Sensory systems, including the olfactory system, encode information across a large dynamic range, making synaptic mechanisms of gain control critical to proper function. Here we demonstrate that a dual-transmitter interneuron in the olfactory bulb controls the gain of intraglomerular afferent input via two distinct mechanisms, presynaptic inhibition as well as inhibition of a principal neuron subtype, and thereby potently controls the synaptic gain of afferent inputs.
Keywords: dopamine, GABA, olfactory bulb, short axon cell, fast-scanning cyclic voltammetry
Abstract
In the olfactory bulb, lateral inhibition mediated by local juxtaglomerular interneurons has been proposed as a gain control mechanism, important for decorrelating odorant responses. Among juxtaglomerular interneurons, short axon cells are unique as dual-transmitter neurons that release dopamine and GABA. To examine their intraglomerular function, we expressed channelrhodopsin under control of the DAT-cre promoter and activated olfactory afferents within individual glomeruli. Optical stimulation of labeled cells triggered endogenous dopamine release as measured by cyclic voltammetry and GABA release as measured by whole cell GABAA receptor currents. Activation of short axon cells reduced the afferent presynaptic release probability via D2 and GABAB receptor activation, resulting in reduced spiking in both mitral and external tufted cells. Our results suggest that short axon cells influence glomerular activity not only by direct inhibition of external tufted cells but also by inhibition of afferent inputs to external tufted and mitral cells.
NEW & NOTEWORTHY Sensory systems, including the olfactory system, encode information across a large dynamic range, making synaptic mechanisms of gain control critical to proper function. Here we demonstrate that a dual-transmitter interneuron in the olfactory bulb controls the gain of intraglomerular afferent input via two distinct mechanisms, presynaptic inhibition as well as inhibition of a principal neuron subtype, and thereby potently controls the synaptic gain of afferent inputs.
in the olfactory bulb, odorant identity is largely encoded in the spatial map of activated glomeruli (Rubin and Katz 1999; Wachowiak and Cohen 2001). One of the computational challenges of encoding odorant identity is the need to discriminate patterns of activated glomeruli, especially at high odor concentrations, where the spatial map is confounded by weak activation of many glomeruli (Cleland 2010). Lateral inhibition between glomeruli may serve this function by filtering out weakly activated glomeruli (Banerjee et al. 2015; Cleland 2010), thereby increasing the signal-to-noise ratio. Short axon cells release both dopamine and GABA and broadly connect multiple glomeruli and thus are well positioned to mediate lateral inhibition across glomerular microcircuits (Banerjee et al. 2015; Borisovska et al. 2013; Kiyokage et al. 2010; Liu et al. 2013; Maher and Westbrook 2008; Whitesell et al. 2013). Although many recent studies have examined the postsynaptic contribution of short axon cells to olfactory processing (Banerjee et al. 2015; Liu et al. 2013, 2016; Whitesell et al. 2013), none has addressed the role of short axon cells in modulating the presynaptic terminal, which expresses D2 and GABAB receptors (Maher and Westbrook 2008). We examined the effects of endogenously released dopamine and GABA on afferent input to the olfactory bulb circuit, using optogenetic targeting in acute mouse brain slices. Endogenous dopamine and GABA reduced the olfactory receptor neuron (ORN)-evoked excitatory postsynaptic current (EPSC) in mitral cells and external tufted cells by a GABAB- and D2-mediated decrease in presynaptic release probability. Our results suggest that short axon cells have two distinct and computationally unique mechanisms to modulate the flow of information into the circuit: inhibition of external tufted cells and inhibition of presynaptic release.
METHODS
Animals.
We used male and female mice (C57Bl/6J; P24–42). To express channelrhodopsin (ChR)2 in dopaminergic short axon cells, a DATIREScre transgenic mouse line was crossed to the Ai32 ChR2-YFP reporter line. Because of a moderate loss of dopamine transporter (DAT) expression in homozygous mice (Bäckman et al. 2006), only heterozygous DATIREScre mice were used. The Oregon Health and Science University Institutional Animal Care and Use Committee approved all animal procedures.
Slice preparation and electrophysiology.
Acute brain slices were prepared as in Vaaga and Westbrook (2016). Whole cell voltage- and current-clamp recordings were made from mitral cells and external tufted cells; cell-attached recordings were made from ChR2+ short axon cells. Mitral cells and external tufted cells were distinguished morphologically as described previously (Hayar et al. 2005). ORN-evoked EPSCs were elicited with a theta electrode as in Vaaga and Westbrook (2016), with an interstimulus interval of 10 s. To optically stimulate ChR2+ short axon cells, LED illumination (2 ms, 470 nm; 16 mW/mm2) was provided through a ×40 objective, such that the maximal area of illumination was ~450 µm in diameter. Given that a single glomerulus is ~100 µm in diameter (Shepherd 2004), this field illumination is predicted to activate short axon cells associated with the intraglomerular (target) glomerulus ±2 glomeruli in either direction. Therefore, this illumination pattern is predicted to strongly activate intraglomerular inhibition from short axon cells associated with the target glomerulus. Trials to optically activate short axon cells included five LED flashes (2 ms each) at 10 Hz, 300 ms before ORN stimulation, unless otherwise noted. ORN-evoked responses were recorded with a potassium-based internal solution that contained (in mM) 130 K-gluconate, 20 KCl, 10 HEPES, 0.1 EGTA, 4 MgATP, 0.3 NaGTP, and 0.07–0.1 Alexa 594 hydrazide (osmolality adjusted to 295 mosM, pH adjusted to 7.2 with KOH). GABAergic currents were recorded with a cesium-based internal solution, which contained (in mM) 125 CsCl, 10 HEPES, 10 EGTA, 2 MgATP, 0.3 NaGTP, 10 phosphocreatine, and 0.07–0.1 Alexa 594 hydrazide (osmolality adjusted to 290 mosM, pH adjusted to 7.2 with CsOH). We made no correction for the liquid junction potential (−7 mV). The intracellular sodium channel blocker QX-314-Cl was included (5 mM) in voltage-clamp experiments. Cell-attached recordings were made with the K-gluconate internal and the pipette held at −70 mV after a gigaohm seal was achieved. All recordings were done at room temperature. Unless otherwise noted, cells were voltage clamped at −70 mV. Data were acquired with a Multiclamp 700B amplifier and AxographX acquisition software. Data was digitized at 10 kHz with a 4-kHz low-pass Bessel filter. Series resistance was continually monitored with a hyperpolarizing voltage step, and cells with >30% change were excluded from analysis. For current-clamp recordings, a hyperpolarizing bias current (−130 to −200 pA) was injected to maintain the membrane voltage at −60 ± 5 mV.
All drugs were bath applied by a recirculating pump. The drugs (Abcam Biosciences and Tocris Biochemicals) included SR95531 [2-(3-carboxypropyl)-3-amino-6(4 methoxyphenyl)pyridazinium bromide; 10 µM], CGP55845 {(2S)-3[((1S)-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid hydrochloride; 200 nM}, sulpiride {(±)-5-(aminosylfonyl)-N-[(1-ethyl-2-pyrrolidinyl)methyl]-2-methoxybenzamide; 500 nM}, NBQX (2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide; 10 µM), CPP [3-((R)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid; 10 µM], SKF97541 {1-[2-[3-(4-methoxyphenyl)propoxy]-2-(4-methyoxyphenyl)ethyl]-1H-imidazole hydrochloride}, quinpirole {(4-aR-trans)-4-4a,5,6,7,8,8a,9-octahydro-5-propyl-1H-pyrazolo[3,4-g]quinolone hydrochloride}, and SCH23390 [(R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride; 1 µM). All drugs were prepared as stock solutions according to manufacturer specifications.
Fast-scanning cyclic voltammetry.
Olfactory bulb slices were prepared as above; dorsolateral striatum slices were prepared as in Ford et al. (2010). Voltammetry recordings were collected and analyzed with Demon Voltammetry and Analysis (Yorgason et al. 2011) and IGOR Pro (WaveMetrics, Lake Oswego, OR). With DIC optics, carbon fiber electrodes (7 µm × 150 µm) were placed either in a glomerulus or into the dorsolateral striatum. The voltage across the carbon fiber electrode was linearly ramped in a triangular waveform (−0.4 V to 1.2 V) at a scan rate of 400 V/s. Cyclic voltammograms were recorded at 10 Hz and used to generate current traces by plotting the oxidation peak current (current at 0.6 V) as a function of time. Dopamine release was stimulated every 2–3 min optogenetically with a 20-pulse, 10-Hz, 2-ms LED protocol. Voltammetry current traces and cyclic voltammograms represent the average of at least three sweeps.
Immunofluorescence.
DATIREScre/WT;Rosa26LSL-ChR2-YFP/WT mice were anesthetized with an intraperitoneal injection of 2% 2,2,2-tribromoethanol and then transcardially perfused with saline followed by 4% paraformaldehyde (10–12 ml). According to standard immunohistochemistry procedures (Chatzi et al. 2015), sections (100 µm) were incubated in a mouse anti-tyrosine hydroxylase (TH) antibody (Sigma; monoclonal, 1:20,000) overnight at 4°C and then incubated with secondary antibodies (Life Technologies; goat anti-mouse, Alexa Fluor 555, 1:200) and a GFP antibody (Life Technologies; rabbit anti-GFP, Alexa Fluor 488, 1:500) for 2 h at room temperature. Sections were imaged on a Zeiss 780 confocal laser-scanning microscope.
Data analysis.
Electrophysiological data were analyzed in AxographX and IGOR Pro (version 6.22A, WaveMetrics). Current-clamp recordings were imported and analyzed with the Igor Neuromatic plugin (Jason Rothman, http://www.neuromatic.thinkrandom.com). All voltage-clamp traces represent the average of at least 10 sweeps after baseline subtraction. The peak EPSC amplitude was calculated with a built-in routine in AxographX. Action potentials were detected with a threshold criterion in Igor. At least 10 sweeps from a single cell in the control condition were averaged and used to normalize subsequent, within-cell manipulations. The onset latency (10% of peak amplitude) for GABAergic currents was calculated with a built-in Axograph routine from the time the LED stimulus terminated. Confocal data were analyzed and prepared in ImageJ (https://imagej.nih.gov/). For colocalization cell counts, random glomeruli were imaged and ChR2+/TH+ cell counts were performed on all imaged glomeruli from a single confocal section.
Statistics.
All data are reported as means ± SE unless otherwise noted. Statistical analyses were performed in Prism6 (GraphPad Software, La Jolla, CA). Distributions were tested for normality with the Shapiro-Wilk test for normality. Normally distributed data were analyzed with paired or unpaired t-tests as appropriate. Nonnormally distributed data were analyzed by Mann-Whitney test (unpaired data) or Wilcoxon matched-pairs signed-rank test (paired data). For repeated-measures experiments, an ANOVA (with a Dunnett’s post hoc test) or Friedman’s test (with a Dunn’s post hoc test) was performed. Sample sizes were chosen to detect an effect size of 20% and a power of 0.8. In all experiments, α was set to P < 0.05 and adjusted for multiple comparisons after post hoc tests.
RESULTS
Characterization and validation of ChR2 expression.
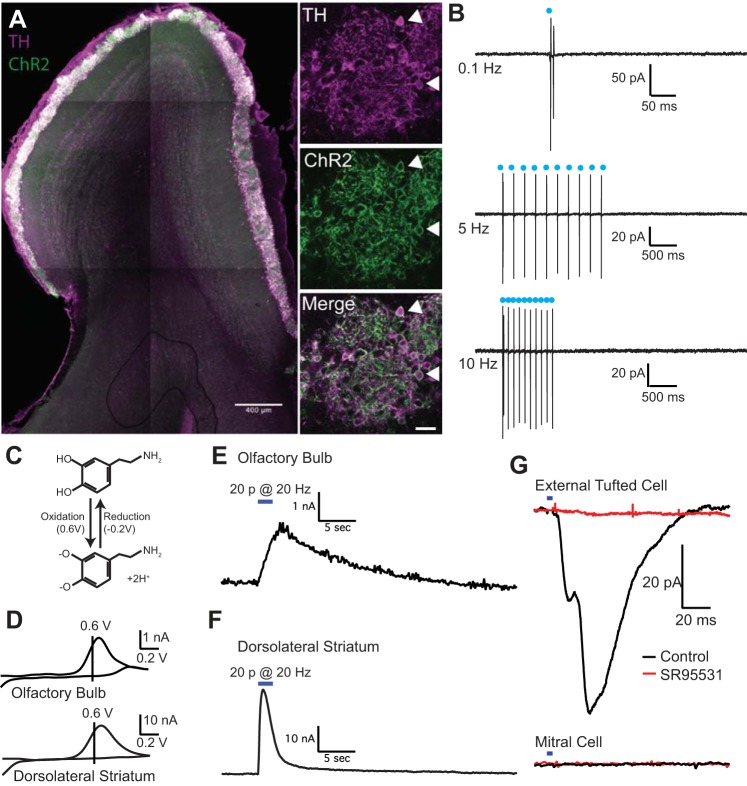
To ensure that ChR was properly targeted to dopaminergic short axon cells, we counterstained tissue from DATIREScre/WT;Rosa26LSL-ChR2-YFP/WT mice with antibodies against TH. As expected, ChR2 was expressed predominantly in the glomerular layer (Fig. 1A; Gall et al. 1987; Maher and Westbrook 2008), with 86.4 ± 1.0% of ChR2+ neurons colocalized with TH immunoreactivity (n = 641 cells, 4 animals) and 83.0 ± 1.1% of TH+ neurons (same cohort) colocalized with ChR2 (Fig. 1A). Consistent with the expression of TH in a subpopulation of external tufted cells (Gall et al. 1987), some external tufted cells also expressed ChR2 (Fig. 1A).
Fig. 1.
Optogenetic activation of short axon cells elicits endogenous dopamine and GABA release. A: expression of channelrhodopsin (ChR2, green) in short axon cells counterstained with tyrosine hydroxylase (TH, magenta). Double-labeled cells (white, indicated by arrowheads) were primarily located in the glomerular layer, with some TH+ external tufted cells in the juxtaglomerular external plexiform layer. B: cell-attached recordings from ChR2+ short axon cells. Optical stimulation (2 ms) reliably evoked spiking in cell-attached recordings at frequencies up to 10 Hz. C: electrochemical reaction demonstrating the cyclic oxidation and reduction of dopamine (top) to dopamine-o-quinone at characteristic voltages, which can be detected as a current with fast-scanning cyclic voltammetry. D: average cyclic voltammograms in olfactory bulb and dorsolateral striatum with oxidation and reduction peaks typical of dopamine. E and F: average oxidation current as a function of time in the olfactory bulb (E) and dorsolateral striatum (F) after 20 LED pulses at 10 Hz; the responses are plotted on the same timescale. G: optogenetic activation of short axon cells elicits a GABAA receptor mediated IPSC in external tufted cells (top, black) but not mitral cells (bottom, black). The IPSC is blocked by GABAA receptor antagonist SR95531 (red).
To determine how effectively ChR2 elicited spiking in ChR2+ cells, we made cell-attached recordings from ChR2+ short axon cells (Fig. 1B). A spike fidelity of 1 was defined as a single action potential per LED stimulus. At low stimulation frequencies (0.1 Hz), LED stimulation elicited multiple action potentials (cell-attached: 2.5 ± 0.5, n = 6 cells; Fig. 1B). At higher frequencies (10 Hz), the spiking was closer to a spike fidelity of 1 (10 Hz: 1.6 ± 0.3 spikes, n = 7 cells; Fig. 1B). Therefore, for all subsequent experiments, we used an LED frequency of 10 Hz, to ensure a high fidelity of action potential generation.
Endogenous release of dopamine and GABA.
To detect endogenous dopamine release from short axon cells we used fast-scanning cyclic voltammetry, which measures the cyclic oxidation and reduction of dopamine at characteristic voltages (Fig. 1C). Carbon fiber electrodes were placed in the tissue of interest with DIC optics; in the olfactory bulb, the electrode was placed in the center of a single glomerulus at an oblique angle. In both the olfactory bulb and dorsolateral striatum, optogenetic stimulation (20 pulses at 10 Hz) elicited cyclic voltammograms with oxidation peaks at 0.6 V and reduction peaks at −0.2 V, consistent with endogenous dopamine release (Fig. 1D). Interestingly, the voltammetric signals recorded in the olfactory bulb were much smaller (1.08 ± 0.22 nA; n = 12 slices from 3 animals) and slower (τ: 7.82 ± 1.04 s; n = 12 slices from 3 animals) than in the dorsolateral striatum (amplitude: 21.4 ± 4.4 nA; n = 6 slices from 1 animal; Mann-Whitney test: P = 0.001; τ: 1.2 ± 0.1 s; Mann-Whitney test: P = 0.001; Fig. 1, E and F).
To detect GABA release, we made whole cell recordings from external tufted cells or mitral cells. Optogenetic activation of short axon cells elicited an inward current in external tufted cells (41.1 ± 12.1 pA, n = 5 cells), which was blocked by the GABAA receptor antagonist SR95531 (2.2 ± 0.4 pA, n = 5 cells, Wilcoxon matched-pairs signed-rank test: P = 0.031). The kinetics of the GABAergic inhibitory postsynaptic current (IPSC) were consistent with monosynaptic GABAergic transmission (10% onset latency: 6.3 ± 0.8 ms). Interestingly, in five of six mitral cells examined, optogenetically evoked GABAergic currents were not detected [Mann-Whitney test (comparing external tufted cell and mitral cell IPSCs): P = 0.043; Fig. 1G], consistent with previous reports (Banerjee et al. 2015; Whitesell et al. 2013; but see Liu et al. 2016). Together, these data indicate that short axon cells release both dopamine and GABA but dendrodendritic activation of postsynaptic GABAA receptors is primarily restricted to external tufted cells.
Presynaptic GABAB and D2 attenuation by intraglomerular short axon cells.
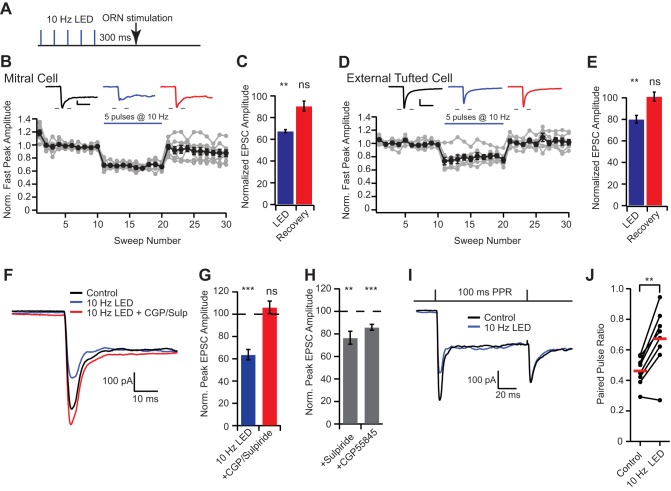
D2 and GABAB receptors are expressed on presynaptic terminals of olfactory receptor neurons, and exogenous agonist application can reduce release probability (Aroniadou-Anderjaska et al. 2000; Ennis et al. 2001; Hsia et al. 1999; Maher and Westbrook 2008; Wachowiak et al. 2005). To determine whether endogenous release from short axon cells can access the axodendritic glomerular compartment and alter ORN-evoked EPSCs, we paired optogenetic stimulation (5 pulses at 10 Hz) of short axon cells centered around the target glomerulus with theta electrode stimulation of ORN axons (0.1 ms, 100 V) at a delay of 300 ms to allow for sufficient G protein-coupled receptor activation (Fig. 2A). In mitral cells, ORN stimulation elicited a biphasic EPSC with a fast peak of 433.0 ± 76.7 pA (n = 8 cells), which was reversibly attenuated by optogenetic activation of short axon cells (10-Hz LED: 289.5 ± 48.4 pA, 67.4 ± 1.3% of control; Dunnett’s post hoc test: P < 0.01; recovery: 382.7 ± 60.1 pA, 90.6 ± 4.7% of control, Dunnett’s post hoc test: P > 0.05; Fig. 2, B and C). Similarly, in external tufted cells, ORN stimulation elicited an EPSC (930.1 ± 136.6 pA) that was reversibly attenuated by optogenetic activation of short axon cells (752.4 ± 116.6 pA, 80.2 ± 3.6% of control, n = 8 cells, Dunnett’s post hoc test: P < 0.01; recovery: 932.7 ± 128.9 pA; 101.2 ± 4.1% of control, Dunnett’s post hoc test: P > 0.05; Fig. 2, D and E). Interestingly, optogenetic stimulation of short axon cells did not significantly reduce the slow component (measured at 200 ms after stimulus) of the mitral cell EPSC (10-Hz LED: 88.9 ± 4.1% control, Dunnett’s post hoc test: P > 0.05; recovery: 93.9 ± 3.4% control, Dunnett’s post hoc test: P > 0.05) or external tufted cell EPSC (10-Hz LED: 84.6 ± 8.8% control, Dunnett’s post hoc test: P > 0.05; recovery: 105.5 ± 7.4% control; Dunnett’s post hoc test: P > 0.05). The selective attenuation of the peak EPSC suggests that D2 and GABAB activation alters the afferent ORN synapse without altering dendrodendritic release, which is consistent with the idea that the slow dendrodendritic current is all or none given sufficient afferent input (Carlson et al. 2000).
Fig. 2.
Short axon cells inhibit the presynaptic ORN terminal via D2 and GABAB metabotropic receptors. A: optogenetic protocol: 5 LED pulses at 10 Hz followed by ORN stimulation [300-ms interstimulus interval (ISI)]. This stimulation protocol was used for all subsequent experiments. B–E: diary plot of the normalized ORN-evoked fast EPSC amplitude (10-s ISI; LED stimulation as in A). Activation of short axon cells elicits a reversible attenuation in the ORN-evoked EPSC in mitral cells (B and C) and external tufted cells (D and E). F and G: short axon cell inhibition of ORN-evoked currents was blocked by GABAB and D2 receptor antagonists (100 nM CGP55845 and 500 nM sulpiride). H: short axon cell activation was capable of eliciting inhibition in the presence of either CGP55845 or sulpiride. I and J: activation of short axon cells alters the paired pulse ratio (PPR), suggesting changes in release probability from the ORN. Scale bar in B: 100 pA; 20 ms; scale bar in D: 200 pA; 20 ms. **P < 0.01; ***P < 0.001; ns, not significant.
In mitral cells, the peak attenuation was blocked by GABAB and D2 receptor antagonists CGP55845 (200 nM) and sulpiride (500 nM), respectively (LED: 63.7 ± 4.6 % of control, Dunnett’s post hoc test: P < 0.001; LED+CPG55845/sulpiride: 106.2 ± 5.6% of control, Dunnett’s post hoc test: P > 0.05, n = 7 cells; Fig. 2, F and G). Both receptors contributed to inhibition, as EPSCs were also reduced in either CGP55845 (LED+CGP55845: 85.9 ± 2.6% of control; Wilcoxon matched-pairs signed-rank test: P = 0.0002, n = 13 cells; Fig. 2H) or sulpiride (LED+sulpiride: 76.6 ± 5.7% of control; Wilcoxon matched-pairs signed-rank test: P = 0.008, n = 8 cells; Fig. 2H). Consistent with a presynaptic site of action, optogenetic stimulation of short axon cells significantly increased the paired-pulse ratio (control: 0.46 ± 0.04, LED: 0.67 ± 0.08, paired t-test: P = 0.002, n = 8 cells; Fig. 2, I and J).
Short axon cell activation elicits postsynaptic rebound firing in external tufted cells (Liu et al. 2013), which could activate periglomerular neurons, resulting in GABA release. To ensure that the presynaptic ORN inhibition observed was not a result of polysynaptic activation of periglomerular neurons, we repeated the experiments in the presence of the D1 receptor antagonist SCH23390 (1 µM), which blocks rebound spiking in external tufted cells. The presence of the D1 antagonist did not reduce the attenuation following short axon cell activation (65.1 ± 3.7% of control; n = 3 external tufted cells), suggesting that the GABAergic inhibition is a not a result of presynaptic pathways involving periglomerular cells.
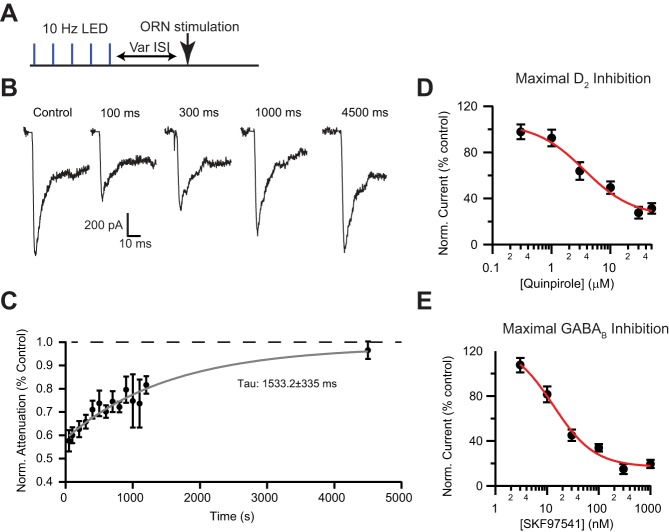
To examine the time course of the endogenous attenuation, we optogenetically activated short axon cells (5 pulses at 10 Hz) and then waited a variable time before stimulating the ORN afferents (50–4,500 ms; Fig. 3A). The onset of inhibition was fully developed by 50 ms, the shortest interval used. Consistent with a metabotropic response, the EPSC attenuation persisted for many hundreds of milliseconds (recovery time constant: 1,533.2 ± 335 ms; Fig. 3, B and C).
Fig. 3.
Time course of endogenous inhibition and maximal pharmacological inhibition. A: optogenetic stimulation protocol: 5 LED pulses at 10 Hz followed by ORN stimulation at various intervals (50–4,500 ms). B and C: time course of attenuation by optogenetic stimulation of short axon cells. Inhibition was maximal at the shortest intervals tested (50 ms) and recovered with a single exponential with a time constant (τ) of 1,533.2 ± 335 ms. Attenuation recovered to baseline levels by ~4,500 ms. D: pharmacological inhibition of afferent input by the D2 agonist quinpirole at various concentrations. E: pharmacological inhibition of afferent input by the GABAB agonist SKF97541.
To determine the relative strength of the endogenous inhibition, we compared endogenous inhibition with pharmacological activation of either D2 or GABAB receptors (quinpirole or SKF97541, respectively). Quinpirole produced a maximal inhibition of 69.7 ± 4.5% (Fig. 3D), whereas SKF97541 produced a maximal inhibition of 81.4 ± 3.6% (Fig. 3E). Therefore the maximal inhibition via presynaptic receptors is ~80%, as both D2 and GABAB are Gi/o-coupled receptors and likely act through the same presynaptic signaling cascade (Wachowiak et al. 2005). These data suggest that the endogenous inhibition (~40%) in our slice experiments is submaximal.
Effect of ORN attenuation on spiking patterns in mitral cells.
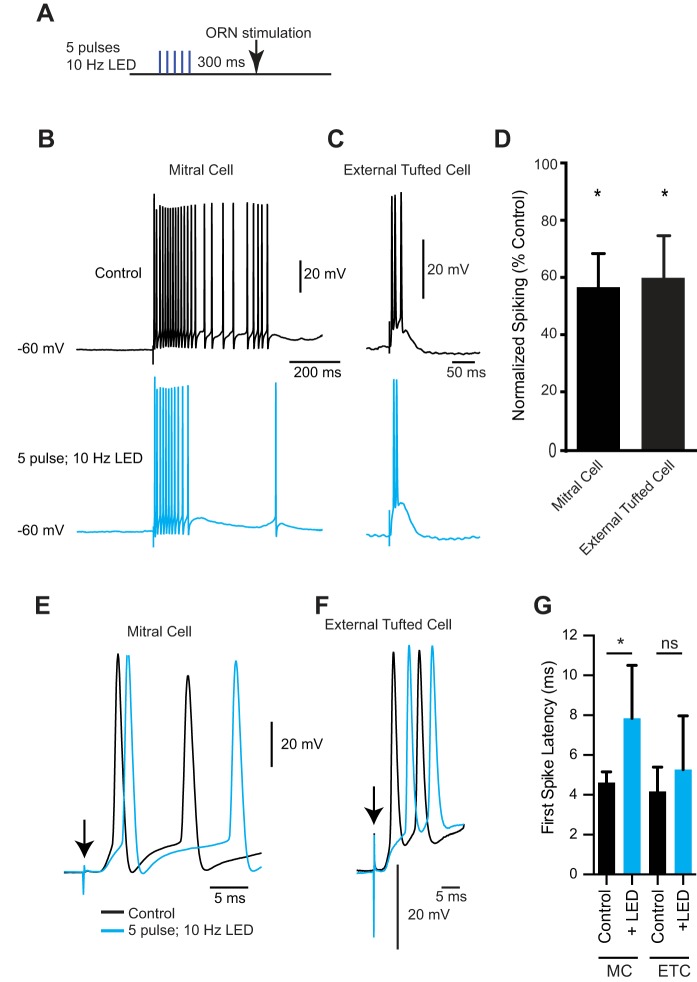
Activation of short axon cells in vivo can suppress action potential generation in mitral cells (Banerjee et al. 2015), which has been attributed to reductions in disynaptic activation of mitral cells. To determine whether presynaptic inhibition of ORNs also affects spiking in mitral cells, we utilized an optogenetic protocol (300 ms between LED and ORN stimulation) following the full decay of GABAA receptor-mediated currents (Fig. 4A). In external tufted cells, optogenetically evoked GABAergic inhibitory postsynaptic potentials (IPSPs) decayed with a time constant of 34.7 ± 2.8 ms and five IPSPs at 10 Hz decayed back to baseline within 107.3 ± 6.5 ms (n = 3 cells), well short of the 300-ms interval between optogenetic and electrical stimulation. Therefore, this optogenetic protocol was well suited to isolate the effects of metabotropic receptor-mediated responses on cell spiking. In mitral cells, optogenetic stimulation reduced the number of action potentials from 22.3 ± 6.3 to 16.9 ± 6.2 (44.4 ± 11.7% reduction; n = 8 cells; Wilcoxon matched-pairs signed-rank test: 0.016; Fig. 4, B and D) and was accompanied by a shift in the first spike latency (control: 4.6 ± 0.2 ms; 10-Hz LED: 7.8 ± 1.1 ms; Wilcoxon matched-pairs signed-rank test: 0.031; Fig. 4, E and G).
Fig. 4.
Presynaptic inhibition of mitral cell and external tufted cell afferents reduces spiking. A: optogenetic protocol: 5 LED pulses at 10 Hz followed by ORN stimulation (300-ms ISI). B: mitral cell spiking induced by ORN stimulation in control condition (top, black) and after short axon cell activation (bottom, blue). C: external tufted cell spiking induced by ORN stimulation in control condition (top, black) and after short axon cell activation (bottom, blue). D: optogenetic stimulation of short axon cells significantly reduced the ORN-evoked spiking in both mitral and external tufted cells. E and F: higher temporal resolution of the first couple of spikes elicited in control (black) and after LED stimulation (blue) in mitral cells (E) and external tufted cells (F). G: optogenetic stimulation of short axon cells significantly increased the first spike latency in mitral cells (MC) but not external tufted cells (ETC); *P < 0.05..
Similarly, in external tufted cells, optogenetic stimulation reduced the number of action potentials from 4.6 ± 1.3 to 3.6 ± 1.4 (32.0 ± 14.6% reduction; n = 5 cells, Wilcoxon matched-pairs signed-rank tests: 0.031; Fig. 4, C and D). This was accompanied by a trend toward a longer first spike latency; however, this was not statistically significant (control: 4.2 ± 0.5 ms; 10-Hz LED: 5.3 ± 1.1 ms, n = 7 cells; Wilcoxon matched-pairs signed-rank test: 0.094; Fig. 4, F and G) Together these results suggest that endogenous short axon cell activation can result in reduced ORN-evoked spiking in mitral and external tufted cells.
DISCUSSION
Our results demonstrate that short axon cells directly inhibit the presynaptic ORN terminal via metabotropic D2 and GABAB receptors as well as inhibiting external tufted cells via GABAAR-mediated currents (as in Whitesell et al. 2013; Liu et al. 2013). These two forms of inhibition are distinct because of their location of expression, predicted effects on circuit dynamics, and kinetic profiles. In summary, short axon cell activation may potently control the strength of afferent input, through both dendrodendritic and axodendritic synapses.
Short axon cell inhibition across timescales.
Short axon cell activation can inhibit the olfactory bulb circuit over multiple timescales. Ionotropic GABAergic inhibition of external tufted cells persists for a few tens of milliseconds. Functionally, external tufted cells not only project to higher areas of olfactory cortex (Igarashi et al. 2012) but also provide extensive feedforward excitation to local glomerular interneurons and mitral cells (Hayar et al. 2005). Therefore, the rapid, GABAA receptor-mediated inhibition of external tufted cells not only inhibits external tufted cell output directly but may also reduce disynaptic activation of mitral cells, a critical component of synaptic amplification within the circuit. On a timescale of hundreds of milliseconds, our experiments show that endogenous dopamine and GABA release can activate presynaptic D2 and GABAB metabotropic receptors, reducing glutamate release from the presynaptic terminal, most likely by reducing calcium currents in ORNs (Wachowiak et al. 2005). Our data suggest that presynaptic inhibition of the ORN reduces spike generation in both mitral and external tufted cells. By inhibiting monosynaptic responses in both mitral and external tufted cells, presynaptic inhibition provides a distinct form of inhibition on a longer timescale than ionotropic GABA conductances.
This temporal disparity may be further accentuated by the long-lasting vesicular release of dopamine from short axon cells, which in cultured short axon cells lasts for many hundreds of milliseconds (Borisovska et al. 2013). The extended time course of dopamine release may explain the slow kinetics of voltammetric dopamine signals observed in the olfactory bulb. Whether dopamine acts via point-to-point or volume transmission in the olfactory bulb is not known; however, the slow envelope of the voltammetric signal suggests that dopamine may inhibit the circuit for many seconds, prolonging presynaptic inhibition. Furthermore, in the olfactory bulb dopamine uptake may be minimal and clearance may be mediated by catechol-O-methyltransferase, further slowing the dopamine signal (Cockerham et al. 2016).
The strength of presynaptic inhibition by short axon cells may be dynamically modulated over slow timescales. The expression level of TH in short axon cells is dependent on olfactory activity (Baker et al. 1983, 1993), suggesting that the overall dopamine tone within the olfactory bulb may serve as a mechanism to control the gain of afferent olfactory input on a timescale of days to weeks. Consistent with this hypothesis, increases in dopamine cell density in sporadic Parkinson’s disease are accompanied by olfactory deficits including anosmia (Doty 2012; Huisman et al. 2004; Mundiñano et al. 2011). Our data suggest that the endogenous short axon cell inhibition is not saturated, at least under our experimental conditions. Although endogenous release may never fully saturate presynaptic receptors, these data suggest that the dynamic range of presynaptic inhibition may be quite large. Future studies examining whether short axon cell inhibition is larger when the density of TH+ short axon cells is higher, as found in Parkinson’s disease patients, could provide novel insights into the mechanism of anosmia in Parkinson’s disease.
Short axon cell inhibition in multiple glomerular compartments.
Multiple experimental factors may account for the relatively modest presynaptic inhibition seen in our experiments. Although individual short axon cells connect between 5 and 100 glomeruli (Kiyokage et al. 2010), some have hypothesized that functionally short axon cells form an all-to-all inhibitory network by heavily interconnecting multiple glomeruli (Cleland 2010). Such an arrangement may provide a neurophysiological mechanism to produce odorant decorrelation, by inhibiting inputs of weakly activated glomeruli, thereby increasing the odorant-evoked signal-to-noise ratio across glomeruli. Such a circuit arrangement has been proposed, in part, because odorant chemotopy at the level of individual glomeruli is not present (Cleland 2010; Soucy et al. 2009). Therefore, a neurophysiological mechanism other than nearest-neighbor lateral inhibition may account for odorant decorrelation (Cleland 2010; Yokoi et al. 1995). More work is needed to determine whether short axon cells form a functional, all-to-all inhibitory network, or if short axon cell inhibition produces more targeted inhibition to specific glomeruli.
Consistent with the activation of multiple short axon cells across glomeruli, activation of single short axon cells has little effect on the ORN-evoked EPSC amplitude (unpublished observation); thus activation of a large ensemble of short axon cells may be necessary for maximizing inhibition. Therefore, in our experiments, limitations from slice preparation, namely severing dendritic arbors, and the number of labeled and activated ChR2+ cells may underestimate the extent of presynaptic inhibition achieved in vivo. It is also worth noting that in our experiments the optogenetic stimulation was centered above the target glomerulus; therefore we predominantly activated intraglomerular circuitry. Other studies, both in vitro and in vivo, have examined the function of short axon cells in inhibition across glomeruli (Banerjee et al. 2015; Liu et al. 2013; Whitesell et al. 2013). It will be important for future experiments to determine the relative contribution of inter- and intraglomerular presynaptic inhibition mediated by short axon cells.
Short axon cells can be activated by feedforward excitation by external tufted cells or by direct ORN input (Kiyokage et al. 2010). Dendrodendritic and axodendritic synapses, however, occupy distinct compartments of the glomerulus: dendrodendritic synapses are in the core of the glomerulus, whereas axodendritic synapses are localized to the shell (Kasowski et al. 1999; Pinching and Powell 1971). Because short axon cells can inhibit both external tufted cells and ORN presynaptic terminals, the dendrites of short axon cells either exist in both glomerular compartments or neurotransmitters released in the dendrodendritic core can diffuse into the axodendritic shell. Despite their name, short axon cells release neurotransmitter from dendrites (Schoppa and Urban 2003); therefore it is reasonable to assume that external tufted cell-driven short axon cells form reciprocal dendrodendritic synapses whereas ORN-driven short axon cells make dendroaxonic synapses back to the ORN. Such an arrangement may explain why short axon cells only inhibit external tufted cells (but see Liu et al. 2016).
Physiological impact of activation of short axon cells.
The ability of short axon cells to inhibit principal neurons via two temporally and mechanistically distinct pathways may result in divergent effects on the glomerular circuit. The functional impact of short axon cell activation on external tufted cells involves more than GABAergic inhibition, as D1 receptor activation in external tufted cells enhances rebound spiking by modulating Ih currents (Liu et al. 2013). This pause-burst firing pattern is predicted to affect glomerular circuitry by engaging inhibitory periglomerular neurons and producing feedforward excitation (Kiyokage et al. 2010; Najac et al. 2011). Some recent evidence also suggests that short axon cells may directly inhibit mitral cells (Liu et al. 2016); however, we (and others) have failed to detect this current (i.e., Banerjee et al. 2015; Whitesell et al. 2013). Our results are consistent with previous reports that short axon cell activation robustly inhibits mitral cell responses both in vitro and in vivo, suggesting powerful inhibitory control over principal neuron firing (Banerjee et al. 2015; Liu et al. 2016).
The impact of short axon cells on the presynaptic ORN has not been as well characterized. Although bath application of D2 and GABAB receptor agonists reduces presynaptic glutamate release (Aroniadou-Anderjaska et al. 2000; Ennis et al. 2001; Hsia et al. 1999; Maher and Westbrook 2008; Wachowiak et al. 2005), the extent and magnitude to which this occurs have not been explored with physiological stimulation of short axon cells. Although there is in vivo evidence to suggest that interglomerular inhibition of the ORN may be modest (McGann et al. 2005), these studies used a combination of two odorants to examine interglomerular inhibition. Such limited odorant mixtures may not be strong enough to engage robust interglomerular inhibition, because the connection probability of any two glomeruli chosen at random is presumably low. Conversely, more recent in vivo experiments have demonstrated that short axon cell activation in distant glomeruli strongly inhibits odorant-evoked responses in mitral cells (Banerjee et al. 2015). Whether this suppression involved presynaptic inhibition was not evaluated. It is worth noting that mitral cells and external tufted cells have unique cellular morphologies and responses to ORN input. External tufted cells, located around each glomerulus, have a much smaller cell body than mitral cells. Furthermore, mitral cells are connected to glomeruli via a long apical dendrite. Therefore, the relative strength of presynaptic inhibition may also vary between cell types, as mitral cells have a smaller monosynaptic ORN current (Vaaga and Westbrook 2016) and therefore may be more sensitive to small changes in glutamate release.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant NS-26494 (G. L. Westbrook), National Science Foundation Graduate Research Fellowship DGE-0925180 (C. E. Vaaga), NIH Grant DA-040409 (J. T. Yorgason), NIH Grant DA-004523 (J. T. Williams), and a NIH P30 imaging grant (NS-061800).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.E.V. and J.T.Y. performed experiments; C.E.V. analyzed data; C.E.V. interpreted results of experiments; C.E.V. prepared figures; C.E.V. and G.L.W. drafted manuscript; C.E.V., J.T.Y., J.T.W., and G.L.W. edited and revised manuscript; C.E.V., J.T.Y., J.T.W., and G.L.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the members of the Westbrook lab for their helpful comments.
REFERENCES
- Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABAB heteroreceptors. J Neurophysiol 84: 1194–1203, 2000. [DOI] [PubMed] [Google Scholar]
- Bäckman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, Tomac AC. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis 44: 383–390, 2006. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- Baker H, Kawano T, Margolis FL, Joh TH. Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J Neurosci 3: 69–78, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H, Morel K, Stone DM, Maruniak JA. Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res 614: 109–116, 1993. doi: 10.1016/0006-8993(93)91023-L. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Marbach F, Anselmi F, Koh MS, Davis MB, Garcia da Silva P, Delevich K, Oyibo HK, Gupta P, Li B, Albeanu DF. An interglomerular circuit gates glomerular output and implements gain control in the mouse olfactory bulb. Neuron 87: 193–207, 2015. doi: 10.1016/j.neuron.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisovska M, Bensen AL, Chong G, Westbrook GL. Distinct modes of dopamine and GABA release in a dual transmitter neuron. J Neurosci 33: 1790–1796, 2013. doi: 10.1523/JNEUROSCI.4342-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, Shipley MT, Keller A. Long-lasting depolarizations in mitral cells of the rat olfactory bulb. J Neurosci 20: 2011–2021, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzi C, Schnell E, Westbrook GL. Localized hypoxia within the subgranular zone determines the early survival of newborn hippocampal granule cells. eLife 4: e08722, 2015. doi: 10.7554/eLife.08722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA. Early transformations in odor representation. Trends Neurosci 33: 130–139, 2010. doi: 10.1016/j.tins.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerham R, Liu S, Cachope R, Kiyokage E, Cheer JF, Shipley MT, Puche AC. Subsecond regulation of synaptically released dopamine by COMT in the olfactory bulb. J Neurosci 36: 7779–7785, 2016. doi: 10.1523/JNEUROSCI.0658-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. Olfaction in Parkinson’s disease and related disorders. Neurobiol Dis 46: 527–552, 2012. doi: 10.1016/j.nbd.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Zhou FM, Ciombor KJ, Aroniadou-Anderjaska V, Hayar A, Borrelli E, Zimmer LA, Margolis F, Shipley MT. Dopamine D2 receptor-mediated presynaptic inhibition of olfactory nerve terminals. J Neurophysiol 86: 2986–2997, 2001. [DOI] [PubMed] [Google Scholar]
- Ford CP, Gantz SC, Phillips PE, Williams JT. Control of extracellular dopamine at dendrite and axon terminals. J Neurosci 30: 6975–6983, 2010. doi: 10.1523/JNEUROSCI.1020-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall CM, Hendry SH, Seroogy KB, Jones EG, Haycock JW. Evidence for coexistence of GABA and dopamine in neurons of the rat olfactory bulb. J Comp Neurol 266: 307–318, 1987. doi: 10.1002/cne.902660302. [DOI] [PubMed] [Google Scholar]
- Hayar A, Shipley MT, Ennis M. Olfactory bulb external tufted cells are synchronized by multiple intraglomerular mechanisms. J Neurosci 25: 8197–8208, 2005. doi: 10.1523/JNEUROSCI.2374-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia AY, Vincent JD, Lledo PM. Dopamine depresses synaptic inputs into the olfactory bulb. J Neurophysiol 82: 1082–1085, 1999. [DOI] [PubMed] [Google Scholar]
- Huisman E, Uylings HB, Hoogland PV. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson’s disease. Mov Disord 19: 687–692, 2004. doi: 10.1002/mds.10713. [DOI] [PubMed] [Google Scholar]
- Igarashi KM, Ieki N, An M, Yamaguchi Y, Nagayama S, Kobayakawa K, Kobayakawa R, Tanifuji M, Sakano H, Chen WR, Mori K. Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex. J Neurosci 32: 7970–7985, 2012. doi: 10.1523/JNEUROSCI.0154-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasowski HJ, Kim H, Greer CA. Compartmental organization of the olfactory bulb glomerulus. J Comp Neurol 407: 261–274, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- Kiyokage E, Pan Y-Z, Shao Z, Kobayashi K, Szabo G, Yanagawa Y, Obata K, Okano H, Toida K, Puche AC, Shipley MT. Molecular identity of periglomerular and short axon cells. J Neurosci 30: 1185–1196, 2010. doi: 10.1523/JNEUROSCI.3497-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Plachez C, Shao Z, Puche A, Shipley MT. Olfactory bulb short axon cell release of GABA and dopamine produces a temporally biphasic inhibition-excitation response in external tufted cells. J Neurosci 33: 2916–2926, 2013. doi: 10.1523/JNEUROSCI.3607-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Puche AC, Shipley MT. The interglomerular circuit potently inhibits olfactory bulb output neurons by both direct and indirect pathways. J Neurosci 36: 9604–9617, 2016. doi: 10.1523/JNEUROSCI.1763-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher BJ, Westbrook GL. Co-transmission of dopamine and GABA in periglomerular cells. J Neurophysiol 99: 1559–1564, 2008. doi: 10.1152/jn.00636.2007. [DOI] [PubMed] [Google Scholar]
- McGann JP, Pírez N, Gainey MA, Muratore C, Elias AS, Wachowiak M. Odorant representations are modulated by intra- but not interglomerular presynaptic inhibition of olfactory sensory neurons. Neuron 48: 1039–1053, 2005. doi: 10.1016/j.neuron.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Mundiñano IC, Caballero MC, Ordóñez C, Hernandez M, DiCaudo C, Marcilla I, Erro ME, Tuñon MT, Luquin MR. Increased dopaminergic cells and protein aggregates in the olfactory bulb of patients with neurodegenerative disorders. Acta Neuropathol 122: 61–74, 2011. doi: 10.1007/s00401-011-0830-2. [DOI] [PubMed] [Google Scholar]
- Najac M, De Saint Jan D, Reguero L, Grandes P, Charpak S. Monosynaptic and polysynaptic feed-forward inputs to mitral cells from olfactory sensory neurons. J Neurosci 31: 8722–8729, 2011. doi: 10.1523/JNEUROSCI.0527-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinching AJ, Powell TP. The neuropil of the glomeruli of the olfactory bulb. J Cell Sci 9: 347–377, 1971. [DOI] [PubMed] [Google Scholar]
- Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron 23: 499–511, 1999. doi: 10.1016/S0896-6273(00)80803-X. [DOI] [PubMed] [Google Scholar]
- Schoppa NE, Urban NN. Dendritic processing within olfactory bulb circuits. Trends Neurosci 26: 501–506, 2003. doi: 10.1016/S0166-2236(03)00228-5. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. Synaptic Organization of the Brain. Oxford, UK: Oxford Univ. Press, 2004, p. 165–216. doi: 10.1093/acprof:oso/9780195159561.003.0005 [DOI] [Google Scholar]
- Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci 12: 210–220, 2009. doi: 10.1038/nn.2262. [DOI] [PubMed] [Google Scholar]
- Vaaga CE, Westbrook GL. Parallel processing of afferent olfactory sensory information. J Physiol 594: 6715–6732, 2016. doi: 10.1113/JP272755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron 32: 723–735, 2001. doi: 10.1016/S0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, McGann JP, Heyward PM, Shao Z, Puche AC, Shipley MT. Inhibition [corrected] of olfactory receptor neuron input to olfactory bulb glomeruli mediated by suppression of presynaptic calcium influx. J Neurophysiol 94: 2700–2712, 2005. doi: 10.1152/jn.00286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell JD, Sorensen KA, Jarvie BC, Hentges ST, Schoppa NE. Interglomerular lateral inhibition targeted on external tufted cells in the olfactory bulb. J Neurosci 33: 1552–1563, 2013. doi: 10.1523/JNEUROSCI.3410-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci USA 92: 3371–3375, 1995. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods 202: 158–164, 2011. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]