With the use of a large database, this study establishes that neighboring homotypic striatal spiny projection neurons have a 50% chance to form one-way collateral inhibitory connection, a substantially higher rate than previous estimates. This study also shows that dopamine denervation may alter presynaptic dopamine receptor function such that dopaminergic treatment of Parkinson's disease can weaken the surround inhibition and may reduce the contrast of the striatal outputs, potentially contributing to dopamine's profound motor and nonmotor behavioral effects.
Keywords: basal ganglia, dopamine receptor supersensitivity, homotypic preference, l-3 4-dihydroxyphenylalanine, medium spiny neuron, Parkinson's disease, surround inhibition
Abstract
The striatal medium spiny neurons (MSNs) are critical to both motor and cognitive functions. A potential regulator of MSN activity is the GABAergic collateral axonal input from neighboring MSNs. These collateral axon terminals are further under the regulation of presynaptic dopamine (DA) receptors that may become dysfunctional when the intense striatal DA innervation is lost in Parkinson's disease (PD). We show that DA D1 receptor-expressing MSNs (D1-MSNs) and D2 receptor-expressing MSNs (D2-MSNs) each formed high-rate, one-way collateral connections with a homotypic preference in both normal and DA-denervated mouse striatum. Furthermore, whereas the homotypic preference, one-way directionality and the basal inhibitory strength were preserved, DA inhibited GABA release at the D2-MSN→D2-MSN collateral synapse in a supersensitive manner in the DA-denervated striatum. In contrast, for D1-MSN-originated collateral connections, whereas D1 agonism facilitated D1-MSN→D1-MSN collateral inhibition in the normal striatum, this presynaptic D1R facilitation of GABA release was lost in the parkinsonian striatum. These results indicate that in the parkinsonian striatum, dopaminergic treatment can presynaptically weaken the D2-MSN→D2-MSN collateral inhibition and disinhibit the surrounding D2-MSNs, whereas the D1-MSN→D1-MSN collateral inhibition is weakened by the loss of the presynaptic D1 receptor facilitation, disinhibiting the surrounding D1-MSNs. Together, these newly discovered effects can disrupt the MSN circuits in the parkinsonian striatum and may contribute to dopaminergic treatment-induced aberrant motor and nonmotor behaviors in PD.
NEW & NOTEWORTHY With the use of a large database, this study establishes that neighboring homotypic striatal spiny projection neurons have a 50% chance to form one-way collateral inhibitory connection, a substantially higher rate than previous estimates. This study also shows that dopamine denervation may alter presynaptic dopamine receptor function such that dopaminergic treatment of Parkinson's disease can weaken the surround inhibition and may reduce the contrast of the striatal outputs, potentially contributing to dopamine's profound motor and nonmotor behavioral effects.
the medium spiny neurons (MSN) are the projection neurons of the striatum and are critical to multiple important brain functions as indicated by the profound behavioral consequences of Huntington's disease (Glass et al. 2000) and Parkinson's disease (PD) (Kish et al., 1988). One group of MSNs heavily express D1 receptors (D1Rs: termed D1-MSNs) and project to the globus pallidus internal segment (GPi) and the substantia nigra pars reticulata (SNr), the output nuclei of the basal ganglia, forming the direct pathway (Gerfen and Bolam 2016; Zhou 2016). The other group of MSNs heavily express D2Rs (D2-MSNs) and project to the globus pallidus external segment (GPe), forming the indirect pathway. Evidence indicates that D1-MSN activity and the consequent striatonigral output facilitate movement, whereas D2-MSN activity and the striatopallidal output inhibit movement (Friend and Kravitz 2014; Kravitz et al. 2010; Redgrave et al. 2010; Sano et al. 2013; Sippy et al. 2015). Evidence also indicates that MSN activity is critical to cognition (Rothwell et al. 2014; Simpson et al. 2010), habit learning and formation (Graybiel and Grafton 2015), multisensory integration (Coffey et al. 2016; Reig and Silberberg 2014), and emotional and motivational regulation (Francis et al. 2015; Haber and Knutson 2010; Ikemoto et al. 2015; Lobo et al. 2013, Révy et al. 2014; Sesack and Grace 2010).
Several mechanisms control MSN activity. In addition to their intrinsic ion channels, cortical and thalamic glutamatergic inputs, and GABAergic input from striatal GABAergic interneurons, MSNs also receive inhibitory synaptic inputs from the axonal collaterals of neighboring MSNs, forming the so-called MSN collateral inhibition, also known as surround inhibition (Dobbs et al. 2016; Moyer et al. 2014; Wickens et al. 2007; Wilson 2007). Anatomical studies show that individual MSNs have an extensive intrastriatal axonal arborization, in addition to their main axons projecting to GPe, GPi, and SNr (Chang et al. 1981; Fujiyama et al. 2011; Kawaguchi et al. 1989, 1990; Preston et al. 1980; Wilson and Groves 1980). Electrophysiological evidence indicates that MSNs receive collateral GABAergic inputs (Chuhma et al. 2011; Guzmán et al. 2003; López-Huerta et al. 2013; Taverna et al. 2008; Tepper et al. 2004; Tunstall et al. 2002).
Given that MSNs receive an intense DA innervation, a natural question is, Does DA denervation alter the collateral connection among MSNs? Published data on the collateral inhibition in the DA-depleted striatum are scarce. To our knowledge, the only study using paired recording (required to examine this question) reported that DA loss decreased the connection rate and strength of the MSN collateral inhibition (Taverna et al. 2008). Given the potential importance of the collateral inhibition in the striatal neuronal network in motor and other brain functions (Dobbs et al. 2016), follow-up studies are needed to more firmly establish the DA denervation-induced alterations in MSN neuron collateral connection.
In addition to their heavy expression in the somatodendritic area and the projection axon terminal (Levey et al. 1993, Yung et al. 1995), D1Rs and D2Rs also are likely expressed in their collateral axon terminals (Guzmán et al. 2003; Tecuapetla et al. 2007, 2009). Thus an equally important question is, Does DA denervation alter the functionality of D1 and D2 receptors on collateral axon terminals? This question is highly relevant to the pathophysiology of PD, because severe DA loss in the striatum leads to D1R and D2R supersensitivity in both animal PD models (Creese et al. 1977; LaHoste and Marshall 1994; Staunton et al. 1982; Trugman and James 1992; Wei et al. 2013) and PD patients (Corvol et al. 2004; Pifl et al. 1992). We have performed paired recording experiments to address these questions.
MATERIALS AND METHODS
Transcription Factor Pitx3 Null Mutant Mice
Pitx3 is required for the survival of most DA neurons in the substantia nigra, whereas about 50% of DA neurons in the ventral tegmental area do not require Pitx3 to survive; hence these mice have a severe and selective DA neuron loss in the substantia nigra and a severe DA denervation in the dorsal striatum, resembling the DA loss pattern in the PD brain (Ding et al. 2015a; Li et al. 2013, 2015; Li and Zhou 2013; Nunes et al. 2003; Smidt et al. 2004; van den Munckhof et al. 2003; Wei et al. 2013). Two breeding pairs of heterozygous Pitx3+/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME), resulting a small colony of homozygous Pitx3−/− (Pitx3Null), heterozygous Pitx3+/−, and wild-type Pitx3+/+ (Pitx3WT) mice. Pitx3Null mice are aphakic and thus clearly identifiable (Wei et al. 2013). The genotypes were also determined by PCR-based genotyping to identify WT, homozygotes, and heterozygotes (Li et al. 2013). Additionally, two pairs of heterozygous BAC D2-GFP+/− breeder mice (Gong et al. 2003) were purchased from the Mutant Mouse Research Resource Centers and crossed to Pitx3WT and Pitx3Null mice, eventually producing Pitx3WT/D2-GFP and Pitx3Null/D2-GFP mice. Mice had free access to food and water. All procedures were approved by The Institutional Animal Care and Use Committee of The University of Tennessee Health Science Center.
Brain Slice Preparation
Brain slices containing the anterior striatum were prepared for electrophysiological recording. Male and female juvenile (postnatal day 19–22) Pitx3Null/D2-GFP or Pitx3WT/D2-GFP mice were killed by decapitation, and brains were dissected out quickly and immediately immersed for 2 min in oxygenated ice-cold cutting solution containing (in mM) 220 glycerol, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, and 20 d-glucose. Coronal brain slices (300 μm thick) were cut using a Leica Zero Z VT1200S vibratome (Leica Microsystems, Wetzlar, Germany). The brain slices were transferred to a holding chamber filled with an artificial cerebrospinal fluid (aCSF) containing (in mM) 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 2.5 CaCl2, 1.3 MgCl2, and 10 d-glucose, which were continuously bubbled with 95% O2 and 5% CO2. The holding chamber was first maintained at 34°C for 30 min and then kept at room temperature (22°C). For recording, a single brain slice was transferred to the recording chamber maintained at 32°C. To avoid any bias, experiments were performed in a double-blind manner: the electrophysiologists were kept blind as to the sources (DA-intact Pitx3WT or DA-deficient Pitx3Null mice) of brain slices until the data were analyzed and tabulated.
D1- and D2-MSN Identification
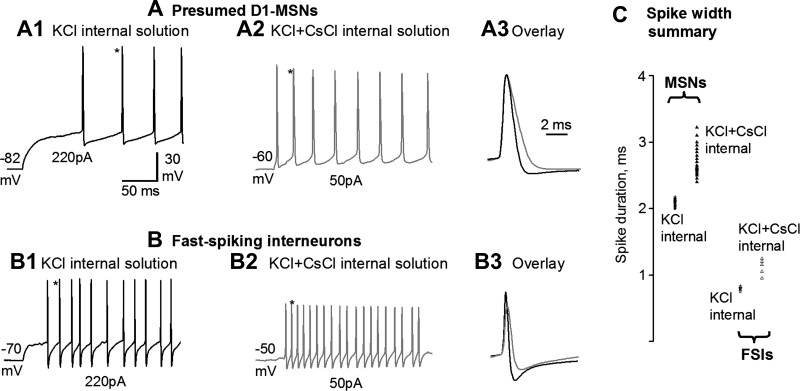
D2-MSNs were identified by D2-GFP fluorescence in D2-eGFP mice. Following the established methods of Taverna et al. (2008), we identified D1-MSNs as D2-GFP negative, nonfluorescent cells with typical MSN electrophysiological properties (Nisenbaum and Wilson 1995). This practice is valid for four reasons. First, D1 receptors and D2 receptors, and also D1- and D2-associated GFP, are overwhelmingly, if not completely, segregated in direct pathway D1-MSNs and indirect pathway D2-MSNs in the dorsal striatum (Gerfen and Surmeier 2011; Valjent et al. 2009). Second, our detected homotypic preference for collateral connection is robust; in particular, the homotypic preference among the presumed D1-MSNs is higher than that among D2-MSNs, indicating that our D1-MSN identification is reliable; otherwise, the homotypic preference would be weak or even nonexistent. Additionally, our detected presynaptic D1R facilitation was only in presumed D1-MSN-originated pairs, and D2R inhibition was only in D2-MSN-originated pairs, strongly indicating our D1-MSN identification was reliable. Third, the rare GABAergic interneurons in the striatum are easily identified by their combined membrane and spike properties that are very different from those of MSNs (Kawaguchi 1993 1997; Koos and Tepper 1999). The spikes in MSNs and fast-spiking GABAergic interneurons (FSIs) were still strikingly different with the use of a partially CsCl-containing pipette solution (see below) within a few minutes after a whole cell recording mode was formed (Fig. 1): the spike duration was still distinctively short for FSIs. Fourth, striatal GABAergic interneurons are also larger than MSNs (Kawaguchi 1993) and can be spotted and avoided by a pair of trained eyes; the cholinergic interneurons are distinctively large among striatal neurons and can be easily avoided. Our combined use of these four parameters ensured that D1- and D2-MSN identification in this study was reliable.
Fig. 1.
Distinct spikes in presumed (D2-GFP negative) D1-MSNs and fast-spiking GABAergic interneurons (FSIs) shortly after whole cell recording mode is formed with a CsCl-containing pipette solution. A1: with a 135 mM KCl-based pipette solution, a presumed D1-MSN displayed current injection-evoked, nonadapting spikes typical of MSNs reported in the literature. A2: with a 67.5 mM KCl and 67.5 mM CsCl-based pipette solution, a presumed D1-MSN displayed current injection-evoked, broadened spikes. These broadened spikes were still distinct from the broadened spikes in FSIs (but see below). A3: overlay of the spikes indicated by asterisks in A1 and A2 showing the broadened spike with a 67.5 mM KCl and 67.5 mM CsCl-based pipette solution in the presumed D1-MSN. B1: with a 135 mM KCl-based pipette solution, an FSI displayed current injection-evoked spikes typical of FSIs reported in the literature. B2: with a 67.5 mM KCl and 67.5 mM CsCl-based pipette solution, an FSI displayed current injection-evoked, broadened spikes that were still fast. B3: overlay of the spikes indicated by asterisks in B1 and B2 showing the broadened spike with a 67.5 mM KCl and 67.5 mM CsCl-based pipette solution in an FSI. C: summary of the spike width of presumed D1-MSNs recorded with a 135 mM KCl-based pipette solution [2.09 ± 0.02 ms (range: 2.00–2.17 ms, all measured at the base), n = 10 (5 each from WT and Pitx3Null mice)] and with a 67.5 mM KCl and 67.5 mM CsCl-based pipette solution [2.72 ± 0.06 ms (range: 2.40–3.22 ms), n = 20 (10 each from WT and Pitx3Null mice)], and summary of the spike width of FSIs recorded with 135 mM KCl-based pipette solution [0.80 ± 0.01 ms (range: 0.75–0.83 ms), n = 5 (3 from WT and 2 from Pitx3Null mice)] and with a 67.5 mM KCl and 67.5 mM CsCl-based pipette solution [1.12 ± 0.05 ms (range: 0.95–1.25 ms), n = 5 (2 from WT and 3 from Pitx3Null mice)]. Recordings indicate were made about 2 min after whole cell mode was reached.
Electrophysiology
A Multiclamp 700B amplifier, pClamp 9.2 software, and Digidata 1322A interface (Molecular Devices, Sunnyvale, CA) were used to acquire data (Ding et al. 2013, 2015a, 2015b; Wei et al. 2013). Patch pipettes were pulled from borosilicate glass capillary tubing (KG-33; 1.1-mm inner diameter, 1.65-mm outer diameter; King Precision Glass, Claremont, CA) using a PC-10 puller (Narishige, Tokyo, Japan) and had resistances of 2–3 MΩ. Two types of intracellular or pipette solutions were used. The first pipette solution contained (in mM) 67.5 KCl, 67.5 CsCl, 0.5 EGTA, 10 HEPES, 2 Mg-ATP, 0.2 Na-GTP, and 4 Na2-phosphocreatine with pH at 7.25 and osmolarity at 280–290 mosM. The high Cl− concentration and the inclusion of CsCl were designed to increase the inhibitory postsynaptic current (IPSC) amplitude and thus facilitate the detection of collateral connections, and the 67.5 mM CsCl-containing pipette solution still allowed us to distinguish the different firing properties of MSNs and GABAergic interneurons within a few minutes after whole cell mode was reached (Fig. 1). In addition, we used a 135 mM KCl-based pipette solution to record normal spikes for data presented in Fig. 1.
Recordings were made in the dorsal striatum (within 400 μm below the corpus callosum), where the DA loss is 95–99% and DA receptors are supersensitive in Pitx3Null mice (Ding et al. 2015a; Li et al. 2013; Wei et al. 2013). Under fluorescence illumination, a pair of MSNs with the somata in the same focal plane and a lateral distance between somata of 30–50 μm were chosen. This distance is short such that the collateral axons and the overlapping dendrites have a relatively high probability to form synapses (Wickens et al. 2007) and might have survived the tissue slicing procedure, but this distance is long enough that the maneuvering of the two recording patch electrodes did not disrupt each other. The microscope illumination was then switched to the bright field differential interference contrast mode for conventional whole cell patch clamping. The first cell in the pair was held in a cell-attached mode until the second cell was in a whole cell mode. For each cell, brief current-clamp recording was made to determine the firing properties and to exclude fast-spiking interneurons. The synaptic connections were tested by recording IPSCs (holding potential −70 mV) in the potential postsynaptic cell that were time-locked with the current injection-evoked spikes or depolarizing voltage pulses in the presynaptic cell. When no IPSC was evoked and also when searching for reciprocal connections, the protocol was repeated in a reversed order.
After a determination of collateral connection, both the presynaptic MSN and postsynaptic MSN were voltage-clamped at −70 mV. Depolarizing pulses (5 ms to 20 mV) were delivered every 20 s to the presynaptic MSN, as in a published study (Tecuapetla et al. 2009). Access resistance was monitored by detecting the stability of current responses to brief voltage pulses (−10 mV from the holding potential of −70 mV, 40 ms) and was not compensated. Postsynaptic cells in which the cell capacitance transient decreased by >15% were discarded. Signals were filtered at 10 kHz using the built-in four-pole low-pass Bessel filter in the patch-clamp amplifier and digitized at 20 kHz. Recordings were made at 32°C, maintained by an automatic temperature controller (TC-324B; Warner Instrument).
Pharmacology
After a stable 5- to 10-min baseline recording was obtained, DA or D1R or D2R agonists were bath-applied for 5 min. The drugs were then either washed out or followed by application of D1R or D2R antagonist. Only the data showing at least a partial recovery upon washout were included for analysis. Ionotropic glutamate receptors were blocked by 20 μM 2-amino-5-phosphonopentanoic acid (APV) and 10 μM 6-cyano-309 7-nitroquinoxaline-2,3-dione (CNQX) to prevent spontaneous glutamatergic inward synaptic currents from contaminating MSN collateral IPSCs that were inward when recorded with a high-Cl−-based pipette solution at −70 mV. Picrotoxin was tested in a subset of cells and blocked to MSN collateral IPSCs, verifying the expected mediation of collateral IPSCs by GABAA receptors.
Data Analysis and Statistics
Data were analyzed using Clampfit 9 (Molecular Devices, CA). Peak IPSC conductance was estimated using Ohm's law: conductance = current/driving force. The driving force was 70 mV in our experiments where conductance was calculated. Numerical values are expressed as means ± SE. The chi-square test was used to determine the difference in the connection rates between different types of MSNs in Pitx3WT and Pitx3Null mice. The paired Student's t-test was used to determine the significance of the changes before and after drug administration within the same group. One-way ANOVA or unpaired Student's t-test was used to compare the changes among different groups.
RESULTS
D1-MSN-Originated Collateral Connection Rate, Strength, Preference, and Direction in Normal and DA-Denervated Striatum
Connection rate.
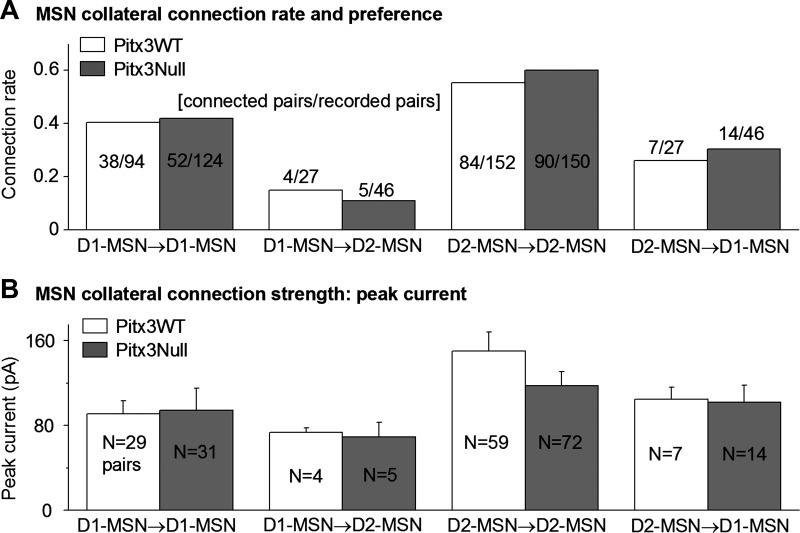
We first characterized the homotypic collateral connections among D1-MSNs. As shown in Fig. 2A, we recorded 94 D1-MSN→D1-MSN pairs in the dorsal striatum in Pitx3WT mice and found that 38 of these pairs were connected, a connection rate of 40.4%. We also recorded 124 D1-MSN→D1-MSN pairs in the dorsal striatum in Pitx3Null mice and found that 52 of these pairs were connected, a connection rate of 41.9%. These two homotypic connection rates were not significantly different (chi-square test: χ2 = 0.00442 < χ0.05,12 = 3.84, P > 0.05), indicating that in Pitx3Null mice, the D1-MSN→D1-MSN collateral connection rate in the dorsal striatum was not disrupted by DA deficiency.
Fig. 2.
MSN collateral connection rates, homotypic preference, and strength in the normal and DA-denervated striatum. A: collateral connection rates for D1-MSN→D1-MSN, D1-MSN→D2 MSN, D2-MSN→D2-MSN, and D2-MSN→D1 MSN pairs in the dorsal striatum in Pitx3WT and Pitx3Null mice. B: peak amplitudes of the collateral IPSCs of D1-MSN→D1-MSN, D1-MSN→D2 MSN, D2-MSN→D2-MSN, and D2-MSN→D1-MSN pairs in Pitx3WT and Pitx3Null mice. Pair numbers (N) are smaller in B than in A because only voltage-clamp recordings are included in B, whereas both voltage- and current-clamp recordings are counted in A. For clarity, the results of statistic comparisons are not marked in the figure but are described in the text.
We next studied D1-MSN→D2-MSN heterotypic collateral connections. We found that among 27 pairs of recorded D1-MSN→D2-MSN pairs, only 4 pairs were connected, at a connection rate of only 14.8%, in the dorsal striatum in Pitx3WT mice; and among 46 pairs of recorded D1-MSN→D2-MSN pairs, only 5 pairs were connected, at a connection rate of only 10.9%, in the dorsal striatum in Pitx3Null mice (Fig. 2A). These two heterotypic collateral connection rates also were not different (chi-square test: χ2 = 0.32 < χ0.05,12 = 3.84, P > 0.05). However, compared with the D1-MSN→D2-MSN connection rate, the D1-MSN→D1-MSN connection rate was significantly higher in both Pitx3WT mice and Pitx3Null mice (40.4% vs. 14.8% in Pitx3WT mice, chi-square test: χ2 = 11.74 > χ0.05,12 = 3.84, P < 0.05; 41.9% vs. 10.9% in Pitx3Null mice, chi-square test: χ2 = 8.81 > χ0.05,12 = 3.84, P < 0.05).
Connection strength.
We evaluated the D1-MSN collateral connection strength by using the collateral IPSC peak amplitude as an indicator. The D1-MSN→D1-MSN unitary IPSC peak amplitude was 91.1 ± 12.3 pA (n = 29 pairs) in Pitx3WT mice and 94.4 ± 10.8 pA (n = 31 pairs) in Pitx3Null mice; these peak amplitudes were similar (P > 0.05, unpaired t-test; Fig. 2B). To further estimate the connection strength, we calculated the conductance of the unitary collateral IPSCs (Fig. 2C). Because the extracellular and intracellular Cl− concentrations were symmetric and the cells were voltage-clamped at −70 mV, the somatically measured unitary IPSC conductance activated by a single neighboring D1-MSN was estimated to be 1.3 nS (Fig. 2C). The true unitary collateral IPSC conductance is likely larger because collateral synapses are commonly formed on dendrites, whereas the recording was made in the soma, leading to dendritic filtering and an underestimated conductance (Wilson 2007; Wilson and Groves 1980).
Connection preference and direction.
As shown in Fig. 2A, compared with the D1-MSN→D2 MSN connection rate, the D1-MSN→D1-MSN connection rate was significantly higher in both Pitx3WT mice and Pitx3Null mice (40.4% vs. 14.8% in Pitx3WT mice, chi-square test: χ2 = 11.74 > χ0.05,12 = 3.84, P < 0.05; 41.9% vs. 10.9% in Pitx3Null mice, chi-square test: χ2 = 8.81 > χ0.05,12 = 3.84, P < 0.05). These results indicate that D1-MSNs preferentially formed axon collateral connections with neighboring D1-MSNs over D2-MSNs.
In addition, no reciprocal connection was detected in 24 D1-MSN→D1-MSN pairs in the DA-intact dorsal striatum in WT mice and 28 D1-MSN→D1-MSN pairs in DA-denervated dorsal striatum in Pitx3Null mice, indicating that collateral inhibition flows in one way only or from upstream MSNs to downstream MSNs, an indication of anatomical organization and functional importance of collateral inhibition. This one-way connection and the homotypic preference indicate that D1-MSN collateral innervation is built in a way that is conducive for receiving and processing cortical and thalamic inputs and then producing appropriate outputs.
DA Modulation of D1-MSN-Originated Collateral Inhibition in Normal and DA-Denervated Striatum
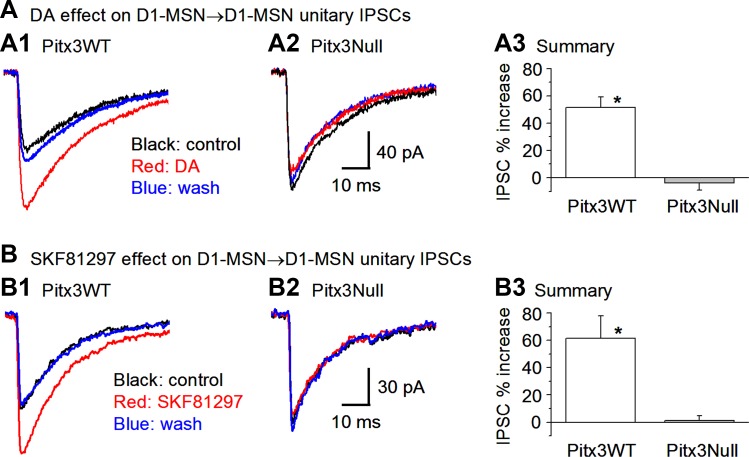
Because D1Rs are highly expressed in D1-MSNs and their projection axon terminals (Levey et al. 1993; Yung et al. 1995; Zhou 2016) and facilitate GABA release at the striatonigral axon terminals (Ding et al. 2015a; Zhou 2016), we predicted that D1Rs might also facilitate GABA release at the D1-MSN-originated collateral axon terminals. To test this idea, we first examined the potential effects of DA on D1-MSN→D1-MSN IPSCs in Pitx3WT mice. As expected, DA (25 μM) significantly increased the D1-MSN→D1-MSN IPSC peak amplitude by 51.6 ± 7.6% in Pitx3WT mice: the unitary IPSC amplitude was 75.2 ± 5.5 pA under control, 113.9 ± 7.5 pA during 25 μM DA application, and 82.3 ± 8.3 pA after washout (n = 12 pairs, P < 0.05, paired t-test; Fig. 3, A1 and A3).
Fig. 3.
DA facilitation of D1-MSN→D1-MSN collateral inhibition in normal and DA-denervated dorsal striatum. A: bath application of 25 μM DA enhanced D1-MSN→D1-MSN collateral average IPSC peak amplitude in Pitx3WT mice (A1) but not in Pitx3Null mice (A2). Pooled data are shown in A3. *P < 0.05. B: the D1 agonist SKF81297 mimicked the effects of DA on D1-MSN→D1-MSN collateral inhibition. Bath application of 1 μM SKF81297 increased the peak amplitude of the D1-MSN→D1-MSN collateral average IPSCs in Pitx3WT mice (B1) but not in Pitx3Null mice (B2). Summary data are shown in B3. *P < 0.05.
We next studied DA regulation of D1-MSN→D1-MSN collateral inhibition in the DA-denervated dorsal striatum in Pitx3Null mice. We expected to see an enhanced or supersensitive D1 facilitation because presynaptic D1Rs in D1-MSN projection axon terminals are supersensitive in Pitx3Null mice (Ding et al. 2015a). To our surprise, a high dose of DA (25 μM) did not induce a detectable facilitation of the D1-MSN→D1-MSN collateral IPSCs in Pitx3Null mice: the IPSC peak amplitude was 74.2 ± 18.9 pA in control and 72.7 ± 20.1 pA under 25 μM DA, a change of −3.8 ± 5.3 (n = 9 pairs, P > 0.05; Fig. 3, A2 and A3).
Because only (or predominately) D1Rs are expressed in D1-MSNs, we reasoned that the facilitatory DA effect on D1-MSN→D1-MSN IPSCs in Pitx3WT mice was mediated by D1Rs, and thus a D1R agonist should mimic DA's facilitatory effect. As shown in Fig. 3, B1 and B3, bath application of 1 μM D1R agonist SKF81297 increased the IPSC peak amplitude by 61.4 ± 16.7% in Pitx3WT mice (from 60.2 ± 9.6 to 94.0 ± 13.8 pA, n = 4 pairs, P < 0.05); in Pitx3Null mice, 1 μM SKF81297 did not increase the D1-MSN→D1-MSN IPSC: the peak amplitude was 79.3 ± 10.4 pA before 1 μM SKF81297 and 80.5 ± 10.8 pA during 1 μM SKF81297 bath application (n = 4 pairs, P > 0.05, Fig. 3, B2 and B3). Furthermore, the D1R antagonist SKF83566 (1 μM) blocked the facilitatory effect of 1 μM SKF81297 on D1-MSN→D1-MSN IPSCs in Pitx3WT mice: the IPSC peak was 94.0 ± 20.8 pA during 1 μM SKF81297 application and 76.7 ± 20.2 pA during 1 μM SKF83566 + 1 μM SKF81297 coapplication (n = 4 pairs, P < 0.05). The fact that SKF81297 mimicked and SKF83566 blocked the effect of DA on D1-MSN→D1-MSN IPSCs in Pitx3WT mice confirms that the DA effect was mediated by D1Rs.
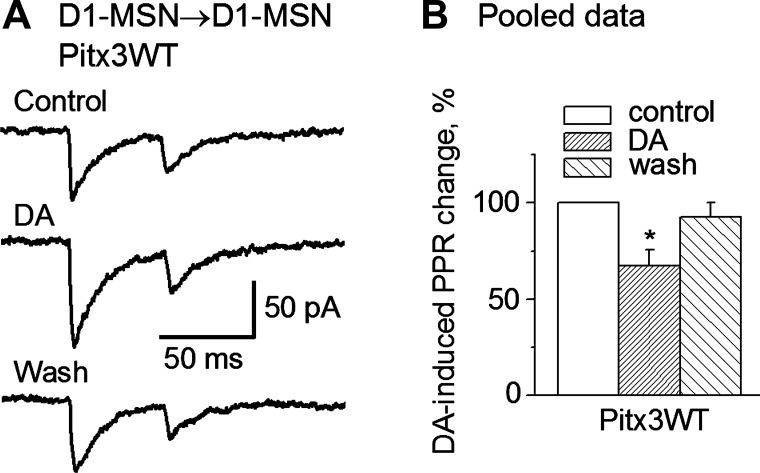
To determine if the D1R agonism-induced facilitation of D1-MSN→D1-MSN IPSCs in Pitx3WT mice was of a pre- or postsynaptic origin, we performed paired-pulse experiments. Because an alteration in the paired-pulse ratio (PPR) of the synaptic currents is an indication of changes in neurotransmitter release or a presynaptic origin (Fioravante and Regehr 2011; Thomson 2000), we examined DA's effects on the PPR in D1-MSN→D1-MSN collateral IPSCs. Whereas the first IPSC was enhanced as described above, bath application of 25 μM DA (5 pairs) or 1 μM SKF81297 (4 pairs) decreased the PPR of the D1-MSN→D1-MSN collateral IPSCs by ∼ 33% in Pitx3WT mice, indicating that the increase in the first IPSC was induced by an increase in GABA release from the collateral axon terminal (Fig. 4, A and B), a presynaptic mechanism. In Pitx3Null mice, 25 μM DA had no effect on the PPR of the D1-MSN→D1-MSN IPSCs (4 pairs); this was expected because DA did not affect D1-MSN→D1-MSN collateral IPSCs in Pitx3Null mice, as described above (Fig. 3, A2 and B2).
Fig. 4.
DA decreases the paired-pulse ratio (PPR) of D1-MSN→D1-MSN collateral IPSCs. A: bath application of 25 μM DA increased the first averaged IPSC more strongly than the second averaged IPSC, and this effect was recovered upon washout. B: pooled data showing that DA decreased the PPR of D1-MSN→D1-MSN collateral IPSCs. *P < 0.05.
Different Release Properties at D1-MSN Collateral Axon Terminals and Projection Axon Terminals
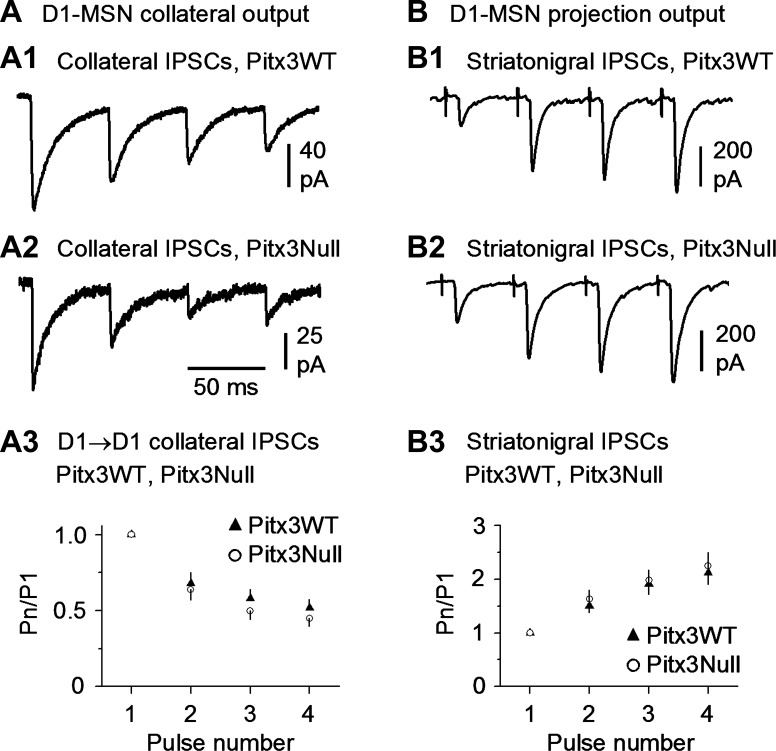
To test the release properties of the D1-MSN→D1-MSN collateral synapse, we used a train of 4 depolarizing pulses with the interpulse interval being 50 ms or 20 Hz, mimicking a common phasic firing in MSNs (Miller et al. 2008; unpublished data, Zhou FM). The bathing solution was the standard normal extracellular solution with 2.5 mM Ca2+. Under this condition, the 4-pulse stimulation train in presynaptic D1-MSNs induced depressing collateral IPSCs in postsynaptic D1-MSNs in both the dorsal striatum in Pitx3WT mice (5 D1-MSN→D1-MSN pairs; Fig. 5A1) and the DA-denervated dorsal striatum in Pitx3Null mice (5 D1-MSN→D1-MSN pairs; Fig. 5A2). In separate experiments, we used an extracellular bipolar stimulating electrode placed in the dorsal striatum to evoke D1-MSN projection (i.e., striatonigral) IPSCs in the GABA projection neurons in the substantia nigra pars reticulata, using the methods described by Ding et al. (2015a). As shown in Fig. 5B, a 20-Hz 4-pulse stimulation train evoked facilitating striatonigral IPSCs in both Pitx3WT mice (n = 5 cells) and Pitx3Null mice (n = 5 cells). These results indicate that axon collateral terminals and the main projection axon terminals have different release properties and may serve different functions.
Fig. 5.
D1-MSN→D1-MSN intrastriatal collateral axon terminals and extrastriatal projection axon terminals have opposite release properties during repetitive stimulation. A: D1-MSN→D1-MSN collateral unitary IPSCs were depressing during 20-Hz, 4-pulse repetitive stimulation in the dorsal striatum in both DA-intact WT mice (A1) and DA-denervated Pitx3Null mice (A2). Summary data are shown in A3. B: dorsal striatum-evoked striatonigral IPSCs were facilitating during 20-Hz, 4-pulse repetitive release in both DA-intact WT mice (B1) and DA-denervated Pitx3Null mice (B2). Also note that the repetitive release properties were similar in DA-intact WT and DA-deficient Pitx3Null mice. Summary data are shown in B3.
D2-MSN-Originated Collateral Connection Rate, Strength, Preference, and Direction in Normal and DA-Denervated Striatum
Connection rate.
In the normal dorsal striatum in Pitx3WT mice (Fig. 2A), we recorded 152 D2-MSN→D2-MSN pairs and found that 84 pairs were connected, a connection rate of 55.3%. In the DA-denervated dorsal striatum in Pitx3Null mice (Fig. 2A), we recorded 150 D2-MSN→D2-MSN pairs and found that 90 pairs were connected, a connection rate of 60%. These connection rates were not statistically different (chi-square test: χ2 = 0.6515 < χ0.05,12 = 3.84, P > 0.05 ). We also found 7 connected D2-MSN→D1-MSN pairs in a total of 27 D2-MSN→D1-MSN pairs in Pitx3WT mice and 14 connected D2-MSN→D1-MSN pairs in a total of 46 D2-MSN→D1-MSN pairs in Pitx3Null mice, respectively (Fig. 2A). These connection rates (25.9% in Pitx3WT and 30.4% in Pitx3Null mice) were not statistically different (chi-square test: χ2 = 0.1040 < χ0.05,12 = 3.84, P > 0.05). However, the D2-MSN→D2-MSN connection rate was significantly higher than the D2-MSN→D1-MSN connection rate in both Pitx3WT mice and Pitx3Null mice (55.3% vs. 25.9% in Pitx3WT mice, chi-square test: χ2 = 6.38 > χ0.05,12 = 3.84, P < 0.05; 60% vs. 30.4% in Pitx3Null mice, chi-square test: χ2 = 4.37 > χ0.05,12 = 3.84, P < 0.05).
Connection strength.
We evaluated the D2-MSN collateral connection strength in the DA-denervated dorsal striatum in Pitx3Null mice by using the collateral IPSC peak amplitude as an indicator. The D2-MSN→D2-MSN IPSC peak amplitude was 150.1 ± 18.0 pA (n = 59 pairs) in the DA-intact dorsal striatum in Pitx3WT mice and 117.6 ± 13.1 pA (n = 72 pairs) in DA-denervated dorsal striatum in Pitx3Null mice, and the values were not statistically different in the two genotypes (P > 0.05, unpaired t-test; Fig. 2B). Similarly, D2-MSN→D1-MSN collateral IPSC peak amplitude was 140.0 ± 31.4 pA (n = 5 pairs) in Pitx3WT mice and 110.0 ± 33.5 pA (n = 7 pairs) in Pitx3Null mice, and these IPSC amplitudes were not statistically different in the two genotypes (P > 0.05, unpaired t-test; Fig. 2B). To further estimate the connection strength, we calculated the conductance of the unitary collateral IPSCs (Fig. 2C). Since the extracellular and intracellular Cl− concentrations were symmetric and the cells were voltage-clamped at −70 mV, the somatically measured unitary IPSC conductance activated by a single neighboring D2-MSN was estimated to be ∼2 nS (Fig. 2C); the true unitary collateral IPSC conductance was likely larger because collateral synapses are commonly formed on dendrites (Wilson and Groves 1980; Wilson, 2007), whereas the recording was somatic, leading to a dendritic filtering and attenuation of the recorded signal.
Connection preference and direction.
As shown Fig. 2A, the D2-MSN→D2-MSN connection rate was significantly higher than the D2-MSN→D1-MSN connection rate in both Pitx3WT mice and Pitx3Null mice (55.3% vs. 25.9% in Pitx3WT mice, chi-square test: χ2 = 6.38 > χ0.05,12 = 3.84, P < 0.05; 60% vs. 30.4% in Pitx3Null mice, chi-square test: χ2 = 4.37 > χ0.05,12 = 3.84, P < 0.05). These results indicate a homotypic collateral connection preference among neighboring D2-MSNs over neighboring D1-MSNs. Additionally, no reciprocal connection was detected in 27 D2-MSN→D2-MSN pairs in the DA-intact dorsal striatum in WT mice and 23 D2-MSN→D2-MSN pairs DA-denervated dorsal striatum in Pitx3Null mice. These results indicate that D2-MSN collateral innervation is built in a way that is conducive for receiving and processing cortical and thalamic inputs and then producing appropriate outputs, although little is known about this hierarchy due to the lack of a recognizable anatomical pattern.
DA Modulation of D2-MSN-Originated Collateral Inhibition in Normal and DA-Denervated Striatum
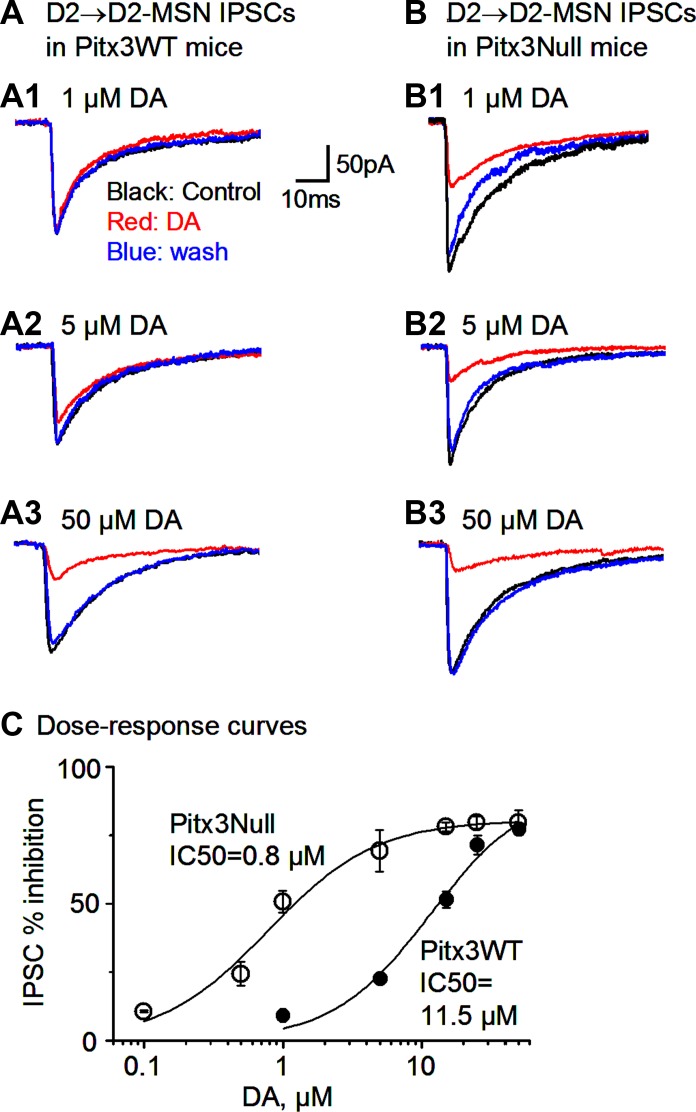
Next, we examined the DA modulation of the inhibition originated from D2-MSNs in normal and DA-denervated striatum. Because D2Rs are the only or the predominant DA receptors expressed in the D2-MSNs, we predicted that DA might inhibit the collateral IPSCs originated from D2-MSNs. Indeed, bath application of 50 μM and 25 μM DA reduced the D2-MSN→D2-MSN IPSC peak amplitude by 77.2 ± 1.8% and 71.4 ± 3.4% in Pitx3WT mice, respectively, and these effects recovered after DA was washed out DA: the IPSC amplitude was 96.9 ± 30.0, 22.1 ± 7.3, and 65.6 ± 26.4 pA before, during, and after 50 μM DA application, respectively (n = 7 pairs, P < 0.05, paired t-test), and 105.0 ± 23.5, 29.5 ± 6.2, and 75.0 ± 17.1 pA before, during, and after 25 μM DA application, respectively (n = 9 pairs, P < 0.05, paired t-test; Fig. 6A).
Fig. 6.
Supersensitive DA inhibition of D2-MSN→D2-MSN collateral inhibition in Pitx3Null mice. A: D2-MSN→D2-MSN average IPSCs before, during, and after bath application of 1 (A1), 5 (A2), and 50 μM DA (A3) in the dorsal striatum in Pitx3WT mice. B: D2-MSN→D2-MSN average IPSCs before, during, and after bath application of 1 (B1), 5 (B2), and 50 μM DA (B3) in the parkinsonian dorsal striatum in Pitx3Null mice. C: dose-response curves of DA inhibiting D2-MSN→D2-MSN collateral IPSCs in Pitx3WT and Pitx3Null mice. Solid curves are data points fitted to the Hill equation. The IC50 value is indicated for each genotype.
In Pitx3Null mice, 50 and 25 μM DA reduced the peak amplitude of D2-MSN-originated collateral IPSCs by 77.1 ± 3.5% and 76.0 ± 4.4%, respectively: the IPSC amplitude was 101.8 ± 29.0 and 26.1 ± 10.0 pA before and during 50 μM DA, respectively (n = 7 pairs), and 117.4 ± 35.9 and 23.1 ± 5.5 pA before and during 25 μM DA, respectively (n = 7 pairs; Fig. 6B). DA-induced inhibition was recovered upon washout. As shown in Fig. 6, the inhibitory effects were similar for 25 and 50 μM DA in both Pitx3WT and Pitx3Null mice [F(3,26) = 0.66, P = 0.57, one-way ANOVA], indicating that 25 μM DA was saturating in both Pitx3WT and Pitx3Null mice and that the maximal D2R inhibition of the D2-MSN-originated IPSCs was similar in WT mice and DA-deficient mice, but lower doses of DA may induce different effects.
Thus we used lower doses (0.1 to 15 μM) of DA to inhibit the D2-MSN-originated collateral IPSCs. We found that the D2-MSN-originated collateral IPSCs in Pitx3Null mice were more sensitive to lower concentrations of DA than those in Pitx3WT mice (Fig. 6C). Hill equation fitting indicates the IC50 was 0.8 μM for Pitx3Null mice and 11 μM for Pitx3WT mice, whereas the slope factor was similar: 1.2 for Pitx3Null mice and 1.1 for Pitx3WT mice (Fig. 6C). These results indicate that D2Rs on the D2-MSN axon collaterals had a higher affinity to and were supersensitive to DA after striatal DA denervation, although the maximal D2R inhibition was similar in the two genotypes.
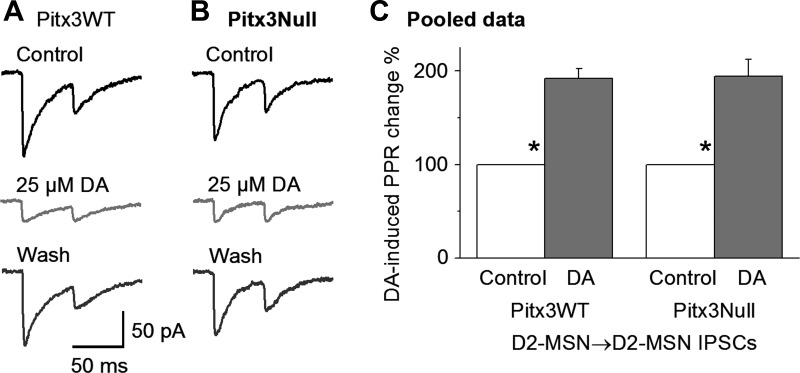
To determine if the DA inhibition of D2-MSN→D2-MSN collateral IPSCs was of presynaptic origin, we examined DA's effects on the PPR in D2-MSN→D2-MSN collateral IPSCs. Whereas the first IPSC was reduced as described above, 25 μM DA increased the PPR by 91.9 ± 10.6% and 94.2 ± 17.8% in Pitx3WT (n = 13 pairs, P < 0.05, paired t-test, before and during DA) and Pitx3Null mice (n = 11 pairs, P < 0.05, paired t-test, before and during DA), respectively (Fig. 7, A–C). These results indicate that the DA inhibition was a presynaptic effect, mediated by reducing GABA release from the D2-MSN→D2-MSN collateral axon terminal.
Fig. 7.
DA increases the paired-pulse ratio (PPR) of D2-MSN→D2-MSN collateral IPSCs. A and B: bath application of 25 μM DA strongly reduced the peak amplitude of the first average IPSC but weakly reduced the peak amplitude of the second average IPSC, thus increasing the PPR of the D2-MSN→D2-MSN IPSCs, in the dorsal striatum in Pitx3WT (A) and Pitx3Null mice (B). C: pooled data showing that 25 μM DA increased the PPR of D2-MSN→D2-MSN collateral IPSCs. *P < 0.05, paired t-test.
Taken together, these results indicate that in both Pitx3WT mice and Pitx3Null mice, DA inhibits the D2-MSN→D2-MSN IPSC peak amplitude through presynaptic D2Rs and that the presynaptic D2Rs on the D2-MSN→D2-MSN axon collaterals are supersensitive in the DA-denervated dorsal striatum in Pitx3Null mice.
Different Release Properties at D2-MSN Collateral Axon Terminals and Projection Axon Terminals
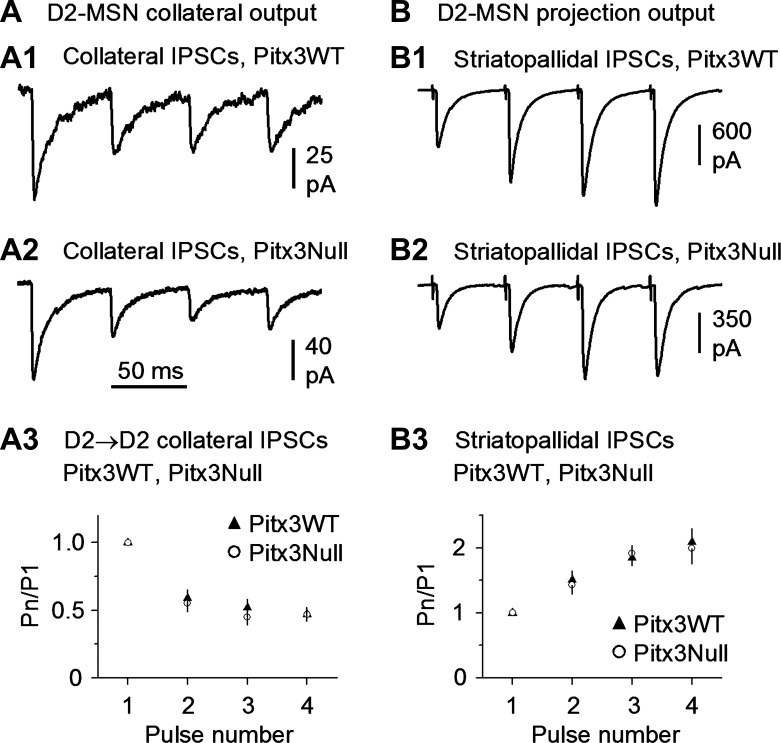
To test the release properties of the D2-MSN→D2-MSN collateral synapse, we used a train of 4 depolarizing pulses with the interpulse interval being 50 ms or 20 Hz, a common frequency for MSN phasic firing (Miller et al. 2008). The bathing solution was the standard normal extracellular solution with 2.5 mM Ca2+. Under these conditions, the 4-pulse stimulation train in presynaptic D2-MSNs consistently induced depressing IPSCs in postsynaptic MSNs in both the dorsal striatum in Pitx3WT mice (5 D2-MSN→D2-MSN pairs; Fig. 8A1) and the DA-denervated dorsal striatum in Pitx3Null mice (5 D2-MSN→D2-MSN pairs; Fig. 8A2). In separate experiments, we used an extracellular bipolar stimulating electrode placed in the dorsal striatum to evoke D2-MSN striatopallidal projection IPSCs in the presumed GABA neurons in the globus pallidus, using the methods described by Wei et al. (2013). As shown in Fig. 8, B1 and B2, a 20-Hz, 4-pulse stimulation train evoked facilitating striatopallidal IPSCs in both Pitx3WT (n = 5 cells) and Pitx3Null mice (n = 5 cells). These results indicate that D2-MSN→D2-MSN axon collateral terminals and the main projection axon terminals have opposite release properties during repetitive firing and thus can serve different functional needs for the circuits.
Fig. 8.
D2-MSN→D2-MSN intrastriatal collateral axon terminals and extrastriatal projection axon terminals have opposite release properties during repetitive stimulation. A: D2-MSN→D2-MSN collateral unitary IPSCs were depressing during repetitive release at 20 Hz, a common MSN phasic firing frequency, in both DA-intact dorsal striatum in WT mice (A1) and DA-denervated dorsal striatum in Pitx3Null mice (A2). Summary data are shown in A3. B: dorsal striatum-evoked striatopallidal IPSCs were facilitating during repetitive release at 20 Hz in both DA-intact WT mice (B1) and DA-denervated Pitx3Null mice (B2). Also note that the repetitive release properties were similar in DA-intact WT and DA-deficient Pitx3Null mice. Summary data are shown in B3.
DISCUSSION
Our main findings are that neighboring homotypic MSNs have a 50% chance of forming one-way collateral connections and that DA loss alters presynaptic DA regulation of MSN collateral inhibition, potentially contributing to the profound behavioral importance of the striatum.
High-Rate, One-Way, and Homotypically Preferential MSN Collateral Connections in Normal and Parkinsonian Striatum
We recorded a combined total of 666 MSN pairs in normal and DA-denervated striatum, probably the largest such data set in the literature. We detected a 40% connection rate among neighboring D1-MSNs and a 60% connection rate among neighboring D2-MSNs in brain slices from both WT mice and DA-denervated Pitx3Null mice when the two chosen MSNs were within 50 μm of each other in both normal and parkinsonian striatum. These estimates from DA-intact WT mice are higher than previous ones obtained in the same DA-intact D2-GFP mice (26% for D1-D1 pairs, 34% for D2-D2 pairs; Taverna et al. 2008). We argue that the higher rates detected in the present study are more reliable than previous estimates because collateral connections can only be lost during brain slice preparation and the recording procedure. We also found that in the dorsal striatum, the D1-MSNs preferentially form collateral connections with neighboring D1-MSNs and the D2-MSNs preferentially form collateral connections with neighboring D2-MSNs in both Pitx3WT and Pitx3Null mice, partially consistent with the findings of Taverna et al. (2008).
We need to emphasize that the high MSN collateral connection rates in Pitx3Null mice are not due to the perinatal onset DA loss, because the connection rates are similar in Pitx3Null and WT mice. We have established that the behavioral phenotypes are similar in Pitx3Null mice with perinatal onset DA loss and in WT mice with adult onset DA loss when the DA loss severity and pattern are similar (Li et al. 2015), demonstrating that for the consequences of DA loss, the severity and pattern are the determining factors and the timing is not.
The high MSN collateral connection rate detected in our present study is also unlikely due to mistaking GABAergic interneurons for D1- and D2-MSNs, because 1) D2-GFP is specific for D2-MSNs and GABAergic interneurons do not express D2 receptors, 2) the spiking properties of GABAergic interneurons are different from those of MSNs even with a partially CsCl-containing pipette solution, and 3) assuming all our cell identification methods fail, an unlikely scenario, GABAergic interneurons are so rare that at most they may add noise (e.g., a few outlying cells) to our large data sets but cannot alter the overall characteristics of these data sets.
Additionally, we found that the D1-MSN→D1-MSN and D2-MSN→D2-MSN collateral connection rates were similar in Pitx3WT mice and Pitx3Null mice, indicating that the axon collateral connections in the dorsal striatum were not disrupted after DA denervation. There also were no significant differences in the collateral IPSC peak amplitudes and shape between Pitx3WT and Pitx3Null mice. Our results indicate that DA depletion in the dorsal striatum does not affect the formation, maintenance, and basic properties of MSN collateral synapses, consistent with our prior studies showing that DA depletion does not affect the basic properties of striatonigral and striatopallidal synapses (Ding et al. 2015a; Wei et al. 2013). Our results are also consistent with those of Zhou and Palimiter (1995), who concluded that DA is required for the normal operation of the striatum but is not required for the normal development of the striatum. However, our present finding is inconsistent with the report by Taverna et al. (2008) that acute DA depletion with reserpine or 6-OHDA lesion attenuates the collateral connectivity among D1-MSNs and among D2-MSNs. Although the reasons for this inconsistency is not known, and future studies using paired recording in identical animal models are needed to resolve this issue, the fact that dyskinesias and impulsive-compulsive behaviors are triggered by DA replacement therapy (Bastide et al. 2015) is consistent with our present finding that the basal collateral connection is normal but the DA regulation of the collateral connection is abnormal. See additional discussion on this point below.
Our data also show that despite the high D1-MSN→D1-MSN and D2-MSN→D2-MSN connection rates, no reciprocal connection was detected in the DA-intact and the DA-denervated dorsal striatum, consistent with the literature (Koos et al. 2004; Taverna et al. 2004). This one-way direction and the homotypic preference, together, indicate that there is a currently hidden hierarchy in MSN collateral connections that needs to be studied in the future.
Loss of Presynaptic D1R Facilitation of D1-MSN-Originated Collateral Inhibition in DA-Denervated Striatum
Presynaptic D1R activation is known to facilitate neurotransmitter release, probably via the cAMP pathway (Ding et al. 2015a). We observed in this study that in normal mice, DA and the D1R agonist SKF81297 each facilitated the D1-MSN→D1-MSN collateral IPSCs, consistent with a previous study in normal animals (Tecuapetla et al. 2007). We have also documented that D1Rs at the striatonigral axon terminals facilitate GABA release in a supersensitive manner in DA-deficient Pitx3Null mice (Ding et al. 2015a). In our present study, to our surprise, the D1R facilitation at the D1-MSN→D1-MSN collateral synapse was lost, rather than upregulated as expected, in the DA-denervated dorsal striatum in drug-naive Pitx3Null mice. The underlying mechanism is not known, but we speculate that the cell membrane expression of D1Rs at the collateral axon terminals may be eliminated after DA denervation; future ultrastructural studies are needed to resolve this issue. Additionally, different DA loss-induced alterations at collateral and projection axon terminals are possible because these axon terminals are different even under the baseline condition; this is further discussed below.
Supersensitive Presynaptic D2R Inhibition of D2-MSN-Originated Collateral Inhibition in DA-Denervated Striatum
We have documented that in DA-denervated striatum in Pitx3Null mice and in WT mice with reserpine-induced DA depletion, D2Rs on the projection axon terminals of the D2-MSNs inhibit the striatopallidal IPSCs in a supersensitive manner (Wei et al. 2013). Our present study clearly shows that D2Rs at the D2-MSN→D2-MSN axon collaterals were supersensitive, with a smaller IC50, in the DA-denervated dorsal striatum in Pitx3Null mice. The most likely mechanism for the D2R supersensitivity is an increased coupling efficiency to G proteins, not increased D2R expression, since there was no change in the total D2R mRNA and the total D2R bindings in the striatum in Pitx3Null mice (Cremer et al. 2015; Li et al. 2013). Furthermore, the supersensitive presynaptic D2R inhibition was observed in drug-naive Pitx3Null mice, indicating that DA loss is sufficient to trigger the molecular process that upregulates D2Rs in the collateral axon terminals, consistent with our behavioral data showing that the first dose of l-3,4-dihydroxyphenylalanine (l-DOPA) stimulates both normal and abnormal motor and nonmotor activities (Li and Zhou 2013).
MSN Collateral Axon Terminals and Projection Axon Terminals Have Opposite Release Properties
Because MSN axonal collaterals and the projection axons originate from the same axon trunk, one may assume that they have identical GABA release properties. Our data indicate that this assumption is incorrect for both D1- and D2-MSNs. We found that GABA release from the collateral axon terminals of D1- and D2-MSNs is depressing during repetitive stimulation in WT and Pitx3Null mice. This synaptic depression is not due to the whole cell stimulation configuration, because a similar synaptic depression was observed when the collateral IPSCs were evoked by antidromic stimulation in the globus pallidus (Tecuapetla et al. 2007). In contrast, GABA release from the D1-MSN striatonigral axon terminals and D2-MSN striatopallidal axon terminals is facilitatory during repetitive stimulation. These results indicate that MSN axon collateral terminals and their projection axon terminals have opposite release properties during repetitive MSN firing, likely serving different functional needs for collateral and projection synapses. For example, the projection axons of D1- and D2-MSNs serve to strongly inhibit their long-distance target neurons in the GPe, GPi, and SNr; thus the self-facilitating GABA release is a built-in mechanism to achieve this strong inhibition. In contrast, the MSN collateral inhibition probably serves to fine-tune the function of the MSN network by gently and briefly inhibiting neighboring MSNs, and the self-depressing GABA release is a built-in safety mechanism to ensure that the collateral inhibition is not too strong to prevent a stall of the MSN network.
Functional Significance of the Changes of DA Modulation on the Axon Collateral Inhibition Within the Striatum
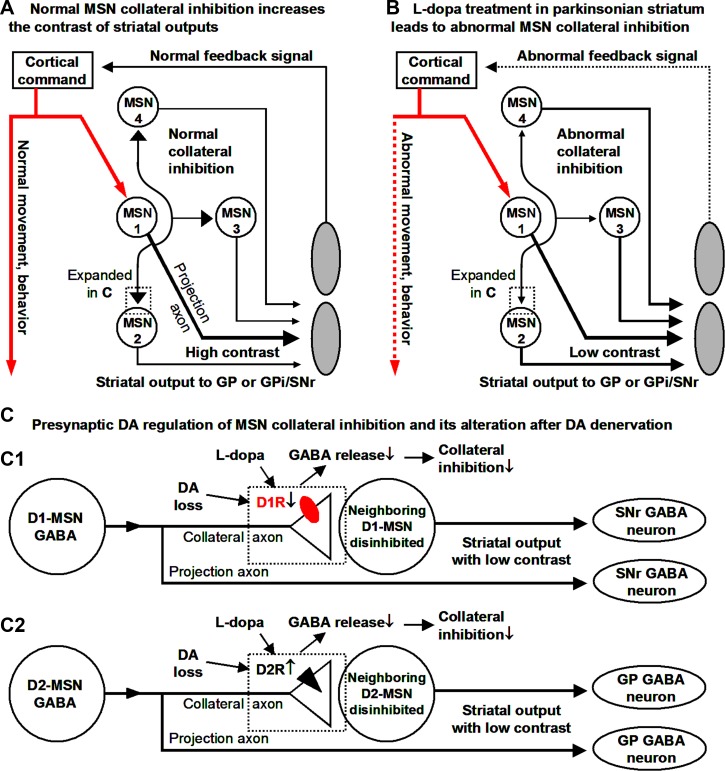
First, the high-rate, one-way, and homotypically preferential MSN collateral inhibition originating from cortically and thalamically activated, behaviorally important MSN activity can inhibit downstream MSNs that may be spuriously active, thus improving the contrast of the striatal and basal ganglia output signal for the desired movement and behavior (Fig. 9A). This effect can be substantial when multiple presynaptic D1- or D2-MSNs are activated within a short time window to produce summating IPSPs, an entirely possible scenario given that a single MSN receives collateral synaptic inputs from several hundred MSNs (Wilson 2007). Second, in DA-denervated striatum, both the elimination of presynaptic D1R-mediated facilitation at D1-MSN→D1-MSN collaterals and the upregulation of presynaptic D2R inhibition at D2-MSN→D2-MSN collaterals lead to a DA-induced reduction of collateral inhibition during l-DOPA treatment of PD (Fig. 9, B and C). Thus, whereas normal MSN collateral inhibition helps the basal ganglia focus onto a particular motor or behavioral task by inhibiting competing motor or behavioral tasks in a normal brain (Mink 1996), this focusing function is diminished during dopaminergic treatment in PD, potentially contributing to the dopaminergic induction of aberrant motor and nonmotor behaviors in PD patients (Bastide et al. 2015).
Fig. 9.
In the parkinsonian striatum, the altered presynaptic DA regulation weakens the collateral inhibition and may reduce the contrast of striatal outputs. The contrast could be defined as the ratio of the GABA release from a behaviorally activated MSN to the GABA release from a neighboring, nonbehaviorally activated MSN. A: cortical input excites the target MSN (MSN 1), which produces a long-distance GABA output projecting to and inhibiting GP or SNr and also a local collateral output inhibiting downstream neighboring MSNs (MSNs 2-4) that are not the target of the cortical input, reducing the output from these 3 MSNs and hence increasing the contrast between MSN 1 outputs and the outputs of MSNs 2–4. B: MSN collateral inhibition is diminished during dopaminergic treatment in the parkinsonian striatum, disinhibiting downstream neighboring MSNs 2–4 and hence decreasing the contrast between MSN 1 outputs and the outputs of MSNs 2–4. C: DA denervation-induced loss of D1R function in the D1-MSN-originated collateral axon terminals (C1) and DA denervation-induced supersensitivity of D2Rs in the D2-MSN-originated collateral axon terminals (C2) lead to diminished MSN collateral inhibition during dopaminergic treatment.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants R01NS097671 and R03NS085380 (to F.-M. Zhou).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.W. and F.-M.Z. conceived and designed research; W.W., S.D., and F.-M.Z. performed experiments; W.W. and F.-M.Z. analyzed data; W.W. and F.-M.Z. interpreted results of experiments; W.W. and F.-M.Z. prepared figures; W.W. and F.-M.Z. drafted manuscript; W.W. and F.-M.Z. edited and revised manuscript; W.W., S.D., and F.-M.Z. approved final version of manuscript.
REFERENCES
- Bastide MF, Meissner WG, Picconi B, Fasano S, Fernagut PO, Feyder M, Francardo V, Alcacer C, Ding Y, Brambilla R, Fisone G, Jon Stoessl A, Bourdenx M, Engeln M, Navailles S, De Deurwaerdère P, Ko WK, Simola N, Morelli M, Groc L, Rodriguez MC, Gurevich EV, Quik M, Morari M, Mellone M, Gardoni F, Tronci E, Guehl D, Tison F, Crossman AR, Kang UJ, Steece-Collier K, Fox S, Carta M, Angela Cenci M, Bézard E. Pathophysiology of l-dopa-induced motor and non-motor complications in Parkinson's disease. Prog Neurobiol 132: 96–168, 2015. [DOI] [PubMed] [Google Scholar]
- Chang HT, Wilson CJ, Kitai ST. Single neostriatal efferent axons in the globus pallidus: a light and electron microscopic study. Science 213: 915–918, 1981. [DOI] [PubMed] [Google Scholar]
- Chuhma N, Tanaka KF, Hen R, Rayport S. Functional connectome of the striatal medium spiny neuron. J Neurosci 31: 1183–1192, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey KR, Nader M, West MO. Single body parts are processed by individual neurons in the mouse dorsolateral striatum. Brain Res 1636: 200–207, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvol JC, Muriel MP, Valjent E, Féger J, Hanoun N, Girault JA, Hirsch EC, Hervé D. Persistent increase in olfactory type G-protein alpha subunit levels may underlie D1 receptor functional hypersensitivity in Parkinson disease. J Neurosci 24: 7007–7014, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science 197: 596–598, 1977. [DOI] [PubMed] [Google Scholar]
- Cremer JN, Amunts K, Graw J, Piel M, Rösch F, Zilles K. Neurotransmitter receptor density changes in Pitx3ak mice–a model relevant to Parkinson's disease. Neuroscience 285: 11–23, 2015. [DOI] [PubMed] [Google Scholar]
- Ding S, Li L, Zhou FM. Presynaptic serotonergic gating of the subthalamonigral glutamatergic projection. J Neurosci 33: 4875–4885, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Li L, Zhou FM. Nigral dopamine loss induces a global upregulation of presynaptic dopamine D1 receptor facilitation of the striatonigral GABAergic output. J Neurophysiol 113: 1697–1711, 2015a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Li L, Zhou FM. Robust presynaptic serotonin 5-HT1B receptor inhibition of the striatonigral output and its sensitization by chronic fluoxetine treatment. J Neurophysiol 113: 3397–3409, 2015b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs LK, Kaplan AR, Lemos JC, Matsui A, Rubinstein M, Alvarez VA. Dopamine regulation of lateral inhibition between striatal neurons gates the stimulant actions of cocaine. Neuron 90: 1100–1113, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Curr Opin Neurobiol 21: 269–274, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, Brooks JM, Iñiguez SD, O'Donnell P, Kravitz A, Lobo MK. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry 77: 212–222, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DM, Kravitz AV. Working together: basal ganglia pathways in action selection. Trends Neurosci 37: 301–303, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama F, Sohn J, Nakano T, Furuta T, Nakamura KC, Matsuda W, Kaneko T. Exclusive and common targets of neostriatofugal projections of rat striosomes neurons: a single neuron-tracing study using a viral vector. Eur J Neurosci 33: 668–677, 2011. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Bolam JP. The neuroanatomical organization of the basal ganglia. In: Handbook of Basal Ganglia Structure and Function (2nd ed) edited by Steiner H, Tseng KY. London: Academic, 2016, p. 3–30. [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34: 441–466, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. The pattern of neurodegeneration in Huntington's disease: a comparative study of cannabinoid, dopamine, adenosine and GABAA receptor alterations in the human basal ganglia in Huntington's disease. Neuroscience 97: 505–519, 2000. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425: 917–925, 2003. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Grafton ST. The striatum: where skills and habits meet. Cold Spring Harb Perspect Biol 7: a021691, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán JN, Hernández A, Galarraga E, Tapia D, Laville A, Vergara R, Aceves J, Bargas J. Dopaminergic modulation of axon collaterals interconnecting spiny neurons of the rat striatum. J Neurosci 23: 8931–8940, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35: 4–26, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Yang C, Tan A. Basal ganglia circuit loops, dopamine and motivation: a review and enquiry. Behav Brain Res 290: 17–31, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci 13: 4908–4923, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Neostriatal cell subtypes and their functional roles. Neurosci Res 27: 1–8, 1997. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol 62: 1052–1068, 1989. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci 10: 3421–3438, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med 318: 876–880, 1988. [DOI] [PubMed] [Google Scholar]
- Koós T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci 2: 467–472, 1999. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM, Wilson CJ. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J Neurosci 24: 7916–7922, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466: 622–626, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaHoste GJ, Marshall JF. Rapid development of D1 and D2 dopamine receptor supersensitivity as indicated by striatal and pallidal Fos expression. Neurosci Lett 179: 153–156, 1994. [DOI] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci USA 90: 8861–8865, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Qiu G, Ding S, Zhou FM. Serotonin hyperinnervation and upregulated 5-HT2A receptor expression and motor-stimulating function in nigrostriatal dopamine-deficient Pitx3 mutant mice. Brain Res 1491: 236–250, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Sagot B, Zhou FM. Similar L-dopa-stimulated motor activity in mice with adult-onset 6-hydroxydopamine-induced symmetric dopamine denervation and in transcription factor Pitx3 null mice with perinatal-onset symmetric dopamine denervation. Brain Res 1615: 12–21, 2015. [DOI] [PubMed] [Google Scholar]
- Li L, Zhou FM. Parallel dopamine D1 receptor activity dependence of l-Dopa-induced normal movement and dyskinesia in mice. Neuroscience 236: 66–76, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, Nugent A, Finkel E, Chaudhury D, Chandra R, Riberio E, Rabkin J, Mouzon E, Cachope R, Cheer JF, Han MH, Dietz DM, Self DW, Hurd YL, Vialou V, Nestler EJ.. ΔFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci 33: 18381–18395, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Huerta VG, Carrillo-Reid L, Galarraga E, Tapia D, Fiordelisio T, Drucker-Colin R, Bargas J. The balance of striatal feedback transmission is disrupted in a model of parkinsonism. J Neurosci 33: 4964–4975, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Walker AG, Shah AS, Barton SJ, Rebec GV. Dysregulated information processing by medium spiny neurons in striatum of freely behaving mouse models of Huntington's disease. J Neurophysiol 100: 2205–2216, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50: 381–425, 1996. [DOI] [PubMed] [Google Scholar]
- Moyer JT, Halterman BL, Finkel LH, Wolf JA. Lateral and feedforward inhibition suppress asynchronous activity in a large, biophysically-detailed computational model of the striatal network. Front Comput Neurosci 8: 152, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum ES, Wilson CJ. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J Neurosci 15: 4449–4463, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes I, Tovmasian LT, Silva RM, Burke RE, Goff SP. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci USA 100: 4245–4250, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifl C, Nanoff C, Schingnitz G, Schütz W, Hornykiewicz O. Sensitization of dopamine-stimulated adenylyl cyclase in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated rhesus monkeys and patients with idiopathic Parkinson's disease. J Neurochem 58: 1997–2004, 1992. [DOI] [PubMed] [Google Scholar]
- Preston RJ, Bishop GA, Kitai ST. Medium spiny neuron projection from the rat striatum: an intracellular horseradish peroxidase study. Brain Res 183: 253–263, 1980. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso JA. Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat Rev Neurosci 11: 760–772, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig R, Silberberg G. Multisensory integration in the mouse striatum. Neuron 83: 1200–1212, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Révy D, Jaouen F, Salin P, Melon C, Chabbert D, Tafi E, Concetta L, Langa F, Amalric M, Kerkerian-Le Goff L, Marie H, Beurrier C. Cellular and behavioral outcomes of dorsal striatonigral neuron ablation: new insights into striatal functions. Neuropsychopharmacology 39: 2662–2672, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PE, Fuccillo MV, Maxeiner S, Hayton SJ, Gokce O, Lim BK, Fowler SC, Malenka RC, Südhof TC. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell 158: 198–212, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Chiken S, Hikida T, Kobayashi K, Nambu A. Signals through the striatopallidal indirect pathway stop movements by phasic excitation in the substantia nigra. J Neurosci 33: 7583–7594, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology 35: 27–47, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron 65: 585–596, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippy T, Lapray D, Crochet S, Petersen CC. Cell-type-specific sensorimotor processing in striatal projection neurons during goal-directed behavior. Neuron 88: 298–305, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidt MP, Smits SM, Bouwmeester H, Hamers FP, van der Linden AJ, Hellemons AJ, Graw J, Burbach JP. Early developmental failure of substantia nigra dopamine neurons in mice lacking the homeodomain gene Pitx3. Development 131: 1145–1155, 2004. [DOI] [PubMed] [Google Scholar]
- Staunton DA, Magistretti PJ, Koob GF, Shoemaker WJ, Bloom FE. Dopaminergic supersensitivity induced by denervation and chronic receptor blockade is additive. Nature 299: 72–74, 1982. [DOI] [PubMed] [Google Scholar]
- Taverna S, van Dongen YC, Groenewegen HJ, Pennartz CM. Direct physiological evidence for synaptic connectivity between medium-sized spiny neurons in rat nucleus accumbens in situ. J Neurophysiol 91: 1111–1121, 2004. [DOI] [PubMed] [Google Scholar]
- Taverna S, Ilijic E, Surmeier DJ. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson's disease. J Neurosci 28: 5504–5512, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Carrillo-Reid L, Bargas J, Galarraga E. Dopaminergic modulation of short-term synaptic plasticity at striatal inhibitory synapses. Proc Natl Acad Sci USA 104: 10258–10263, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Koós T, Tepper JM, Kabbani N, Yeckel MF. Differential DArgic modulation of neostriatal synaptic connections of striatopallidal axon collaterals. J Neurosci 29: 8977–8990, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Koós T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci 27: 662–669, 2004. [DOI] [PubMed] [Google Scholar]
- Thomson AM. Molecular frequency filters at central synapses. Prog Neurobiol 62: 159–196, 2000. [DOI] [PubMed] [Google Scholar]
- Trugman JM, James CL. Rapid development of dopaminergic supersensitivity in reserpine-treated rats demonstrated with 14C-2-deoxyglucose autoradiography. J Neurosci 12: 122875–122879, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall MJ, Oorschot DE, Kean A, Wickens JR. Inhibitory interactions between spiny projection neurons in the rat striatum. J Neurophysiol 88: 1263–1269, 2002. [DOI] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Hervé D, Fisone G, Girault JA. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci 32: 538–547, 2009. [DOI] [PubMed] [Google Scholar]
- van den Munckhof P, Luk KC, Ste-Marie L, Montgomery J, Blanchet PJ, Sadikot AF, Drouin J. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development 130: 2535–2542, 2003. [DOI] [PubMed] [Google Scholar]
- Wei W, Li L, Yu G, Ding S, Li C, Zhou FM. Supersensitive presynaptic dopamine D2 receptor inhibition of the striatopallidal projection in nigrostriatal dopamine-deficient mice. J Neurophysiol 110: 2203–2216, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens JR, Arbuthnott GW, Shindou T. Simulation of GABA function in the basal ganglia: computational models of GABAergic mechanisms in basal ganglia function. Prog Brain Res 160: 313–329, 2007. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. GABAergic inhibition in the neostriatum. Prog Brain Res 160: 91–110, 2007. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J Comp Neurol 194: 599–615, 1980. [DOI] [PubMed] [Google Scholar]
- Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience 65: 709–730, 1995. [DOI] [PubMed] [Google Scholar]
- Zhou FM. The substantia nigra pars reticulata. In: Handbook of Basal Ganglia Structure and Function (2nd ed), edited by Steiner H, Tseng KY. London: Academic, 2016, p. 293–316. [Google Scholar]
- Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83: 1197–1209, 1995. [DOI] [PubMed] [Google Scholar]