Abstract
Benefiting from strong coordination ability and unique vascular structure, EDTA modified L. cylindrica opens up an alternative way for uranium recovery from seawater. However, limitations, such as poor adsorption capacity and slow adsorption rate due to low graft ratio of EDTA via one-step esterification block its practical application. Here, a strategy for increasing the graft ratio is proposed in order to improve the adsorption performance. The strategy initially involves immobilization of epichlorohydrin (EPI) onto L. cylindrica and then ethylenediamine (EDA) is introduced via facile ring-opening reaction. EPI and EDA serve as a bridge between L. cylindrica and EDTA. The graft ratio is promoted (15.01 to 21.44%) contributing to the smaller steric hindrance of EPI and EDA than EDTA and improvement in adsorption performance. In addition, the adsorbent prepared by the new strategy exhibits excellent adsorption properties in simulated seawater.
Recovery of uranium from seawater has attracted great interest as a hot research topic recently, because of the heavy demand for uranium used in nuclear reactors1,2,3,4. In seawater, the total amount of uranium is about 4.5 billion tons, one thousand times more than the amount found in mineral ores on land5,6. However, its concentration is extremely low (~3.3 μg/L) while other metal ions in seawater have relatively high concentrations7,8. Therefore, the development of technologies and materials for realizing the selective extraction of uranyl ion from seawater is necessary and urgent. Many methods have been tried, including adsorption9,10,11, ion exchange12,13,14, chemical reduction15,16, biological processes17,18 and membrane processes19,20,21. Of these methods, adsorption is often preferred for its easy operation and lower cos; various kinds of organic adsorbents (such as such as amidoxime22,23,24, amine25,26,27, carboxylates28,29,30 functionalized adsorbents31) and inorganic adsorbents (such as mesoporous Mg(OH)232,33,34, zero-valent iron35,36 and other functionalized inorganic adsorbents37,38,39,40,41,42) have been developed. However, limitations such as aggregation, complex post-treatment and nonbiodegradability precluded their practical applications in seawater.
Modified L. cylindrica has been used as an efficient adsorbent for the removal of heavy metal from aqueous solutions43,44,45. L. cylindrica is a natural porous fibrous vascular system. The fibers are disposed in a multidirectional array forming a mat-like structure. Because of its unique structure, L. cylindrica can deal with seawater efficiently and it is easy to implement. Moreover, due to abundant hydrophilic hydroxyl groups on the surface of fibers, selective functional groups are readily introduced to enhance the affinity and adsorption capacity for uranium46. However, to the best of our knowledge, the use of L. cylindrica for the recovery of uranium from seawater has not been investigated.
EDTA is a chelating agent which is widely used to sequester metal ions owing to its role as a hexadentate ligand (two amines and four carboxylate groups)47. It is well known that EDTA could form stable (1:1) complexes with rare earth ions in aqueous solution48. Inspired by the above, a facile route was developed for introducing EDTA onto the surface of L. cylindrica via esterification as in previous works49,50,51. However, the products prepared via the one-step method have a low graft ratio and poor adsorption. We reasoned that a high steric hindrance of EDTA caused a low graft ratio to be unfavorable for adsorption. To resolve this problem, a new strategy was proposed in which EPI and EDA were introduced to minimize space steric hindrance and improve graft ratio, which served as a bridge between L. cylindrica and EDTA. The effect of graft ratio of EDTA on the adsorption of U(VI) was discussed by comparing two routes. Adsorption kinetics and various isotherm models were evaluated to better understand the adsorption process. Finally, the adsorbent was employed to adsorb of U (VI) from simulated seawater so as to explore its potential application under real seawater.
Result and Discussion
Characterizations
The synthetic routes are illustrated in Fig. 1. In route1, ELC1 was prepared by reaction of EDTA dianhydride with LC directly. In the second route, EDTA was introduced onto L. cylindrica fibers by a series of chemical modifications, as listed below: (1) introduction of epoxy groups via reaction with L. cylindrica and EPI; (2) aminolyzation via a ring-opening reaction between the epoxy group and EDA; (3) immobilization of EDTA via facial acid-base reaction. EPI and EDA served as a bridge between L. cylindrica and EDTA.
Figure 1. Steps involved in the preparation of EDTA modified LC by two routes.
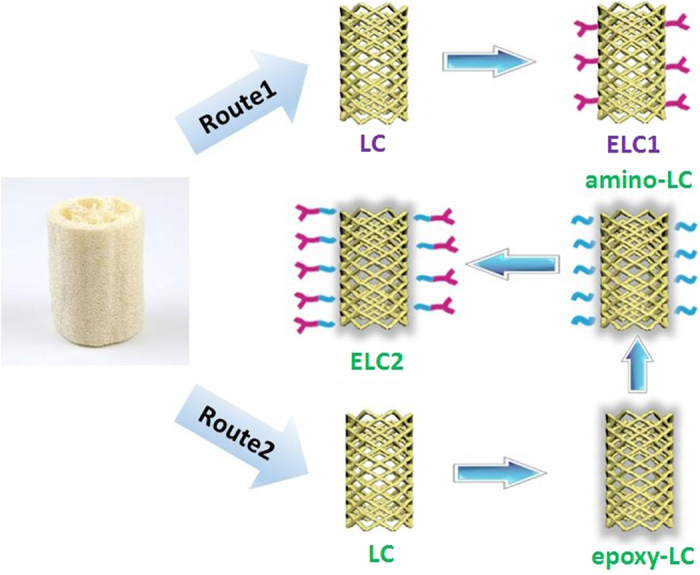
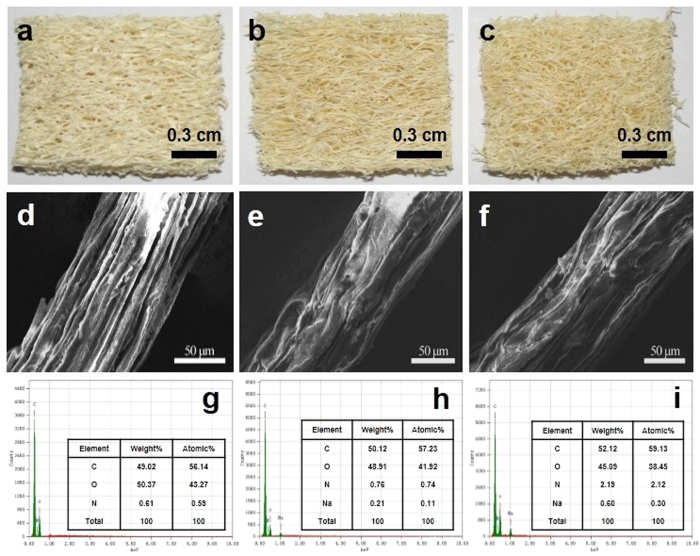
Figure 2(a) and (c) shows the digital photos of LC, ELC1 and ELC2. The fibers are disposed in a multidirectional array forming a natural mat-like structure. It is obviously see that the color of fibers becomes darker after modification. The SEM images of LC, ELC1 and ELC2 are shown in (d), (e) and (f). The surface morphology of LC appears smooth and neat. However, after grafting onto the matrix, the surface morphology of ELC1 and ELC2 turned out to be rough. As shown in EDX spectra of LC (g), ELC1 (h) and ELC2 (i), the atomic% of O decreases from 43.27 to 41.92 and 38.45 and N increases from 0.59 to 0.74 and 2.21 during route1 and route2.
Figure 2.
Digital photos of LC (a), ELC1 (b) and ELC2 (c); SEM of LC (d), ELC1 (e) and ELC2 (f); EDX spectra of LC (g), ELC1 (h) and ELC2 (i).
As shown in Fig. S1, the FTIR spectra of LC (a), ELC1 (b) and ELC2 (c). Intense bands around 3400 and 1060 cm−1 are indicative of the existing hydroxyl groups in LC. In ELC1 the shift of the characteristic peak from 1632 to1643 cm−1 indicates the substitution of −COO−Na+ group on starch molecular chain during esterification of LC with EDTA52. The increasing intensity at 1740 cm−1 and the appearance of new peaks at 1261 cm−1 in ELC2 are attributed to the stretching vibration of C=O and C-N of -NHCO- derived from the reaction of amino and EDTA dianhydride.
The XRD pattern of LC, ELC1 and ELC2 were shown in Fig. S2. The characteristics peaks of LC were observed at 20.10° and 14.50° with relative intensities at 1341 and 808, respectively. The peaks of ELC1 and ELC2 were found at 20.12°, 14.48° and 20.24°, 14.46° with relative intensities of 933, 633 and 875, 625, respectively. The decrease of peak intensity indicates grafting to the backbone impairing the crystallinity of the fiber which might reduce hardness and increase flexibility.
Elemental analysis was used to further verify the preparation of ELC1 and ELC2 and calculated the graft ratios. Table 1 shows the results of LC, ELC1, epoxy-LC, amino-LC, and ELC2. The obvious increase of nitrogen content in ELC1 confirms the successful introduction of EDTA dianhydride onto LC; during the process of grafting EPI, the nitrogen content decreases and then increases for the reaction of epoxy rings with EDA. After introducing EDTA dianhydride, the nitrogen content continues to increase from 0.93 to 1.75%. The changes of nitrogen content indicate successful preparation of ELC2. By calculation the graft ratio of ELC1 and ELC2 are 15.01 and 21.44%, respectively. The results show that the new synthetic route effectively improves the graft ratio. The effect of graft ratio on adsorption of U(VI) is discussed latter.
Table 1. Elemental analysis results of raw-LC, LC, ELC1, epoxy-LC, amino-LC, and ELC2.
| Compound | Elemental content % | ||
|---|---|---|---|
| C | N | H | |
| LC | 43.81 | 0.23 | 6.14 |
| ELC1 | 42.43 | 0.66 | 6.03 |
| epoxy-LC | 44.13 | 0.19 | 6.12 |
| amino-LC | 43.97 | 0.93 | 6.33 |
| ELC2 | 42.24 | 1.75 | 6.00 |
Adsorption analysis
The effect of pH on the adsorption capacity of U(VI) by ELC1 and ELC2 was evaluated in a pH range of 2.0–8.0. As shown in Fig. S3, it is clear that the adsorption capacity of both ELC1 and ELC2 toward U(VI) strongly depends on solution pH. The pHzpc (pH zero point charge, Fig. S4) of ELC1 and ELC2 is found to be 2.0 and 2.1. When pH > 2.1 the surface charge of ELC1 and ELC2 is negative, which is prone to adsorb positively charged metal ions on their surfaces. However, at low pH abundant of hydronium ion (H3O+) competed with U(VI) for binding on the functional groups (binding sites). With the increase of pH, the adsorption capacity increases and reaches maximum at pH 6.0. When pH values are greater than 6.0, the prominent U(VI) species are multi-nuclear hydroxide and carbonate complexes such as (UO2)2(OH)22+, (UO2)3(OH)5+ and (UO2)(CO3)34−, leading to the decreased adsorption of U(VI)53,54. Therefore, an optimum pH value for effective adsorption was chosen as 6.0 for further studies.
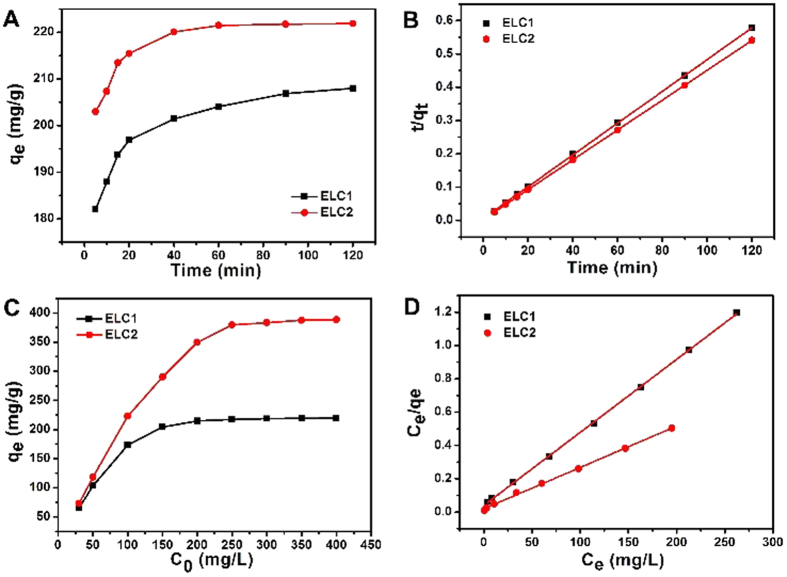
The effect of contact time was investigated in a kinetics study of the adsorption process. Figure 3A shows the time profile of U(VI) adsorption onto ELC1 and ELC2 in terms of adsorption capacity. It is observed that adsorption reaches an equilibrium in 60 min and 40 min (shorter than 120 min for LC) with an adsorption efficiency of 73.2% and 88.7% for ELC1 and ELC2, respectively. The results indicate that the new synthesis route not only increases the adsorption rate but also the adsorption efficiency.
Figure 3.
Effect of contact time of ELC1 and ELC2 (A) and their corresponding pseudo second-order kinetics (B); effect of initial concentration of ELC1 and ELC2 (C) and their corresponding Langmuir adsorption isotherms (D).
The following pseudo-first-order55 (Fig. S5) and pseudo-second-order56 (Fig. 3B) models are employed to further interpret the kinetic data:
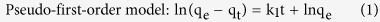 |
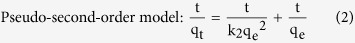 |
where qe and qt (mg/g) are the adsorption capacities at equilibrium and at time t (min), respectively; k1 and k2 are the rate constant of the pseudo-first-order and pseudo-second-order model.
The corresponding kinetic parameters from both models are listed in Table S1. Higher correlation coefficient (R2 > 0.99 for both) as well as value of qe,cal (209.6 and 223.2 mg/g) of ELC1 and ELC2, approximating to qe,exp (208.0 and 221.9 mg/g), indicate that a pseudo-second-order model describes the adsorption process better. This model is based on the assumption that the rate-limiting step of the reaction is due to chemical adsorption. Thus, more grafting EDTA is favorable for adsorption.
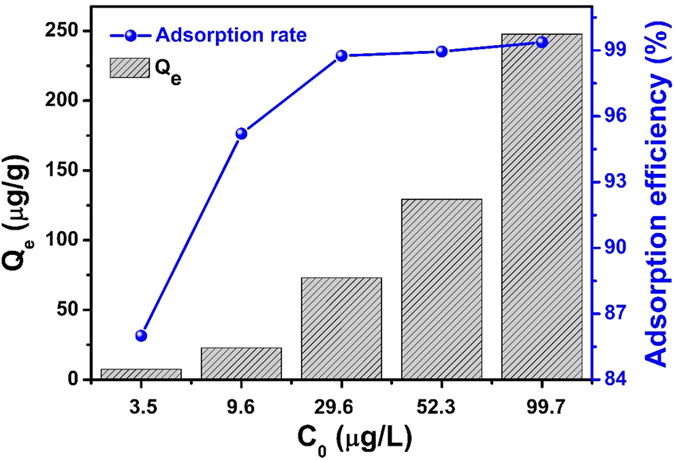
Figure 3C shows the equilibrium adsorption capacity of U(VI) onto ELC1 and ELC2 as a function of different initial uranium concentrations. The adsorption capacity increases rapidly with the initial concentration increasing until equilibrium is reached. The equilibrium adsorption capacities of ELC1 and ELC2 for U(VI) on are 219.25 and 388.25 mg/g.
The equilibrium adsorption isotherm is fundamental to describe the interactive behavior between the solution and adsorbent. The adsorption equilibrium data of U (VI) onto adsorbent is analyzed in terms of Langmuir and Freundlich models in this paper. The Langmuir isotherm assumes monolayer coverage on the surface of the adsorbent and no subsequent interaction among adsorbed molecules57. The linear Langmuir isotherm equation is given as:
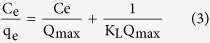 |
where Qmax (mg/g) is the maximum absorption capacity; KL is Langmuir constant. The values of Qmax and KL are calculated from the slope and intercept respectively from the linear plot of Ce/qe versus Ce (Fig. 3D).
Freundlich isotherm is an empirical equation which is used to describe adsorption at the multilayer and taking place on heterogeneous surfaces58. The equation of Freundlich isotherm is as follows:
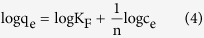 |
where qe (mg/g) and Ce (mg/L) are the adsorption capacity and the equilibrium concentration of U(VI), respectively; KF and n are Freundlich constants. The experimental data are plotted as log qe versus log Ce (Fig. S6).
The model parameters obtained by Langmuir and Freundlich models are given in Table S2. The present adsorption process is likely dominated by a monolayer adsorption because the Langmuir isotherm fits a higher correlation coefficient for ELC1 and ELC2. This, as well as the calculated adsorption capacity (Qmax, 228.3 and 416.7 mg/g), exhibit good agreement with the experimental values. As shown in Table S2, the grafting of EDTA effectively increases the adsorption capacity of LC; U(VI) coordinates with EDTA sodium to form a stable composite59. By comparison with ELC1, ELC2 had a bridge between LC and EDTA. But from the calculation of the ratio of Qmax,L and graft ratio compared with ELC1, adsorption of ELC2 is also mainly dependent on EDTA. The result indicates that the increase of the adsorption capacity is due to the increase of the graft ratio of EDTA.
Furthermore, the essential characteristics of the Langmuir isotherm are described by a separation factor, RL60, which is defined as follows:
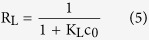 |
where KL is the Langmuir equilibrium constant and C0 is the initial concentration of metal ion. The values of RL give an indication for the possibility of the adsorption process to proceed: RL > 1.0, unfavorable; RL = 1, linear; 0 < RL < 1, suitable; RL = 0, irreversible61. The values of RL lie between 0.1022 and 0.0084, indicating the suitability of ELC2 as adsorbent for U(VI) from aqueous solution.
To determine the effect of temperature on U(VI) adsorption, adsorption experiments were conducted at 298, 308 and 318 K. The equilibrium adsorption capacity of ELC1 and ELC2 increases with the increase of temperature (from 219.2 and 388.2 to 254.5 and 458.7 mg/g), indicating that the adsorption of U(VI) onto EDTA modified LC is an endothermic process.
The thermodynamic parameters, enthalpy (ΔH°) and entropy (ΔS°), associated with the adsorption process were calculated from the Van’t Hoff equation:
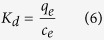 |
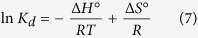 |
where ΔH° (kJ mol−1) and ΔS° (J mol−1 K−1) are enthalpy and entropy, respectively; R (8.314 J mol−1 K−1) is the universal gas constant; T is the absolute temperature (K); qe (mg g−1) and Ce (mg L−1) are the adsorption capacity and the concentration of U(VI) at equilibrium, respectively. The plot of ln Kd as a function of 1/T yields a straight line from which ΔH° and ΔS° are calculated from the slope and intercept, respectively. The thermodynamic parameters calculated are tabulated in Table S3.
The positive value of ΔH° shows the endothermic nature of adsorption process, coinciding with the practical result. A higher temperature favors adsorption. The Gibbs free energy of specific adsorption (ΔG°) was calculated using the equation:
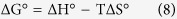 |
The negative values of ΔG° indicate that the adsorption reaction of EDTA modified LC is spontaneous.
According to the previous result, ELC1 and ELC2 have poor U(VI) adsorption ability under acid conditions. HNO3 (0.1–1.0 mol/L) was used to evaluate the reutilization performance of ELC1 and ELC2. The results obtained are given in Table S4. The elution efficiency is above 95% when the acid concentration is greater than 0.6 mol/L, suggesting that HNO3 is an effective eluent for the recovery of the adsorbent. Therefore, reusability experiments were subsequently performed using 0.6 mol/L HNO3 (Fig. 4). It is observed that regenerated ELC2 still had high adsorption capacity even after 5 cycles of adsorption/desorption, indicating that ELC2 is a stable and recyclable adsorbent. The decrease in adsorption capacity of ELC1 is probably due to hydrolysis of the ester bond between EDTA and LC fiber as well as ELC1 disintegration at high acidity.
Figure 4. Regeneration studies of ELC1 and ELC2.
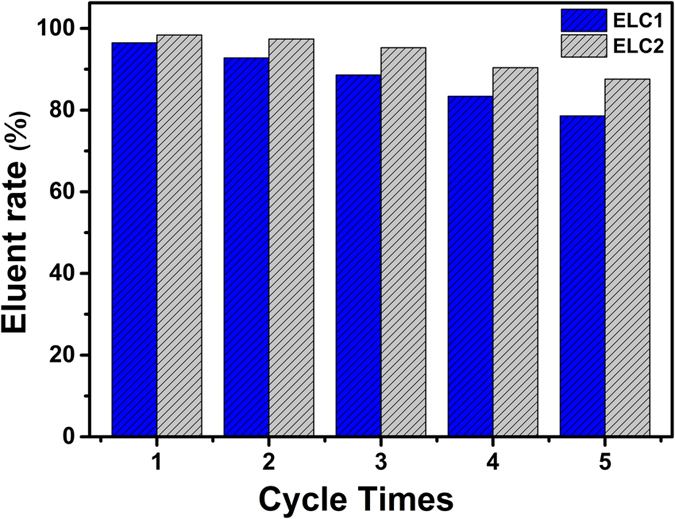
According to the above, ELC2 has better adsorption properties than ELC1, including shorter equilibrium time, higher adsorption capacity and better reutilization performance. The results show that the new synthetic route of grafting EDTA onto LC fiber successfully improves not only the ratio of grafting, but also the adsorption property.
Uranium adsorption tests in simulated seawater
To further investigate whether ELC2 is suitable for the extraction of uranium from seawater, we examined the applicability of adsorbent in the extraction of low concentration U(VI) from aqueous (U(VI) only) and simulated seawater (U(VI) and other metals ions with similar or higher concentrations). As shown in Table S5, ELC2 shows excellent adsorptive capacity from aqueous, even at extremely low concentration of 3 μg/L (residual concentration <0.1 μg/L). In simulated seawater, U(VI) is still effectively adsorbed onto ELC2 and the adsorption rate is above 85% (Fig. 5). The results show that ELC2 can be able to extract U(VI) in the presence of other metals ions. It is interesting to note that residual U(VI) in simulated seawater is almost the same, at an initial concentration of 100 μg/L as well as 3 μg/L (Table S6). The possible reason for this is that the adsorption of U(VI) on ELC2 in solution reaches an equilibrium from which little further progress is made.
Figure 5. The adsorption rate of U (VI) by ELC2 in simulated seawater.
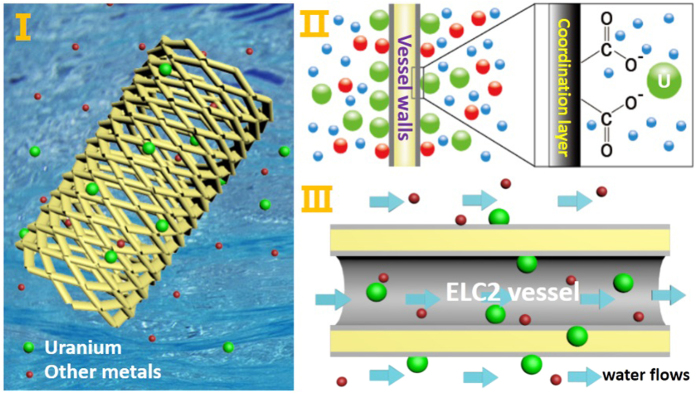
According to previous studies, it is reasonable to attribute such high adsorption ability of ELC2 for U(VI) to two factors (Fig. 6). On the one hand, the new approach of grafting EDTA onto LC generates a greater graft ratio and EDTA exhibits strong coordination to U(VI) (Fig. 6II). On the other hand, L. cylindrica has a highly porous fibrous vascular system in which the fibers are disposed in a multidirectional array forming a natural mat-like structure (Fig. S7 and Table S7). The unique hydrophilic structure makes seawater quickly flow from the vascular and promoted the coordination to U(VI) (Fig. 6III).
Figure 6.
U(VI) adsorption on ELC2 in simulated seawater (I); U(VI) adsorb on the coordination layer of vessel walls (II); water flows from vessel (III).
Conclusions
In summary, we demonstrate a better strategy for introducing EDTA onto L. cylindrica than one-step esterification. The new route in this paper effectively improved not only the graft ratio (5.48 to 10.46%), but also adsorption properties, such as shorter equilibrium time (60 min to 40 min), higher adsorption capacity (219.25 to 388.25 mg/g) and better reutilization performance (fifth cycle 78.6% to 87.6%). Moreover, combining the merits of a unique porous fibrous vascular structure of L. cylindrica, the adsorbent, as prepared by this new synthetic route, makes efficient contact with a low concentration of U(VI) in simulated seawater. We conclude that EDTA modified L. cylindrica will be potentially advantageous in the extraction of uranium from seawater.
Methods
Materials
L. cylindrica was purchased from Handan, China. Ethylenediamine tetraacetic acid (EDTA) was purchased from Xiya (China). Other organic reagents, including EPI and EDA were purchased from Fuyu (China). NaOH, HCl and NaHCO3 were supplied by Zhiyuan (China). All reagents were of AR grade and were used without further purification.
Preparation of adsorbent
Pretreatment of L. cylindrica
L. cylindrica fibers were obtained from a dried fruit and the outer mat was utilized in this study. Firstly, L. cylindrica was immersed in fresh water at room temperature for about 4 days to remove dirt and soluble impurities. Then it was treated with 10% sodium hydroxide for 60 min to increase its hydrophilicity. The alkali treated fibers were washed thoroughly with distilled water until the pH was close to neutral. The fibers were finally dried in an oven at 328 K for 12 h and denoted LC.
Preparation of EDTA-LC
Route 1
EDTA was first made into the form of acid anhydride following the methodology described by Capretta et al.62. Then the prepared EDTA dianhydride (1.0 g) and LC (1.0 g) were added to N,N-dimethylformamide (DMF) (80 mL) in a flask. After the mixture was stirred at 338 K for 4 h, the product was washed with DMF, saturated sodium bicarbonate solution and deionized water, successively. The EDTA modified LC fibers thus obtained were dried at 328 K for 12 h in an oven and denoted as ELC1.
Route 2
Preparation of epoxy-LC
LC (1.0 g) was immersed in NaOH solution (50 ml, 5%), EPI (15 ml) and alcohol (15 ml) to introduce epoxy groups onto the on the surface of fibers. The mixture was left under stirring at 328 K for 5 h. The treated fibers were washed with distilled water to a neutral pH and then dried in an oven at 328 K63,64.
Preparation of amino-LC
The amino groups were anchored onto the fiber by aminolyzation via a ring-opening reaction between the epoxy group and EDA. Briefly, epoxy-LC (1 g), EDA (5 ml) and Na2CO3 solution (50 ml, 1%) were added to a round-bottom flask and stirred at 328 K for 2 h. Then the product was filtered, rinsed thoroughly with water and dried at 328 K65,66.
Preparation of EDTA-LC
After EDA modification, amino-LC (1 g) and EDTA dianhydride (1.0 g) were added to DMF (80 mL) (the same amount of reactants as Route 1). The mixture was stirred at 338 K for 2 h. The product was filtered and washed with DMF, saturated sodium bicarbonate solution and deionized water, successively. The final EDTA modified LC was dried in an oven for 12 h at 328 K and denoted as ELC2.
Batch Equilibrium U(VI) Adsorption Experiments
Individual stock solution of 1000 mg/L U(VI) was prepared by dissolving UO2(NO3)2·6H2O in deionized water. Adsorption experiments were performed in a 50 mL U(VI) solution with initial concentrations of 30, 50, 100, 200, 250, 300, 350, or 400 mg/L, 20 mg adsorbent and the solution pH adjusted to 6 with HNO3 or NaOH. The effect of solution pH was evaluated in the case of 100 mg/L U(VI), 20 mg adsorbent and where the contact time was 120 min. For kinetics studies, the solution was shaken at 200 rpm for 5, 10, 15, 20, 40, 60, 90 or 120 min. After adsorption, the adsorbent was collected and regenerated by using 50 mL HNO3. An enhanced simulated seawater test was performed following our previous work23. ELC2 was added into 50 mL simulated seawater containing U(VI) with initial concentrations of 3, 10, 30, 50 or 100 μg/L, after shaking at 200 rpm for 120 min, the residual U(VI) ions in solution were analysed by ICP-MS.
Characterization methods
Qualitative chemical structure assessment was done by FT-IR analysis (PerkinElmer Spectrum 100) in a range of 4000–500 cm−1. The element content was measured by element analyzer (Vario Macro). Inductively coupled plasma-atomic emission spectroscope (ICP-AES, Optima-7000DV) was used to analyze the initial and equilibrium concentration of U(VI). The concentration of trace U(VI) was analyzed using inductively coupled plasma mass spectrometry (ICP-MS, Bruker 820-MS).
Additional Information
How to cite this article: Su, S. et al. Enhancing adsorption of U(VI) onto EDTA modified L. cylindrica using epichlorohydrin and ethylenediamine as a bridge. Sci. Rep. 7, 44156; doi: 10.1038/srep44156 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China (NSFC 51402065), Fundamental Research Funds of the Central University (HEUCFZ), Natural Science Foundation of Heilongjiang Province (B2015021), International Science & Technology Cooperation Program of China (2015DFR50050) and the Magor Project of Science and Technology of Heilongjiang Province (GA14A101).
Footnotes
The authors declare no competing financial interests.
Author Contributions S.Z.S. and J.W. contributed equally to this work. They conceived the idea, designed the experiments and wrote the paper. Q.L., J.Y.L. and H.S.Z. performed the material preparation, characterization, and adsorption tests. R.M.L. and X.Y.J. analyzed and interpreted the data. All authors discussed the results and commented on the manuscript.
References
- Sun X. Q., Xu C., Tian G. X. & Rao L. F. Complexation of glutarimidedioxime with Fe(III), Cu(II), Pb(II), and Ni(II), the competing ions for the sequestration of U(VI) from seawater. Dalton T 42, 14621–14627 (2013). [DOI] [PubMed] [Google Scholar]
- Lu C. H. et al. Boron doped g-C3N4 with enhanced photocatalytic UO22+ reduction performance. Appl Surf Sci 360, 1016–1022 (2016). [Google Scholar]
- Zhang X. R., Yuan L. Y., Chai Z. F. & Shi W. Q. A new solvent system containing N,N ′-diethyl-N,N ′-ditolyl-2,9-diamide-1,10-phenanthroline in 1-(trifluoromethyl)-3-nitrobenzene for highly selective UO22+ extraction. Sep Purif Technol 168, 232–237 (2016). [Google Scholar]
- Ashry A., Bailey E. H., Chenery S. R. N. & Young S. D. Kinetic study of time-dependent fixation of U-VI on biochar. J Hazard Mater 320, 55–66 (2016). [DOI] [PubMed] [Google Scholar]
- Davies R. V., Kennedy J., McIlroy R. W., Spence R. & Hill K. M. Extraction of uranium from sea water. Nature 203, 1110–1115 (1964). [Google Scholar]
- Saito K. et al. Recovery of uranium from seawater using amidoxime hollow fibers. AIChE J 34, 411–416 (1988). [Google Scholar]
- Kobuke Y. et al. Recovery of uranium from seawater by composite fiber adsorbent. Ind Eng Chem Res 29, 1662–1668 (1990). [Google Scholar]
- Takeda T. et al. Adsorption and elution in hollow-fiber-packed bed for recovery of uranium from seawater. Ind Eng Chem Res 30, 185–190 (1991). [Google Scholar]
- Manos M. J. & Kanatzidis M. G. Layered Metal Sulfides Capture Uranium from Seawater. J Am Chem Soc 134, 16441–16446 (2012). [DOI] [PubMed] [Google Scholar]
- Carboni M., Abney C. W., Liu S. B. & Lin W. B. Highly porous and stable metal-organic frameworks for uranium extraction. Chem Sci 4, 2396–2402 (2013). [Google Scholar]
- Zhou L. et al. A protein engineered to bind uranyl selectively and with femtomolar affinity. Nat Chem 6, 236–241 (2014). [DOI] [PubMed] [Google Scholar]
- Zhu X. P. & Alexandratos S. D. Development of a new ion-exchange/coordinating phosphate ligand for the sorption of U(VI) and trivalent ions from phosphoric acid solutions. Chem Eng Sci 127, 126–132 (2015). [Google Scholar]
- Shokobayev N. M., Bouffier C. & Dauletbakov T. S. Rare earth metals sorption recovery from uranium in situ leaching process solutions. Rare Metals 34, 195–201 (2015). [Google Scholar]
- Gu B. H., Ku Y. K. & Jardine P. M. Sorption and binary exchange of nitrate, sulfate, and uranium on an anion-exchange resin. Environ Sci Technol 38, 3184–3188 (2004). [DOI] [PubMed] [Google Scholar]
- Eglizaud N., Miserque F., Simoni E., Schlegel M. & Descostes M. Uranium(VI) interaction with pyrite (FeS2): Chemical and spectroscopic studies. Radiochim Acta 94, 651–656 (2006). [Google Scholar]
- Hua B. & Deng B. L. Reductive Immobilization of Uranium(VI) by Amorphous Iron Sulfide. Environ Sci Technol 42, 8703–8708 (2008). [DOI] [PubMed] [Google Scholar]
- Hua B. & Deng B. L. Comment on “Reductive Immobilization of Uranium(VI) by Amorphous Iron Sulfide” Response. Environ Sci Technol 43, 1237–1238 (2009). [Google Scholar]
- Finneran K. T., Anderson R. T., Nevin K. P. & Lovley D. R. Potential for Bioremediation of uranium-contaminated aquifers with microbial U(VI) reduction. Soil Sediment Contam 11, 339–357 (2002). [Google Scholar]
- Villalobos-Rodriguez R. et al. Iron influence on uranium removal from water using cellulose acetate membranes doped with activated carbon. Desalin Water Treat 56, 3476–3485 (2015). [Google Scholar]
- Gao M. W., Zhu G. R., Wang X. H., Wang P. & Gao C. J. Preparation of short channels SBA-15-PVC membrane and its adsorption properties for removal of uranium(VI). J Radioanal Nucl Ch 304, 675–682 (2015). [Google Scholar]
- Kryvoruchko A. P., Yu L., Atamanenko I. D. & Kornilovich B. Y. Ultrafiltration removal of U(VI) from contaminated water. Desalination 162, 229–236 (2004). [Google Scholar]
- Yue Y. F. et al. Seawater Uranium Sorbents: Preparation from a Mesoporous Copolymer Initiator by Atom-Transfer Radical Polymerization. Angew Chem Int Edit 52, 13458–13462 (2013). [DOI] [PubMed] [Google Scholar]
- Wang F. H. et al. A graphene oxide/amidoxime hydrogel for enhanced uranium capture. Sci Rep-Uk 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T. et al. Uranium recovery from seawater: development of fiber adsorbents prepared via atom-transfer radical polymerization. J Mater Chem A 2, 14674–14681 (2014). [Google Scholar]
- Sun X. Q., Zanonato P. L., Di Bernardo P., Zhang Z. C. & Rao L. F. Sorption of Uranium and other Metal Ions on Amine-Functionalized Silica Materials. Sep Sci Technol 50, 2769–2775 (2015). [Google Scholar]
- Yousif A. M., El-Afandy A. H., Wahab G. M. A., Mubark A. E. & Ibrahim A. Selective separation of uranium(VI) from aqueous solutions using amine functionalized cellulose. J Radioanal Nucl Ch 303, 1821–1833 (2015). [Google Scholar]
- Peng G. W. et al. Adsorption of uranium ions from aqueous solution by amine-group functionalized magnetic Fe3O4 nanoparticle. J Radioanal Nucl Ch 301, 781–788 (2014). [Google Scholar]
- Xie Y., Helvenston E. M., Shilller-Nickles L. C. & Powell B. A. Surface Complexation Modeling of Eu(III) and U(VI) Interactions with Graphene Oxide. Environ Sci Technol 50, 1821–1827 (2016). [DOI] [PubMed] [Google Scholar]
- Vivero-Escoto J. L., Carboni M., Abney C. W., deKrafft K. E. & Lin W. B. Organo-functionalized mesoporous silicas for efficient uranium extraction. Micropor Mesopor Mat 180, 22–31 (2013). [Google Scholar]
- Duan G. J., Liu T. H., Wu W. S. & Yang Y. Adsorption of UO22+ from aqueous solution onto copolymers of styrene and maleic anhydride. J Radioanal Nucl Ch 295, 2193–2201 (2013). [Google Scholar]
- Sun X., Huang X., Liao X. P. & Shi B. Adsorptive recovery of UO22+ from aqueous solutions using collagen-tannin resin. J Hazard Mater 179, 295–302 (2010). [DOI] [PubMed] [Google Scholar]
- Yan H. J. et al. High U(VI) adsorption capacity by mesoporous Mg(OH)(2) deriving from MgO hydrolysis. Rsc Adv 3, 23278–23289 (2013). [Google Scholar]
- Chen Z. et al. Adsorption-Induced Crystallization of U-Rich Nanocrystals on Nano-Mg(OH)(2) and the Aqueous Uranyl Enrichment. Acs Appl Mater Inter 6, 1301–1305 (2014). [DOI] [PubMed] [Google Scholar]
- Zhuang Z. Y. et al. Interfacial Engineering Improved the Selective Extraction of Uranyl from Saline Water by Nano-Mg(OH)(2) and the Underlying Mechanism. Acs Sustain Chem Eng 4, 801–809 (2016). [Google Scholar]
- Zhang Z. B. et al. Comparison of U(VI) adsorption onto nanoscale zero-valent iron and red soil in the presence of U(VI)-CO3/Ca-U(VI)-CO3 complexes. J Hazard Mater 300, 633–642 (2015). [DOI] [PubMed] [Google Scholar]
- Crane R. A., Pullin H. & Scott T. B. The influence of calcium, sodium and bicarbonate on the uptake of uranium onto nanoscale zero-valent iron particles. Chem Eng J 277, 252–259 (2015). [Google Scholar]
- Wu L. P., Lin X. Y., Zhou X. B. & Luo X. G. Removal of uranium and fluorine from wastewater by double-functional microsphere adsorbent of SA/CMC loaded with calcium and aluminum. Appl Surf Sci 384, 466–479 (2016). [Google Scholar]
- Zhu K. R., Lu S. H., Gao Y., Zhang R., Tan X. L. & Chen C. L. Fabrication of hierarchical core-shell polydopamine@MgAl-LDHs composites for the efficient enrichment of radionuclides. Appl Surf Sci 396, 1726–1735 (2017). [Google Scholar]
- Zhang R., Chen C. L., Li J. & Wang X. K. Preparation of montmorillonite@carbon composite and its application for U(VI) removal from aqueous solution. Appl Surf Sci 349, 129–137 (2015). [Google Scholar]
- Guimaraes V., Rodriguez-Castellon E., Algarra M., Rocha F. & Bobos I. Influence of pH, layer charge location and crystal thickness distribution on U(VI) sorption onto heterogeneous dioctahedral smectite. J Hazard Mater 317, 246–258 (2016). [DOI] [PubMed] [Google Scholar]
- Feng Y. F. et al. Metal-organic frameworks HKUST-1 for liquid-phase adsorption of uranium. Colloid Surface A 431, 87–92 (2013). [Google Scholar]
- Ma S. L. et al. Efficient Uranium Capture by Polysulfide/Layered Double Hydroxide Composites. J Am Chem Soc 137, 3670–3677 (2015). [DOI] [PubMed] [Google Scholar]
- Liu C. et al. Simple preparation and enhanced adsorption properties of loofah fiber adsorbent by ultraviolet radiation graft. Mater Lett 157, 303–306 (2015). [Google Scholar]
- Verma D. K., Hasan S. H., Ranjan D. & Banik R. M. Modified biomass of Phanerochaete chrysosporium immobilized on luffa sponge for biosorption of hexavalent chromium. Int J Environ Sci Te 11, 1927–1938 (2014). [Google Scholar]
- Gupta V. K., Agarwal S., Singh P. & Pathania D. Acrylic acid grafted cellulosic Luffa cylindrical fiber for the removal of dye and metal ions. Carbohyd Polym 98, 1214–1221 (2013). [DOI] [PubMed] [Google Scholar]
- Altinisik A., Gur E. & Seki Y. A natural sorbent, Luffa cylindrica for the removal of a model basic dye. J Hazard Mater 179, 658–664 (2010). [DOI] [PubMed] [Google Scholar]
- Repo E., Warchol J. K., Bhatnagar A., Mudhoo A. & Sillanpaa M. Aminopolycarboxylic acid functionalized adsorbents for heavy metals removal from water. Water Res 47, 4812–4832 (2013). [DOI] [PubMed] [Google Scholar]
- Zhao F. P. et al. An EDTA-beta-cyclodextrin material for the adsorption of rare earth elements and its application in preconcentration of rare earth elements in seawater. J Colloid Interf Sci 465, 215–224 (2016). [DOI] [PubMed] [Google Scholar]
- Zhao F. P. et al. EDTA-Cross-Linked beta-Cyclodextrin: An Environmentally Friendly Bifunctional Adsorbent for Simultaneous Adsorption of Metals and Cationic Dyes. Environ Sci Technol 49, 10570–10580 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang Y. S. et al. Synthesis of EDTAD-modified magnetic baker’s yeast biomass for Pb2+ and Cd2+ adsorption. Desalination 278, 42–49 (2011). [Google Scholar]
- Labidi A., Salaberria A. M., Fernandes S. C. M., Labidi J. & Abderrabba M. Adsorption of copper on chitin-based materials: Kinetic and thermodynamic studies. J Taiwan Inst Chem E 65, 140–148 (2016). [Google Scholar]
- Basri S. N., Zainuddin N., Hashim K. & Yusof N. A. Preparation and characterization of irradiated carboxymethyl sago starch-acid hydrogel and its application as metal scavenger in aqueous solution. Carbohyd Polym 138, 34–40 (2016). [DOI] [PubMed] [Google Scholar]
- Sureshkumar M. K., Das D., Mallia M. B. & Gupta P. C. Adsorption of uranium from aqueous solution using chitosan-tripolyphosphate (CTPP) beads. J Hazard Mater 184, 65–72 (2010). [DOI] [PubMed] [Google Scholar]
- Han R. P., Zou W. H., Wang Y. & Zhu L. Removal of uranium(VI) from aqueous solutions by manganese oxide coated zeolite: discussion of adsorption isotherms and pH effect. J Environ Radioactiv 93, 127–143 (2007). [DOI] [PubMed] [Google Scholar]
- Chang Y. H., Huang C. F., Hsu W. J. & Chang F. C. Removal of Hg2+ from aqueous solution using alginate gel containing chitosan. J Appl Polym Sci 104, 2896–2905 (2007). [Google Scholar]
- Atia A. A., Donia A. M. & Shahin A. E. Studies on the uptake behavior of a magnetic Co3O4-containing resin for Ni(II), Cu(II) and Hg(II) from their aqueous solutions. Sep Purif Technol 46, 208–213 (2005). [Google Scholar]
- Aksu Z. Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of nickel(II) ions onto Chlorella vulgaris. Process Biochem 38, 89–99 (2002). [Google Scholar]
- Li Y. H. et al. Adsorption thermodynamic, kinetic and desorption studies of Pb2+ on carbon nanotubes. Water Res 39, 605–609 (2005). [DOI] [PubMed] [Google Scholar]
- Pan N. et al. Preparation of graphene oxide-manganese dioxide for highly efficient adsorption and separation of Th(IV)/U(VI). J Hazard Mater 309, 107–115 (2016). [DOI] [PubMed] [Google Scholar]
- Nadeem R. et al. Physical and chemical modification of distillery sludge for Pb(II) biosorption. J Hazard Mater 150, 335–342 (2008). [DOI] [PubMed] [Google Scholar]
- Dahiya S., Tripathi R. M. & Hegde A. G. Biosorption of heavy metals and radionuclide from aqueous solutions by pre-treated arca shell biomass. J Hazard Mater 150, 376–386 (2008). [DOI] [PubMed] [Google Scholar]
- Capretta A., Maharajh R. B. & Bell R. A. Synthesis and characterization of cyclomaltoheptaose-based metal chelants as probes for intestinal permeability. Carbohydr Res 267, 49–63 (1995). [Google Scholar]
- Zhu Y. L. & Wang D. F. Rapid Detection of Enterobacter Sakazakii in milk Powder using amino modified chitosan immunomagnetic beads. Int J Biol Macromol 93, 615–622 (2016). [DOI] [PubMed] [Google Scholar]
- Keranen A., Leiviska T., Hormi O. & Tanskanen J. Removal of nitrate by modified pine sawdust: Effects of temperature and co-existing anions. J Environ Manage 147, 46–54 (2015). [DOI] [PubMed] [Google Scholar]
- Song Y. et al. Surface-Initiated ARGET ATRP of Poly(Glycidyl Methacrylate) from Carbon Nanotubes via Bioinspired Catechol Chemistry for Efficient Adsorption of Uranium Ions. Acs Macro Lett 5, 382–386 (2016). [DOI] [PubMed] [Google Scholar]
- Shan W. J. et al. Equilibrium, Kinetics, and Thermodynamics Studies on the Recovery of Rhenium(VII) and Molybdenum(VI) from Industrial Wastewater by Chemically Modified Waste Paper Gel. J Chem Eng Data 57, 290–297 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.