Abstract
Background
Mesothelium VCAM-1 expression in the metastatic epithelial ovarian cancer (EOC) microenvironment is induced by tumor and mediates tumor cell invasion. VCAM-1 imaging suggests expression during treatment is an indicator of platinum resistance. Here, we assess the potential prognostic significance of mesothelium VCAM-1 expression and prospectively evaluate whether soluble VCAM-1 (sVCAM-1) is a surrogate for mesothelium expression.
Methods
A retrospective review of EOC patients was performed to evaluate outcomes with mesothelium VCAM-1 expression determined by immunohistochemistry of peritoneum or omentum specimens. A prospective cohort of EOC patients was identified and followed through primary treatment. Serum for sVCAM-1 evaluation, determined by ELISA, was collected prior to surgery or neoadjuvant chemotherapy and at each treatment cycle. Peritoneal specimens were obtained during debulking to assess mesothelial VCAM-1 expression.
Results
Retrospective review identified 54 advanced stage EOC patients. Patients expressing mesothelium VCAM-1 had shortened overall (44 vs 79 months, p=0.035) and progression-free survival (18 vs 67 months, p=0.010); median time to platinum-resistance was 36 months for VCAM-1 expressing patients and not yet determined for the VCAM-1 negative group. In our prospective observational cohort, eighteen EOC patients completed primary treatment; 3 were negative for mesothelium VCAM-1 expression, and sVCAM-1 did not vary between groups.
Conclusions
Mesothelium VCAM-1 expression negatively associates with progression-free and overall survival in EOC. This is especially compelling in light of prior data suggesting that persistent VCAM-1 expression during treatment is an indicator of platinum resistance. Our pilot study had insufficient cases to determine whether sVCAM-1 would substitute for mesothelium expression.
Keywords: Epithelial Ovarian Cancer, Vascular Cell Adhesion Molecule-1, Mesothelium, Metastasis, Survival, Tumor Microenvironment
INTRODUCTION
Despite years of developing aggressive surgical techniques and novel adjuvant therapy strategies, epithelial ovarian cancer (EOC) remains the most lethal of the gynecologic malignancies (1). Since the likelihood of developing robust whole population screening programs in the near future is low (2, 3), it is imperative that the mechanisms of peritoneal seeding and tumor spread are better defined in order to direct therapeutic strategies. Furthermore, identification of specific biomarkers of disease response and recurrence may aid in the development of more personalized treatment strategies earlier in the course of treatment.
Current primary treatment strategies for EOC combine surgical resection and systemic platinum based chemotherapy. The order and approach to these treatment modalities have been modified over the last several decades with some promise; in general, 80% of women will experience a response to treatment with the majority achieving remission (4–6). Unfortunately, approximately 80% of patients will experience a recurrence within 24 months of completion of primary therapy. Not surprisingly, the time to recurrence dramatically impacts prognosis and future response to therapy; however, currently no reliable method or biomarker exists to identify patients most likely to recur in short order or those unlikely to respond to primary therapy from the outset. Therefore, while the exact combination and timing may vary, the approach to primary chemotherapy and surgery for EOC is somewhat standard for all patients. Attempts to predict time to recurrence, the utility of maintenance chemotherapy, or identification of early signs of recurrent disease have failed to identify any reliable biomarkers of disease behavior, and as a result, have not provided a significant improvement in survival (7, 8).
We identified vascular cell adhesion molecule-1 (VCAM-1) as a regulator of ovarian cancer peritoneal metastasis (9). VCAM-1 is consistently over-expressed on the mesothelium, a single cell layer of mesothelial cells lining the peritoneal cavity and surrounding organs within it, in the setting of advanced disease (10), which represents the majority of EOC patients at diagnosis. Furthermore, the frequency of VCAM-1 expression increases with the extent of peritoneal tumor involvement; however, not all patients with peritoneal metastases display VCAM-1 positivity (10). In our prior assessment of advanced-stage EOC patients, the majority of those lacking mesothelium VCAM-1 expression had previously been exposed to neoadjuvant chemotherapy (NACT), and our pre-clinical models employing VCAM-1 targeted imaging demonstrated that expression during treatment is an indicator of platinum resistance (10). Taken together, our findings implicate VCAM-1 as a potential marker of platinum responsiveness.
While mechanistic studies of the impact of VCAM-1 expression in the EOC microenvironment are ongoing, the clinical impact of its presence as a potential therapeutic target and a molecular indicator of treatment sensitivity is compelling. In the current study, we employed a retrospective cohort of ovarian cancer patients to assess whether mesothelium VCAM-1 expression is associated with prognosis, thus further strengthening our hypothesis that it is a potential biomarker of platinum responsiveness in EOC. Since mesothelium VCAM-1 expression segregates advanced-stage ovarian cancer patients who respond to standard-of-care chemotherapy from those who do not, and VCAM-1 is also found as a soluble form (sVCAM-1) in serum that has been investigated as a component of a biomarker panel for the detection of EOC (11), we investigated whether sVCAM-1, like its mesothelium counterpart, would also reflect the presence of advanced disease burden and serve as a less invasive surrogate for mesothelium expression. We report the results of a prospective observational cohort study investigating serum sVCAM-1 levels in EOC patients over the course of standard treatment as compared to healthy volunteer controls.
METHODS
The University of Virginia institutional review board approved all endeavors described below.
Retrospective Review
Retrospective review of patients undergoing surgery for EOC at the University of Virginia Health System from 1999 to 2008 identified 54 advanced-stage patients from whom clinical records and paraffin-embedded tissue blocks of omentum or peritoneum were obtained. H & E stained sections were evaluated to ensure the presence of mesothelial cells. The Biorepository Tissue Research Facility at the University of Virginia stained additional sections immunohistochemically for VCAM-1 as described previously using anti- human VCAM-1 antibodies (Abcam) according to the manufacturer’s instructions (9, 10). Expression was scored as high if >50% and a contiguous string of ≥ 10 mesothelial cells displayed membranous staining with 3+ intensity or low if <50% or fewer than 10 contiguous cells stained with 3+ intensity. Slides were read and scored by a single pathologist (KAA) at our institution. Expression classification was evaluated with respect to clinical data including demographics, tumor stage, tumor histology, treatment options, and outcomes including overall survival, progression-free survival and time to platinum resistance.
Prospective Serum VCAM-1 detection
Subject identification
EOC patients were identified at the time of diagnosis and following consent, were assigned to one of two cohorts on the basis of treatment approach, which was determined at the discretion of the treating physician: primary debulking surgery + adjuvant chemotherapy (PDS) or neoaduvant chemotherapy + interval debulking (NACT). A third cohort of healthy volunteer control participants with no active gynecologic problem, autoimmunity or known inflammatory condition was included to obtain non-disease sVCAM-1 levels. These patients were not matched on the basis of age. EOC patients received a standard six-cycle platinum/taxane-based chemotherapy regimen. Those receiving NACT underwent interval debulking surgery after the third cycle of chemotherapy and completed three additional cycles postoperatively. Serum was collected at the time of routine monthly chemotherapy labs for each treatment cycle and at one month and three months post treatment. Peritoneum and omentum samples were collected from each patient at the time of primary or interval surgery. Healthy volunteer control participants consented to 4 separate monthly blood draws for soluble VCAM-1 (sVCAM-1) assessment.
Sample size
The prospective observational pilot study set target sample size at 24 subjects, 9 for each cohort of ovarian cancer patients (18 total) and 6 volunteer healthy control subjects. Based on previous data assessing sVCAM-1 levels in gastric cancer patients vs. controls (12), the total sample of 24 subjects would achieve approximately 90% power to detect a difference in baseline sVCAM-1 expression of at least 242 using the Dunnett (with control) multiple comparison test at a 4% significance level, which corresponded to the minimum difference observed in sVCAM-1 expression and the largest observed within group standard deviation of 78 (12). Thirty newly diagnosed EOC patients were identified and consented to participate in this observational study. Twelve patients were excluded: three for final diagnosis not consistent with EOC, one declined further treatment, one patient died of disease during primary therapy and seven patients elected to receive chemotherapy closer to home due to geographic constraints. Eighteen patients were enrolled for NACT (n=9) or PDS (n=9), based on their clinical presentation and discretion of the treating physician. All patients completed primary treatment.
Specimen collection and evaluation
Serum (10 ml/draw) was collected in red top silica clot activator tubes with a conventional stopper, assigned a unique identifier, transported to the Biorepository and Tissue Research Facility at the University of Virginia within 1 hour of collection and stored at −80° C. ELISA analysis of sVCAM-1 was performed by the Center for Research in Reproduction Ligand Assay and Analysis Core at the University of Virginia. Tissue specimens were handled as per institutional pathology protocol. Peritoneum and omentum specimens not necessary for diagnosis were paraffin embedded and stained for VCAM-1 expression as described above.
Statistical considerations
ANOVA using Dunnett’s multiple comparison test was used to assess differences in serum sVCAM-1 at baseline among the three groups. Repeated measure models were used to assess the pattern of change in serum sVCAM-1 levels over time in the cancer patients and to determine if patterns differed by cohort for the prospective observational study. Time to event distributions were estimated by the method of Kaplan and Meier, and the Logrank test was used to test for differences.
RESULTS
Retrospective cohort analysis
Fifty-four advanced-stage (primarily III and IV) EOC patients were identified retrospectively; three were eliminated for ambiguous VCAM-1 staining and one for the lack of follow-up data (Table 1). Median age, stage and histology were all consistent with the literature. Optimal cytoreduction, which we defined as leaving no more than 1 cm of residual tumor, portends a favorable prognosis for advanced-stage ovarian cancer patients (4, 5). Within this cohort, 76% of patients were optimally debulked and had a median survival of 74 months compared to 23 months for the 22% of patients who were suboptimally debulked (Figure 1). Sixty-five percent of the patients received primary debulking surgery (PDS) followed by adjuvant chemotherapy; the remaining 35% received neoadjuvant chemotherapy (NACT) with interval debulking surgery. The most commonly cited reason for pursuing NACT in this setting was medical comorbidity/poor functional status. Survival analysis was performed on the basis of treatment approach (Figure 1). In keeping with the evolving literature regarding treatment approaches in ovarian cancer (13, 14), there was no evidence of a survival difference between patients receiving NACT compared to PDS in this cohort (Figure 1).
Table 1.
Patient Demographics
| VCAM-1 POS | VCAM-1 NEG | All Patients | p-valuea | ||
|---|---|---|---|---|---|
| Number | 30 | 20 | 50 | ||
| Median Age | 61 | 63 | 62 | ||
| White | 27 (90%) | 17 (85%) | 44 (88%) | 0.18 | |
| Black | 1 (3%) | 3 (15%) | 4 (8%) | ||
| Other | 2 (7%) | 0 | 2 (4%) | ||
| Stage | Stage IIB | 0 | 1 (5%) | 1 (2%) | 0.59 |
| Stage IIIA | 1 (3%) | 2 (10%) | 3 (6%) | ||
| Stage IIIB | 3 (10%) | 1 (5%) | 4 (8%) | ||
| Stage IIIC | 18 (60%) | 11 (55%) | 29 (58%) | ||
| Stage IV | 8 (27%) | 5 (25%) | 13 (26%) | ||
| Histology | High Grade Serous | 17 (57%) | 15 (75%) | 32 (64%) | 0.3 |
| Low Grade Serous | 3 (9%) | 0 | 3 (6%) | ||
| Mucinous | 1 (3%) | 0 | 1 (2%) | ||
| Endometrioid | 0 | 2 (10%) | 2 (4%) | ||
| Clear Cell | 4 (13%) | 1 (5%) | 5 (10%) | ||
| Adeno. NOSb | 2 (6%) | 1 (5%) | 3 (6%) | ||
| Other | 3 (9%) | 1 (5%) | 4 (8%) | ||
| Treatment | NACTc | 5 (17%) | 13 (65%) | 18 (35%) | 0.0005 |
| Upfront Surgery | 25 (83%) | 7 (35%) | 32 (64%) | ||
| Surgical Outcomed | Optimal Cytoreduction | 20 (67%) | 18 (90%) | 38 (76%) | 0.16 |
| Suboptimal Cytoreduction | 9 (30%) | 2 (10%) | 11 (22%) |
Comparing VCAM-1 positive to VCAM-1 negative using Chi-squared test
Adeno. NOS – adenocarcinoma not otherwise specified
NACT – neoadjuvant chemotherapy
Surgical outcome data missing for 1 patient
Figure 1. Survival analysis based on treatment.
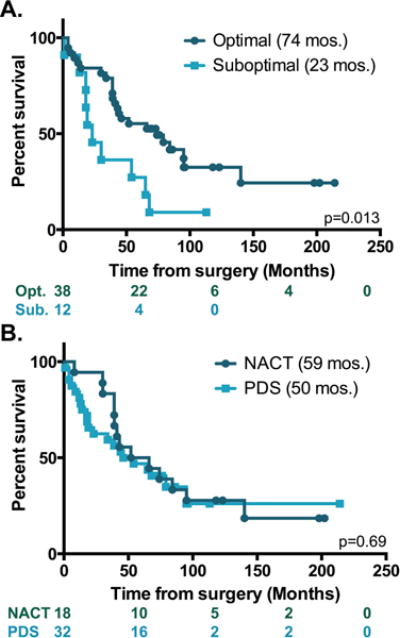
Kaplan-Meier plots showing survival in months of patients with (dark teal) or without (light teal) optimal cytoreduction (A) or receiving neoadjuvant chemotherapy (NACT, dark teal) versus upfront surgery (light teal) (B). Log rank test was used to evaluate significance. The number of subjects at risk is indicated. p values shown on each graph.
Peritoneum or omentum tissue samples were evaluated for membranous VCAM-1 staining on mesothelial cells. We found that the mesothelium VCAM-1 expression level was either high (3+) or absent; intermediate levels of expression were not observed (see Supporting Information). Therefore, expression was scored as positive or negative. Additionally, half of the tissue blocks contained tumor cells, and VCAM-1 expression did not depend on their presence (not shown). We reported previously that patients receiving NACT were less likely to express VCAM-1 on the mesothelium (10) (Table 1). Indeed, among the patient demographic information presented in Table 1, treatment approach was the only factor that associated significantly with VCAM-1 expression. Pre-clinical data demonstrate that VCAM-1 expression reflects chemoresponsiveness and resistance to platinum-based therapy (10). As platinum resistance has a known negative impact on survival of EOC, we evaluated whether baseline mesothelium VCAM-1 expression itself was associated with overall prognosis. Within this cohort, overall survival was significantly less in patients expressing mesothelium VCAM-1 (median of 44 mos. vs. 79 mos., Logrank p=0.035) with a minimum of 5 years of follow-up (Figure 2). The 5-year survival for VCAM-1 negative patients was 55% compared to 37% for VCAM-1 positive patients. Moreover, progression-free survival was shorter for VCAM-1 positive patients than for the negative group (median of 18 mos. vs. 67 mos., Logrank p=0.01, Figure 3) in the subset of 41 patients where progression or lack of progression information was known.
Figure 2. Survival based on mesothelium VCAM-1 expression.
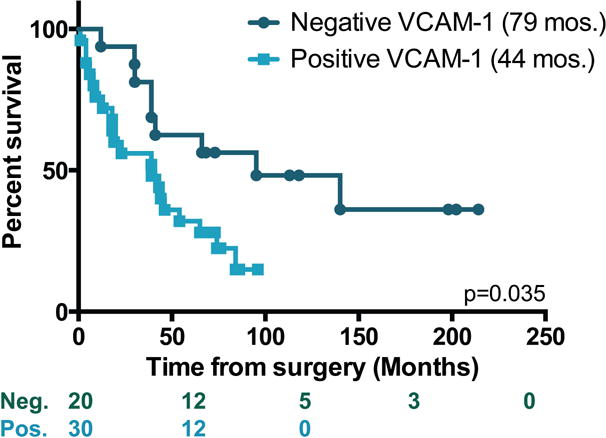
Kaplan-Meier plots showing survival in months of patients who were positive (light teal) or negative (dark teal) for VCAM-1 expression on the mesothelium. Log rank test was used to evaluate significance. The number of subjects at risk is indicated below the graph.
Figure 3. Progression-free survival based on mesothelium VCAM-1 expression.
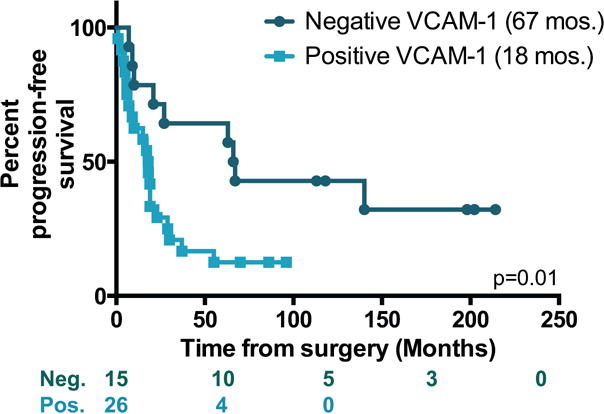
Kaplan-Meier plots showing survival in months of patients who were positive (light teal) or negative (dark teal) for mesothelium VCAM-1 expression. Log rank test was used to evaluate significance. The number of subjects at risk is indicated below the graph.
To further assess the influence of VCAM-1 expression on chemosensitivity, we analyzed patients on the basis of primary platinum resistance. Information regarding platinum responsiveness was available for 35 patients in this cohort. Development of platinum resistance was determined from the date of surgery irrespective of the treatment approach and included all patients regardless of their initial response to treatment (i.e., platinum refractory, platinum resistant, and development of resistance after initial sensitivity). The median time to develop platinum resistance was 36 months for patients with positive mesothelium VCAM-1 expression and not yet reached for those who were negative (p=0.052, Figure 4). Together, these observations support the notion that mesothelium expression of VCAM-1 at the time of debulking surgery identifies patients who are less likely to respond favorably to standard-of-care treatment and have an unfavorable prognosis.
Figure 4. Time-to-platinum-resistance based on mesothelium VCAM-1 expression.
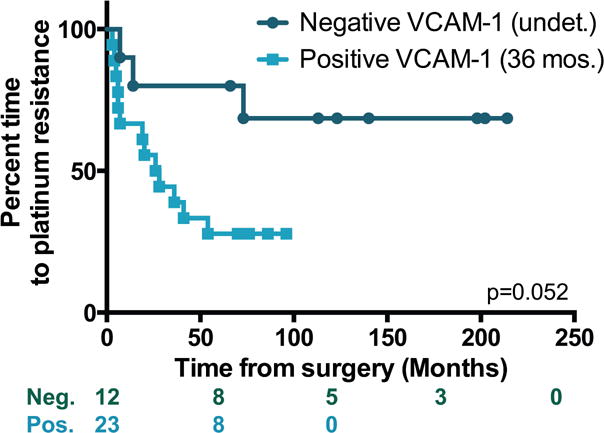
Kaplan-Meier plots showing survival in months of patients who were positive (light teal) or negative (dark teal) for mesothelium VCAM-1 expression. Log rank test was used to evaluate significance. The number of subjects at risk is indicated below the graph.
Prospective sVCAM-1 pilot study
The pilot prospective observational study was designed as described to obtain preliminary data on 1) whether sVCAM-1 levels differed between malignant and benign conditions and 2) whether the levels among women with malignancies were reflective of mesothelium expression of VCAM-1. Based on our retrospective findings indicating that mesothelium VCAM-1 expression was inversely associated with outcome, we sought to identify a less invasive surrogate that could be followed easily and potentially clinically actionable over a standard EOC treatment course. Baseline characteristics are shown in Table 2. The median age of all eighteen EOC patients was 61 years with median ages of 60 and 64 for the PDS and NACT groups, respectively (Table 2). The cited reason for utilization of NACT in this study was medical comorbidity and the general performance status of the patient. Use of NACT at our institution at the time of this study was approximately 20% and was generally reserved for women felt to be at high risk of surgical morbidity/mortality at the time of diagnosis. Ninety-four percent of patients were Stage IIIC or greater, and 72% of patients had high-grade serous disease (data not shown). A similar distribution of optimal vs. suboptimal cytoreduction was obtained for both groups (Table 2), and all patients received treatment consisting of a combination of platinum and taxane.
Table 2.
Prospective sVCAM-1 study
| Control | PDSa | NACTb | Mesothelial VCAM-1 | |||
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Number | 6 | 9 | 9 | |||
| Median Age | 60 | 64 | 66 | 56 | ||
| Surgical Outcome | Opt. | N/Ac | 7 | 7 | 9 | 3 |
| Sub-opt. | N/A | 2 | 2 | 3 | 0 | |
| Mesothelial VCAM-1 | Pos. | 6 | 6 | 12 | ||
| Neg. | 1 | 2 | 3 | |||
| NDd | 6 | 2 | 1 | |||
PDS – primary debulking surgery
NACT – neoadjuvant chemotherapy
N/A – not applicable
ND – no data. Samples were not collected from the control group; specimens from upfront surgery or NACT had insufficient mesothelial cells to evaluate.
Mesothelium VCAM-1 staining was obtained for 7 PDS and 8 NACT patients; one patient in the PDS group and 2 NACT patients were negative for VCAM-1 (Table 2). The mean sVCAM-1 concentration for PDS (813 ± 58.8 ng/ml) and NACT (906.1 ± 102.6 ng/ml) groups taken at the time of surgery did not differ from each other or control (764.5 ± 84.5 ng/ml) (Dunnett’s p=0.55) nor did they vary over time (F-test p=0.8) or between groups over time (F-test p=0.72) (Figure 5). With only 3 patients identified as negative for mesothelium VCAM-1 expression, there were insufficient data to assess the association with sVCAM-1 (Table 2). Therefore, sVCAM-1 was unable to distinguish women with or without ovarian cancer, and this pilot study was unable to identify sVCAM-1 as a surrogate for mesothelium expression. Moreover, the data indicate a high level of variability in sVCAM-1 measurements over time that should be considered in the design of future studies.
Figure 5. Serum sVCAM-1 concentration.
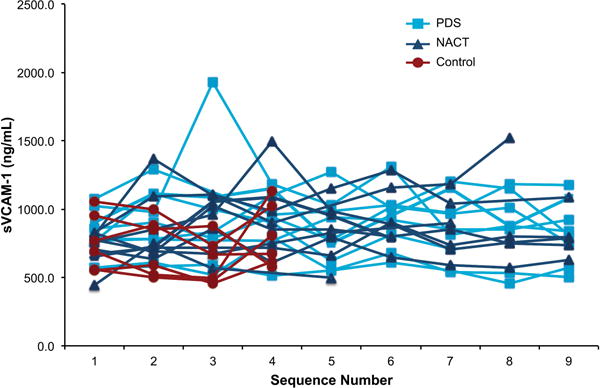
Levels of sVCAM-1 were determined from serum of ovarian cancer patients undergoing PDS (light blue squares) or NACT (dark blue triangles) taken just prior to each chemotherapy session (sequences 1–6) and during 3 follow-up visits. VCAM-1 concentration was determined from serum withdrawn from women free of disease at 4 monthly intervals (control, red circles).
DISCUSSION
We previously identified mesothelium VCAM-1 as an indicator of peritoneal metastasis where it regulated ovarian cancer cell invasion. Here, we found that mesothelium expression of VCAM-1 is negatively associated with progression-free and overall survival in EOC. Additionally, patients expressing VCAM-1 develop resistance to platinum agents more rapidly than the low expressing cohort. However, our pilot study evaluating sVCAM-1 as a potential surrogate for mesothelium expression was unable to distinguish cancer patients from healthy individuals or correlate serum levels with mesothelium expression. Together these observations provide the first evidence that VCAM-1 expressed in the microenvironment may have prognostic significance for EOC.
The a priori goal of our retrospective study was to assess whether there was an association between mesothelium VCAM-1 expression and EOC outcome. The identification of a negative association between expression and progression-free or overall survival among advanced-stage EOC patients is compelling. The number of observed events within our patient population guided the decision to limit the number of evaluable characteristics to three and ruled out the ability to assess interaction effects. It should be noted that there was a disparity in VCAM-1 negative staining between the retrospective cohort (40%) and our prospective cohort (20%). The lower incidence of VCAM-1 negative staining in our observational study raises the question as to whether NACT truly impacts VCAM-1 expression; however, with only eight patients analyzed in the prospective group, cross comparisons between cohorts are less likely to generate meaningful conclusions. Additional larger studies will be necessary to resolve these discrepancies as well as to determine whether mesothelium VCAM-1 expression predicts outcomes and whether it does so independently of other variables, including age at diagnosis, histological subtype, and surgical timing and outcome among others.
While the incidence of VCAM-1 expression is lower among patients who received NACT and preclinical imaging data in mouse models indicate that mesothelium VCAM-1 expression reflects tumor response to treatment, it is unclear whether VCAM-1 expression changes in EOC patients and what impact changing expression might indicate about prognosis. The pilot prospective observational study evaluating sVCAM-1 over the course of treatment was an attempt to address this issue; however, sVCAM-1 did not segregate malignant versus benign conditions or change significantly over time irrespective of disease status. At the time of study design, there were no clearly defined parameters for serum sVCAM-1 in a normal state of health. Additionally, we observed a higher degree of variability than that which was reported previously (12). The inability to monitor mesothelium VCAM-1 expression by measuring sVCAM-1 lends support to develop VCAM-1 targeting imaging probes to monitor expression in EOC patients over the course of treatment.
VCAM-1 has been identified as a prognostic indicator in other disease sites, including gastric, colon, breast, renal, melanoma, non-Hodgkin’s and Hodgkin’s lymphoma, and chronic B-cell lymphoma (12, 15–24). Expression of VCAM-1 on the stroma in colon cancer portended an unfavorable outcome and was inversely related to sVCAM-1 levels (16). However, others have shown that elevated sVCAM-1 is associated with increased tumor size and metastasis (15) and predicts recurrence in colorectal cancer (18). Tumor cell expression of VCAM-1 is associated with increased survival in renal cell carcinoma (25) and a worse outcome in ovarian cancer (26). While VCAM-1 expression on tumor cells was not observed (data not shown), our data linking mesothelium VCAM-1 expression to poor survival are consistent with the reported association between tumor expression and outcome (26). Importantly, mesothelial cells are not under the evolutionary pressure of genetically mutable tumors; VCAM-1 expression is not affected by exposure to platinum agents (10). It is unclear whether tumor cell expression responds to treatment.
Increased sVCAM-1 levels have been linked with increased tumor burden in colon cancer and chronic B-cell lymphoma (17, 22) or poor survival in gastric, breast, melanoma, and non-Hodgkin’s and Hodgkin’s lymphoma (15, 19–21, 23, 24). One small cohort study of 36 ovarian cancer patients identified significantly elevated sVCAM-1 in 5 patients who died within 11 months of follow-up compared to the 31 who were alive (27). Another report found no association between sVCAM-1 and outcome among ovarian cancer patients (n=50) but was able to identify patients with metastatic disease (28). The primary objective of our study was to link sVCAM-1 to mesothelium VCAM-1 expression with the expectation that it could serve as a surrogate prognosticator. Several limitations potentially impeded our ability to achieve this objective. Other conditions contribute to sVCAM-1 expression including chronic inflammatory conditions such as atherosclerosis or autoimmune disease. This study did not account for different treatment approaches such as intraperitoneal chemotherapy or the use of bevacizumab, and the sample size was too small to stratify based on additional variables. Additionally, the small sample size restricted our ability to determine whether outcomes such as survival or development of platinum resistance would correlate with sVCAM-1 levels. While additional studies would be necessary, it is possible that sVCAM-1 will not reflect mesothelium VCAM-1 expression; sVCAM-1 and tissue VCAM-1 expression have not correlated in other cancers (16, 21, 22).
The ability of mesothelium VCAM-1 expression to identify advanced-stage ovarian cancer patients who are likely to respond to current standard-of-care chemotherapy and discriminate outcomes provides an opportunity to impact clinical management. While upfront treatment options are limited to surgery and platinum/taxane-based chemotherapy, newer drugs including bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor,(29) and olaparib, an inhibitor of poly (ADP-ribose) polymerase (PARP) (30), have been approved for recurrent ovarian cancer, and VEGF and PARP inhibitors are currently being tested in multiple clinical trials for upfront treatment and in maintenance therapy. Immunotherapy is another realm of developing therapeutics in EOC. It would be interesting to know whether VCAM-1 expressing patients respond to these classes of drugs.
As the use of NACT evolves, many academic centers are now performing diagnostic laparoscopy prior to primary cytoreductive surgery to more accurately predict those patients for whom a primary surgical effort will be successful. This procedure allows appropriate triage of patients who cannot have a successful surgery due to disease burden to neoadjuvant therapy, and also allows the collection of a sufficient tumor biopsy for histology as well as next generation sequencing and potentially VCAM-1 measurement. The VCAM-1 results would then allow patients to be appropriately stratified to clinical trials, which for example, utilize biologics or immunotherapy to potentially prevent or reverse the development of platinum resistance.
Supplementary Material
Supplementary Figure. Representative images of omentum specimens showing VCAM-1 negative (A) or positive (B) mesothelium (single cell layer indicated by arrows) staining. Adi – adipocytes.
Acknowledgments
This work was supported by NIH/NCI R01 CA142783 (JKS-D) and a pilot award from the University of Virginia Cancer Center and Women’s Oncology Research Fund (JMS, LD, JKS-D). E. Saks was the 2014–2015 Orr Scholar and partially supported by the James and Vicki Orr Endowed Award in Women’s Oncology. The Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) Grant U54-HD28934. Supported in part by the University of Virginia Cancer Center Biostatistics Shared Resource (P30 CA044579).
Footnotes
There are no conflicts of interest to disclose.
Author contributions: Concept and design – J.M. Scalici, L.R. Duska, J.K. Slack-Davis; Formal analysis – G. Petroni; Investigation – S. Arapovic, E.J. Saks, K.A. Atkins; Supervision – L.R. Duska, J.K. Slack-Davis; Writing – J.M. Scalici, G. Petroni, L.R. Duska, J.K. Slack-Davis
References
- 1.http://seer.cancer.gov/statfacts/html/ovary.html
- 2.Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)01224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan RJ, Jr, Alvarez RD, Armstrong DK, Boston B, Burger RA, Chen LM, et al. Epithelial ovarian cancer. J National Comprehensive Cancer Network: JNCCN. 2011;9:82–113. doi: 10.6004/jnccn.2011.0008. [DOI] [PubMed] [Google Scholar]
- 4.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 5.Chang SJ, Bristow RE, Chi DS, Cliby WA. Role of aggressive surgical cytoreduction in advanced ovarian cancer. J Gynecol Oncol. 2015;26:336–42. doi: 10.3802/jgo.2015.26.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horowitz NS, Miller A, Rungruang B, Richard SD, Rodriguez N, Bookman MA, et al. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: an analysis of GOG 182. J Clin Oncol. 2015;33:937–43. doi: 10.1200/JCO.2014.56.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mueller JJ, Zhou QC, Iasonos A, O’Cearbhaill RE, Alvi FA, El Haraki A, et al. Neoadjuvant chemotherapy and primary debulking surgery utilization for advanced-stage ovarian cancer at a comprehensive cancer center. Gynecol Oncol. 2016;140:436–42. doi: 10.1016/j.ygyno.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vergote I, Trope CG, Amant F, Ehlen T, Reed NS, Casado A. Neoadjuvant chemotherapy is the better treatment option in some patients with stage IIIc to IV ovarian cancer. J Clin Oncol. 2011;29:4076–8. doi: 10.1200/JCO.2011.36.9785. [DOI] [PubMed] [Google Scholar]
- 9.Slack-Davis JK, Atkins KA, Harrer C, Hershey ED, Conaway M. Vascular cell adhesion molecule-1 is a regulator of ovarian cancer peritoneal metastasis. Cancer Res. 2009;69:1469–76. doi: 10.1158/0008-5472.CAN-08-2678. [DOI] [PubMed] [Google Scholar]
- 10.Scalici JM, Thomas S, Harrer C, Raines TA, Curran J, Atkins KA, et al. Imaging VCAM-1 as an indicator of treatment efficacy in metastatic ovarian cancer. J Nucl Med. 2013;54:1883–9. doi: 10.2967/jnumed.112.117796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yurkovetsky Z, Skates S, Lomakin A, Nolen B, Pulsipher T, Modugno F, et al. Development of a multimarker assay for early detection of ovarian cancer. J Clin Oncol. 2010;28:2159–66. doi: 10.1200/JCO.2008.19.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding YB, Chen GY, Xia JG, Zang XW, Yang HY, Yang L. Association of VCAM-1 overexpression with oncogenesis, tumor angiogenesis and metastasis of gastric carcinoma. World J Gastroenterol. 2003;9:1409–14. doi: 10.3748/wjg.v9.i7.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–57. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 14.Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. New Engl J Med. 2010;363:943–53. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 15.Velikova G, Banks RE, Gearing A, Hemingway I, Forbes MA, Preston SR, et al. Circulating soluble adhesion molecules E-cadherin, E-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) in patients with gastric cancer. Br J Cancer. 1997;76:1398–404. doi: 10.1038/bjc.1997.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okugawa Y, Miki C, Toiyama Y, Koike Y, Yokoe T, Saigusa S, et al. Soluble VCAM-1 and its relation to disease progression in colorectal carcinoma. Exp Ther Med. 2010;1:463–9. [Google Scholar]
- 17.Dymicka-Piekarska V, Guzinska-Ustymowicz K, Kuklinski A, Kemona H. Prognostic significance of adhesion molecules (sICAM-1, sVCAM-1) and VEGF in colorectal cancer patients. Thromb Res. 2012;129:e47–50. doi: 10.1016/j.thromres.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Yamada Y, Arao T, Matsumoto K, Gupta V, Tan W, Fedynyshyn J, et al. Plasma concentrations of VCAM-1 and PAI-1: a predictive biomarker for post-operative recurrence in colorectal cancer. Cancer Sci. 2010;101:1886–90. doi: 10.1111/j.1349-7006.2010.01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franzke A, Probst-Kepper M, Buer J, Duensing S, Hoffmann R, Wittke F, et al. Elevated pretreatment serum levels of soluble vascular cell adhesion molecule 1 and lactate dehydrogenase as predictors of survival in cutaneous metastatic malignant melanoma. Br J Cancer. 1998;78:40–5. doi: 10.1038/bjc.1998.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christiansen I, Sundstrom C, Enblad G, Totterman TH. Soluble vascular cell adhesion molecule-1 (sVCAM-1) is an independent prognostic marker in Hodgkin’s disease. Br J Haematol. 1998;102:701–9. doi: 10.1046/j.1365-2141.1998.00840.x. [DOI] [PubMed] [Google Scholar]
- 21.Christiansen I, Sundstrom C, Kalkner KM, Bring J, Totterman TH. Serum levels of soluble vascular cell adhesion molecule-1 (sVCAM-1) are elevated in advanced stages of non-Hodgkin’s lymphomas. Eur J Haematol. 1999;62:202–9. doi: 10.1111/j.1600-0609.1999.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 22.Christiansen I, Sundstrom C, Totterman TH. Elevated serum levels of soluble vascular cell adhesion molecule-1 (sVCAM-1) closely reflect tumour burden in chronic B-lymphocytic leukaemia. Br J Haematol. 1998;103:1129–37. doi: 10.1046/j.1365-2141.1998.01110.x. [DOI] [PubMed] [Google Scholar]
- 23.Shah N, Cabanillas F, McIntyre B, Feng L, McLaughlin P, Rodriguez MA, et al. Prognostic value of serum CD44, intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 levels in patients with indolent non-Hodgkin lymphomas. Leukemia & Lymphoma. 2012;53:50–6. doi: 10.3109/10428194.2011.616611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Hanlon DM, Fitzsimons H, Lynch J, Tormey S, Malone C, Given HF. Soluble adhesion molecules (E-selectin, ICAM-1 and VCAM-1) in breast carcinoma. Eur J Cancer. 2002;38:2252–7. doi: 10.1016/s0959-8049(02)00218-6. [DOI] [PubMed] [Google Scholar]
- 25.Shioi K, Komiya A, Hattori K, Huang Y, Sano F, Murakami T, et al. Vascular cell adhesion molecule 1 predicts cancer-free survival in clear cell renal carcinoma patients. Clin Cancer Res. 2006;12:7339–46. doi: 10.1158/1078-0432.CCR-06-1737. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Zhang J, Li H, Lu Z, Shan W, Mercado-Uribe I, et al. VCAM1 expression correlated with tumorigenesis and poor prognosis in high grade serous ovarian cancer. Am J Transl Res. 2013;5:336–46. [PMC free article] [PubMed] [Google Scholar]
- 27.Jakimovska M, Cerne K, Verdenik I, Kobal B. Circulating serum sVCAM-1 concentration in advanced ovarian cancer patients: correlation with concentration in ascites. Radiol Oncol. 2014;48:307–13. doi: 10.2478/raon-2013-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tas F, Karabulut S, Serilmez M, Ciftci R, Duranyildiz D. Clinical significance of serum epithelial cell adhesion molecule (EPCAM) and vascular cell adhesion molecule-1 (VCAM-1) levels in patients with epithelial ovarian cancer. Tumour Biol. 2014;35:3095–102. doi: 10.1007/s13277-013-1401-z. [DOI] [PubMed] [Google Scholar]
- 29.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–8. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 30.Kim G, Ison G, McKee AE, Zhang H, Tang S, Gwise T, et al. FDA Approval Summary: Olaparib Monotherapy in Patients with Deleterious Germline BRCA-Mutated Advanced Ovarian Cancer Treated with Three or More Lines of Chemotherapy. Clin Cancer Res. 2015;21:4257–61. doi: 10.1158/1078-0432.CCR-15-0887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure. Representative images of omentum specimens showing VCAM-1 negative (A) or positive (B) mesothelium (single cell layer indicated by arrows) staining. Adi – adipocytes.


