncated discussion of Mucoepidermoid carcinoma and Pleomorphic adenoma [62, 63]. Tumours of the salivary glands in Chapter 7 cover both these entities in more detail including illustrations.
Haematolymphoid Tumours
Similar to the section on salivary-type tumors, this section has also been trimmed as these entities are discussed more fully in the chapter on haematolymphoid tumours. CD30-positive T-cell lymphoproliferative disorder (TLPD) now has an expanded dedicated section reflecting the increased knowledge of this disease since 2005 [64]. TLPD is a neoplasm of CD30-positive T-cells arising mainly in the oral cavity but occasionally occurs in other mucosal sites of the head and neck [65–67]. The disease has a male:female ratio of 2:1 and typically presents in the sixth decade [67]. TLPD is analogous to primary cutaneous CD30-positive TLPD and represents a clinicopathologic spectrum. Rarely secondary oral involvement by anaplastic large cell lymphoma can occur and must be ruled out. TLPD is considered an indolent lesion and most cases show complete resolution with excision [66, 67]. Spontaneous regression has also been reported. The tumor typically presents as a mass lesion with ulceration (Fig. 5). Large, atypical lymphoid cells with atypical nuclei grow in a diffuse pattern with a mixed inflammatory background including eosinophils (Fig. 6) [66–68]. CD30 immunohistochemistry highlights the atypical cells and is strongly and diffusely positive. TLPD must be distinguished from reactive lesions, in particular, traumatic ulceration with stromal eosinophilia (TUGSE) [69]. Similar to TLPD, TUGSE is typically a self-limited lesion with rapid healing after biopsy or excision. The underlying pathogenesis of TUGSE is unknown and studies have shown some case to have TCRγ gene rearrangement. TUGSE is not diffusely CD30 positive compared to TLPD. As both TUGSE and TLPD are managed clinically similar and follow an indolent course it is important to recognize these entities to avoid possible overtreatment resulting from a diagnosis of anaplastic large cell lymphoma.
Fig. 5.
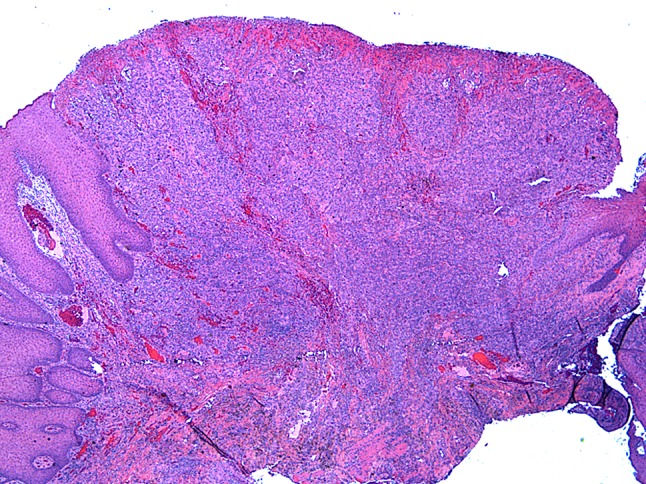
Primary CD30-positive T-cell lymphoproliferative disorder of the tongue. Extensive surface ulceration with a deeply infiltrative lesion composed of mixed inflammation supporting large atypical lymphoid cells (×20)
Fig. 6.
Primary CD30-positive T-cell lymphoproliferative disorder. a Large, atypical lymphoid cells that are mitotically active (arrows) (×400). b Numerous scattered eosinophils (arrows) with a mixed inflammatory background. CD30 immunostaining should be strong in contradistinction to traumatic ulcerative granuloma with stromal eosinophilia (×400)
Plasmablastic lymphoma (PBL) has also been expanded from the 3rd edition [70]. PBL, an aggressive high-grade B-cell non-Hodgkin lymphoma with plasma cell immunophenotype, is strongly associated with HIV and is an AIDS-defining neoplasm (Fig. 7) [71, 72]. PBL also occurs in non-HIV immunosuppressed patients and in older patients (>60 years of age), more commonly female [71]. Other head and neck mucosal sites affected by PBL in addition to the oral cavity include oropharynx, nasopharynx, and sinonasal tract [73]. Lymph nodes may be involved but usually in the HIV-negative patient. PBL is associated with Epstein Barr virus (EBV) but the precise pathogenesis is currently unknown. EBV can cause a surge in plasmablasts and in addition, MYC gene rearrangements may be an important pathogenic mechanism. The histology of PBL shows a proliferation of large lymphoid cells with immunoblastic and plasmablastic features. The cells have moderately abundant basophilic cytoplasm with a central round nuclei, large nucleoli and occasional paranuclear hofs (Fig. 8a). PBL expresses plasma cell markers (CD79a, CD38, MUM1-IRF4, CD138) and lacks B-cell markers (CD19, CD20, PAX5, ALK) [74]. Ki-67 index is uniformly high (>80%) and EBV-encoded RNA (EBER) is >70% in HIV-positive and transplant associated cases and in about 50% on HIV-negative cases (Fig. 8b) [71]. The prognosis is poor with a median survival of 15 months [75 ].
Fig. 7.

Plasmablastic lymphoma in a 53-year-old HIV-positive black male presenting as a mass on the maxillary alveolar mucosa
Fig. 8.
Plasmablastic lymphoma in an HIV-positive male. a Sheets of large lymphoma cells with immunoblastic features: round to oval nuclei, prominent nucleoli and moderately abundant cytoplasm and occasional paranuclear hofs (×400). b EBV-encoded small RNA (EBER) in situ hybridization is positive, a finding in >70% of HIV-associated and post-transplant cases (×400). Images courtesy of Dr. Sonja Boy
Omitted from this section is a discussion of follicular dendritic cells sarcoma/tumour. Also omitted from the updated 4th edition is the section on secondary tumors which reviewed metastases to bone and soft tissue in the oral cavity and mobile tongue.
Footnotes
Special Issue: World Health Organization Classification Update
References
- 1.Tumours of the oral cavity and mobile tongue. In: el-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors, WHO classification of tumours of the head and neck. 4th ed. Lyon: IARC Press; 2017.
- 2.Sloan P, Gale N, Hunter K, et al. Malignant surface epithelial tumours: Squamous cell carcinoma. In: el-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, et al., editors. WHO classification of tumours of the head and neck. 4th ed. Lyon: IARC Press; 2017. [Google Scholar]
- 3.Dahlgren L, Dahlstrand HM, Lindquist D, et al. Human papillomavirus is more common in base of tongue than in mobile tongue cancer and is a favorable prognostic factor in base of tongue cancer patients. Int J Cancer. 2004;112:1015–1019. doi: 10.1002/ijc.20490. [DOI] [PubMed] [Google Scholar]
- 4.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33:3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lingen MW, Xiao W, Schmitt A, et al. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2013;49:1–8. doi: 10.1016/j.oraloncology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Sgaramella N, Coates PJ, Strindlund K, et al. Expression of p16 in squamous cell carcinoma of the mobile tongue is independent of HPV infection despite presence of the HPV-receptor syndecan-1. Br J Cancer. 2015;113:321–326. doi: 10.1038/bjc.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zafereo ME, Xu L, Dahlstrom KR, Viamonte CA, et al. Squamous cell carcinoma of the oral cavity often overexpresses p16 but is rarely driven by human papillomavirus. Oral Oncol. 2016;56:47–53. doi: 10.1016/j.oraloncology.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reuschenbach M, Kansy K, Garbe K, et al. Lack of evidence of human papillomavirus-induced squamous cell carcinomas of the oral cavity in southern Germany. Oral Oncol. 2013;49(9):937–942. doi: 10.1016/j.oraloncology.2013.03.451. [DOI] [PubMed] [Google Scholar]
- 9.Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015;112:580–593. doi: 10.1038/bjc.2014.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein BY, Chang SC, Hashibe M, et al. Alcohol consumption and cancers of the oral cavity and pharynx from 1988 to 2009: an update. Eur J Cancer Prev. 2010;19:431–465. doi: 10.1097/CEJ.0b013e32833d936d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IARC Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum. 2010;96:1–1428. [PMC free article] [PubMed] [Google Scholar]
- 12.Llewellyn CD, Johnson NW, Warnakulasuriya KA. Risk factors for squamous cell carcinoma of the oral cavity in young people–a comprehensive literature review. Oral Oncol. 2001;37:401–418. doi: 10.1016/S1368-8375(00)00135-4. [DOI] [PubMed] [Google Scholar]
- 13.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 14.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon: IARC. 2012. http://www.globocan.iarc.fr. Accessed 3 Mar 2016.
- 15.Fritsch VA, Gerry DR, Lentsch EJ. Basaloid squamous cell carcinoma of the oral cavity: an analysis of 92 cases. Laryngoscope. 2014;124:1573–1578. doi: 10.1002/lary.24384. [DOI] [PubMed] [Google Scholar]
- 16.Bice TC, Tran V, Merkley MA, et al. Disease-Specific survival with spindle cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2015;153:973–980. doi: 10.1177/0194599815594360. [DOI] [PubMed] [Google Scholar]
- 17.Kass JI, Lee SC, Abberbock S, et al. Adenosquamous carcinoma of the head and neck: Molecular analysis using CRTC-MAML FISH and survival comparison with paired conventional squamous cell carcinoma. Laryngoscope. 2015;125:E371–E376. doi: 10.1002/lary.25519. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Kuyama K, Burkhardt A, Yamamoto H. Clinicopathological evaluation of carcinoma cuniculatum: a variant of oral squamous cell carcinoma. J Oral Pathol Med. 2012;41:303–308. doi: 10.1111/j.1600-0714.2011.01116.x. [DOI] [PubMed] [Google Scholar]
- 19.Mallick S, Breta M, Gupta SD, et al. Angiogenesis, proliferative activity and DNA ploidy in oral verrucous carcinoma: a comparative study including verrucous hyperplasia and squamous cell carcinoma. Pathol Oncol Res. 2015;21:1249–1257. doi: 10.1007/s12253-014-9856-9. [DOI] [PubMed] [Google Scholar]
- 20.Samman M, Wood HM, Conway C, et al. A novel genomic signature reclassifies an oral cancer subtype. Int J Cancer. 2015;137:2364–2373. doi: 10.1002/ijc.29615. [DOI] [PubMed] [Google Scholar]
- 21.Garcia C, Crowson AN. Acantholytic squamous cell carcinoma: is it really a more aggressive tumor? Dermatol Surg. 2011;37:353–356. doi: 10.1111/j.1524-4725.2011.01886.x. [DOI] [PubMed] [Google Scholar]
- 22.Rytkönen AE, Hirvikoski PP, Salo TA. Lymphoepithelial carcinoma: two case reports and a systematic review of oral and sinonasal cases. Head Neck Pathol. 2011;5:327–334. doi: 10.1007/s12105-011-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Bai S, Carroll W, Dayan D, et al. Validation of the risk model: high-risk classification and tumor pattern of invasion predict outcome for patients with low-stage oral cavity squamous cell carcinoma. Head Neck Pathol. 2013;7:211–223. doi: 10.1007/s12105-012-0412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woolgar JA. Histopathological prognosticators in oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2006;42:229–39. [DOI] [PubMed]
- 26.Woolgar JA, Triantafyllou A. Squamous cell carcinoma and precursor lesions: clinical pathology. Periodontol. 2011;57:51–72. doi: 10.1111/j.1600-0757.2011.00389.x. [DOI] [PubMed] [Google Scholar]
- 27.Almangush A, Bello IO, Coletta RD, et al. For early-stage oral tongue cancer, depth of invasion and worst pattern of invasion are the strongest pathological predictors for locoregional recurrence and mortality. Virchows Arch. 2015;467:39–46. doi: 10.1007/s00428-015-1758-z. [DOI] [PubMed] [Google Scholar]
- 28.Ridge JA, Lydiatt WM, Patel SG, et al. In: Head and NeckAJCC cancer staging manual. 8th ed. Amin MB, Edge S, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR, et al., editors. New York: Springer; 2017. pp. 79–94. [Google Scholar]
- 29.Reibel J, Gale N, Hille J, et al. Oral potentially malignant disorders and oral epithelial dysplasia. In: el-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, et al., editors. WHO classification of tumours of the head and neck. 4th ed. Lyon: IARC Press; 2017. [Google Scholar]
- 30.Kuribayashi Y, Tsushima F, Morita K, et al. Long-term outcome of non-surgical treatment in patients with oral leukoplakia. Oral Oncol. 2015;51:1020–1025. doi: 10.1016/j.oraloncology.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Petti S. Pooled estimate of world leukoplakia prevalence: a systematic review. Oral Oncol. 2003;39:770–780. doi: 10.1016/S1368-8375(03)00102-7. [DOI] [PubMed] [Google Scholar]
- 32.Gale N, Blagus R, El-Mofty SK, et al. Evaluation of a new grading system for laryngeal squamous intraepithelial lesions—a proposed unified classification. Histopathology. 2014;65:456–464. doi: 10.1111/his.12427. [DOI] [PubMed] [Google Scholar]
- 33.Woo SB, Cashman EC, Lerman MA. Human papillomavirus-associated oral intraepithelial neoplasia. Mod Pathol. 2013;26:1288–1297. doi: 10.1038/modpathol.2013.70. [DOI] [PubMed] [Google Scholar]
- 34.Vigneswaran N, Carlos R, Lippman S, et al. Multifocal epithelial hyperplasia. In: el-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, et al., editors. WHO classification of tumours of the head and neck. 4th ed. Lyon: IARC Press; 2017. [Google Scholar]
- 35.Carlos R, Sedano HO. Multifocal papilloma virus epithelial hyperplasia. Oral Surg Oral Med Oral Pathol. 1994;77:631–635. doi: 10.1016/0030-4220(94)90325-5. [DOI] [PubMed] [Google Scholar]
- 36.King MD, Reznik DA, O’Daniels CM, et al. Human papillomavirus-associated oral warts among human immunodeficiency virus-seropositive patients in the era of highly active antiretroviral therapy: an emerging infection. Clin Infect Dis. 2002;34:641–648. doi: 10.1086/338637. [DOI] [PubMed] [Google Scholar]
- 37.Said AK, Leao JC, Fedele S, Porter SR. Focal epithelial hyperplasia—an update. J Oral Pathol Med. 2013;42:435–442. doi: 10.1111/jop.12009. [DOI] [PubMed] [Google Scholar]
- 38.Archard HO, Heck JW, Stanley HR. Focal epithelial hyperplasia: an unusual oral mucosal lesion found in Indian children. Oral Surg Oral Med Oral Pathol. 1965;20:201–212. doi: 10.1016/0030-4220(65)90192-1. [DOI] [PubMed] [Google Scholar]
- 39.Henke RP, Guèrin-Reverchon I, Milde-Langosch K, et al. In situ detection of human papillomavirus types 13 and 32 in focal epithelial hyperplasia of the oral mucosa. J Oral Pathol Med. 1989;18:419–421. doi: 10.1111/j.1600-0714.1989.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 40.Bishop J, Gnepp DR, Ro JY. Ectomesenchymal chondromyxoid tumour. In: el-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of tumours of the head and neck. 4th ed. Lyon: IARC Press; 2017. [Google Scholar]
- 41.Aldojain A, Jaradat J, Summersgill K, Bilodeau EA. Ectomesenchymal chondromyxoid tumor: a series of seven cases and review of the literature. Head Neck Pathol. 2015;9:315–322. doi: 10.1007/s12105-014-0578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nigam S, Dhingra KK, Gulati A. Ectomesenchymal chondromyxoid tumor of the hard palate–a case report. J Oral Pathol Med. 2006;35:126–128. doi: 10.1111/j.1600-0714.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 43.Goveas N, Ethunandan M, Cowlishaw D, Flood TR. Ectomesenchymal chondromyxoid tumour of the tongue: unlikely to originate from myoepithelial cells. Oral Oncol. 2006;42:1026–1028. doi: 10.1016/j.oraloncology.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Smith BC, Ellis GL, Meis-Kindblom JM, Williams SB. Ectomesenchymal chondromyxoid tumor of the anterior tongue. Nineteen cases of a new clinicopathologic entity. Am J Surg Pathol. 1995;19:519–530. doi: 10.1097/00000478-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Bullerdiek J, Ro JY. Thompson LDR. rhabdomyoma. In: el-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of tumours of the head and neck. 4th ed. Lyon: IARC Press; 2017. [Google Scholar]
- 46.Hettmer S, Teot LA, van Hummelen P, et al. Mutations in Hedgehog pathway genes in fetal rhabdomyomas. J Pathol. 2013;231:44–52. doi: 10.1002/path.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bullerdiek J, Flucke U. Lymphangioma. In: el-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of tumours of the head and neck. 4th ed. Lyon: IARC Press; 2017. [Google Scholar]
- 48.Kardon DE, Wenig BM, Heffner DK, Thompson LD. Tonsillar lymphangiomatous polyps: a clinicopathologic series of 26 cases. Mod Pathol. 2000;13:1128–1133. doi: 10.1038/modpathol.3880208. [DOI] [PubMed] [Google Scholar]
- 49.Bruder E, Alaggio R, Kozakewich HP, et al. Vascular and perivascular lesions of skin and soft tissues in children and adolescents. Pediatr Dev Pathol. 2012;15:26–61. [DOI] [PubMed]
- 50.Kalof AN, Cooper K. D2-40 immunohistochemistry–so far! Adv Anat Pathol. 2009;16:62–64. doi: 10.1097/PAP.0b013e3181915e94. [DOI] [PubMed] [Google Scholar]
- 51.Mardekian S, Karp JK. Lymphangioma of the palatine tonsil. Arch Pathol Lab Med. 2013;137:1837–1842. doi: 10.5858/arpa.2012-0678-RS. [DOI] [PubMed] [Google Scholar]
- 52.Rekhi B, Sethi S, Kulkarni SS, Jambhekar NA. Kaposiform hemangioendothelioma in tonsil of a child associated with cervical lymphangioma: a rare case report. World J Surg Oncol. 2011;9:57. doi: 10.1186/1477-7819-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindhurst MJ, Sapp JC, Teer JK, et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N Engl J Med. 2011;365:611–619. doi: 10.1056/NEJMoa1104017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bullerdiek J, Flucke U. Haemangioma. In: el-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of tumours of the head and neck. 4th ed. Lyon: IARC Press; 2017. [Google Scholar]
- 55.Açikgöz A, Sakallioglu U, Ozdamar S, Uysal A. Rare benign tumours of oral cavity–capillary haemangioma of palatal mucosa: a case report. Int J Paediatr Dent. 2000;10:161–165. doi: 10.1046/j.1365-263x.2000.00188.x. [DOI] [PubMed] [Google Scholar]
- 56.Bonet-Coloma C, Mínguez-Martínez I, Palma-Carrió C, et al. Clinical characteristics, treatment and outcome of 28 oral haemangiomas in pediatric patients. Med Oral Patol Oral Cir Bucal. 2011;16:e19–e22. doi: 10.4317/medoral.16.e19. [DOI] [PubMed] [Google Scholar]
- 57.Maaita JK. Oral tumors in child- ren: a review. J Clin Pediatr Dent. 2000;24:133–135. [PubMed] [Google Scholar]
- 58.Sato M, Tanaka N, Sato T, Amagasa T. Oral and maxillofacial tumours in children: a review. Br J Oral Maxillofac Surg. 1997;35:92–95. doi: 10.1016/S0266-4356(97)90682-3. [DOI] [PubMed] [Google Scholar]
- 59.Flucke U, Franchi A. Myofibroblastic sarcoma. In: el-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of tumours of the head and neck. 4th ed. Lyon: IARC Press; 2017. [Google Scholar]
- 60.Fisher C. Low-grade sarcomas with CD34-positive fibroblasts and low-grade myofibroblastic sarcomas. Ultrastruct Pathol. 2003;428:291–305. doi: 10.1080/019131290882187. [DOI] [PubMed] [Google Scholar]
- 61.Mentzel T, Dry S, Katenkamp D, Fletcher CD. Low-grade myofibroblastic sarcoma: analysis of 18 cases in the spectrum of myofibroblastic tumors. Am J Surg Pathol. 1998;22:1228–1238. doi: 10.1097/00000478-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Inagaki H, Bell D, Brandwein-Gensler M. Mucoepidermoid Carcinoma. In: el-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of tumours of the head and neck. 4th ed. Lyon: IARC Press; 2017. [Google Scholar]
- 63.Bell D, Brandwein-Gensler M, Chiosea S. Pleomorphic Adenoma. In: el-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of tumours of the head and neck. 4th ed. Lyon: IARC Press;; 2017. [Google Scholar]
- 64.Feldman AL, Boy S, Ferry JA, et al. CD30-positive T-cell lymphoproliferative disorder. In: el-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, et al., editors. WHO classification of tumours of the head and neck. 4th ed. Lyon: IARC Press; 2017. [Google Scholar]
- 65.Rosenberg A, Biesma DH, Sie-Go DM, Slootweg PJ. Primary extranodal CD30-positive T-cell non-Hodgkins lymphoma of the oral mucosa. Report of two cases. Int J Oral Maxillofac Surg. 1996;25:57–59. doi: 10.1016/S0901-5027(96)80013-0. [DOI] [PubMed] [Google Scholar]
- 66.Sciallis AP, Law ME, Inwards DJ, et al. Mucosal CD30-positive T-cell lymphoproliferations of the head and neck show a clinicopathologic spectrum similar to cutaneous CD30-positive T-cell lymphoproliferative disorders. Mod Pathol. 2012;25:983–992. doi: 10.1038/modpathol.2012.38. [DOI] [PubMed] [Google Scholar]
- 67.Wang W, Cai Y, Sheng W, Lu H, Li X. The spectrum of primary mucosal CD30-positive T-cell lymphoproliferative disorders of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:96–104. doi: 10.1016/j.oooo.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 68.Alobeid B, Pan LX, Milligan L, et al. Eosinophil-rich CD30+ lymphoproliferative disorder of the oral mucosa. A form of “traumatic eosinophilic granuloma”. Am J Clin Pathol. 2004;121:43–50. doi: 10.1309/JQFXPND6DBLF6B9U. [DOI] [PubMed] [Google Scholar]
- 69.Hirshberg A, Amariglio N, Akrish S, et al. Traumatic ulcerative granuloma with stromal eosinophilia: a reactive lesion of the oral mucosa. Am J Clin Pathol. 2006;126:522–529. doi: 10.1309/AFHA406GBT0N2Y64. [DOI] [PubMed] [Google Scholar]
- 70.Boy S, Ferry JA. Plasmablastic lymphoma. In: el-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of tumours of the head and neck. 4th ed. Lyon: IARC Press; 2017. [Google Scholar]
- 71.Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125:2323–2330. doi: 10.1182/blood-2014-10-567479. [DOI] [PubMed] [Google Scholar]
- 72.Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: lessons learned from 112 published cases. Am J Hematol. 2008;83:804–809. doi: 10.1002/ajh.21250. [DOI] [PubMed] [Google Scholar]
- 73.Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89:1413–1420. [PubMed] [Google Scholar]
- 74.Boy S, van Heerden M, Pool R, et al. Plasmablastic lymphoma versus diffuse large B-cell lymphoma with plasmablastic differentiation: proposal for a novel diagnostic scoring system. J Hematop. 2015;8:3–11. doi: 10.1007/s12308-014-0227-y. [DOI] [Google Scholar]
- 75.Loghavi S, Alayed K, Aladily TN, et al. Stage, age, and EBV status impact outcomes of plasmablastic lymphoma patients: a clinicopathologic analysis of 61 patients. J Hematol Oncol. 2015;8:65. doi: 10.1186/s13045-015-0163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]