Spinal cord direct current stimulation (sDCS) modulates spinal functions and shows potential for neural rehabilitation after motor systems injury. Using a multichannel electrode array, we found that cathodal DCS enhanced, and anodal depressed, M1-evoked local field potentials, network oscillations, neuronal activity, and neuronal synchrony, especially in the ventral horn. With this new understanding, it is hoped that sDCS can be developed into a tunable spinal neuromodulatory tool for promoting function after brain or spinal injury.
Abstract
Spinal cord direct current stimulation (sDCS) has the potential for promoting motor function after injury through its modulatory actions on sensory processing, reflex functions, the motor cortex (M1) motor map, and motor output. Here we addressed systems-level mechanisms underlying sDCS neuromodulation of spinal circuits activated by M1 and peripheral forelimb electrical stimulation in anesthetized healthy rats. We determined the effects of cathodal and anodal sDCS (c- and a-sDCS) on local field potentials (LFP) and single-unit activity recorded at 32 sites simultaneously within the sixth cervical segment using a silicon multielectrode array. M1 stimulation produced distinctive dorsomedial and ventral LFP responses that showed polarity-dependent sDCS modulation. c-sDCS enhanced and a-sDCS depressed significantly ventral M1 responses; neither modulated dorsal responses significantly. Using evoked changes in β- and γ-oscillations to assay network function, c-sDCS enhanced and a-sDCS reduced oscillation power ventrally. c-sDCS increased and a-sDCS decreased background firing and firing synchrony of recorded pairs of single units. Peripheral stimulation produced a region-dependent response that showed polarity-dependent sDCS modulation. The dorsomedial LFP was unaffected by c-sDCS and weakly suppressed with a-sDCS. Peripheral-evoked unit responses showed limited polarity dependence. Our findings stress that ventral motor network behavior is enhanced by the neuromodulatory actions of c-sDCS. The combined actions of c-sDCS on M1-evoked neural responses and network behavior in the cervical spinal cord help explain the reported enhanced motor effects of this neuromodulation approach and inform the mechanisms of sDCS for promoting motor rehabilitation after spinal cord or brain injury.
NEW & NOTEWORTHY Spinal cord direct current stimulation (sDCS) modulates spinal functions and shows potential for neural rehabilitation after motor systems injury. Using a multichannel electrode array, we found that cathodal DCS enhanced, and anodal depressed, M1-evoked local field potentials, network oscillations, neuronal activity, and neuronal synchrony, especially in the ventral horn. With this new understanding, it is hoped that sDCS can be developed into a tunable spinal neuromodulatory tool for promoting function after brain or spinal injury.
spinal direct current stimulation (sDCS) has been shown to modulate motor output (Ahmed 2011; Bocci et al. 2014), sensory input (Aguilar et al. 2011; Cogiamanian et al. 2008), as well as spinal reflex function (Cogiamanian et al. 2011; Perrotta et al. 2015) in a polarity-dependent manner. Recently we also showed in rats that the motor cortex (M1) forelimb motor map is expanded during cathodal direct current stimulation (DCS) applied to the skin surface (Song et al. 2015). Thus sDCS shows potential for neural rehabilitation after stroke or spinal cord injury (Heide et al. 2014; Wallace et al. 1987). How this promising neuromodulatory approach facilitates spinal circuits, which is the principal intended target of its actions, is poorly understood. Several recent studies have provided key insights into some of the basic mechanisms (Ahmed 2014; Bolzoni and Jankowska 2015; Jankowska et al. 2016; Niérat et al. 2014). Although computational models of the sDCS provide valuable information on the current density distribution within the spinal cord (Hernández-Labrado et al. 2011; Song et al. 2015), many questions remain. Importantly, our knowledge of which laminar regions of the spinal cord contain neurons that modulate their action during sDCS is incomplete.
In this study, we address two major questions regarding sDCS neuromodulation of spinal circuits using the novel implementation of recording local field potential (LFP) and single-unit activity simultaneously from 32 spinal gray matter sites. First, how do neurons across the different dorsoventral and mediolateral regions respond to activation by two major inputs to spinal circuits: the corticospinal tract (CST), in response to M1 stimulation, and peripheral afferent stimulation? This is the first report using high-resolution spinal multielectrode recordings to examine simultaneously these kinds of spinal responses. Second, what are the underlying changes in spinal neural activity—modulation of LFP response amplitude and oscillations, activity of individual units, and synchrony between unit pairs—by which cathodal-sDCS (c-sDCS) enhances and anodal-sDCS (a-sDCS) depresses M1-evoked muscle responses (Song et al. 2015)? We examined the putative mono- and oligosynaptic spinal responses produced by M1 stimulation. In addition, we hoped to understand if the same sDCS that modulates M1-evoked motor responses also affected peripheral sensory-evoked cortical potentials to a similar extent.
Based on our initial study (Song et al. 2015), we hypothesized that sDCS will preferentially modulate motor responses within the different laminae and regions of the spinal gray matter and, in particular, would modulate ventral horn excitability during c-sDCS. We found that M1 stimulation activates virtually the entire contralateral cervical gray matter within 10–20 ms, indicating the capacity for the CST to enhance the excitability of diverse spinal circuits transiently. We found that sDCS modulated motor-evoked more than sensory-evoked responses. The motor responses in the ventral horn were strongly enhanced by c-sDCS and suppressed by a-sDCS, suggesting that motoneurons are an important target of sDCS. Our findings stress that ventral motor network behavior is enhanced by the neuromodulatory actions of c-sDCS and provide important new insights for understanding the neural underpinnings of sDCS and informs further the mechanisms of this neuromodulatory rehabilitation tool.
METHODS
A total of nine adult Sprague-Dawley rats (250~320 g) provided data for this study. Among these, one animal was used for histology to validate the electrode array placement, and three rats were used to test the differential effect of stimulation on muscle and subcutaneous afferents. Care and treatment of the animals conformed to protocols approved by the Institutional Animal Care and Use Committee of the City College of New York.
Surgery, M1 electrode placement, and M1 stimulation.
After anesthetizing with a mixture of ketamine and xylazine (80 mg:5 mg/kg), each rat was placed in a stereotaxic frame (Kopf Instruments) with normal body temperature maintained with a circulating water bath heating pad (39 ± 1°C). A craniotomy (5 mm × 5 mm) was made over the right forelimb representation area of M1, and a PlasticOne bipolar stimulating electrode (PlasticOne Com.) was placed epidurally over the distal forelimb movement area (Brus-Ramer et al. 2009). We optimized electrode placement and standardized the current used across all experiments, according the following protocol. We first placed the electrode sterotactically in the center of the forelimb field and determined the movement threshold in respond to a train of stimuli (13 biphasic pulses of duration 200 µs at 333 Hz; ≤1.5 mA). This train, which had the same parameters as commonly used in M1 mapping experiments, produced a small contralateral distal forelimb movement. The electrode was repositioned if necessary to stimulate at the site with the lowest movement threshold. Ipsilateral forelimb and hindlimb movements were not produced by this stimulation. These observations indicate that, despite the milliampere-level epidural current threshold, current did not spread beyond the M1 forelimb area. The milliampere threshold currents reflect the epidural electrode placement. When we used a microelectrode in M1 at the same site as the epidural electrode, the thresholds were in the microampere range (data not shown). For all of the experiments described here, we used this threshold current, but changed from the 13-pulse train of stimuli to a single stimulus pulse to evoke spinal responses. This single stimulus did not produce an observable movement, but did produce a small distal forelimb muscle-evoked potential (MEP; Fig. 1A shows setup; Fig. 1B). We obtained similar movement thresholds for contralateral forelimb responses in a recent publication (Song et al. 2016). Next, a laminectomy was made at C5 and C6; the T1 vertebra was fixated with a spinal clamp. The anesthesia level was checked throughout the entire procedure by monitoring the breathing rate, the absence of vibrissae whisking and the absence of hindlimb withdrawal to foot pinch. Supplemental doses of ketamine (25 mg/kg) were administered to maintain the required anesthetic depth during the experiment. Spinal dura and pia over C6 were cut to facilitate spinal electrode array insertion.
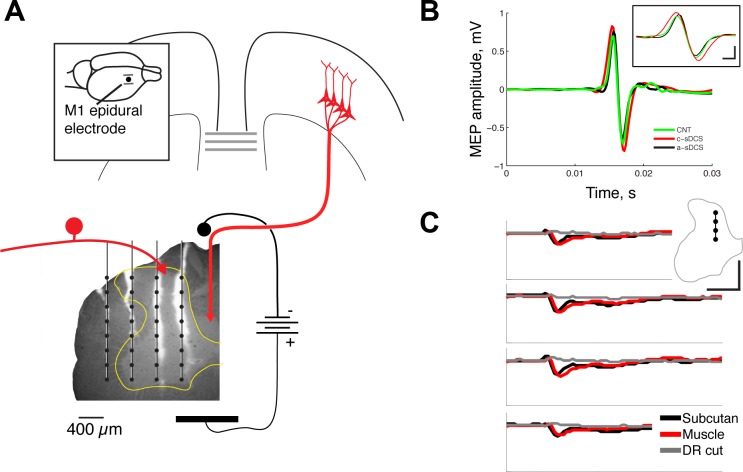
Fig. 1.
Experimental setup and confirmation of effects. A: spinal direct current stimulation electrode was put over C7, and a four-shank silicon recording electrode array (4 × 8) was inserted transversely into the left side of the spinal cord (C6) at a depth of 2 mm from the pial surface. The fluorescence micrograph shows an unstained section through the spinal cord with the tracks of the electrodes marked with the dye diI. Each vertical line indicates a recording tract of one shank, and each dot, a recording site. The configuration of M1 and peripheral stimulation is shown schematically. The inset shows the location of the M1 electrodes. Calibration: 0.4 mm. B: muscle-evoked potentials (MEPs) recorded from the extensor carpi radialis muscle in response to the same M1 stimulation as was used for producing the spinal potentials. The inset shows the same recorded MEP but at a faster time course to show the effects of cathodal and anodal stimulation. Calibration: inset 0.75 mV, 1 ms. C: electrical stimulation using subcutaneous (black line) or muscle (red line) electrodes produced the same initial negative response in the medial dorsal horn. Cutting the C6 dorsal root (DR) eliminated the potential (gray line). Calibration: 10 ms, 0.1 mV.
Spinal recordings and peripheral stimulation.
A four-shank silicon array (interrow electrode contact distance: 0.2 mm; intershank distance: 0.4 mm; NeuroNexus) was inserted transversely into the left side of the spinal cord (contralateral to the cortical stimulation electrode; Fig. 1A). The electrode array was positioned 0.2 mm from the midline and inserted to a depth of 2 mm from the surface, as measured by the initial pial contact with the electrode array. Electrode positioning was carefully performed in all animals under a dissecting microscope to minimize interanimal electrode position differences. In one experiment, we coated the array with a dye (diI; Molecular Probes), and this was used to localize the array within spinal cord gray and white matter landmarks. A stainless steel disk (diameter 5 mm) was used as the active sDCS electrode and was placed above a piece of saline-soaked gelfoam over the C7 vertebrae. The reference sDCS electrode was modified by cutting a surface patch-electrode (StimTent Com.) into a rectangular shape (15 mm × 20 mm), and placed over the chest before surgery and after the hair was removed. The polarity of the sDCS current was referred to as the active electrode. Direct current (±1.5 mA) was delivered through an analog isolated stimulator (A-M Systems, 2100).
To stimulate peripheral afferents, two 7-strand stainless steel wires (deinsulated 5 mm at the end) were inserted subcutaneously at the wrist of the left forearm. The electrodes were placed dorsal and ventral to the forelimb extensor and flexor muscle compartments. We intended to activate preferentially muscle/deep afferent fibers from the forelimb at threshold as an assay for the effects of sDCS. Figure 1C shows representative evoked responses within a single electrode track within the dorsal horn, which is within the primary termination field of forelimb peripheral afferents (Jiang et al. 2016). As described in the results, ventral responses were dominated by initially positive current sources (see Fig. 5 for ventral responses). Responses evoked by the typical stimulating electrode (placed subcutaneously dorsal and ventral to the forelimb extensor and flexor muscle compartments, respectively; Fig. 1C, red) are compared with those evoked by selective extensor carpi radialis muscle stimulation (black). The initial response latency and time to peak negativity for the two electrode placements appeared to be the same. We noted variability between different animals in later responses with the dorsoventral electrode pair. However, this did not affect our results, because we only examined quantitatively the initial afferent response. Furthermore, analysis of the 95% confidence limits within and across animals showed minimal variability (e.g., Figs. 2, B and C, and 5, C and D). Section of the C6 dorsal roots eliminated the evoked negativity (Fig. 1C, gray).
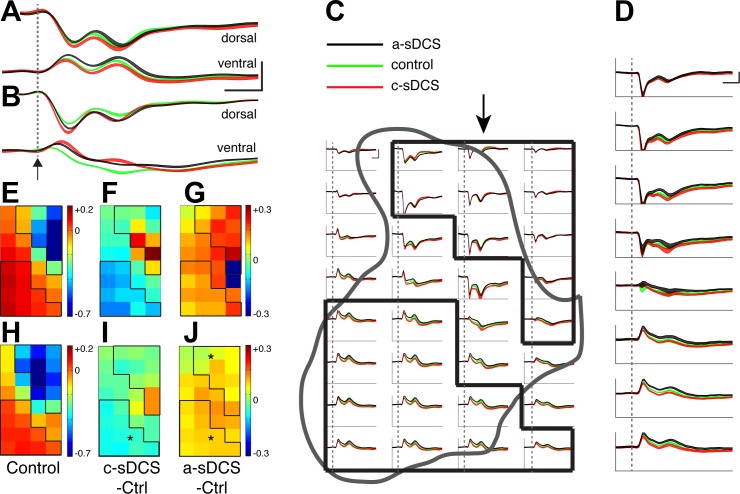
Fig. 5.
Afferent stimulation-evoked spinal potentials under control and sDCS. A and B: sample recordings from a dorsal and a ventral site in the control (green) and two sDCS conditions (c-sDCS, red; a-sDCS, black) from two animals, respectively. The 95% confidence limits are indicated by the colored bands. Dotted line and arrow show stimulus onset. Dotted lines indicate stimulus onset. Calibration: 10 ms, 0.1 mV. C: recordings from all 32 sites from a single representative experiment. The black outlines correspond to the dorsal and ventral region of interests (ROIs). The 95% confidence limits are indicated by the colored bands. Calibration: 10 ms, 0.1 mV. D: averaged responses from all animals (n = 5) for responses along the second electrode tract from the midline (marked by arrow in C). Dotted line indicate stimulus onset. Calibration: 10 ms, 0.1 mV. Sensory map of control condition (E), and maps of changes induced by c-sDCS (F) and a-sDCS (G) from an individual animal are shown. H: averaged (n = 5 rats) control peak first negative response amplitude from each recording site. I and J: average maps of changes induced by c-sDCS and a-sDCS, respectively. Calibrations for D and G: mV2/Hz. The mean response amplitudes under the three condition (G–I) are as follows: control, −234 ± 28 µV; cathodal sDCS, −252 ± 25 µV; and anodal sDCS, −148 ± 30 µV. *Significant changes from control in the ROIs (I and J).
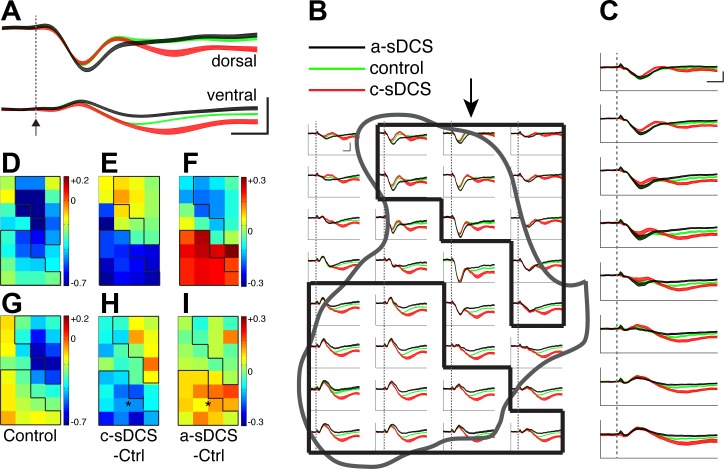
Fig. 2.
M1-evoked spinal potentials under control and sDCS. A: sample recordings from a dorsal and a ventral site in the control (green) and two sDCS conditions (c-sDCS, red; a-sDCS, black). The 95% confidence limits are indicated by the colored bands. Dotted line and arrow show stimulus onset. Calibration: 10 ms, 0.1 mV. B: recordings from all 32 sites from a single representative experiment. The 95% confidence limits are indicated by the colored bands. Dotted lines indicate stimulus onset. Calibration: 10 ms, 0.1 mV. C: averaged responses from all animals (n = 5) for responses along the second electrode tract from the midline (marked by arrow in B). The 95% confidence limits are indicated by the colored bands. Dotted line indicate stimulus onset. Calibration: 10 ms, 0.1 mV. D: data from a representative animal plotting the control peak first negative response amplitude from each recording site. The black outlines correspond to the dorsal (dROI) and ventral region of interests (vROI). Calibration: mV2/Hz. E and F: maps of changes induced by c-sDCS and a-sDCS, respectively. G: averaged (n = 5 rats) control peak first negative response amplitude from each recording site. The black outlines correspond to the dROI and vROI. Calibration: mV2/Hz. H and I: average maps of changes induced by c-sDCS and a-sDCS, respectively. The mean response amplitudes under the three condition (C–E) are as follows: control, −323 ± 19 µV; during c-sDCS, −381 ± 17 µV; during a-sDCS, −262 ± 22 µV. *Significant changes from control within the ROIs (H and I).
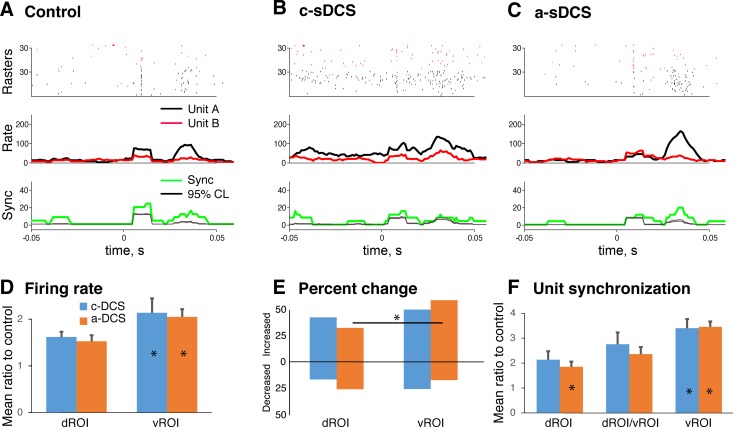
A unity gain head-stage was connected via flexible cables to the spinal recording array, then the wideband signals were amplified (×1,000) and split into low-pass (0.1–300 Hz) signal for LFP recordings and high-pass (300 Hz to 10 kHz) signal for unit activity recordings with the OmniPlex-D system (Plexon). The LFPs were digitized at a sampling frequency of 1 kHz and were notch-filtered offline to remove 60-Hz noise (iirnotch, Matlab). Unit activity was digitized at a sampling frequency of 40 kHz, and spikes were detected online when the amplitude was above a threshold, which was defined as greater than three times the SD of the baseline level. Online-detected spike waveforms were further classified offline using Offline Sorter (Plexon). Well-isolated units were defined subjectively by the presence of a clear and consistent waveform shape with an interspike interval >1.6 ms, and a good separation in the principal components feature space. Typical waveforms of two isolated units are shown in Fig. 4A.
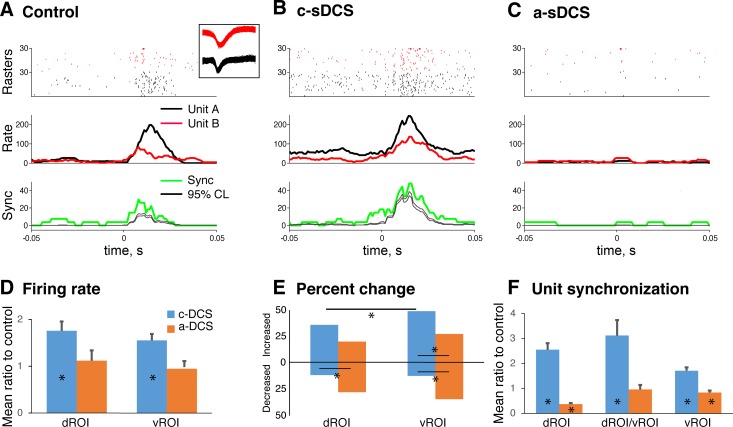
Fig. 4.
Changes in M1-evoked unit activity in response to sDCS. A–C: example of raster plots of two single units (top), poststimulus time histograms of spiking (PSTH; middle), and synchrony between the unit pair (bottom) under control (A), c-sDCS (B), and a-sDCS (C). Red and black indicate two units, and each row in the raster plot in A, B, and C shows spikes from one trial. A total of 30 trials comprise each raster plot for each unit, plotted in order of data collection. Unit waveforms are shown in inset. The PSTH of the synchrony is shown between the two units, which is defined as simultaneous firing (within 1 ms) between the unit pairs. The upper and lower 95% confidence levels (thin black line) show the trial-shuffled PSTH (n = 100). This indicates that synchrony is not simply arising from the rate increase. Rate is in spikes/second; synchrony is occurrences/second (upper and lower of 95% confidence levels). D: bar graphs plotting mean (±SE) of the ratio of the integrated firing rate under sDCS and control conditions for the dROI and vROI. E: bar graphs plotting the percent of dROI and vROI units showing an increase or decrease in activity under the two sDCS conditions. F: bar graphs plotting mean (±SE) of the ratio of the unit-pair synchronization rate under sDCS and control conditions for units with the dROI and vROI, and for one unit in the dROI and the other in the vROI.
Motor- and sensory-evoked spinal potentials and response maps.
M1 stimulation-evoked spinal potentials were assessed by electrical stimulation through the epidural cortical electrode. Thirty single pulses (duration 200 µs; at the 14-pulse motor movement threshold) were delivered at a frequency of 0.5 Hz, and the evoked potentials from each site of the array were averaged to get a motor-evoked spinal potential for individual recording sites/channels (see Fig. 2B). The stimulation parameters were kept the same during sDCS and control conditions for each animal. Similar to the motor-evoked spinal potentials, spinal potentials evoked by the peripheral stimulation were recorded at each site in response to peripheral electrical stimulation (30 pulses; 0.5 Hz frequency; duration: 200 µs; intensity at threshold, which was ~0.1 mA). This stimulation could induce sensory-evoked spinal potentials reliably without noticeable muscle contractions.
The spinal recording array was held in the same position during motor- and sensory-evoked spinal potential testing and associated single unit recordings. The first peak of the downward deflection (see Figs. 2A and 5, A and B) was used as the marker of local depolarization from the underlying neurons (Destexhe et al. 1999; Fu et al. 1974). In one session, we also validated that c-sDCS augmented and a-sDCS reduced the M1-evoked muscle potentials (MEPs) during sDCS stimulation (see Fig. 1B), which is consistent with our other finding (Song et al. 2015, 2016). Then the average first negative deflection peak values were determined for each recording channel of the array under the following three conditions: M1 and/or afferent stimulation alone, with c-sDCS, and with a-sDCS. These values were averaged across a subset of animals in the study (n = 5) in which all conditions were successfully recorded. The values were arranged as a two-dimensional map based on the relative position of each recording channels within spinal cord, to present an ensemble map of mean values. Averages ±95% confidence limits are plotted for each recording site.
Spectral power maps of motor- and sensory-evoked spinal potentials.
Neural oscillations reflect the interactions between neurons within the local or long-range circuits, based on studies of cortex (Pfurtscheller and Lopes da Silva 1999). Different frequency bands are thought to represent distinct networks (Rosanova et al. 2009) that, also based on studies in cortex, might play different functional roles. For these reasons, the oscillatory activity of spinal neurons was analyzed with the event-related spectrum perturbation (Makeig 1993), which was calculated as a time-frequency representation of wavelet decomposition of individual responses from each stimulation trial in each channel of motor- and sensory-evoked spinal potential. We choose continuous wavelet transform to achieve optimized frequency resolution at different time scales. Then we calculated the mean power for the β- (13–30 Hz) and γ-frequency bands (30–80 Hz) from each of the time-frequency motor- and sensory-evoked spinal potential spectral power representations for each recording channel for 200 ms following each stimulus. Similar to the initial negative deflection peak values of the evoked responses, the oscillatory spectral power maps of the β- and γ-bands were compared under different conditions (control without sDCS, c-sDCS, and a-sDCS).
Unit activity analysis.
To test the effect of sDCS on modulation of single units within the spinal cord, we constructed peristimulus time histograms (PSTH) of neural firing for individual units and PSTHs of the rate of synchronization between unit pairs. Synchrony was defined as simultaneous firing of two units within a 1-ms time window. The PSTHs were smoothed (9-ms window length) to obtain the firing rate and synchrony rate (Song and Francis 2013). Then the smoothed PSTHs were examined from the baseline window (50-ms period before stimulation onset) and the response window (50-ms period after stimulation onset), as shown in Figs. 4 and Fig. 7. The effect of sDCS was analyzed with the area-under-response curve (within the response window), which is thought to be related to the functional activation/synaptic transmission within the neural circuit (Abeles 1991).
Fig. 7.
Changes in afferent stimulation-evoked unit activity in response to sDCS. A–C: example of raster plots of two single units (top), poststimulus time histograms of spiking (PSTH; middle), and synchrony between the unit pair (bottom) under control (A), c-sDCS (B), and a-sDCS (C). Same units as in Fig. 4. Raster plot shows 30 trials for each unit. The PSTH of the synchrony between the two units is shown, which is defined as simultaneous firing (within 1 ms) between the unit pairs. The upper and lower 95% confidence levels (thin black line) show the trial-shuffled PSTH (n = 100), similar to Fig. 4. Rate is spikes/second; synchrony is occurrences/second. D: bar graphs plotting mean (±SE) of the ratio of the integrated firing rate under sDCS and control conditions for the dROI and vROI. E: bar graphs plotting the percent of dROI and vROI units showing an increase or decrease in activity under the two sDCS conditions. F: bar graphs plotting mean (±SE) of the ratio of the unit-pair synchronization rate under sDCS and control conditions for units within the dROI and vROI and for one unit in the dROI and the other in the vROI.
Statistical analysis.
To test the effects of sDCS on spinal neurons at the different recording sites in the context of interanimal map variability, we analyzed data within two regions of interest (ROIs; see Fig. 2B, solid lines) corresponding to the dorsal (dROI) and ventral horns (vROI). Statistical analyses are based on these two locations, instead of comparisons of each point in the map. The effects of sDCS within the dROI and vROI were tested by parametric (t-test, MATLAB) or nonparametric tests (rank sum or signed rank, MATLAB) for data sets with a normal or nonnormal distribution, respectively. A Bonferroni correction was used for multiple comparisons. After rearranging the entire data set from each recording site within the dROI and vROI in the five rats subjected to the complete M1 and peripheral stimulation protocols, we further tested the effects of sDCS (anodal and cathodal) and ROI (dorsal and ventral), as well as the interaction between the two factors on the amplitude of the spinal responses using a two-way ANOVA (anovan, MATLAB). A Fisher’s exact test was used to determine significance of changes in background unit firing in response to c-sDCS and a-sDCS. The significance level was set at 0.05. All data analyses were performed using MATLAB (Math Works).
RESULTS
For LFPs, we monitored changes both in response amplitude and oscillatory power at different frequency bands. For single units, we assessed firing rate changes of individual units, as well as the synchrony between unit pairs. We first present results of M1-evoked changes in LFP and then single unit activity, following which we present the effects on peripheral stimulation-evoked activity.
Broad segmental response to M1 stimulation.
M1 stimulation at threshold evoked responses (Fig. 2A; green trace, control, no stimulation) that were distributed broadly throughout the spinal cord, but differed in relation to electrode location (Fig. 2, B and C, single animal; F, population). The location of each trace in Fig. 2B corresponds to the recording site on the electrode array (see Fig. 1A). As described in the methods, we focused on the initially negative (i.e., downward) response, since this corresponds to local spinal depolarization (Fu et al. 1974). Dorsally, M1 stimulation evoked an initially negative deflection with a short latency and short time-to-response peak. By contrast, the ventral negative response was less phasic, with a longer latency and longer time to peak. Note that the ventral negative response was preceded at many recording sites by an initially positive (i.e., upward) response that is the inverse of the dorsal negative response and has an identical time course from onset to peak as the dorsal response. The positive peak aligns with the negative dorsal peak along the electrode track at all locations ventral to the central canal (Fig. 2B; see Fig. 2C for responses across subjects). This positive response is consistent with a more ventral current source for the dorsal depolarization. The dorsomedial recording zone (Fig. 2B) corresponds well with the published distribution of the anatomically labeled CST termination field of forelimb M1 (Jiang et al. 2016).
The mean time to peak dorsal negative response is 15 ± 0.3 (SE) ms. Estimates based on the averages are similar to published accounts (Nielsen et al. 2007). The presence of a short latency on the averages, the absence of an earlier response preceding the phasic negative response, the initially-negative deflection consistent with local depolarization, and localization within the predominantly dorsal termination field of the CST suggest that the dorsal negative response is the monosynaptic CST depolarizing response.
The initially ventral-positive response obscures the onset of the ventral-negative response. The mean time to peak ventral negative response is 32 ± 1.4 (SE) ms. Inspection of individual animal data revealed sites where there was little or no initial positive deflection. At those sites, the onset latency lags that of more dorsal sites (e.g., Fig. 2B; ventrolateral sites). Based on it being a later event and the absence of significant CST projections ventrally in the rat (Jiang et al. 2016), the negative ventral deflection is consistent with an oligosynaptic synaptic response. The possible origin of the ventral response is considered further in the discussion. The recorded responses were highly consistent from animal to animal, as shown by responses along a single track averaged across all animals (Fig. 2C; track location indicated in B by arrow; n = 5).
We constructed average response maps for the entire recording array, and represent the first negative deflection peak, both dorsally and ventrally (i.e., initial ventral positive peak was not measured), as a color scale (Fig. 2D for single animal, G for all animals). Based on the locations of the recording sites, we established a dorsal horn ROI (dROI) and a ventral horn ROI (vROI) to quantify the responses further (solid lines in each figure; data values presented in the next section). In addition to the high-resolution topographic maps, these ROIs approximated the dorsal and ventral horns and permitted statistical comparison of recorded events within each area. Baseline responses were largest and most phasic within the dROI and smaller and less phasic within vROI, as revealed in a representative single animal (Fig. 2D) and across the population of animals studied (Fig. 2G). Our findings using an array of 32 simultaneous recordings show that within 10–20 ms after stimulation, M1 stimulation evoked robust activation throughout the contralateral C6 gray matter.
c-sDCS enhanced and a-sDCS reduced M1-evoked responses.
We next examined the effects of a- and c-sDCS on the motor-evoked spinal potential. Overall, c-sDCS increased responses, and a-sDCS decreased responses. Based on averages of all 32 sites, the change in value during c-sDCS was −54 ± 14 µV (negative, meaning more depolarizing), and the change in value during a-sDCS was 51 ± 11 µV (positive, less depolarizing). To determine the spatial distribution of effects of sDCS, we plot the mean change in the amplitude of the response recorded from each electrode site during c- and a-sDCS in a representative animal (Fig. 2, E and F) and for the population studied (Fig. 2, H and I). Since we plot only the first negative deflection peak in response to M1 stimulation, augmentation of the negative deflection will result in a shift to a cooler color. Negative deflection augmentation (i.e., increased activation) is the case only for cathodal stimulation ventrally, which is very robust in the individual animal shown (Fig. 2E, c-sDCS; see also Fig. 2A). For the population, this shift is consistent and significant (Fig. 2H; signed rank, P < 0.05; see also Fig. 2C). Conversely, reduction of the negative response (i.e., decreased activation) is shown as a shift to warmer colors, which is the case only for anodal stimulation ventrally. This too is a very strong effect in the individual animal (Fig. 2F, a-sDCS) and significant across the group (Fig. 2I; signed rank, P < 0.05). Remarkably, the dorsal response was not significantly affected (signed rank, P > 0.05). Overall, a two-way ANOVA showed significant effects of the two main factors (sDCS and ROI) and an interaction between them (FsDCS = 22.28, P = 0; FROI = 7.04, P = 0.009; Finteraction = 41.23, P = 0). This suggests that the LFP response was ROI specific, and modulation was polarity dependent, and that the two factors (ROI and sDCS) interact each other.
Our findings show that c-sDCS robustly augmented the ventral depolarizing response, whereas a-sDCS significantly suppressed the ventral responses. However, this anodal suppression was much smaller compared with cathodal augmentation. Cathodal augmentation of the ventral motor-evoked spinal potential is similar to cathodal augmentation of MEPs, as shown in Fig. 1B and studied extensively in our other reports (Song et al. 2015; 2016). The cathodal neuromodulatory effect on the ventral horn would thus be expected to enhance motor output.
c-sDCS enhanced and a-sDCS reduced motor-evoked oscillation power.
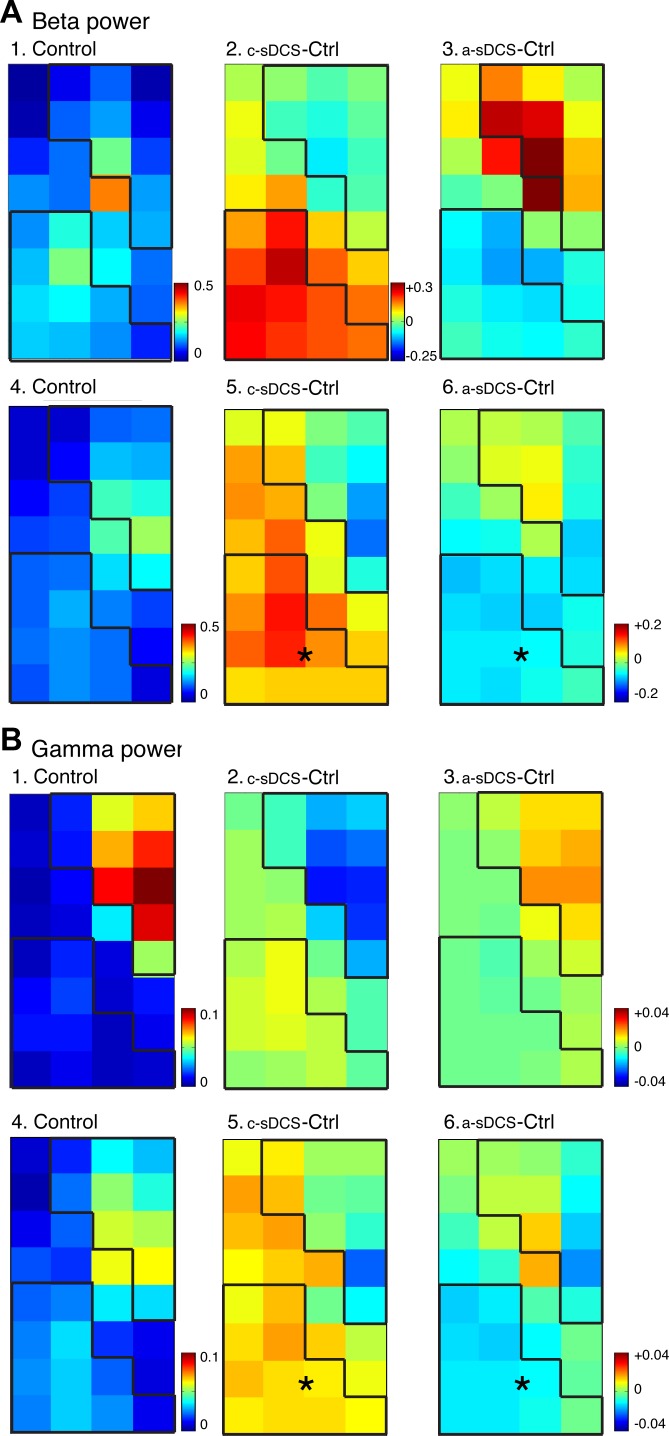
To determine whether sDCS changed spinal neuronal oscillations, which are thought to represent interactions between neurons within local and distant circuits (Pfurtscheller and Lopes da Silva 1999), we measured the power within the β- and γ-frequency bands during the 200-ms period after M1 stimulation. We mapped the changes in oscillations at each electrode recording site (Fig. 3). Note that the oscillation color scale (left column) is from 0 (blue) to larger values (red). We found that β-oscillations were somewhat greater than γ-oscillations overall (Fig. 3, A and B) in the representative individual animal (top row; A1–3; B1–3) and in the population studied (bottom row; A4–6; B4–6). For both frequency bands, the locations of maximal oscillation power were localized in the dROI in the control condition, suggesting an important drive by the direct CST projection into this territory. Two-way ANOVA of β-power showed significant effects of the two main factors (sDCS and ROI) and an interaction between them (FsDCS = 20.6, P = 0; FROI = 4.27, P = 0.04; Finteraction = 31.64, P = 0). This suggests that β-power was polarity dependent and ROI specific, and that the two factors (ROI and sDCS) interact with each other. γ-Power showed sDCS specificity, but did not show a region-specific effect from the two-way ANOVA (FsDCS = 17.59, P = 0; FROI = 1.07, P = 0.302; Finteraction = 13.51, P = 0.0003). Across all sites, cathodal stimulation increased β-oscillations (0.047 ± 0.01 mV2/Hz), and anodal stimulation decreased (−0.033 ± 0.008 mV2/Hz) β-oscillation power compared with control. The same pattern was observed for γ-oscillation power (cathodal: 0.007 ± 0.002 mV2/Hz; anodal: −0.006 ± 0.002 mV2/Hz). Difference maps are shown in the center and right columns. As with response amplitude, significant changes were observed within the vROI for both c- and a-sDCS (signed rank; P < 0.05), but not within the dROI. The direction of changes produced by the sDCS neuromodulation paralleled the amplitude changes presented earlier.
Fig. 3.
Topographic effects of sDCS on spinal oscillations evoked by M1 electrical stimulation in the β- (A) and γ-ranges (B). Top rows for A and B show data from a representative animal, and the bottom rows show averaged data (n = 5 rats). A1: control map of β-oscillation power. A2 and A3: changes induced by c-sDCS and a-sDCS, respectively. A4: average control map of β-oscillation power. A5 and A6: average maps of changes induced by c-sDCS and a-sDCS, respectively. B: same as A but for γ-band oscillations. Calibrations: A1, 4. 0–0.5 mV2/Hz; A2 and A3, −0.25 to +0.3 mV2/Hz; A5 and A6, −0.2 to +0.2 mV2/Hz; B1, 4. 0–0.1 mV2/Hz; B2, B3, B5, and B6, −0.04 to +0.04 mV2/Hz. *Significant changes from control in the ROIs.
c-sDCS enhanced and a-sDCS reduced background firing rate of individual units.
To characterize the effect of sDCS on individual units, we selected units that showed an excitatory response to both motor and sensory stimulation that we were able to record during the three conditions (control, a-sDCS and c-sDCS). The relevance of these criteria is described in the next section. We first examined changes in background firing. Among units recorded (n = 75), 52 increased their background firing with c-sDCS, and 23 decreased. When the same units were tested with a-sDCS, 29 showed an increased firing rate, and 46 a decrease. This association between current polarity and background firing rate was significant (X2 = 14.198; P = 0.0002). Thus c-sDCS increased the background firing rate of 69.3% of the units, whereas a-sDCS decreased the firing rate of 61.3%. The same trend was observed for units located dorsally (n = 34) or ventrally (n = 41). For dorsal units, 67% increased and 33% decreased their background firing rates with c-sDCS; whereas 59% decreased and 41% increased their background rates with a-sDCS. For ventral units, 71% increased and 29% decreased their background firing rates with c-sDCS, whereas 63% decreased and 37% increased their background rates with a-sDCS. Overall, c-sDCS increased and a-sDCS decreased background firing suggested opposing effects on spinal neuron excitability.
c-sDCS enhanced motor-evoked firing rate of individual units and synchrony between unit pairs.
To characterize the effect of sDCS on evoked changes in firing rate of individual units and synchrony between unit pairs, we selected units that showed an excitatory response to both motor and sensory stimulation that we were able to record during the three conditions (control, a-sDCS and c-sDCS). An evoked unit response was defined as having a peak firing rate within the response time window (50 ms from stimulation) that was greater than 2 SDs over background (prestimulus activity). Representative raster plots of unit pairs are shown (Fig. 4A, top) under control, c-sDCS, and a-sDCS conditions. Complementary PSTHs of the M1-evoked response (middle) and synchrony between the unit pairs (bottom) are also presented. These traces show examples of response augmentation with cathodal stimulation and response reduction with anodal stimulation, which was the general trend for unit recordings. Similar changes were observed for synchrony between unit pairs. Importantly, the increase in synchrony rate did not simply arise from the increased firing rate, as the rate of synchrony is significantly above the rate predicted on the basis of the spike train shuffled in trials (n = 100; gray line in Fig. 4A).
To quantify M1-evoked unit responses across the population of recorded neurons (dROI: n = 24; vROI: n = 34), we measured the area under the PSTH curve within the response window for each unit to determine the evoked response size and the effect on unit synchrony. The ratio of this measure for the control condition and during sDCS was computed to obtain an estimate of the effect of sDCS. For both the dROI and vROI, c-sDCS significantly augmented response amplitude (Fig. 4D; unit firing), whereas a-sDCS had no significant effect. We found that there were significantly more units whose activity was augmented by c-sDCS in the vROI than in the dROI (Fig. 4E). Importantly, in the ventral horn, significantly more units were activated by c-sDCS than a-sDCS, whereas more units showed decreased activity by a-sDCS than c-sDCS. This stronger and complementary vROI modulatory response is similar to the effects of sDCS on LFPs.
Finally, unit pair synchrony across the population (Fig. 4F) was also enhanced to a greater extent with c-sDCS than a-sDCS. As indicated above, these changes are not due to simple activity changes, since the rates are over 95% confidence interval of the rate from trial shuffled testing (n = 100). Interestingly, synchrony between units in the dROI and vROI also was significantly increased with c-sDCS (Fig. 4F, middle bars). Neural synchronization is thought to play an important role in motor output and could bind the neural circuits during sensorimotor tasks (van Wijk et al. 2012). Our results stress that c-sDCS augments the firing rates and increased unit pair synchronization of spinal cord neurons, both dorsally and ventrally.
Sensory-evoked spinal potentials were localized to the dorsomedial gray matter and inconsistently modulated by sDCS.
Similar to the motor-evoked response, peripheral stimulation evoked a short-latency dorsomedial potential at threshold, which comprised a consistent early negative response that peaked at 9 ± 0.55 ms, followed by small oscillations (Fig. 5, A and B). The initial response likely corresponds to a group I monosynaptic response because group I fibers have the lowest response threshold (Pierrot-Deseilligny and Burke 2005). Whereas the later responses varied in amplitude between animals (Fig. 5, A and B), the 95% confidence limits for the population of rats (n = 5) shows a prominent initial negative response and a second response with reduced amplitude (see Fig. 5D; responses are from the track marked with the arrow in C; green traces are without sDCS). We focused only on the initial negative response. The initial negative sensory spinal potential was largest medially in the dorsal horn and distributed, with attenuation, to the ventral horn (Fig. 5C; data from animal shown in B). Given that stimulation was at threshold, the dorsal late negative-positive waves more likely reflect an oligosynaptic group I response than a monosynaptic response of a slower-conducting afferent fiber group.
We then tested the effects of sDCS on sensory-evoked potentials within each ROI. Over sites ventrally and laterally (Fig. 5C), the initial response was positive (Fig. 5, A and B; lower trace of each pair), and the first negative response followed this positive peak. As with the M1-evoked potential, the initial positive sensory response likely reflects the current source for the early dorsomedial negativity since its timing was identical to the dorsal negativity (Fig. 5, A, B, and D), and, when present along an electrode track, this did not change (Fig. 5, C and D). In a minority of ventral sites, the initial response was negative, but, since these sites were sparse, we did not analyze them further. As with the motor potential, we plot the topographic distribution of the initial negative component of sensory responses throughout the gray matter for a representative individual animal (Fig. 5E) and for the group (Fig. 5H).
For sDCS modulation of the sensory-evoked spinal potential, the dominant effect on the population of recordings was anodal suppression of the response (i.e., less activation). Whereas anodal stimulation diminished the response significantly (87 ± 5 µV; signed rank, P < 0.05), the action of c-sDCS on the dorsal response was not significant (−19 ± 9 µV). The effects of c-sDCS and a-sDCS on the distribution of sensory responses in a typical animal and population are shown in Fig. 5, F and G and I and J, respectively. The negative deflection peak is color-coded according to the stimulation site. The dorsomedial region of the initial large negative deflection corresponds closely to the afferent fiber termination field (Jiang et al. 2016). The initial negative deflection laterally and ventrally tended to be greater than zero, hence the red color. As described above, this likely reflects changes in the ventral-lateral current source (i.e., repolarization) for the early dorsal negative response. Similar to the M1-evoked response, a two-way ANOVA of the peripheral stimulation-evoked response, showed significant effects for the two main factors (sDCS and ROI) and an interaction between them (FsDCS = 74.11, P = 0; FROI = 4.13, P = 0.043; Finteraction = 16.55, P = 0.0001). This suggests that the sensory LFP response was ROI specific, and amplitude modulation was polarity dependent, and that the two factors (ROI and sDCS) interact with each other.
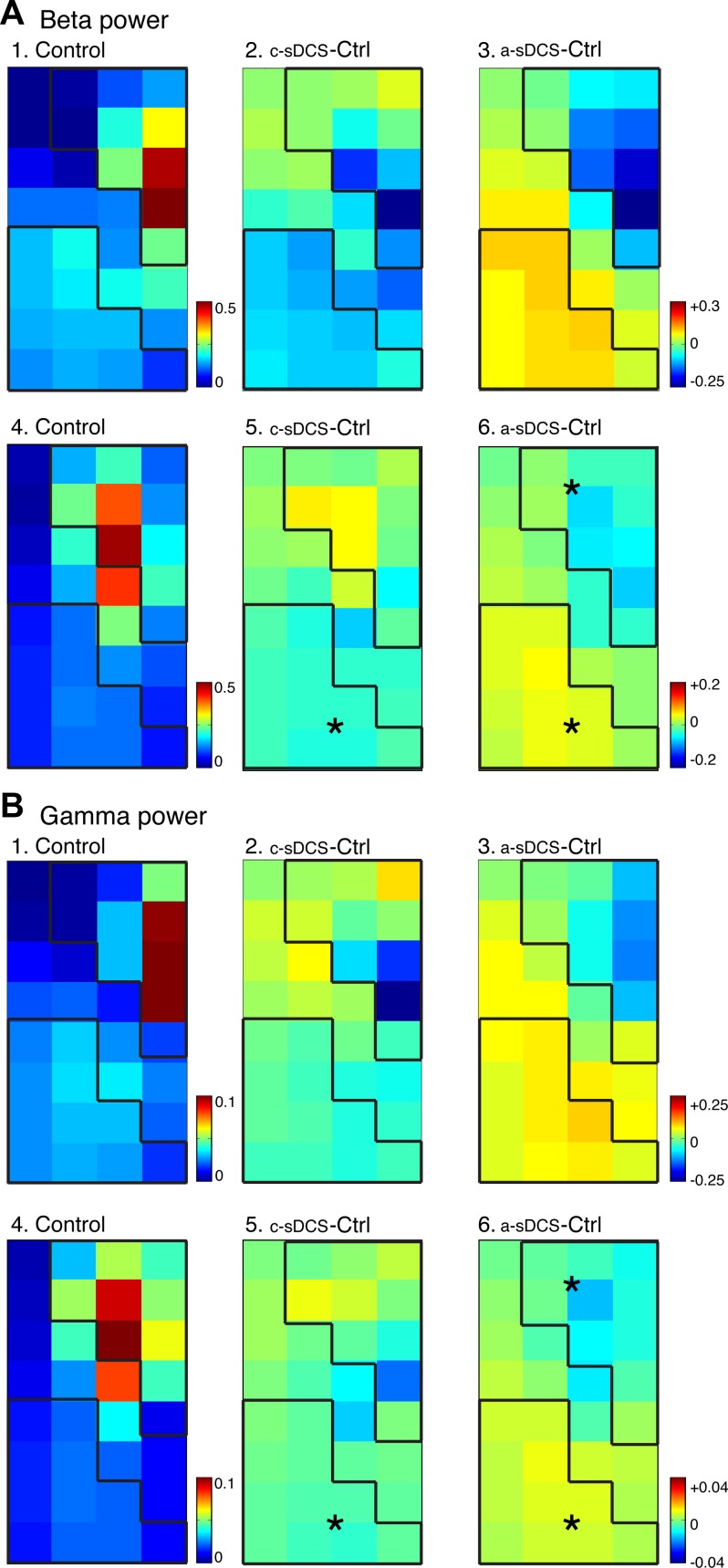
β-Oscillations were stronger than γ-oscillations and, for both, were localized in the dROI (Fig. 6, A1 and B1, for a representative animal; and A4 and B4 for the group). This suggests, as for M1-evoked changes, that sensory-evoked changes in oscillations are dominated by the direct projection of afferent fibers. The mean spectral power of the entire map after afferent stimulation was somewhat larger than after M1 stimulation in the control condition (afferent: β, 0.146 ± 0.015 mV2/Hz; γ, 0.031 ± 0.003 mV2/Hz; M1: β; 0.117 ± 0.008 mV2/Hz; γ, 0.028 ± 0.002 mV2/Hz). a-sDCS significantly reduced β-(−0.036 ± 0.012 mV2/Hz) and γ-power (−0.007 ± 0.001 mV2/Hz) within the dROI (A5 and A6; B5 and B6; signed rank, P < 0.05), and both polarities modulated ventral β-power (a-sDCS: 0.032 ± 0.004 mV2/Hz; c-sDCS: −0.032 ± 0.005 mV2/Hz) and γ-power (a-sDCS: 0.006 ± 0.001 mV2/Hz; c-sDCS: −0.004 ± 0.001 mV2/Hz). Only γ-power showed ROI effects from the two-way ANOVA, while both β- and γ-power showed significant interactions between ROI and sDCS (β: FsDCS = 1.57, P = 0.212; FROI = 3.16, P = 0.077; Finteraction = 38.55, P = 0; γ: FsDCS = 2.4, P = 0.123; FROI = 11.92, P = 0.0007; Finteraction = 21.31, P = 0).
Fig. 6.
Topographic effects of sDCS on spinal oscillations evoked by afferent electrical stimulation in the β- (A) and γ-ranges (B). Top rows for A and B show data from a representative animal, and the bottom rows show averaged data (n = 5 rats). A1: control map of β-oscillation power. A2 and A3: changes induced by c-sDCS and a-sDCS, respectively. A4: average control map of β-oscillation power. A5 and A6: average maps of changes induced by c-sDCS and a-sDCS, respectively. B: same as A but for γ-band oscillations. Calibrations: A1, 4. 0–0.5 mV2/Hz; A2, A3, A5, and A6, −0.2 to +0.2 mV2/Hz; B1, 4. 0–0.1 mV2/Hz; B2, B3, B5, and B6, −0.04 to +0.04 mV2/Hz. *Significant changes from control in the ROIs.
To characterize the effect of sDCS on firing rate of individual units and synchrony between unit pairs, we constructed PSTHs of individual unit activity and synchrony between unit pairs. Representative samples are shown (Fig. 7, A–C). In contrast to responses to M1 stimulation, where there were polarity-dependent differences, generally the effects of c-sDCS and a-sDCS were of a similar direction for the sensory responses. And, in contrast to the M1-evoked unit activity (Fig. 4), where the dominant effect was anodal suppression, for sensory-evoked unit firing we noted increased activity with each polarity. Other more subtle differences between motor and sensory responses were noted, including sDCS increased firing rate and synchrony of sensory responses in the vROI (Fig. 7, D and F), and more units were excited in vROI than dROI (Fig. 7F), while there was significant decrease in synchrony between unit pairs in the dROI during a-sDCS (Fig. 7E, signed rank, P < 0.05).
DISCUSSION
Transspinal DCS is a therapeutic neuromodulatory tool that can be applied to the surface of the body. Our study advances our understanding of the mechanism of action of transspinal DCS on corticospinal motor system function. From M1 to muscle, the responses of the circuit are augmented by cathodal DCS. The firing rate of single units to M1 stimulation is enhanced, similar to changes in background firing, and there is greater polarity-dependent synchronization of activity. M1-evoked LFPs showed a polarity-dependent and region-specific enhancement. β- and γ-M1-evoked oscillations were enhanced. With increased oscillations, we hypothesize that overall network function also is enhanced, which is suggested by the task-dependent changes in β- and γ-frequency band coherence between sensorimotor cortex and spinal motoneurons during precision control of motor output (Mehrkanoon et al. 2014; Omlor et al. 2011). During the period of c-sDCS, this set of actions strengthens a spinal motor output triggered by M1. Anodal stimulation had a weaker effect compared with cathodal stimulation and, when present, the effect was generally to suppress activity.
We also examined the cervical response to proprioceptive system activation. Focusing on the dorsal horn, which is both the major recipient of this afferent input and is the major origin of ascending afferent projection systems (Brown 1981), LFPs were unchanged during cathodal stimulation and suppressed during anodal stimulation. Network oscillations showed a small increase with anodal stimulation. Since a-sDCS suppressed afferent-evoked LFPs, the stronger oscillations with this stimulation may be due to disinhibition (i.e., reduced responses of spinal inhibitory interneurons). Surprisingly, single-unit peripheral-evoked responses were facilitated with either polarity.
Multisite recording of the spinal cord in response to M1 stimulation.
This is the first in vivo multisite recording of M1-evoked responses in the spinal cord. One goal of this study was to determine the simultaneous actions of M1/CST on the various laminae of the contralateral cervical spinal cord. The distribution of large-amplitude initially negative deflection is located dorsomedial, within the territory of densest CST projections from the forelimb representation of M1 (Jiang et al. 2016). It is likely that this initial response is dominated by the monosynaptic CST connection. It should be noted that M1 also projects to brain stem targets that, in turn, project to the spinal cord, and contributions by indirect pathways cannot be excluded. The ventrolateral response is localized within the motor pools and ventral lamina 7, which is outside the territory of dense CST projections (Jiang et al. 2016). Since there is no significant monosynaptic CST responses on motoneurons in rats (Jiang et al. 2016; Yang and Lemon 2003), this ventral response likely reflects oligosynaptic CST excitation of motoneurons. Our findings show that virtually the entire contralateral cervical gray matter becomes transiently activated after a single M1 epidural stimulus pulse.
The second, and principal, goal of this study was to use the multiarray recording to inform the action of sDCS on two major inputs to cervical segmental circuits. We were particularly interested in why c-sDCS can augment M1 motor output (Ahmed 2011; Song et al. 2015; Wallace et al. 1987). We were also interested in understanding why c-sDCS neuromodulation, together with phasic M1 stimulation, promoted CST sprouting and skilled locomotor recovery after pyramidal tract lesion (Song et al. 2016). To this end, we found that c-sDCS increased dorsal and ventral unit activity and unit pair synchrony in response to M1 stimulation. Ventral, not dorsal, initially-negative LFPs were robustly augmented, as were oscillations in the β- and γ-bands. Together, these effects of c-sDCS would be expected to increase M1 throughput, defined as the ability of M1 stimulation to activate spinal motor circuits and produce a motor response. For long-term therapeutic effects, we hypothesize that increased throughput during daily treatment sessions summate and, together with sDCS aftereffects, result in stronger and more extensive CST connections (Song et al. 2016).
a-sDCS suppresses sensory-evoked responses.
Afferent fiber stimulation, to activate skin and muscle fibers, produces an initial dorsal response that is well localized to the distribution of afferent terminations in the dorsal horn (Brown 1981; Jiang et al. 2016; Tan et al. 2012). The major effect on sensory potentials was suppression during anodal stimulation. Dorsomedially, this may be a reduction in the strength of the monosynaptic afferent fiber connection. Ventrally/ventrolaterally, the origin of the response is not clear, as there are both direct excitatory ventral afferent projection as well as oligosynaptic excitatory and inhibitory connections. Bolzoni and Jankowska noted that c-sDCS applied locally to the recording site enhanced the 1a EPSP in lumbar motoneurons (Bolzoni and Jankowska 2015). Our study stresses minimal changes to afferent signals relative to the robust facilitation of M1-evoked responses. Local application of sDCS, as in the Bolzoni and Jankowska (2015) study, vs. a more distant site, as in ours, may contribute to this difference. But it is important to consider that we examined the effect of DC current at the population level, which may not detect localized synaptic changes. To tease out these complex effects will require better modeling of current flow during sDCS (Song et al. 2015) and selective activation of particular excitatory and inhibitory spinal networks. The effects of sDCS on afferent-evoked unit activity and synchrony between unit pairs were complex and did not necessarily parallel changes in LFPs. It remains to be determined how the biophysical actions of neuromodulatory sDCS differentially affect unit activity and LFP/synaptic activity. However, the paucity of modulation of afferent-evoked LFP activity in this study is paralleled by a similar lack of a strong effect of c-sDCS on afferent transmission (Song et al. 2015).
c-DCS enhances motor-evoked spinal neural oscillations.
We measured spinal neural oscillations in response to M1 and afferent stimulation as a way to assess the effects of sDCS modulation on spinal motor and sensory networks. This is the first study to examine spinal oscillations, and we do so by simultaneously recording from different layers of spinal neurons. Neural oscillations are thought to reflect interactions between neurons comprising the active network (Pfurtscheller and Lopes da Silva 1999). Based on studies in cortex, oscillations in the γ-band are thought to arise mostly from local network actions and, in the β-band, longer distance interactions (Buzsáki et al. 2012). Enhanced network oscillations are thought to confer stronger communication and improved network function, as well as the excitability of the neurons themselves. Slow cortical oscillations, for example, enhance visual cortex neuron responses (Haider et al. 2007). The reciprocal changes in background unit activity with a-sDCS and c-sDCS may reflect network function secondary to neuromodulation. We envision a similar network structure for the spinal cord. Spinal γ-oscillations, which are likely to be local, based on cortical modeling studies (Paik et al. 2009), might function to synchronize motor output and binding with descending input, similar to what has been described in cortex (Paik et al. 2009). Since c-sDCS enhanced β- and γ-oscillations following M1 stimulation, we hypothesize that this contributes to the enhanced ventral neural responses and, in consequence, stronger M1-to-muscle throughput. Compared with the effect of ROI on the M1-evoked spinal oscillation, the effect of sDCS is more consistent and stronger (see F values in the two-way ANOVA of both β- and γ-power). In contrast, peripheral stimulation-evoked effects on spinal oscillations show a more consistent and stronger effect of ROI than that of sDCS. Together this stresses the efficacy of sDCS in corticospinal motor circuit neuromodulation.
M1-evoked changes in unit activity during sDCS parallel the effects on EMG activation.
The analysis of unit activity and synchrony between neurons provides additional information to help understand the effect of the sDCS on spinal networks. The area under the PSTH response curve is thought to represent the efficacy of synaptic transmission (Abeles 1991), which agrees with the LFP amplitude change and the increase in network oscillations. Both would tend to facilitate M1-to-muscle efficacy, which we assayed using M1 stimulation. Firing rate (background and evoked) and unit pair synchrony represent different circuit mechanisms. The polarity-dependent effect of sDCS was not only on the firing rate of individual units, but also in the synchrony between unit pairs, where c-sDCS increased and a-sDCS decreased the responses. Synchrony of unit pair and LFP oscillations are strongly correlated (Engel et al. 2001), and thus the polarity-dependent influence on LFPs agrees with the synchrony between unit pairs. Increased motor unit synchronization, which reflects increased synchronization between motoneurons, was found to increase EMG activity (Yao et al. 2000). This too could explain the polarity-dependent effect of sDCS on motor-evoked potentials in muscle (Song et al. 2016).
Systems-level mechanisms of sDCS neuromodulation of motor output.
The changes in the evoked motor and sensory responses produced by anodal and cathodal currents have clinical significance. Potentiating motor strength with cathodal stimulation can be used to augment weakened M1-to-muscle circuit connections after a spinal cord injury, much like epidural stimulation (Angeli et al. 2014). Our findings suggest that ventral horn neurons are an important locus for mediating this effect. A preferential ventral spinal action for c-sDCS provides further justification for combining this neuromodulatory approach with therapeutic M1 stimulation to target spinal neuron and CST structural plasticity, respectively (Song et al. 2016). This ventral action may reflect hyperpolarization of distal dendrites of motoneurons and depolarization of the cell body, similar to the simulated polarization of layer 5 pyramidal neurons produced by transcranial DCS (Rahman et al. 2013). Because the actions of c-sDCS on the afferent responses were minimal, it is unlikely that c-sDCS would mitigate therapeutic strengthening of the corticospinal motor system. Of potential clinical relevance is the use of anodal stimulation, which reduced peripheral-evoked LFP responses in the dorsal horn, to abrogate maladaptive sensory responses that contribute to muscle afferent fiber sprouting and hyperreflexia after CST injury (Tan et al. 2012). With a greater understanding of the patterns of spinal current flow (Song et al. 2015) and the regional physiological actions of sDCS, it is hoped that sDCS can be developed into a tunable spinal neuromodulatory tool for promoting function after spinal injury.
GRANTS
This work was supported by the National Institute of Neurological Disorders and Stroke (2R01NS-064004; J. H. Martin), the Craig H. Neilsen Foundation (no. 261214; J. H. Martin), and New York School Department of Health Spinal Cord Injury Board (C30606GG and C30835GG; J. H. Martin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.S. and J.H.M. conceived and designed research; W.S. performed experiments; W.S. analyzed data; W.S. and J.H.M. interpreted results of experiments; W.S. and J.H.M. prepared figures; W.S. and J.H.M. drafted manuscript; W.S. and J.H.M. edited and revised manuscript; W.S. and J.H.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Xiuli Wu for histology support.
REFERENCES
- Abeles M. Corticonics: Neural Circuits of the Cerebral Cortex. Cambridge, UK: Cambridge University Press, 1991. doi: 10.1017/CBO9780511574566 [DOI] [Google Scholar]
- Aguilar J, Pulecchi F, Dilena R, Oliviero A, Priori A, Foffani G. Spinal direct current stimulation modulates the activity of gracile nucleus and primary somatosensory cortex in anaesthetized rats. J Physiol 589: 4981–4996, 2011. doi: 10.1113/jphysiol.2011.214189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z. Trans-spinal direct current stimulation modulates motor cortex-induced muscle contraction in mice. J Appl Physiol (1985) 110: 1414–1424, 2011. doi: 10.1152/japplphysiol.01390.2010. [DOI] [PubMed] [Google Scholar]
- Ahmed Z. Trans-spinal direct current stimulation modifies spinal cord excitability through synaptic and axonal mechanisms. Physiol Rep 2: e12157, 2014. doi: 10.14814/phy2.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137: 1394–1409, 2014. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci T, Vannini B, Torzini A, Mazzatenta A, Vergari M, Cogiamanian F, Priori A, Sartucci F. Cathodal transcutaneous spinal direct current stimulation (tsDCS) improves motor unit recruitment in healthy subjects. Neurosci Lett 578: 75–79, 2014. doi: 10.1016/j.neulet.2014.06.037. [DOI] [PubMed] [Google Scholar]
- Bolzoni F, Jankowska E. Presynaptic and postsynaptic effects of local cathodal DC polarization within the spinal cord in anaesthetized animal preparations. J Physiol 593: 947–966, 2015. doi: 10.1113/jphysiol.2014.285940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG. The Organization of the Spinal Cord: The Anatomy and Physiology of Identified Neurones. New York: Springer, 1981. doi: 10.1007/978-1-4471-1305-8 [DOI] [Google Scholar]
- Brus-Ramer M, Carmel JB, Martin JH. Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. J Neurosci 29: 6196–6206, 2009. doi: 10.1523/JNEUROSCI.5852-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat Rev Neurosci 13: 407–420, 2012. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogiamanian F, Vergari M, Pulecchi F, Marceglia S, Priori A. Effect of spinal transcutaneous direct current stimulation on somatosensory evoked potentials in humans. Clin Neurophysiol 119: 2636–2640, 2008. doi: 10.1016/j.clinph.2008.07.249. [DOI] [PubMed] [Google Scholar]
- Cogiamanian F, Vergari M, Schiaffi E, Marceglia S, Ardolino G, Barbieri S, Priori A. Transcutaneous spinal cord direct current stimulation inhibits the lower limb nociceptive flexion reflex in human beings. Pain 152: 370–375, 2011. doi: 10.1016/j.pain.2010.10.041. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J Neurosci 19: 4595–4608, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2: 704–716, 2001. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Fu TC, Santini M, Schomburg ED. Characteristics and distribution of spinal focal synaptic potentials generated by group II muscle afferents. Acta Physiol Scand 91: 298–313, 1974. doi: 10.1111/j.1748-1716.1974.tb05686.x. [DOI] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, Yu Y, McCormick DA. Enhancement of visual responsiveness by spontaneous local network activity in vivo. J Neurophysiol 97: 4186–4202, 2007. doi: 10.1152/jn.01114.2006. [DOI] [PubMed] [Google Scholar]
- Heide AC, Winkler T, Helms HJ, Nitsche MA, Trenkwalder C, Paulus W, Bachmann CG. Effects of transcutaneous spinal direct current stimulation in idiopathic restless legs patients. Brain Stimulat 7: 636–642, 2014. doi: 10.1016/j.brs.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Hernández-Labrado GR, Polo JL, López-Dolado E, Collazos-Castro JE. Spinal cord direct current stimulation: finite element analysis of the electric field and current density. Med Biol Eng Comput 49: 417–429, 2011. doi: 10.1007/s11517-011-0756-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Kaczmarek D, Bolzoni F, Hammar I. Evidence that some long-lasting effects of direct current in the rat spinal cord are activity-independent. Eur J Neurosci 43: 1400–1411, 2016. doi: 10.1111/ejn.13238. [DOI] [PubMed] [Google Scholar]
- Jiang YQ, Zaaimi B, Martin JH. Competition with primary sensory afferents drives remodeling of corticospinal axons in mature spinal motor circuits. J Neurosci 36: 193–203, 2016. doi: 10.1523/JNEUROSCI.3441-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr Clin Neurophysiol 86: 283–293, 1993. doi: 10.1016/0013-4694(93)90110-H. [DOI] [PubMed] [Google Scholar]
- Mehrkanoon S, Breakspear M, Boonstra TW. The reorganization of corticomuscular coherence during a transition between sensorimotor states. Neuroimage 100: 692–702, 2014. doi: 10.1016/j.neuroimage.2014.06.050. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Perez MA, Oudega M, Enriquez-Denton M, Aimonetti JM. Evaluation of transcranial magnetic stimulation for investigating transmission in descending motor tracts in the rat. Eur J Neurosci 25: 805–814, 2007. doi: 10.1111/j.1460-9568.2007.05326.x. [DOI] [PubMed] [Google Scholar]
- Niérat MC, Similowski T, Lamy JC. Does trans-spinal direct current stimulation alter phrenic motoneurons and respiratory neuromechanical outputs in humans? A double-blind, sham-controlled, randomized, crossover study. J Neurosci 34: 14420–14429, 2014. doi: 10.1523/JNEUROSCI.1288-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omlor W, Patino L, Mendez-Balbuena I, Schulte-Mönting J, Kristeva R. Corticospinal beta-range coherence is highly dependent on the pre-stationary motor state. J Neurosci 31: 8037–8045, 2011. doi: 10.1523/JNEUROSCI.4153-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik SB, Kumar T, Glaser DA. Spontaneous local gamma oscillation selectively enhances neural network responsiveness. PLOS Comput Biol 5: e1000342, 2009. doi: 10.1371/journal.pcbi.1000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotta A, Bolla M, Anastasio MG, Serrao M, Sandrini G, Pierelli F. Modulation of temporal summation threshold of the nociceptive withdrawal reflex by transcutaneous spinal direct current stimulation in humans. Clin Neurophysiol 127: 755–761, 2016. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110: 1842–1857, 1999. doi: 10.1016/S1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord. Cambridge, UK: Cambridge University Press, 2005, p. 642. doi: 10.1017/CBO9780511545047 [DOI] [Google Scholar]
- Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, Bikson M. Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol 591: 2563–2578, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural frequencies of human corticothalamic circuits. J Neurosci 29: 7679–7685, 2009. doi: 10.1523/JNEUROSCI.0445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Amer A, Ryan D, Martin JH. Combined motor cortex and spinal cord neuromodulation promotes corticospinal system functional and structural plasticity and motor function after injury. Exp Neurol 277: 46–57, 2016. doi: 10.1016/j.expneurol.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Francis JT. Tactile information processing in primate hand somatosensory cortex (S1) during passive arm movement. J Neurophysiol 110: 2061–2070, 2013. doi: 10.1152/jn.00893.2012. [DOI] [PubMed] [Google Scholar]
- Song W, Truong DQ, Bikson M, Martin JH. Transspinal direct current stimulation immediately modifies motor cortex sensorimotor maps. J Neurophysiol 113: 2801–2811, 2015. doi: 10.1152/jn.00784.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Chakrabarty S, Kimura H, Martin JH. Selective corticospinal tract injury in the rat induces primary afferent fiber sprouting in the spinal cord and hyperreflexia. J Neurosci 32: 12896–12908, 2012. doi: 10.1523/JNEUROSCI.6451-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk BC, Beek PJ, Daffertshofer A. Neural synchrony within the motor system: what have we learned so far? Front Hum Neurosci 6: 252, 2012. doi: 10.3389/fnhum.2012.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MC, Tator CH, Piper I. Recovery of spinal cord function induced by direct current stimulation of the injured rat spinal cord. Neurosurgery 20: 878–884, 1987. doi: 10.1227/00006123-198706000-00010. [DOI] [PubMed] [Google Scholar]
- Yang HW, Lemon RN. An electron microscopic examination of the corticospinal projection to the cervical spinal cord in the rat: lack of evidence for cortico-motoneuronal synapses. Exp Brain Res 149: 458–469, 2003. doi: 10.1007/s00221-003-1393-9. [DOI] [PubMed] [Google Scholar]
- Yao W, Fuglevand RJ, Enoka RM. Motor-unit synchronization increases EMG amplitude and decreases force steadiness of simulated contractions. J Neurophysiol 83: 441–452, 2000. [DOI] [PubMed] [Google Scholar]