Abstract
Gastrointestinal stromal tumors (GISTs) are the most common type of gastrointestinal mesenchymal tumors, although metastasis to the perigastric lymph nodes is relatively rare, compared with liver or peritoneal metastasis. In this report, we describe a case of stomach GIST with a solitary simultaneous metastasis in the left axillary lymph node. A 68-year-old man was diagnosed with a large upper-stomach GIST, and computed tomography and positron emission tomography revealed masses in the left axilla and right mediastinum. We did not detect evidence of metastases to the liver, or other sites including the perigastric lymph nodes, although findings from the surgically resected axillary lymph nodes were compatible with GIST metastasis. Treatment using imatinib markedly reduced the gastric and mediastinal lesions, and this response persisted for 3 years. The patient subsequently experienced rapid growth of the gastric lesion without mediastinal or axilla recurrence, which required palliative surgery. Despite continuing medical treatment (sunitinib and regorafenib), the patient died of liver metastases 23 mo after the surgery. Based on our findings, it appears that the axillary lymph nodes can be a potential metastatic site for GIST metastasis.
Keywords: Gastrointestinal stromal tumor, Axillary, Lymph node, Metastasis, Imatinib
Core tip: Gastrointestinal stromal tumors (GISTs) are the most common type of gastrointestinal mesenchymal tumors, although metastasis to the perigastric lymph nodes is relatively rare, compared to liver or peritoneal metastasis. In this report, we describe a case of stomach GIST with a solitary simultaneous metastasis in the left axillary lymph node. Based on our findings, it appears the axillary lymph nodes can be a potential metastatic site of GIST metastasis.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most common type of gastrointestinal mesenchymal tumor, although GIST only accounts for approximately 1% of gastric malignancies[1]. GISTs frequently metastasize to the liver or peritoneum, although lymph node metastasis is very rare[2], even to the perigastric area. Therefore, we report the first documented case of stomach GIST with simultaneous axillary lymph node metastasis.
CASE REPORT
A 68-year-old man was admitted to our hospital after complaining of anorexia and obvious weight loss. Gastroscopy revealed a large tumor with ulceration in the upper stomach body (Figure 1), and histological evaluation confirmed a diagnosis of a GIST (positive for c-kit and CD34). The mitotic index was 5/50 in high-power field and the MIB-1 labeling index was 10%. Computed tomography (CT) and positron emission tomography revealed a primary gastric tumor (diameter: 5 cm), a right mediastinal tumor (diameter: 2 cm), and a left axilla mass (diameter: 1 cm) (Figure 2). Based on these findings, we performed a complete gross excision of the left axilla mass. The specimen was 1.4 cm in diameter, and there was no extranodal extension, it exhibited monotonous spindle cells (Figure 3A-D) and was diagnosed as a metastasis of the GIST, because it exhibited positive immunohistochemical staining for c-kit (Figure 3E) and DOG1 (Figure 3F), the mitotic index was 15/50 in high-power field and the MIB-1 labeling index was 10%. Based on these findings, we started treatment using oral imatinib (400 mg/d), and in the next year, after starting imatinib, we followed up the patient every 3 mo by using CT and every 6 mo by using PET. The 6-mo follow-up revealed rapid response of the primary lesion and complete remission in the mediastinal lymph nodes. However, after 3 years of imatinib treatment, the primary gastric tumor exhibited rapid growth that resulted in obstructive symptoms and continuous bleeding, although we did not detect recurrence in the mediastinal and axillary lymph nodes. We performed total gastrectomy as palliative surgery, and all 13 resected perigastric lymph nodes were negative for metastasis; the mitotic index was 20/50 in high-power field and the MIB-1 labeling index was 30%. The patient subsequently recovered, and we started postoperative adjuvant treatment using imatinib. However, liver metastases appeared after 13 mo of treatment using imatinib, which we attempted to treat using regorafenib because gene sequence analysis of the tumor showed a KIT exon 11 mutation. The liver metastasis increased 2 mo later, and we started treatment with sunitinib. However, the patient developed renal dysfunction, and died of the liver metastasis 23 mo after the surgery with gastretomy.
Figure 1.
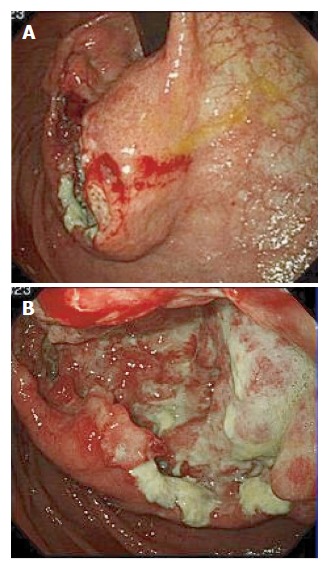
Gastroscopy revealed a large tumor with ulceration in the upper body of the stomach.
Figure 2.
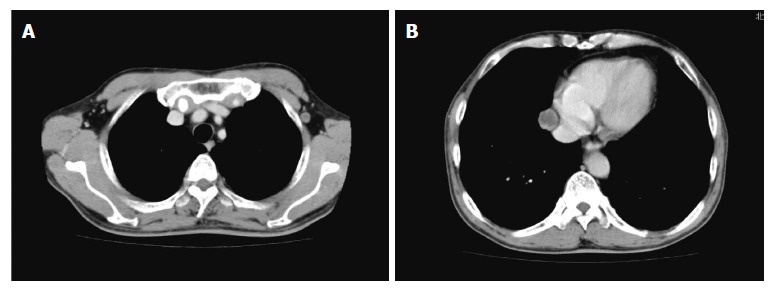
Computed tomography reveals a tumor in the left axilla (A, diameter: 1 cm) and a tumor in the right mediastinum (B, diameter: 2 cm).
Figure 3.
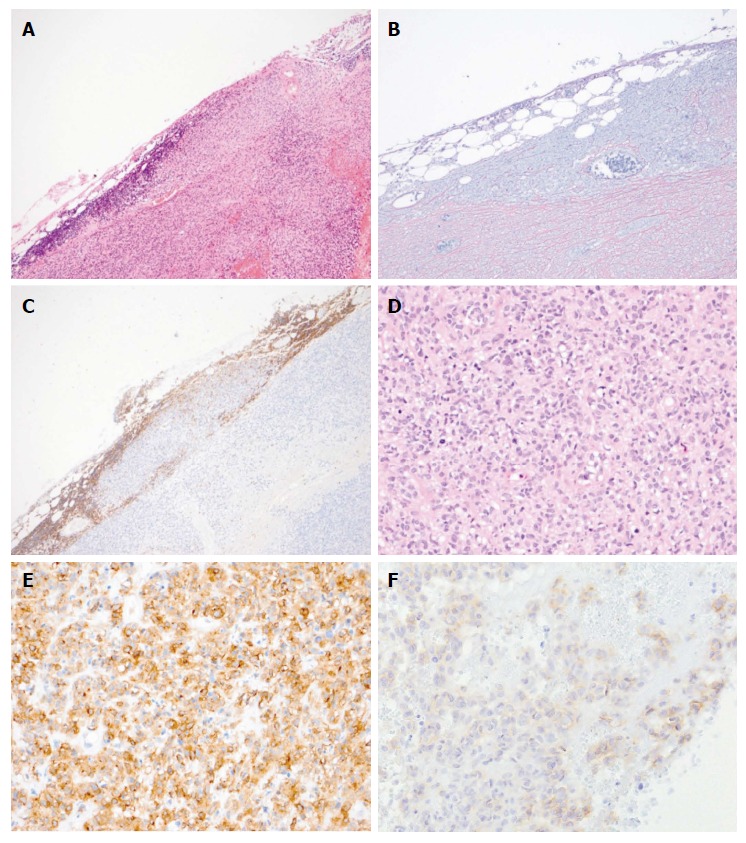
Pathological findings of the biopsied left axilla lymph node. Analysis of the tumor revealed tunicate formation and the survival of lymphoid tissue [hematoxylin and eosin staining(A), silver impregnation (B), and Leukocyte common antigen (C) (magnification × 40)]. The tumor exhibited monotonous spindle cells (D, hematoxylin and eosin staining), and the cells were positive for c-kit (E) and DOG1 (F, magnification × 100).
DISCUSSION
GIST is the most common type of gastrointestinal mesenchymal tumor, and commonly exhibits mutations in the c-kit proto-oncogene[3]. GIST frequently metastasizes to the liver or peritoneum, although nodal metastasis is very rare[2]. Among 200 reported patients with digestive tract GIST, 94 patients exhibited metastasis, which included 61 liver metastases (65%), 20 peritoneal metastases (21%), and only 6 lymph node metastases (6%)[4]. To the best of our knowledge, there is only one documented case of distant lymph node metastasis from a stomach GIST, and that case involved inguinal lymph node metastasis[5]. Although a few cases of peripheral lymph nodes metastasis from GIST have been reported[6], the present case is the first documented case of a GIST presenting with solitary axillary lymph node metastasis.
The three major routes of metastasis to the axillary lymph nodes are clearly documented in lung cancer[7,8]. The first route involves newly developed lymphatic channels that arise from the pleural lesions of adhesive lung tumors. The second route involves retrograde spread in the presence of supraclavicular lymph node metastasis. The third route involves systemic axial lymph node metastasis. In this context, systemic metastases could be caused by the primary tumor invading nearby blood vessels and the subsequent dissemination of tumor cells into the venous system through the thoracic duct[9,10]. In the present case, the tumor was likely caused by systemic metastasis, as the recurrence was only detected in the right mediastinum, without lung or supraclavicular lymph node metastases.
Tumor size, location, mitotic rate, and C-KIT and PDGFRA genotype are the major determinants of the malignant potential of the tumor, and have significant impact on prognosis[11]. In the TNM (tumor-node-metastasis) system for GISTs, the presences of lymph node metastasis is classified as stage IV, which generally portends a poor prognosis, but cases with long-term survival have also been reported[5,12]. Valadao reported that lymph node metastasis is not related to poor prognosis; however, the study included a small number of patients[13]. Furthermore, it is unclear whether there is a difference of prognosis according to the site of lymph node metastasis, because reports of distant lymph node metastasis are very rare.
The appropriate therapy for GIST with distant metastasis remains controversial. Surgery is recommended if curative resection is possible, although imatinib therapy is occasionally selected in cases that have complications or may require expansive surgery. In the present case, we performed complete gross excision of the left axilla mass, and imaging revealed that only right mediastinum metastasis remained. However, we selected imatinib therapy, based on the tumor's stage and the patient's surgical stress. Six months of imatinib therapy markedly reduced the gastric lesion and the mediastinal lesion completely disappeared. In this context, resection of residual disease after imatinib pre-treatment is feasible in patients with metastatic GIST, even those with advanced hepatic and peritoneal metastasis[14], and surgery after achieving the best clinical response may be associated with a survival benefit (vs historical patients who were treated using imatinib alone)[15]. Therefore, we assumed that the distant metastasis had been controlled by imatinib and that curative resection was possible. However, the patient refused to undergo gastrectomy and elected to continue receiving imatinib. Unfortunately, the primary lesion exhibited rapid regrowth after 3 years of imatinib treatment without reappearance of the mediastinal lesion or other distinct metastases, which led to obstructive symptoms and continuous bleeding. At this point, we performed palliative total gastrectomy and provided ongoing medical treatment (sunitinib and regorafenib), although the patient ultimately died of liver metastases at 23 mo after the surgery. Nevertheless, the patient did not exhibit signs of distant lymph node metastasis.
In conclusion, the axillary lymph nodes can be a site of GIST metastasis, and imatinib chemotherapy may be useful for controlling distant lymph node metastasis from GIST. Although we performed a resection for the original lesion because the distant metastasis had been controlled by imatinib, the appropriate therapy for GIST with distant metastasis remains controversial. Further studies are needed to clarify the duration of chemotherapy and an appropriate surgical intervention that will be effective for treating distant lymph node metastasis.
ACKNOWLEDGMENTS
We thank Dr. M Fujiwara, Department of Pathology, Ina central hospital for his pathological diagnosis.
COMMENTS
Case characteristics
A 68-year-old man was admitted to our hospital after complaining of anorexia obvious weight loss.
Clinical diagnosis
The patient was diagnosed with malignancies in the stomach.
Differential diagnosis
Gastric cancer.
Imaging diagnosis
Gastroscopy revealed a large tumor with ulceration in the upper stomach body. Computed tomography and positron emission tomography revealed a primary gastric tumor and a left axilla mass.
Pathological diagnosis
Gastrointestinal stromal tumor of the stomach with axillary lymph node metastasis.
Treatment
Surgical resection and chemotherapy (imatinib, regorafenib and sunitinib).
Related reports
There is only one documented case of distant lymph node metastasis from a stomach gastrointestinal stromal tumor (GIST), and that case involved inguinal lymph node metastasis.
Term explanation
The axillary lymph nodes can be a site of GIST metastasis.
Experiences and lessons
Imatinib chemotherapy may be useful for controlling distant lymph node metastasis from GIST.
Peer-review
This is the interesting case report. The authors described lymph node metastasis of GIST is very rare; therefore, the appropriate therapy for GIST with lymph node metastasis remains controversial.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This case report was exempt from the Institutional Review Board standard at the Ina Central Hospital.
Informed consent statement: The patient has died no consent obtained.
Conflict-of-interest statement: The authors have no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 1, 2016
First decision: December 2, 2016
Article in press: January 18, 2017
P- Reviewer: Ding XW, Luchini C, Niimi K S- Editor: Yu J L- Editor: A E- Editor: Liu WX
References
- 1.Lehnert T. Gastrointestinal sarcoma (GIST)--a review of surgical management. Ann Chir Gynaecol. 1998;87:297–305. [PubMed] [Google Scholar]
- 2.Miettinen M, Furlong M, Sarlomo-Rikala M, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol. 2001;25:1121–1133. doi: 10.1097/00000478-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813–3825. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 4.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Q, Yu JW, Yang WL, Liu XS, Yu JR. Gastrointestinal stromal tumor of stomach with inguinal lymph nodes metastasis: a case report. World J Gastroenterol. 2010;16:1808–1810. doi: 10.3748/wjg.v16.i14.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canda AE, Ozsoy Y, Nalbant OA, Sagol O. Gastrointestinal stromal tumor of the stomach with lymph node metastasis. World J Surg Oncol. 2008;6:97. doi: 10.1186/1477-7819-6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riquet M, Le Pimpec-Barthes F, Danel C. Axillary lymph node metastases from bronchogenic carcinoma. Ann Thorac Surg. 1998;66:920–922. doi: 10.1016/s0003-4975(98)00556-6. [DOI] [PubMed] [Google Scholar]
- 8.Marcantonio DR, Libshitz HI. Axillary lymph node metastases of bronchogenic carcinoma. Cancer. 1995;76:803–806. doi: 10.1002/1097-0142(19950901)76:5<803::aid-cncr2820760514>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Ku YC, Chang CC. Tumor cells in the thoracic duct lymph in carcinoma of esophagus and cardia of stomach. Chin Med J. 1966;85:258–263. [PubMed] [Google Scholar]
- 10.Stevens WM. The dissemination of intra-abdominal malignant disease by means of the lymphatics and thoracic duct. Br Med J. 1907;1:306–310. doi: 10.1136/bmj.1.2406.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dematteo RP, Gold JS, Saran L, Gönen M, Liau KH, Maki RG, Singer S, Besmer P, Brennan MF, Antonescu CR. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST) Cancer. 2008;112:608–615. doi: 10.1002/cncr.23199. [DOI] [PubMed] [Google Scholar]
- 12.Sato T, Kanda T, Nishikura K, Hirota S, Hashimoto K, Nahagawa S, Ohashi M, Hatakeyama K. Two cases of gastrointestinal stromal tumor of the stomach with lymph node metastasis. Hepatogastroenterology. 2007;54:1057–1060. [PubMed] [Google Scholar]
- 13.Valadão M, de Mello EL, Lourenço L, Vilhena B, Romano S, Castro Ldos S. What is the prognostic significance of metastatic lymph nodes in GIST? Hepatogastroenterology. 2008;55:471–474. [PubMed] [Google Scholar]
- 14.Bauer S, Hartmann JT, de Wit M, Lang H, Grabellus F, Antoch G, Niebel W, Erhard J, Ebeling P, Zeth M, et al. Resection of residual disease in patients with metastatic gastrointestinal stromal tumors responding to treatment with imatinib. Int J Cancer. 2005;117:316–325. doi: 10.1002/ijc.21164. [DOI] [PubMed] [Google Scholar]
- 15.Mussi C, Ronellenfitsch U, Jakob J, Tamborini E, Reichardt P, Casali PG, Fiore M, Hohenberger P, Gronchi A. Post-imatinib surgery in advanced/metastatic GIST: is it worthwhile in all patients? Ann Oncol. 2010;21:403–408. doi: 10.1093/annonc/mdp310. [DOI] [PubMed] [Google Scholar]


