Abstract
Neurological disorders account for a majority of non-malignant disability in humans and are often associated with dysfunction of the blood-brain barrier (BBB). Recent evidence shows that despite apparent variation in the origin of neural damage, the central nervous system has a common injury response mechanism involving platelet-derived growth factor (PDGF)-CC activation in the neurovascular unit and subsequent dysfunction of BBB integrity. Inhibition of PDGF-CC signaling with imatinib in mice has been shown to prevent BBB dysfunction and have neuroprotective effects in acute damage conditions, including traumatic brain injury, seizures or stroke, as well as in neurodegenerative diseases that develop over time, including multiple sclerosis and amyotrophic lateral sclerosis. Stroke and traumatic injuries are major risk factors for age-associated neurodegenerative disorders and we speculate that restoring BBB properties through PDGF-CC inhibition might provide a common therapeutic opportunity for treatment of both acute and progressive neuropathology in humans. In this review we will summarize what is known about the role of PDGF-CC in neurovascular signaling events and the variety of seemingly different neuropathologies it is involved in. We will also discuss the pharmacological means of therapeutic interventions for anti-PDGF-CC therapy and ongoing clinical trials. In summary: inhibition of PDGF-CC signaling can be protective for immediate injury and decrease the long-term neurodegenerative consequences.
Keywords: Blood-brain barrier, PDGF-CC, tPA, neurodegeneration, traumatic brain injury, stroke
1. Introduction
The 2013 Global Burden of Disease Study showed that cerebrovascular disease and traumatic injuries are among the top five leading causes for disability incidence worldwide regardless of country wealth (Murray et al., 2015) and are the main drivers for increase in years lived with disability (Vos et al., 2015). It is expected that the prevalence of cerebrovascular disabilities including stroke and neurodegenerative diseases will expand due to an aging population and lead to increased costs for direct and follow-up patient care (Olesen, Gustavsson, Svensson, Wittchen, & Jönsson, 2012). Apart from rare genetic predispositions or aging, which are the most evident risk factors for neurodegeneration, an additional aspect can contribute to increased prevalence of neurodegenerative diseases, namely early traumatic events and blood-brain barrier (BBB) dysfunction. It has become increasingly apparent that even mild cerebral traumatic events occurring early in life can lead to BBB dysfunction and thereby an increased risk for developing neurological disorders later in life (Gardner & Yaffe, 2015; Gardner et al., 2015; Johnson, Stewart, & Smith, 2012; Kang & Lin, 2012; Lunny, Fraser, & Knopp-Sihota, 2014; Seelen et al., 2014; Sivanandam & Thakur, 2012). Along with these epidemiological studies recent literature has identified various molecular signaling pathways governing BBB formation and maintenance. In this review we will focus on the platelet-derived growth factor (PDGF)-CC signaling pathway, since inhibition of this pathway has been shown to minimize BBB dysfunction in both acute and progressive experimental neuropathology models. We will first outline different neurological disorders and their link to BBB dysfunction, and then we will look into the mechanisms of PDGF-CC activation in light of its role in regulation of BBB integrity. Lastly, we will discuss the therapeutic attempts of PDGF-CC inhibition in neurological diseases with focus on future human therapy.
2. Neurological disorders, their association with BBB dysfunction and the role of PDGF-CC
a. Overview of neurological disorders and their association with BBB dysfunction
In order to improve treatments for acute neurologic conditions as well as neurodegenerative diseases it is necessary to understand the mechanisms leading to development of these disorders. A growing body of epidemiological evidence shows that apart from genetic predisposition and advanced age, previous incidence of cerebral trauma is associated with increased risk for neurodegeneration in the elderly.
For example: traumatic brain injury (TBI) with its clinical spectrum (Jordan, 2013) and acute pathophysiology (DeKosky, Blennow, Ikonomovic, & Gandy, 2013; Prins, Greco, Alexander, & Giza, 2013) can have a wide array of long-term consequences. Initial head concussions induce chronic traumatic encephalopathy (CTE) with a broad pathology spectrum of amyloid, tau and TDP-43 inclusions together with behavioral and cognitive impairment (McKee et al., 2013). The widespread accumulations of tau and amyloid pathology can persist for years after TBI (Johnson et al., 2012) together with sustained inflammation and white matter degeneration (Johnson et al., 2013). The long term consequences of TBI incidence leads to an increased risk for developing Alzheimer’s disease (AD) (Sivanandam & Thakur, 2012), dementia (Johnson & Stewart, 2015), Parkinson’s disease (PD) (Gardner et al., 2015) multiple sclerosis (MS) (Kang & Lin, 2012; Lunny et al., 2014) and amyotrophic lateral sclerosis (ALS) (Seelen et al., 2014). In a similar fashion, the incidence of stroke, along with its immediate cellular and clinical consequences (Moskowitz, Lo, & Iadecola, 2010; van der Worp & van Gijn, 2007), has also been shown to increase the risk for Alzheimer’s disease (Thiel, Cechetto, Heiss, Hachinski, & Whitehead, 2014; Torre, 2013), ALS (Turner, Goldacre, Talbot, & Goldacre, 2015), vascular dementia (Allan et al., 2011; Savva & Stephan, 2010) and can lead to vascular forms of Parkinson’s disease (Korczyn, 2015). This causal relationship between early trauma and neurodegeneration is not well understood. However, both the acute and the long-term degenerative events in the brain, despite their variety of origins, share common mechanisms of cerebral injury response that are associated with increased BBB dysfunction.
Blood vessels in the brain have unique properties with a tight barrier, known as the BBB separating circulating plasma contents from the brain parenchyma, and BBB dysfunction defined either as serum protein leak, hemorrhage or infiltration is thought to be a significant component leading to neurodegeneration (Obermeier, Daneman, & Ransohoff, 2013; Zlokovic, 2010). On the cellular level the formation of the physical barrier is manifested in the endothelial cells lining the vessel wall, with specialized tight junctions to prevent paracellular outflow, low vesicle transcytosis and active efflux mechanisms, which together minimize transcellular transport of plasma contents. These physical properties of the brain endothelium are induced and maintained by molecular signaling pathways (reviewed by (Chow & Gu, 2015)) as well as cellular interactions with perivascular cells including pericytes or smooth muscle cells (generally referred to as vascular mural cells) and astrocytes (reviewed by (Abbott, Rönnbäck, & Hansson, 2006; Obermeier et al., 2013; Zhao, Nelson, Betsholtz, & Zlokovic, 2015)).
Recent studies have increased our understanding of the contribution of the BBB to acute and long-term neuropathologies. For instance, an acute traumatic blow to the head or spinal cord results in an immediate BBB dysfunction (Sharma, 2011), followed by an increase in cerebral edema and inflammation (Donkin & Vink, 2010; Woodcock & Morganti-Kossmann, 2013). Therefore restoration of the BBB control is considered an important protective target for TBI therapy (Shlosberg, Benifla, Kaufer, & Friedman, 2010). Similarly, ischemic stroke results in an abrupt increased of BBB permeability followed by cerebral edema (Ayata & Ropper, 2002; Yang & Rosenberg, 2011) and in severe cases hemorrhagic transformation (Hom et al., 2010). The mechanisms leading to ischemic BBB opening are intensely studied for their therapeutic potential (Jin, Yang, & Li, 2010)
Apart from acute injury, BBB dysfunction is also reported in neurodegenerative diseases where a variety of contributing factors result in a slowly progressive disease course (Zlokovic, 2011). BBB dysfunction has also been described in patients with Alzheimer’s disease (Sengillo et al., 2013; van Assema et al., 2012), ALS (Donnenfeld, Kascsak, & Bartfeld, 1984; Miyazaki et al., 2011), MS (Gaitán et al., 2011) and PD (Bartels et al., 2008; Kortekaas et al., 2005). Among several pathways described to play a role in the development and maintenance of the BBB (reviewed by (Chow & Gu, 2015; Obermeier et al., 2013; Zhao et al., 2015)), in this review we will focus on the role of PDGF-CC in regulation of BBB integrity in various neuropathologies and the potential therapeutic applications of targeting this pathway.
b. PDGF-CC as a regulator of BBB integrity
PDGF-CC belongs to a family of secreted proteins which stimulate PDGF receptors α and β tyrosine kinase activity and have important roles in development and homeostasis of several tissues (reviewed by (Andrae, Gallini, & Betsholtz, 2008)). In the developing CNS the PDGFs have been shown to stimulate cell migration, formation of cranial neural crest and meningeal basement membranes (Andrae et al., 2016, 2008; Smith & Tallquist, 2010). In the adult CNS PDGF-CC, and later also PDGF-BB, have been shown to affect BBB permeability, the latter through maintenance of the pericyte-endothelial interaction (Armulik et al., 2010; Armulik, Genové, & Betsholtz, 2011; Su et al., 2008). Intraventricular injection of active PDGF-CC protein is sufficient to induce BBB opening (Su et al., 2008) and its inhibition was shown to reduce BBB dysfunction and indicated promising therapeutic effects in experimental ischemic stroke (Su et al., 2008), spinal cord injury (Abrams et al., 2012; Sharp, Yee, & Steward, 2014), TBI (Su et al., 2015), MS (Adzemovic, Zeitelhofer, Eriksson, Olsson, & Nilsson, 2013), seizures (Fredriksson et al., 2015) and ALS neurodegeneration (Lewandowski et al., 2016). In order to better understand the role of PDGF-CC in regulation of BBB integrity we will first describe its structure and mechanisms of activation.
3. PDGF-CC structure and mechanisms of activation
a. Gene and protein organization
PDGF-CC was discovered as a novel member of the PDGF family (Li et al., 2000) about three decades after the identification of PDGF-AA and PDGF-BB (Johnsson, Heldin, Westermark, & Wasteson, 1982; Kohler & Lipton, 1974). The PDGF family has since been extended with an additional member, PDGF-DD (Bergsten et al., 2001; LaRochelle et al., 2001). All the members of this family contain a structurally well-conserved growth factor domain (GFD), that is characterized by a pattern of eight invariant cysteine residues (reviewed in (Fredriksson, Li, & Eriksson, 2004)) (Figure 1). PDGF-CC and PDGF-DD contain an additional unique structural module, referred to as the CUB (Complement subcomponents C1r/C1s, Urchin EGF-like protein, Bone morphogenic protein-1) domain (Bergsten et al., 2001; Li et al., 2000) (Figure 1).
Figure 1. Putative structure of the human PDGF-C CUB and GF domains.
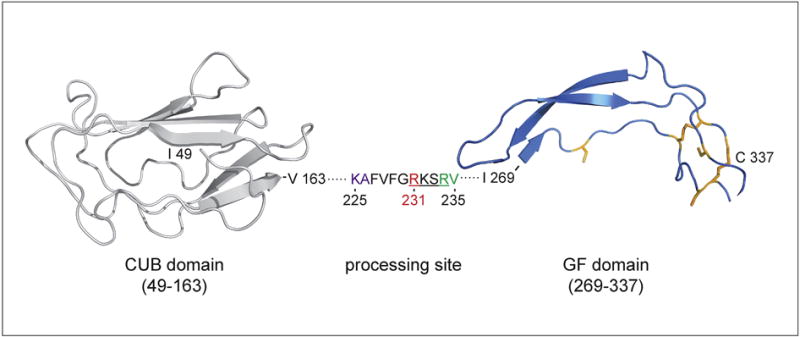
Domains are modeled separately and based on homology with human Cubulin (PDB: 3KQ4, residues 935–1042) and human PDGF-A (PDB: 3MJK, residues 118–179). The segment between CUB and GF domains is represented as dots with displayed cleavage site sequence. RV – predicted and KA – observed cleavage site (Gilbertson et al. JBC 2001), RKSR – putative processing site (Li et al. Nat. Cell. Biol. 2000), R231 – essential for tPA-mediated cleavage (Fredriksson et al. JBC 2005). The disulfide bond-forming cysteines conserved in the GF domain of PDGF family are highlighted in orange.
The human PDGFC gene is located on chromosome 4 and spans around 200 Kb of genomic DNA (Uutela et al., 2001). It consists of 6 coding exons, where exon 1 encodes the signal peptide, and exons 2 and 3 encode the structural CUB domain. Exon 4 in the PDGFC gene encodes the region between the two structural domains in the growth factor and exons 5 and 6 encode the GFD (Uutela et al., 2001). PDGFC promoter is similar to the one of PDGFA with binding sites for Sp1, Egr-1, Smad and Ets transcription factors and was shown to be stimulated by binding of Sp1 and Egr-1 proteins (Midgley & Khachigian, 2004; Sanchez-Guerrero, Midgley, & Khachigian, 2008) (Figure 2). The Egr-1 and Sp1 binding sites at −986 contain a C>T single nucleotide polymorphism termed rs28999109. It abolishes the Sp1 and Egr-1 consensus binding motif and was shown to decrease promoter activity (Choi et al., 2009) (Figure 2). The mRNA encoding PDGF-C results in a polypeptide chain of 345 amino acids in length that assembles into a PDGF-CC, homodimer through intra- and inter-chain disulphide bond formation between cysteine residues in the GFD (Figure 1). The GFD is around 100 amino acid residues in length and crystallization studies have shown that the two GFD in a PDGF-BB homodimer are arranged in an anti-parallel fashion (Oefner, D’Arcy, Winkler, Eggimann, & Hosang, 1992). Given the close similarities in the primary structures of the GFD in all the PDGFs, it is likely that also PDGF-CC adopts this anti-parallel organization during dimerization. The N-terminal of the GFD in the PDGF-C polypeptide chain is the CUB domain (Li et al., 2000). It is now widely accepted that one of the most important roles of the CUB domains in PDGF-CC is to prevent the full-length dimer from binding its cognate receptor by sterically blocking the receptor binding surfaces in the GFDs. PDGF-CC is thus secreted as a latent factor requiring proteolytic removal of the CUB domains to release the GFD dimer, which can subsequently bind and activate the tyrosine kinase receptor PDGFR-α (discussed in further detail below) to regulate various biological processes. Another important function of the CUB domains in PDGF-CC is that they play a role in the binding and positioning of its activating enzyme, tissue-type plasminogen activator (tPA) (Fredriksson, Ehnman, Fieber, & Eriksson, 2005; Fredriksson, Li, Fieber, Li, & Eriksson, 2004). CUB domains have been found in many different proteins where they are involved in protein – protein or protein – carbohydrate interactions (Bork & Beckmann, 1993) and it has therefore been proposed that the CUB domains in PDGF-CC, as well as PDGF-DD, might confer retention of the growth factors to the pericellular space after secretion (Li et al., 2000). Studies on PDGF-DD do not however support this hypothesis, instead these studies suggest that the CUB domain in PDGF-CC and PDGF-DD conceals a retention motif that is exposed by proteolytic cleavage (Ehnman, Li, Fredriksson, Pietras, & Eriksson, 2009). Recent results have suggested that the CUB domain in PDGF-CC transduce intracellular signaling events independently of the GFD (Hayes et al., 2015). This notion is not supported by studies in mice where overexpression of only the GFD in heart induced cardiac fibrosis, hypertrophy, and dilated cardiomyopathy whereas overexpression of only the CUB domain did not (Pontén et al., 2003).
Figure 2. Overview of PDGF-CC signaling.
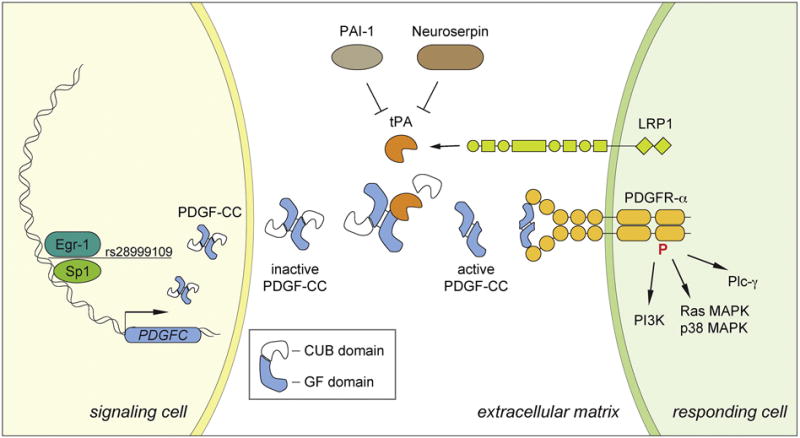
PDGFC gene expression in the signaling cell is regulated by the Egr-1 and Sp1 transcription factors. SNP rs28999109 disrupts the consensus binding sequence for Egr-1 and Sp1 and decreases promoter activity. In the extracellular matrix the inactive PDGF-CC homodimer containing the CUB domains is cleaved by tPA to release the active dimer consisting of growth factor domains. PAI-1 is a tPA inhibitor in plasma fibrinolysis and Neuroserpin inhibits tPA in the CNS parenchyma. PDGFR-α is present in the responding cell together with LRP1, a co-receptor that facilitates efficient cleavage of PDGF-CC by tPA. Upon ligand binding PDGFR-α becomes phosphorylated and activates intracellular signaling pathways including PI3K, Ras MAPK, p38 MAPK and Plc-γ.
b. Mechanisms of activation
PDGF-CC is secreted as an inactive dimer of about 90 kDa in size that requires extracellular proteolytic activation through removal of its N-terminal CUB domains (Li et al., 2000) (Figure 2). We have previously shown that the trypsin-like serine protease, tissue-plasminogen activator (tPA), is a potent activating enzyme of PDGF-CC (Fredriksson, Li, Fieber, et al., 2004) and this has later been confirmed by others (Hurst, Najy, Ustach, Movilla, & Kim, 2012; Lei et al., 2007; Riehle et al., 2014). It has since been shown that PDGF-DD is activated through proteolysis by the other known plasminogen activator, urokinase plasminogen activator (uPA) (Ehnman et al., 2009; Ustach & Kim, 2005). Cleavage of latent PDGF-CC by tPA releases a GFD dimer of approximately 35 kDa in size (Fredriksson, Li, Fieber, et al., 2004) (Figure 2). Identification of tPA as the catalytic enzyme responsible for PDGF-CC activation was rather unexpected because tPA is best known for its role in fibrinolysis within blood plasma, where it cleaves and activates plasminogen into the broad specificity protease plasmin (Collen, 2001). However, especially in the CNS it is evident that tPA regulates biologic processes that are not mediated through activation of plasminogen, but through other downstream substrates (Lemarchant et al., 2012; Su, Fredriksson, Schielke, Eriksson, & Lawrence, 2009).
Based on alignments with the other PDGFs, a highly conserved tribasic sequence has been identified in all four chains (Bergsten et al., 2001; Li et al., 2000). These sites are located approximately 15 amino acids upstream of the first cysteine residue of the GFDs. In human PDGF-C this tribasic sequence is RKSR234 and using mutagenesis of this tribasic sequence in PDGF-CC it was confirmed that tPA-mediated cleavage depends on this site with R231 in human PDGF-CC being of particular importance (Fredriksson et al., 2005) (Figure 1). The cleavage site of human PDGF-C was predicted to be at R234-V235 and observed at K225-A226 (Gilbertson et al., 2001). Cleavage of PDGF-AA and PDGF-BB is also known to occur at these sites, whereas cleavage of PDGF-DD has been reported to occur further away from the GFD (LaRochelle et al., 2001; Ustach & Kim, 2005). tPA-mediated cleavage of PDGF-CC depends not only on this conserved tribasic site, but also on correct positioning of tPA through interaction between the second kringle domain of the protease with both the CUB and GF domains in PDGF-CC (Fredriksson et al., 2005; Fredriksson, Li, Fieber, et al., 2004).
Apart from tPA, other proteases, including plasmin, uPA and matriptase have been shown to cleave PDGF-CC in in vitro biochemical assays and experimental disease models (Lei, Velez, Hovland, Hirose, & Kazlauskas, 2008; Li et al., 2000; Riehle et al., 2014). The existence of additional PDGF-CC activating proteases is supported by recent findings illustrating an incomplete phenotypic overlap in the brains of PDGF-CC and tPA deficient mice, but it is considered unlikely that broad specificity proteases, such as plasmin and matriptase will be relevant for regulation of PDGF-CC activity in adult physiology (Fredriksson, Li, Fieber, et al., 2004). Nevertheless it is certainly plausible that these proteases might play a role in activation of the growth factor during developmental processes and in pathologic conditions, although this requires further investigation.
The activation of PDGF-CC by tPA is regulated by a number of serine protease inhibitors (serpins) and co-receptors (Su et al., 2009). Outside the CNS, tPA activity is mainly regulated through inhibition by plasminogen activator inhibitor-1 (PAI-1, SERPINE1) (Collen, 2001), whereas in the CNS, neuroserpin (SERPINI1) was recently found to be the physiologic relevant inhibitor of tPA activity (Fredriksson et al., 2015) (Figure 2). In the CNS, where tPA is believed to exert unique functions distinct from its role in fibrinolysis, we have previously demonstrated that activation of PDGF-CC in the neurovascular interface requires existence of LDL receptor-related protein (LRP1) (Su et al., 2008) (Figure 2). Thus, we speculate that efficient activation of PDGF-CC in the neurovascular unit and subsequent signaling through PDGFR-α depends on the formation of a multimeric protein complex involving, at least, tPA, PDGF-CC, LRP1, and PDGFR-α (Su et al., 2009) (Figure 2).
c. Protein function in physiology and disease
PDGF signaling regulates diverse cellular functions in connective tissue cells, such as fibroblasts and smooth muscle cells, as well as in cells of neuroectodermal origin (Andrae et al., 2008). PDGF signaling is important for recruitment of supportive cells during the process of angiogenesis, but these molecules also display other functions. It is now widely appreciated that the PDGF ligand – receptor systems are essential for embryogenesis (Betsholtz, 2004), and that overactivity of PDGF signaling correlates to several pathological conditions (Heldin, 2013, 2014). This has led to investigations targeting the PDGF pathway in various diseases including pulmonary arterial hypertension (Antoniu, 2012), cancers (Östman, 2004) as well as fibrotic diseases and systemic sclerosis (Trojanowska, 2008). Considering that PDGF-CC signaling has also been linked to both fibrotic disease (Eitner et al., 2008) and breast cancer (Hurst et al., 2012) it is possible that targeting this growth factor in these non-neurologic disorders by the means summarized below may be an effective therapeutic approach, however, this review is focused only on the potential indications in the CNS.
Deletion of the genes encoding the various PDGF ligands and receptors has provided invaluable information about their biological function during embryonic development. Deletion of Pdgfra gene (Soriano, 1997), encoding the receptor for PDGF-AA and PDGF-CC, results in a phenotype that is similar, but more severe than elimination of either Pdgfa (Boström et al., 1996) or Pdgfc (Ding et al., 2004) alone. Combined deletion of Pdgfa and Pdgfc was found to recapitulate the Pdgfra knockout phenotype and indicates that PDGF-AA and PDGF-CC are the primary PDGFR-α ligands, at least during embryonic development (Ding et al., 2004). Our previous data showed that Pdgfc deficiency on a C57BL/6 background is largely compatible with postnatal life and offered the opportunity to study physiologic and pathophysiologic roles of this protein in the adult mouse (Fredriksson et al., 2012). These Pdgfc deficient mice have numerous congenital defects, including cerebral ventricular malformations with a distorted ependymal lining and abnormal cerebral vascularization, features that we recently found also to be associated with ablation of tPA (Stefanitsch, Lawrence, Olverling, Nilsson, & Fredriksson, 2015), which suggests a direct in vivo link between PDGF-CC signaling and its activating enzyme in CNS development. More recently, studies utilizing Pdgfc deficient mice with reduced Pdgfra levels have identified additional roles for PDGF-CC in CNS development including abnormal meningeal formation and neuronal over-migration in the cerebral cortex (Andrae et al., 2016). Pdgfc deletion studies in mice have also revealed that PDGF-CC is essential for palatogenesis (Ding et al., 2004). Interestingly, human linkage and association studies show strong linkage between a 30-cM region on human chromosome 4, where the PDGFC gene maps, or the rs28999109 polymorphism in the PDGFC promoter and occurrence of cleft lip with or without cleft palate (CL/P) (Choi et al., 2009; Marazita et al., 2002), which suggests that PDGF-CC has role a in palatogenesis also in humans (Calcia et al., 2013; Choi et al., 2009; Jugessur et al., 2009).
The role of PDGF-CC in adult physiology is much less well understood. tPA was identified as the activating enzyme of PDGF-CC (Fredriksson, Li, Fieber, et al., 2004) and has been demonstrated to be both necessary and sufficient to increase vascular permeability in the brain through control of BBB integrity (Yepes et al., 2003). This tPA-induced opening of the BBB was reported to depend on binding of tPA to the cell surface receptor LRP1 and cleavage of an unknown substrate (Yepes et al., 2003) which was later shown to be PDGF-CC (Su et al., 2008). In addition, LRP1 had been shown to act as a co-receptor of PDGF receptor signaling in an experimental model of atherosclerosis (Boucher, 2003; Loukinova et al., 2002). Based on these findings it was hypothesized that PDGF-CC might be a downstream substrate of tPA within the neurovascular unit involved in regulation of BBB integrity, which was later confirmed by studies in mice (Su et al., 2008). It was demonstrated that both tPA and LRP1 act upstream of active PDGF-CC in controlling cerebrovascular permeability and that LRP1 expressed on the cell surface enhances tPA-mediated activation of latent PDGF-CC and subsequently PDGFR-α signaling expressed on perivascular astrocytes (Su et al., 2008). Activation of PDGFR upon ligand binding depends on receptor dimerization and subsequent phosphorylation of the intracellular domains (reviewed by (Heldin, Östman, & Rönnstrand, 1998)). Activated PDGFR-α and -β receptors stimulate several downstream signaling pathways including Ras and p38 MAPK (mitogen–activated protein kinase), PI3K and Plc-γ (reviewed by (Andrae et al., 2008; Demoulin & Essaghir, 2014)) (Figure 2) with the p38 MAPK activity being particularly relevant for BBB integrity upon PDGFR-α stimulation (Ma et al., 2011).
4. tPA – PDGF-CC – PDGFR-α signaling axis in the CNS: Means of pharmacological inhibition and therapeutic applications
a. Means of pharmacological PDGF-CC inhibition
As mentioned above PDGF-CC exerts its function by binding to and activating PDGFR-α signaling. Signal transduction can be inhibited by several approaches (reviewed by (Andrae et al., 2008)) and here we will review the means reported to inhibit PDGF-CC – PDGFR-α signaling that are applicable for translational therapies. First, the ligand can be blocked from interacting with the receptor by antibodies targeting the PDGF-CC protein. This has been illustrated in a number of studies using anti-PDGF-CC neutralizing antibodies in models of experimental stroke (Su et al., 2008), fibrosis (Eitner et al., 2008; Martin et al., 2013) and macrophage survival (Son, Na, Hwang, & Seok, 2014) (Figure 3 and Table 1). Second, antibodies directed against the ligand-binding domain of the PDGFR-α have been successfully used to inhibit receptor activation in myocardial infarction (Zymek et al., 2006) and cancer (Dolloff, Russell, Loizos, & Fatatis, 2007; Laing et al., 2013; Loizos et al., 2005; Taniguchi et al., 2008) (Figure 3 and Table 1). Antibody-based methods have the advantage of target specificity and low toxicity, but may be costly to manufacture. Lastly, small molecules, including tyrosine kinase inhibitors (TKIs), can be used to prevent the intracellular domain phosphorylation of PDGFR-α after ligand binding. Tyrosine kinase inhibitors with high specificity for PDGFR-α/β inhibition and IC50 values below 400 nM include imatinib, sunitinib, sorafenib, pazopanib and nilotinib (Heldin, 2013, 2014) (Figure 3 and Table 1). These inhibitors all have other targets beyond PDGFR and inhibit several other tyrosine kinases. Indeed, most were developed as inhibitors of other target receptors. For example imatinib was developed as an inhibitor of Abl kinase and sunitinib can also inhibit VEGFR. Numerous other TKIs have been designed to inhibit PDGFRs and are currently tested by pharmaceutical companies for therapeutic applications (Dai, 2010). For broader description on TKI selectivity and sensitivity see the reviews by (Heldin, 2014; Levitzki, 2013). The advantage of TKIs is that they have been approved by the FDA for the treatment of human diseases and can provide options for faster translation into therapy. Taking into consideration the rather broad receptor selectivity of many of the TKIs development of antibodies for specific PDGF ligands and receptors could become a more precise therapeutic approach with minimal off-target effects. Below we will describe the neurological conditions where inhibition of the PDGF-CC signaling has been associated with restoration of the BBB with beneficial neuroprotective effects.
Figure 3. Pharmacological means of inhibition for PDGF-CC – PDGFR-α signaling.
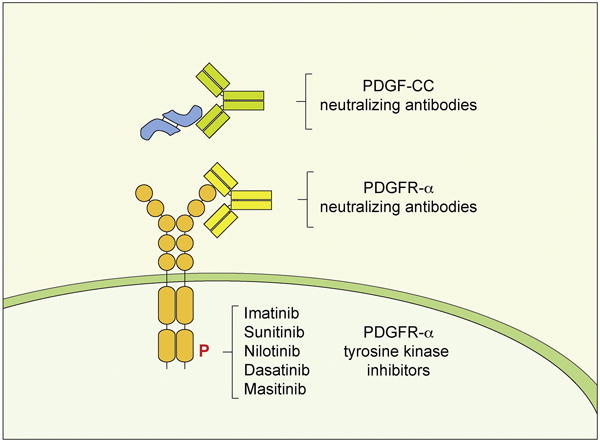
Active PDGF-CC ligand can be targeted with neutralising antibodies to prevent receptor binding. In similar fashion ligand binding to PDGFR-α can be prevented by antibodies targeting the extracellular domains of the receptor. PDGFR-α intracellular domain phosphorylation and signaling activity can be reduced with tyrosine kinase inhibitors. For detailed description see Table 1.
Table 1.
Pharmacological methods of targeting the PDGF-CC – PDGFR-α pathway.
| Means of inhibition | Effect on PDGF-CC pathway | Other biological functions tested | Effect on BBB | Effect on neurodegeneration | Reference | |
|---|---|---|---|---|---|---|
| Anti PDGFR-α antibodies | PDGF-CC 615 Ab (rabbit) | Inhibits PDGF-CC induced PDGR-α phosphorylation | Inhibits TPA induced BBB dysfunction | Reduces tPA induced BBB dysfunction | - N.S. (not studied) | (Su et al., 2008) |
| PDGF-CC Ab (sheep) | Inhibits PDGFC induced PDGR-α phosphorylation | Reduction of kidney fibrosis and myofibroblast accumulation. | - N.S. | - N.S. | (Eitner et al., 2008) (Martin et al., 2013) | |
| PDGF-CC HH1–57 Ab (mouse) | Inhibits PDGF-CC induced PDGFR-α phosphorylation | PDGFR-α activation assay in vitro | - N.S. | - N.S. | (Lei et al., 2009) | |
| Anti PDGFR-α antibodies | PDGFR-α 3G3 Ab (mouse) | Blocks ligand binding to PDGFR-α. | Inhibits growth of glioblastoma leiomyosarcoma xenografts | - N.S. | - N.S. | (Loizos et al., 2005) |
| Inhibits Akt pathway in prostate cancer cells | - N.S. | - N.S. | (Dolloff et al., 2007) | |||
| Decreased proliferation and survival of hepatoma cell lines | - N.S. | - N.S. | (Stock et al., 2007) | |||
| Reduced growth of ovarian cancer | - N.S. | - N.S. | (Matei et al., 2005) | |||
| PDGFR-α MEDI-575 Ab (mouse) | Blocks ligand binding to PDGFR-α. | Reduced stromal fibroblast content and signaling in non-small cell lung cancer xenografts. | - N.S. | - N.S. | (Laing et al., 2013) | |
| PDGFR-αAF1062 Ab (goat) | Reduced rhabdomyosarcom a progression in vivo. Prevented ciliary instability in PDGF-AA stimulated MEFs. | - N.S. | - N.S. | (Taniguchi et al., 2008) (Jacoby et al., 2009) | ||
| PDGFR-α αR1 Ab (mouse) | Blocks ligand binding to PDGFR-α. | Inhibits PDGF-AA mediated growth of hematopoietic cells. | - N.S. | - N.S. | (LaRochelle et al., 1993) (Yu, Mahadevan, LaRochelle, Pierce, & Heidaran, 1994) | |
| PDGFR-α tyrosine kinase inhibitors | Imatinib (STI571) | - Inhibits ligand induced PDGFR-α phosphorylation - | -Inhibits growth of nerve sheath tumors. | - N.S. | - N.S. | - (Holtkamp et al., 2005) |
| - Reduced stroke induced BBB opening | - Reduced stroke infarct size edema and hemorrhage. | - (Su et al., 2008) (Ma et al., 2011) (Zhan et al., 2015) | ||||
| - N.S. | Reduced kainate induced seizure onset and progression. | -(Fredriksson et al., 2015) | ||||
| -Reduced spinal cord trauma induced BBB opening | - Reduced glial scar, improved locomotor performance | -(Abrams et al., 2012) (Sharp et al., 2014) | ||||
| -Reduced TBI induced BBB opening | - Reduced edema, lesion volume, tissue and memory loss | -(Su et al., 2015) | ||||
| -Reduced ALS associated BBB dysfunction | - Reduced motor neuron loss and delayed ALS onset | -(Lewandowski et al., 2016) | ||||
| - Reduced EAE associated BBB dysfunction | - Reduced CNS inflammation and demyelination | -(Adzemovic et al., 2013) | ||||
| Sunitinib (SU11248) | - Inhibits PDGFR-α auto-phosphorylation | -Inhibits growth of transformed Ba/F3 cells | - N.S. | - N.S. | -(Prenen, 2006) | |
| - N.S. | -Reduces hypoxia induced BBB opening and edema | -(Saraswat et al., 2015) | ||||
| Nilotinib (AMN107) | - Inhibits PDGFR-α auto-phosphorylation | - Inhibits growth of transformed Ba/F3 cells | - N.S. | -N.S. | -(Stover et al., 2005) | |
| - N.S | - Reduces neuronal loss, motor and cognitive decline in ALS mice | -(Wenqiang et al., 2014) | ||||
| - N.S | - Neuroprotective and improves motor performance in α-synuclein PD model | - (Hebron et al., 2013) | ||||
| - N.S | -Prevents neuronal loss and behavioral deficits in MPTP induced PD model | -(Karuppagounder et al., 2014) | ||||
| Dasatinib (BMS- 354825) | - Inhibits PDGFR-α auto-phosphorylation | - Inhibits growth of PDGFR-α ΔDIM842-844 transformed Ba/F3 cells | - N.S. | - N.S. | - (Dewaele et al., 2008) | |
| - N.S | - Improves memory, decreased microglia activation in AD mice. | - (Dhawan & Combs, 2012) | ||||
| - N.S | - Delays onset of EAE | - (Azizi et al., 2015) | ||||
| - N.S | - Extends survival and motor skills in ALS mice | -(Katsumata et al., 2012) | ||||
| Masitinib (AB1010) | - Inhibits PDGFR-α auto-phosphorylation | - Inhibits growth of FIP1L1- PDGFR-α transformed Ba/F3 cells | - N.S. | - N.S. | - (Sadovnik et al., 2014) | |
| - N.S. | - Neuroprotective in ischemic stroke | - (Kocic et al., 2015) | ||||
| - N.S. | - Beneficial in AD trial | - (Piette et al., 2011) | ||||
| - N.S. | - Beneficial in MS trial | - (Vermersch et al., 2012) | ||||
| - N.S. | - Report of use in an ALS trial | - (Salvado et al., 2015) |
i. Inhibition of PDGF-CC signaling restores BBB and is neuroprotective in acute trauma
The discovery that PDGF-CC can induce BBB permeability was made when it was shown that injection of active PDGF-CC or tPA proteins into the cerebral spinal fluid (CSF) of the cerebral ventricles of mice was sufficient to cause increased BBB permeability (Su et al., 2008; Yepes et al., 2003). Further, tPA-induced BBB opening could be decreased with neutralizing anti-PDGF-CC antibodies, indicating that PDGF-CC acts downstream of tPA in the CNS. BBB dysfunction during cerebral ischemia was also associated with increased phosphorylation of PDGFR-α and provided the mechanistic outline for a tPA – PDGF-CC – PDGFR-α signaling axis in vivo. The effect of BBB disruption after intracerebral injection of PDGF-CC was confirmed in another study (Jackman et al., 2013), which showed that genetic deficiency for progranulin (Pgrn) exacerbates the degree of PDGF-CC – induced BBB dysfunction. Together, these findings provide a novel concept to pharmacologically target the PDGF-CC pathway to prevent or reduce BBB dysfunction in a range of neurological disorders.
Ischemic stroke results in a rapid increase in perivascular tPA activity and increased BBB permeability (Yang & Rosenberg, 2011; Yepes et al., 2003). Restoration of BBB integrity is therefore considered an important therapeutic target (Jin et al., 2010). Our group showed that inhibition of the PDGF-CC signaling by neutralizing antibodies or imatinib reduced BBB dysfunction in stroke and that this was associated with significantly reduced infarct size and hemorrhage (Su et al., 2008). These findings were confirmed by independent studies in mice (Ma et al., 2011) or rats (Zhan et al., 2015) which showed that inhibition of PDGFR-α with imatinib reduced BBB opening and cerebral edema. It was demonstrated that p38 MAPK is responsible for PDGFR-α mediated increase in BBB permeability since the use of SB203580, a p38 MAPK inhibitor, decreased BBB dysfunction induced by a PDGFR-α ligand (Ma et al., 2011). Another study also supported the role of PDGF-CC in hemorrhagic transformation in humans by showing that increased levels of PDGF-CC in the plasma of stroke patients were associated with increased hemorrhagic transformation after thrombolytic therapy with tPA (Rodríguez-González et al., 2013). These findings reinforced the concept that the BBB may be a therapeutic target in the treatment of stroke, and opened up the possibility that similar protective effects of PDGF-CC pathway inhibition could also be present in other forms of traumatic injuries to the CNS.
The early manifestation of seizures is associated with BBB dysfunction (Marchi, Granata, Ghosh, & Janigro, 2012) and is suggested to be sufficient to induce onset (Marchi et al., 2007) or contribute to progression to epilepsy (van Vliet et al., 2007). Intriguingly, tPA was one of the first genes recognized to be activated early after seizure onset (Qian, Gilbert, Colicos, Kandel, & Kuhl, 1993) and its genetic deficiency raises threshold for seizure induction (Tsirka, Gualandris, Amaral, & Strickland, 1995; Yepes et al., 2002). In a recent study we showed that imatinib delays onset and progression of kainate-induced seizures in wild-type mice but not in tPA knockout mice (Fredriksson et al., 2015). This lack of cumulative protection of imatinib treatment in tPA knockout mice suggests that during seizures both tPA and PDGFR-α operate in the same signaling pathway. Moreover, inducible deletion of recombinant PDGFR-α in perivascular astrocytes reduced progression of convulsive seizure behavior (Fredriksson et al., 2015) and indicates that intervention at the PDGFR-α, acting downstream of tPA, is sufficient to have an effect on seizure progression.
Traumatic spinal cord injuries also lead to abrupt BBB dysfunction (Sharma, 2011) and restoration of barrier integrity is considered a vital therapeutic target (Shlosberg et al., 2010). Our group has shown that rats with spinal cord injury treated with imatinib display improved BBB function, and reduced formation of glial scar and deposition of chondroid sulphate proteoglycans (Abrams et al., 2012). These beneficial effects on histological preservation resulted in an improved locomotor and sensimotor hindlimb performance together with preserved bladder function after injury (Abrams et al., 2012). Our findings were confirmed by an independent re-assessment study where imatinib treatment preserved bladder function (Sharp et al., 2014). In a similar fashion imatinib treatment was proven to improve outcome of traumatic brain injury in a mouse model (Su et al., 2015). Imatinib delivered 45 min after TBI reduced BBB dysfunction, edema, lesion size, and was able to preserve cognitive function in the Morris water maze. Importantly, patients with TBI had increased concentrations of PDGF-CC in the CSF in proportion with increased severity of trauma, which would suggest its use as a marker for neurological injury.
ii. Inhibition of PDGF-CC signaling restores BBB properties and is neuroptotective in neurodegenerative disorders
Apart from targeting the PDGF-CC pathway to restore BBB in acute cerebral trauma, such therapeutic approach might also be applied to other neuropathologies where CNS injury develops over time. For example imatinib treatment of rats with experimental autoimmune encephalopathy (EAE) model of MS reduced BBB dysfunction, CNS inflammation and demyelination (Adzemovic et al., 2013). In this study imatinib was also able to suppress the peripheral immune response and reduce T-cell recruitment, which together with BBB restoration significantly improved EAE scores. Another example of PDGF-CC pathway activation during long-term neurodegeneration was found in ALS. ALS is a progressive neurodegenerative disorder associated with aging and, apart from mutations causing the familiar forms of the disease, factors triggering sporadic ALS are poorly understood. Dysfunction of the BBB was proposed as one of the elements contributing to onset of ALS since it was described in humans (Halliday et al., 2015; Leonardi, Abbruzzese, Arata, Cocito, & Vische, 1984; Miyazaki et al., 2011) and was present before the onset of disease in transgenic ALS mice models (Miyazaki et al., 2011; Zhong et al., 2008). We have shown that presymptomatic activation of the PDGF-CC pathway leads to dysfunction of the BBB and accelerates onset of ALS neurodegeneration (Lewandowski et al., 2016). Spinal cords of presymptomatic SOD1G93A ALS mice had increased expression and activity of tPA together with accumulation of cleaved PDGF-CC protein. Moreover, the presymptomatic mice showed signs of BBB dysfunction on vessels surrounded with PDGFR-α positive astrocytes with overall increase of PDGFR-α phosphorylation in tissue lysates (Lewandowski et al., 2016). We found similar evidence for activation of the PDGF-CC pathway in motor neurons from spinal cords of sporadic ALS patients where gene expression of both PDGFC and PLAT was increased. Inhibition of the PDGF-CC pathway by either imatinib treatment or crossing with Pdgfc null strain restored BBB properties, reduced neuron degeneration and delayed the onset of disease in SOD1G93A ALS mice. The effect of Pdgfc ablation on onset was the strongest reported delay for a genetic intervention in this strain and outperformed transgenic overexpression of parvalbumin (Beers et al., 2001) or glutamate transporter EAAT2 (Guo et al., 2003). Analogous result was found in neuronal cells from sporadic ALS patients where decreased expression of PGDFC or PLAT was significantly correlated with increased latency to disease onset (Lewandowski et al., 2016). The beneficial effects of BBB restoration in EAE and ALS suggest that, apart from immediate injury response, inhibition of PDGF-CC can be applied as a therapy of neurodegenerative diseases that develop over time. Moreover, activation of the PDGF-CC pathway and subsequent BBB dysfunction can provide a mechanistic explanation for increased risk of neurodegenerative diseases after traumatic events.
b. PDGF-CC as a common target for preventive treatments in neurodegeneration
The similarity of PDGF-CC induced BBB dysfunction in both acute and long-term neurological conditions provides a plausible mechanistic explanation why cerebral trauma leads to increased risk for neurodegeneration in later life and can be outlined in three steps. First, initial injury by direct trauma, ischemia or seizures results in early expression and increase of tPA activity from neurons (Qian et al., 1993; Sashindranath et al., 2012; Yepes et al., 2003). Second, as a consequence of tPA activity in stroke or trauma, PDGF-CC becomes cleaved producing an active ligand and its levels in the CSF correspond to the degree of cerebral injury (Su et al., 2008, 2015). Third, PDGF-CC mediated PDGFR-α activation is sufficient to induce BBB opening known to exacerbate the downstream consequences of injury like cerebral edema, neuroinflammation and neuronal death (Ma et al., 2011; Su et al., 2008) (Figure 4). As a consequence PDGF-CC induced BBB dysfunction, apart from the immediate consequences, can provide additional stressor stimuli for long-term neurological conditions where cumulative effects of environmental factors and genetic predisposition can lead to sporadic neurodegeneration.
Figure 4. Schematic diagram of the neurovascular unit in CNS injury.
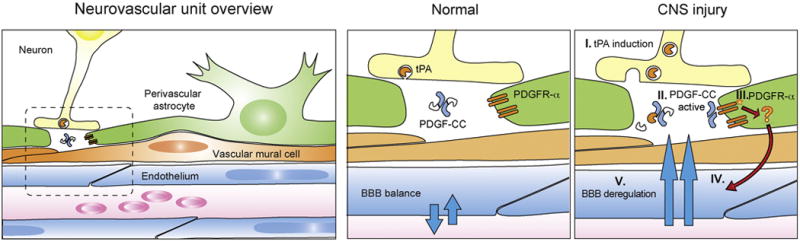
(I) Expression of tPA protein is induced and it becomes available in the extracellular matrix. (II) Proteolytically active tPA cleaves off the regulatory CUB domain (white) from the PDGF-CC dimer core (blue) yielding an active ligand. (III) Activation of PDGFR-α located on the perivascular astrocytes exerts influence on endothelial uptake by an unknown mechanism (IV) and results in BBB deregulation (V).
The concept of BBB dysfunction as contributor to a spectrum of cerebral diseases is supported by epidemiological evidence where previous history of trauma or stroke increases the risk for various forms of neurodegeneration (Gardner & Yaffe, 2015; Perry et al., 2016). In particular, prior incidence of trauma was shown to increase the risk of ALS in the general population up to 11 fold (Chen, Richard, Sandler, Umbach, & Kamel, 2007; Pupillo et al., 2012; Seelen et al., 2014; Turner et al., 2015) and is thought to be the cause for the high ALS incidence in military servicemen (Beard et al., 2016; Horner et al., 2003; Seals, Kioumourtzoglou, Gredal, Hansen, & Weisskopf, 2015; Weisskopf et al., 2005) and professional football players (Chio, 2005; Lehman, Hein, Baron, & Gersic, 2012). Similarly, the principle of PDGF-CC induced BBB opening might hypothetically also be in play in other neurodegenerative disorders including AD, PD or vascular dementia where BBB dysfunction and a history of trauma has been described as a risk factor (Allan et al., 2011; Gardner et al., 2015; Johnson & Stewart, 2015; Korczyn, 2015; Savva & Stephan, 2010; Sivanandam & Thakur, 2012; Thiel et al., 2014; Torre, 2013).
BBB restoration was most extensively studied with the use of imatinib as a means to inhibit the PDGFR-α signaling. Other TKIs like sunitinib, nilotinib, dasatinib and masitinib have similar potency to inhibit the PDGFR-α and have been used in numerous preclinical studies in the context of neurodegeneration. We will now describe several instances where the beneficial effects of these TKIs on neurodegeneration are likely to have an effect on inhibition of the PDGFR-α and restoration of the BBB. For instance: sunitinib was reported to reduce hypoxia-induced cerebral dysfunction and edema formation in rat brains (Saraswat, Nehra, Chaudhary, & Prasad, 2015). Nilotinib was reported to reduce neurodegeneration in a TDP-43 induced model of ALS (Wenqiang et al., 2014), AD model (Lonskaya, Hebron, Selby, Turner, & Moussa, 2015), as well as MPTP-induced and α-synuclein overexpression PD models (Hebron, Lonskaya, & Moussa, 2013; Karuppagounder et al., 2014). Dasatinib was shown to improve memory and decrease microglia activation in AD mice (Dhawan & Combs, 2012), to delay the onset of EAE (Azizi, Goudarzvand, Afraei, Sedaghat, & Mirshafiey, 2015), and to extend survival and motor skills in SOD1G93A ALS mice (Katsumata et al., 2012). Masitinib was shown to be neuroprotective in ischemic stroke (Kocic et al., 2015) and is being tested in a clinical trial for MS, ALS and AD (Piette et al., 2011; Salvado et al., 2015; Vermersch et al., 2012). These studies assume other protein kinases as primary targets for TKIs and do not analyze BBB as a measurable outcome, yet it is probable that observed beneficial effects include or are partly due to restoration of the BBB. The beneficial effects of these TKIs warrant future studies where BBB restoration should be a measurable outcome and allow us to better understand their therapeutic potential.
5. Conclusions and future perspectives
Despite increasing knowledge of the cellular and molecular mechanisms driving the cerebral injury response the therapeutic options for treatment of both acute and progressive forms of neuropathology remain limited. Ischemic injury and TBI still remain the leading causes for premature death and long-term disability with high costs of secondary care (Ma, Chan, & Carruthers, 2014; Olesen et al., 2012; Vos et al., 2015). At the same time reliable standardized diagnosis, particularly for mild traumatic events, is scarce and as a result they are underrepresented in the epidemiological statistics for associations with long-term consequences of cerebral injury (Roozenbeek, Maas, & Menon, 2013; Rusnak, 2013). Therefore development of reliable methods for diagnosis of acute BBB dysfunction is needed to fully appreciate its long-term effects. For example, improved imaging techniques (Mac Donald et al., 2011; Weissberg et al., 2014) together with novel CSF protein biomarkers of injury (DeKosky et al., 2013; Zetterberg, Smith, & Blennow, 2013) could lead to increased awareness of BBB changes in cerebral injury. The presence of PDGF-CC in CSF after trauma could likely become a very informative biomarker since its direct consequences on BBB dysfunction are now more evident. More importantly, PDGF-CC signaling can become a common therapeutic target to prevent BBB dysfunction and improve recovery in for both acute forms of injury seen in TBI, stroke or seizures as well as in progressive neurodegenerative disorders such as MS and ALS. Imatinib is an FDA-approved drug, and can be administered orally as a tablet, it is most suitable for short-term treatments in acute forms of cerebral injury and is currently evaluated in clinical trial for ischemic stroke (EudraCT Number: 2010-019014-25). However, due to its effects on other signaling pathways, development of antibody-based therapeutic strategies might be more suitable for long term treatments of progressive neurodegenerative disorders.
Acknowledgments
The authors would like to thank Dr. Robert Schnell for generation of protein structure images used in Figure 1. The structural models of PDGF-C CUB domain were based on human Cubulin (residues 935–1042, PDB: 3KQ4, (Andersen, Madsen, Storm, Moestrup, & Andersen, 2010)) and GF domain was based on human PDGF-A (residues 118–179. PDB: 3MJK, (Hye-Ryong Shim et al., 2010)) obtained from the MODBASE database (Haas et al., 2013). The authors would like to acknowledge grant support from the following agencies: the Björklunds fund by the Swedish Society of Medicine SLS-499431 (S.A.L.), the Swedish Agency for Innovation Systems, Grant 2011-03503 (LF), the Swedish Research Council, Grant 2012-1853 (LF), the National Institutes of Health Grants HL055374 and NS079639 (DAL), the Swedish Heart and Lung Foundation 2012-0077 (U.E.), the Swedish Cancer Foundation 2014/630 (U.E.), and the Swedish Research Council 2011-3861 (U.E.).
Abbreviations
- BBB
Blood-brain barrier
- PDGF
Platelet-derived growth factor
- PDGFR
Platelet-derived growth factor receptor
- CNS
Central nervous system
- tPA
Tissue plasminogen activator
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- ALS
Multiple sclerosis (MS), Amyotrophic lateral sclerosis
- TBI
Traumatic brain injury
- CTE
chronic traumatic encephalopathy
- TKI
Tyrosine kinase inhibitor
- CSF
cerebral spinal fluid
- EAE
Experimental autoimmune encephalopathy
- GFD
Growth factor domain
- CUB
Complement subcomponents C1r/C1s, Urchin EGF-like protein, Bone morphogenic protein-1
- LRP
LDL receptor-related protein
- CL/P
Cleft lip with or without cleft palate
- MAPK
Mitogen-activated protein kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement.
Drs. U. Eriksson, D.A. Lawrence, and L. Fredriksson are named inventors on a patent on modulating blood-neural barrier using PDGF-CC and PDGFR-α antagonist. The other author declares no potential conflict of interest.
References
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nature Reviews Neuroscience. 2006;7(1):41–53. doi: 10.1038/nrn1824. http://doi.org/10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Abrams MB, Nilsson I, Lewandowski SA, Kjell J, Codeluppi S, Olson L, Eriksson U. Imatinib enhances functional outcome after spinal cord injury. PloS One. 2012;7(6):e38760. doi: 10.1371/journal.pone.0038760. http://doi.org/10.1371/journal.pone.0038760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzemovic MZ, Zeitelhofer M, Eriksson U, Olsson T, Nilsson I. Imatinib ameliorates neuroinflammation in a rat model of multiple sclerosis by enhancing blood-brain barrier integrity and by modulating the peripheral immune response. PloS One. 2013;8(2):e56586. doi: 10.1371/journal.pone.0056586. http://doi.org/10.1371/journal.pone.0056586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan LM, Rowan EN, Firbank MJ, Thomas AJ, Parry SW, Polvikoski TM, Kalaria RN. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain. 2011;134(12):3716–3727. doi: 10.1093/brain/awr273. http://doi.org/10.1093/brain/awr273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen CBF, Madsen M, Storm T, Moestrup SK, Andersen GR. Structural basis for receptor recognition of vitamin-B12–intrinsic factor complexes. Nature. 2010;464(7287):445–448. doi: 10.1038/nature08874. http://doi.org/10.1038/nature08874. [DOI] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes & Development. 2008;22(10):1276–1312. doi: 10.1101/gad.1653708. http://doi.org/10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrae J, Gouveia L, Gallini R, He L, Fredriksson L, Nilsson I, Betsholtz C. A role for PDGF-C/PDGFRα signaling in the formation of the meningeal basement membranes surrounding the cerebral cortex. Biology Open. 2016 doi: 10.1242/bio.017368. http://doi.org/10.1242/bio.017368. [DOI] [PMC free article] [PubMed]
- Antoniu SA. Targeting PDGF pathway in pulmonary arterial hypertension. Expert Opinion on Therapeutic Targets. 2012;16(11):1055–63. doi: 10.1517/14728222.2012.719500. http://doi.org/10.1517/14728222.2012.719500. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Developmental Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. http://doi.org/10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468(V):557–561. doi: 10.1038/nature09522. http://doi.org/10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Ayata C, Ropper AH. Ischaemic brain oedema. Journal of Clinical Neuroscience. 2002;9(2):113–124. doi: 10.1054/jocn.2001.1031. http://doi.org/10.1054/jocn.2001.1031. [DOI] [PubMed] [Google Scholar]
- Azizi G, Goudarzvand M, Afraei S, Sedaghat R, Mirshafiey A. Therapeutic effects of dasatinib in mouse model of multiple sclerosis. Immunopharmacology and Immunotoxicology. 2015;37(3):287–94. doi: 10.3109/08923973.2015.1028074. http://doi.org/10.3109/08923973.2015.1028074. [DOI] [PubMed] [Google Scholar]
- Bartels AL, Willemsen ATM, Kortekaas R, de Jong BM, de Vries R, de Klerk O, Leenders KL. Decreased blood–brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. Journal of Neural Transmission. 2008;115(7):1001–1009. doi: 10.1007/s00702-008-0030-y. http://doi.org/10.1007/s00702-008-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard JD, Engel LS, Richardson DB, Gammon MD, Baird C, Umbach DM, Kamel F. Military service, deployments, and exposures in relation to amyotrophic lateral sclerosis etiology. Environment International. 2016;91:104–115. doi: 10.1016/j.envint.2016.02.014. http://doi.org/10.1016/j.envint.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers DR, Ho BK, Siklós L, Alexianu ME, Mosier DR, Mohamed HA, Appel SH. Parvalbumin overexpression alters immune-mediated increases in intracellular calcium, and delays disease onset in a transgenic model of familial amyotrophic lateral sclerosis. Journal of Neurochemistry. 2001;79(3):499–509. doi: 10.1046/j.1471-4159.2001.00582.x. http://doi.org/10.1046/j.1471-4159.2001.00582.x. [DOI] [PubMed] [Google Scholar]
- Bergsten E, Uutela M, Li X, Pietras K, Ostman A, Heldin CH, Eriksson U. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nature Cell Biology. 2001;3(5):512–6. doi: 10.1038/35074588. http://doi.org/10.1038/35074588. [DOI] [PubMed] [Google Scholar]
- Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine & Growth Factor Reviews. 2004;15(4):215–28. doi: 10.1016/j.cytogfr.2004.03.005. http://doi.org/10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Bork P, Beckmann G. The CUB domain. A widespread module in developmentally regulated proteins. Journal of Molecular Biology. 1993;231(2):539–45. doi: 10.1006/jmbi.1993.1305. http://doi.org/10.1006/jmbi.1993.1305. [DOI] [PubMed] [Google Scholar]
- Boström H, Willetts K, Pekny M, Levéen P, Lindahl P, Hedstrand H, Betsholtz C. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85(6):863–73. doi: 10.1016/s0092-8674(00)81270-2. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8681381. [DOI] [PubMed] [Google Scholar]
- Boucher P. LRP: Role in Vascular Wall Integrity and Protection from Atherosclerosis. Science. 2003;300(5617):329–332. doi: 10.1126/science.1082095. http://doi.org/10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- Calcia A, Gai G, Di Gregorio E, Talarico F, Naretto VG, Migone N, Brusco A. Bilaterally cleft lip and bilateral thumb polydactyly with triphalangeal component in a patient with two de novo deletions of HSA 4q32 and 4q34 involving PDGFC, GRIA2, and FBXO8 genes. American Journal of Medical Genetics. Part A. 2013;161A(10):2656–62. doi: 10.1002/ajmg.a.36146. http://doi.org/10.1002/ajmg.a.36146. [DOI] [PubMed] [Google Scholar]
- Chen H, Richard M, Sandler DP, Umbach DM, Kamel F. Head injury and amyotrophic lateral sclerosis. American Journal of Epidemiology. 2007;166(7):810–6. doi: 10.1093/aje/kwm153. http://doi.org/10.1093/aje/kwm153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio A. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005;128(3):472–476. doi: 10.1093/brain/awh373. http://doi.org/10.1093/brain/awh373. [DOI] [PubMed] [Google Scholar]
- Choi SJ, Marazita ML, Hart PS, Sulima PP, Field LL, McHenry TG, Hart TC. The PDGF-C regulatory region SNP rs28999109 decreases promoter transcriptional activity and is associated with CL/P. European Journal of Human Genetics. 2009;17(6):774–784. doi: 10.1038/ejhg.2008.245. http://doi.org/10.1038/ejhg.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BW, Gu C. The Molecular Constituents of the Blood-Brain Barrier. Trends in Neurosciences. 2015;38(10):598–608. doi: 10.1016/j.tins.2015.08.003. http://doi.org/10.1016/j.tins.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collen D. Ham-Wasserman Lecture: Role of the Plasminogen System in Fibrin-Homeostasis and Tissue Remodeling. Hematology. 2001;2001(1):1–9. doi: 10.1182/asheducation-2001.1.1. http://doi.org/10.1182/asheducation-2001.1.1. [DOI] [PubMed] [Google Scholar]
- Dai Y. Platelet-derived growth factor receptor tyrosine kinase inhibitors: a review of the recent patent literature. Expert Opinion on Therapeutic Patents. 2010;20(7):885–97. doi: 10.1517/13543776.2010.493559. http://doi.org/10.1517/13543776.2010.493559. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Blennow K, Ikonomovic MD, Gandy S. Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nature Reviews. Neurology. 2013;9(4):192–200. doi: 10.1038/nrneurol.2013.36. http://doi.org/10.1038/nrneurol.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoulin JB, Essaghir A. PDGF receptor signaling networks in normal and cancer cells. Cytokine & Growth Factor Reviews. 2014;25(3):273–83. doi: 10.1016/j.cytogfr.2014.03.003. http://doi.org/10.1016/j.cytogfr.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Dewaele B, Wasag B, Cools J, Sciot R, Prenen H, Vandenberghe P, Debiec-Rychter M. Activity of Dasatinib, a Dual SRC/ABL Kinase Inhibitor, and IPI-504, a Heat Shock Protein 90 Inhibitor, against Gastrointestinal Stromal Tumor-Associated PDGFRAD842V Mutation. Clinical Cancer Research. 2008;14(18):5749–5758. doi: 10.1158/1078-0432.CCR-08-0533. http://doi.org/10.1158/1078-0432.CCR-08-0533. [DOI] [PubMed] [Google Scholar]
- Dhawan G, Combs CK. Inhibition of Src kinase activity attenuates amyloid associated microgliosis in a murine model of Alzheimer’s disease. Journal of Neuroinflammation. 2012;9:117. doi: 10.1186/1742-2094-9-117. http://doi.org/10.1186/1742-2094-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Wu X, Boström H, Kim I, Wong N, Tsoi B, Nagy A. A specific requirement for PDGF-C in palate formation and PDGFR-alpha signaling. Nature Genetics. 2004;36(10):1111–1116. doi: 10.1038/ng1415. http://doi.org/10.1038/ng1415. [DOI] [PubMed] [Google Scholar]
- Dolloff NG, Russell MR, Loizos N, Fatatis A. Human bone marrow activates the Akt pathway in metastatic prostate cells through transactivation of the alpha-platelet-derived growth factor receptor. Cancer Research. 2007;67(2):555–62. doi: 10.1158/0008-5472.CAN-06-2593. http://doi.org/10.1158/0008-5472.CAN-06-2593. [DOI] [PubMed] [Google Scholar]
- Donkin JJ, Vink R. Mechanisms of cerebral edema in traumatic brain injury: therapeutic developments. Current Opinion in Neurology. 2010;23(3):293–9. doi: 10.1097/WCO.0b013e328337f451. http://doi.org/10.1097/WCO.0b013e328337f451. [DOI] [PubMed] [Google Scholar]
- Donnenfeld H, Kascsak RJ, Bartfeld H. Deposits of IgG and C3 in the spinal cord and motor cortex of ALS patients. Journal of Neuroimmunology. 1984;6(1):51–57. doi: 10.1016/0165-5728(84)90042-0. http://doi.org/10.1016/0165-5728(84)90042-0. [DOI] [PubMed] [Google Scholar]
- Ehnman M, Li H, Fredriksson L, Pietras K, Eriksson U. The uPA/uPAR system regulates the bioavailability of PDGF-DD: implications for tumour growth. Oncogene. 2009;28(4):534–44. doi: 10.1038/onc.2008.410. http://doi.org/10.1038/onc.2008.410. [DOI] [PubMed] [Google Scholar]
- Eitner F, Bucher E, van Roeyen C, Kunter U, Rong S, Seikrit C, Ostendorf T. PDGF-C Is a Proinflammatory Cytokine that Mediates Renal Interstitial Fibrosis. Journal of the American Society of Nephrology. 2008;19(2):281–289. doi: 10.1681/ASN.2007030290. http://doi.org/10.1681/ASN.2007030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson L, Ehnman M, Fieber C, Eriksson U. Structural requirements for activation of latent platelet-derived growth factor CC by tissue plasminogen activator. The Journal of Biological Chemistry. 2005;280(29):26856–62. doi: 10.1074/jbc.M503388200. http://doi.org/10.1074/jbc.M503388200. [DOI] [PubMed] [Google Scholar]
- Fredriksson L, Li H, Eriksson U. The PDGF family: Four gene products form five dimeric isoforms. Cytokine and Growth Factor Reviews. 2004;15(4):197–204. doi: 10.1016/j.cytogfr.2004.03.007. http://doi.org/10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Fredriksson L, Li H, Fieber C, Li X, Eriksson U. Tissue plasminogen activator is a potent activator of PDGF-CC. The EMBO Journal. 2004;23(19):3793–802. doi: 10.1038/sj.emboj.7600397. http://doi.org/10.1038/sj.emboj.7600397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson L, Nilsson I, Su EJ, Andrae J, Ding H, Betsholtz C, Lawrence DA. Platelet-derived growth factor C deficiency in C57BL/6 mice leads to abnormal cerebral vascularization, loss of neuroependymal integrity, and ventricular abnormalities. The American Journal of Pathology. 2012;180(3):1136–44. doi: 10.1016/j.ajpath.2011.12.006. http://doi.org/10.1016/j.ajpath.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson L, Stevenson TK, Su EJ, Ragsdale M, Moore S, Craciun S, Lawrence DA. Identification of a neurovascular signaling pathway regulating seizures in mice. Annals of Clinical and Translational Neurology. 2015;2(7):722–38. doi: 10.1002/acn3.209. http://doi.org/10.1002/acn3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitán MI, Shea CD, Evangelou IE, Stone RD, Fenton KM, Bielekova B, Reich DS. Evolution of the blood-brain barrier in newly forming multiple sclerosis lesions. Annals of Neurology. 2011;70(1):22–9. doi: 10.1002/ana.22472. http://doi.org/10.1002/ana.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Burke JF, Nettiksimmons J, Goldman S, Tanner CM, Yaffe K. Traumatic brain injury in later life increases risk for Parkinson disease. Annals of Neurology. 2015;77(6):987–95. doi: 10.1002/ana.24396. http://doi.org/10.1002/ana.24396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Molecular and Cellular Neuroscience. 2015;66(Pt B):75–80. doi: 10.1016/j.mcn.2015.03.001. http://doi.org/10.1016/j.mcn.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson DG, Duff ME, West JW, Kelly JD, Sheppard PO, Hofstrand PD, Hart CE. Platelet-derived growth factor C (PDGF-C), a novel growth factor that binds to PDGF alpha and beta receptor. The Journal of Biological Chemistry. 2001;276(29):27406–27414. doi: 10.1074/jbc.M101056200. http://doi.org/10.1074/jbc.M101056200. [DOI] [PubMed] [Google Scholar]
- Guo H, Lai L, Butchbach MER, Stockinger MP, Shan X, Bishop GA, Lin CLG. Increased expression of the glial glutamate transporter EAAT2 modulates excitotoxicity and delays the onset but not the outcome of ALS in mice. Human Molecular Genetics. 2003;12(19):2519–2532. doi: 10.1093/hmg/ddg267. http://doi.org/10.1093/hmg/ddg267. [DOI] [PubMed] [Google Scholar]
- Haas J, Roth S, Arnold K, Kiefer F, Schmidt T, Bordoli L, Schwede T. The Protein Model Portal–a comprehensive resource for protein structure and model information. Database. 2013;2013(0):bat031–bat031. doi: 10.1093/database/bat031. http://doi.org/10.1093/database/bat031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, Zlokovic BV. Accelerated pericyte degeneration and blood–brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. Journal of Cerebral Blood Flow & Metabolism. 2015:1–9. doi: 10.1038/jcbfm.2015.44. (November 2014) http://doi.org/10.1038/jcbfm.2015.44. [DOI] [PMC free article] [PubMed]
- Hayes BJ, Riehle KJ, Gilbertson DG, Curtis WR, Kelly EJ, Treuting PM, Campbell JS. The PDGFC CUB Domain Enhances Survival in PDGFC Mutant Mice. SOJ Immunology. 2015;3(3) http://doi.org/10.15226/soji/3/3/00133. [Google Scholar]
- Hebron ML, Lonskaya I, Moussa CEH. Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of α-synuclein in Parkinson’s disease models. Human Molecular Genetics. 2013;22(16):3315–28. doi: 10.1093/hmg/ddt192. http://doi.org/10.1093/hmg/ddt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH. Targeting the PDGF signaling pathway in tumor treatment. Cell Communication and Signaling. 2013;11(1):97. doi: 10.1186/1478-811X-11-97. http://doi.org/10.1186/1478-811X-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH. Targeting the PDGF Signaling Pathway in the Treatment of Non-Malignant Diseases. Journal of Neuroimmune Pharmacology. 2014;9(2):69–79. doi: 10.1007/s11481-013-9484-2. http://doi.org/10.1007/s11481-013-9484-2. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Östman A, Rönnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochimica et Biophysica Acta. 1998;1378(1):F79–113. doi: 10.1016/s0304-419x(98)00015-8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9739761. [DOI] [PubMed] [Google Scholar]
- Holtkamp N, Okuducu AF, Mucha J, Afanasieva A, Hartmann C, Atallah I, von Deimling A. Mutation and expression of PDGFRA and KIT in malignant peripheral nerve sheath tumors, and its implications for imatinib sensitivity. Carcinogenesis. 2005;27(3):664–671. doi: 10.1093/carcin/bgi273. http://doi.org/10.1093/carcin/bgi273. [DOI] [PubMed] [Google Scholar]
- Hom J, Dankbaar JW, Soares BP, Schneider T, Cheng SC, Bredno J, Wintermark M. Blood-Brain Barrier Permeability Assessed by Perfusion CT Predicts Symptomatic Hemorrhagic Transformation and Malignant Edema in Acute Ischemic Stroke. American Journal of Neuroradiology. 2010;32(1):41–8. doi: 10.3174/ajnr.A2244. http://doi.org/10.3174/ajnr.A2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RD, Kamins KG, Feussner JR, Grambow SC, Hoff-Lindquist J, Harati Y, Kasarskis EJ. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61(6):742–9. doi: 10.1212/01.wnl.0000069922.32557.ca. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14504315. [DOI] [PubMed] [Google Scholar]
- Hurst NJ, Najy AJ, Ustach CV, Movilla L, Kim HRC. Platelet-derived growth factor-C (PDGF-C) activation by serine proteases: implications for breast cancer progression. The Biochemical Journal. 2012;441(3):909–18. doi: 10.1042/BJ20111020. http://doi.org/10.1042/BJ20111020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hye-Ryong Shim A, Liu H, Focia PJ, Chen X, Lin PC, He X. Structures of a platelet-derived growth factor/propeptide complex and a platelet-derived growth factor/receptor complex. Proceedings of the National Academy of Sciences. 2010;107(25):11307–11312. doi: 10.1073/pnas.1000806107. http://doi.org/10.1073/pnas.1000806107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman K, Kahles T, Lane D, Garcia-Bonilla L, Abe T, Capone C, Iadecola C. Progranulin Deficiency Promotes Post-Ischemic Blood-Brain Barrier Disruption. Journal of Neuroscience. 2013;33(50):19579–19589. doi: 10.1523/JNEUROSCI.4318-13.2013. http://doi.org/10.1523/JNEUROSCI.4318-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, Schurmans S. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nature Genetics. 2009;41(9):1027–31. doi: 10.1038/ng.427. http://doi.org/10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G. Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: Critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiology of Disease. 2010;38(3):376–385. doi: 10.1016/j.nbd.2010.03.008. http://doi.org/10.1016/j.nbd.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136(1):28–42. doi: 10.1093/brain/aws322. http://doi.org/10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W. Traumatic brain injury: age at injury influences dementia risk after TBI. Nature Reviews. Neurology. 2015;11(3):128–30. doi: 10.1038/nrneurol.2014.241. http://doi.org/10.1038/nrneurol.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH. Widespread Tau and Amyloid-Beta Pathology Many Years After a Single Traumatic Brain Injury in Humans. Brain Pathology. 2012;22(2):142–149. doi: 10.1111/j.1750-3639.2011.00513.x. http://doi.org/10.1111/j.1750-3639.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson A, Heldin CH, Westermark B, Wasteson A. Platelet-derived growth factor: identification of constituent polypeptide chains. Biochemical and Biophysical Research Communications. 1982;104(1):66–74. doi: 10.1016/0006-291x(82)91941-6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7073684. [DOI] [PubMed] [Google Scholar]
- Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nature Reviews. Neurology. 2013;9(4):222–30. doi: 10.1038/nrneurol.2013.33. http://doi.org/10.1038/nrneurol.2013.33. [DOI] [PubMed] [Google Scholar]
- Jugessur A, Shi M, Gjessing HK, Lie RT, Wilcox AJ, Weinberg CR, Murray JC. Genetic determinants of facial clefting: analysis of 357 candidate genes using two national cleft studies from Scandinavia. PloS One. 2009;4(4):e5385. doi: 10.1371/journal.pone.0005385. http://doi.org/10.1371/journal.pone.0005385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Lin HC. Increased risk of multiple sclerosis after traumatic brain injury: a nationwide population-based study. Journal of Neurotrauma. 2012;29(1):90–5. doi: 10.1089/neu.2011.1936. http://doi.org/10.1089/neu.2011.1936. [DOI] [PubMed] [Google Scholar]
- Karuppagounder SS, Brahmachari S, Lee Y, Dawson VL, Dawson TM, Ko HS. The c-Abl inhibitor, nilotinib, protects dopaminergic neurons in a preclinical animal model of Parkinson’s disease. Scientific Reports. 2014;4:4874. doi: 10.1038/srep04874. http://doi.org/10.1038/srep04874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumata R, Ishigaki S, Katsuno M, Kawai K, Sone J, Huang Z, Sobue G. c-Abl inhibition delays motor neuron degeneration in the G93A mouse, an animal model of amyotrophic lateral sclerosis. PloS One. 2012;7(9):e46185. doi: 10.1371/journal.pone.0046185. http://doi.org/10.1371/journal.pone.0046185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocic I, Kowianski P, Rusiecka I, Lietzau G, Mansfield C, Moussy A, Dubreuil P. Neuroprotective effect of masitinib in rats with postischemic stroke. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2015;388(1):79–86. doi: 10.1007/s00210-014-1061-6. http://doi.org/10.1007/s00210-014-1061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler N, Lipton A. Platelets as a source of fibroblast growth-promoting activity. Experimental Cell Research. 1974;87(2):297–301. doi: 10.1016/0014-4827(74)90484-4. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4370268. [DOI] [PubMed] [Google Scholar]
- Korczyn AD. Vascular parkinsonism–characteristics, pathogenesis and treatment. Nature Reviews. Neurology. 2015;11(6):319–26. doi: 10.1038/nrneurol.2015.61. http://doi.org/10.1038/nrneurol.2015.61. [DOI] [PubMed] [Google Scholar]
- Kortekaas R, Leenders KL, van Oostrom JCH, Vaalburg W, Bart J, Willemsen ATM, Hendrikse NH. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Annals of Neurology. 2005;57(2):176–9. doi: 10.1002/ana.20369. http://doi.org/10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- Laing N, McDermott B, Wen S, Yang D, Lawson D, Collins M, Blakey D. Inhibition of platelet-derived growth factor receptor α by MEDI-575 reduces tumor growth and stromal fibroblast content in a model of non-small cell lung cancer. Molecular Pharmacology. 2013;83(6):1247–56. doi: 10.1124/mol.112.084079. http://doi.org/10.1124/mol.112.084079. [DOI] [PubMed] [Google Scholar]
- LaRochelle WJ, Jeffers M, McDonald WF, Chillakuru RA, Giese NA, Lokker NA, Lichenstein HS. PDGF-D, a new protease-activated growth factor. Nature Cell Biology. 2001;3(5):517–21. doi: 10.1038/35074593. http://doi.org/10.1038/35074593. [DOI] [PubMed] [Google Scholar]
- LaRochelle WJ, Jensen R, Heidaran M, May-Siroff M, Wang L, Aaronson S, Pierce J. Inhibition of platelet-derived growth factor autocrine growth stimulation by a monoclonal antibody to the human alpha platelet-derived growth factor receptor. Cell Growth & Differentiation. 1993;4(7):547. Retrieved from http://cgd.aacrjournals.org/cgi/content/abstract/4/7/547. [PubMed] [Google Scholar]
- Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology. 2012;79(19):1970–4. doi: 10.1212/WNL.0b013e31826daf50. http://doi.org/10.1212/WNL.0b013e31826daf50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Hovland P, Velez G, Haran A, Gilbertson D, Hirose T, Kazlauskas A. A potential role for PDGF-C in experimental and clinical proliferative vitreoretinopathy. Investigative Ophthalmology & Visual Science. 2007;48(5):2335–42. doi: 10.1167/iovs.06-0965. http://doi.org/10.1167/iovs.06-0965. [DOI] [PubMed] [Google Scholar]
- Lei H, Velez G, Hovland P, Hirose T, Gilbertson D, Kazlauskas A. Growth factors outside the PDGF family drive experimental PVR. Investigative Ophthalmology & Visual Science. 2009;50(7):3394–403. doi: 10.1167/iovs.08-3042. http://doi.org/10.1167/iovs.08-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Velez G, Hovland P, Hirose T, Kazlauskas A. Plasmin is the major protease responsible for processing PDGF-C in the vitreous of patients with proliferative vitreoretinopathy. Investigative Ophthalmology & Visual Science. 2008;49(1):42–8. doi: 10.1167/iovs.07-0776. http://doi.org/10.1167/iovs.07-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarchant S, Docagne F, Emery E, Vivien D, Ali C, Rubio M. tPA in the injured central nervous system: different scenarios starring the same actor? Neuropharmacology. 2012;62(2):749–56. doi: 10.1016/j.neuropharm.2011.10.020. http://doi.org/10.1016/j.neuropharm.2011.10.020. [DOI] [PubMed] [Google Scholar]
- Leonardi A, Abbruzzese G, Arata L, Cocito L, Vische M. Cerebrospinal fluid (CSF) findings in amyotrophic lateral sclerosis. Journal of Neurology. 1984;231(2):75–8. doi: 10.1007/BF00313720. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6737012. [DOI] [PubMed] [Google Scholar]
- Levitzki A. Tyrosine kinase inhibitors: views of selectivity, sensitivity, and clinical performance. Annual Review of Pharmacology and Toxicology. 2013;53:161–85. doi: 10.1146/annurev-pharmtox-011112-140341. http://doi.org/10.1146/annurev-pharmtox-011112-140341. [DOI] [PubMed] [Google Scholar]
- Lewandowski SA, Nilsson I, Fredriksson L, Lönnerberg P, Muhl L, Zeitelhofer M, Eriksson U. Presymptomatic activation of the PDGF-CC pathway accelerates onset of ALS neurodegeneration. Acta Neuropathologica. 2016;131(3):453–64. doi: 10.1007/s00401-015-1520-2. http://doi.org/10.1007/s00401-015-1520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Pontén A, Aase K, Karlsson L, Abramsson A, Uutela M, Eriksson U. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nature Cell Biology. 2000;2(5):302–9. doi: 10.1038/35010579. http://doi.org/10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- Loizos N, Xu Y, Huber J, Liu M, Lu D, Finnerty B, Kussie P. Targeting the platelet-derived growth factor receptor alpha with a neutralizing human monoclonal antibody inhibits the growth of tumor xenografts: implications as a potential therapeutic target. Molecular Cancer Therapeutics. 2005;4(3):369–79. doi: 10.1158/1535-7163.MCT-04-0114. http://doi.org/10.1158/1535-7163.MCT-04-0114. [DOI] [PubMed] [Google Scholar]
- Lonskaya I, Hebron ML, Selby ST, Turner RS, Moussa CEH. Nilotinib and bosutinib modulate pre-plaque alterations of blood immune markers and neuro-inflammation in Alzheimer’s disease models. Neuroscience. 2015;304:316–27. doi: 10.1016/j.neuroscience.2015.07.070. http://doi.org/10.1016/j.neuroscience.2015.07.070. [DOI] [PubMed] [Google Scholar]
- Loukinova E, Ranganathan S, Kuznetsov S, Gorlatova N, Migliorini MM, Loukinov D, Strickland DK. Platelet-derived growth factor (PDGF)-induced tyrosine phosphorylation of the low density lipoprotein receptor-related protein (LRP). Evidence for integrated co-receptor function betwenn LRP and the PDGF. The Journal of Biological Chemistry. 2002;277(18):15499–506. doi: 10.1074/jbc.M200427200. http://doi.org/10.1074/jbc.M200427200. [DOI] [PubMed] [Google Scholar]
- Lunny CA, Fraser SN, Knopp-Sihota JA. Physical trauma and risk of multiple sclerosis: a systematic review and meta-analysis of observational studies. Journal of the Neurological Sciences. 2014;336(1–2):13–23. doi: 10.1016/j.jns.2013.08.011. http://doi.org/10.1016/j.jns.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Ma Q, Huang B, Khatibi N, Rolland W, Suzuki H, Zhang JH, Tang J. PDGFR-α inhibition preserves blood-brain barrier after intracerebral hemorrhage. Annals of Neurology. 2011;70(6):920–31. doi: 10.1002/ana.22549. http://doi.org/10.1002/ana.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma VY, Chan L, Carruthers KJ. Incidence, Prevalence, Costs, and Impact on Disability of Common Conditions Requiring Rehabilitation in the United States: Stroke, Spinal Cord Injury, Traumatic Brain Injury, Multiple Sclerosis, Osteoarthritis, Rheumatoid Arthritis, Limb Loss, and Back Pa. Archives of Physical Medicine and Rehabilitation. 2014;95(5):986–995.e1. doi: 10.1016/j.apmr.2013.10.032. http://doi.org/10.1016/j.apmr.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, Brody DL. Detection of blast-related traumatic brain injury in U.S. military personnel. The New England Journal of Medicine. 2011;364(22):2091–100. doi: 10.1056/NEJMoa1008069. http://doi.org/10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazita ML, Field LL, Cooper ME, Tobias R, Maher BS, Peanchitlertkajorn S, Liu Y. Genome scan for loci involved in cleft lip with or without cleft palate, in Chinese multiplex families. American Journal of Human Genetics. 2002;71(2):349–64. doi: 10.1086/341944. http://doi.org/10.1086/341944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Angelov L, Masaryk T, Fazio V, Granata T, Hernandez N, Janigro D. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48(4):732–42. doi: 10.1111/j.1528-1167.2007.00988.x. http://doi.org/10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Granata T, Ghosh C, Janigro D. Blood-brain barrier dysfunction and epilepsy: pathophysiologic role and therapeutic approaches. Epilepsia. 2012;53(11):1877–86. doi: 10.1111/j.1528-1167.2012.03637.x. http://doi.org/10.1111/j.1528-1167.2012.03637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin IV, Borkham-Kamphorst E, Zok S, van Roeyen CRC, Eriksson U, Boor P, Ostendorf T. Platelet-derived growth factor (PDGF)-C neutralization reveals differential roles of PDGF receptors in liver and kidney fibrosis. The American Journal of Pathology. 2013;182(1):107–17. doi: 10.1016/j.ajpath.2012.09.006. http://doi.org/10.1016/j.ajpath.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Matei D, Emerson RE, Lai YC, Baldridge LA, Rao J, Yiannoutsos C, Donner DD. Autocrine activation of PDGFRα promotes the progression of ovarian cancer. Oncogene. 2005;25(14):2060–2069. doi: 10.1038/sj.onc.1209232. http://doi.org/10.1038/sj.onc.1209232. [DOI] [PubMed] [Google Scholar]
- McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, Cantu RC. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(1):43–64. doi: 10.1093/brain/aws307. http://doi.org/10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley VC, Khachigian LM. Fibroblast growth factor-2 induction of platelet-derived growth factor-C chain transcription in vascular smooth muscle cells is ERK-dependent but not JNK-dependent and mediated by Egr-1. The Journal of Biological Chemistry. 2004;279(39):40289–95. doi: 10.1074/jbc.M406063200. http://doi.org/10.1074/jbc.M406063200. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Ohta Y, Nagai M, Morimoto N, Kurata T, Takehisa Y, Abe K. Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. Journal of Neuroscience Research. 2011;89(5):718–728. doi: 10.1002/jnr.22594. http://doi.org/10.1002/jnr.22594. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010;67(2):181–198. doi: 10.1016/j.neuron.2010.07.002. http://doi.org/10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJL, Barber RM, Foreman KJ, Ozgoren AA, Abd-Allah F, Abera SF, Vos T. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. The Lancet. 2015;386(10009):2145–2191. doi: 10.1016/S0140-6736(15)61340-X. http://doi.org/10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nature Medicine. 2013;19(12):1584–96. doi: 10.1038/nm.3407. http://doi.org/10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oefner C, D’Arcy A, Winkler FK, Eggimann B, Hosang M. Crystal structure of human platelet-derived growth factor BB. The EMBO Journal. 1992;11(11):3921–6. doi: 10.1002/j.1460-2075.1992.tb05485.x. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=556902&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jönsson B. The economic cost of brain disorders in Europe. European Journal of Neurology. 2012;19(1):155–162. doi: 10.1111/j.1468-1331.2011.03590.x. http://doi.org/10.1111/j.1468-1331.2011.03590.x. [DOI] [PubMed] [Google Scholar]
- Östman A. PDGF receptors-mediators of autocrine tumor growth and regulators of tumor vasculature and stroma. Cytokine & Growth Factor Reviews. 2004;15(4):275–86. doi: 10.1016/j.cytogfr.2004.03.002. http://doi.org/10.1016/j.cytogfr.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Perry DC, Sturm VE, Peterson MJ, Pieper CF, Bullock T, Boeve BF, Welsh-Bohmer KA. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. Journal of Neurosurgery. 2016;124(2):511–26. doi: 10.3171/2015.2.JNS14503. http://doi.org/10.3171/2015.2.JNS14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette F, Belmin J, Vincent H, Schmidt N, Pariel S, Verny M, Hermine O. Masitinib as an adjunct therapy for mild-to-moderate Alzheimer’s disease: a randomised, placebo-controlled phase 2 trial. Alzheimer’s Research & Therapy. 2011;3(2):16. doi: 10.1186/alzrt75. http://doi.org/10.1186/alzrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontén A, Li X, Thorén P, Aase K, Sjöblom T, Ostman A, Eriksson U. Transgenic overexpression of platelet-derived growth factor-C in the mouse heart induces cardiac fibrosis, hypertrophy, and dilated cardiomyopathy. The American Journal of Pathology. 2003;163(2):673–82. doi: 10.1016/S0002-9440(10)63694-2. http://doi.org/10.1016/S0002-9440(10)63694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenen H. Efficacy of the Kinase Inhibitor SU11248 against Gastrointestinal Stromal Tumor Mutants Refractory to Imatinib Mesylate. Clinical Cancer Research. 2006;12(8):2622–2627. doi: 10.1158/1078-0432.CCR-05-2275. http://doi.org/10.1158/1078-0432.CCR-05-2275. [DOI] [PubMed] [Google Scholar]
- Prins M, Greco T, Alexander D, Giza CC. The pathophysiology of traumatic brain injury at a glance. Disease Models & Mechanisms. 2013;6(6):1307–15. doi: 10.1242/dmm.011585. http://doi.org/10.1242/dmm.011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupillo E, Messina P, Logroscino G, Zoccolella S, Chiò A, Calvo A, Beghi E. Trauma and amyotrophic lateral sclerosis: a case-control study from a population-based registry. European Journal of Neurology. 2012;19(12):1509–17. doi: 10.1111/j.1468-1331.2012.03723.x. http://doi.org/10.1111/j.1468-1331.2012.03723.x. [DOI] [PubMed] [Google Scholar]
- Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361(6411):453–7. doi: 10.1038/361453a0. http://doi.org/10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- Riehle KJ, Johnson MM, Johansson F, Bauer RL, Hayes BJ, Gilbertson DG, Campbell JS. Tissue-type plasminogen activator is not necessary for platelet-derived growth factor-c activation. Biochimica et Biophysica Acta. 2014;1842(2):318–325. doi: 10.1016/j.bbadis.2013.11.013. http://doi.org/10.1016/j.bbadis.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-González R, Blanco M, Rodríguez-Yáñez M, Moldes O, Castillo J, Sobrino T. Platelet derived growth factor-CC isoform is associated with hemorrhagic transformation in ischemic stroke patients treated with tissue plasminogen activator. Atherosclerosis. 2013;226(1):165–71. doi: 10.1016/j.atherosclerosis.2012.10.072. http://doi.org/10.1016/j.atherosclerosis.2012.10.072. [DOI] [PubMed] [Google Scholar]
- Roozenbeek B, Maas AIR, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nature Reviews. Neurology. 2013;9(4):231–6. doi: 10.1038/nrneurol.2013.22. http://doi.org/10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- Rusnak M. Traumatic brain injury: Giving voice to a silent epidemic. Nature Reviews. Neurology. 2013;9(4):186–7. doi: 10.1038/nrneurol.2013.38. http://doi.org/10.1038/nrneurol.2013.38. [DOI] [PubMed] [Google Scholar]
- Sadovnik I, Lierman E, Peter B, Herrmann H, Suppan V, Stefanzl G, Valent P. Identification of Ponatinib as a potent inhibitor of growth, migration, and activation of neoplastic eosinophils carrying FIP1L1-PDGFRA. Experimental Hematology. 2014;42(4):282–293. doi: 10.1016/j.exphem.2013.12.007. e4. http://doi.org/10.1016/j.exphem.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvado M, Vargas V, Vidal M, Simon-Talero M, Camacho J, Gamez J. Autoimmune-like hepatitis during masitinib therapy in an amyotrophic lateral sclerosis patient. World Journal of Gastroenterology. 2015;21(36):10475–9. doi: 10.3748/wjg.v21.i36.10475. http://doi.org/10.3748/wjg.v21.i36.10475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guerrero E, Midgley VC, Khachigian LM. Angiotensin II induction of PDGF-C expression is mediated by AT1 receptor-dependent Egr-1 transactivation. Nucleic Acids Research. 2008;36(6):1941–1951. doi: 10.1093/nar/gkm923. http://doi.org/10.1093/nar/gkm923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswat D, Nehra S, Chaudhary K, Prasad SC. Novel vascular endothelial growth factor blocker improves cellular viability and reduces hypobaric hypoxia-induced vascular leakage and oedema in rat brain. Clinical and Experimental Pharmacology and Physiology. 2015;42(5):475–484. doi: 10.1111/1440-1681.12387. http://doi.org/10.1111/1440-1681.12387. [DOI] [PubMed] [Google Scholar]
- Sashindranath M, Sales E, Daglas M, Freeman R, Samson AL, Cops EJ, Medcalf RL. The tissue-type plasminogen activator-plasminogen activator inhibitor 1 complex promotes neurovascular injury in brain trauma: evidence from mice and humans. Brain. 2012;135(11):3251–3264. doi: 10.1093/brain/aws178. http://doi.org/10.1093/brain/aws178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savva GM, Stephan BCM. Epidemiological Studies of the Effect of Stroke on Incident Dementia: A Systematic Review. Stroke. 2010;41(1):e41–e46. doi: 10.1161/STROKEAHA.109.559880. http://doi.org/10.1161/STROKEAHA.109.559880. [DOI] [PubMed] [Google Scholar]
- Seals RM, Kioumourtzoglou MA, Gredal O, Hansen J, Weisskopf MG. ALS and the Military. Epidemiology. 2015;27(2):1. doi: 10.1097/EDE.0000000000000417. http://doi.org/10.1097/EDE.0000000000000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelen M, van Doormaal PTC, Visser AE, Huisman MHB, Roozekrans MHJ, de Jong SW, van den Berg LH. Prior medical conditions and the risk of amyotrophic lateral sclerosis. Journal of Neurology. 2014;261(10):1949–56. doi: 10.1007/s00415-014-7445-1. http://doi.org/10.1007/s00415-014-7445-1. [DOI] [PubMed] [Google Scholar]
- Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in alzheimer’s disease. Brain Pathology. 2013;23(3):303–310. doi: 10.1111/bpa.12004. http://doi.org/10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma HS. Early microvascular reactions and blood–spinal cord barrier disruption are instrumental in pathophysiology of spinal cord injury and repair: novel therapeutic strategies including nanowired drug delivery to enhance neuroprotection. Journal of Neural Transmission. 2011;118(1):155–176. doi: 10.1007/s00702-010-0514-4. http://doi.org/10.1007/s00702-010-0514-4. [DOI] [PubMed] [Google Scholar]
- Sharp KG, Yee KM, Steward O. A re-assessment of treatment with a tyrosine kinase inhibitor (imatinib) on tissue sparing and functional recovery after spinal cord injury. Experimental Neurology. 2014;254:1–11. doi: 10.1016/j.expneurol.2013.12.019. http://doi.org/10.1016/j.expneurol.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nature Reviews. Neurology. 2010;6(7):393–403. doi: 10.1038/nrneurol.2010.74. http://doi.org/10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanandam TM, Thakur MK. Traumatic brain injury: a risk factor for Alzheimer’s disease. Neuroscience and Biobehavioral Reviews. 2012;36(5):1376–81. doi: 10.1016/j.neubiorev.2012.02.013. http://doi.org/10.1016/j.neubiorev.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Smith CL, Tallquist MD. PDGF function in diverse neural crest cell populations. Cell Adhesion and Migration. 2010;4(4):561–566. doi: 10.4161/cam.4.4.12829. http://doi.org/10.4161/cam.4.4.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son D, Na YR, Hwang ES, Seok SH. Platelet-derived growth factor-C (PDGF-C) induces anti-apoptotic effects on macrophages through Akt and Bad phosphorylation. The Journal of Biological Chemistry. 2014;289(9):6225–35. doi: 10.1074/jbc.M113.508994. http://doi.org/10.1074/jbc.M113.508994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997;124(14):2691–700. doi: 10.1242/dev.124.14.2691. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9226440. [DOI] [PubMed] [Google Scholar]
- Stefanitsch C, Lawrence ALE, Olverling A, Nilsson I, Fredriksson L. tPA Deficiency in Mice Leads to Rearrangement in the Cerebrovascular Tree and Cerebroventricular Malformations. Frontiers in Cellular Neuroscience. 2015;9:456. doi: 10.3389/fncel.2015.00456. http://doi.org/10.3389/fncel.2015.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock P, Monga D, Tan X, Micsenyi A, Loizos N, Monga SPS. Platelet-derived growth factor receptor-alpha : a novel therapeutic target in human hepatocellular cancer. Molecular Cancer Therapeutics. 2007;6(7):1932–1941. doi: 10.1158/1535-7163.MCT-06-0720. http://doi.org/10.1158/1535-7163.MCT-06-0720. [DOI] [PubMed] [Google Scholar]
- Stover EH, Chen J, Lee BH, Cools J, McDowell E, Adelsperger J, Gilliland DG. The small molecule tyrosine kinase inhibitor AMN107 inhibits TEL-PDGFRbeta and FIP1L1-PDGFRalpha in vitro and in vivo. Blood. 2005;106(9):3206–13. doi: 10.1182/blood-2005-05-1932. http://doi.org/10.1182/blood-2005-05-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Lawrence DA. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nature Medicine. 2008;14(7):731–7. doi: 10.1038/nm1787. http://doi.org/10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su EJ, Fredriksson L, Kanzawa M, Moore S, Folestad E, Stevenson TK, Lawrence DA. Imatinib treatment reduces brain injury in a murine model of traumatic brain injury. Frontiers in Cellular Neuroscience. 2015;9:385. doi: 10.3389/fncel.2015.00385. http://doi.org/10.3389/fncel.2015.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su EJ, Fredriksson L, Schielke GP, Eriksson U, Lawrence DA. Tissue plasminogen activator-mediated PDGF signaling and neurovascular coupling in stroke. Journal of Thrombosis and Haemostasis. 2009;7(SUPPL. 1):155–158. doi: 10.1111/j.1538-7836.2009.03402.x. http://doi.org/10.1111/j.1538-7836.2009.03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi E, Nishijo K, McCleish AT, Michalek JE, Grayson MH, Infante AJ, Keller C. PDGFR-A is a therapeutic target in alveolar rhabdomyosarcoma. Oncogene. 2008;27(51):6550–60. doi: 10.1038/onc.2008.255. http://doi.org/10.1038/onc.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel A, Cechetto DF, Heiss WD, Hachinski V, Whitehead SN. Amyloid Burden, Neuroinflammation, and Links to Cognitive Decline After Ischemic Stroke. Stroke. 2014;45(9):2825–2829. doi: 10.1161/STROKEAHA.114.004285. http://doi.org/10.1161/STROKEAHA.114.004285. [DOI] [PubMed] [Google Scholar]
- Torre JC de la. How do heart disease and stroke become risk factors for Alzheimer’s disease? Neurological Research. 2013 doi: 10.1179/016164106X130362. Retrieved from http://www.tandfonline.com/doi/citedby/10.1179/016164106X130362. [DOI] [PubMed]
- Trojanowska M. Role of PDGF in fibrotic diseases and systemic sclerosis. Rheumatology. 2008;47(Supplement 5):v2–v4. doi: 10.1093/rheumatology/ken265. http://doi.org/10.1093/rheumatology/ken265. [DOI] [PubMed] [Google Scholar]
- Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377(6547):340–4. doi: 10.1038/377340a0. http://doi.org/10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- Turner MR, Goldacre R, Talbot K, Goldacre MJ. Cerebrovascular injury as a risk factor for amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry. 2015 doi: 10.1136/jnnp-2015-311157. jnnp–2015–311157 http://doi.org/10.1136/jnnp-2015-311157. [DOI] [PMC free article] [PubMed]
- Ustach CV, Kim HRC. Platelet-derived growth factor D is activated by urokinase plasminogen activator in prostate carcinoma cells. Molecular and Cellular Biology. 2005;25(14):6279–88. doi: 10.1128/MCB.25.14.6279-6288.2005. http://doi.org/10.1128/MCB.25.14.6279-6288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uutela M, Laurén J, Bergsten E, Li X, Horelli-Kuitunen N, Eriksson U, Alitalo K. Chromosomal location, exon structure, and vascular expression patterns of the human PDGFC and PDGFD genes. Circulation. 2001;103(18):2242–7. doi: 10.1161/01.cir.103.18.2242. http://doi.org/10.1161/01.CIR.103.18.2242. [DOI] [PubMed] [Google Scholar]
- van Assema DME, Lubberink M, Bauer M, van der Flier WM, Schuit RC, Windhorst AD, van Berckel BNM. Blood-brain barrier P-glycoprotein function in Alzheimer’s disease. Brain. 2012;135(1):181–189. doi: 10.1093/brain/awr298. http://doi.org/10.1093/brain/awr298. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, van Gijn J. Clinical practice. Acute ischemic stroke. The New England Journal of Medicine. 2007;357(6):572–9. doi: 10.1056/NEJMcp072057. http://doi.org/10.1056/NEJMcp072057. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, da Costa Araujo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130(2):521–534. doi: 10.1093/brain/awl318. http://doi.org/10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- Vermersch P, Benrabah R, Schmidt N, Zéphir H, Clavelou P, Vongsouthi C, Hermine O. Masitinib treatment in patients with progressive multiple sclerosis: a randomized pilot study. BMC Neurology. 2012;12:36. doi: 10.1186/1471-2377-12-36. http://doi.org/10.1186/1471-2377-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, Murray CJ. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. http://doi.org/10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissberg I, Veksler R, Kamintsky L, Saar-Ashkenazy R, Milikovsky DZ, Shelef I, Friedman A. Imaging blood-brain barrier dysfunction in football players. JAMA Neurology. 2014;71(11):1453–5. doi: 10.1001/jamaneurol.2014.2682. http://doi.org/10.1001/jamaneurol.2014.2682. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, O’Reilly EJ, McCullough ML, Calle EE, Thun MJ, Cudkowicz M, Ascherio A. Prospective study of military service and mortality from ALS. Neurology. 2005;64(1):32–7. doi: 10.1212/01.WNL.0000148649.17706.D9. http://doi.org/10.1212/01.WNL.0000148649.17706.D9. [DOI] [PubMed] [Google Scholar]
- Wenqiang C, Lonskaya I, Hebron ML, Ibrahim Z, Olszewski RT, Neale JH, Moussa CEH. Parkin-mediated reduction of nuclear and soluble TDP-43 reverses behavioral decline in symptomatic mice. Human Molecular Genetics. 2014;23(18):4960–9. doi: 10.1093/hmg/ddu211. http://doi.org/10.1093/hmg/ddu211. [DOI] [PubMed] [Google Scholar]
- Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Frontiers in Neurology. 2013;4:18. doi: 10.3389/fneur.2013.00018. http://doi.org/10.3389/fneur.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Rosenberg GA. Blood-Brain Barrier Breakdown in Acute and Chronic Cerebrovascular Disease. Stroke. 2011;42(11):3323–3328. doi: 10.1161/STROKEAHA.110.608257. http://doi.org/10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Coleman TA, Moore E, Wu JY, Mitola D, Lawrence DA. Regulation of seizure spreading by neuroserpin and tissue-type plasminogen activator is plasminogen-independent. The Journal of Clinical Investigation. 2002;109(12):1571–8. doi: 10.1172/JCI14308. http://doi.org/10.1172/JCI14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. The Journal of Clinical Investigation. 2003;112(10):1533–40. doi: 10.1172/JCI19212. http://doi.org/10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JC, Mahadevan D, LaRochelle WJ, Pierce JH, Heidaran MA. Structural coincidence of alpha PDGFR epitopes binding to platelet-derived growth factor-AA and a potent neutralizing monoclonal antibody. The Journal of Biological Chemistry. 1994;269(14):10668–74. Retrieved from http://www.jbc.org/content/269/14/10668.abstract. [PubMed] [Google Scholar]
- Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nature Reviews. Neurology. 2013;9(4):201–10. doi: 10.1038/nrneurol.2013.9. http://doi.org/10.1038/nrneurol.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Krafft PR, Lekic T, Ma Q, Souvenir R, Zhang JH, Tang J. Imatinib preserves blood-brain barrier integrity following experimental subarachnoid hemorrhage in rats. Journal of Neuroscience Research. 2015;93(1):94–103. doi: 10.1002/jnr.23475. http://doi.org/10.1002/jnr.23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and Dysfunction of the Blood-Brain Barrier. Cell. 2015;163(5):1064–1078. doi: 10.1016/j.cell.2015.10.067. http://doi.org/10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O’Banion MK, Zlokovic BV. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nature Neuroscience. 2008;11(4):420–422. doi: 10.1038/nn2073. http://doi.org/10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Neurodegeneration and the neurovascular unit. Nature Medicine. 2010;16(12):1370–1371. doi: 10.1038/nm1210-1370. http://doi.org/10.1038/nm1210-1370. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature Reviews. Neuroscience. 2011;12(12):723–38. doi: 10.1038/nrn3114. http://doi.org/10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zymek P, Bujak M, Chatila K, Cieslak A, Thakker G, Entman ML, Frangogiannis NG. The role of platelet-derived growth factor signaling in healing myocardial infarcts. Journal of the American College of Cardiology. 2006;48(11):2315–23. doi: 10.1016/j.jacc.2006.07.060. http://doi.org/10.1016/j.jacc.2006.07.060. [DOI] [PubMed] [Google Scholar]


