Abstract
The endosymbiont Wolbachia wAlbB induces refractoriness to Plasmodium falciparum in Anopheles stephensi, the primary mosquito vector of human malaria in the Middle East and South Asia. However, it remains unknown whether such refractoriness can be extended to other malaria species. In particular, it was reported that under very specific conditions, wAlbB can enhance Plasmodium infection in some hosts. Here, we measured the impact of wAlbB on the rodent malaria parasite Plasmodium berghei in A. stephensi by comparing the load of oocysts and sporozoites in midguts and salivary glands, respectively, between wAlbB-infected and -uninfected mosquitoes. To investigate whether wAlbB modulated mosquito immune defense against parasites, we compared the expression of the immune genes, which were previously reported to involve in antimalarial response, in both midguts and the remaining carcass tissues of mosquitoes. The stable association of wAlbB with A. stephensi resulted in reduction of parasites by more than half at the oocyst stage, and up to 91.8% at the sporzoite stage. The anti-plasmodium immune genes, including TEP1, LRIM1, Toll pathway gene Rel1 and the effector Defensin 1, were induced by wAlbB in different mosquito body tissues. These findings suggest that immune priming is a potential cause of wAlbB-mediated antimalarial response in A. stephensi. More importantly, no evidence was found for any enhancement of Plasmodium infection in A. stephensi stably infected with wAlbB. We discuss these findings with possible implementations of Wolbachia for malaria control in disease endemic areas.
Keywords: Wolbachia, Plasmodium, malaria, population replacement, vector-borne disease, innate immunity
Introduction
Transmitted by Anopheles mosquitoes, malaria is one of the world’s deadliest diseases caused by protozoan parasites of the genus Plasmodium. Although significant efforts and resources have been devoted to malaria control, especially over the past decade, there are still 3.2 billion people currently living in areas of high malaria risk, with about 214 million cases of clinical malaria and 438,000 malaria-related deaths in WHO (2015). Given the lack of a highly effective vaccine and the development of drug resistance in parasites and insecticide resistance in mosquito vectors, there is an urgent need for novel control strategies to target the vectors that are difficult to control by the existing methods (The malERA Consultative Group on Vector Control, 2011). One of the potential approaches is to suppress or modify mosquito population using either genetically modified techniques or the endosymbiotic bacterium Wolbachia (Ito et al., 2002; The malERA Consultative Group on Vector Control, 2011), which was recently tested for proof-of-concept through field release to control mosquito-borne diseases (Hoffmann et al., 2011; Carvalho et al., 2015; Mains et al., 2016).
Wolbachia spp. are intracellular alpha-proteobacteria closely related to Rickettsia. Maternally inherited infections with Wolbachia occur in an estimated 40–66% of insect species, and approximately 28% of surveyed mosquito species (Kittayapong et al., 2000; Ricci et al., 2002; Hilgenboecker et al., 2008; Zug and Hammerstein, 2012). No native Wolbachia had been found in the 38 surveyed Anopheles species until a recent report identified a novel Wolbachia strain, related to but distinct from strains infecting other arthropods, in Anopheles gambiae (Kittayapong et al., 2000; Baldini et al., 2014; Bourtzis et al., 2014). Through the cytoplasmic incompatibility (CI) mechanism, some Wolbachia strains induce early embryonic death when a Wolbachia-infected male mates with an uninfected female (Sinkins, 2004; Werren et al., 2008). Thus, Wolbachia-infected male mosquitoes are proposed to be released to induce incompatible (or sterile) mating and reduce vector density below a threshold required for disease transmission (Laven, 1967; Brelsfoard et al., 2008). Because Wolbachia-infected females produce viable offspring whether they mate with uninfected or infected males, CI provides the infected females an advantage in reproduction over the uninfected female, allowing Wolbachia to spread into an uninfected population. With the ability of Wolbachia to directly reduce malaria parasites in the Anopheles mosquito (Bian et al., 2013), invasion of Wolbachia into vector populations theoretically will reduce mosquito vector competence for malaria parasites, resulting in intervention of disease transmission. The feasibility of the above two strategies in disease control is currently being tested to combat Zika and dengue through field trials in many countries, including Australia, USA, China and Brazil, using Aedes mosquitoes carrying different type of Wolbachia (Hoffmann et al., 2011; Bourtzis et al., 2014; Xi and Joshi, 2015; Dutra et al., 2016; Mains et al., 2016).
We previously generated the first maternally inheritable Wolbachia infection in an Anopheles malaria vector by transferring Wolbachia wAlbB from Aedes albopictus into A. stephensi through embryonic microinjection, resulting in establishment of the transinfected LB1 strain in 2011 (Bian et al., 2013). Since then, wAlbB has been stably maintained in the A. stephensi LB1 strain in the laboratory for more than 5 years, displaying both perfect maternal transmission and the ability to induce a nearly complete CI. Furthermore, wAlbB induces refractoriness to the human malaria parasite Plasmodium falciparum in the LB1 mosquito (Bian et al., 2013). This is consistent with the observation that a stable wMelPop infection reduced the infection of avian malaria parasite, P. gallinaceum, in Aedes aegypti (Moreira et al., 2009). Without a stable infection in Anopheles gambiae, a transient Wolbachia infection was used as a model to study Wolbachia–Plasmodium interactions in this mosquito species (Kambris et al., 2010; Hughes et al., 2011). This transient infection differed from a stable infection in that it was generated through adult intrathoracic injection and the infection was lost in the subsequent generations. Consistently, a transient wMelPop infection significantly reduced both P. falciparum and Plasmodium berghei in A. gambiae (Kambris et al., 2010; Hughes et al., 2011). Suppression of P. falciparum was also observed in A. gambiae with a transient wAlbB infection (Hughes et al., 2011). In addition, the observed reduction was associated with induction of anti-Plasmodium immune genes, including TEP1 (Kambris et al., 2010).
However, the impact of Wolbachia on Plasmodium in mosquito may differ, depending on the strain of Wolbachia, the species of parasite and the environmental temperature. In contrast to the pathogen interference described above, a native wPip infection was claimed to enhance the avian malaria parasite P. relictum in Culex pipiens mosquito (Zele et al., 2014). A transient wAlbB infection was also reported to increase the rodent parasite P. berghei oocyst load in the midgut of A. gamibiae, and the P. yoelii oocyst load at 24°C in A. stephensi (Hughes et al., 2012; Murdock et al., 2014). It is known that P. falciparum and P. berghei interact differently with mosquito hosts and the other four human malaria species, P. malariae, P. ovale, P. knowlesi, and P. vivax are phylogenetically more closely related to P. berghei than they are to P. falciparum (Hughes et al., 2014). This raises concerns whether Wolbachia-based population replacement may enhance transmission of those parasites (Hughes et al., 2014). Thus, it is essential to validate the impact of a stable wAlbB infection on mosquito vector competence for additional Plasmodium species in Anopheles mosquitoes.
In this study, we compared the vector competence for P. berghei between of Wolbachia-infected and -uninfected A. stephensi by examining oocyst and sporozoite loads in mosquito midguts and salivary glands, respectively. To confirm whether Wolbachia was able to regulate expression of the host immune genes, we compared the expression of several immune genes, which are known to be involved in antimalarial response, in both midguts and the remaining carcass tissues between the LB1 strain and Wolbachia-uninfected A. stephensi. We observed that wAlbB significantly reduced P. berghei at both oocyst and sporozoite stage in LB1 mosquitoes. This reduction was associated with induction of a number of anti-Plasmodium immune genes, including TEP1, Rel1 and Defensin 1, in either midgut or the carcass. Consistent with our previous studies using blood-fed mosquitoes, there was no impact of wAlbB on mosquito life span after taking P. berghei-infected blood meal.
Materials and Methods
Mosquito Rearing
The wild-type A. stephensi LIS strain (Wolbachia-free), A. stephensi LB1 strain (Wolbachia-infected) and the aposymbiotic line LBT strain (Wolbachia-free; generated by tetracycline treatment of the LB1 strain to remove wAlbB) were described previously (Bian et al., 2013) and maintained on sugar solution at 27°C and 85% humidity, with a 12-h/12-h light/dark cycle, according to standard rearing procedures (Joshi et al., 2014). Before the infection assay, females of both LBT and LB1 were outcrossed with LIS males for >4 generations. For colony maintenance and Plasmodium infection assay, adult females were fed on the blood of anesthetized mice (BALB/c) according to a protocol (03/14-036-00) approved by Michigan State University Institutional Animal Care and Use Committees.
P. berghei Infection Assay
Plasmodium berghei (ANKA 2.34 strain) parasites from frozen stocks were administered intraperitoneally to donor mice. When the parasitemias of donor mice reached 10–20%, infected blood was collected by heart puncture, washed and diluted 2.5 times with PBS. Then, 200 μl of this diluted blood was transferred to naive mice via intraperitoneal injection. All mice were 4- to 6-week-old BALB/c females. Parasitemia and exflagellation rates were assessed by light microscopy inspection of Giemsa-stained thin smears obtained by tail snips before mice were used for mosquito feeding. At 7–8 days post-emergence, 60–70 females were transferred into a 0.5-lt mesh-covered cardboard cup and were deprived of sucrose solution for 1–2 h, then allowed to feed on anesthetized mice infected with P. berghei that exhibited 1–3 exflagellation events per field, as previously described (Billker et al., 1997). All P. berghei-infected mosquitoes and corresponding control mosquitoes were kept at 21°C and 80% humidity. At 10 days post-blood feeding, mosquito midguts were dissected and stained in 0.05% mercurochrome for at least 10–30 min, and the loads of P. berghei oocysts were quantified under light microscopy at 10X magnification. At 21 days post-infection, mosquito salivary glands were dissected to quantify the infection intensity at the stage of sporzoite. The salivary glands were transferred into a microfuge tube containing 120 μl of PBS and homogenized gently for 30 s with a hand held pestle. After centrifugation at 8,000 rpm for 10 min, 90 μl of the supernatant was discarded. The sporozoites were resuspended in a final volume of 30 μl of PBS, and 10 μl of this suspension was used to count the sporozoites as described previously (Bian et al., 2013).
RNA Extraction, cDNA Synthesis, and qRT PCR
Seven- to nine-day-old non-blood fed females were dissected in PBS and midguts and the remaining carcass tissues were collected in TRIzol® reagent (Life technologies) and stored at -80°C until RNA extraction. Each tissue had eight replicates, with samples of five females from a cohort of mosquitoes pooled together to make one replicate. The cDNA transcript was produced using Reverse Transcription Kit (Invitrogen). Real time PCR was performed using SYBR green kit (Qiagen sciences) and ABI Prism 7900HT Sequence Detection System (Applied Biosystems). The data were processed and analyzed with Applied Biosystems SDS2.3 software and the obtained CT values were exported into the Excel program to calculate the relative fold changes. The ribosomal protein S6 (RPS6) was used as an internal control for normalization of cDNA templates (Bian et al., 2013). Relative fold changes in gene expression values between Wolbachia-infected (LB1) and uninfected (LBT) tissues were obtained by using 2-ΔΔCT method (Livak and Schmittgen, 2001). All primers used for real-time PCR are listed in Supplementary Table S1.
Life Span Assays
After feeding on P. berghei-infected mice, LB1, LIS, and LBT mosquitoes were sorted and transferred into 0.5-lt mesh-covered cardboard cups, with at least 20 females for each of 2–4 replicates. Cups were changed every 2 days to avoid the impact of fungi, which may grow on the dead mosquitoes, on the data. Mortality was recorded on daily basis until day 9 post-infection, when over 70% of death had occurred.
Statistical Analyses
Prior to analyses, the normality of the data sets was checked using D’Agostino and Pearson omnibus normality test. The oocyst and sprozoite data were not normally distributed. Thus, the Mann–Whittney U-test based on median values were used for analysis. The prevalence rates in mosquitoes, in terms of midgut and salivary gland infection rates, were analyzed using Fisher’s exact test and the expression values of the genes were analyzed using Student’s t-test. Log-rank test was used to compare the survivorship after mosquitoes took P. berghei-infected blood. All the analysis was done using GraphPad Prism version 5.00.
Results
wAlbB Reduces P. berghei Oocyst Loads in Mosquito Midguts
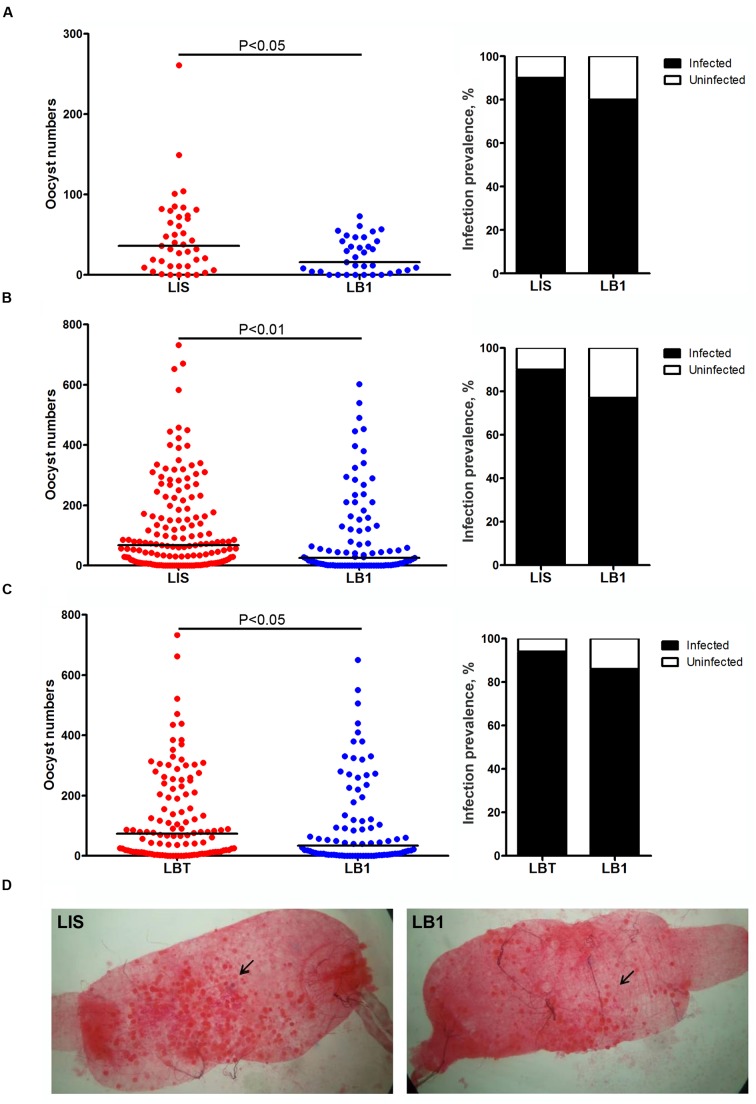
We previously showed that a stable wAlbB infection reduced P. falciparum oocyst loads in midguts of A. stephensi (Bian et al., 2013). However, a transient wAlbB infection was reported to increase P. berghei oocyst load in midguts of A. gambiae (Hughes et al., 2012). In order to characterize the effect of wAlbB on vector competence of A. stephensi for P. berghei, we compared the oocyst levels between the wild-type LIS strain (Wolbachia-free) and LIS-derived LB1 strain (wAlbB -infected) or between LB1 strain and its aposymbiotic LBT strain at day 10 after they took an infectious blood meal from mice. In all three experiments, LB1 mosquitoes displayed a significant reduction in the oocyst loads, with a trend of reduction in the infection prevalence, compared to the LIS or LBT mosquitoes (Figure 1). Specifically, a significant reduction in oocyst load was observed in midguts of LB1 mosquitoes compared to those of LIS mosquitoes in both experiments 1 and 2 (Mann–Whitney U-test, P < 0.05 and < 0.01 in experiments 1 and 2, respectively) (Figures 1A,B and Supplementary Table S2). The median number of oocysts was reduced by 55.6 and 63% in LB1 midguts compared to LIS midguts in experiments 1 and 2, respectively. In experiment 3, a similar experiment was performed to compare oocyst development in LB1 mosquitoes and LBT strain. Consistently, there was a significant reduction in oocyst load in midguts of LB1 mosquitoes compared to those of LBT mosquitoes (Mann–Whitney U-test, P < 0.05) (Figure 1C and Supplementary Table S2). These results indicate that wAlbB interferes with P. berghei oocyst development in midguts of LB1 mosquitoes.
FIGURE 1.
Wolbachia wAlbB-mediated reduction in Plasmodium berghei oocyst load in LB1 midguts. P. berghei oocyst loads in midguts of Anophelus stephensi LB1 strain and its infection prevalence (the percentage of mosquitoes that were infected at any level) are compared to those of LIS (A,B) and LBT strains (C). Points represent the number of parasites from an individual mosquito and horizontal lines indicate the median number of parasites per tissue. P-value is indicated based on Mann–Whitney test. (D) One representative midgut from LIS and LB1 strain is shown, with an arrow indicating the oocyst.
wAlbB Reduces P. berghei Sporozoite Loads in Mosquito Salivary Glands
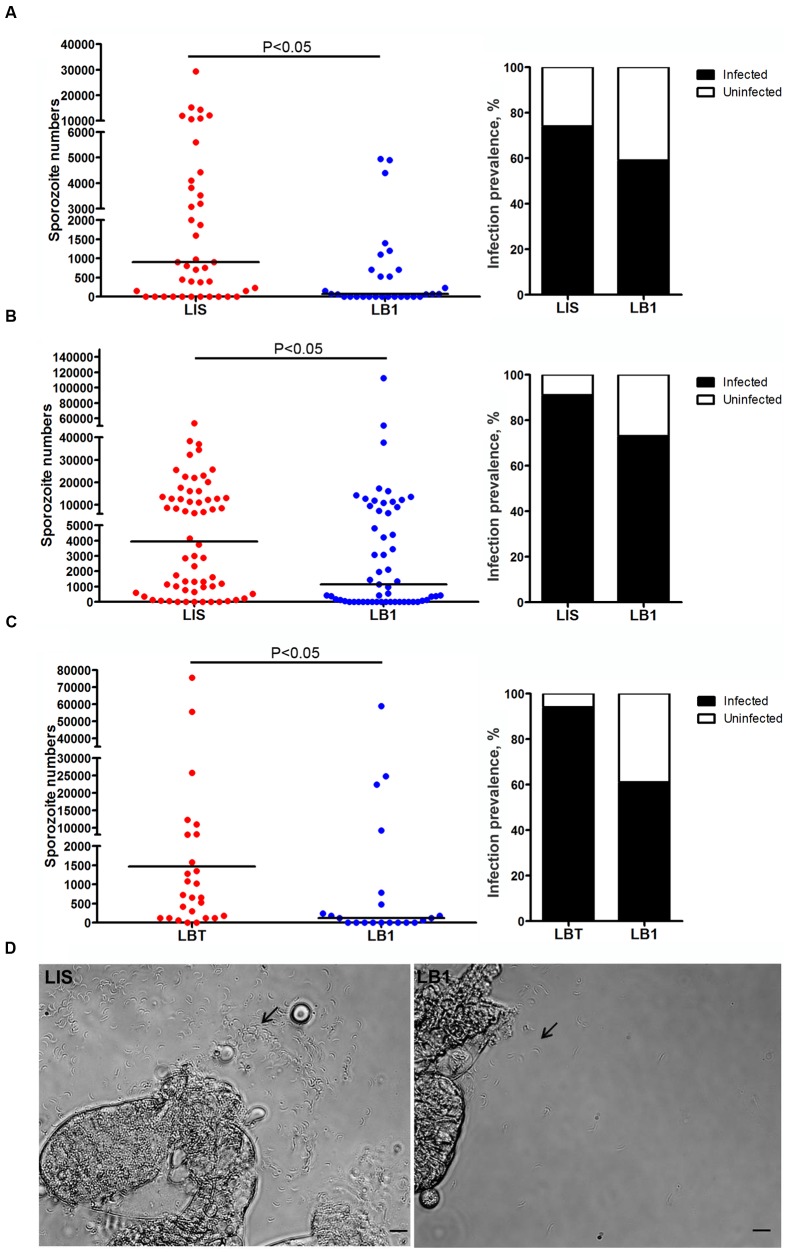
To further test whether mosquito’s potential to transmit P. berghei was reduced by wAlbB, we compared the number of sporozoites in the salivary glands of LB1 and LIS or LBT mosquitoes at 21 days post-infection. In all three experiments, there were significantly lower numbers of sporozoites, with a trend of reduction in the infection prevalence, in the salivary glands of LB1 mosquitoes than those of LIS or LBT mosquitoes (Mann–Whitney U-test, P < 0.05) (Figure 2 and Supplementary Table S2). In experiments 1 and 2, the median number of sporozoites was reduced by 91.7 and 60.2%, respectively, in LB1 mosquitoes compared to LIS mosquitoes (Figures 2A,B and Supplementary Table S2). In experiment 3, the median number of sporozoites was reduced by 91.8% in LB1 mosquitoes compared to LBT mosquitoes (Figure 2C and Supplementary Table S2). These results indicate wAlbB interferes with P. berghei sporozoite development in mosquito salivary glands.
FIGURE 2.
Wolbachia wAlbB-mediated reduction in P. berghei sporozoite loads in LB1 salivary glands. P. berghei sporozoite loads in salivary glands of A. stephensi LB1 strain and its infection prevalence are compared to those of LIS (A,B) and LBT strains (C). Points represent the number of parasites from an individual mosquito and horizontal lines indicate the median number of parasites per tissue. P-value is indicated based on Mann–Whitney test. (D) One representative salivary glands from LIS and LB1 strain is shown, with an arrow indicating the sporozoite.
wAlbB Induces Expression of Anti-Plasmodium Immune Factors in Mosquito
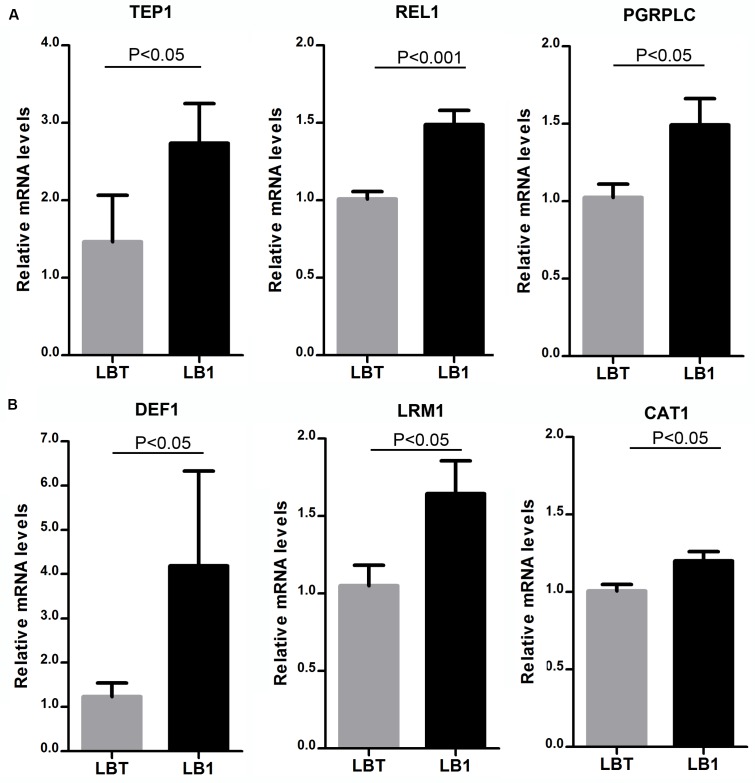
To explore the potential mechanism underlying wAlbB-mediated Plasmodium interference, we selected six immune genes that were previously reported to play roles in anti-Plasmodium response (Blandin et al., 2004; Frolet et al., 2006; Povelones et al., 2009) and compared their expression in both midguts and the remaining carcass tissues of non-blood-fed mosquito LB1 and LBT strains using qRT-PCR. As a result, we found that expression of TEP1, Rel1, and PGRP-LC genes were significantly induced by wAlbB in midguts (Figure 3A) and expression of Def1, LRIM1, and CAT genes were significantly induced in the carcass tissues (Figure 3B). These results indicate that wAlbB may induce parasite interference through priming the mosquito’s anti-Plasmodium immune system.
FIGURE 3.
Up-regulation of anti-Plasmodium immune genes by wAlbB in LB1 mosquito. qRT-PCR was used to assess the expression of each of the selected anti-Plasmodium immune genes in either midguts (A) or the remaining carcass tissues (B), with the host RPS6 as an internal reference control to normalize the data. 2-ΔΔCT method was used to calculate fold change for each gene and significance was determined based on comparison of ΔCT of each gene in LB1 and LBT. P-value is indicated based on Mann–Whitney test.
wAlbB Does Not Change the Life Span of P. berghei-Infected Mosquitoes
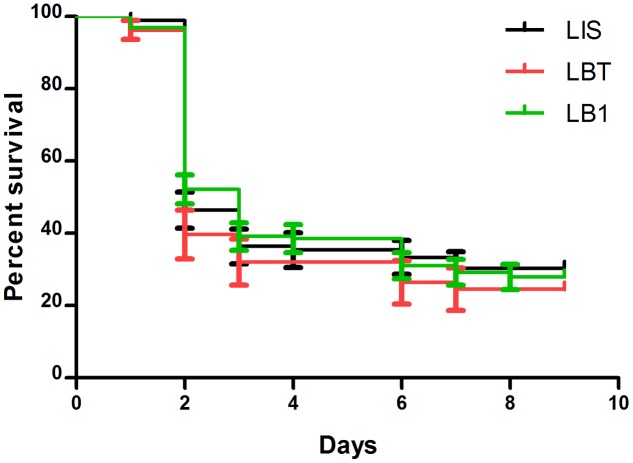
We previously observed that wAlbB increased longevity of LB1 mosquitoes when they were maintained using 10% sugar solution (Joshi et al., 2014), resulting in a concern because infectious mosquitoes with increased longevity may facilitate disease transmission (Killeen et al., 2013). Thus, we compared the life spans of LB1, LIS, and LBT mosquitoes after they took the P. berghei-infected blood meal. All three strains showed a high mortality within 2 days after taking the blood meal and thereafter maintained a low mortality (Figure 4). There were no statistical differences in survivorship among LB1, LIS, and LBT mosquitoes (log-rank test, P > 0.05). Taken together with the observation of no change in LB1 female longevity after taking uninfected blood (Joshi et al., 2014), our results confirm that wAlbB-associated increase in mosquito survivorship will occur only in males or females that have not taken blood.
FIGURE 4.
The impact of wAlbB on the life span of LB1 after taking the P. berghei-infected blood meal. After LB1, LIS, and LBT mosquitoes were infected by P. berghei, dead mosquitoes were removed and recorded every day. The curves represent the mean percentage of mosquitoes surviving from different biological replicates each day. There was no significant difference in the life span of the infected females between LB1, LIS, and LBT mosquitoes.
Discussion
We have previously shown that wAlbB interferes with P. falciparum in the A. stephensi LB1 strain (Bian et al., 2013). In order to better characterize the spectrum of wAlbB-mediated Plasmodium interference, we examined the impact of wAlbB on P. berghei development in LB1 mosquitoes in this study. We found that wAlbB reduced both oocyst and sporozoite loads in midguts and salivary glands, respectively, of LB1 mosquitoes. Furthermore, this reduction was associated with up-regulation of a number of anti-Plasmodium immune genes, including TEP1, Rel1 and Def1, in LB1 mosquitoes. We also showed that wAlbB infection did not increase the life span of mosquitoes after taking P. berghei -infected blood. These results support the potential to develop Wolbachia-based strategy for malaria control.
The strength of wAlbB-mediated reduction in P. berghei infection is similar to what was previously observed on P. falciparum infection (Bian et al., 2013). The oocyst load of both P. berghei and P. falciparum was reduced by more than half in LB1 midguts compared to LIS midguts. For both parasites, there was stronger reduction in the sporozoite stage than in the oocyst stage. The reduction in P. berghei sporozoite levels ranged between 71.1 and 91.8% as shown in the three experiments while P. falciparum sporozoite levels were reduced by 70.9% in the previous studies. Thus, these results indicate that wAlbB reduces P. berghei infection as effectively as P. falciparum infection in LB1 mosquitoes.
The ability of wAlbB to reduce both P. berghei and P. falciparum in A. stephensi indicates that wAlbB may target common host factors that are essential for Plasmodium development in mosquitoes. Two main hypotheses have been proposed to understand the mechanism of Wolbachia-mediated pathogen interference in insect hosts (Bourtzis et al., 2014; Xi and Joshi, 2015). First, Wolbachia primes host immunity such that it can effectively react to inhibit the subsequent pathogen invasion. This is supported by the fact that a number of immune genes, including Toll pathway genes and redox genes, were induced by Wolbachia in the transinfected lines (Pan et al., 2012). Second, Wolbachia outcompetes pathogens in utilizing host metabolic pathway/components for its intracellular growth. For example, Wolbachia replication is cholesterol-dependent, requiring cholesterol-rich host membranes to form the vacuole surrounding each bacterium (Caragata et al., 2013). This could lead to a competition for cholesterol between Wolbachia and pathogens (Atella et al., 2009).
To test the first hypothesis, we compared the expression of six immune genes that were reported to be involved in blocking of human and mouse malaria in A. gambiae (Blandin et al., 2004; Frolet et al., 2006; Povelones et al., 2009). We found that TEP1, PGRP-LC, and REL1 were induced in the midgut while CAT1, Def1, and LRIM1 were up-regulated in the carcass. Among them, TEP1 and Def1 were induced 2.7- and 4.2-fold, respectively, by wAlbB in LB1 mosquitoes. These results are consistent with the previous studies using A. gambiae with a transient somatic wMelPop infection and a stably infected cell line, in which wMelPop strongly induced the expression of TEP1, LRIM1, and Def1 (Kambris et al., 2010). Further studies show that a recombinant Wolbachia surface protein (WSP) is sufficient to induce the expression of those malaria-related immune genes in both Anopheles and Aedes cell lines (Pinto et al., 2012).
As a well-characterized complement-like molecule in the anti-parasitic defense of mosquitoes, TEP1 plays a central role in defense against gram negative bacteria, P. falciparum and P. berghei in Anopheles mosquitoes. With the capacity to reduce the oocyst burden by either direct killing or melanization process, TEP1 was described as major determinant of vectorial capacity for Plasmodium in A. gambiae (Blandin et al., 2004). Knocking down TEP1 gene expression has resulted in several fold increase in the midgut oocyst load (Dong et al., 2006; Frolet et al., 2006). In A. gambiae, Leucine-rich Repeat (LRR) proteins, LRIM1, and APL1C, form complexes to facilitate recognition of the parasite by TEP1, resulting in parasite killing either by a lytic mechanism or by arresting their development through melanization (Fraiture et al., 2009; Povelones et al., 2009). Consistently, LRIM1 was observed to be up-regulated by wAlbB in the carcass of LB1 mosquitoes. However, the orthologes of the A. gambiae sub family APL genes (APL1-A, APL1-B, and APL1-C) are absent in the genome of A. stephensi (Jiang et al., 2014). It is unknown whether another molecule plays a role similar to APL1C to form a comparable complex in A. stephensi. Evidence has shown that boosting NF-Kappa B factors (REL1 and REL2) induces production of TEP1 in A. gambiae (Frolet et al., 2006). We observed that REL1 and IMD pathway gene PGRP-LC were also up-regulated by wAlbB in LB1 mosquitoes. Activation of Toll and IMD pathways by wAlbB was further supported by a strong up-regulation of expression of the antimicrobial peptide DEF1. DEF1 has a profound effect on development of oocysts and sporozoites. Previous studies have shown that either injection or over-expression of defensin in A. aegypti reduced both Plasmodium oocyst in midgut and sporozoite in salivary gland (Shahabuddin et al., 1998; Kokoza et al., 2010).
We previously found that the levels of ROS were significantly higher in midguts and fat bodies of LB1 mosquitoes than in those of LIS mosquitoes and nearly twofold higher in the whole body of LB1 than in that of LIS mosquitoes (Bian et al., 2013). Interestingly, midgut epithelial nitration has been shown to work as an opsonization system that promotes activation of the mosquito complement cascade (Kumar et al., 2010). It would be interested to know whether epithelial nitration is increased in Plasmodium-infected midguts of LB1 mosquitoes, resulting in enhancement of TEP1-mediated lysis for anti-Plamsmodial immunity. Increased production of ROS can lead to melanization of parasites in an A. gabmbiae refractory strain (L3 strain) (Kumar et al., 2003). However, we did not observe the deposition of the melanin pigment on the surface of the oocysts in midguts of the LB1 strain.
While we saw that wAlbB reduced P. berghei at both oocyst and sporozoite stages in LB1 mosquito, in contrast it was reported that A. gambiae with a transient wAlbB infection enhanced P. berghei oocyst load (Hughes et al., 2012). The same group also reported that the number of P. yoelii oocyst increased in A. stephensi with a transient wAlbB infection at 24°C although wAlbB reduced the parasite at warmer temperatures (Murdock et al., 2014). We think that they are likely to be artifacts associated with their used transient infection system, in which Wolbachia infection cannot pass to mosquito offspring because a barrier appears to block the ability of Wolbachia to infect the germ line. Mosquito lines with stable maternal inheritable infections are generated through embryonic microinjection, during which Wolbachia is introduced into germ plasm before the germ cells are formed in the early embryo. Because only a small percentage of the surviving individuals will have acquired a germ-line Wolbachia infection through this process, a further intensive screen is carried out to identify a stably infected line, which can maternally transmit Wolbachia to the next generations at 100% efficiency. Thus, a stable infection system has passed through a restricted selection process that results in both Wolbachia and its host being able to adapt to each other and to maintain their co-existence. It is possible that only a subset of the variants in a population of Wolbachia have been selected to form the symbiosis in the stable infection system. Some Wolbachia may not be able to form symbiosis due to inability to adapt to the new host, which usually occurs when Wolbachia is transferred between phylogenetically distant hosts. The requirement for embryonic injection to generate a stable infection in mosquito may indicate that early contact between Wolbachia and the host (i.e., earlier than the development of the host immune system) may be important in shaping the host’s immune system so that Wolbachia can be persistently maintained in a new host. In a transient infection system, the process of selection, adaptation, symbiosis formation, and shaping of the host immune system is avoided, thus, it can represent a very different type of system than that of a stably infected system.
When evaluating the impact of Wolbachia on mosquito vector competence, we think that two gold standards should be used. First, only mosquito with a stable Wolbachia infection should be used to provide conclusive evidence on whether pathogen interference will occur. This is because only stably infected mosquito can be developed for implementation in disease control, and as described above, the transient infection system does not accurately mimic the stable infection system. Thus conflicting results can be misleading and damage the public perception about the ongoing field trials and future implementation of this control method. Second, the impact of Wolbachia on mosquito vector competence should focus on the pathogen transmission stage. This means direct measurement of the number and infectiousness of pathogens that will be transmitted from mosquito to vertebrate host, such as those in mosquito saliva or salivary gland. When Plasmodia enter and develop in the midgut, disseminate through hemolymph, and infect the salivary gland, they will be attacked by Wolbachia-mediated interference at each stage because Wolbachia has preoccupied those tissues and induced hostile environment either in tissue-specific (e.g., in midgut and salivary gland) or systemic (e.g., in fat body) ways. An accumulated effect will be observed best at the final transmission stage. It is possible that no reduction or even slight enhancement could be observed in the midgut but strong inhibition still occurs in saliva or salivary gland.
Anopheles stephensi is an interesting model species for these studies as its delay in producing a peritrophic matrix (PM, chitin/protein matrix that forms around the blood meal in the gut), which is more than twice as long as that of A. gambiae (Freyvogel and Staeubli, 1965). This PM barrier delay results in many more motile ookinetes escaping the gut to form oocysts. Presence of these large number of oocysts (>100s) in a mosquito is laboratory effect because in the field (as seen with other Anopheles species and human Plasmodium sp.) only one or a couple of oocysts are normally found on the midgut in a potentially infectious field collected Anopheles (Sinden et al., 2004). Thus a reduction in oocysts by 50% can mean a lot in the field – however as an oocyst can produce over 1,000 sporozoites, it is likely not the number of oocysts that make a mosquito infective. It would be interested to determine the level of inhibition that is sufficient for Wolbachia to interfere with disease transmission in the field.
The two stable Wolbachia-infected mosquitoes examined to date show resistances to malaria parasites, including P. falciparum, P. berghei, and P. gallinaceum (Moreira et al., 2009; Bian et al., 2013). It is argued that this resistance occurs only in recent Wolbachia-host associations and will disappear during the long-term evolution of novel Wolbachia-host associations in mosquitoes. A recent study has discovered a marginally higher number of the avian malaria parasite P. relictum in Culex pipiens mosquito with native wPip infection as compared to its aposymbiotic strain derived from antibiotic treatment, resulting in a speculation that those transinfected mosquitoes may evolve to be a better malaria vector (Zele et al., 2014). We think that it is too early to make this prediction because many other reasons, such as host body size and genetic background, cannot be excluded to cause the subtle increase observed in that study. In addition, antibiotic treatment to remove Wolbachia has a systemic effect on the whole microbiome of an organism and may result in a multitude of effects especially on the immune system and infection responses. Currently, it is difficult to design laboratory experiments to mimic this long-term evolution process. What we know, however, is that Wolbachia-mediated pathogen interference has been maintained for >12 years in A. aegypt and >5 years in A. stephensi in laboratory conditions. Here, we may be able to learn from the Mycobacterium bovis bacille Calmette–Guérin (BCG) vaccine against tuberculosis (TB). Although BCG is protective for only 10–20 years, it has maintained its position as the world’s most widely used vaccine (Andersen and Doherty, 2005). The ability to both generate a better mosquito strain with improved pathogen blocking and repeatedly spread novel Wolbachia into population will allow us to develop either a better strain to replace the old one or a method to boost its effectiveness should the pathogen interference decline over time.
Conclusion
We have shown that a maternal inheritable wAlbB infection can reduce P. berghei in A. stephensi. This pathogen interference is associated with up-regulation of TEP1, LRIM1, REL1 and the other anti-Plasmodium immune genes. wAlbB has no impact on the longevity of females after taking infected blood meal although it increases male mosquito survivorship (Joshi et al., 2014). Future studies will investigate the contribution of those up-regulated immune genes to the overall effect of Wolbachia-mediated Plasmodium interference. Understanding of the mechanism of Wolbachia-host interactions will facilitate the development of transinfected mosquito strains with strong pathogen blocking, low fitness cost, stable maternal transmission and complete CI expression. Because there are overlaps in distribution of the parasite P. vivax and A. stephensi, future studies should also characterize the impact of wAlbB on vector competence for P. vivax. Since the endosymbiotic bacterium Wolbachia was introduced into the primary dengue vector, a global effort, with field trials ongoing in eight countries, has been initiated to develop Wolbachia for dengue control. Like the primary dengue vector A. aegypti, A. stephensi is an urban vector, which allows the transfer our experience from the dengue control field trials to malaria control field trials with this mosquito species more likely to succeed. The ability of wAlbB to stably infect an Anopheles malaria vector, induce a complete CI and confer mosquito resistance to malaria parasite has opened an exciting opportunity to develop Wolbachia-based strategy for malaria control.
Author Contributions
Conceived and designed the experiments: DJ and ZX. Performed the experiments: DJ, XP, MM, DB, XL, and PL. Analyzed the data: DJ and ZX. Contributed reagents/materials/analysis tools: DJ. Wrote the paper: DJ, ST, and ZX.
Conflict of Interest Statement
ZX is affiliated with Guangzhou Wolbaki Biotech, Co., Ltd. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Professor George Dimopoulos for providing us P. berghei (ANKA 2.34 strain) and Dr. Yuemei Dong for providing technical support to the experiment.
Footnotes
Funding. This work was supported by NIH grants R01AI080597, Guangdong Innovative Research Team Program (No. 2011S009), Michigan State University Strategic Partnership Grant (15-SPG-Full-3109), and a grant from the Foundation for the NIH through the Grand Challenges in Global Health Initiative of the Bill and Melinda Gates Foundation.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2017.00366/full#supplementary-material
References
- Andersen P., Doherty T. M. (2005). The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 3 656–662. 10.1038/nrmicro1211 [DOI] [PubMed] [Google Scholar]
- Atella G. C., Bittencourt-Cunha P. R., Nunes R. D., Shahabuddin M., Silva-Neto M. A. (2009). The major insect lipoprotein is a lipid source to mosquito stages of malaria parasite. Acta Trop. 109 159–162. 10.1016/j.actatropica.2008.10.004 [DOI] [PubMed] [Google Scholar]
- Baldini F., Segata N., Pompon J., Marcenac P., Robert Shaw W., Dabire R. K., et al. (2014). Evidence of natural Wolbachia infections in field populations of Anopheles gambiae. Nat. Commun. 5:3985 10.1038/ncomms4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian G., Joshi D., Dong Y., Lu P., Zhou G., Pan X., et al. (2013). Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science 340 748–751. 10.1126/science.1236192 [DOI] [PubMed] [Google Scholar]
- Billker O., Shaw M. K., Margos G., Sinden R. E. (1997). The roles of temperature, pH and mosquito factors as triggers of male and female gametogenesis of Plasmodium berghei in vitro. Parasitology 115(Pt 1), 1–7. 10.1017/S0031182097008895 [DOI] [PubMed] [Google Scholar]
- Blandin S., Shiao S. H., Moita L. F., Janse C. J., Waters A. P., Kafatos F. C., et al. (2004). Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116 661–670. 10.1016/S0092-8674(04)00173-4 [DOI] [PubMed] [Google Scholar]
- Bourtzis K., Dobson S. L., Xi Z., Rasgon J. L., Calvitti M., Moreira L. A., et al. (2014). Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop. 132(Suppl.), S150–S163. 10.1016/j.actatropica.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Brelsfoard C. L., Sechan Y., Dobson S. L. (2008). Interspecific hybridization yields strategy for South Pacific filariasis vector elimination. PLoS Negl. Trop. Dis. 2:e129 10.1371/journal.pntd.0000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caragata E. P., Rances E., Hedges L. M., Gofton A. W., Johnson K. N., O’Neill S. L., et al. (2013). Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 9:e1003459 10.1371/journal.ppat.1003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho D. O., McKemey A. R., Garziera L., Lacroix R., Donnelly C. A., Alphey L., et al. (2015). Suppression of a field population of aedes aegypti in brazil by sustained release of transgenic male mosquitoes. PLoS Negl. Trop. Dis. 9:e0003864 10.1371/journal.pntd.0003864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Aguilar R., Xi Z., Warr E., Mongin E., Dimopoulos G. (2006). Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2:e52 10.1371/journal.ppat.0020052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra H. L., Rocha M. N., Dias F. B., Mansur S. B., Caragata E. P., Moreira L. A. (2016). Wolbachia blocks currently circulating zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe 19 771–774. 10.1016/j.chom.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraiture M., Baxter R. H., Steinert S., Chelliah Y., Frolet C., Quispe-Tintaya W., et al. (2009). Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe 5 273–284. 10.1016/j.chom.2009.01.005 [DOI] [PubMed] [Google Scholar]
- Freyvogel T. A., Staeubli W. (1965). The formation of the peritrophic membrane in culicidae. Acta Trop. 22 118–147. [PubMed] [Google Scholar]
- Frolet C., Thoma M., Blandin S., Hoffmann J. A., Levashina E. A. (2006). Boosting NF-kappaB-dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity 25 677–685. 10.1016/j.immuni.2006.08.019 [DOI] [PubMed] [Google Scholar]
- Hilgenboecker K., Hammerstein P., Schlattmann P., Telschow A., Werren J. H. (2008). How many species are infected with Wolbachia?–A statistical analysis of current data. FEMS Microbiol. Lett. 281 215–220. 10.1111/j.1574-6968.2008.01110.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A. A., Montgomery B. L., Popovici J., Iturbe-Ormaetxe I., Johnson P. H., Muzzi F., et al. (2011). Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476 454–457. 10.1038/nature10356 [DOI] [PubMed] [Google Scholar]
- Hughes G. L., Koga R., Xue P., Fukatsu T., Rasgon J. L. (2011). Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in anopheles gambiae. PLoS Pathog. 7:e1002043 10.1371/journal.ppat.1002043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes G. L., Rivero A., Rasgon J. L. (2014). Wolbachia can enhance Plasmodium infection in mosquitoes: implications for malaria control? PLoS Pathog. 10:e1004182 10.1371/journal.ppat.1004182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes G. L., Vega-Rodriguez J., Xue P., Rasgon J. L. (2012). Wolbachia strain wAlbB enhances infection by the rodent malaria parasite Plasmodium berghei in Anopheles gambiae mosquitoes. Appl. Environ. Microbiol. 78 1491–1495. 10.1128/AEM.06751-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Ghosh A., Moreira L. A., Wimmer E. A., Jacobs-Lorena M. (2002). Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature 417 452–455. 10.1038/417452a [DOI] [PubMed] [Google Scholar]
- Jiang X., Peery A., Hall A. B., Sharma A., Chen X. G., Waterhouse R. M., et al. (2014). Genome analysis of a major urban malaria vector mosquito. Anopheles stephensi. Genome Biol. 15 459 10.1186/s13059-014-0459-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi D., McFadden M. J., Bevins D., Zhang F., Xi Z. (2014). Wolbachia strain wAlbB confers both fitness costs and benefit on Anopheles stephensi. Parasit Vectors 7:336 10.1186/1756-3305-7-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambris Z., Blagborough A. M., Pinto S. B., Blagrove M. S., Godfray H. C., Sinden R. E., et al. (2010). Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae. PLoS Pathog. 6:e1001143 10.1371/journal.ppat.1001143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen G. F., Barillas-Mury C., Thomas M. B., Greenwood B. (2013). Modulating malaria with Wolbachia. Nat. Med. 19 974–975. 10.1038/nm.3298 [DOI] [PubMed] [Google Scholar]
- Kittayapong P., Baisley K. J., Baimai V., O’Neill S. L. (2000). Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae). J. Med. Entomol. 37 340–345. 10.1093/jmedent/37.3.340 [DOI] [PubMed] [Google Scholar]
- Kokoza V., Ahmed A., Woon Shin S., Okafor N., Zou Z., Raikhel A. S. (2010). Blocking of Plasmodium transmission by cooperative action of Cecropin A and Defensin A in transgenic Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. U.S.A. 107 8111–8116. 10.1073/pnas.1003056107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Christophides G. K., Cantera R., Charles B., Han Y. S., Meister S., et al. (2003). The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc. Natl. Acad. Sci. U.S.A. 100 14139–14144. 10.1073/pnas.2036262100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Molina-Cruz A., Gupta L., Rodrigues J., Barillas-Mury C. (2010). A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science 327 1644–1648. 10.1126/science.1184008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laven H. (1967). Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature 216 383–384. 10.1038/216383a0 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Mains J. W., Brelsfoard C. L., Rose R. I., Dobson S. L. (2016). Female adult Aedes albopictus suppression by Wolbachia-infected male mosquitoes. Sci. Rep. 6:33846 10.1038/srep33846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira L. A., Iturbe-Ormaetxe I., Jeffery J. A., Lu G., Pyke A. T., Hedges L. M., et al. (2009). A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139 1268–1278. 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- Murdock C. C., Blanford S., Hughes G. L., Rasgon J. L., Thomas M. B. (2014). Temperature alters Plasmodium blocking by Wolbachia. Sci. Rep. 4:3932 10.1038/srep03932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Zhou G., Wu J., Bian G., Lu P., Raikhel A. S., et al. (2012). Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 109 E23–E31. 10.1073/pnas.1116932108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S. B., Mariconti M., Bazzocchi C., Bandi C., Sinkins S. P. (2012). Wolbachia surface protein induces innate immune responses in mosquito cells. BMC Microbiol. 12(Suppl. 1):S11 10.1186/1471-2180-12-S1-S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povelones M., Waterhouse R. M., Kafatos F. C., Christophides G. K. (2009). Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science 324 258–261. 10.1126/science.1171400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci I., Cancrini G., Gabrielli S., D’Amelio S., Favi G. (2002). Searching for Wolbachia (Rickettsiales: Rickettsiaceae) in mosquitoes (Diptera: Culicidae): large polymerase chain reaction survey and new identifications. J. Med. Entomol. 39 562–567. 10.1603/0022-2585-39.4.562 [DOI] [PubMed] [Google Scholar]
- Shahabuddin M., Fields I., Bulet P., Hoffmann J. A., Miller L. H. (1998). Plasmodium gallinaceum: differential killing of some mosquito stages of the parasite by insect defensin. Exp. Parasitol. 89 103–112. 10.1006/expr.1998.4212 [DOI] [PubMed] [Google Scholar]
- Sinden R. E., Alavi Y., Raine J. D. (2004). Mosquito–malaria interactions: a reappraisal of the concepts of susceptibility and refractoriness. Insect Biochem. Mol. Biol. 34 625–629. 10.1016/j.ibmb.2004.03.015 [DOI] [PubMed] [Google Scholar]
- Sinkins S. P. (2004). Wolbachia and cytoplasmic incompatibility in mosquitoes. Insect Biochem. Mol. Biol. 34 723–729. 10.1016/j.ibmb.2004.03.025 [DOI] [PubMed] [Google Scholar]
- The malERA Consultative Group on Vector Control (2011). A research agenda for malaria eradication: vector control. PLoS Med. 8:e1000401 10.1371/journal.pmed.1000401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren J. H., Baldo L., Clark M. E. (2008). Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6 741–751. 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- WHO (2015). World Malaria Report 2015. Geneva: WHO Press. [Google Scholar]
- Xi Z., Joshi D. (2015). “Genetic control of malaria and dengue using Wolbachia,” in Genetic Control of Malaria and Dengue, ed. Adelman Z. N. (Amsterdam: Elsevier Inc; ), 305–333. [Google Scholar]
- Zele F., Nicot A., Berthomieu A., Weill M., Duron O., Rivero A. (2014). Wolbachia increases susceptibility to Plasmodium infection in a natural system. Proc. Biol. Sci. 281:20132837 10.1098/rspb.2013.2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zug R., Hammerstein P. (2012). Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 7:e38544 10.1371/journal.pone.0038544 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.