Abstract
Background
Participation rates in bowel cancer screening programs in Germany continue to be low. In a model project, a logistically simple procedure for inviting patients to participate was tested as a means of increasing participation.
Methods
A randomized trial was performed involving persons residing in the German federal state of Saarland who had either their 50th or their 55th birthday in the year beginning on 1 April 2012 (18 560 and 16 824 persons, respectively). The 50-year-olds received a written invitation to undergo a test for blood in the stool, either with or without a stool test attached, or else no invitation at all. The 55-year-olds received either an invitation to undergo colonoscopy or no invitation. Participation rates within one year were determined from billing data of the Saarland Association of Statutory Health. Insurance Physicians. The trial was registered in the German Registry of Clinical Trials, no. DRKS00006098.
Results
A written invitation to undergo testing of the stool for blood, together with an accompanying test, increased the participation rate within one year by 62% (from 15% to 25%, p <0.001, especially among men (+158% vs. +39% for women). The participation rate was higher in general among women than among men (33% vs. 17%). On the other hand, a written invitation with no accompanying test did not increase the participation rate. A written invitation to undergo colonoscopic screening increased the participation rate within one year by 32% (5.9% vs 4.4%, p <0.001).
Conclusion
Targeted invitations can markedly increase participation rates in cancer screening. Written invitations to undergo stool testing for blood should be accompanied by an actual test. Further trials should also include information about the number of adenomas and carcinomas detected by screening.
Bowel cancer ranks third among both cancer diagnoses and cancer deaths in Germany. The annual incidence of bowel cancer exceeds 60 000, and around 26 000 deaths each year are attributable to the consequences of this disease (1). The potential to lower the incidence and mortality of bowel cancer by annual tests for blood in the feces has long been confirmed by randomized intervention studies (2). There are not yet any data from randomized trials on the efficacy of colonoscopy in bowel cancer screening (3). However, the published epidemiological studies, among them a meta-analysis, unanimously indicate that sharp reductions in both incidence and mortality can be expected (4– 8).
Since October 2002, all residents of Germany with health insurance aged 50 to 54 years have been entitled to an annual fecal blood test, and from the age of 55 years upward, screening colonoscopy. However, despite intensive publicity and motivational campaigns by, among other institutions, the Lebensblicke foundation, the Felix Burda Foundation, and regional organizations, the rates of participation in bowel cancer screening remain low (9). Each year only 2 to 3% of men and women entitled to screening colonoscopy take advantage of the offer. Over a period of 10 years, the interval between screenings recommended by the relevant professional bodies, this amounts to 20 to 30%. In the year 2014, around 15% of the entitled 50- to 54-year-olds took up the offer of testing for fecal blood (9).
Experience in other countries has consistently shown that much higher participation rates can be achieved by organized screening with written personal invitations to take part and, in the case of testing for fecal occult blood, simultaneous provision of test materials (10, 11). Data on screening mammography indicate that in Germany too, organized screening with personal invitation can achieve high participation rates (12). There are no corresponding data for bowel cancer screening in Germany. However, pilot studies in the federal states of North Rhine–Westphalia and Saarland show that even sending a single invitation letter to each eligible person clearly increases the utilization of screening colonoscopy. We therefore designed and conducted a model project to increase participation of the target population in Saarland in bowel cancer screening by means of an uncomplicated personal invitation procedure.
Methods
Study design and study population
The SAMS study (Saarland gegen Darmkrebs – Machen Sie mit!; Saarland Against Cancer—Join In!) was carried out as an individually randomized intervention trial. Included were all persons born in 1962/1963 and 1957/1958 with their principal residence in Saarland who reached the age of 50 or 55 years, respectively, between 1 April 2012 and 31 March 2013. The target population was identified and the necessary data (name, postal address, sex, date of birth) provided by the Saarland central residents’ registration office. The study was approved by the internal review boards of the medical faculty of the University of Heidelberg and the Saarland Medical Association and it was registered with the German Clinical Trials Register (DRKS): DRKS00006098.
Intervention
The 50-year-olds were assigned randomly and in equal numbers to one of two intervention groups (invitation to a test for fecal blood with or without the test kit enclosed) or to a control group (no invitation) (figure 1). The probands in the intervention group with test kit received a guaiac-based fecal occult blood test (gFOBT; test kit for two samples from each of three consecutive defecations) and were asked to mail the samples to the study center in a franked envelope provided with the kit, or alternatively to give the test kit to their primary care physician. The 55-year-olds were assigned randomly and in equal numbers to an intervention group (invitation for screening colonoscopy) or a control group (no invitation).
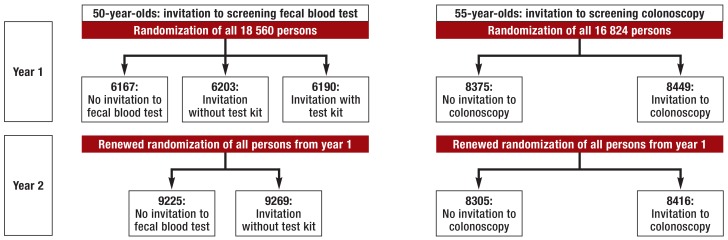
Figure 1.
Randomization design of the SAMS study: The numbers of persons randomized are smaller in year 2 than in year 1 because some members of the population had died or moved away from Saarland.
To ascertain the benefit of repeated written contact, the persons born in the years specified above who were still registered in Saarland on their 51st or 56th birthday, respectively, were sent another letter—regardless of whether or not they had taken advantage of bowel cancer screening in the previous year. However, this second letter stated that the repeat invitation was invalid if the proband had taken up the first offer of screening in the meantime. The younger age group was therefore again invited to take up the offer of a fecal blood test, provided no such test had been carried out in the current calendar year. These 51-year-old probands were randomized anew, this time into only one intervention group and a control group. No test kits were sent to those in the intervention group, because it could be assumed that some of them had already received such a kit in the current calendar year. Repeat testing of these probands in the same calendar year would have been pointless, and in any case they had no entitlement to a second test. The 56-year-olds were again invited for screening colonoscopy, provided no such examination had yet been carried out. The letters of invitation contained information sheets on the services offered (13). Details thereof can be found in eBox 1.
eBox 1. Information materials.
Each person assigned to an intervention group received a personal letter from the Saarland Minister of Education. The letter began with birthday congratulations and went on to invite the recipient to bowel cancer screening. Information about the services on offer was included. The invitation sent to the 50- or 51-year-old probands focused on testing for blood in the feces. In addition to providing information about this screening service and an invitation to participate, the letter was accompanied by an attractively presented and readily understandable leaflet with background data about the test, details of how it is carried out and its predictive value, and useful links to further information sources on the internet, e.g., the cancer information service of the German Center for Cancer Research (Deutsches Krebsforschungszentrums, DKFZ). The invitation and leaflet sent to the 55- or 56-year-old probands focused on screening colonoscopy. This group also received a frequently updated list of physicians authorized to perform screening colonoscopy. The continuing entitlement to testing for fecal blood was also clearly stated. Both leaflets included all the information contained in the brochure “Patienteninformation Darmkrebsfrüherkennung” (Bowel Cancer Screening: Information for Patients) issued by the Joint Federal Committee (13).
Utilization of screening services
To determine whether a study participant had taken advantage of the offer of gFOBT (EBM [Einheitlicher Bewertungsmaßstab, German physicians’ fee scale] code 01734) or screening colonoscopy (EBM code 01741), his/her data were compared with the invoicing data of the Saarland Association of Statutory Health Insurance Physicians (Kassenärztliche Vereinigung, KV) for bowel cancer screening. The linkage took place by means of generated control numbers and was thus anonymized for all intents and purposes (ebox 2). If the letter of invitation contained a test kit, the latter could be sent directly to the study center at the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) for analysis. Participants who did this received a reply with the test result, which, if they had so requested, was also sent to a physician of their choice.
eBox 2. Linkage of study participants’ data and invoicing data.
The study participants’ data and the screening services performed were virtually anonymized by means of control numbers and linked with the aid of deterministic and probabilistic record-linkage procedures. Control numbers are encrypted identity markers from which it is impossible to ascertain the clear-text identity. Safe assignment is possible because the same identity markers in clear text always yield the same control numbers. Control numbers and record-linkage procedures are standard tools for cancer registration in Germany (e1).
The control numbers were generated from the personal identification markers family name, given name(s), and the phonetic codes thereof after each monthly mailing and before provision of the invoicing data by the Saarland Association of Statutory Health Insurance Physicians (Kassenärztliche Vereinigung, KV). The control numbers, together with birthday, sex, date of birth, and residential postal code, provided the basis for record linkage. These markers, along with the remaining characteristics, e.g., serial number, day of screening, date and result of randomization in the first and second year, and date of mailing the invitation, were saved in the study database.
Preparatory analyses carried out by the Cancer Registry Saarland to ascertain the parameters and assignment threshold values required for record linkage showed that there is a very high level of agreement between the KV’s non-inpatient invoicing data and residents’ registration office data for constituent parts of names, sex, date of birth, and residential address. With regard to constituent parts of names, the differences are mainly due to the fact that given names are frequently not recorded in full in the invoicing data.
The data were linked using the record-linkage program Merge ToolBox (MTB) (version 0.742) and the deterministic and stochastic record-linkage routines implemented therein (e2). Altogether, data on 35 911 recipients of invitation letters were linked with 126 763 data sets from fecal blood tests (Physicians’ Fee Scale [EBM] code 01734) and screening colonoscopies (EBM code 01741) carried out between 1 April 2012 and 30 June 2014. By this means, 10 561 services rendered were assigned to the study participants. 125 assignments were based on phonetic codes that took account of variations in spelling of constituent parts of names. The assignment of 109 services rendered was uncertain, and these services were excluded from analysis.
Statistical analysis
For both age cohorts the statistical analysis consisted primarily of comparing the utilization of bowel cancer screening in the intervention and control groups within a year of the first letter of invitation or first randomization to the control group. The data of KV Saarland include persons with statutory health insurance (ca. 86% of those to whom letters were sent), but not the privately insured; however, the latter have access to comparable screening services. To render the numerators and denominators of the participation rates comparable, the number of persons invited was multiplied by the proportion of statutory insurees among the 50- to 54-year-old and 55- to 59-year-old men (81.6% and 82.7%, respectively) and women (89.9% and 89.0%) resident in Saarland in 2013 (Tables 1 and 2). Contingency table comparison of the participation rates was performed (relative participation rate in the intervention group compared with the control group, two-sided chi-square testing of differences in participation rates, a = 0,05, percentage differences). The cumulative monthly utilization of screening services was expressed in terms of the median follow-up period after invitation (20 months; study period: 1 April 2012 to 30 June 2014). Percentage changes in utilization following the invitation letters compared with the non-invited population were calculated on a monthly basis. The statistical analyses were carried out using SAS version 9.4.
Table 1. Utilization of screening fecal blood test within a year of receiving a letter of invitation with or without a test kit enclosed, or after randomization.
|
No letter (N = 6167) NSHI = 5283* |
Letter (N = 6203) NSHI = 5322* |
Difference | P value for heterogeneity |
Letter + kit (N = 6190) NSHI = 5301* |
Difference | P value for heterogeneity | |
| Utilization of screening gFOBT | 809 (15%) | 781 (15%) | −4% | 0.358 | 1319 (25%) | +62% | <0.001 |
| – Men | 165 (6%) | 147 (5%) | − 9% | 0.418 | 430 (17%) | +158% | <0.001 |
| – Women | 644 (23%) | 634 (22%) | − 5% | 0.258 | 889 (33%) | +39% | <0.001 |
| By county (% of Saarland population) | |||||||
| – Merzig-Wadern (10%) | 86 (15%) | 83 (14%) | − 5% | 0.711 | 142 (24%) | +60% | <0.001 |
| – Neunkirchen (13%) | 112 (15%) | 118 (15%) | +3% | 0.810 | 182 (24%) | +61% | <0.001 |
| – Saarbrücken region (33%) | 222 (14%) | 194 (12%) | − 14% | 0.103 | 386 (23%) | +68% | <0.001 |
| – Saarlouis (20%) | 194 (18%) | 190 (17%) | − 2% | 0.841 | 304 (29%) | +61% | <0.001 |
| – Saarpfalz (15%) | 135 (17%) | 129 (17%) | − 1% | 0.936 | 185 (25%) | +42% | <0.001 |
| – Sankt Wendel (9%) | 60 (12%) | 67 (13%) | +8% | 0.646 | 120 (24%) | +101% | <0.001 |
gFOBT, Guaiac-based fecal occult blood test; NSHI, number of persons with statutory health insurance in the population; SHI, statutory health insurance
*NSHI was established on the basis of the proportion of 50- to 54-year-olds with SHI in Saarland in 2013 (men: 81.6%; women: 89.9%). The utilization rates were calculated with the number of SHI insurees as denominator.
Table 2. Utilization of screening colonoscopy within a year of receiving a letter of invitation, or after randomization.
|
No letter (N = 8375) NSHI = 7 88* |
Letter (N = 8449) NSHI = 7251* |
Difference | P value for heterogeneity | |
| Utilization of screening colonoscopy | 323 (4.4%) | 430 (5.9%) | +32% | <0.001 |
| – Men | 155 (4.4%) | 199 (5.6%) | +27% | 0.021 |
| – Women | 168 (4.5%) | 231 (6.2%) | +36% | 0.002 |
| By county (% of Saarland population) | ||||
| – Merzig-Wadern (10%) | 36 (4.9%) | 47 (6.2%) | +26% | 0.276 |
| – Neunkirchen (13%) | 54 (5.3%) | 67 (6.7%) | +26% | 0.190 |
| – Saarbrücken region (33%) | 90 (4.1%) | 147 (6.4%) | +56% | <0.001 |
| – Saarlouis (20%) | 59 (4.0%) | 71 (4.9%) | +23% | 0.226 |
| – Saarpfalz (15%) | 63 (5.8%) | 71 (6.6%) | +14% | 0.424 |
| – Sankt Wendel (9%) | 21 (3.1%) | 25 (3.7%) | +19% | 0.535 |
NSHI, number of persons with statutory health insurance in the population; SHI, statutory health insurance
*NSHI was established on the basis of the proportion of 55- to 59-year-olds with SHI in Saarland in 2013 (men: 82.7%; women: 89.0%). The utilization rates were calculated with the number of SHI insurees as denominator.
Results
A total of 18 560 persons who reached the age of 50 years and 16 824 persons who reached the age of 55 years in the period from 1 April 2012 to 31 March 2013 were included in the randomization (figure 1).
Invitation to fecal blood test
In the intervention group comprising persons who received a letter of invitation with no test kit enclosed, the proportion of those who took advantage of the offer of a fecal blood test within a year was not appreciably greater than in the control population (both 15%, p = 0.358) (table 1). Utilization of this screening service also did not improve in the subgroups defined by sex or county of residence.
In contrast, an invitation letter with a test kit enclosed increased participation by 62% compared with the control population (25% versus 15%, p <0.001), particularly in men (+158%; women: +39%). Even after receiving a test kit with the invitation, 75% did not take up the offer. Despite the higher percentage increase in uptake by men, the participation rate was almost twice as high in women than in men (33% versus 17%). Utilization of the screening fecal test improved in all counties of Saarland, the increase ranging from 42% to 101%. Ten letters of invitation with a test kit enclosed were sent for each additional test carried out.
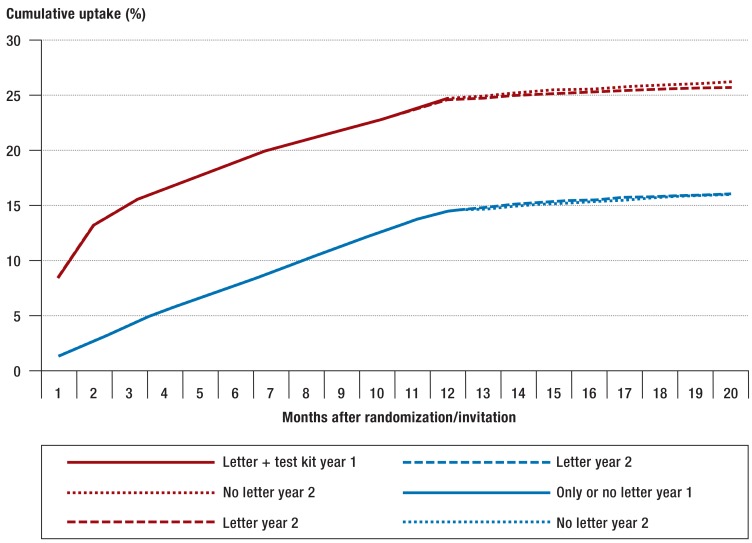
The cumulative participation rate over the whole observation period rose immediately after the invitations including a test kit were mailed (figure 2). After around 3 months, no further increase was seen. The repeat invitation after a year without a test kit enclosed did not increase the uptake.
Figure 2.
Cumulative uptake of the test for fecal occult blood (gFOBT) during the whole observation period, taking into account the renewed randomization of the study population with mailing of invitations after the end of year 1. Because of the almost identical pattern of utilization within the first 12 months without enclosure of a test kit, the groups “Only year 1” and “No letter year 1” were amalgamated.
Invitation to colonoscopy
The utilization of screening colonoscopy within a year of receiving a letter of invitation was 32% higher than in the control group (5.9% versus 4.4%, p <0.001) (table 2). The increase was greater in women than in men (36% versus 27%). Sixty-nine invitation letters with information about bowel cancer screening were sent for each additional screening colonoscopy performed.
The uptake of screening colonoscopy was higher in the intervention group than in the control group in all counties of Saarland. From different baseline rates, increases of between 14% and 56% were observed. Owing to the low case numbers in the individual counties, however, the difference was statistically significant only in the Saarbrücken area.
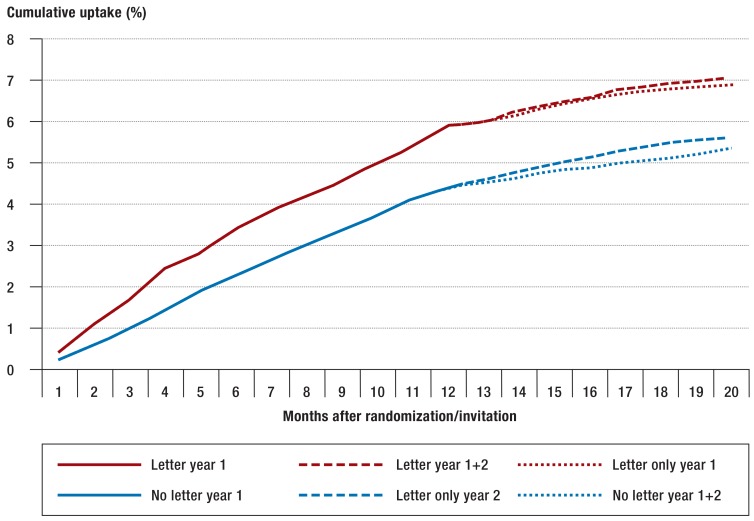
Mailing of another invitation a year later after the second randomization resulted in a slight increase in uptake among the 56-year-olds (figure 3).
Figure 3.
Cumulative uptake of screening colonoscopy during the whole observation period, taking into account the renewed randomization of the study population with mailing of invitations after the end of year 1
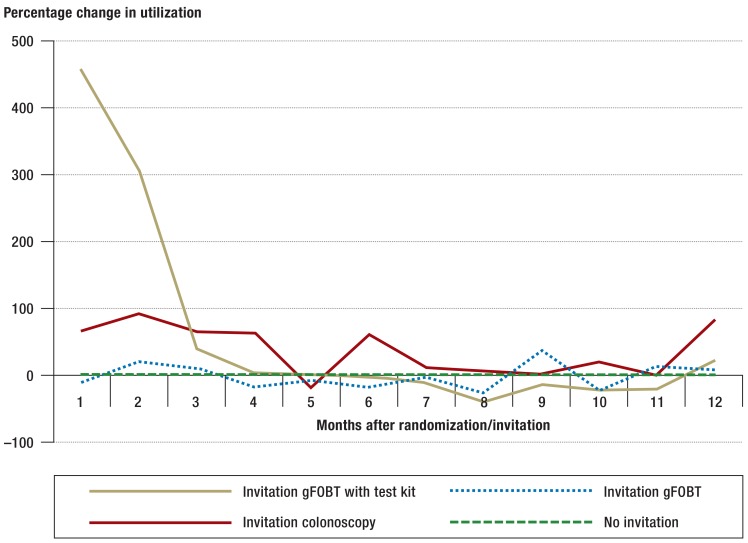
In the first year, improvements in the utilization of screening services were observed up to 6 months after invitation (efigure).
eFigure.
Percentage change in monthly utilization of the screening fecal blood test (gFOBT) or screening colonoscopy by invited persons within a year of invitation in comparison with persons who did not receive an invitation
Discussion
This randomized intervention study has shown that the utilization of bowel cancer screening services in Germany can, in some segments, be increased considerably by sending letters of invitation. Invitation letters raised the 1-year uptake of screening colonoscopy by 32%. Sending a second invitation a year later led only to a slight increase in utilization. The number of screening tests for fecal blood rose by 62%—but only when a test kit was enclosed with the invitation. Inclusion of the test kit was particularly effective in men, in whom the participation rate was increased from 6% to 17% by this means. Without such encouragement, men take advantage of the fecal blood test much less than women, who often have the test ordered by their gynecologist. In contrast, uptake of the screening fecal blood test did not increase when an invitation letter was sent without a test kit enclosed.
This is the first study to compare the effect of sending a letter of invitation with or without a fecal blood test kit. Despite the clear increase in uptake after the inclusion of a test kit (and after sending an invitation to screening colonoscopy) the participation rate, at 25% (5.9%), is still lower than those achieved in other countries.
Unexpectedly, sending a letter of invitation without a fecal blood test kit enclosed did not increase the rate of uptake. In previous pilot projects, e.g. in Saarland and among AOK insurees in the Rhineland region, higher participation was reported after a simple invitation (15). The members of our intervention group without enclosure of a test kit may have decided, after consultation with a physician or informing themselves elsewhere, that they would prefer to have the fecal immunochemical test (FIT; an immunological test for fecal occult blood), which has recently been demonstrated to be greatly superior to the conventional gFOBT (16). At the time of our study, however, FIT was not yet covered by health insurance and thus not included in the KV invoicing data.
Organized bowel cancer screening programs usually achieve much greater uptake than opportunistic programs. In the English national colorectal cancer screening program, for example, 54% of the first 2.6 million people who received an invitation with a gFOBT kit enclosed underwent screening (10). Moreover, numerous studies, including a recently published article from England evaluating the data of the screening program there, report higher uptake for FIT than for gFOBT (17, 18). In the Netherlands, three successive rounds of an organized program concentrating solely on screening for fecal blood achieved participation rates of 56 to 60% (19). In that program, mailing of the FIT test kits was announced in letters sent 2 weeks before. Those who did send samples for testing were sent a reminder. The quantitative FIT was carried out in specialized laboratories.
In the USA, 10-year rates of participation in screening colonoscopy upwards of 60% (e.g., 61.7% in 2012) are reached even without organized programs, thanks to extensive publicity campaigns (20). However, the number of people in Germany who have ever had a colonoscopy is much higher than the uptake of screening colonoscopy would lead one to suspect. According to a survey carried out in the period 2008 to 2011, 55% of 55- to 79-year olds had undergone colonoscopy in the previous 10 years (21). This means that a large proportion of the colonoscopies in Germany are performed for diagnostic reasons, e.g., in the attempt to establish the cause of pain or frank fecal blood, and are therefore not reflected in the screening statistics. Diagnostic colonoscopies also play an important part in reducing the incidence of bowel cancer (8, 22).
Our study revealed considerable variation from county to county in the uptake of screening colonoscopy. This may be attributable to differences in the accessibility of gastroenterological services or in the health-related behavior of the respective residential populations.
To ensure that our calculation of the participation rates was as accurate as possible, the proportion of probands with statutory health insurance was used in the denominator, because the utilization of screening services by the privately insured persons (around 14% of the population in this age group) who also received invitations was not included in the KV invoicing data. In interpreting the findings of our study, it should be borne in mind that insufficient data were available on the performance of fecal blood tests and diagnostic colonoscopy in our randomized population before the ages of 50 and 55, respectively. An additional screening procedure would not have been appropriate for most of the persons who had undergone colonoscopy in the last few years before receiving our invitation. The actual target population is therefore smaller than assumed. Furthermore, around 43 000 persons (ca. 5% of the population) insured by the Knappschaft (a health insurance fund that offers some additional benefits to its members) could potentially have taken advantage of services that were not documented in the invoicing data available to us. Both of these factors result in underestimation of the rate of uptake. We had no access to the findings of screening or diagnostic colonoscopy. Future studies should include details of the adenomas and carcinomas detected, to enable calculation of the number of invitations per prevented or detected malignancy.
Overall, our model project showed that also in Germany, invitation letters are a simple and inexpensive means of attaining a considerable increase in the uptake of bowel cancer screening services in the form of colonoscopy and tests for occult fecal blood. The invitation to have a fecal blood test should be accompanied by a test kit. Rather than the gFOBT used in our project, however, the kit enclosed should be the FIT, which is now covered by statutory health insurance. Nevertheless, the participation rates remained below those achieved with organized screening programs in other countries. Our findings therefore not only underline the efficacy of personal invitation, but also emphasize the need for comprehensive efforts to increase both awareness and prevention of bowel cancer through organized screening programs.
Key Messages.
The utilization of bowel cancer screening services in Germany is very low.
The Joint Federal Committee was tasked with introducing an organized program for bowel cancer screening featuring personal written invitation of all members of the population entitled to screening.
This statewide randomized intervention study in Saarland included more than 35 000 persons aged 50 or 55 years and showed that personal invitation can considerably increase the utilization of screening colonoscopy and testing for occult fecal blood.
However, the invitation to have a fecal blood test was effective only when a test kit was enclosed.
Despite the demonstrated efficacy of a single invitation, the rates of participation were still low compared with other countries. In an organized program the invitations should be accompanied by comprehensive efforts to increase both awareness and prevention of bowel cancer in order to improve participation further.
Acknowledgments
We are grateful to the Saarland Association of Statutory Health Insurance Physicians (represented by the chairman of the board, Dr. Gunter Hauptmann, and his deputy, Dr. Joachim Meiser) for the superlative assistance and cooperation rendered in the course of this model project.
Funding
The study was funded by the German Federal Ministry of Health (chapter 1501 project 54401).
Study registration
German Clinical Trials Register (DRKS): DRKS00006098
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
Translated from the original German by David Roseveare
References
- 1.Robert Koch-Institut, Gesellschaft der epidemiologischen Krebsregister in Deutschland e. V. (eds.) Häufigkeiten und Trends. 10. Berlin: 2015. Krebs in Deutschland 2011/2012. [Google Scholar]
- 2.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (Hemoccult): an update. Am J Gastroenterol. 2008;103:1541–1549. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 3.Robertson DJ, Kaminski MF, Bretthauer M. Effectiveness, training and quality assurance of colonoscopy screening for colorectal cancer. Gut. 2015;64:982–990. doi: 10.1136/gutjnl-2014-308076. [DOI] [PubMed] [Google Scholar]
- 4.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 5.Walsh JM, Terdiman JP. Colorectal cancer screening: scientific review. JAMA. 2003;289:1288–1296. doi: 10.1001/jama.289.10.1288. [DOI] [PubMed] [Google Scholar]
- 6.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 7.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014;348 doi: 10.1136/bmj.g2467. g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenner H, Schrotz-King P, Holleczek B, Katalinic A, Hoffmeister M. Declining bowel cancer incidence and mortality in Germany. Dtsch Arztebl Int. 2016;113:101–106. doi: 10.3238/arztebl.2016.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altenhofen L. Zentralinstitut für die kassenärztliche Versorgung in Deutschland. Berlin/Köln: 2016. Projekt Wissenschaftliche Begleitung von Früherkennungs-Koloskopien in Deutschland, Berichtszeitraum 2014 12. Jahresbericht, Version 2. [Google Scholar]
- 10.von Wagner C, Baio G, Raine R, et al. Inequalities in participation in an organized national colorectal cancer screening programme: results from the first 2.6 million invitations in England. Int J Epidemiol. 2011;40:712–718. doi: 10.1093/ije/dyr008. [DOI] [PubMed] [Google Scholar]
- 11.Malila N, Palva T, Malminiemi O, et al. Coverage and performance of colorectal cancer screening with the faecal occult blood test in Finland. J Med Screen. 2011;18:18–23. doi: 10.1258/jms.2010.010036. [DOI] [PubMed] [Google Scholar]
- 12.Kooperationsgemeinschaft Mammographie (eds.) Evaluationsbericht 2005-2012. Ergebnisse des Mammographie-Screening-Programms in Deutschland. Kooperationsgemeinschaft Mammographie, 2015. http://newsroom.mammo-programm.de/download/fachpublikation/Mammographiescreening_Evaluations_bericht_2005%20bis%202012.pdf (last accessed 9 January 2017) [Google Scholar]
- 13.Gemeinsamer Bundesausschuss (G-BA) Patienteninformation Darmkrebs-Früherkennung. www.g-ba.de/downloads/17-98-2233/ 2010-07-27_Merkblatt%2520Darmkrebs_WZ.pdf (last accessed 9 January 2017) [Google Scholar]
- 14.Bundesministerium für Gesundheit Mitgliederstatistik KM6, 2013. www.bundesgesundheitsministerium.de/krankenversicherung/zahlen-und-fakten-zur-krankenversicherung/mitglieder-und-versicherte.html (last accessed 9 January 2017) [Google Scholar]
- 15.Nationaler Krebsplan. Handlungsfeld 1, Ziele-Papier 2b: Bericht zum Workshop am 13. 11. 2009. www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/N/Nationaler_Krebsplan/Ziel_2b_Weiterentwicklung_der_Darmkrebsfrueherkennung.pdf (last accessed 9 January 2017) [Google Scholar]
- 16.Brenner H, Tao S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer. 2013;49:3049–3054. doi: 10.1016/j.ejca.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Moss S, Mathews C, Day TJ, et al. Increased uptake and improved outcomes of bowel cancer screening with a faecal immunochemical test: results from a pilot study within the national screening programme in England. Gut 2016; doi: 10.1136/gutjnl-2015-310691. [Epub ahead of print] doi: 10.1136/gutjnl-2015-310691. [DOI] [PubMed] [Google Scholar]
- 18.Vart G, Banzi R, Minozzi S. Comparing participation rates between immunochemical and guaiac faecal occult blood tests: a systematic review and meta-analysis. Prev Med. 2012;55:87–92. doi: 10.1016/j.ypmed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Stegeman I, van Doorn SC, Mundt MW, et al. Participation, yield, and interval carcinomas in three rounds of biennial FIT-based colorectal cancer screening. Cancer Epidemiol. 2015;39:388–393. doi: 10.1016/j.canep.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) Vital signs: colorectal cancer screening test use—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:881–888. [PMC free article] [PubMed] [Google Scholar]
- 21.Starker A, Sass AC. [Participation in cancer screening in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1)] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:858–867. doi: 10.1007/s00103-012-1655-4. [DOI] [PubMed] [Google Scholar]
- 22.Brenner H, Chang-Claude J, Jansen L, Knebel P, Stock C, Hoffmeister M. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology. 2014;146:709–717. doi: 10.1053/j.gastro.2013.09.001. [DOI] [PubMed] [Google Scholar]
- E1.Hentschel S, Katalinic A, editors. Zuckschwerdt Verlag. München: 2008. Das Krebsregister-Manual der Gesellschaft der epidemiologischen Krebsregister in Deutschland e. V. [Google Scholar]
- E2.German Record Linkage Center. Merge ToolBox (MTB) www.record-linkage.de/-Downloads–software.htm (9 January 2017) [Google Scholar]