Abstract
AIM
To evaluate the prognostic value of the phase angle (PA) obtained from bioelectrical impedance analysis (BIA) for mortality prediction in patients with cirrhosis.
METHODS
In total, 134 male cirrhotic patients prospectively completed clinical evaluations and nutritional assessment by BIA to obtain PAs during a 36-mo follow-up period. Mortality risk was analyzed by applying the PA cutoff point recently proposed as a malnutrition marker (PA ≤ 4.9°) in Kaplan-Meier curves and multivariate Cox regression models.
RESULTS
The patients were divided into two groups according to the PA cutoff value (PA > 4.9°, n = 73; PA ≤ 4.9°, n = 61). Weight, height, and body mass index were similar in both groups, but patients with PAs > 4.9° were younger and had higher mid-arm muscle circumference, albumin, and handgrip-strength values and lower severe ascites and encephalopathy incidences, interleukin (IL)-6/IL-10 ratios and C-reactive protein levels than did patients with PAs ≤ 4.9° (P ≤ 0.05). Forty-eight (35.80%) patients died due to cirrhosis, with a median of 18 mo (interquartile range, 3.3-25.6 mo) follow-up until death. Thirty-one (64.60%) of these patients were from the PA ≤ 4.9° group. PA ≤ 4.9° significantly and independently affected the mortality model adjusted for Model for End-Stage Liver Disease score and age (hazard ratio = 2.05, 95%CI: 1.11-3.77, P = 0.021). In addition, Kaplan-Meier curves showed that patients with PAs ≤ 4.9° were significantly more likely to die.
CONCLUSION
In male patients with cirrhosis, the PA ≤ 4.9° cutoff was associated independently with mortality and identified patients with worse metabolic, nutritional, and disease progression profiles. The PA may be a useful and reliable bedside tool to evaluate prognosis in cirrhosis.
Keywords: Bioelectrical impedance analysis, Body composition, Phase angle, Nutritional assessment, Liver disease, Cirrhosis, Mortality
Core tip: This article provides original data displaying the good performance of the phase angle (PA) obtained by bioelectrical impedance analysis in the evaluation of mortality prognosis in patients with cirrhosis. The findings suggest that the PA is a safe, practical, and inexpensive tool for the prediction of mortality potentially associated with malnutrition.
INTRODUCTION
Liver transplantation (LT) is the best option for patients with advanced cirrhosis, but its clinical application is often limited by the low availability of organ donors, risk of organ rejection, and implied high cost[1,2]. Consequently, the control and treatment of cirrhosis-associated complications remains the mainstay for this population. Malnutrition is a major complication often observed in patients with cirrhosis, and it has been associated with more severe disease, the manifestation of other cirrhosis-associated complications, and mortality[3]. Early diagnosis of malnutrition in patients with cirrhosis is important for prompt management and to improve quality of life[4-7].
In general, ascites, edema, and other chronic liver disease-associated complications (i.e., altered immunocompetence, decreased protein synthesis, and renal failure) can impair the performance of traditionally applied criteria for nutritional assessment (NA)[8]. Consequently, weight loss, anthropometric measurements, the creatinine-height index, nitrogen balance, lymphocyte count, and serum albumin, transferrin, prealbumin, and retinol-bound protein levels should be interpreted with restrictions when assessing the nutritional status of cirrhotic patients[9]. In this scenario, a gold standard NA method is required for the proper diagnosis of malnutrition in this patient population[10-15].
The phase angle (PA) obtained from bioimpedance analysis (BIA) has been proposed as a nutritional status marker, with low values associated with malnutrition and nutritional risk at the time of hospital admission[16]. The PA reflects the relationship between the resistance component (R), meaning tissue opposition to the passage of electric current, and reactance (Xc), meaning the resistance effect produced by the interface of tissues and cell membranes[17]. A main advantage of the use of PA is that it can be applied even under unstable tissue hydration conditions, such as edema and ascites[18].
By potentially reflecting malnutrition, the PA can be a useful prognostic marker in several clinical settings[16,18-29]. As with any biological marker, the PA is influenced by the specific characteristics of each clinical population and may vary according to sex and age. Thus, specific PA reference and cutoff values have been proposed to establish prognoses for different diseases[16,18-26,30-34]. Recently, the 4.9° PA value was identified as the best cut-off for malnutrition associated to disease severity of patients with liver cirrhosis and shown to have important prognostic value for malnutrition-associated mortality in this patient population[35].
In this study, we aimed to test whether this PA cutoff (≤ 4.9°) had prognostic value for mortality in a population of patients with cirrhosis of different ethnicity than used for its initial identification.
MATERIALS AND METHODS
Patients
This study included 134 male patients with biopsy-proven cirrhosis who were recruited prospectively from the Digestive Tract Surgery Service at the Hospital das Clínicas of the University of São Paulo Medical School between January 2012 and December 2014. Exclusion criteria were alcohol abuse; human immunodeficiency virus positivity; cancer diagnosis, acute liver failure, or chronic or acute disease of the lung, kidney, or heart; previous LT; orthopedic prosthesis use; and dementia. All patients provided written informed consent before trial participation.
Protocol design
Our protocol was designed to determine whether the PA has prognostic value for mortality in male patients with cirrhosis, by considering the PA cutoff point proposed by Ruiz-Margáina et al[35] (PA ≤ 4.9°) as a malnutrition marker. All recruited subjects were instructed to refrain from excessive physical activity, diuretic use, and alcohol consumption for 24 h before the assessment, which was performed in a 4-h fasting state[36]. Demographic data were recorded for all subjects. Death events were recorded for all patients with cirrhosis during the 36-mo follow-up period. A single trained technician performed all study procedures according to the ethical standards of the Declaration of Helsinki of the World Medical Association. All procedures were approved by the Institutional Ethics Review Board (0646/11) and registered at https://www.clinicaltrials.gov/ (NCT02421848).
Demographic and clinical data collection
The following demographic, clinical, inflammatory, and anthropometric data were collected: Age, liver cirrhosis etiology, Child-Pugh and Model for End-Stage Liver Disease (MELD) scores, presence of severe ascites, presence of encephalopathy, interleukin (IL)-6/IL-10 ratio, C-reactive protein (CRP) level, body weight and height, body mass index (BMI), non-dominant handgrip-strength (ND-HGS), and mid-arm muscle circumference (MAMC). Body weight was measured with the participant standing in the center of a single electronic scale platform (ADP; BOD PODTM BC system device; Life Measurement Instruments, Concord, CA, United States) while barefoot and wearing only light clothes[37]. Height was measured with a single stadiometer (Sanny, São Paulo, SP, Brazil) with the individual standing barefoot with the heels together, back upright, and arms extended next to the body[38]. BMI was calculated as weight divided by height squared (kg/m2). ND-HGS was measured using a digital dynamometer (Charder Co. Ltd., Taichung City, Taiwan), as described previously[39]. Arm circumference (AC) was measured around the mid-upper arm, between the shoulder and elbow, using a flexible tape. Triceps skinfold thickness (TST) was assessed and MAMC was calculated using the formula: MAMC = AC (cm) = π × [TST (mm)/10].
Phase angle estimation
The PA was assessed by whole-body BIA[40] at 50 kHz (Bodystat 4000 model; Bodystat Ltd., Douglas, Isle of Man, British Isles) with APEX software (version 4.02; Hologic Inc., Bedford, MA, United States). Participants removed all metal objects and other items that might interfere with the scan and were instructed to empty the bladder. Each participant was positioned supine in the center of the scanning table with the palms down and the arms beside the body. His age, height, weight, sex, and ethnicity were entered into the computer. The PA value was calculated as PA = arc tangent Xc/R × 180/π. Patients were grouped according to PA value (PA > 4.9°, PA ≤ 4.9°)[35].
Survival
Death events were assessed by telephone calls at the end of the study period. Only deaths related directly to cirrhosis complications were counted. The prognostic value of the PA for mortality prediction was evaluated in mortality models adjusted for variables potentially impacting nutritional status and/or cirrhosis severity (age, Child-Pugh and MELD score)[35,41,42]. A longitudinal analysis of mortality was used to assess the prognostic value of malnutrition.
Sample size
The sample size required to analyze the prognostic value of the PA for mortality was calculated using the G Power software package (version 3.1.9.2; Heinrich Heine University, Dusseldorf, Germany). A sample size of 134 patients was obtained from a Cox proportional-hazards regression model, considering a significance level of 5% and rate of 36% at 36 mo of follow-up, with 80% power to detect a hazard ratio (HR) of 2.50 for mortality prediction.
Statistical analysis
Survival probabilities were estimated by the Kaplan-Meier method, compared using the log-rank test, and estimated in terms of the failure rate according to independent and multiple models of Cox proportional hazards. The mortality models included PA values and were adjusted for MELD score and age. Data were expressed as means ± SDs, medians, interquartile ranges (IQRs; 25th-75th percentile), or percentages, depending on the normality of distribution and type of variable. Data were analyzed using the R software package (version 3.1.3, 2015; R Core Team, Vienna, Austria) and a significance level of 5%.
RESULTS
Patient characteristics
A total of 134 patients (mean age, 54.30 ± 10.10 years) with cirrhosis of different etiologies (59.80% alcoholic, 20.10% viral, 10.40% cryptogenic, and 9.70% other), presenting as 17.90% Child A, 54.50% Child B, and 27.60% Child C and with a mean MELD score of 14.11 ± 4.95, were enrolled in the study. Of these patients, 73 (54.48%) were assigned to the PA > 4.9° group and 61 (45.52%) were assigned to the PA ≤ 4.9° group. Weight, height, and BMI were similar in both groups, but patients from the PA > 4.9° group were younger and had higher MAMC, albumin, and ND-HGS values and lower severe ascites and encephalopathy incidences, IL-6/IL-10 ratios, and CRP levels than did patients from the PA ≤ 4.9° group (Table 1).
Table 1.
Baseline characteristics and body composition of patients with cirrhosis
| Variable | PA > 4.9º (n = 73) | PA ≤ 4.9º (n = 61) | Total (n = 134) | P valuea |
| Age (yr) | 52.10 ± 9.80 | 56.90 ± 9.80 | 54.30 ± 10.10 | 0.0051 |
| Weight (kg) | 76.60 ± 13.10 | 76.40 ± 15.30 | 76.50 ± 14.10 | 0.9191 |
| Height (m) | 1.70 ± 0.10 | 1.70 ± 0.10 | 1.70 ± 0.10 | 0.5361 |
| Child Pugh A (%) | 25 | 10 | 18 | |
| Child Pugh B (%) | 45 | 65 | 55 | |
| Child Pugh C (%) | 30 | 25 | 27 | 0.0313 |
| Model for end-stage liver disease score | 13.41 ± 5.11 | 14.95 ± 4.65 | 14.11 ± 4.95 | 0.0733 |
| Severe ascites (%) | 10.00 | 29.00 | 18.20 | 0.0163 |
| Encephalopathy (%) | 40.00 | 60.00 | 50.00 | 0.0443 |
| Body mass index (kg/m2) | 26.70 ± 4.10 | 26.40 ± 5.00 | 26.60 ± 4.50 | 0.6831 |
| Mid-arm muscle circumference (cm) | 25.80 ± 3.20 | 23.20 ± 3.10 | 24.70 ± 3.40 | < 0.0011 |
| Handgrip strength (kg) | 31.80 ± 7.00 | 24.40 ± 8.90 | 28.60 ± 8.70 | < 0.0011 |
| IL-6/IL-10 ratio (pg/mL) | 1.10 (0.51; 2.35) | 1.29 (0.71; 4.68) | 1.17 (0.58; 2.68) | 0.0862 |
| C-reactive protein (mg/dL) | 0.88 (0.42; 1.96) | 1.20 (0.60; 4.72) | 1.09 (0.54; 2.62) | 0.0302 |
| Albumin (g/dL) | 3.90 (3.40; 4.30) | 3.50 (2.90; 3.80) | 3.60 (3.20; 4.20) | 0.0022 |
PA > 4.9º vs PA ≤ 4.9º;
Student’s t test;
Mann-Whitney test;
χ2 test. Data are presented as mean ± SD (confidence interval), or percentage. PA: Phase angle; IL: Interleukin.
Prognostic value of malnutrition, identified by the phase angle
The mean follow-up duration was 25 mo (median, 32.1 mo). Of the 134 patients included in the mortality prediction analysis, 48 (35.80%) died due to cirrhosis, with a median of 18 mo (IQR, 3.3; 25.6 mo) of follow-up until death. Thirty-one (64.60%) patients who died were from the PA ≤ 4.9° group.
The Child-Pugh score had no significant effect in the initial mortality model and was not included in the final model (Table 2). PA values ≤ 4.9° significantly affected the mortality model adjusted for MELD score and age (HR = 2.05, 95%CI: 1.11-3.77, P = 0.021). In addition, the mortality prediction was not influenced by MELD or age. Patients from the PA ≤ 4.9° group were significantly more likely to die, as demonstrated by Kaplan-Meier curves (Figure 1). In the median follow-up period of 18 mo, the incidence ratios of death were 27.10% for patients from the PA ≤ 4.9° group and 9.90% for those from the PA > 4.9° group.
Table 2.
Mortality estimates for patients with cirrhosis from a multiple Cox regression model
| Variable | HR (95%CI) | P value |
| Age (yr) | 1.03 (1.00, 1.06) | 0.042 |
| MELD score | 1.10 (1.05, 1.16) | 0.001 |
| Phase angle 50 kHz (< 4.9º) | 2.05 (1.11, 3.77) | 0.021 |
P values for independent Cox regression models refer to three models explained by age, MELD score, and phase angle. HR: Hazard ratio; MELD: Model for end-stage liver disease.
Figure 1.
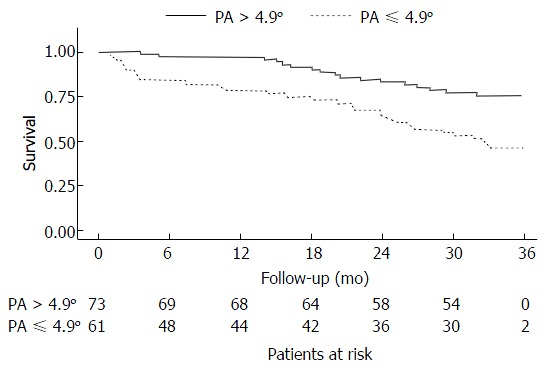
Kaplan-Meier survival curves for 134 patients with cirrhosis, obtained using cutoff scores based on phase angle obtained by bioelectrical impedance analysis (PA < 4.9º, n = 61; PA > 4.9º, n = 73). PA: Phase angle.
DISCUSSION
Although malnutrition implies a poor prognosis for patients with cirrhosis, its diagnosis has been masked in this population due to the unavailability of a clinically accessible method that is not affected by edema and/or ascites[18]. The PA is not affected by hydric changes and was recently proposed as a good tool for malnutrition diagnosis in patients with cirrhosis, with a cutoff value of ≤ 4.9°[35]. Here, we showed that PA ≤ 4.9° predicted mortality in male cirrhotic patients, in a model adjusted for age and MELD score.
We identified four studies evaluating the prognostic value of the PA in Brazilian (n = 2), German, and, more recently, Mexican patients with cirrhosis. These studies showed that PA cutoff values of 5.18°, 5.44°, 5.4° and 4.9°, respectively, were related to disease severity and even mortality, when controlling for age and other nutritional variables[14,18,35,43]. Here, we applied the PA cutoff value proposed recently by Ruiz-Margáin et al[35] (≤ 4.9°), which was further used to establish malnutrition with good prognostic value for mortality in a cohort of Mexican cirrhotic patients.
In our study, the prognostic value of this PA cutoff was tested in mortality models adjusted for age and MELD score, as the main markers of PA performance and disease severity, respectively. Age has been proposed as the main indicator for PA determination in women and men, and the MELD score has been considered a good predictor of short-term mortality in patients with cirrhosis[35,41,42].
The Child-Pugh score was added to our initial mortality model because it may reflect the progression of liver damage and indirectly detect metabolic changes that may influence the prognosis of the disease[42]. However, it had no significant effect on mortality prediction. Notably, the MELD score has been validated as a good predictor of the survival of adult patients on the LT list, and has been found to better predict short-term results than does the CP score[44]. This difference in performance may explain the significant value of the MELD score, and not the CP score, for mortality prediction in our initial model. Data from the final mortality model support the prognostic value of PA ≤ 4.9°, as it was associated independently with mortality. Furthermore, our HR for mortality was similar to that reported by Ruiz-Margáin et al[35].
Results from some studies suggest that malnutrition is related strongly to mortality and cirrhosis-related complications[14,18,27,35,43,44]. Despite evidence suggesting the utility of the PA as a nutritional marker, its validity has been questioned. According to our data, the PA ≤ 4.9° cutoff was able to identify patients with significant changes in inflammatory and nutritional markers highly indicative of catabolism and malnutrition (i.e., increased IL-6/IL-10 ratio and CRP level and decreased albumin level and HGS, a relevant marker of muscle loss associated largely with poor prognosis in cirrhosis). The notably increased mortality rate observed in our patients with PAs ≤ 4.9° may be associated closely with the deleterious effects of malnutrition.
PA values change in response to nutritional interventions, with greater sensitivity than achieved with other nutritional markers[45]. Thus, even if the PA cannot actually represent the nutritional status of a patient, it seems to adequately reflect minimal changes in this clinical variable. In this scenario, the PA could be applied for nutritional monitoring of patients for whom the risk of malnutrition could significantly influence clinical outcomes. For instance, the incidences of severe ascites and encephalopathy complications were significantly higher among patients with PAs ≤ 4.9° than among those with PAs above this cutoff in our study, in response to the metabolic consequences of the disease.
Patients with cirrhosis often display circulatory dysfunction with portal hypertension, leading to vasodilatation of splanchnic vessels and favoring decreased peripheral resistance and effective central blood volume, with consequent arterial hypotension and hyperdynamic circulation. These abnormalities result in the activation of vasoconstrictor systems through the renin-angiotensin-aldosterone system and of the sympathetic nervous system, with increased levels of antidiuretic hormone and renal vasoconstriction that culminate in ascites and/or edema[46]. These altered physiological states limit the application of available methods to evaluate nutritional status[47].
Indeed, as a result of ascites and/or edema, anthropometric measures such as BMI usually overestimate lean mass in patients with end-stage liver disease who require LT[3]. Consequently, although easier, traditional NA may underestimate the prevalence and severity of malnutrition in patients with cirrhosis[13]. Moreover, the presence of body fluid changes, mainly ascites, may explain the marked discrepancies in malnutrition frequencies (ranging from 5.4% to 68.2%) among NA methods in patients with cirrhosis[12,47-54]. As PA values are not influenced by unstable hydration, we suggest that this tool is useful for nutritional monitoring of patients with cirrhosis, and that the PA cutoff value proposed by Ruiz-Margáina et al[35] can identify those at high risk of death if not nutritionally treated.
One limitation of our study was the inclusion of solely male patients. We assessed only male patients to make our sample as uniform as possible, as liver cirrhosis per se is a progressive disease and hepatic damage may differ, even slightly, among patients. In addition, cirrhosis is more common in men and malnutrition seems to have greater prognostic value for disease progression in men than in women. The prognostic ability of the studied cutoff value for phase angle is associated directly with malnutrition. Thus, by evaluating only men, we were able to access not only a more uniform sample, but also the population most susceptible to the studied disease and its associated nutritional complications. Ruiz-Margáin et al[35] did not specify the sex of the cirrhotic patients with which the studied PA cutoff value was developed. Thus, we cannot confirm whether this value performs similarly in the prediction of malnutrition-associated mortality in women. We can conclude that the PA ≤ 4.9° cutoff was associated independently with mortality in male patients with cirrhosis, potentially associated to malnutrition. The PA may be a useful and reliable bedside tool to evaluate prognosis in cirrhosis.
ACKNOWLEDGMENTS
The authors thank the patients and nurses who participated in the study.
COMMENTS
Background
Liver transplantation is the best option for patients with advanced cirrhosis, but its clinical application is often limited. Malnutrition is a major complication often observed in patients with cirrhosis. Early diagnosis of malnutrition in patients with cirrhosis is important. In general, ascites, edema, and other chronic liver disease-associated complications can impair the performance of traditionally applied criteria for nutritional assessment (NA). Consequently, weight loss, anthropometric measurements, the creatinine-height index, nitrogen balance, lymphocyte count, and serum albumin, transferrin, prealbumin, and retinol-bound protein levels should be interpreted with restrictions when assessing the nutritional status of cirrhotic patients. In this scenario, a gold standard NA method is required for the proper diagnosis of malnutrition in this patient population.
Research frontiers
The phase angle (PA) obtained from bioimpedance analysis has been proposed as a nutritional status marker, with low values associated with malnutrition and nutritional risk at the time of hospital admission. The PA reflects the relationship between the resistance component, meaning tissue opposition to the passage of electric current, and reactance, meaning the resistance effect produced by the interface of tissues and cell membranes. A main advantage of the use of PA is that it can be applied even under unstable tissue hydration conditions, such as edema and ascites.
Innovations and breakthroughs
This article provides original data displaying the good performance of the PA obtained by bioelectrical impedance analysis in the evaluation of mortality prognosis in patients with cirrhosis.
Applications
The findings suggest that the PA is a safe, practical, and inexpensive tool for the prediction of mortality potentially associated with malnutrition.
Peer-review
The authors aim to explore the potential value of PA in cirrhosis. In general, the topic is interesting, and the design is sound.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Institutional Ethics Review Board (0646/11) of the Hospital das Clínicas (São Paulo, SP, Brazil).
Clinical trial registration statement: This clinical trial was registered at ClinicalTrials.gov with the identifier NCT02421848. Details can be found at https://clinicaltrials.gov/ct2/show/NCT02421848.
Informed consent statement: All study participants, or their legal guardians, provided written consent prior to study enrollment.
Conflict-of-interest statement: The authors of this manuscript have no conflict of interest to disclose.
Data sharing statement: No additional data are available.
Peer-review started: August 2, 2016
First decision: September 8, 2016
Article in press: January 14, 2017
P- Reviewer: Ikura Y, Zheng MH S- Editor: Kong JX L- Editor: A E- Editor: Li D
References
- 1.Habka D, Mann D, Landes R, Soto-Gutierrez A. Future Economics of Liver Transplantation: A 20-Year Cost Modeling Forecast and the Prospect of Bioengineering Autologous Liver Grafts. PLoS One. 2015;10:e0131764. doi: 10.1371/journal.pone.0131764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axelrod DA, Dzebisashvili N, Lentine K, Segev DL, Dickson R, Tuttle-Newhall E, Freeman R, Schnitzler M. Assessing variation in the costs of care among patients awaiting liver transplantation. Am J Transplant. 2014;14:70–78. doi: 10.1111/ajt.12494. [DOI] [PubMed] [Google Scholar]
- 3.Ritter L, Gazzola J. [Nutritional evaluation of the cirrhotic patient: an objective, subjective or multicompartmental approach?] Arq Gastroenterol. 2006;43:66–70. doi: 10.1590/s0004-28032006000100016. [DOI] [PubMed] [Google Scholar]
- 4.Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–173, 173.e1. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Kalafateli M, Mantzoukis K, Choi Yau Y, Mohammad AO, Arora S, Rodrigues S, de Vos M, Papadimitriou K, Thorburn D, O’Beirne J, et al. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J Cachexia Sarcopenia Muscle. 2016 doi: 10.1002/jcsm.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masuda T, Shirabe K, Ikegami T, Harimoto N, Yoshizumi T, Soejima Y, Uchiyama H, Ikeda T, Baba H, Maehara Y. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl. 2014;20:401–407. doi: 10.1002/lt.23811. [DOI] [PubMed] [Google Scholar]
- 7.Pinzani M, Rosselli M, Zuckermann M. Liver cirrhosis. Best Pract Res Clin Gastroenterol. 2011;25:281–290. doi: 10.1016/j.bpg.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21:113–117. doi: 10.1016/j.nut.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Barbosa-Silva MC, de Barros AJ. [Subjective global assessment: Part 2. Review of its adaptations and utilization in different clinical specialties] Arq Gastroenterol. 2002;39:248–252. doi: 10.1590/s0004-28032002000400008. [DOI] [PubMed] [Google Scholar]
- 10.Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, Takai K, Shimizu M, Moriwaki H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193–199. doi: 10.1016/j.nut.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Johnson TM, Overgard EB, Cohen AE, DiBaise JK. Nutrition assessment and management in advanced liver disease. Nutr Clin Pract. 2013;28:15–29. doi: 10.1177/0884533612469027. [DOI] [PubMed] [Google Scholar]
- 12.Figueiredo FA, De Mello Perez R, Kondo M. Effect of liver cirrhosis on body composition: evidence of significant depletion even in mild disease. J Gastroenterol Hepatol. 2005;20:209–216. doi: 10.1111/j.1440-1746.2004.03544.x. [DOI] [PubMed] [Google Scholar]
- 13.Figueiredo FA, Perez RM, Freitas MM, Kondo M. Comparison of three methods of nutritional assessment in liver cirrhosis: subjective global assessment, traditional nutritional parameters, and body composition analysis. J Gastroenterol. 2006;41:476–482. doi: 10.1007/s00535-006-1794-1. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes SA, Bassani L, Nunes FF, Aydos ME, Alves AV, Marroni CA. Nutritional assessment in patients with cirrhosis. Arq Gastroenterol. 2012;49:19–27. doi: 10.1590/s0004-28032012000100005. [DOI] [PubMed] [Google Scholar]
- 15.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 16.Kyle UG, Genton L, Pichard C. Low phase angle determined by bioelectrical impedance analysis is associated with malnutrition and nutritional risk at hospital admission. Clin Nutr. 2013;32:294–299. doi: 10.1016/j.clnu.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Baumgartner RN, Chumlea WC, Roche AF. Bioelectric impedance phase angle and body composition. Am J Clin Nutr. 1988;48:16–23. doi: 10.1093/ajcn/48.1.16. [DOI] [PubMed] [Google Scholar]
- 18.Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol. 2002;86:509–516. doi: 10.1007/s00421-001-0570-4. [DOI] [PubMed] [Google Scholar]
- 19.Krause L, Becker MO, Brueckner CS, Bellinghausen CJ, Becker C, Schneider U, Haeupl T, Hanke K, Hensel-Wiegel K, Ebert H, et al. Nutritional status as marker for disease activity and severity predicting mortality in patients with systemic sclerosis. Ann Rheum Dis. 2010;69:1951–1957. doi: 10.1136/ard.2009.123273. [DOI] [PubMed] [Google Scholar]
- 20.Faisy C, Rabbat A, Kouchakji B, Laaban JP. Bioelectrical impedance analysis in estimating nutritional status and outcome of patients with chronic obstructive pulmonary disease and acute respiratory failure. Intensive Care Med. 2000;26:518–525. doi: 10.1007/s001340051198. [DOI] [PubMed] [Google Scholar]
- 21.Maggiore Q, Nigrelli S, Ciccarelli C, Grimaldi C, Rossi GA, Michelassi C. Nutritional and prognostic correlates of bioimpedance indexes in hemodialysis patients. Kidney Int. 1996;50:2103–2108. doi: 10.1038/ki.1996.535. [DOI] [PubMed] [Google Scholar]
- 22.Ott M, Fischer H, Polat H, Helm EB, Frenz M, Caspary WF, Lembcke B. Bioelectrical impedance analysis as a predictor of survival in patients with human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:20–25. [PubMed] [Google Scholar]
- 23.Schwenk A, Ward LC, Elia M, Scott GM. Bioelectrical impedance analysis predicts outcome in patients with suspected bacteremia. Infection. 1998;26:277–282. doi: 10.1007/BF02962247. [DOI] [PubMed] [Google Scholar]
- 24.Schwenk A, Beisenherz A, Römer K, Kremer G, Salzberger B, Elia M. Phase angle from bioelectrical impedance analysis remains an independent predictive marker in HIV-infected patients in the era of highly active antiretroviral treatment. Am J Clin Nutr. 2000;72:496–501. doi: 10.1093/ajcn/72.2.496. [DOI] [PubMed] [Google Scholar]
- 25.Gupta D, Lammersfeld CA, Burrows JL, Dahlk SL, Vashi PG, Grutsch JF, Hoffman S, Lis CG. Bioelectrical impedance phase angle in clinical practice: implications for prognosis in advanced colorectal cancer. Am J Clin Nutr. 2004;80:1634–1638. doi: 10.1093/ajcn/80.6.1634. [DOI] [PubMed] [Google Scholar]
- 26.Gupta D, Lammersfeld CA, Vashi PG, King J, Dahlk SL, Grutsch JF, Lis CG. Bioelectrical impedance phase angle as a prognostic indicator in breast cancer. BMC Cancer. 2008;8:249. doi: 10.1186/1471-2407-8-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alberino F, Gatta A, Amodio P, Merkel C, Di Pascoli L, Boffo G, Caregaro L. Nutrition and survival in patients with liver cirrhosis. Nutrition. 2001;17:445–450. doi: 10.1016/s0899-9007(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 28.Bosy-Westphal A, Danielzik S, Dörhöfer RP, Piccoli A, Müller MJ. Patterns of bioelectrical impedance vector distribution by body mass index and age: implications for body-composition analysis. Am J Clin Nutr. 2005;82:60–68. doi: 10.1093/ajcn.82.1.60. [DOI] [PubMed] [Google Scholar]
- 29.Roman M, Torres S, Casanova M. 1999. Bases físicas del análisis de la impedância bioeléctrica. Universidad de Cádiz; pp. 39–143. [Google Scholar]
- 30.Llames L, Baldomero V, Iglesias ML, Rodota LP. [Values of the phase angle by bioelectrical impedance; nutritional status and prognostic value] Nutr Hosp. 2013;28:286–295. doi: 10.3305/nh.2013.28.2.6306. [DOI] [PubMed] [Google Scholar]
- 31.Norman K, Stobäus N, Zocher D, Bosy-Westphal A, Szramek A, Scheufele R, Smoliner C, Pirlich M. Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. Am J Clin Nutr. 2010;92:612–619. doi: 10.3945/ajcn.2010.29215. [DOI] [PubMed] [Google Scholar]
- 32.Souza Thompson Motta R, Alves Castanho I, Guillermo Coca Velarde L. CUTOFF POINT OF THE PHASE ANGLE IN PRE-RADIOTHERAPY CANCER PATIENTS. Nutr Hosp. 2015;32:2253–2260. doi: 10.3305/nh.2015.32.5.9626. [DOI] [PubMed] [Google Scholar]
- 33.Kyle UG, Soundar EP, Genton L, Pichard C. Can phase angle determined by bioelectrical impedance analysis assess nutritional risk? A comparison between healthy and hospitalized subjects. Clin Nutr. 2012;31:875–881. doi: 10.1016/j.clnu.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 34.da Silva TK, Berbigier MC, Rubin Bde A, Moraes RB, Corrêa Souza G, Schweigert Perry ID. Phase angle as a prognostic marker in patients with critical illness. Nutr Clin Pract. 2015;30:261–265. doi: 10.1177/0884533615572150. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz-Margáin A, Macías-Rodríguez RU, Duarte-Rojo A, Ríos-Torres SL, Espinosa-Cuevas Á, Torre A. Malnutrition assessed through phase angle and its relation to prognosis in patients with compensated liver cirrhosis: a prospective cohort study. Dig Liver Dis. 2015;47:309–314. doi: 10.1016/j.dld.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 36.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gómez J, Lilienthal Heitmann B, Kent-Smith L, Melchior JC, Pirlich M, et al. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23:1430–1453. doi: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Ginde SR, Geliebter A, Rubiano F, Silva AM, Wang J, Heshka S, Heymsfield SB. Air displacement plethysmography: validation in overweight and obese subjects. Obes Res. 2005;13:1232–1237. doi: 10.1038/oby.2005.146. [DOI] [PubMed] [Google Scholar]
- 38.McDowell MA, Fryar CD, Ogden CL, Flegal KM. Hyattsville, MD: National Center for Health Statistics;; 2008. Anthropometric reference data for children and adults: United States, 2003-2006. National Health Statistics Report; no 10. Available from: https://www.cdc.gov/nchs/data/nhsr/nhsr010.pdf. [PubMed] [Google Scholar]
- 39.Gottschall CA, Álvares-da-Silva MR, Camargo AC, Burtett RM, Silveira TR. Avaliação nutricional de pacientes com cirrose pelo vírus da hepatite C: a aplicação da calorimetria indireta. Arq Gastroenterol. 2004;41:220–224. doi: 10.1590/s0004-28032004000400004. [DOI] [PubMed] [Google Scholar]
- 40.Barbosa-Silva MCG, Barros AJD. Bioelectrical impedance analysis in clinical practice: a new perspective on its use beyond body composition equations. Curr Opin Clin Nutr Metab Care. 2005;8:311–317. doi: 10.1097/01.mco.0000165011.69943.39. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez MC, Barbosa-Silva TG, Bielemann RM, Gallagher D, Heymsfield SB. Phase angle and its determinants in healthy subjects: influence of body composition. Am J Clin Nutr. 2016;103:712–716. doi: 10.3945/ajcn.115.116772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 43.Peres WA, Lento DF, Baluz K, Ramalho A. Phase angle as a nutritional evaluation tool in all stages of chronic liver disease. Nutr Hosp. 2012;27:2072–2078. doi: 10.3305/nh.2012.27.6.6015. [DOI] [PubMed] [Google Scholar]
- 44.Sam J, Nguyen GC. Protein-calorie malnutrition as a prognostic indicator of mortality among patients hospitalized with cirrhosis and portal hypertension. Liver Int. 2009;29:1396–1402. doi: 10.1111/j.1478-3231.2009.02077.x. [DOI] [PubMed] [Google Scholar]
- 45.Norman K, Stübler D, Baier P, Schütz T, Ocran K, Holm E, Lochs H, Pirlich M. Effects of creatine supplementation on nutritional status, muscle function and quality of life in patients with colorectal cancer--a double blind randomised controlled trial. Clin Nutr. 2006;25:596–605. doi: 10.1016/j.clnu.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Urrunaga NH, Magder LS, Weir MR, Rockey DC, Mindikoglu AL. Prevalence, Severity, and Impact of Renal Dysfunction in Acute Liver Failure on the US Liver Transplant Waiting List. Dig Dis Sci. 2016;61:309–316. doi: 10.1007/s10620-015-3870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgan MY, Madden AM, Soulsby CT, Morris RW. Derivation and validation of a new global method for assessing nutritional status in patients with cirrhosis. Hepatology. 2006;44:823–835. doi: 10.1002/hep.21358. [DOI] [PubMed] [Google Scholar]
- 48.Shawcross DL, Shabbir SS, Taylor NJ, Hughes RD. Ammonia and the neutrophil in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology. 2010;51:1062–1069. doi: 10.1002/hep.23367. [DOI] [PubMed] [Google Scholar]
- 49.Putadechakum S, Klangjareonchai T, Soponsaritsuk A, Roongpisuthipong C. Nutritional status assessment in cirrhotic patients after protein supplementation. ISRN Gastroenterol. 2012;2012:690402. doi: 10.5402/2012/690402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pirlich M, Schütz T, Spachos T, Ertl S, Weiss ML, Lochs H, Plauth M. Bioelectrical impedance analysis is a useful bedside technique to assess malnutrition in cirrhotic patients with and without ascites. Hepatology. 2000;32:1208–1215. doi: 10.1053/jhep.2000.20524. [DOI] [PubMed] [Google Scholar]
- 51.Chang WT, Ker CG, Hung HC, Lee KT, Chen LS, Chiang HC, Huang MC. Albumin and prealbumin may predict retinol status in patients with liver cirrhosis. Hepatogastroenterology. 2008;55:1681–1685. [PubMed] [Google Scholar]
- 52.Masuda T, Shirabe K, Yoshiya S, Matono R, Morita K, Hashimoto N, Ikegami T, Yoshizumi T, Baba H, Maehara Y. Nutrition support and infections associated with hepatic resection and liver transplantation in patients with chronic liver disease. JPEN J Parenter Enteral Nutr. 2013;37:318–326. doi: 10.1177/0148607112456041. [DOI] [PubMed] [Google Scholar]
- 53.Stephenson GR, Moretti EW, El-Moalem H, Clavien PA, Tuttle-Newhall JE. Malnutrition in liver transplant patients: preoperative subjective global assessment is predictive of outcome after liver transplantation. Transplantation. 2001;72:666–670. doi: 10.1097/00007890-200108270-00018. [DOI] [PubMed] [Google Scholar]
- 54.Merli M, Giusto M, Gentili F, Novelli G, Ferretti G, Riggio O, Corradini SG, Siciliano M, Farcomeni A, Attili AF, et al. Nutritional status: its influence on the outcome of patients undergoing liver transplantation. Liver Int. 2010;30:208–214. doi: 10.1111/j.1478-3231.2009.02135.x. [DOI] [PubMed] [Google Scholar]


