Abstract
Background
Identifying undiagnosed HIV infection is necessary for the elimination of HIV transmission in the United States. The present study evaluated the efficacy of three community-based approaches for uncovering undiagnosed HIV among heterosexuals at high-risk (HHR), who are mainly African American/Black and Hispanic. Heterosexuals comprise 24% of newly reported HIV infections in the U.S, but experience complex multi-level barriers to HIV testing.
Methods
We recruited African American/Black and Hispanic HHR in a discrete urban area with both elevated HIV prevalence and poverty rates. Approaches tested were: 1) respondent-driven sampling (RDS) and confidential HIV testing in two sessions (n=3116); 2) RDS and anonymous HIV testing in one session (n=498); and 3) venue-based sampling (VBS) and HIV testing in a single session (n=403). The main outcome was newly diagnosed HIV infection.
Results
RDS with anonymous testing and one session reached HHR with less HIV testing experience and more risk factors than the other approaches. Further, RDS with anonymous (4.0%) and confidential (1.0%) testing yielded significantly higher rates of newly diagnosed HIV than VBS (0.3%).
Conclusion
Peer-referral approaches were more efficacious than VBS for uncovering HHR with undiagnosed HIV, particularly a single-session/anonymous strategy, and have a vital role to play in efforts to eliminate HIV transmission.
Keywords: undiagnosed HIV, respondent-driven sampling, venue-based sampling, high-risk heterosexuals, HIV testing, HIV care cascade
INTRODUCTION
Despite a decreasing trend in HIV transmission rates in the United States (US), an estimated 44,000 individuals are infected with HIV each year.1 HIV disproportionately affects African American/Black and Hispanic populations from the lower socio-economic strata, due in part to insufficient HIV diagnostic testing.2 Approximately 15% of persons living with HIV (PLWH) are unaware of their status,3 yet a third of HIV transmission events are linked to those with undiagnosed HIV infection.4 Uncovering these undiagnosed cases, the first step to engagement along the HIV care continuum, remains both a public health priority and a significant challenge.5
Nationally, heterosexual sex is the second most common route of HIV transmission after male-to-male sexual contact, accounting for an estimated 24% of newly reported infections annually, and is the main route of transmission among women.1 Consistent with the CDC’s National HIV Behavioral Surveillance (NHBS) system, we define heterosexuals at high-risk (HHR) for HIV as adults who are heterosexually active and socially networked within geographic areas with both excess HIV burden and socioeconomic disadvantage, referred to as “high-risk areas” (HRAs).6 HIV prevalence among heterosexuals overall is 0.1%, and 2.0% among HHR.7 In New York City (NYC), data from the most recent NHBS cycle with published data estimated HIV prevalence among HHR at 9.6%.8
Heterosexuals in the US are less likely to be tested for HIV over their lifetimes compared to other risk groups such as MSM (43.5% [heterosexuals] vs. 69% [MSM]).9,10 Moreover, rates of regular, annual HIV testing are insufficient among HHR (approximately one third test annually).11 As a result, an estimated 25% of HIV-infected heterosexuals are undiagnosed.9 Furthermore, late HIV diagnosis is common among heterosexuals.12 Barriers at individual/attitudinal-, social-, and structural-levels of influence impede access to and uptake of HIV testing among HHR, including insufficient knowledge of HIV, substance use, fear of stigma, distrust of medical settings, social norms that deter HIV testing, and poor access to settings where high-quality HIV testing is offered.13,14 Thus potent, culturally appropriate, active approaches to seeking out HHR, reducing barriers to HIV testing, and providing high-quality HIV testing are critically needed.
Identifying HHR is challenging because many HHR are embedded within large urban populations of lower-risk heterosexual adults. Therefore, efficient methods are needed to identify persons among whom HIV prevention activities will yield significant impact. Recruitment methods such as respondent-driven sampling (RDS), a network-based peer-to-peer recruitment strategy, and venue-based sampling (VBS), have been used in numerous research studies and intervention delivery settings for other high-risk populations such as MSM and persons who inject drugs (PWID).15 The CDC’s NHBS projects use RDS for recruiting HHR populations nationally,3,16 but the efficacy of RDS has not yet been directly tested alongside VBS for the purposes of identifying HHR with undiagnosed HIV.
This study compares the efficacy of three behavioral interventions to uncover undiagnosed HIV among HHR within an urban HRA in the US as part of the National Institute on Drug Abuse’s (NIDA) “Seek, Test, Treat, and Retain” (STTR) initiative. The present study describes the “Seek/Test” phase of these three interventions. Each intervention was guided by the Theory of Triadic Influence, a multi-level social-cognitive theory,17 integrated with Self Determination Theory18; was culturally appropriate for African American/Black and Hispanic HHR; addressed the multi-level barriers to HIV testing experienced by HHR described above; and had specific design features and advantages/disadvantages. Motivational Interviewing was used as the counseling approach in each intervention.19 To locate HHR within an urban HRA, RDS was used as the sampling method for two interventions, which provided either confidential or anonymous HIV testing, and a third used VBS.
METHODS
The present study used a non-randomized parallel study design. Similar to past NHBS studies,6,20 the present study was conducted in a well-defined HRA in central Brooklyn in New York City. As described elsewhere,21 for each Brooklyn ZIP code, an “HRA index” was calculated by combining Census-based poverty levels and case surveillance-based heterosexual HIV prevalence, with each standardized to the values of those variables for all Brooklyn ZIP codes together, and then ranked. Next, a core HRA (7 ZIP codes) was identified using the local indicators of spatial association (LISA) procedure.21 To reduce artificial restrictions on RDS recruitment, a larger HRA was then demarcated, adding remaining ZIP codes in the top 50% of the empirical distribution of the HRA index (12 additional ZIP codes). The three interventions were conducted in the same HRA during the same time period (2012–2015). A study field site was established in the core HRA. The study received ethical approval from the New York University School of Medicine Institutional Review Board and was registered with ClinicalTrials.gov (NCT01607541, NCT02421159).
Description of the sampling methods and interventions
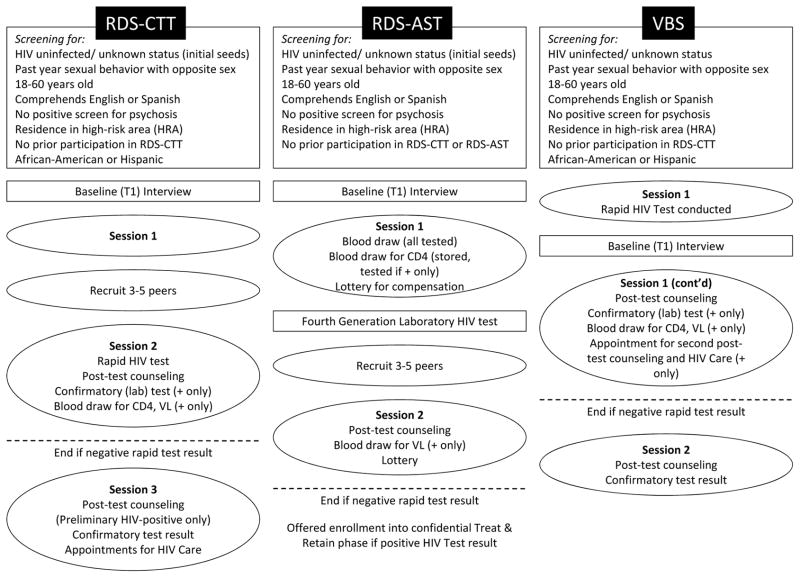
The first intervention used RDS as its recruitment method and consisted of two sessions, one to orient/engage participants and train them on peer recruitment, and the second providing confidential HIV counseling and rapid HIV testing (see Fig. 1). We refer to this as RDS-CTT (“Confidential Two-session Testing”). RDS-CTT was comparable to HIV testing services provided in a medical/clinical setting. The second intervention was planned and implemented mid-way through RDS-CTT. It addressed early findings from RDS-CTT, such as perceived HIV stigma acting as a barrier to peer recruitment and that ~25% of participants in RDS-CTT did not return for session 2. This second intervention also used RDS as its recruitment method and was designed as a flexible, relatively brief, low-threshold approach to provide easy-access to HIV counseling and testing, and using anonymous HIV testing to reduce perceived stigma (called RDS-AST, “Anonymous Single-session Testing”). A laboratory-based blood test was conducted in the first session of RDS-AST to increase rates of test acceptance/completion. The third intervention enrolled participants using VBS and provided confidential HIV counseling and rapid HIV testing in a single session. Components were culturally targeted to the barriers African American/Black and Hispanic HHR experience to HIV testing (e.g., low perceived risk, fear of HIV stigma) and carried out by trained, mainly master’s level staff. 21,22 The study’s primary outcome was the proportion of newly diagnosed HIV infections identified. All three Seek/Test interventions included components to refer those testing HIV-negative to prevention services, and link newly diagnosed individuals to HIV primary care (i.e. a “Treat & Retain” study phase),21,22 not described here.
Figure 1.
Schematic of study activities
RDS resembles other social network strategies typically conducted within community-based organizations that use members of high-risk networks, extensive training of recruiters, and compensation for recruitment to identify other potentially high-risk persons.23 However, RDS is a less labor-intensive method than these social network strategies embedded in organizations. RDS is generally conducted in street or community-based settings, uses a short and streamlined peer-recruitment training protocol, and establishes upper limits on the numbers of peers that can be recruited in order to reach members of the population of interest who may be more easily missed, in part because they do not present to, or even actively avoid, organizational settings.24 The RDS aspect of the two RDS-based interventions were consistent with standard RDS procedures (e.g., number of recruits was rationed, relationships between recruiters and recruits were assessed).24
Based on local NHBS studies published at the time the study was planned25 and the literature on the effectiveness of peer recruitment in reaching vulnerable populations,5,15,26 we hypothesized RDS-CTT and RSD-AST would be more effective than VBS in accessing populations at high-risk for undiagnosed HIV, and also that RDS-AST would yield a higher proportion of newly diagnosed HIV-infected persons (estimated 8%) with a shorter intervention than RDS-CTT (estimated 5%), with VBS showing the lowest yield (estimated 3%). Further, to allow comparisons to past surveillance studies, we assessed HIV prevalence (i.e., both previously diagnosed and newly identified HIV infections) for RDS-CTT and RDS-AST. Thus the two RDS studies included those with previously diagnosed HIV to estimate HIV prevalence, but also as a means of identifying undiagnosed HIV infections among peers and to improve the validity of self-reported HIV status at initial study screening, because prior HIV diagnosis did not preclude study participation. Procedures are described in more detail elsewhere.21,22
Sampling
“Initial seeds” starting the RDS peer recruitment chains were recruited in, and VBS events were conducted in, the same core HRA in order to access HHR with comparable socio-demographic characteristics. Sample size goals were approximately 400 for VBS, 3000 for RDS-CTT, and 500 for RDS-AST. For RDS-CTT, a total of 107 initial seeds, selected to vary in age, gender, and race/ethnicity, were directly recruited by study staff in 2012–2014 from public and street venues within the core HRA. Each seed was encouraged to start a recruitment chain by recruiting 3–5 peers. These peers then entered the study and recruited their own peers until the sample size goals were met. RDS recruitment proceeds in “waves,” that is, a set of all peers recruited by the previous set, and these waves form recruitment chains, with equilibrium, or stability, on key variables generally reached after 5–6 waves.24 Most (66.0%) participants in RDS-CTT recruited ≥1 peer (mean = 1.6; median = 1). Recruitment chain lengths ranged from 1 (just the seed, and no recruits) to 1184 (mean length = 28, SD = 119). RDS-AST began with 7 diverse initial seeds who recruited a total of 491 peers; 66.1% recruited at least one peer (mean = 1.3; median = 1) with recruitment chain lengths ranging from 2 to 171 (mean length = 69, SD = 69). The longest recruitment chains were 25 and 21 waves deep for RDS-CTT and RDS-AST, respectively. Both RDS-CTT and RDS-AST initial seeds and peers are included as participants in the present study.
VBS was conducted by randomly selecting venue-day-time units from a pre-specified sampling frame of all possible venues in the core HRA (with a venue defined as a city block with >70% commercial activity).21 The VBS intervention was conducted over 71 separate recruitment events, with the number of participants recruited at each event ranging from 1 to 13 (mean recruits per event = 6, SD = 3). RDS-CTT and VBS were conducted simultaneously in 2012–2015, and RDS-AST was conducted in 2015 over six months after RDS-CTT enrollment was completed. Procedures were designed to prevent cross-enrollment in the interventions.21,22
Eligibility
The main study eligibility criteria were: age 18–60 years, sexually active with ≥1 opposite sex partner in the past year, resides in the HRA, comprehends English or Spanish, no prior participation in the study, and not actively psychotic (see Fig. 1). Additional inclusion criteria for VBS and initial seeds for RDS (but not peers) included negative/unknown HIV status by self-report and residence in the core HRA. Peers with a previous HIV diagnosis were enrolled in the RDS studies, as described above. VBS and RDS-CTT enrolled participants from African American/Black and Hispanic racial/ethnic backgrounds only. However, in keeping with RDS-AST as a type of adapted intervention, and to further reduce artificial restriction of recruitment chains, initial seeds for RDS-AST were African American/Black and Hispanic but there was no race/ethnicity criterion for peers, although we estimated <4% would be White based on past studies.25 Procedures in the RDS studies were constructed to increase veracity of self-reported HIV status at screening, including providing comparable activities and compensation rates for those with negative/unknown and previously diagnosed HIV, a design element that participants were trained to communicate to their social network members during peer recruitment. (Those with previous HIV diagnoses are not included in the present paper as described below.)
Procedures
Participants were either directly recruited in venues by staff (VBS) or by peers (RDS, with the exception of initial seeds). Those recruited by peers presented to the study with a coded recruitment coupon. Potential participants (in both RDS and VBS) were then guided through a verbal consent process and screened for eligibility with a brief structured assessment (10 minutes). Those found eligible provided signed informed consent for remaining study activities and then completed a structured baseline interview lasting 30–60 minutes using an audio, computer-assisted self-interviewing (ACASI) program. Participants received compensation for recruitment of peers ($15/eligible peer), $15 for screening interviews, and $30 for baseline interviews and interventions session(s), as well as funds for public transportation. Assessment instruments were drawn from a set of harmonized measures developed for the NIDA-funded STTR studies, and included socio-demographic/background factors, HIV testing history, sexual behavior, and drug and alcohol use patterns.27 VBS and RDS-AST used an abbreviated version of the full assessment battery. RDS-CTT and VBS used the OraQuick ADVANCE® Rapid HIV-1/2 Antibody Test, followed by the OraSure confirmatory test, both with oral fluid. RDS-AST used the combination Antigen/Antibody Preliminary Test with Cascade Reflex to Supplementary Testing, a 4th generation test. (Because 4th generation tests detect HIV earlier than OraQuick/OraSure, we conducted OraQuick rapid tests in RDS-AST to allow direct comparison of results across studies. No discrepancies between test types were found.)
Statistical Analysis
The prevalence of previously undiagnosed HIV infection, the primary outcome, was estimated separately for each Seek/Test approach using RDS weights and bootstrapping, as implemented in the RDS package28 of the R statistical computing environment.29 Gile’s (2011) successive sampling (SS) approach was used for weighting, with one thousand SS samples per iteration.30 Ninety-eight thousand bootstrap samples were taken to estimate confidence intervals and make pairwise comparisons of Seek/Test approaches. The population size (residents of the seven HRA ZIP codes 18 years of age or older) was estimated as four hundred thousand.31 Interval estimates of relative risk were estimated using the method of variance estimates recovery (MOVER) approach.32 The same methods were used for when comparing approaches on secondary outcomes, demographic, background, health, relationship, and HIV testing history variables. Because efficiently reaching people at higher risk for HIV infection is part of how one seek strategy may be more effective than another, we do not include socio-demographic and other control variables when comparing Seek/Test strategies. All tests of statistical significance were two-tailed, and P < 0.05 was considered significant.
RESULTS
A total of 3116, 498, and 403 participants were enrolled in RDS-CTT, RDS-AST, and VBS approaches, respectively. As shown in Table 1, the prevalence of confirmed newly diagnosed HIV infection was higher among RDS-AST and RDS-CTT participants than among VBS participants, with prevalence nearly fifteen times higher in RDS-AST (RR = 14.89, 95% CI: 1.01 – 48.18, P < .01) and more than three times higher in RDS-CTT (RR = 3.84, 95% CI: 1.05 – 11.36, P = .03). Prevalence of newly diagnosed infection also was almost one-quarter as high among RDS-CTT (1%) than RDS-AST (4%), but this difference only approached statistical significance (RR = 0.26, 95% CI: 0.08 – 3.60, P = .06). The overall prevalence rates (i.e., previously and newly diagnosed) in the RDS cohorts were comparable: 7.4% (RDS-CTT) and 10.3% (RDS-AST).
Table 1.
Participant flow and primary and secondary outcomes: New York, NY, 2012–2015
| Three Seek-Test Approaches | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 RDS-CTT (N=3005) |
2 RDS-AST (N=484) |
3 VBS (N=403) |
|||||||
| CTT vs. AST Risk Ratio 95% CI |
CTT vs. AST p-value |
CTT vs. VBS Risk Ratio 95% CI |
CTT vs. VBS p-value |
AST vs. VBS Risk Ratio 95% CI |
AST vs. VBS p-value |
||||
| Primary outcome | |||||||||
| Confirmed newly diagnosed HIV-infection A | 29 1.0% |
14 4.0% |
2 0.3% |
0.26 (0.08–3.60) | 0.06 | 3.84 (1.05–11.36) | 0.03 | 14.89 (1.01–48.18) | <.01 |
| Secondary outcomes | |||||||||
| Screened for eligibility | 5057 | 661 | 565 | ||||||
| Found eligible B | 3445 66.3% |
525 79.1% |
428 71.5% |
0.84 (0.78–0.90) | <.01 | 0.93 (0.84–1.03) | 0.17 | 1.11 (0.98–1.25) | 0.09 |
| Enrolled into the study and completed baseline interview C | 3005 83.3% |
484 87.4% |
403 92.6% |
0.95 (0.88–1.04) | 0.26 | 0.90 (0.85–0.96) | <.01 | 0.94 (0.86–1.03) | 0.20 |
| Offered HIV testing D | 2380 78.9% |
484 100% |
403 100% |
||||||
| Accepted HIV testing/tested E | 2297 96.7% |
477 97.8% |
385 97.1% |
0.99 (0.96–1.01) | 0.36 | 1.00 (0.97–1.02) | 0.70 | 1.01 (0.98–1.04) | 0.60 |
| Overall HIV prevalence (previously and newly diagnosed) F | 140 7.4% |
28 10.3% |
2 0.3% |
0.72 (0.39–1.84) | 0.40 | 27.34 (10.36–75.86) | <.01 | 38.08 (10.77–112.20) | <.01 |
- Among tested
- Among screened
- Among eligible
- Among enrolled with baseline interview
- Among offered testing
- Among tested and previously diagnosed
We examined rates of engagement in study activities, which differed among the three approaches. Those in RDS-CTT were less likely than RDS-AST to be found eligible (RR = 0.84, 95% CI: 0.78 – 0.90, P < .01). RDS-CTT participants found eligible were less likely than eligible persons in VBS to enroll and complete baseline interviews (RR = 0.90, 95% CI: 0.85 – 0.96, P < .01). All enrolled VBS and RDS-AST participants were offered an HIV test, which took place in the first intervention session. Among those offered an HIV test, almost all accepted it (≥ 96.7% accepted), with no significant differences by intervention approach.
As shown in Table 2, participants recruited by the three intervention approaches differed in a number of respects, with participants recruited by RDS-AST being least likely to have regular, annual HIV testing and most likely to have both sexual and substance use risk factors compared to participants recruited by RDS-CTT and VBS.
Table 2.
Socio-demographic/background characteristics, risk factors, health, and HIV testing history: New York, NY, 2012–2015
| Three Seek-Test Approaches | ||||||
|---|---|---|---|---|---|---|
| 1 RDS-CTT (N=3005) |
2 RDS-AST (N=484) |
3 VBS (N=403) |
CTT vs. AST Risk Ratio 95% CI |
CTT vs. VBS Risk Ratio 95% CI |
AST vs. VBS Risk Ratio 95% CI |
|
| HIV infected at baseline (previously diagnosed) | 5.0 N=111 |
6.4 N=14 |
0.0 N=0 |
0.78 (0.35–8.50) | ||
| Age ≥ 40 Years | 52.4 | 61.9 | 30.7 | 0.85 (0.72–1.01) | 1.71 (1.34–2.32) | 2.02 (1.52–2.81) |
| Male Gender | 57.6 | 50.0 | 49.6 | 1.15 (0.97–1.40) | 1.16 (0.98–1.42) | 1.01 (0.79–1.29) |
| African-American, Not Hispanic | 70.6 | 69.3 | 66.4 | 1.02 (0.89–1.18) | 1.06 (0.93–1.24) | 1.04 (0.87–1.26) |
| Hispanic | 25.7 | 24.1 | 30.0 | 1.07 (0.77–1.66) | 0.86 (0.64–1.22) | 0.80 (0.49–1.26) |
| Married or in long-term relationship | 33.0 | 29.0 | 28.5 | 1.14 (0.90–1.50) | 1.16 (0.89–1.61) | 1.02 (0.71–1.49) |
| No High School Diploma | 38.2 | 44.1 | 39.6 | 0.87 (0.72–1.06) | 0.97 (0.78–1.24) | 1.11 (0.85–1.48) |
| Current Full-Time or Part-Time Work | 18.5 | 16.4 | 35.6 | 1.12 (0.81–1.71) | 0.52 (0.40–0.69) | 0.46 (0.29–0.68) |
| Unable to pay for necessities in past year | 79.8 | 84.1 | 75.4 | 0.95 (0.88–1.03) | 1.06 (0.95–1.19) | 1.12 (0.99–1.27) |
| Portion of income includes gov’t benefits | 32.3 | 32.7 | 28.9 | 0.99 (0.78–1.31) | 1.12 (0.84–1.62) | 1.13 (0.78–1.71) |
| Ever homeless | 57.5 | 61.6 | 44.3 | 0.93 (0.81–1.08) | 1.30 (1.07–1.64) | 1.39 (1.11–1.79) |
| Currently homeless | 23.8 | 22.3 | 20.0 | 1.07 (0.76–1.67) | 1.19 (0.80–2.16) | 1.11 (0.63–2.14) |
| Ever been incarcerated for > 24 hours | 57.7 | 64.7 | 47.8 | 0.89 (0.78–1.04) | 1.21 (1.01–1.48) | 1.35 (1.09–1.71) |
| Incarcerated in the past year for > 24 hours | 22.3 | 36.6 | 21.0 | 0.61 (0.48–0.79) | 1.06 (0.77–1.61) | 1.74 (1.20–2.72) |
| Health and health-related factors | ||||||
| Currently has health insurance | 85.8 | 84.3 | 83.5 | 1.02 (0.95–1.10) | 1.03 (0.95–1.11) | 1.01 (0.91–1.12) |
| Ever had STI testing | 64.3 | -- | 72.3 | 0.89 (0.80–1.00) | ||
| Ever had STI diagnosis | 25.0 | 35.4 | 27.1 | 0.71 (0.56–0.92) | 0.92 (0.69–1.35) | 1.31 (0.91–1.98) |
| Ever had hepatitis C virus diagnosis | 5.8 | 13.4 | 4.9 | 0.43 (0.28–0.76) | 1.19 (0.59–12.38) | 2.74 (1.18–28.75) |
| Clinically signif. depressive symptoms (CESD, PHQ-2) | 58.7 | 36.4 | -- | 1.61 (1.33–2.04) | ||
| Substance use | ||||||
| Ever Injected Drugs Not for a Medical Reason | 7.8 | 18.2 | 6.5 | 0.43 (0.30–0.66) | 1.19 (0.66–4.21) | 2.80 (1.43–10.01) |
| Injected Drugs in the Past Month | 1.5 | 4.7 | 1.5 | 0.32 (0.12–0.79) | 0.98 (0.33–5.12) | 3.08 (1.16–15.98) |
| Any Drug Use in the Past Month | 31.6 | 64.4 | 29.7 | 0.49 (0.42–0.58) | 1.07 (0.83–1.45) | 2.17 (1.66–2.98) |
| Meets AUDIT Criterion for Alcohol Problem – past year | 26.4 | 41.4 | -- | 0.64 (0.51–0.81) | ||
| Meets TCU Criterion for Drug Problem – past year | 22.2 | 45.1 | -- | 0.49 (0.39–0.63) | ||
| Meets Criteria for Drug or Alcohol Problem | 36.9 | 60.1 | -- | 0.61 (0.52–0.73) | ||
| Drug Use Weekly or More Often in Past Month | 20.5 | 56.2 | 20.7 | 0.36 (0.30–0.44) | 0.99 (0.74–1.43) | 2.71 (2.00–3.94) |
| Sexual behavior | ||||||
| Sex without a condom in the past month | 58.7 | 69.3 | 53.4 | 0.85 (0.75–0.97) | 1.10 (0.94–1.32) | 1.30 (1.07–1.59) |
| More than one partner past month | 27.3 | 60.7 | 22.3 | 0.45 (0.38–0.54) | 1.23 (0.9–1.85) | 2.72 (1.98–4.14) |
| Lifetime transactional sex | 29.2 | 45.6 | -- | 0.64 (0.52–0.81) | ||
| Lifetime MSM/FSF | 20.3 | -- | 14.8 | 1.37 (0.92–2.54) | ||
| HIV testing history | ||||||
| Ever tested for HIV | 91.9 | 81.1 | 95.5 | 1.13 (1.05–1.23) | 0.96 (0.93–0.99) | 0.85 (0.78–0.92) |
| HIV Test Within the Past Year | 43.7 | 32.4 | 53.7 | 1.35 (1.06–1.81) | 0.81 (0.69–0.98) | 0.60 (0.44–0.80) |
| Tests Annually Since 2006 | 23.3 | 13.0 | 21.8 | 1.79 (1.25–2.97) | 1.07 (0.8–1.55) | 0.60 (0.34–0.97) |
Notes: Numbers in the first three columns are percentages. Bold font indicates a variable for which at least one risk ratio 95% confidence interval does not include 1.0.
Compared with VBS, RDS-AST participants were more likely to be at least 40 years old (RR = 2.02), and to have been homeless (RR = 1.39). RDS-AST participants were also more likely than VBS participants to have lifetime (RR = 1.35) and recent (RR = 1.74) incarceration, hepatitis C virus infection (RR = 2.74), lifetime (RR = 2.80) and recent injection drug use (RR = 3.08), drug use by any route of administration in the past month (RR = 2.17), drug use weekly or more often in the past month (RR = 2.71), condomless sex (RR = 1.30), and multiple sex partners (RR = 2.72). RDS-AST participants were less likely than VBS participants to be employed (RR = 0.46) and to have lifetime (RR = 0.85), past-year (RR = 0.60), and regular, annual HIV testing (RR = 0.60).
Compared with VBS, RDS-CTT participants were more likely to be at least 40 years old (RR = 1.71) and were more likely to have been homeless (RR = 1.30). RDS-CTT participants were also more likely than VBS participants to have lifetime incarceration (RR = 1.21). RDS-CTT participants were less likely than VBS participants to be employed (RR = 0.52) and to have lifetime (RR = 0.96) or past-year HIV testing (RR = 0.81).
Compared with RDS-AST, RDS-CTT participants were more likely to have clinically significant levels of depressive symptoms (RR = 1.61) as well as lifetime (RR = 1.13), past-year (RR = 1.35), and annual HIV testing (RR = 1.79). RDS-CTT participants were less likely than RDS-AST participants to have past-year incarceration (RR = 0.61), a lifetime STI diagnosis (RR = 0.71), hepatitis C infection (RR = 0.43), lifetime (RR = 0.43) and recent (RR = 0.32) injection drug use, drug use by any route of administration in the past month (RR = 0.49), an alcohol (RR = 0.64), drug use (RR = 0.49), or substance use problem (RR = 0.61), drug use weekly or more often in the past month (RR = 0.36), condomless sex (RR = 0.85), multiple sex partners (RR = 0.45), and lifetime transactional sex/exchanging sex for money/drugs (RR = 0.64).
In sensitivity analysis for the primary outcome of confirmed newly diagnosed HIV infection, we excluded all participants with lifetime injection drug use (n = 390). Among VBS participants who did not report lifetime injection drug use, none tested positive for HIV infection (i.e., both VBS participants with newly diagnosed HIV infection had injected drugs). With people who had injected drugs excluded, prevalence of confirmed newly diagnosed HIV infection was still more than four times higher among RDS-AST participants (3.98%) than among RDS-CTT participants (0.93%), but this difference was not statistically significant (RR = 4.26, 95% CI: 0.11 – 17.57, P = .11). In other words, excluding participants with lifetime injection drug use led to a small reduction in the yield of newly diagnosed infections in both RDS-AST and RDS-CTT, with RDS-AST showing the same sizeable but not statistically significant advantage over RDS-CTT.
DISCUSSION
Efficient and potent active approaches to detect undiagnosed HIV among HHR are sorely needed to achieve the goal of elimination of HIV transmission in the US, and the present study addresses this gap in available HIV prevention programs. We examined the efficacy of three intervention strategies to uncover undiagnosed HIV infection among HHR by seeking them out in their communities and providing HIV counseling and testing. Moreover, this is the first direct comparison of RDS and VBS to identify undiagnosed HIV prevalence among HHR.
All three approaches yielded samples with high rates of serious risk factors such as incarceration, unemployment, and homelessness, particularly those in RDS-AST. Importantly, more than half the RDS-AST sample (60.1%) experienced substance use problems in the past year, and recent drug use was common (64.4%). Moreover, the RDS-AST sample had the lowest rates of past-year and annual HIV testing compared to the other groups – risk and behavioral factors that likely contribute substantially to ongoing undiagnosed HIV in the population. RDS-AST and RDS-CTT yielded comparable HIV prevalence rates (10.3%, 7.4%), higher than national estimates for HHR (2.0%)7 and roughly comparable to past local NHBS surveillance studies.8 Of the three interventions, RDS-AST yielded the highest rate of newly diagnosed HIV (4.0%), followed by RDS-CTT (1.0%), and then VBS (0.3%). Retention to study activities was acceptable in all three interventions, and almost all (>95%) accepted HIV testing when it was offered. Past research has noted that successful HIV testing programs are convenient, address confidentiality issues, and have credibility.33 The low-threshold approach used in RDS-AST in particular appears to have met these standards and as a result produced the highest yield of newly diagnosed individuals. On the other hand, RDS-CTT may have created barriers to study completion and reached a lower-risk sample. In future research we will examine the efficacy of the two RDS studies with respect to HIV care linkage, retention in care, and health outcomes.
We found VBS was feasible but yielded a substantially lower proportion of newly diagnosed HIV than the two RDS approaches. This suggests VBS is not an optimal STTR approach for HHR, perhaps because heterosexuals at high-risk for HIV are embedded in physical spaces that include lower-risk individuals, and VBS is an inefficient means of gaining access to those at highest risk. These findings, on the other hand, underscore the utility of peer-recruitment methods such as RDS, particularly in conjunction with interventions that provide HIV testing at the first contact, and which directly address potential issues of perceived stigma, for example, through anonymous testing. This may be in part because peers have credibility that fosters the engagement of hidden and at-risk members of social networks, who may not be present in social venues, including service organizations, and/or who are not ordinarily willing to engage with HIV testing programs in a setting such as VBS.26 The yield of newly diagnosed HIV in the three interventions was lower than hypothesized based on local surveillance studies.25 These lower than expected proportions may reflect truly lower rates, due to the active, comprehensive, and rapidly evolving local HIV prevention strategies implemented in New York City.34 As of 2012, New York State had one of the lowest rates of undiagnosed HIV (estimated 92.9% of persons living with diagnosed HIV).35 Thus the RDS approaches tested here are promising, particularly RDS-AST, and, we postulate, would have an even greater yield in a context with higher rates of undiagnosed HIV. Alternately, lower rates of newly diagnosed HIV in RDS-CTT may have emerged from departures from NHBS methodologies, such as testing for HIV in the second session. This suggests variations in the components and timing of seek/test strategies can impact sample composition with respect to risk factors and yield of undiagnosed HIV infection. Nonetheless, NYC has nearly 3,000 new HIV cases each year, almost all from African American/Black and Hispanic backgrounds.34 Thus STTR approaches are vital, albeit challenging, even in contexts with relatively low rates of undiagnosed HIV such as NYC.
Limitations
Study limitations include the use of self-reported HIV status to identify undiagnosed HIV infection. Indeed, other studies have found nondisclosure of HIV status, particularly when participants would be denied financial compensation if they so disclosed.36 The present study was designed to reduce psychosocial incentives for participants to mask their HIV-infected status at enrollment as described above, yet it is possible some individuals classified as newly diagnosed individuals were previously aware of their HIV infection. Anonymous HIV testing might increase rates of testing acceptance but precludes linkage to the local HIV surveillance registry to verify whether the individual received an HIV diagnosis. Yet recently low-cost screening for antiretroviral (ART) use has emerged as a potentially valuable objective biomarker assessment of knowledge of HIV infection, although capturing only those on ART.36 Further, despite its advantages, RDS is subject to potential biases; for example, by potentially over-recruiting from social networks that differ in key respects from the underlying target population.20 RDS sampling in the present study proceeded over a large number of recruitment waves, thereby increasing the probability that the samples are broadly representative of the underlying population.24 Also, the study did not randomize individuals to the different methods of recruitment. However, some situations are not amenable to randomized designs, such as the public health and scientific questions examined here.37 Further, power to detect differences among Seek/Test approaches on the primary outcome was reduced by the prevalence of newly diagnosed HIV infection, which was lower than expected based on previous work.
Public health implications
This study is the first to prospectively demonstrate the value of potentially replicable Seek/Test interventions to identify HHR with undiagnosed HIV, namely, interventions that use peer-referral, focus on a large socially networked population, are located in high HIV prevalence areas, and include well-trained staff and culturally focused components to motivate peer recruitment and foster engagement in study activities. As such, it provides further support for the utility of social network methods to identify undiagnosed HIV. The three interventions were successful in finding and engaging a high-risk population of HHR with suboptimal rates of past-year HIV testing. The brief, single session RDS-AST intervention yielded the greatest proportion of those newly diagnosed with HIV, and VBS yielded the lowest proportion. Because the factors that impede HIV testing among HHR are complex, serious, and long-standing, community-based STTR approaches are needed, with an emphasis on active outreach, low-threshold, and peer-based methods. Implemented on a continual basis in urban HRAs, such approaches can complement institutionally based HIV testing programs and play a vital role in eliminating HIV transmission by promoting regular HIV testing among populations at high risk for HIV, including substance users.
Footnotes
Conflicts of Interest and Source of Funding: This study was supported by the National Institute on Drug Abuse (R01DA032083, PI: Gwadz; P30DA011041, PIs: Deren and Hagan). The authors declare no financial conflicts of interest.
Contributor Information
Marya Gwadz, Center for Drug Use and HIV Research, Rory Meyers College of Nursing, New York University, New York, NY.
Charles M Cleland, Center for Drug Use and HIV Research, Rory Meyers College of Nursing, New York University, New York, NY.
David C Perlman, Center for Drug Use and HIV Research and Icahn School of Medicine at Mount Sinai, New York, NY.
Holly Hagan, Center for Drug Use and HIV Research, Rory Meyers College of Nursing, New York University, New York, NY.
Samuel M Jenness, Rollins School of Public Health, Emory University, Atlanta, GA.
Noelle R Leonard, Center for Drug Use and HIV Research, Rory Meyers College of Nursing, New York University, New York, NY.
Amanda S Ritchie, Center for Drug Use and HIV Research, Rory Meyers College of Nursing, New York University, New York, NY.
Alexandra Kutnick, Center for Drug Use and HIV Research, Rory Meyers College of Nursing, New York University, New York, NY.
References
- 1.Centers for Disease Control and Prevention. Diagnoses of HIV Infection in the United States and Dependent Areas, 2014. Vol. 26 Atlanta, GA: U. S. Department of Health and Human Services; 2015. HIV Surveillance Report. [Google Scholar]
- 2.Chopel AM, Minkler M, Nuru-Jeter A, Dunbar M. Social determinants of late stage HIV diagnosis and its distributions among African Americans and Latinos: A critical literature review. J Health Dispar Res Pract. 2014;8(4):1–29. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data - United States and 6 dependent areas, 2012. Vol. 19. Atlanta, GA: US Department of Health and Human Services; 2014. Report Number 3. [Google Scholar]
- 4.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human Immunodeficiency Virus Transmission at Each Step of the Care Continuum in the United States. Human Immunodeficiency Virus Transmission at Each Step of the Care Continuum in the United States. 2015;175(4):588–596. doi: 10.1001/jamainternmed.2014.8180. [DOI] [PubMed] [Google Scholar]
- 5.Burns D, Degruttola V, Pilcher C, et al. Towards an Endgame: Finding and Engaging People Unaware of their HIV-1 Infection in Treatment and Prevention. AIDS Res Hum Retroviruses. 2014;30(3):217–224. doi: 10.1089/aid.2013.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenness SM, Neaigus A, Murrill CS, Wendel T, Forgione L, Hagan H. Estimated HIV incidence among high-risk heterosexuals in New York City, 2007. J Acquir Immune Defic Syndr. 2011;56(2):193–197. doi: 10.1097/QAI.0b013e318202a9c4. [DOI] [PubMed] [Google Scholar]
- 7.Lansky A, Johnson C, Oraka E, et al. Estimating the Number of Heterosexual Persons in the United States to Calculate National Rates of HIV Infection. PloS one. 2015;10(7):e0133543. doi: 10.1371/journal.pone.0133543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenness SM, Neaigus A, Wendel T, Gelpi-Acosta C, Hagan H. Spatial recruitment bias in respondent-driven sampling: Implications for HIV prevalence estimation in urban heterosexuals. AIDS Behav. 2014;18(12):2366–2373. doi: 10.1007/s10461-013-0640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linley L, An Q, Song R. HIV testing experience before HIV diagnosis among men who have sex with men - 21 jurisdictions, United States, 2007–2013. MMWR Morb Mortal Wkly Rep. 2016;65(37):999–1003. doi: 10.15585/mmwr.mm6537a3. [DOI] [PubMed] [Google Scholar]
- 10.Woodring JV, Kruszon-Moran D, Oster AM, McQuillan GM. Did CDC’s 2006 revised HIV testing recommendations make a difference? Evaluation of HIV testing in the US household population, 2003–2010. J Acquir Immune Defic Syndr. 2014;67(3):331–340. doi: 10.1097/QAI.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sionean C, Le BC, Hageman K, et al. HIV risk, prevention, and testing behaviors among heterosexuals at increased risk for HIV infection--National HIV Behavioral Surveillance System, 21 U.S. cities, 2010. MMWR Surveill Summ. 2014;63(14):1–39. [PubMed] [Google Scholar]
- 12.Hall HI, Tang T, Espinoza L. Late Diagnosis of HIV Infection in Metropolitan Areas of the United States and Puerto Rico. AIDS Behav. 2016;20(5):967–972. doi: 10.1007/s10461-015-1241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahajan AP, Sayles JN, Patel VA, et al. Stigma in the HIV/AIDS epidemic: a review of the literature and recommendations for the way forward. AIDS. 2008;22(Suppl 2):S67–79. doi: 10.1097/01.aids.0000327438.13291.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarcz S, Richards TA, Frank H, et al. Identifying barriers to HIV testing: personal and contextual factors associated with late HIV testing. AIDS Care. 2011;23(7):892–900. doi: 10.1080/09540121.2010.534436. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. [Accessed May 26, 2016];National HIV Behavioral Surveillance (NHBS) http://www.cdc.gov/hiv/statistics/systems/nhbs/
- 16.Centers for Disease Control and Prevention. HIV infection, risk, prevention, and testing behaviors among heterosexuals at increased risk of HIV infection - National HIV Behavioral Surveillance, 20 U.S. cities, 2013. Atlanta, GA: U. S. Department of Health and Human Services; 2015. HIV Surveillance Special Report 13. [Google Scholar]
- 17.Flay BR, Petraitis J. The theory of triadic influence: A new theory of health behavior with implications for preventive interventions. In: Albrecht GL, editor. Advances in Medical Sociology: Volumn IV. A Reconsideration of Models of Health Behavior Change. Greenwich, CT: JAI Press; 1994. pp. 19–44. [Google Scholar]
- 18.Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self-determination of behavior. Psychol Inq. 2000;11(4):227–268. [Google Scholar]
- 19.Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3. New York, NY: Guilford Press; 2012. [Google Scholar]
- 20.Reilly KH, Neaigus A, Jenness SM, Hagan H, Wendel T, Gelpi-Acosta C. High HIV prevalence among low-income, Black women in New York City with self-reported HIV negative and unknown status. J Womens Health. 2013;22(9):745–754. doi: 10.1089/jwh.2013.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gwadz M, Cleland CM, Hagan H, et al. Strategies to uncover undiagnosed HIV infection among heterosexuals at high risk and link them to HIV care with high retention: a “seek, test, treat, and retain” study. BMC Public Health. 2015;15(1):481. doi: 10.1186/s12889-015-1816-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwadz M, Cleland C, Leonard N, et al. Hybrid STTR intervention for heterosexuals using anonymous HIV testing and confidential linkage to care: A single arm exploratory trial using respondent-driven sampling. BMC Public Health. 2015;15:1133. doi: 10.1186/s12889-015-2451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCree DH, Millett G, Baytop C, et al. Lessons learned from use of social network strategy in HIV testing programs targeting African American men who have sex with men. Am J Public Health. 2013;103(10):1851–1856. doi: 10.2105/AJPH.2013.301260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magnani R, Sabin K, Saidel T, Heckathorn D. Review of sampling hard-to-reach and hidden populations for HIV surveillance. AIDS. 2005;19(Suppl 2):S67–72. doi: 10.1097/01.aids.0000172879.20628.e1. [DOI] [PubMed] [Google Scholar]
- 25.Jenness SM, Murrill CS, Liu KL, Wendel T, Begier E, Hagan H. Missed opportunities for HIV testing among high-risk heterosexuals. Sex Transm Dis. 2009;36(11):704–710. doi: 10.1097/OLQ.0b013e3181ab375d. [DOI] [PubMed] [Google Scholar]
- 26.Johnston LG, Sabin K. Sampling hard-to-reach populations with respondent driven sampling. Methodological Innovations Online. 2010;5(2):38–48. [Google Scholar]
- 27.Chandler RK, Kahana SY, Fletcher B, et al. Data Collection and Harmonization in HIV Research: The Seek, Test, Treat, and Retain Initiative at the National Institute on Drug Abuse. Data Collection and Harmonization in HIV Research: The Seek, Test, Treat, and Retain Initiative at the National Institute on Drug Abuse. 2015;105(12):2416–2422. doi: 10.2105/AJPH.2015.302788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handcock MS, Fellows IE, Gile KJ. RDS: Respondent-Driven Sampling. [computer program]. Version 0.7–6. Los Angeles, CA: 2016. [Google Scholar]
- 29.R Core Team. R: A language and environment for statistical computing [computer program] Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 30.Gile KJ. Improved Inference for Respondent-Driven Sampling Data With Application to HIV Prevalence Estimation. J Am Stat Assoc. 2011;106(493):135–146. [Google Scholar]
- 31.United States Census Bureau. [Accessed May 24, 2016];American Fact Finder: Community Facts. http://factfinder.census.gov/faces/nav/jsf/pages/community_facts.xhtml.
- 32.Newcombe RG. MOVER-R confidence intervals for ratios and products of two independently estimated quantities. Stat Methods Med Res. 2013;0(0):1–5. doi: 10.1177/0962280213502144. [DOI] [PubMed] [Google Scholar]
- 33.Angotti N, Bula A, Gaydosh L, Kimchi EZ, Thornton RL, Yeatman SE. Increasing the acceptability of HIV counseling and testing with three C’s: convenience, confidentiality and credibility. Soc Sci Med. 2009;68(12):2263–2270. doi: 10.1016/j.socscimed.2009.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.New York City Department of Health and Mental Hygiene. [Accessed May 24, 2016];HIV/AIDS Annual Surveillance Statistics. 2015 https://www1.nyc.gov/site/doh/data/data-sets/hiv-aids-annual-surveillance-statistics.page.
- 35.Hall HI, An Q, Tang T, et al. Prevalence of Diagnosed and Undiagnosed HIV Infection - United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2015;64(24):657–662. [PMC free article] [PubMed] [Google Scholar]
- 36.Marzinke MA, Clarke W, Wang L, et al. Nondisclosure of HIV status in a clinical trial setting: antiretroviral drug screening can help distinguish between newly diagnosed and previously diagnosed HIV infection. Clin Infect Dis. 2014;58(1):117–120. doi: 10.1093/cid/cit672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West SG, Duan N, Pequegnat W, et al. Alternatives to the randomized controlled trial. Am J Public Health. 2008;98(8):1359–1366. doi: 10.2105/AJPH.2007.124446. [DOI] [PMC free article] [PubMed] [Google Scholar]